Abstract
T cell activation is tightly regulated to avoid autoimmunity. Gene related to anergy in lymphocytes (GRAIL, encoded by Rnf128) is an E3 ubiquitin ligase associated with T cell tolerance. Here we generated and analyzed GRAIL-deficient mice and found they were resistant to immune tolerance induction and exhibited greater susceptibility to autoimmune diseases than wild-type mice. GRAIL-deficient naïve T cells, after activation, exhibited increased proliferation and cytokine expression than controls and did not depend on costimulation for effector generation. Moreover, GRAIL-deficient regulatory T (Treg) cells displayed reduced suppressive function, associated with increased Th17 cell-related gene expression. GRAIL-deficient naïve and Treg cells were less efficient in downregulating T cell receptor (TCR)-CD3 expression after activation, and exhibited increased NFATc1 transcription factor expression; GRAIL expression promoted CD3 ubiquitinylation. Our results indicate that GRAIL, by mediating TCR-CD3 degradation, regulates naïve T cell tolerance induction and Treg cell function.
Introduction
T cell activation is tightly regulated to ensure effective elimination of invading pathogens as well as maintaining tolerance against self-tissues. T cells are regulated by extracellular signals, especially the positive and negative costimulatory molecules on antigen presenting cells (APCs), and also by delicate intracellular signal transducers and regulators. E3 ubiquitin ligases, including cbl-b and Itch, have been shown to play important roles in regulation of T cell tolerance (Heissmeyer and Rao, 2004; Liu et al., 2005). GRAIL is a type I transmembrane protein localized to endosomal compartment with homology to RING finger proteins whose expression was previously associated with T cell anergy induction (Anandasabapathy et al., 2003; Heissmeyer et al., 2004; Seroogy et al., 2004). The GRAIL mRNA was initially determined to be induced in anergic T helper 1 (Th1) cells (Anandasabapathy et al., 2003). Previously, we reported that T cells activated in the absence of both CD28 and ICOS costimulation developed into tolerant T cells, associated with markedly upregulated GRAIL expression (Nurieva et al., 2006). Consistent with the notion that GRAIL regulates T cell anergy, overexpression of GRAIL in T cell hybridomas or in primary cells reduced T cell cytokine expression (Anandasabapathy et al., 2003). Moreover, expression of an enzymatic inactive form of GRAIL in primary T cells prevented T cell anergy (Seroogy et al., 2004). In addition to anergic CD4+ T cells, enhanced amount of GRAIL was detected in regulatory T (Treg) cells and over-expression of GRAIL in Ova-specific CD4+ T cell line was reported to convert these cells to a regulatory phenotype in the absence of detectable Foxp3 expression (MacKenzie et al., 2007). Despite the above interesting preliminary data on GRAIL expression and function, the physiological function of GRAIL in immune regulation is not well understood, in part due to lack of genetic studies.
In the current study, we generated and analyzed mice deficient in Rnf128 (encoding GRAIL). GRAIL-deficient mice exhibited impairments in peripheral tolerance induction and greater susceptibility to autoimmune diseases. Naïve T cells lacking GRAIL showed greatly enhanced proliferation and cytokine production after T cell receptor (TCR) activation and did not depend on CD28 and ICOS for their effector cytokine expression. We also found that lack of GRAIL abrogated suppressive function of regulatory T (Treg) cells in an interleukin-21 (IL-21)-dependent manner. Both naïve and Treg cells from GRAIL-deficient mice were less efficient in down-regulation of their TCR-CD3 expression and exhibited increased NFATc1 transcription factor expression after TCR activation. Moreover, GRAIL promotes CD3 ubiquitinylation. Our results thus indicate GRAIL as an essential regulator of T cell tolerance by regulating naïve T cell tolerance and Treg cell function.
Results
Generation of GRAIL-deficient mice
In order to understand the physiological function of GRAIL, we generated mice deficient in Rnf128 by replacing part of exon 4 and all of exons 5 and 6 with the neomycin-resistant gene, which removes amino acids 283–385 encompassing most of the RING domain (amino acids 277–317) (Supplementary Figure 1A). Rnf128 gene targeting was confirmed by Southern blot and PCR analysis of genomic DNA from several embryonic stem cell clones (Supplementary Figure 1B–C). The targeted ES cells were used to generate GRAIL-deficient mice. RT-PCR analysis of Rnf128 expression in various tissues revealed that the appropriate regions of Rnf128 were deleted (Supplementary Figure 1D).
GRAIL-deficient male and female mice were viable and fertile, and grossly normal. Analysis of spleen and thymus of 6–8 week old mice indicated a normal ratio of CD4+ and CD8+ T cells (Supplementary Figure 1E). In addition, CD4+ and CD8+ T cells from spleen of GRAIL-deficient mice at this age did not show altered expression of activation markers CD69, CD44 and CD25 and naïve T cells marker CD62L (Supplementary Figure 1G). Furthermore, GRAIL-deficient mice displayed the same percentages of Treg cells in spleen and thymus as wild-type mice (Supplementary Figure 1F). Thus, T cells in young GRAIL-deficient mice appear to develop normally.
GRAIL is required in immune tolerance induction in vivo
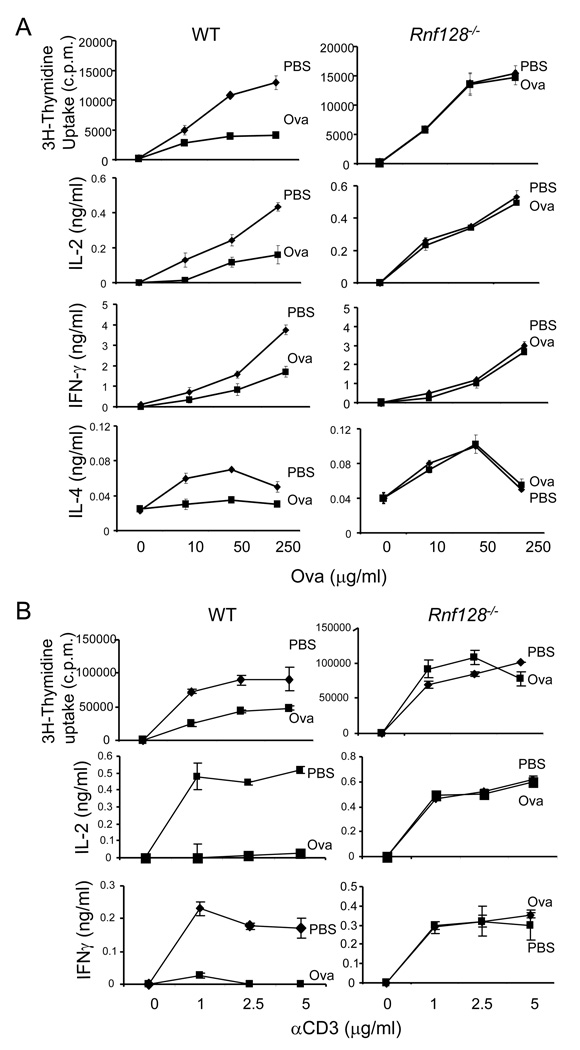
Next we examined the role of GRAIL in oral tolerance, a form of peripheral tolerance. Wild-type (WT) and Rnf128−/− mice were given daily doses of 2 mg of ovalbulim (Ova) protein intragastrically for a total 5 times, after subcutaneous immunization with Ova protein emulsified in complete Freud’s adjuvant (CFA). 7 days after immunization, splenocytes were restimulated with different concentrations of Ova protein, and proliferation and cytokine production were examined. Whereas WT T cells from the Ova-fed group exhibited markedly reduced proliferation and IL-2, IFN-γ and IL-4 production upon Ova protein restimulation, proliferation and cytokine production by GRAIL-deficient T cells from Ova- and PBS-fed mice were indistinguishable (Figure 1A).
Figure 1. GRAIL is required for T cell tolerance induction in vivo.
(A) WT and Rnf128−/− mice were fed five times with OVA or PBS. Seven days after the last feeding, all mice were immunized with OVA in CFA. Seven days later, mice were sacrificed and analyzed. Spleen cells from these mice were stimulated with the indicated concentration of Ova. Proliferation was assayed after 3 days of treatment by adding [3H]-thymidine to the culture for the last 8 h. IL-2 was measured 1 day later, and effector cytokines (IFN-γ and IL-4) were measured after 4 days of treatment. Each experimental group consisted of three mice. The graph shows means ± standard deviation (SD). Data are a representative of two individual experiments. (B) WT and Rnf128−/− OT-II TcR transgenic mice (3 mice per group) were injected twice with 500 µg of soluble Ova peptide to induce T cell tolerance or with PBS as control. 7 days after the second administration of the peptide, flow cytometry-sorted Vα2+CD44+CD4+ T cells were isolated from spleen and restimulated with different concentrations of plate-bound anti-CD3. Proliferation, IL-2 production and secretion of IFN-γ were assessed as in (A). The graph shows means ± standard deviation (SD). Data are a representative of two individual experiments with consistent results.
To further ascertain the role of GRAIL in induction of CD4+ T cell tolerance, Rnf128−/− mice were bred with OT-II TCR transgenic mice and both WT and Rnf128−/− OT-II TCR transgenic mice were injected twice with a high dose of soluble Ova peptide to induce T cell tolerance or with PBS as control. 7 days after the second administration of the peptide, clonotypic CD4+ T cells were isolated from spleen and restimulated with different concentrations of plate-bound anti-CD3. Although wild-type T cells from Ova-sensitized mice showed decreased proliferation, accompanied with markedly reduced IL-2 and IFN-γ production, Rnf128−/− T cells from Ova- or PBS-treated mice were identical in their responses (Figure 1B). These data indicated that GRAIL controls the induction of antigen-specific CD4+ T cell tolerance.
Rnf128−/− mice exhibit increased susceptibility to autoimmune diseases
Because GRAIL plays a critical role in regulating T cell tolerance in experimental models, we further investigated if deficiency of GRAIL leads to spontaneous autoimmunity in aged mice. Rnf128−/− mice on C57BL/6 × 129 background at age of 2, 18–20, 26–28 months, together with age- and sex-matched wild-type mice (Figure 2A–B), and Rnf128−/− mice on C57BL/6 background at age 18-12 months (Figure 2C–D) were examined. Rnf128−/− and WT mice had similar sizes of spleens and mesenteric lymph nodes at 2 months of age. However, at 18–20 or 26–28 months of age, 27 to 50 percent, respectively, of Rnf128−/− mice exhibited splenomegaly and 27 to 75 percent, respectively, increased sizes of mesenteric lymph nodes (Figure 2A), but not other lymph nodes (data not shown). Histological analysis showed massive infiltration of lymphocytes in the lungs of all GRAIL-deficient mice at 18–20 months of age (Supplementary Figure 2A and B). Moreover, kidneys in these mice were infiltrated by mononuclear cells as well, although variable in severity but consistently present in all GRAIL-deficient mice (Supplementary Figure 2D and E). In addition, minor infiltration could be detected in the liver of Rnf128−/− mice (Supplementary Figure 2G and H), but other organs examined were free of infiltration. In wild-type littermates, no infiltration could be detected in any organ. In addition, Rnf128−/− mice developed higher titers of dsDNA antibodies in their sera starting at 18–20 months when compared with wild-type mice (Figure 2A and C). Splenic CD4+ T cells from aged Rnf128−/− mice also showed significantly enhanced levels of pro-inflammatory cytokine IFN-γ and IL-17 expression (Figure 2B and D, Supplementary Figure 2J and K).
Figure 2. GRAIL deficiency leads to autoimmune symptoms.
(A) Percentages of WT and Rnf128−/− (−/−) mice on C57BL/6 × 129 background at 2 [n=8 (WT), n=8 (−/−)], 18–20 [n=7 (WT), n=10 (−/−)] or 26–28 [(n=6 (WT), n=4 (−/−)] months of age with enlarged sizes of spleen and mLN. (A and C) Sera were collected from WT and Rnf128−/− (−/−) mice on C57BL/6 × 129 background (A) or from 18–20 month old WT and Rnf128−/− mice on C57BL/6 background [n=5 (WT), n=5 (−/−)] and anti-dsDNA IgG was measured by ELISA. The graph shows means ± SD. p values were calculated with the t test by comparison of the concentration of ds-DNA in sera WT and Rnf128−/− mice. (B and D) Splenocytes from 18–20 month old WT or Rnf128−/− mice on 129×B6 background (B) and on B6 background (D) were assessed for IL-17 and IFN-γ using intracellular cytokine staining. p values were calculated with the t test by comparison of the percentage of CD4+IFN-γ+ or CD4+IL-17+ T cells between WT and Rnf128−/− mice.
The above results suggest a critical role of GRAIL in preventing lymphoproliferative and autoimmune responses. To further examine the role of GRAIL in autoimmune diseases, we immunized female Rnf128+/+, Rnf128+/−, Rnf128−/− mice on C57BL/6 × 129 mixed background with MOG peptide emulsified with CFA to induce experimental autoimmune encephalomyelitis (EAE). WT mice developed very mild disease, due to their partial 129 genetic background (Figure 3A). In contrast, Rnf128−/− mice showed markedly higher scores of EAE. Interestingly, Rnf128+/− mice with mixture of T cells containing WT or Rnf128−/− allele of GRAIL due to X-chromosome inactivation also developed marked symptoms of EAE. Consistent with development of EAE symptoms, Rnf128−/− mice accumulated more CD4+ T cells in the central nervous system (CNS) (Figure 3B). We further analyzed the cytokine production profiles by CD4+ cells infiltrating into the CNS and in spleen. CNS-infiltrating and splenic CD4+ T cells from Rnf128+/− and Rnf128−/− mice produced increased amounts of IL-17 and IFN-γ (Figure 3B, Supplementary Figure 3A). In addition, splenic cells from Rnf128−/− mice exhibited increased proliferation in response to MOG restimulation (Supplementary Figure 3A). Our results indicate a critical role of GRAIL in controlling autoimmune responses by negatively regulating autoreactive T cell proliferation and IFN-γ and IL-17 expression.
Figure 3. Rnf128−/− mice exhibit exacerbated EAE.
(A and B) WT, Rnf128+/−, Rnf128−/− mice were immunized with MOG peptide to induce EAE. The result (means ± SD) shown is a representative of two independent experiments with similar results [n=8 (WT), n=7 (+/−), n=11 (−/−)]. (C and D) CD4+ T cells from WT and Rnf128−/− mice were i.v. transferred into Rag1−/− mice. The recipient mice were induced EAE and disease incidence and score were measured daily and means ± SD of all mice in each group were shown [n=11 (WT), n=11 (−/−)]. Data represent two independent experiments with consistent results. (B and D) Mononuclear cells isolated from spinal cords and brains of experimental mice were stained with anti-CD4 and CD11b, and analyzed by FACS. Mononuclear cells from CNS were stimulated for 5 hours with PMA and ionomycine and spleenocytes were restimulated with MOG peptide for 24 hours, followed by intracellular staining of IL-17 and IFNγ, and analyzed in CD4+ gate. Numbers in dot plot quadrants represent the percentages.
To determine whether expression of GRAIL in T cells is critical for controlling EAE, we adoptively transfer CD4+ T cells from WT or Rnf128−/− mice on B6 background into Rag1−/− mice. Upon immunization with MOG, recipient mice containing Rnf128−/− T cells developed EAE 6 days earlier compared to recipients of WT cells (Figure 3C). Moreover, the clinical scores of EAE were higher in mice with Rnf128−/− T cells than in those with WT cells. In addition, IL-17 and IFN-γ expression by CNS and spleen cells was higher in Rnf128−/− than WT CD4+ T cell populations (Figure 3D, Supplementary Figure 3B). Further examination of MOG-reactive T cells revealed the enhanced proliferation by Rnf128−/− T cells when compared to WT cells, suggesting an intrinsic role of GRAIL in CD4+ T cells in controlling autoimmune responses.
Rnf128−/− T cells are hyper-responsive to TCR stimulation
To understand GRAIL function in T cells, we first re-examined Rnf128 expression. Flow cytometry-sorted CD62LhiCD44loCD25− naïve CD4+ T cells were activated with plate-bound anti-CD3 or anti-CD3 and anti-CD28 for 1, 2 or 3 days and GRAIL mRNA expression was analyzed by real-time RT-PCR. GRAIL mRNA expression was detected on day 0, which was upregulated on day 2 after anti-CD3 stimulation and further increased on day 3, whereas costimulation with anti-CD28 potentiated GRAIL expression on day 2, which was downregulated on day 3 (Supplementary Figure 4A). Because T cells activated under these conditions were not tolerant, our results suggest that GRAIL may be involved in normal naïve T cell activation.
To further determine the role of GRAIL during T cell activation, flow cytometry-sorted naïve CD4+ T cells from Rnf128+/+ and Rnf128−/− mice on B6 background were activated with plate-bound anti-CD3 alone or together with anti-CD28. Rnf128−/− CD4+ T cells exhibited greatly enhanced proliferation and cytokine (IL-2 and IFN-γ) production when compared to WT cells in response to anti-CD3 stimulation (Figure 4A). Addition of anti-CD28 enhanced T cell proliferation and cytokine production in wild-type and Rnf128−/− T cells. Thus, these data indicate that GRAIL negatively regulates TCR signaling strength. We also use Rnf128−/− mice bred with OT-II TCR transgenic mice. Consistent with the above results, Rnf128−/− OT-II cells exhibited greater proliferation compared with wild-type OT-II cells when activated with OVA peptide and WT APCs (Figure 4B). More significant differences in IL-2 production between WT and Rnf128−/− OT-II cells were observed in the presence of low concentrations of Ova peptide compared to higher concentrations. Next, we assessed if Rnf128−/− CD4+ T cells can be tolerized when activated in the absence of costimulatory signals. Naïve CD4+ T cells from OT-II TCR transgenic mice or Rnf128−/− OT-II mice were activated in the presence of OT-II peptide and irradiated APCs from WT mice or those deficient in B7.1, B7.2 and B7h (TKO). As we reported before (Nurieva et al., 2006), naïve wild-type OT-II cells when activated with TKO APCs were impaired in proliferation and IL-2 production, and exhibited absence of effector cytokine expression in response to anti-CD3 restimulation (Figure 4B). In contrast, Rnf128−/− OT-II T cells activated with TKO APCs expressed these cytokines. Thus, the deficiency in GRAIL leads to T cell hyper-activation and their independency on costimulation for activation.
Figure 4. Rnf128−/− T cells are hyper-responsive to TCR signaling.
(A) Flow cytometry-sorted naïve CD4+ T cells were activated with plate-bound anti-CD3 alone or together with anti-CD28. Proliferation was assayed after 3 days of treatment by adding [3H]-thymidine to the culture for the last 8 h. IL-2 was measured 1 day later, and IFN-γ were measured after 4 days of treatment. The graph shows means ± standard deviation (SD). The results shown are a representative of at least two independent experiments. (B) Flow cytometry-sorted naïve wild-type or Rnf128−/− OT-II cells were activated with Ova peptide and irradiated APCs from WT or B7.1/B7.2/B7h KO (TKO) mice for 5 days. IL-2 production was determined 24 h after T cell activation by ELISA. Proliferation was assayed 3 days after treatment by adding [3H]-thymidine to the culture for the last 8 hours. 5 days later, differentiated T cells were restimulated with anti-CD3 for 24 hours, and effector cytokine production was measured by ELISA. The graph shows means ± standard deviation (SD). P values were calculated with the t test by comparing the IL-2 production by WT and Rnf128−/− CD4+ T cells activated with Ova peptide and WT APCs and are indicated as followed: asterisk, P < 0.01; two asterisks, P < 0.001. The results shown are a representative of at least three independent experiments. (C) Flow cytometry-sorted naïve CD4+ T cells from WT and Rnf128−/− mice were polarized under Th1, Th2 and Th17 conditions. 5 days later, effector cytokine production was analyzed by intracellular staining and ELISA. The graph shows means ± standard deviation (SD). The results shown are a representative of at least two independent experiments.
To further characterize the role of GRAIL in T cell effector function, naïve CD4+ T cells from WT and Rnf128−/− mice were differentiated in vitro under Th1, Th2 or Th17 cell conditions for 5 days and analyzed for their cytokine production by intracellular cytokine staining and ELISA. IFN-γ and IL-17 expression by Rnf128−/− Th cells activated under Th1 and Th17 cell condition, respectively, was markedly enhanced compared to wild-type cells (Figure 4C). However, under Th2 cell polarizing conditions, Rnf128−/− T cells exhibited decreased percentages of IL-4-producing cells compared to WT cells, whereas IL-5 production appeared to be normal (Figure 4C). Thus, GRAIL may negatively regulate IFN-γ and IL-17 expression.
To further analyze T cell responses in vivo, Rnf128−/− and their wild-type littermate mice on C57BL/6 × 129 mixed background were immunized with Ova protein emulsified in CFA. 7 days after immunization, splenocytes were restimulated with Ova peptide, and cytokine production was examined. T cells from Rnf128−/− mice exhibited enhanced proliferation, IL-2 secretion, and production of Th1 (IFN-γ) and Th17 (IL-17, IL-21 and IL-22) cell cytokines; expression of Th2 cell cytokines (IL-4, IL-5 and IL-13) were decreased in Rnf128−/− mice, which might have been caused by increased IFN-γ expression in vivo (Supplementary Figure 4B). These results indicate that GRAIL negatively regulates T cell activation and Th1-Th17 effector function in vitro and in vivo.
We also investigated the role of GRAIL in an asthma model induced by an exogenous antigen. Wild-type or Rnf128−/− mice were intraperitoneally sensitized twice with Ova protein in alum with 2-week intervals. Ten days later, mice received daily intranasal Ova protein for three days. There was no significant difference in total BALF cell numbers between WT and Rnf128−/− mice (Supplementary Figure 4C). Ova-specific IgG1 was very moderately enhanced in sera from Rnf128−/− mice as compared with controls. Anti-Ova IgG production was the same between WT and mutant groups. Interestingly, anti-Ova IgM was substantially elevated in Rnf128−/− mice (Supplementary Figure 4D). When we examined cytokine expression by splenocytes after ex vivo OVA restimulation, Rnf128−/− cells exhibited enhanced proliferation and all effector cytokine production by CD4+ T cells (Supplementary Figure 4E). GRAIL thus also negatively regulates T cell activation and cytokine expression in this asthma model.
GRAIL regulates TCR and CD3 expression
From above, the deficiency in GRAIL leads to T cell hyper-activation and their independency on costimulation for activation. Because other E3 ubiquitin ligases have been implicated in degrading signaling components downstream of TCR (Heissmeyer and Rao, 2004; Liu et al., 2005), we compared the expression of these molecules in WT and Rnf128−/− T cells. First, we activated WT and Rnf128−/− OT-II T cells with Ova peptide and WT APCs for different time points and analyzed changes in the TCRβ surface expression by flow cytometry. Ova peptide stimulation led to TCR down-modulation on wild-type T cells, whereas TCR down-modulation was markedly attenuated in the Rnf128−/− cells (Figure 5A). After 24 hr of stimulation, WT cells lost approximately 63.4 percents of their TCRβ surface expression. In contrast, Rnf128−/− CD4+ T cells retained about 65 percent of the TCR levels. Thus, GRAIL-deficient T cells are more resistant to activation-induced TCR down-modulation than wild-type cells. To determine whether GRAIL over-expression is sufficient to enhance TCRβ down-regulation, TCRβ expression was assayed after TCR engagement in CD4+ T cells retrovirally transduced to express WT GRAIL or a GRAIL mutant (lacking functional RING finger domain). In T cells expressing WT GRAIL, we observed markedly enhanced TCRβ down-regulation in response to anti-CD3 treatment when compared to T cells transduced with an empty vector (Supplementary Figure 5A). In contrast, over-expression of the GRAIL mutant delayed TCRβ down-regulation, suggesting an important role of GRAIL E3 ligase activity in regulation of cell-surface TCRβ expression.
Figure 5. GRAIL negatively regulates TCR/CD3 expression.
(A and C) Splenic T cells from wild-type and Rnf128−/− OT-II mice were stimulated with OT-II peptide in the absence (A) or presence of MG-132 (C), and the expression of TCRβ was determined by flow cytometry. Percentage of CD4+TCRβ+ cells is shown. The results shown are an average of three independent experiments. (B) Naïve CD4+ T cells from WT or Rnf128−/− mice were activated with plate-bond anti-CD3 for 24 hrs. Total cell lysates from naïve or activated CD4+ T cells were probed with antibodies to TCRβ, TCRζ, Lck, Zap-70, PKCθ and LyGDI. β-actin was used as loading control. Relative Western blotting signals were inducted. The results shown are a representative of at least three independent experiments. (D) 293T cells were transfected with vectors encoding CD3ζ, HA-Ub, and either GRAIL or a GRAIL mutant (GRAIL-m). The lysates were subject to immunoprecipitation (i.p.) using an anti-CD3ζ antibody. The blot was probed with anti-HA-HRP, and re-probed with anti-CD3ζ-HRP. (E) CD4+ T cells from WT and Rnf128−/− mice were untreated or activated with anti-CD3 for 6 hours. MG-132 was added to all samples for 6 hours. The cell lysates were ip using anti-CD3ζ antibodies. The blot was probed with anti-Ub antibodies, and re-probed with anti-CD3ζ-HRP. The results shown are a representative of at least three independent experiments. Relative Western blotting signals were calculated as a ratio of ubiquitinated and non-ubiquitinated CD3ζ, and shown as average of thee experiments.
In addition to surface TCR expression, we also compared the protein expression of TCR signaling components in naïve WT and Rnf128−/− CD4+ T cells or after they were activated for 24 hr with plate-bound anti-CD3. Consistent with increased TCR surface expression, TCRβ and CD3ζ protein expression was also enhanced in activated Rnf128−/− T cells (Figure 5B). In contrast, expression of PKCθ was not markedly altered and that of Lck, Zap-70 (both of which are TCR signaling components) and LyGDI (a potential substrate of GRAIL) (Su et al., 2006), was reduced, the basis of which is unknown at this stage. Thus, these results indicate that deficiency of GRAIL results in selective upregulation of TCR protein expression. To determine whether GRAIL regulates the expression of TCR through proteosome-mediated degradation, OT-II T cells from WT or Rnf128−/− mice were activated with Ova peptide and WT APC in the presence of proteosome inhibitor MG-132 or lysosome inhibitor chloroquine. Addition of MG-132 led to complete inhibition of TCR down-modulation in both of WT and Rnf128−/− T cells (Figure 5C), whereas chloroquine had no significant effect (data not shown). Thus, GRAIL may target endocytosed TCR-CD3 complex via proteosome-mediated degradation.
Because TCRα and β chains do not have extensive cytoplasmic regions, we hypothesize that GRAIL may regulate the ubiquitinylation of CD3 molecules. CD3ζ together with WT or GRAIL mutant were co-expressed in 293T cells along with HA-tagged ubiquitin. After immunoprecipitation of CD3ζ and probing with HA-HRP antibodies, we observed the formation of high molecular weight ubiquitin conjugates in the present of WT GRAIL, indicating polyubiquitin chain formation (Figure 5D). Ubiquitinylation effect was greatly diminished in the presence of GRAIL mutant. Thus, CD3ζ is a potential substrate for GRAIL-mediated ubiquitination. The CD3ζ intracellular domain contains 8 highly conserved lysine residues and mutagenesis of each revealed that lysine 116 (K116) and possibly K118 might be necessary for GRAIL-mediated ubiquitination of CD3ζ (Supplementary Figure 5B). To investigate further, we analyzed the ubiquitinylation of CD3ζ in CD4+ T cells from WT and Rnf128−/− mice, 6 hours after activation in the presence of MG132 to prevent degradation, T cell lysates were subject to immunoprecipitation with CD3ζ antibodies and immunoblotting with Ub antibodies. In comparison to WT cells, ubiquitination of CD3ζ in Rnf128−/− T cells was substantially reduced (Figure 5E). Thus, GRAIL down-modulates the expression of TCR-CD3 complex through ubiquitin-dependent proteosome degradation pathway.
Impaired suppressive function in Rnf128−/− Treg cells
We also assessed the expression and function of GRAIL in Treg cell generation and function. Consistent with a recent report (MacKenzie et al., 2007), we found that GRAIL mRNA expression was up-regulated in naturally occurring as well as TGF-β-induced Treg cells (data not shown). Because GRAIL is expressed in TGFβ-induced Foxp3-expressing Treg (iTreg) cells, we examined the role of GRAIL in the generation of this cell subset. Naïve CD4+ T cells from WT and Rnf128−/− mice were activated with plate-bound anti-CD3 and anti-CD28 in the presence of exogenous TGF-β. GRAIL deficiency did not affect the generation of iTreg or natural Treg (nTreg) cells, (Supplementary Figure 6A). We then tested the function of GRAIL in Treg-mediated suppression of proliferation. In contrast to cbl-b-deficient T cells (Wohlfert et al., 2004; Wohlfert et al., 2006), naïve Rnf128−/− CD4+ T cells could be suppressed by WT or Rnf128−/− nTreg cells (Supplementary Figure 6B). However, Rnf128−/− CD4+CD25+ nTreg cells exhibited reduced suppression of WT naïve CD4+ T cells when compared with WT Treg cells (Supplementary Figure 6B).
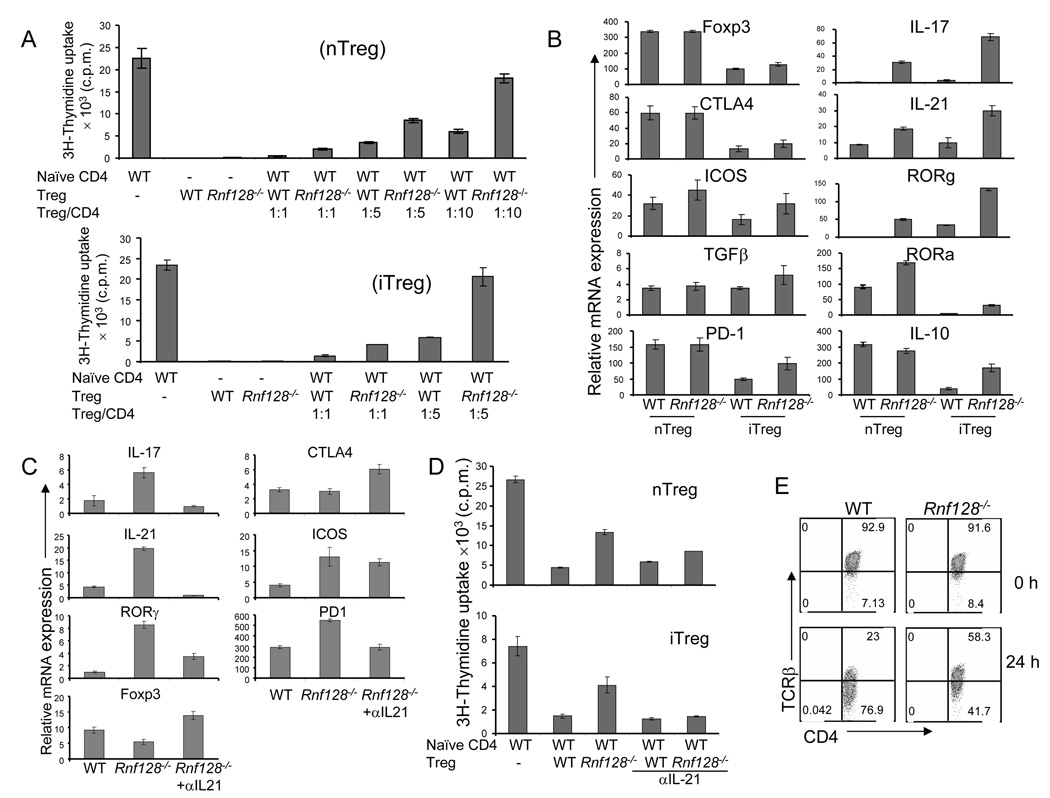
To avoid possible contamination of effector or memory cells in the CD25+ T cell population, we crossed Rnf128−/− mice to Foxp3-GFP reporter mice (Fontenot et al., 2005). Similar to the above results, CD4+ Foxp3-GFP+ T cells from Rnf128−/− mice exhibited reduced suppression activity compared to WT Treg cells (Figure 6A). Furthermore, using naïve T cells from these animals for induction and purification of iTreg cells, Rnf128−/− iTreg also had reduced ability to inhibit the proliferation of naïve CD4+ T cells (Figure 6A). Thus, GRAIL is not required for generation of natural or inducible Treg cells, but is required for their suppression functions.
Figure 6. GRAIL is required for suppressive function of regulatory T cells.
(A) Naïve WT CD4+ T cells were cultured with or without flow cytometry-sorted WT or Rnf128−/− Foxp3-GFP+CD4+ nTreg cells or iTreg in triplicate wells with plate-bound anti-CD3 and irradiated WT APC. Proliferation was assayed 72 h after treatment by adding [3H]thymidine to the culture for the last 8 h. (B) mRNA expression of various genes in FACS-sorted Foxp3-GFP+CD4+ nTreg and iTreg cells from WT or Rnf128−/− KO mice was analyzed by real-time RT-PCR after restimulation with anti-CD3 for 4 hrs. The graph shows means ± standard deviation (SD). The results shown are representative of at least two independent experiments. (C) mRNA expression of various genes in flow cytometry-sorted WT Foxp3-GFP+CD4+ iTreg and Rnf128−/− Foxp3-GFP+CD4+ iTreg generated in the presence or absence of an anti-IL-21 blocking antibody was analyzed by real-time RT-PCR after restimulation with anti-CD3 for 4 hrs. The graph shows means ± standard deviation (SD). The results shown are a representative of at least two independent experiments. (D) Naïve WT CD4+ T cells were cultured with or without flow cytometry-sorted WT or Rnf128−/− Foxp3-GFP+CD4+ nTreg cells or iTreg in triplicate wells with plate-bound anti-CD3, irradiated WT APC and in the presence or absence of blocking antibodies to IL-21. Proliferation was assayed 72 h after treatment by adding [3H]thymidine to the culture for the last 8 h. (E) Splenic T cells from wild-type and Rnf128−/− were stimulated with anti-CD3 for 24 hrs and the expression of TCRβ on gated CD4+Foxp3+ T cells was determined by flow cytometry. Numbers in dot plot quadrants represent the percentages. The results shown are a representative of at least two independent experiments.
To further examine why Rnf128-/- nTreg and iTreg cells were impaired in their suppressive function, we compared the expression of genes between WT and Rnf128−/− Treg cells after activation with anti-CD3 for 4 hours by real-time RT–PCR. Rnf128−/− Treg cells did not show any defect in Foxp3, CTLA4, ICOS, IL-10, PD-1 and TGF-β expression (Figure 6B). In contrast, expression of Th17 cell-specific genes, such as IL-17, IL-21, RORα and ROR-γ, were markedly augmented in Rnf128−/− Treg cells compared to WT Treg cells.
Because Rnf128−/− iTreg and nTreg cells expressed more IL-21 than WT cells and IL-21 regulates Th17 cell-related gene expression in naïve and Treg cells (Nurieva et al., 2007a; Yang et al., 2008), we examined whether this cytokine accounts for IL-17 expression by Rnf128−/− Treg cells and their diminished suppression activity. Addition of an IL-21 blocking antibody during the generation of iTreg cells suppressed the expression of Th17 cell-specific genes, such as those encoding IL-17, IL-21 and RORγ in Rnf128−/− cells (Figure 6C). In addition, blockade of IL-21 signaling in suppression assays using Rnf128−/− nTreg and iTreg cells restored their function to the levels of WT Treg cells (Figure 6D). These results indicate that GRAIL is necessary for the function of Treg cells by suppressing the Th17 cell-related genes.
We further assessed whether impaired suppressive function of Rnf128−/− Treg cells is associated with their inefficiency in TCR down-modulation. Anti-CD3 stimulation led to TCR down-modulation on WT Treg cells, whereas TCR down-modulation was markedly attenuated in the Rnf128−/− Treg cells (Figure 6E). Thus, GRAIL regulates the function of Treg cells possible through mediating downregulation of TCR-CD3 complex.
GRAIL deficiency causes enhanced NFATc1 expression in naïve T and Treg cells
Our above results indicate that a defect in Rnf128−/− Treg cell activity was functionally associated with increased IL-21 expression. IL-21 has been shown to be regulated by transcription factor NFATc1 (Kim et al., 2005). To further address the mechanism whereby GRAIL controls IL-21 expression, Foxp3-GFP+ nTreg cells from WT and Rnf128−/− mice were activated with plate-bound anti-CD3 for 6 hours. Real-time RT-PCR analysis revealed that whereas WT cells upregulated NFATc1 mRNA expression, there was a marked enhancement of its expression in Grail-deficient Treg cells after but not before activation (Figure 7A). In contrast, expression of other transcription factors downstream of TCR signaling such as JunB, c-Jun, c-Fos and c-Rel in Rnf128−/− Treg cells was similar as that in WT cells (Figure 7A). Similar to Rnf128−/− Treg cells, WT Treg cells treated with MG-132 and Latrunculin B, an endocytosis inhibitor that prevents TCR downregulation in naïve and Treg cells after TCR engagement (data not shown), also expressed increased amounts of NFATc1 as well as IL-21, IL-17 and RORγt mRNA (Supplementary Figure 7A and B), suggesting that GRAIL-mediated proteosome-dependent TCR-CD3 degradation prevents NFATc1 overexpression. NFATc1 autoregulates its own expression by binding to the P1 promoter and costimulation greatly enhances this autoregulatory circuit (Chuvpilo et al., 2002; Nurieva et al., 2007b). To examine whether NFATc1 autoregulation is required for expression of IL-17 and IL-21 in Rnf128−/− Treg cells, WT and Rnf128−/− Treg cells were treated with CsA. We found that blockade of NFAT activities by CsA markedly decreased the expression of NFATc1 and Th17-specific genes such as IL-21, IL-17 and RORγt (Figure 7B). Interestingly, blockade of IL-21 did not alter NFATc1 expression, but led to inhibition of IL-17 and RORγ expression (Figure 7B).
Figure 7. GRAIL negatively regulates NFATc1 expression in activated naïve and regulatory T cells.
(A and B) mRNA expression of indicated genes in flow cytometry-sorted Foxp3-GFP+CD4+ nTreg from WT and Rnf128−/− mice activated with anti-CD3 for 6 hours in the absence or presence of CsA, anti-IL-2 and anti-IL-21 antibodies were analyzed by real-time RT-PCR. The graph shows means ± standard deviation (SD). The results shown are a representative of at least two independent experiments. (C and D) mRNA expression of various genes in naïve CD4+ T cells from WT or Rnf128−/− mice activated with anti-CD3 for 24 hours in the absence or presence of CsA, anti-IL-2 and anti-IL-21 antibodies was analyzed by real-time RT-PCR. The graph shows means ± standard deviation (SD). The results shown are a representative of at least two independent experiments.
Analysis of naïve CD4+ T cells from WT and Rnf128−/− mice revealed enhanced NFATc1, IL-2, and IL-21 expression upon anti-CD3 activation for 24 hours in Rnf128−/− cells compared to WT cells (Figure 7C), whereas JunB, c-Jun, c-Fos and c-Rel expression was not affected. Similar to mRNA expression, NFATc1 total protein as well as translocation to the nucleus was markedly enhanced in Rnf128−/− naïve T and Treg cells upon TcR engagement (Supplementary Figure 7F). In addition, when we analyzed the activation of MAP kinase and NF-κB pathways in WT and Rnf128−/− T cells after anti-CD3 treatment, activation of p38 and Erk was not altered in WT and Rnf128−/− T cells after TCR ligation (Supplementary Figure 7G). Degradation of IkBα was not substantially changed in Rnf128−/− cells compare to WT cells at early time points after T cell activation (Supplementary Figure 7H). Thus, enhanced NFATc1 expression and nuclear translocation by Rnf128−/− cells is best associated with enhanced IL-2 and IL-21 production. Similar to Rnf128−/− T cells, we also observed enhanced expression of NFATc1, IL-2, and IL-21 in WT cells treated with MG-132 and Latrunculin B (Supplementary Figure 7C and D). Moreover, we activated naïve T cells from WT and Rnf128−/− mice in the presence of CsA (Figure 7D). Addition of CsA to Rnf128−/− T cells and to WT cells treated with MG-132 inhibited the expression of NFATc1, as well as IL-2 and IL-21 (Figure 7D, Supplementary Figure 7E). Blockade of IL-2 decreased IL-2 and IL-21 mRNA levels, but did not change NFATc1 expression. In addition, blockade of IL-21 impaired the expression of IL-21, but not NFATc1 (Figure 7D). Thus, these results indicate that in naïve T cells, GRAIL regulates T cell activation and cytokine production through negative regulation of NFATc1 expression.
Discussion
The molecular mechanisms underlying immune tolerance have not been well understood. Using Rnf128−/− mice, we found in the current study that GRAIL is required to properly downregulate TCR signaling in recently activated T cells, restrict their cytokine expression and maintain Treg cell function. Lack of GRAIL greatly exacerbates autoimmune diseases.
We found that GRAIL mRNA is up-regulated during normal T cell activation even under non-tolerant conditions. These observations suggest that GRAIL function might not be restricted to T cell anergy, which is supported by an independent study (Kriegel et al., 2009). Our analysis on Rnf128−/− mice showed that they are hyper-responsive to immunization with foreign as well as self antigens. Based on our data, we believe that GRAIL critically controls the thresholds of T cell activation, and as a consequence of GRAIL deficiency, immune responses to exogenous as well as endogenous stimuli are both increased. On one hand, this may have pathological implication in predisposing autoimmune responses. On the other hand, modulation of GRAIL expression and function may help boost immune responses to infection and cancer.
E3 ubiquitin ligases including cbl-b and Itch negatively regulate T cell responses by targeting multiple components of TCR signaling pathway for degradation. We found that hyper-activation of Rnf128−/− T cells are selectively associated with their inefficiency in TCR down-modulation. In contrast, expression of other TCR proximal signaling components, such as PKCθ, PLCγ1 were not increased in the absence of GRAIL. We further determined that TCR downregulation by GRAIL was dependent on the E3 ligase activity and mediated by proteosome-mediated degradation. These results suggest that GRAIL, located in the endosomal compartment, may target endocytosed TCR-CD3 complex via ubiquitination and proteasome-mediated degradation. In Rnf128−/− T cells, early activation of multiple pathways was not defective. Moreover, endocytosis inhibitor could cause similar defects in wild-type T cells seen in the Rnf128−/− cells. GRAIL thus may have more restricted specificity than cbl-b or Itch. Recently, Lineberry et al. proposed CD40L as another potential target for GRAIL (Lineberry et al., 2008). However, we did not detect an increase in CD40L expression in Rnf128−/− cells in comparison to WT cells (data not shown). Furthermore, purified Rnf128−/− T cells were hyper-responsive to TCR signaling, independent of APC, which is unlikely caused by a defective regulation on CD40L.
A previous study indicates the elevated expression of GRAIL in Treg cells (MacKenzie et al., 2007). We found that GRAIL was not necessary for development of Treg cells, but was required for their suppressive function. Unlike cbl-b-deficient T cells (Wohlfert et al., 2004; Wohlfert et al., 2006), naïve Rnf128−/− CD4+ T cells could be suppressed by WT Treg cells, suggesting that GRAIL deficiency might not affect TGF-β signaling. In addition, reduced suppressive function was associated with elevated expression of Th17 cell-specific genes such as those that encode IL-17, IL-21 and RORγ. IL-21 was sufficient in upregulating Th17 cell-specific genes in Treg cells. Inhibition of IL-21 restored suppressive activity and downregulated Th17 cell gene expression in Rnf128−/− Treg cells. Our studies for the first time indicate a critical role of GRAIL in control of IL-21 expression in Treg cells.
NFATc1 is strongly induced upon T cell activation and controls numerous genes that are involved in T cell effector function. Multiple isoforms of NFATc1 have been recently identified that are generated by the activity of two different promoters designated as P1 and P2 (Chuvpilo et al., 2002). The activity of the P1 but not the P2 promoter is highly induced after T cell activation and is regulated by costimulation (Nurieva et al., 2007b). The autoregulation of the P1 promoter by NFAT factors results in strongly increased NFATc1 protein expression. We found that Rnf128−/− naïve and Treg cells after TCR activation expressed substantially higher amounts of mRNA and protein of NFATc1 compared to WT cells, whereas the activation of other factors in AP-1 and NFκB pathways were normal. Our data also suggested that sustained TcR cell-surface expression in the absence of GRAIL led to selective NFATc1 expression in both naïve T cells and Treg cells, which was supported by our data using an endocytosis inhibitor. In contrast to naïve T cells, increased expression of NFATc1 in Rnf128−/− Treg cells was associated with just enhanced expression of Th17 cell genes. This selectivity may be controlled at the epigenetic level, which further supports a close relationship and plasticity between Treg and Th17 cells.
In summary, our results indicate a broad function of GRAIL in regulation of various aspects of T cell activation and function. GRAIL, whose expression is enhanced by TCR signaling, by targeting TCR-CD3 for degradation, restricts NFATc1 expression in recently activated naive and regulatory T cells. This helps the former to prevent their overactivation and maintain costimulation-dependent effector generation. In addition, GRAIL, through controlling NFATc1 expression, inhibits IL-21 production and upregulation of Th17-specific genes in Treg cells. The immune regulation by GRAIL in both naïve and Treg cells is absolutely critical as evidenced by the failure of T cell tolerance induction and greatly increased susceptibility to autoimmune diseases of Rnf128−/− mice.
Experimental Procedures
Mice
A Rnf128 gene-targeting vector was created by replacing part of exon 4 and all of exons 5 and 6 with the neomycin-resistant gene, and electroporated into mouse embryonic stem cells. The targeted clones were identified by Southern blot and PCR analysis and the appropriately targeted ES cells were injected C57BL/6 blastocysts. Mice on C57BL/6 × 129 F2 background and C57BL6 F6 were used for our analysis. OT-II TcR transgenic mice were purchased from Jackson Laboratories. Rnf128−/− mice were crossed with OT-II mice to get Grail-deficient OT-II mice. Rnf128−/− mice were crossed with Foxp3-GFP reporter mice. Mice were housed in the SPF animal facility at M. D. Anderson Cancer Center and the animal experiments were performed using protocols approved by Institutional Animal Care and Use Committee.
T cell function analysis
CD4+Foxp3-GFP−CD62LhiCD44lo cells were flow cytometry-sorted, and activate under Th1, Th2, Th17 and iTreg cell condition as described (Nurieva et al., 2007a; Yang et al., 2008). In Figure 6A, naïve WT or Rnf128−/− CD4+ T cells were cultured with or without WT or Rnf128−/− Foxp3-GFP+CD4+ nTreg or iTreg cells in triplicate wells with plate-bound anti-CD3 and irradiated WT APCs. Proliferation was assayed 72 h after treatment by adding [3H]thymidine to the culture for the last 8 h.
Retroviral transduction
Naïve CD4+CD25−CD62LhiCD44lo T cells from OT-II mice were flow cytometry-sorted and activated with Ova peptide and irradiated wild-type splenic APCs. 24 hours after activation, cells were infected by retroviruses expressing GRAIL, GRAIL mutant or control empty vector (containing only IRES-GFP). 4 days after infection, GFP+ cells were flow cytometry sorted and restimulated with anti-CD3 for 30 minutes, 1, 2, 4 hours after which CD4 and TCRβ-expressing cells were analyzed by flow cytometry.
Quantitative real-time PCR
Total RNA was prepared from T cells using TriZol reagent (Invitrogen). cDNA was synthesized using Superscript reverse transcriptase and oligo(dT) primers (Invitrogen) and gene expression was examined with a Bio-Rad iCycler Optical System using iQ™ SYBR green real-time PCR kit (Bio-Rad Laboratories, Inc.). The data were normalized to Actb reference. The primers for IL-17, IL-17F, IL-21, IL-22, IL-10, Foxp3, TGFβ, RORγ, Junb, NFATcP1, c-Rel, c-Fos, c-Jun and Actb were previously described (Evans and Fox, 2007; Lang et al., 2002; Nurieva et al., 2007a; Rangatia et al., 2003; Rao et al., 2003; Voice et al., 2004; Xia et al., 2001). The following primer pairs for GRAIL mRNA were used: forward: TAGCTGTGCTGTGTGCATTG, reverse: CTTCATGGGGAGAGGCAGTA.
Immunoblot analysis and ubiquitination assay
Nuclear fraction of CD4+ T cells was prepared as described (Nurieva et al., 2006). Whole T cell lysates were prepared by lysing cells in triton lysis buffer. The amounts of protein were determined by Bio-Rad protein assay to ensure equal protein loading for Western blot analysis with antibodies to TCRβ, CD3ζ, Lck, Zap-70, PKCθ, LyGDI, p38, ERK, NFATc1, β-actin, Lamin B (Santa Cruz Biotechnology), pp38 and pERK (Cell Signalling).
For ubiquitination assays, cells were lysed in kinase lysis buffer supplemented with 1 mM N-ethylmaleimide (NEM). CD3ζ or CD3ζ mutants was immunoprecipitated using CD3ζ-specific antibody (Santa Cruz Biotechnology), and the ubiquitin-conjugated CD3ζ was detected by IB using anti-ubiquitin antibody (Santa Cruz Biotechnology).
For the transfection model, 293T cells were transfected in 6-well plates with retroviral expression vector pIB II containing CD3ζ-CFP (kindly provided by Mark Davis, Stanford University), bicistronic retroviral vector pGFP-RV contains Grail or Grail mutant, and pcDNA-HA-ubiquitin. CD3ζ was isolated by IP using anti-CD3ζ followed by detecting ubiquitinated CD3ζ by IB using anti-HA-HRP (Santa Cruz Biotechnology).
Ova Immunization
WT and Rnf128−/− KO mice (6–8 wk old; three per group) were immunized with Ova protein (1.0 mg/ml) emulsified in CFA (0.5 mg/ml) at the base of the tail (100 µl each mouse). Seven days after immunization, these mice were sacrificed and analyzed individually. Spleen cells from Ova-immunized mice were stimulated in 96-well plates as triplicates with or without OT-II peptide. IL-2 production was determined 24 h after T cell activation by ELISA. Proliferation was assayed 3 days after treatment by adding [3H]-thymidine to the culture for the last 8 hours. Effector cytokines were analyzed 4 days later by ELISA (Pharmingen).
Oral tolerance induction
WT and Rnf128−/− mice were daily administrated intragastrically as previously described (Zhang et al., 2006). Control mice were given PBS alone. One week after the last treatment, all mice were immunized subcutaneously with 100 µg of Ova protein emulsified in CFA. Seven days immunization, these mice were sacrificed and spleen cells were stimulated in 96-well plates as triplicates with or without Ova protein. IL-2 production was determined 24 h after T cell activation by ELISA. Proliferation was assayed 3 days after treatment by adding [3H]-thymidine to the culture for the last 8 hours. Effector cytokines were analyzed 4 days later by ELISA (Pharmingen).
Peptide-induced tolerance
WT and Rnf128−/− OT-II TcR transgenic mice were injected intravenously with 500 µg OVA peptide in PBS or PBS alone on day 0 and day 3. On day 10, the mice were sacrificed and analyzed individually. FACS-sorted Vα2+CD44hiD4+ T cells from spleen were restimulated in vitro with plate-bound anti-CD3. IL-2 production was determined 24 h after T cell activation by ELISA. Proliferation was assayed 3 days after treatment by adding [3H]-thymidine to the culture for the last 8 hours. Effector cytokines were analyzed 4 days later by ELISA (Pharmingen).
EAE induction
For the induction of EAE, mice were immunized with the MOG peptide emulsified in CFA as previously described (Zhang et al., 2004). Signs of EAE were assigned scores on a scale of 1–4 as follows: 0, none; 1, limp tail or waddling gait with tail tonicity; 2, wobbly gait; 3, hind limb paralysis; 4, hind limb and forelimb paralysis.
For transfer EAE CD4+ T cells (5–10 × 106 cells) from WT and Rnf128−/− mice were injected in the tail vein of Rag1−/− recipient mice. 24 h after T cell transfer, Rag1−/− recipients were used for EAE induction and analysis as described above.
Anti-dsDNA measurement
dsDNA antibodies in their sera of 2, 18–20, 26–28 months old mice were measured by using mouse Anti-dsDNA Ig's (Total A+G+M) ELISA Kit (Alpha diagnostic International Inc.).
Highlights.
GRAIL inhibits T cell activation.
GRAIL targets TCR-CD3 for degradation.
GRAIL is required for Treg cell function.
GRAIL helps to control autoimmunity.
Supplementary Material
Acknowledgements
We thank MD Anderson Cancer Center Genetic Engineered Mouse Facility for their assistance in generation of GRAIL-deficient mice, Dr. Gagea-Iurascu for help in pathology analysis, and the Dong lab members for their help. The work is supported by research grants from NIH (to GL, CD and RIN) and Leukemia and Lymphoma Society (to CD). RIN was a recipient of a Scientist Development Grant from the American Heart Association, JMR is a recipient of a T32 training grant from the National Cancer Institute, GJM. was a Schissler Foundation Fellow in cancer research, YC is a recipient of postdoctoral fellowship program from the Korea Science and Engineering Foundation, and CD is a Leukemia and Lymphoma Society Scholar and a Trust Fellow of the MD Anderson Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, Seroogy C, Skrenta H, Hollenhorst M, Fathman CG, Soares L. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- Chuvpilo S, Jankevics E, Tyrsin D, Akimzhanov A, Moroz D, Jha MK, Schulze-Luehrmann J, Santner-Nanan B, Feoktistova E, Konig T, et al. Autoregulation of NFATc1/A expression facilitates effector T cells to escape from rapid apoptosis. Immunity. 2002;16:881–895. doi: 10.1016/s1074-7613(02)00329-1. [DOI] [PubMed] [Google Scholar]
- Evans KE, Fox SW. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC cell biology. 2007;8:4. doi: 10.1186/1471-2121-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, Gu H, Liu YC, Dustin ML, Rao A. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nature immunology. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- Heissmeyer V, Rao A. E3 ligases in T cell anergy--turning immune responses into tolerance. Sci STKE. 2004;2004:pe29. doi: 10.1126/stke.2412004pe29. [DOI] [PubMed] [Google Scholar]
- Kim HP, Korn LL, Gamero AM, Leonard WJ. Calcium-dependent activation of interleukin-21 gene expression in T cells. The Journal of biological chemistry. 2005;280:25291–25297. doi: 10.1074/jbc.M501459200. [DOI] [PubMed] [Google Scholar]
- Kriegel MA, Rathinam C, Flavell RA. E3 ubiquitin ligase GRAIL controls primary T cell activation and oral tolerance. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16770–16775. doi: 10.1073/pnas.0908957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R, Rutschman RL, Greaves DR, Murray PJ. Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J Immunol. 2002;168:3402–3411. doi: 10.4049/jimmunol.168.7.3402. [DOI] [PubMed] [Google Scholar]
- Lineberry NB, Su LL, Lin JT, Coffey GP, Seroogy CM, Fathman CG. Cutting edge: The transmembrane E3 ligase GRAIL ubiquitinates the costimulatory molecule CD40 ligand during the induction of T cell anergy. J Immunol. 2008;181:1622–1626. doi: 10.4049/jimmunol.181.3.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Penninger J, Karin M. Immunity by ubiquitylation: a reversible process of modification. Nature reviews. 2005;5:941–952. doi: 10.1038/nri1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie DA, Schartner J, Lin J, Timmel A, Jennens-Clough M, Fathman CG, Seroogy CM. GRAIL is up-regulated in CD4+ CD25+ T regulatory cells and is sufficient for conversion of T cells to a regulatory phenotype. The Journal of biological chemistry. 2007;282:9696–9702. doi: 10.1074/jbc.M604192200. [DOI] [PubMed] [Google Scholar]
- Nurieva R, Thomas S, Nguyen T, Martin-Orozco N, Wang Y, Kaja MK, Yu XZ, Dong C. T-cell tolerance or function is determined by combinatorial costimulatory signals. The EMBO journal. 2006;25:2623–2633. doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007a;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chuvpilo S, Wieder ED, Elkon KB, Locksley R, Serfling E, Dong C. A costimulation-initiated signaling pathway regulates NFATc1 transcription in T lymphocytes. J Immunol. 2007b;179:1096–1103. doi: 10.4049/jimmunol.179.2.1096. [DOI] [PubMed] [Google Scholar]
- Rangatia J, Vangala RK, Singh SM, Peer Zada AA, Elsasser A, Kohlmann A, Haferlach T, Tenen DG, Hiddemann W, Behre G. Elevated c-Jun expression in acute myeloid leukemias inhibits C/EBPalpha DNA binding via leucine zipper domain interaction. Oncogene. 2003;22:4760–4764. doi: 10.1038/sj.onc.1206664. [DOI] [PubMed] [Google Scholar]
- Rao S, Gerondakis S, Woltring D, Shannon MF. c-Rel is required for chromatin remodeling across the IL-2 gene promoter. J Immunol. 2003;170:3724–3731. doi: 10.4049/jimmunol.170.7.3724. [DOI] [PubMed] [Google Scholar]
- Seroogy CM, Soares L, Ranheim EA, Su L, Holness C, Bloom D, Fathman CG. The gene related to anergy in lymphocytes, an E3 ubiquitin ligase, is necessary for anergy induction in CD4 T cells. J Immunol. 2004;173:79–85. doi: 10.4049/jimmunol.173.1.79. [DOI] [PubMed] [Google Scholar]
- Su L, Lineberry N, Huh Y, Soares L, Fathman CG. A novel E3 ubiquitin ligase substrate screen identifies Rho guanine dissociation inhibitor as a substrate of gene related to anergy in lymphocytes. J Immunol. 2006;177:7559–7566. doi: 10.4049/jimmunol.177.11.7559. [DOI] [PubMed] [Google Scholar]
- Voice J, Donnelly S, Dorsam G, Dolganov G, Paul S, Goetzl EJ. c-Maf and JunB mediation of Th2 differentiation induced by the type 2 G protein-coupled receptor (VPAC2) for vasoactive intestinal peptide. J Immunol. 2004;172:7289–7296. doi: 10.4049/jimmunol.172.12.7289. [DOI] [PubMed] [Google Scholar]
- Wohlfert EA, Callahan MK, Clark RB. Resistance to CD4+CD25+ regulatory T cells and TGF-beta in Cbl-b−/− mice. J Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- Wohlfert EA, Gorelik L, Mittler R, Flavell RA, Clark RB. Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-beta sensitivity in vitro and in vivo. J Immunol. 2006;176:1316–1320. doi: 10.4049/jimmunol.176.3.1316. [DOI] [PubMed] [Google Scholar]
- Xia D, Sanders A, Shah M, Bickerstaff A, Orosz C. Real-time polymerase chain reaction analysis reveals an evolution of cytokine mRNA production in allograft acceptor mice. Transplantation. 2001;72:907–914. doi: 10.1097/00007890-200109150-00028. [DOI] [PubMed] [Google Scholar]
- Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Blattman JN, Kennedy NJ, Duong J, Nguyen T, Wang Y, Davis RJ, Greenberg PD, Flavell RA, Dong C. Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature. 2004;430:793–797. doi: 10.1038/nature02764. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chung Y, Bishop C, Daugherty B, Chute H, Holst P, Kurahara C, Lott F, Sun N, Welcher AA, Dong C. Regulation of T cell activation and tolerance by PDL2. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11695–11700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.