Abstract
Objective
To describe the development of nevi from age of 3 to 8 in a birth cohort of children in Colorado, United States.
Design
Longitudinal observational study.
Setting
Large managed care organization, university, private primary care practices.
Participants
Annual convenience samples of children born in 1998, n= 137 to 870 (participation rates 19% to 76%). Recruitment through managed care organization, private primary care practices and community settings.
Main Outcome Measures
Total whole body nevus counts, nevus counts by size (< 2mm, 2 to <5 mm, ≥ 5mm), nevus counts for chronically and intermittently exposed body sites.
Results
Non-Hispanic white children had significantly more nevi than other racial/ethnic groups, and developed an average of 4-6 new nevi per year from age 3 to 8. Non-Hispanic white males had significantly more nevi than females beginning at age 6 (median 21 [inter-quartile range 12 – 30] vs. 17 [inter-quartile range 9 – 26], p=.002). This difference was due to nevi < 2mm and nevi in chronically exposed body sites. Development of new nevi leveled off in chronically exposed body sites at age 7, at a higher level for males than females.
Conclusions
Children in Colorado developed more small nevi and fewer larger nevi compared to children in other regions of the world, highlighting the importance of studying nevus development in various locations where sun exposure patterns and behavioral norms vary. The gender difference in nevus development could be due to variation in sun exposure and/or a biological predisposition of males to develop more nevi. Studies of nevus development can aid in the understanding of the complicated relationship between nevus development and malignant melanoma.
INTRODUCTION
The incidence rate for malignant melanoma nearly tripled for whites in the U.S. between 1975 and 2004,1 making melanoma the cancer with the fastest rising incidence rate in the United States. Melanoma rates in the United States are on a trajectory that mirrors Australia, where melanoma rates are the highest in the world.2 The presence of numerous melanocytic nevi is the strongest risk factor for melanoma.3 Most nevi are developed in childhood, and several factors have been consistently shown to be related to higher numbers of nevi in childhood, including lighter skin, lighter hair color, blue or green eyes, and higher levels of sun exposure.4-8 Because the risk factors for nevi closely match the risk factors for melanoma, and because number of nevi is the strongest risk factor for melanoma, nevi are studied as intermediate markers for melanoma, and understanding nevus development could lead to a better understanding of the causes of melanoma.
Caucasian populations are at considerably higher risk for malignant melanoma than other racial/ethnic groups. In the United States, non-Hispanic whites had an annual incidence rate of 25.1/100,000 for the period 2000-2004, compared to rates of 1.0 for blacks, 4.5 for Hispanic whites, 1.5 for Asians, and 2.9 for American Indian/Alaskan Natives.1 Thus, most previous studies of nevus development have focused exclusively on white/Caucasian populations. Previous nevus studies of children have generally been cross-sectional, examining risk factors for nevi at a single point in time, while a few studies have followed cohorts of children from a baseline measure of nevi to a follow-up measure some 3-5 years later.4,9,10 To our knowledge, there have been no previous published studies that followed children from a single population annually for a period of years prior to age 10 so that annual accumulation of nevi could be studied. Studies that examine nevus development on at least an annual basis are important because they have the potential to identify developmental periods of greatest increase in nevi. Longitudinal studies in different geographic conditions (e.g., latitude, altitude, climate) and in populations with different behavioral patterns (e.g., clothing styles, outdoor activity patterns) are imperative to understanding melanoma risk, including understanding whether there may be a threshold of sun exposure during childhood at which additional exposure conveys no further risk, as has been suggested by researchers in Australia.11 This threshold, if it exists, could be reached at different ages under different geographic and social conditions.
The present study is the first annual longitudinal study of nevus development in children in the United States. Children born in 1998 and residing in the Denver/Boulder, Colorado metropolitan area were examined in 2001, 2002, 2003, 2004, 2005 and 2006 (at ages 3-8 years). We traced nevus development over time by race/ethnicity, and among non-Hispanic white children, by gender for all nevi, nevi < 2mm, nevi ≥ 2mm, nevi on chronically exposed skin, and nevi on intermittently exposed skin.
METHODS
Study population
This nevus study was conducted as part of two behavioral intervention trials aimed at increasing sun protection practices of parents for their young children. Children born between January 1 and September 30 of 1998 were eligible. Initial recruitment in 1998 was conducted through a large managed care organization (MCO) that serves 29% of the insured population of the Denver/Boulder, Colorado area. Hospital birth logs were used to identify all births and mothers were contacted by telephone for enrollment before the child turned 6 months of age. The recruitment interview included a screening of skin cancer risk, and parents whose children had dark skin color, dark eye color, and dark hair color were informed that the program may be of minimal benefit to their child because of low skin cancer risk. Otherwise, all families were invited to participate. From a total of 2,148 births, we contacted 1,177 eligible families and recruited 728 (62%). Skin exams were conducted in 2001 and 2002 (ages 3 and 4) on this sample. Subjects were randomly assigned to a control group or behavioral intervention group according to the managed care clinic at which they were enrolled (7 clinics in each group). The control group received standard care and the intervention group received anticipatory guidance for sun protection at well-child visits between 2 and 36 months of age. 12 The behavioral intervention did not affect nevus development by 3 years of age.12
Additional children were recruited for a second behavioral intervention trial in fall 2003 and winter 2004. Due to new restrictions governing patient privacy (the Health Insurance Portability and Accountability Act), we were unable to directly contact parents from health system enrollment records, so mailings were sent to eligible families attending the MCO described above and seven large private pediatric practices. Parents were asked to return a card if they were interested in participating and then were called by study interviewers who explained the study and followed the same enrollment protocol as described for the 1998 enrollment. Additionally, we recruited some families through community retail sites, recreational facilities, and schools. Participating children were not required to have been born in Colorado; 19% (166/867) were born outside the state. Because the study team was not given access to patient names, the participation rate for the study among those invited is not known.
The second behavioral trial was conducted between 2004 and 2007 and included sun protection educational mailings to parents and children based on the Precaution Adoption Process Model.13 Children also received sun protection resources such as sunhats, swim shirts, sunscreen, tree seeds, a backpack, and sun protection learning activities. Individual children among those newly recruited were randomly assigned to the intervention group or a standard care control group. All children continuing from the sample recruited in 1998 (n=278) were assigned to the intervention group.
All study procedures were reviewed and approved by the Colorado Multiple Institutional Review Board and the Kaiser Permanente of Colorado Institutional Review Board. Parents provided written informed consent prior to skin exams. Beginning at age 7, children provided written assent for participation.
Skin Exams
Skin exams provided information on nevus distribution and size, phenotypic factors, including height, weight, pigmentation of the skin, hair, and eyes, and degree of tanning and freckling. Observation of hair color used hair samples and was coded into five categories: red, blonde, light brown, medium brown, and dark brown or black. Eye color was recorded as blue and/or green, brown, or hazel (any combination of brown with another color). Height and weight were measured using standard clinical procedures. Freckling on the face was assessed using a 10-level chart.14 Skin color and degree of tanning were measured using a Minolta Chroma Meter CF-300 or CR-400, which quantifies color using the three-dimension L a b system. Using the b-dimension,15 base skin color was measured on the unexposed, upper medial arm and degree of tanning was calculated as the difference in b-dimension values in this area and the exposed lateral forearm. Skin color and tanning values were the mean of five measurements.
Nevus exams were conducted each summer by a team of four to seven health care providers who received annual training from the study’s lead dermatologist (JM). The entirety of the body was examined for nevi, with the exception of the scalp, genitals and buttocks. Nevi were defined as any size pigmented macules or papules, excluding freckles, café-au-lait macules and warts. Nevi were differentiated from freckles and café-au-lait macules by the fact that only nevi are raised and that flat, early junctional nevi are dark brown, have regular edges, and do not occur in patches as freckles do. Lentigines were expected to be rare in this young population. Size of nevus was determined using a clear plastic stencil with cutouts for 2mm and 5mm. Nevi were classified as < 2mm, ≥ 2mm to < 5mm, or ≥ 5mm. Nevi were recorded by size on a standardized body map. Congenital nevi were recorded separately and excluded from overall nevus counts. All examiners participated in duplicate exams each year to determine inter-rater reliability. Inter-rater reliability coefficients (calculated using the intra-class correlation) for 2001, 2002, 2003, 2004, 2005, and 2006 were, respectively, 0.82 (n=48), 0.89 (n=22), 0.97 (n=7), 0.89 (n=33), 0.80 (n=48), and 0.85 (n=66).
With the exception of 2003, skin exams were conducted between mid-June and early October each year so that tanning during the warmer months could be observed. In 2003, skin exams were delayed because of funding and study enrollment issues, and were conducted between late October, 2003 and March, 2004. Thus, an interval of approximately 12 months can be assumed between exams for all years except for 2003. In 2003, skin exams were conducted on average 17 months after the 2002 exams and only 7 months prior to the 2004 exams.
Participation rate in skin exams
There were 728 children enrolled at the beginning of the study in 1998. Of these, 281 (39%) participated in skin exams in 2001, and 137 (19%) participated in 2002. Following re-recruitment in 2003-04, a total of 1145 children were enrolled, of which 278 (24%) were from the original sample. Participation rates in the skin exams in subsequent years were: 2003: 25% (n=284); 2004: 75% (n=856); 2005: 76% (n=870); 2006: 75% (n=860). The primary factor that distinguished data collection in 2001 and 2002 from data collection in subsequent years was the offering of an incentive ($25) for attendance in 2003-2006. The lower participation in 2003 was due to the fact that many of the sample of 1145 were recruited in spring, 2004, after the 2003 exams had been completed.
Measurement of other variables
Race/ethnicity was as reported by the parent and categorized as non-Hispanic white, Hispanic white, black, Asian/Pacific Islander, Native American or other.
Body sites were classified as chronically exposed or intermittently exposed based on previous research.16 Chronically exposed sites included: face, anterior neck, lateral forearms, dorsa of hands, and for males, posterior neck. Rarely exposed sites (medial aspects of arms, palms, bottoms of feet) were combined with intermittently exposed sites because they are minimal in body area and due to the small number of nevi present could not be analyzed separately. Intermittently exposed sites were: chest/abdomen, back/shoulders, lateral upper arms, legs, dorsa of feet, and for females only, posterior neck.
Data analysis
Because not all children participated in exams each year, and because of re-recruitment in 2003-04 resulting in the inclusion of 867 new subjects, we conducted this analysis as five separate cross-sectional samples. After verifying the expected large difference in number of nevi by race/ethnicity, subsequent analyses were restricted to non-Hispanic white children (80-90% of the sample, depending on year). For non-Hispanic white children, gender, phenotypic data (eye color, hair color, skin pigmentation), child size (height and weight), and levels of freckling and tanning are presented separately for each annual sample.
Nevus counts showed a positively skewed distribution, with a few children having very high counts; thus, median counts are presented. Nevi measuring ≥ 5mm were very infrequent and were therefore collapsed with nevi ≥ 2mm. Median number of total nevi, median number of two sizes of nevi (< 2mm, ≥ 2mm), and median number of nevi for chronically vs. intermittently exposed skin areas were calculated for each annual sample for non-Hispanic white children by gender. Medians are presented in graphs and significant differences, using the Mann-Whitney test (for two group comparisons) or the Kruskal-Wallis test (for multiple group comparisons), which test for a location shift in the overall distributions, are noted. We elected to analyze nevus counts rather than nevus density because the children were all born within a 9-month time period and thus were relatively similar in body size, and because of the greater ability to interpret and compare counts with other populations.
The prevalence of nevi ≥ 2mm was low. Therefore, data for nevi ≥ 2mm are presented as the proportion of the children with one or more nevi ≥ 2mm in size rather than as medians. To demonstrate the gender difference in posterior neck nevus counts (which is considered chronically exposed for males and intermittently exposed for females), we report the proportion of children with any nevus on the posterior neck by gender for each year. Chi-square tests were used to compare proportions across groups. All statistical tests were two-sided and statistical procedures were implemented using the Statistical Package for the Social Sciences, Version 15.0 for Windows.
Sensitivity analysis
To verify the validity of pooling children recruited at different times and from different sources, we conducted sensitivity analyses as follows. First, we repeated the major analyses reported in this paper on the 57 non-Hispanic white children who attended all six years of exams (2001-2006). Second, we compared subject characteristics and nevus counts for three sub-samples in the 2006 data collection: 1) children enrolled in 1998 from the MCO (n=150); 2) children from the same MCO enrolled in 2003-04 (n=155); and 3) children enrolled from private pediatric practices and community settings in 2003-04 (n=423). To validate the inclusion of both intervention and control group children in this analysis of the natural history of nevus development, we compared nevus counts in 2006 for children randomly assigned to the two study groups.
RESULTS
Table 1 provides characteristics of the study participants for each study year. Race/ethnicity is presented for all participants; additional characteristics are presented only for non-Hispanic white participants. Figure 1 shows median number of moles over time by race/ethnicity. Non-Hispanic white children consistently exhibited a higher number of nevi compared to other racial/ethnic groups, and the differences increased as the children aged. Black children had the fewest nevi, followed by Asians/Pacific Islander children and Hispanic white children. The differences between non-Hispanic whites and all other groups became statistically significant at age 5 and increased in statistical significance from that point forward. Because non-Hispanic whites are known to have a 6- to 25-fold higher incidence of malignant melanoma compared to other racial/ethnic groups1 the remainder of the analysis focuses solely on the non-Hispanic white children.
Table 1.
Characteristics of study participants born in 1998 by year, 2001-2006, age 3-8 years, Colorado, United States
| Race/ethnicity of study sample | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age 3, 2001 (n=281) |
Age 4, 2002 (n=137) |
Age 5, 2003 (n=284) |
Age 6, 2004 (n=856) |
Age 7, 2005 (n=870) |
Age 8, 2006 (n=860) |
|||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| White, non-Hispanic | 232 | 83.8 | 121 | 89.6 | 229 | 81.8 | 720 | 84.6 | 733 | 84.5 | 728 | 84.8 |
| Hispanic white | 29 | 10.5 | 9 | 6.7 | 38 | 13.6 | 80 | 9.4 | 78 | 9.0 | 78 | 9.1 |
| Black | 6 | 2.2 | 1 | 0.7 | 6 | 2.1 | 21 | 2.5 | 24 | 2.8 | 22 | 2.6 |
| Asian/Pacific Islander | 9 | 3.2 | 4 | 3.0 | 5 | 1.8 | 26 | 3.1 | 27 | 3.1 | 24 | 2.8 |
| Native American | 1 | 0.4 | 0 | 0 | 2 | 0.7 | 4 | 0.5 | 5 | 0.6 | 6 | 0.7 |
| Other/missing | 4 | 2 | 4 | 5 | 3 | 2 | ||||||
| Characteristics of White, non-Hispanic study participants | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age 3, 2001 (n=232) |
Age 4, 2002 (n=121) |
Age 5, 2003 (n=229) |
Age 6, 2004 (n=720) |
Age 7, 2005 (n=733) |
Age 8, 2006 (n=728) |
|||||||
| n | value | n | value | n | value | n | value | n | value | n | value | |
| Sex (%) | ||||||||||||
| Male | 115 | 49.6 | 54 | 44.6 | 107 | 46.7 | 350 | 48.6 | 346 | 47.2 | 338 | 46.4 |
| Female | 117 | 50.4 | 67 | 55.4 | 122 | 53.3 | 370 | 51.4 | 387 | 52.8 | 390 | 53.6 |
| Hair color (%) | ||||||||||||
| Blonde | 69 | 29.9 | 21 | 17.4 | 55 | 24.1 | 133 | 18.6 | 49 | 6.7 | 68 | 9.4 |
| Red | 11 | 4.8 | 7 | 5.8 | 7 | 3.1 | 27 | 3.8 | 25 | 3.4 | 34 | 4.7 |
| Light brown | 98 | 42.4 | 64 | 52.9 | 89 | 39.0 | 322 | 45.0 | 351 | 48.0 | 295 | 40.6 |
| Medium brown | 48 | 20.8 | 29 | 24.0 | 74 | 32.5 | 229 | 32.0 | 302 | 41.3 | 327 | 45.0 |
| Dark brown/black | 5 | 2.2 | 0 | 0 | 3 | 1.3 | 4 | 0.6 | 5 | 0.7 | 3 | 0.4 |
| Missing | 1 | 5 | 1 | 1 | ||||||||
| Eye color (%) | ||||||||||||
| Blue/green | 144 | 62.3 | 59 | 48.8 | 132 | 57.9 | 389 | 54.5 | 401 | 54.8 | 370 | 50.9 |
| Brown/black | 57 | 24.7 | 30 | 24.8 | 56 | 24.6 | 172 | 24.1 | 186 | 25.4 | 192 | 26.4 |
| Hazel | 30 | 13.0 | 32 | 26.4 | 40 | 17.5 | 153 | 21.4 | 145 | 19.8 | 165 | 22.7 |
| Missing | 1 | 1 | 1 | |||||||||
| Age 3, 2001 (n=232) |
Age 4, 2002 (n=121) |
Age 5, 2003 (n=229) |
Age 6, 2004 (n=720) |
Age 7, 2005 (n=733) |
Age 8, 2006 (n=728) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | value | n | value | n | value | n | value | n | value | n | Value | |
| Freckling (%) | ||||||||||||
| Face (any) | 38 | 16.7 | 37 | 30.6 | 91 | 39.7 | 349 | 49.4 | 446 | 60.9 | 509 | 69.9 |
| Arms (any) | 1 | 0.4 | 1 | 0.8 | 2 | 0.9 | 10 | 1.4 | 38 | 5.2 | 82 | 11.3 |
| Back (any) | 0 | 0 | 0 | 0 | 3 | 1.3 | 9 | 1.3 | 29 | 4.0 | 52 | 7.1 |
| Base skin color, b scale (mean) |
232 | 11.1 | 121 | 11.3 | 227 | 10.4 | 717 | 11.0 | 732 | 10.8 | 723 | 10.9 |
| Tanning score, b scale (mean) |
231 | 2.3 | 121 | 1.9 | 227 | 1.8 | 717 | 2.8 | 732 | 2.7 | 723 | 2.6 |
| Nevi | ||||||||||||
| Mean | 231 | 6.2 | 121 | 11.8 | 229 | 19.3 | 719 | 21.8 | 732 | 28.8 | 728 | 31.6 |
| Median | 231 | 6.0 | 121 | 11.0 | 229 | 17.0 | 719 | 19.0 | 732 | 25.0 | 728 | 29.0 |
| Range | 231 | 0-22 | 121 | 1-35 | 229 | 1-66 | 719 | 1-92 | 732 | 2-116 | 728 | 2-118 |
| Standard deviation | 231 | 4.1 | 121 | 6.5 | 229 | 11.4 | 719 | 13.6 | 732 | 17.4 | 728 | 18.4 |
| Interquartile range | 231 | 6.0 | 121 | 8.5 | 229 | 14.0 | 719 | 17.0 | 732 | 22.0 | 728 | 22.0 |
| Height (mean, inches) | 231 | 37.4 | 121 | 40.7 | 229 | 45.0 | 715 | 46.6 | 732 | 49.8 | 727 | 51.0 |
| Weight (mean, pounds) | 231 | 32.1 | 121 | 36.3 | 229 | 44.5 | 718 | 48.0 | 732 | 53.8 | 727 | 60.7 |
| Age at time of exam (range - months) |
231 | 33-41 | 121 | 45-53 | 229 | 61-75 | 719 | 69-81 | 732 | 81-93 | 727 | 93-105 |
Figure 1. Median total nevi by race/ethnicity, age 3 – 8, Colorado, United States, 2006.
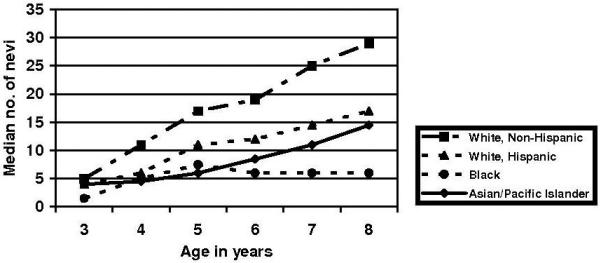
Distribution of non-Hispanic whites significantly different from other groups at age 3 (p=0.03), age 4 (p=0.05), age 5 (p=0.001), ages 6-8 (p<0.001) (Kruskal-Wallis test for all comparisons).
For all study years, our samples were approximately evenly split on gender (Table 1). The proportion of children with hair designated as “blonde” decreased with time, while the proportion with medium brown hair increased. This is mostly likely due to the natural darkening of hair during childhood rather than a change in the children included in each year’s sample. Around half of the children had blue or green eyes. Freckling was relatively infrequent at age 3, and increased steadily over time. By age 8, 70% of the children had facial freckling, 11% had arm freckling, and 7% had back freckling. Base skin color, which ranged from 10.4 to 11.3 on the b scale, confirms that the samples for each of the years were fairly consistent in pigmentation. The tanning score was lower for 2003, when the exams were conducted in winter instead of summer (1.8 compared to 2.6-2.8 for later years). At age 3, children had a median of 6 nevi. Median number of nevi increased by 4-6 nevi per year, with the exception of from age 5 to age 6 when a median of only 2 new moles were gained; however exams were completed only about 7 months apart for that time period.
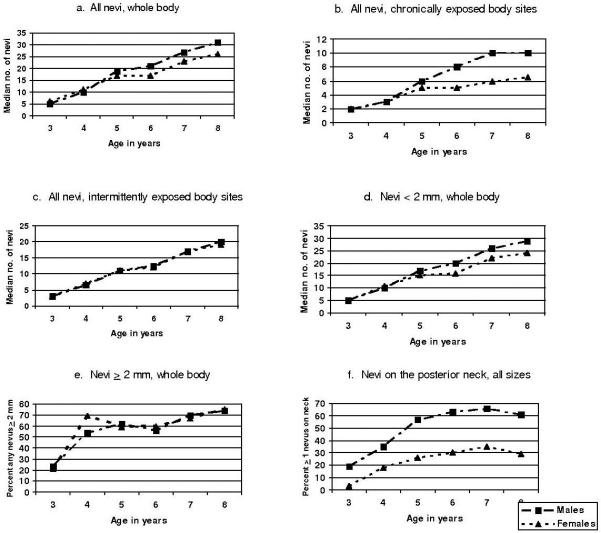
Figure 2 shows increases in number of nevi over time for all nevi, small nevi (< 2mm), larger (≥ 2mm) nevi, nevi in chronically exposed body parts and nevi in intermittently or rarely exposed body parts. Total number of nevi was significantly higher for males compared to females starting at age 6, at which point there was a 4 nevus median difference (p=0.002), with the difference growing to a 5 nevus difference by age 8 (p=0.001). The gender difference is primarily due to small nevi (< 2mm) and nevi in chronically exposed body parts. By age 6, males had a median of 4 more small nevi (whole body) and 3 more nevi (any size) on their chronically exposed body parts, compared to females (p=0.001 and p<0.001, respectively). These gender differences do not appear to exist for nevi ≥ 2mm or for intermittently exposed body parts. The gender difference with regard to chronically exposed body parts is further demonstrated for the posterior neck, which is considered chronically exposed for males and intermittently exposed for females (Figure 2f). While the absolute counts of nevi on the posterior neck were small, at least twice as many males were found to have any nevi on their posterior neck compared to females at all ages studied (all p<0.05). The number of children with any nevi on the posterior neck appears to have leveled off for both genders around age 6. The gender difference in nevi cannot be explained by greater body size; at age 8, males were only 4% larger in body weight and 2% larger in height compared to females.
Figure 2. Median total nevi (whole body), all nevi on chronically exposed parts, all nevi on intermittently exposed parts, nevi < 2 mm (whole body), nevi ≥ 2 mm (whole body), and nevi on the posterior neck, by sex, children age 3-8, Colorado, United States, 2001-2006.
(a) Males significantly different from females at ages 6, 7, 8 (p<0.02, Mann-Whitney test). (b) Males significantly different from females at ages 5, 6, 7, 8 (p≤0.001, Mann-Whitney test). (c) Males and females statistically similar (Mann-Whitney test). (d) Males significantly different from females at ages 6, 7, 8 (p≤ 0.01, Mann-Whitney test). (e) Males and females statistically similar (Mann-Whitney test). (f) Males significantly different from females at ages 3, 5, 6, 7, 8 (p<0.001) and at age 4 (p=0.03, Mann-Whitney test).
Sensitivity analysis
Fifty-seven non-Hispanic white children attended all six skin exam sessions (2001-2006). To investigate whether the addition of children in the 2003-04 recruitment could have substantially altered the trends reported in the main analysis, plots of all nevi, nevi in chronically exposed body sites, nevi in intermittently exposed body sites, and nevi < 2mm in size were constructed (data not shown). All patterns presented in Figure 2 were replicated, including the gender difference for nevi in chronically exposed body sites beginning at age 6, lack of gender difference for intermittently exposed body sites, and the gender difference in nevi < 2mm, although this difference emerged at age 7 rather than at age 6 in this subset of subjects.
Subject characteristics and nevus counts at age 8 were compared for children from three recruitment samples. The three samples were statistically similar (p>0.20) for freckling, hair color, gender, base skin color (b scale), height and weight. Marginally significant differences were found for eye color (a higher proportion of children recruited from the MCO in 2003-04 had blue or green eyes and fewer had hazel eyes compared to the other sub-samples, p=0.09); all nevi (children recruited from the MCO at both time points had somewhat fewer nevi than children recruited from other locations, p=0.18); and tanning (children recruited from the MCO in 1998 had somewhat higher tanning scores compared to other children, p=0.18). Statistically significant differences were found for nevi ≥ 2mm and for nevi in intermittently exposed sites. Children recruited from private practices and community locations had a median of 2 nevi ≥ 2mm compared to medians of 1 nevus for both MCO samples (p=0.02). Children from private practices and community locations had a median of 21 nevi in intermittently exposed sites compared to medians of 18 for both MCO samples of children (p=0.04). In general, intervention group status was not related to number of nevi. There was no statistically significant difference for all nevi, nevi > 2mm, and nevi in intermittent and chronically exposed sites. The Mann-Whitney test showed a significant difference for nevi < 2mm, with children randomly assigned to the control group having fewer nevi (control group median = 23, intervention group median = 26, p=0.04). Because the intervention was intended to decrease UV exposure, it was expected that the intervention would be associated with fewer rather than more nevi.
DISCUSSION
Non-Hispanic white children in Colorado, United States, developed approximately 4-6 new nevi per year on average between the ages of 3 and 8. The majority of these nevi (90-95%) remained less than 2mm in diameter through age 8. The accumulation of nevi was comparable for males and females when nevi in intermittently exposed body sites were considered. However, in chronically exposed areas (the face and front of neck, and lower outer arms for both genders, and back of the neck in males), there was a divergence between genders starting around age 5, with males accumulating more nevi in the chronically exposed areas. By age 8, males had about 20% more total nevi than females, and about 50% more nevi in chronically exposed areas. The greater number of nevi in chronically exposed areas for males was only minimally due to males’ greater numbers on the posterior neck, which is considered to be chronically exposed for males but not females. The data suggest that the number of nevi on chronically exposed sites began to level off for both genders at age 7-8. Our study verifies the considerably higher number of nevi on non-Hispanic white children compared to other groups,17 which mirrors the differences in melanoma incidence by race/ethnicity.1 The sensitivity analysis supports the conclusion that the overall trends found were not affected by the inclusion of children from three different sampling sources. The inclusion of children from private pediatric practices and community sites may have resulted in slightly increased counts for nevi ≥ 2mm and for nevi in intermittently exposed body sites.
The gender difference in nevi that we found in our study population has been noted by researchers studying nevi in other parts of the world, although our finding that the gender difference in moles is limited to chronically exposed body areas differs from findings from other regions. Autier et al. examined 6-7 year olds in four European cities and found that males had slightly more nevi compared to females and that the excess nevi in males was predominantly due to nevi on the head and neck, trunk and shoulders.18 While the head and neck are chronically exposed, the trunk and shoulders are generally not considered chronically exposed. Darlington et al. studied Australian adolescents over five years from ages 12-13 to ages 16-17 and found gender differences for both chronically exposed areas (face/neck) and intermittently exposed areas (shoulder/back).11 Their study provides evidence of a saturation level for the face/neck reached at age 14-15 for both genders, with males topping off at significantly higher counts than females. Children in that study continued to accumulate nevi on the shoulders/back throughout the study. In a separate analysis of children in our study, we found that number of nevi on the face was not associated with sunburns to the face, whereas number of nevi on the back was associated with sunburns to the back.8 This also provides support for the saturation hypothesis, with the faces of our children showing evidence of saturation, whereas the backs of our children did not. However, continuing to follow our cohort into the adolescent years could show that there are multiple periods of plateau in nevus development, followed by periods of increase. These could be due to developmental biology or age-related periods of greater sun exposure, or a combination of these two factors.
In our Colorado cohort, we found that the gender difference for chronically exposed body sites emerged at approximately age 6. In the U.K., Pope et al. also observed the emergence of a gender difference at age 6.7 The fact that the gender difference in Colorado children is limited to chronically exposed body parts suggests that sun exposure plays a key role. This difference could be due to sun exposure alone, or it could be due to an interaction between sun exposure and a biological predisposition of males to develop more nevi.18 However, there has been no biological mechanism proposed for this gender difference. It has been suggested that there is a delay of two years or more between sun exposure and resultant nevus development.19 If the gender difference we observed is due to sun exposure alone, our findings suggest that parents protect their very young male and female children equally from sun exposure until age 3-4 years, at which time parents may become more diligent about the sun protection of their female children compared to their male children, and the subsequent effect on nevus development becomes apparent at 6 years of age. The gender difference in moles may also be influenced by gender differences in childhood activities. Boys may be more involved in outdoor sports and other outdoor activities. There may also be gender differences in clothing styles that lead to the divergence in number of moles, although the pattern of nevus development suggests that both boys and girls get their highest levels of exposure on the lower outer arms and the face and neck. Sun protection studies have shown that males use less sun protection than females,21-23 and the pattern of nevus emergence in our cohort, as well as the divergence as early as age 6 suggests that this gender-based behavioral difference starts young, while the behavior is still in the control of parents rather than children.
Also of note is that nevi in our cohort of Colorado children were mostly less than 2 mm in size. Studies in other regions have found considerably higher levels of larger nevi (≥ 2mm), and in many cases studies have not even counted nevi < 2 mm.4,23-25 For example, in Kallas et al.’s study of children in Estonia, 9 year olds had a median of 26 nevi ≥ 2mm in size.23 Some portion of these differences in findings may be rooted in methodology. We considered nevi to be ≥ 2mm only if they completely filled the 2 mm cut-out in the measurement stencil. Some studies have considered nevi to be ≥ 2mm if any dimension of the nevus is ≥ 2mm. Since many of the nevi measured in our study population were close to 2mm in size, we expect that as the cohort ages those nevi will grow and the apparent differences in nevus size between our cohort and other studies will diminish. It is possible, however, that sun exposure patterns and clothing norms in Colorado lead to a different pattern of nevus development. Autier et al. have suggested that genetically determined characteristics (i.e., skin type and eye color) play a major role in development of smaller nevi (< 5mm) but that intense sun exposure (such as that received on vacations) is necessary for the development of large nevi (> 5mm).26 Bauer et al., however, suggest that both intermittent and chronic sun exposure are important in the development of nevi, with intermittent intense exposure being relatively more important in regions of higher latitude and chronic exposure being more important in regions with lower latitude.5
Compared to our Colorado cohort, studies of similarly aged children in Vancouver, BC9; Bochum, Germany4; and Brisbane, Australia24 have reported higher total nevus counts, while studies in Hamburg, Germany27; Ramat-Gan/Jerusalem, Israel28; and Kaunas, Lithuania10 have reported lower counts than Colorado. Interestingly, two separate U.K. studies, one in West Midlands7 and one in Oxford29, reported counts very similar to ours in Colorado. Sun exposure (both at home and while on vacation), behavioral patterns, and genetic variability are likely to contribute to regional differences in nevus development. Colorado has over 300 sunny days a year and most of the population live at an elevation above 5000 feet. Further, the non-Hispanic white population of Colorado may have greater genetic diversity affecting pigmentation and nevus development than the populations in some other regions previously studied.
Our study has some limitations. Our sample was not randomly selected from the population and therefore may not be representative of the larger population. Most likely, the study attracts participation from families that are more aware of and/or concerned about skin cancer. Thus, families in our study may be more vigilant about sun protection and if so, our study might underestimate nevus prevalence in non-Hispanic white Colorado children. The lower participation rates in skin exams in 2001 and 2002, which are most likely due to the lack of a financial incentive offered in those years, may have resulted in further bias towards the inclusion of children with parents who are more concerned about skin cancer. This bias, if present, could have resulted in greater underestimation of nevi in the first two years compared to later years, if these families were more diligent about sun protection and thus the children developed fewer nevi than other children. Low participation in 2003 was due to delayed recruitment and is not likely to have produced bias in a particular direction. The net effect of the potential bias could have resulted in the appearance of a more dramatic increase in nevus counts from the earlier years (2001-2002) to the later years (2003-2006) than what would have been found in a more representative sample. Despite potential bias in participation that may have differentially affected nevus estimates by year, the comparisons of brown/black eye color and base skin color presented in Table 1 indicate that the samples across all years were similar phenotypically.
A portion of our study population is currently receiving a sun protection intervention that is designed to reduce sun exposure and thus may prevent the development of nevi. However, our analysis generally showed no effect of the intervention on nevus counts, with the exception of nevi < 2 mm, for which the analysis showed lower nevus counts for the control group, contrary to the intention of the intervention.
Rather than following a single sample of children recruited shortly after birth in 1998, our study combined samples recruited in 1998 and in 2003-04, with all children being in the same 1998 birth cohort. This could call into question whether our treatment of the two samples as a single cohort for analysis is appropriate. However, our sensitively analysis showed that inclusion of the two samples strengthens the study by including both a larger number of children and somewhat greater variation in the children studied (e.g., children recruited from private practices and community sites in 2003-04 tended toward development of higher numbers of nevi). Thus, the inclusion of the additional sample of children strengthens the ability to generalize the findings to all Colorado non-Hispanic white children born in 1998.
Except for 2003, during which data collection was delayed due to funding, all data collection occurred during the summer months. Therefore, we are unable to examine the seasonality of nevus development. For example, an interesting question is whether nevi tend to develop during seasons of high sun exposure, or whether they develop some months following sun exposure (e.g., in the winter). Seasonal trends have been detected in melanoma incidence, with more melanomas being detected in the spring and summer months.30 With skin exams occurring at approximately annual intervals, our data cannot speak to this issue.
In conclusion, this study contributes to the body of literature on nevus development in children by adding the first annual longitudinal study in children prior to age 10 and the first U.S. study. Children in Colorado exhibit nevus development patterns similar to those in the U.K., but notably different from children in other Northern European countries and Australia, particularly with respect to the size of nevi and the patterns of nevus development between intermittently and chronically exposed body sites. These differences highlight the importance of studying nevus development in various regions of the world where sun exposure patterns and behavioral norms vary; such studies can aid in the understanding of the complicated relationship between nevus development and malignant melanoma.
Acknowledgments
We are indebted to Dr. H. Alan Arbuckle, Brenda Mokrohisky, Cathi Sommer, and Laura Wilson for conducting skin exams.
Funding/Support: This study was supported in part by a grant to Dr. Crane from the National Cancer Institute (RO1-CA74592) and by bridge funding from the Department of Dermatology, University of Colorado Denver School of Medicine.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Abbreviations
- mm
millimeters
- MCO
managed care organization
Footnotes
All Financial Interests (including pharmaceutical and device products):
Employment ________University of Colorado Denver (Crane, Asdigian, Morelli, Zeng, Aalborg, Burch, Byers, Barón, Dellavalle); Kaiser Foundation Health Plan of Colorado (Mokrohisky); Kaiser Permanente of Colorado (Zeng); Denver VA Medical Center (Dellavalle); AMC Cancer Research Center (Crane); Public Health Research Group (Asdigian); Office of Research, Health Assessment and Data Systems, Boston Public Health Commission (Asdigian); Family Research Laboratory, University of New Hampshire (Asdigian)
1. Consultancies _____World Health Organization (Dellavalle); Cade Pharmaceuticals Inc. (Dellavalle); TEVA Pharmaceuticals (Dellavalle); Boehringer Ingelheim (Dellavalle); American College of Physicians-American Society of Internal Medicine (Dellavalle); Center for Personalized Education for Physicians (Dellavalle); Cephalon, Inc. (Dellavalle); PacifiCare (Dellavalle); Kaiser Permanente of Colorado (Dellavalle); TEVA USA (Dellavalle); Mertz Pharm (Dellavalle)
2. Honoraria _______ Colorado Chapter of the American Academy of Dermatology (Burch); Colorado Dermatologic Society (Burch); Clinics of Dermatology (Dellavalle); National Cancer Institute (Crane); Colorado Action for Health People (Crane); American Cancer Society (Crane); Manilla Consulting, reviewer for National Registry for Evidence Based Programs and Practices (Crane); Colorado Department of Public Health and Environment (Crane); Division of Research Grants, National Institutes of Health (Crane); California Breast Cancer Research Program, State of California (Crane)
3. Stock ownership or options _______ Merck Pharmaceuticals 100 shares common stock in retirement account (Dellavalle)
4. Expert testimony ______none
5. Grants ______ American Cancer Society (Burch); Dermatology Foundation (Burch); Women’s Dermatologic Society (Burch); Pfizer Pharmaceuticals (Dellavalle); National Cancer Institute (Crane); Colorado Department of Public Health and Environment (Crane); Centers for Disease Control and Prevention (Crane); University of Colorado Comprehensive Cancer Center (Crane); Colorado Foundation for Medical Care (Crane); Department of Defense Breast Cancer Research Program (Crane)
6. Patents _____________none
7. Patent applications ___________none
8. Royalties _____UpToDate (Dellavalle)
9. Other ________none RELEVANT TO THIS MANUSCRIPT:
10. Consultancies _____ none
11. Honoraria _______none
12. Stock ownership or options: ____none
13. Expert testimony ____________none
14. Grants ______none
15. Patents __none
16. Patent applications ______none
This research was conducted in the United States of America.
Contributor Information
Lori A. Crane, Department of Preventive Medicine and Biometrics, University of Colorado Denver, Denver, Colorado, USA, lori.crane@uchsc.edu
Stefan T. Mokrohisky, Kaiser Foundation Health Plan of Colorado, Denver, Colorado, USA, stefan.mokrohisky@uchsc.edu
Robert P. Dellavalle, Department of Dermatology, Denver Veteran’s Administration Hospital, Denver, Colorado, USA; Departments of Dermatology and Preventive Medicine and Biometrics, University of Colorado Denver, Denver, CO, U.S.A., robert.dellavalle@uchsc.edu
Nancy Asdigian, Department of Preventive Medicine and Biometrics, University of Colorado Denver, Denver, Colorado, USA, nancy.asdigian@uchsc.edu
Jenny Aalborg, Department of Preventive Medicine and Biometrics, University of Colorado Denver, Denver, Colorado, USA, jenny.aalborg@uchsc.edu
Tim E. Byers, Department of Preventive Medicine and Biometrics, University of Colorado Denver, Denver, Colorado, USA, tim.byers@uchsc.edu
Chan Zeng, Department of Preventive Medicine and Biometrics, University of Colorado Denver, Denver, Colorado, USA, chan.zeng@uchsc.edu
Anna E. Barón, Department of Preventive Medicine and Biometrics, University of Colorado Denver, Denver, Colorado, USA, anna.baron@uchsc.edu
Joanna M. Burch, Department of Dermatology, University of Colorado Denver, Denver, CO, USA, joanna.burch@uchsc.edu
Joseph G. Morelli, Department of Dermatology, University of Colorado Denver, Denver, CO, USA, joseph.morelli@uchsc.edu
REFERENCES
- 1.Ries LAG, Melbert D, Krapcho M, et al. [Accessed October 1, 2007];SEER Cancer Statistics Review, 1975-2004, National Cancer Institute. http://seer.cancer.gov/csr/1975_2004/ (November 2006 SEER data submission) [Google Scholar]
- 2.Coory M, Baade P, Aitken J, Smithers M, McLeod GRC, Ring I. Trends for in situ and invasive melanoma in Queensland, Australia, 1982-2002. Cancer Causes Control. 2006;17:21–27. doi: 10.1007/s10552-005-3637-4. [DOI] [PubMed] [Google Scholar]
- 3.MacKie RM, Freudenberger T, Aitchison TC. Personal risk-factor chart for cutaneous melanoma. Lancet. 1989;2:487–490. doi: 10.1016/s0140-6736(89)92097-7. [DOI] [PubMed] [Google Scholar]
- 4.Luther H, Altmeyer P, Garbe C, et al. Increase of melanocytic nevus counts in children during 5 years of follow-up and analysis of associated factors. Arch Dermatol. 1996;132:1473–1478. [PubMed] [Google Scholar]
- 5.Bauer J, Büttner B, Wiecker TS, Luther H, Garbe C. Risk factors of incident melanocytic nevi: a longitudinal study in a cohort of 1,232 young German children. Int J Cancer. 2005;115:121–126. doi: 10.1002/ijc.20812. [DOI] [PubMed] [Google Scholar]
- 6.Carli P, Naldi L, Lovati S, La Vecchia C, Oncology Cooperative Group of the Italian Group for Epidemiologic Research in Dermatology The density of melanocytic nevi correlates with constitutional variables and history of sunburns: a prevalence study among Italian schoolchildren. Int J Cancer. 2002;101:375–379. doi: 10.1002/ijc.10629. [DOI] [PubMed] [Google Scholar]
- 7.Pope DJ, Sorahan T, Marsden JR, Ball PM, Grimley RP, Peck IM. Benign pigmented nevi in children. Prevalence and associated factors: the West Midlands, United Kingdom Mole Study. Arch Dermatol. 1992;128:1201–1206. doi: 10.1001/archderm.128.9.1201. [DOI] [PubMed] [Google Scholar]
- 8.Dodd AT, Morelli J, Mokrohisky ST, Asdigian N, Byers TE, Crane LA. Melanocytic nevi and sun exposure in a cohort of Colorado children: anatomic distribution and site-specific sunburn. Cancer Epidem Biomark Prev. 2007;16:2136–2143. doi: 10.1158/1055-9965.EPI-07-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA. 2000;283:2955–2960. doi: 10.1001/jama.283.22.2955. [DOI] [PubMed] [Google Scholar]
- 10.Valiukeviciene S, Miseviciene I, Gollnick H. The prevalence of common acquired melanocytic nevi and the relationship with skin type characteristics and sun exposure among children in Lithuania. Arch Dermatol. 2005;141:579–586. doi: 10.1001/archderm.141.5.579. [DOI] [PubMed] [Google Scholar]
- 11.Darlington S, Siskind V, Green L, Green A. Longitudinal study of melanocytic nevi adolescents. J Am Acad Dermatol. 2002;46:715–722. doi: 10.1067/mjd.2002.120931. [DOI] [PubMed] [Google Scholar]
- 12.Crane LA, Deas A, Mokrohisky ST, et al. A randomized intervention study of sun protection promotion in well-child care. Prev Med. 2006;42:162–170. doi: 10.1016/j.ypmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein ND. The Precaution Adoption Process. Health Psychol. 1988;7:355–386. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher RP, McLean DI, Yang P, et al. Suntan, sunburn, and pigmentation factors and the frequency of acquired melanocytic nevi in children. Similarities to melanoma: the Vancouver Mole Study. Arch Dermatol. 1990;126:770–776. [PubMed] [Google Scholar]
- 15.Creech LL, Mayer JA. Ultraviolet radiation exposure in children: a review of measurement strategies. Ann Behav Med. 1997;19:399–407. doi: 10.1007/BF02895159. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher RP, McLean DI, Yang CP, et al. Anatomic distribution of acquired melanocytic nevi in white children. A comparison with melanoma: The Vancouver Mole Study. Arch Dermatol. 1990;126:466–471. [PubMed] [Google Scholar]
- 17.Rampen FH, de Wit PE. Racial differences in mole proneness. Acta Derm Venereol. 1989;69:234–236. [PubMed] [Google Scholar]
- 18.Autier P, Boniol M, Severi G, et al. Sex differences in numbers of nevi on body sites of young European children: implications for the etiology of cutaneous melanoma. Cancer Epidem Biomark Prev. 2004;13:2003–2005. [PubMed] [Google Scholar]
- 19.Milne E, Johnston R, Cross D, Giles-Corti B, English DR. Effect of a school-based sun-protection intervention on the development of melanocytic nevi in children. Am J Epidemiol. 2002;155:739–745. doi: 10.1093/aje/155.8.739. [DOI] [PubMed] [Google Scholar]
- 20.Geller AC, Colditz G, Oliveria S, et al. Use of sunscreen, sunburning rates, and tanning bed use among more than 100,000 US children and adolescents. Pediatrics. 2002;109:1009–1014. doi: 10.1542/peds.109.6.1009. [DOI] [PubMed] [Google Scholar]
- 21.Cokkinides V, Weinstock M, Glanz K, Albano J, Ward E, Thun M. Trends in sunburns, sun protection practices, and attitudes toward sun exposure protection and tanning among US adolescents, 1998-2004. Pediatrics. 2006;118:853–864. doi: 10.1542/peds.2005-3109. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy EM, Ethridge KP, Wagner RF., Jr Beach holiday sunburn: the sunscreen paradox and gender differences. Cutis. 1999;64:37–42. [PubMed] [Google Scholar]
- 23.Kallas M, Rosdahl I, Fredriksson M, Synnerstad I. Frequency and distribution pattern of melanocytic naevi in Estonian children and the influence of atopic dermatitis. J Eur Acad Dermatol Venereol. 2006;20:143–148. doi: 10.1111/j.1468-3083.2005.01379.x. [DOI] [PubMed] [Google Scholar]
- 24.Green A, Sorahan T, Pope D, et al. Moles in Australian and British schoolchildren. Lancet. 1988;2:1497. doi: 10.1016/s0140-6736(88)90980-4. [DOI] [PubMed] [Google Scholar]
- 25.Autier P, Doré J-F, Cattaruzza M, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year old European children. J Natl Cancer Inst. 1998;90:1873–1880. doi: 10.1093/jnci/90.24.1873. [DOI] [PubMed] [Google Scholar]
- 26.Autier P, Severi G, Pedeux R, et al. Number and size of nevi are influenced by different sun exposure components: implications for the etiology of cutaneous melanoma (Belgium, Germany, France, Italy) Cancer Causes Control. 2003;14:453–459. doi: 10.1023/a:1024961100651. [DOI] [PubMed] [Google Scholar]
- 27.Dulon M, Weichenthal M, Blettner M, et al. Sun exposure and number of nevi in 5- to 6-year-old European children. J Clin Epidemio. 2003;55:1075–1081. doi: 10.1016/s0895-4356(02)00484-5. [DOI] [PubMed] [Google Scholar]
- 28.Azizi E, Iscovich J, Pavlotsky F, et al. Use of sunscreen is linked with elevated naevi counts in Israeli school children and adolescents. Melanoma Res. 2000;10:491–498. doi: 10.1097/00008390-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Graham A, Fuller A, Murphy M, Jones M, Forman D, Swerdlow AJ. Maternal and child constitutional factors and the frequency of melanocytic naevi in children. Paediatric Perinatal Epidemiology. 1999;13:316–324. doi: 10.1046/j.1365-3016.1999.00189.x. [DOI] [PubMed] [Google Scholar]
- 30.Boniol M, Armstrong BK, Doré JF. Variation in incidence and fatality of melanoma by season of diagnosis in New South Wales, Australia. Cancer Epidemiol Biomarkers Prev. 2006;15:524–526. doi: 10.1158/1055-9965.EPI-05-0684. [DOI] [PubMed] [Google Scholar]