Abstract
Objective
To test whether black cohosh (BC) exhibits an action on the central endogenous opioid system in postmenopausal women.
Design
A mechanistic study conducted in the same individuals of LH pulsatility with a saline/naloxone (NAL) challenge (n=6) and PET imaging with [11C]carfentanil, a selective μ-opioid receptor radioligand (n= 5), before and after 12 weeks of unblinded treatment with a popular black cohosh daily supplement.
Results
Black cohosh treatment for 12 weeks at a standard dose (Remifemin, 40 mg/day) had no effect on spontaneous LH pulsatility or estrogen concentrations. With NAL blockade, there was an unexpected suppression of mean LH pulse frequency (saline vs NAL = 9.0+.6 vs 6.0+.7 pulses/16 hrs; p= 0.056), especially during sleep when the mean interpulse interval (IPI) was prolonged by approximately 90 minutes (SAL night IPI = 103± 9 mins vs NAL night IPI = 191± 31min, p = 0.03). There were significant increases in μ-opioid receptor binding potential (BP) in the posterior and subgenual cingulate, temporal and orbitofrontal cortex, thalamus and nucleus accumbens ranging from 10% to 61 % across regions - brain regions involved in emotional and cognitive function. In contrast, BP reductions of lesser magnitude were observed in regions known to be involved in the placebo response (anterior cingulate and anterior insular cortex).
Conclusion
Using two different challenge paradigms for the examination of central opioid function, a neuropharmacologic action of black cohosh treatment was demonstrated in postmenopausal women.
Keywords: LH pulsatility, opioid peptides, menopause, PET
INTRODUCTION
Although estrogen therapy is the most effective modality for the relief of menopausal hot flashes and the sequelae of insomnia and impaired quality of life, evidence from the Women’s Health Initiative linking long-term hormone therapy to heart attack, stroke, and breast cancer has left millions of postmenopausal women and breast cancer survivors with few satisfactory treatment alternatives. Hot flash sufferers are resorting to a variety of herbal supplements such as soy and red clover with presumed estrogen activity, but most trials show little support for efficacy over placebos [1, 2].
Preparations made from the rhizomes of the herbal plant, black cohosh (Cimicifuga racemosa, are the most widely studied botanical therapies for relieving hot flashes, with well-designed laboratory studies supporting a favorable safety profile for human use. [3-5]. Despite its popularity, clinical trial results from around the world have been mixed with respect to any benefit above that of placebo [6-12]. Some of the discrepant findings may be due to the high placebo response, typically 30-50%, as well as to the variety of black cohosh compounds, doses, and plant sources used as test agents. The most rigorously tested compound in both US and European studies is the commercially prepared isopropranoloic/ethanolic extract, Remifemin® (Schaper & Brummer, GmBH, Salzgitter, Germany). Among its components thought to have an active therapeutic role (possibly through metabolic activation of estrogen pathways) are the triterpene glycosides, including actein, 27-deoxyactein, and cimifugoside- molecules structurally related to steroids [13].
The mechanism of action of this herbal compound is poorly understood. Studies conducted after 1990 using purified black cohosh free of estrogenic adulterants [14] have generally not demonstrated classical estrogen-like effects on target cell activity in various animal or in vitro models [15-19] or in clinical studies [9, 20], although modest protective effects on bone in postmenopausal women have been reported [11]. Moreover, estrogen receptor binding studies [21-24] have generally failed to reveal any interactions with ligands of the estrogen receptor (ER) in breast and uterine cells. Paradoxically, black cohosh extract has been shown to inhibit the estrogen-dependent MCF-7 mammary tumor cell proliferation and to enhance inhibition with tamoxifen, suggesting an estrogen antagonist, anti-tumor effect on the breast [19, 25, 26].
In a series of studies in ovariectomized rats, black cohosh extract improved bone mineral density, reduced abdominal fat deposition, and dampened LH pulsatility, but failed to increase uterine weight or ER beta gene expression [27-29]. In humans, no change in gonadotropin secretion has been reported with black cohosh [9, 30], although no studies to date have incorporated frequent, serial blood sampling to detect dynamic hypothalamic-pituitary-ovarian (HPO) axis function.
At the same time, a review of 15 animal and 15 in vitro mechanistic studies concluded that C. racemosa possesses central neurotransmitter activity instead of a direct hormonal action [31]. Two recent in vitro studies from the same laboratory provide strong evidence that black cohosh exhibits partial agonist activity in both serotonin [15] and opioid [32] systems. In the ovariectomized rat, several black cohosh extracts failed to demonstrate any estrogenic action on uterine weight, but exhibited strong specific binding to the 5HT1A and 5HT7 receptors [15], serotonin subtypes found predominantly in the hypothalamus, the key area for regulation of body temperature [33]. Additionally, in a Chinese hamster ovary cell system transfected with human μ-opioid receptors, black cohosh served as an effective competitive ligand and activator [32].
Central endogenous opioid peptides (EOPs) are known to mediate the dynamic secretion of GnRH some of the estrogenic effects on both the GnRH system as well as play a role in thermoregulation and the placebo response, making this system a reasonable target for clinical studies of black cohosh mechanisms in women. We, therefore, studied for the first time central parameters of EOP function in postmenopausal women before and after treatment with a popular black cohosh dietary supplement. We hypothesized that if black cohosh (BC) is acting as an opioid ligand in the hypothalamus, it would suppress GnRH activity under basal conditions and stimulate GnRH secretion during opioid blockade. Using state-of-the-art molecular neuroimaging techniques to directly assess regional changes in binding activity of the μ-opioid receptor in the living brain [34, 35], we conducted parallel imaging studies of this neurotransmitter system using positron electron tomography (PET) in a subset of these same individuals.
MATERIALS AND METHODS
Subjects
After permission was obtained from the hospital Institutional Review Board (IRB) and the Radiation Safety Committee for research with human subjects, healthy volunteers willing to undergo black cohosh therapy exclusively for 12 weeks for the treatment of their hot flash symptoms were recruited by electronic, print, television advertisement and from the University of Michigan Women’s Health Registry [36, 37]. All volunteers provided written informed consent.
The inclusion criteria were as follows: spontaneous amenorrhea for at least 12 months in conjunction with a screening FSH value of >40 mIU/ml, and an estradiol value of <20 pg/ml, no use of hormone therapies within 6 months of enrollment, and symptoms of estrogen deficiency (bothersome hot flashes, night sweats or painful intercourse due to vaginal dryness). In all participants, the results of screening values for hemoglobin, hematocrit, liver function, glucose, and prolactin were in the expected ranges for healthy volunteers. All subjects had a normal endocrine screen, a body mass index (BMI) of 20-30 kg/m2, and reported no current history of the following: smoking, vegetarian diet, medical or psychiatric illness, medication usage including oral contraceptives, benzodiazepines, antidepressants, psychostimulants or over-the-counter drugs or herbal supplements, pregnancy or breastfeeding in the past 6 months, sleep disorders, shift work, dieting or excessive exercise or alcohol consumption. Those volunteers who underwent imaging studies had to be right-handed and not have received more than a total of 5 rad to a radiosensitive organ or 15 rad to the body as a whole during a 12-month period As a test of the full protocol, the first volunteer, who had previously demonstrated a postmenopausal pattern of elevated pulsatile LH secretion in an earlier protocol under similar conditions [38], underwent the imaging studies before and after just 4 wks of treatment with black cohosh (as opposed to 12 weeks in the remainder of the subjects), along with the naloxone/saline challenge at the end of treatment. As outcome data from this shortened protocol demonstrated significant changes [39], they were included in the analysis of the entire study group.
Study Protocol
At baseline, volunteers were admitted at noon to the inpatient division of the GCRC of the University of Michigan Hospitals for insertion of a heparinized, indwelling IV catheter into a forearm vein. At 1pm, a 5 ml blood sample was drawn for determinations of FSH, estradiol and progesterone by the hospital ligand facility. Beginning at 3pm, blood was collected every 10 minutes until 7am the next day (16 hrs). For each 10-minute sample, two ml of blood were drawn from the indwelling cannula, then flushed with heparinized saline.
Subjects were ambulatory at will during the day and early evening in a private room, then in bed with lights turned out between 11pm and 7am of the following day. Caffeinated beverages were restricted after 1pm. Subjects were allowed to sleep on their back or on the side opposite to the blood drawing arm, changing position at will.
Presumed sleep was measured by 16 hr records of motor activity, as recorded by an actigraph unit (Mini Mitter Co.) worn on the nondominant wrist [40]. Actigraphy recordings were analyzed for activity and nonactivity using commercially available computer software (Minimitter, Bend, OR) according to the method used previously in sleep studies in menopausal women [41]. As a way to determine the impact of perceived hot flash incidence as a potential confound of sleep quality, and in turn LH pulse frequency, subjective hot flash recordings were obtained during the pulse studies using the actigraph event recorder which permits manual event entry.
A 30-day supply of the study drug was provided by the study coordinator at each monthly visit. Beginning on the morning of discharge after completing the baseline study, subjects were instructed to take one tablet of the study drug (one Remifemin 20 mg) twice daily with meals. Remifemin, contains black cohosh extract (isopropyl alcohol, 40% by volume) equivalent to 20 mg of root per tablet. This brand and dose have been used previously in several clinical trials [8, 9, 42]. Compliance was monitored by pill counts at treatment weeks 4, 8, and 12.
During weeks 11 and 12 of treatment, participants returned to repeat the 16 hr LH pulse studies along with an 8 hr saline or naloxone infusion during the hours of 3pm-11pm. To avoid interaction with the radioligand, the naloxone infusion was always administered at the second treatment visit (wk12). For the challenge test, a second IV line was placed in the opposite arm for infusions of normal saline (0.9% NaCl; 20 ml/hr) or naloxone for 8 hours at a constant rate (1 mg/m2/hr) from 12noon until 8pm with the use of an infusion pump. Naloxone infusion at this dose and duration administered during waking hours elicits LH responsiveness for at least 8 hrs following IV discontinuation [43].
Imaging Methods and Analysis
Two 90 min PET scans per subject (before and after treatment) were acquired with a Siemens (Knoxville, TN) HR+ scanner in 3-D mode (reconstructed full-width half maximum –FWHM- resolution ~5.5 mm in-plane and 5.0 mm axially), with septa retracted and scatter correction. Participants were positioned in the PET scanner gantry, and two intravenous (antecubital) lines were placed. A light forehead restraint was used to eliminate intrascan head movement. [11C]carfentanil was synthesized at high specific activity (> 2000 Ci/mmol) by the reaction of [11C]methyliodide and a nor-methyl precursor as previously described [35]. Ten to 15 mCi were administered in each of the scans, with a mass of carfentanil injected of < 0.03 μg/kg per scan. This ensured that the compound was administered in tracer quantities, i.e., subpharmacological doses occupying less than 1% of the available receptors. Fifty percent of the radiotracer doses were administered as an initial bolus, and the remaining 50% by continuous infusion for the remainder of the study. This procedure compensates for the metabolism of the radiotracer, leading to constant plasma concentrations over time and more rapid equilibration between kinetic compartments. For each study, 21 sets of scans (frames) were acquired over 90 minutes with an increasing duration (30 seconds frames × 4, 1 min × 3, 2.5 min × 2, 5 min × 8, 10 min × 4).
Images were reconstructed using iterative algorithms (brain mode; Fourier rebinning with ordered subsets-expectation maximization, 4 iterations, 16 subsets; no smoothing) into a 128 × 128 pixel matrix in a 28.8 cm diameter field of view. Attenuation correction was performed through a 6-min transmission scan (68Ge source) obtained prior to the PET study, also with iterative reconstruction of the blank/transmission data followed by segmentation of the attenuation image. Small head motions during emission scans were corrected by an automated computer algorithm for each subject before analysis, and the images were co-registered with the same software [44]. Time points were then decay-corrected during reconstruction of the PET data.
Image data were then transformed on a voxel-by-voxel basis into two sets of parametric maps: (a) a tracer transport measure (K1 ratio), and (b) a receptor-related measure (Binding Potential, BP). To avoid the need for arterial blood sampling, these measures were calculated using a modified Logan graphical analysis [45] using the occipital cortex (an area devoid of μ-opioid receptors) as reference region. The slope of the Logan plot is equal to the (f2Bmax/Kd) + 1 for this receptor site (receptor concentration divided by its affinity for the radiotracer) and it has been referred to as the Distribution Volume Ratio, DVR; f2Bmax/Kd (or DVR - 1) is the “receptor related” measure BP[46] or receptor availability in vivo. The term f2 refers to the concentration of free radiotracer in the extracellular fluid and is considered to represent a constant and very small value.
Anatomical MRI scans were acquired prior to PET scanning on a 3 Tesla scanner (General Electric, Milwaukee, WI). Acquisition sequences were axial SPGR Inverse Recovery-Prepared MR [echo time (TE) = 3.4 ms, repetition time (TR) = 10.5 ms, inversion time (TI) = 200 ms, flip angle = 25°, number of excitations (NEX) = 1, using 124 contiguous images, 1.5 mm-thickness). K1 and DVR images for each experimental period and MR images were co-registered both to each other and to the International Consortium for Brain Mapping (ICBM) stereotactic atlas orientation[47]. Statistical parametric maps of differences between conditions were generated by anatomically standardizing the T1-SPGR MRI of each subject to the ICBM stereotactic atlas coordinates, with subsequent application of this transformation to the DA D2/D3 and μ-opioid receptor binding maps. The accuracy of coregistration and non-linear warping algorithms was confirmed for each subject individually by comparing the transformed MRI and PET images both to each other and to the ICBM atlas template. To compensate for small residual anatomic variations across subjects and to improve signal to noise ratios, a three-dimensional Gaussian filter (full width at half- maximum of 6 mm) was applied to each scan.
For each subtraction analysis, two tailed, paired t statistic values were calculated for each voxel using the pooled variance across voxels [48] and the Statistical Parametric Mapping package (SPM2). In view of the small sample size of this pilot study, we accepted a p = value of 0.002 uncorrected as threshold of statistical significance.
Hormone Assays
Plasma LH was measured using an automated chemiluminiscent Immulite system (Diagnostic Products Corp, Los Angeles,CA) as previously described [49]. As a test of the adequacy of the naloxone challenge to hypothalamic function, measures of cortisol and prolactin were determined by chemiluminescent assay using the automated Immulite system, obtained from the 3pm, 11pm and 7am blood samples. All samples from a subject were measured in the same assay.
LH pulse analysis
Cluster analysis was used as the method for objective peak detection, as validated earlier [50]. The highest concentration in a pulse was designated the peak maximum; the lowest interpeak hormone concentration, the nadir; the time (min) separating consecutive peak maxima, the interpulse interval; the number of peaks per 8hr, the frequency.
Mean values for LH from the serial q10 minute measures were determined across the 16-hr sampling interval of the three studies. The distribution of hormone values were assessed for normality and natural log transformations were used to correct skew. Differences in mean concentrations and LH pulse characteristics across study days and conditions were determined by nonparametric tests for paired (Wilcoxon signed rank test) and nonpaired (Mann-Whitney test) observations. The time blocks analyzed in each of the 3 LH pulse studies were 3pm-11pm (day) and 11pm-7am (night), each providing 49 data points for analysis. LH pulse frequency is expressed as the number of LH pulses per 8 hr. For paired, within-subject comparisons of the day vs night conditions, a paired student’s t test was performed on transformed data.Values are reported as the mean ± SE.
Results
Four of seven volunteers who fulfilled all screening criteria (#s 1,2,4,5) underwent the full protocol; two others declined to undergo the neuroimaging studies (#6, 7) and another participant (#3) declined to complete the LH pulse studies. An 8th participant who reported her last menstrual period approximately 12 months prior to enrollment completed all studies but was dropped from the group analysis when she experienced vaginal spotting and presumptive ovulation (serum progesterone values >3 ng/ml) during treatment week 11. Thus, there were usable data from 6 participants for the LH pulse studies and from 5 subjects for the imaging analysis.
The mean age of the cohort was 53.2± 1 yrs; the mean BMI was 24.3 ± 1 kg/m2, and mean years postmenopause was 3.7±1 yrs. Table 1 presents the clinical characteristics of each participant during study. In all individuals, FSH concentrations were ≥ 60 IU/ml, E2 values were ≤ 25 pg/ml, and sleep efficiency rates exceeded 70 % - - a level previously demonstrated to be associated with differences in day-night LH pulsatility [38]. There were no significant differences in the mean number of recorded hot flash events across the three study days, although hot flash events were reduced in all cases during the naloxone challenge vs the saline infusion.
Table 1.
Clinical characteristics of the study participants.
| Baseline Study | Tx Wk 11 (Saline Study) | Tx Wk 12 (Naloxone Study) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Age | BMI | PM | E2 | FSH | %sleep | Hf | E2 | FSH | %Slep | Hf | E2 | FSH | %Sleep | Hf |
| 1 | 57 | 20.5 | 7 | 20 | 87 | --- | --- | 17 | 60 | 83.8% | 3 | 15 | 73 | 83.9% | 1 |
| 2 | 53 | 22.5 | 4 | 10 | 70 | 77.2% | 2 | 10 | 76 | 77.3% | 4 | 11 | 79 | 81.1% | 4 |
| 3 | 51 | 29.0 | 1 | 22 | 61 | --- | --- | 17 | 53 | --- | --- | --- | --- | --- | |
| 4 | 47 | 24.9 | 7 | 17 | 65 | 88% | 6 | 14 | 63 | 81.6% | 7 | 17 | 68 | 83.0% | 3 |
| 5 | 55 | 23.2 | 2 | 12 | 79 | 87.6% | 5 | 12 | 79 | 83.6% | 8 | 17 | 87 | 84.6% | 5 |
| 6 | 53 | 30.0 | 4 | 12 | 61 | 72.4% | 8 | 23 | 60 | 78.2% | 9 | 16 | 66 | 71.6% | 3 |
| 7 | 54 | 24.7 | 1.5 | 14 | 60 | 80.9% | 10 | 12 | 59 | 70.7% | 9 | 18 | 56 | 87.9% | 6 |
PM = postmenopausal; E2= pg/ml; FSH = mIU/ml; hormone values determined from the 1pm blood sample. Hf = hot flash events recorded on actigraph; % sleep = percent of time spent sleeping during 11pm-7am calculated from actigraph data; unavailable for subject 3 and subject 1 (baseline).
LH Pulse Analysis
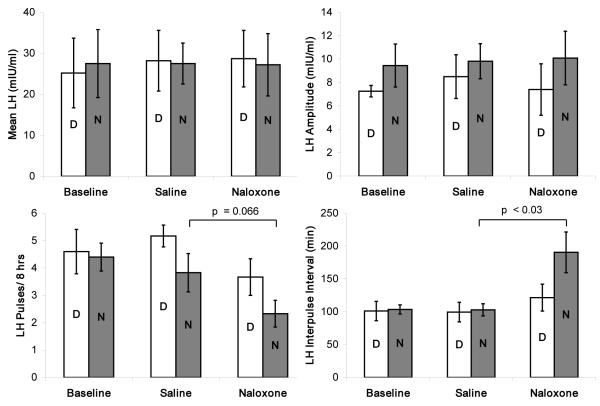
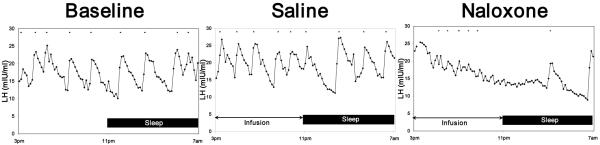
Baseline studies confirmed that the mean LH concentration and pulse pattern of the study group was typical of the postmenopause state (Table 2) and were similar during day and nighttime sampling ( DAY LH mean ± SE = 25.1 ± 8.5 mIU/ml vs NIGHT LH mean±SE = 27.6±8.2 mIU/ml). After 11 wks of black cohosh treatment, no differences in group mean LH pulse parameters were observed compared to baseline. However, with naloxone challenge, there was a trend for a significant suppression of mean LH pulse frequency and prolongation of the IPI. When the 16 hr sampling period was divided into 8 hr day-night intervals (Fig 1), the reduction in pulse frequency with naloxone infusion was most pronounced at night, resulting in a marked prolongation of the IPI by nearly 90 minutes compared to the saline study (SAL night IPI = 103± 9 mins vs NAL night IPI = 191± 31min, p = 0.03). In contrast, the expected increase in cortisol after naloxone vs saline was observed (mean ± SE SAL = 15.3 ±1.5 vs mean ± SE NAL = 16.4±.7 ng/ml; p = 0.05), with a trend for a rise in prolactin (mean ± SE SAL = 9.9±.7 units vs mean ± SE NAL = 12.2±1.1 units; p = 0.08). Figure 2 depicts a representative set of LH pulsatile patterns from the same individual across the three study days to demonstrate the effect observed in the group data.
Table 2.
Black cohosh (BC) effects on spontaneous pulsatile LH secretion and with naloxone blockade.
| Baseline | BC-Saline (wk 11) | BC-Naloxone (wk 12) | |
|---|---|---|---|
| Mean LH (mIU/ml) | 26.4±8.4 | 27.7±6.2 | 27.9±7.3 |
| pulses/16hr* | 9.0±.3 | 9.0±.6 | 6.0±.7 |
| Amp (mIU/ml) | 7.9±1.1 | 8.9±1.7 | 8.4±2.4 |
| IPI (min)** | 96.6±5.7 | 95.8±7.2 | 142.8±11.2 |
p = 0.056, naloxone vs saline; Amp = LH pulse amplitude; IPI = interpulse interval
p = 0.075 naloxone vs saline
Figure 1.
Mean LH pulse features for the day (D) and night (N) 8hr intervals in 6 postmenopause women before (basal), and during treatment with black cohosh at wk 11 (Saline) and wk 12 (Naloxone) infusion studies.
Figure 2.
LH pulsatile patterns from the same individual at baseline, and then during black cohosh (BC) treatment at wk 11 (Saline study) and wk 12 (Naloxone) to demonstrate the suppressive effect of naloxone on LH mean secretion and pulse parameters. At treatment wk 11, the pulsatile pattern was relatively unchanged vs baseline. In contrast, naloxone had a marked effect on pulse amplitude and frequency during both the wake (3pm-11pm) and sleep portions (11pm-7am) of the study, resulting in a suppression in mean LH secretion.
Neuroimaging Studies
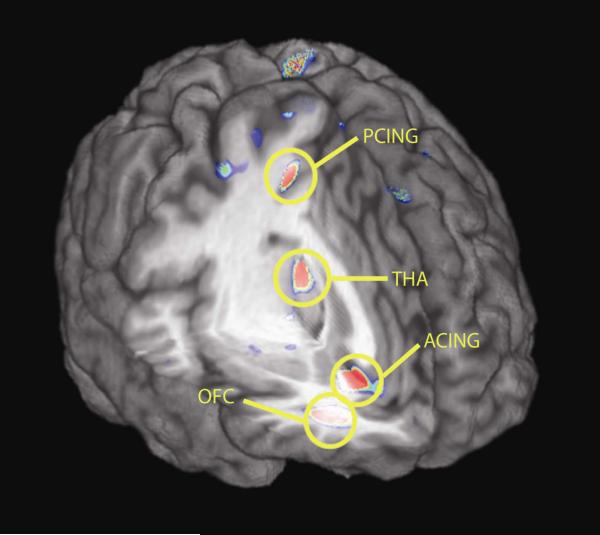
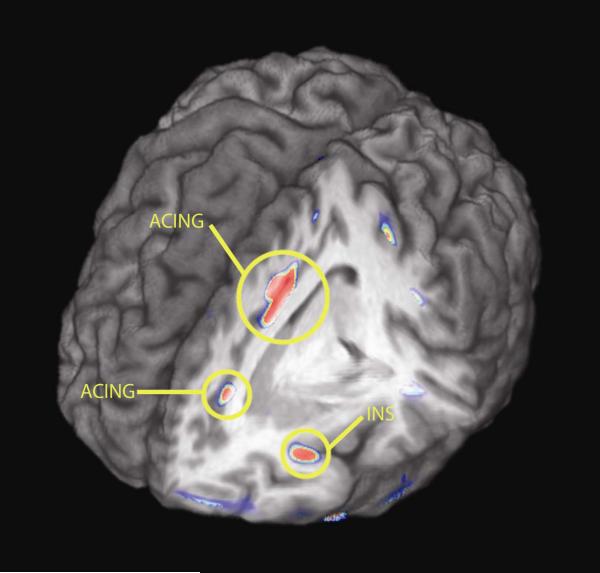
Evidence of increases in μ-opioid receptor availability (binding potential, BP) were obtained in the posterior and subgenual cingulate, orbitofrontal and temporal cortex and the dorsomedial area of the thalamus (Fig 3). Similar effects were obtained in an area that included the medial section of the nucleus accumbens, extending into the hypothalamus, although it did not reach statistical significance (Table 3). Reductions in μ-opioid receptor BP were also observed in some regions (Fig 4), however the average change for these areas was generally of lesser magnitude than the increases shown in Fig 3. Significant reductions in BP were obtained in the dorsal anterior cingulate (two peaks, one rostral, one dorsal). Changes in the same direction and of similar magnitude were also obtained in the anterior insular cortex, but they did not reach statistical significance (Table 4).
Figure 3.
Brain regions showing increases in μ-opioid receptor BP after black cohosh treatment, depicted in a 3D view. Standardized Z scores of statistical significance are represented by a pseudocolor scale with the areas in red showing the most significant differences.
Table 3.
Brain regions demonstrating significant increases in μ-opioid receptor BP after black cohosh treatment.
| Region | Coordinates | Cluster size1 |
Z-score | P- value2 |
% change BP |
|---|---|---|---|---|---|
| Subgenual ant cingulate |
5 26 −6 | 986 | 2.90 | 0.002 | 18% |
| L post cingulate | 14 −13 50 | 196 | 2.88 | 0.002 | 61% |
| L orbitofrontal cortex |
26 43 −3 | 558 | 3.01 | 0.001 | 26% |
| L temporal cortex | 24 −44 −8 | 2909 | 3.33 | 0.000 | 32% |
| M Thalamus | −4 −17 14 | 3882 | 3.96 | 0.000 | 24% |
| R NAC/HYPOTH | −9 6 −6 | 19 | 2.26 | 0.012 | 10% |
Coordinates, in mm, refer to the International Conference on Brain Mapping (also known as Montreal Neurological Institute) x, y, z stereotactic coordinates. Cluster size is expressed in mm3 for voxels with p values <0.01 within the activated area.
voxel-level, uncorrected. NAC/HYPOTH = nucleus accumbens, extending medially into the hypothalamus; M=medial; R=right side; L= left side.
Figure 4.
Brain regions showing reductions in μ-opioid receptor BP after black cohosh treatment, depicted in a 3D view. Standardized Z scores of statistical significance are represented by a pseudocolor scale with the areas in red showing the most significant differences. The most pronounced reductions took place in the anterior insular and cingulate cortex.
Table 4.
Brain regions demonstrating significant reductions in μ-opioid receptor BP after black cohosh treatment.
| Region | Coordinates | Cluster size1 |
Z-score | P- value2 |
% change BP |
|---|---|---|---|---|---|
| R dorsal Ant cingulate (1st peak) |
−12 10 47 | 130 | 2.67 | 0.004 | 14% |
| R dorsal Ant cingulate (2nd peak) |
−12 9 35 | 267 | 2.35 | 0.009 | 16% |
| R Ant insula | −38 22 −13 | 48 | 2.29 | 0.011 | 10% |
Coordinates, in mm, refer to the International Conference on Brain Mapping (also known as Montreal Neurological Institute) x, y, z stereotactic coordinates. Cluster size is expressed in mm3 for voxels with p values <0.01 within the activated area.
voxel-level, uncorrected.
DISCUSSION
Predicated on the results of in vitro and animal work, and using our well-tested approaches, we undertook a pilot study directly assessing for the first time changes in LH pulsatility and μ-opioid receptor binding activity in postmenopausal women treated with black cohosh. After 11 wks of BC treatment, spontaneous pulsatile LH secretion was unchanged compared to baseline, suggesting a lack of estrogenic action on the HPO axis. In contrast, naloxone infusion was associated with an unexpected suppression of LH pulsatility, which was more pronounced during sleep, again inconsistent with typical estrogen action. Furthermore, BC treatment resulted in bi-directional changes in μ-opioid receptor availability in vivo, varying by brain regions. Increases were observed in areas involved in cognitive and emotion processing, such as the posterior and subgenual cingulate, mesial temporal cortex and dorsomedial region of the thalamus. Reductions in availability were registered in the rostral and dorsal anterior cingulate as well as in the anterior insula. Of interest, given the high placebo response in trials with black cohosh [51], reductions in μ-opioid receptor BP have previously been described in these regions during placebo administration [52]. These were interpreted as reflecting the release of endogenous opioids and were related to individual expectations and placebo-induced psychophysical effects in the areas of pain and affective state [52, 53].
From the perspective of the neuroendocrine effects of black cohosh, earlier investigators have failed to demonstrate any effect of naloxone on gonadotropin secretion or hot flashes in postmenopausal women who are estrogen-depleted [54, 55]. However, with estrogen therapy, naloxone blockade will provoke an increase in pulse frequency and a rise in mean LH secretion [56, 57], owing to the presumed action of estrogen to restore opioid activity on the hypothalamic GnRH system. Contrary to these typical effects, here we observed that naloxone administration in the context of BC treatment was associated with LH pulsatile suppression. Thus, a direct central estrogen-like effect of BC resulting in restoration of opioid activity was not identified in our study.
Our findings argue instead for a black cohosh effect on other inhibitory neuromodulators of GnRH secretion which is only unmasked in the absence of significant opioid tone. The unusual LH response to naloxone in postmenopausal women has previously been observed when sodium valproate, presumed to affect the GABAergic system, is given concomitantly with naloxone [58]. Alternatively, serotonergic pathways may link the opioid system and/or estrogen to inhibitory control of LH release [59-62]. Electrical stimulation of the dorsal raphe nucleus in the midbrain of ovariectomized rats inhibits episodic LH release and this inhibitory influence can be reduced by depletion of brain levels of 5HT and inhibition or blockade of brain 5HT receptors [63]. In men, pretreatment with the serotonin reuptake inhibitor, fenfluramine, prevented the naloxone-induced increase in LH concentrations [64], although later studies using fluoxetine and oral naltrexone for 7 days did not replicate this effect [65].
Because this was not designed as a placebo-controlled efficacy trial, we did not obtain daily diaries of hot flash events at baseline and across the 12 wks of treatment. However, as LH pulsatility and hot flashes are believed to be co-occuring epiphenomena [66], we documented self-reported hot flash events during study days as a possible confound on sleep quality, and in turn LH pulsatility. Hot flashes were present during all three study days, with no differences between the incidence observed at baseline vs treatment week 11, suggesting the failure of black cohosh treatment to ameliorate or protect against any stress-induced hot flash trigger brought on by the burden of the frequent blood sampling protocol. Whether the modest reduction in perceived hot flashes which occurred with the naloxone challenge (vs saline) was related to the fall in LH pulses cannot be determined in the present study. Moreover, to what extent the unblinded nature of the protocol contributed to the reduction is symptoms is not known. Despite the presence of hot flashes during the study, sleep efficiency, although reduced as expected under the experimental conditions, was similar to that observed in healthy postmenopausal women without hot flashes undergoing a similar protocol [67].
In parallel with the LH pulse studies, our neuroimaging studies provide evidence for the first time that black cohosh has direct in vivo central effects which are mediated via alterations of the μ-opioid system. Specifically, BC treatment increased μ-opioid receptor availability in vivo in brain areas where estrogen effects in the same direction have been previously observed (thalamus, nucleus accumbens) [68]. In addition, increases in μ-opioid receptor binding were also obtained in the mesial temporal and orbitofrontal cortex, and subgenual and posterior anterior cingulate - - areas typically implicated in cognitive functions and cognitive-emotional integration [69-75].
Unexpectedly, we also describe regional reductions in the BP measure, which under acute challenges are interpreted as indicating the release of endogenous opioid peptides and lesser availability of receptors to bind the radiotracer [74, 76]. After the short-term treatment utilized here, these changes could represent an increase in endogenous opioid tone/release, a downregulation of receptor sites, or both. The regions involved, the rostral and dorsal anterior cingulate and anterior insula, are typically implicated in responses to sensory stimuli, particularly those with emotional significance [77] [78]. Brain regional activity in these areas is further modulated by the administration of placebos, presumably as a result of the expectations associated with the receipt of a potentially therapeutic agent [79, 80] [81]. At least in the context of the limited studies performed in humans examining these processes, the μ-opioid system appears to have a primary role in these effects [52, 82].
Given the high level of placebo responsiveness observed in BC trials for hot flashes, it is tempting to hypothesize that both pharmacological and placebo effects are taking place simultaneously, and affecting the same neurochemical substrate (the endogenous opioid system). Both effects, increases in μ-opioid receptor protein or affinity (BC pharmacological effect) and endogenous opioid release (placebo effect), would potentiate the function of this neurotransmitter system.
The hypothalamus is believed to be central to thermoregulation and the hot flash trigger [83]. Our parallel neuroimaging studies only partially confirmed an opioid receptor effect of black cohosh on the hypothalamus. Increases in the binding measure were obtained in this region below the statistically significant threshold employed, and extended into the medial section of the adjacent nucleus accumbens. Given the small size of this region, which would reduce the probability of finding significant effects, larger samples are required to answer this question.
Recent rCBF PET and fMRI imaging studies of thermoregulatory responses [84] and hot flashes [85] have reported increases in the activity of the anterior cingulate and the anterior insular cortex. These regions fully overlap with those in which the administration of black cohosh was associated with a reduction in μ-opioid receptor availability. This may suggest that hot flashes may be associated with changes in endogenous opioid system function, and that black cohosh has an impact on the same regions and neurotransmitter system. However, this hypothesis would have to be tested in a larger randomized, placebo-controlled study.
The strength of this pilot study is the comprehensive assessment of black cohosh in the same subjects with both traditional neuroendocrine and neuroimaging approaches to evaluate the potential opioidergic effects of BC. The study is however limited by its preliminary nature in a small sample of subjects, studied without placebo control or an objective measure of hot flashes. Furthermore, the effects obtained were complex at both neuroendocrine and receptor levels. In the context of short-term administrations, changes in receptor availability may represent effects on release –reductions or increases in endogenous opioid tone, or alternatively, changes in receptor protein or affinity. Although these data cannot be extrapolated to other study populations, the results obtained are nevertheless suggestive of neurobiological effects of BC affecting systems relevant to the pathophysiology of hot flashes, and warrant further investigation.
ACKNOWLEDGEMENTS
We wish to thank Julie Chilimigras, MPH (study coordinator), Pam Olton, BS (hormone assays), James Lee, BS (cluster analysis), the clinical care staff of the General Clinical Research Center, especially Jamie Frey NP, and Damanjit Grover, PAC, and Glaxo-Smith Kline for providing the study drug. We also appreciate the assistance of Professor Dan Clauw and the resources of the Michigan Institute for Clinical and Health Research (MICHR) for the use of the actigraph wrist monitors and for carrying out the sleep analysis. We are especially grateful to the women who served as research participants.
Supported in part by the University of Michigan General Clinical Research Center (NIH M01-RR00042), the University of Michigan Office of the Vice President for Research Faculty Grants and Awards Program and NIH grants AG15083 (to NKR), NCRR K23RR017043 (to YS), and AT 001415 (to JKZ).
Footnotes
Presented in part as a late-breaking paper at the 17th Annual Meeting of the North American Menopause Society, Nashville, TN, Oct 6, 2006.
References
- 1.Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med. 2002;137(10):805–13. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 2.Tice JA, et al. Phytoestrogen supplements for the treatment of hot flashes: the Isoflavone Clover Extract (ICE) Study: a randomized controlled trial. Jama. 2003;290(2):207–14. doi: 10.1001/jama.290.2.207. [DOI] [PubMed] [Google Scholar]
- 3.Lontos S, et al. Acute liver failure associated with the use of herbal preparations containing black cohosh. Med J Aust. 2003;179(7):390–1. [PubMed] [Google Scholar]
- 4.Dog T. Low, Powell KL, Weisman SM. Critical evaluation of the safety of Cimicifuga racemosa in menopause symptom relief. Menopause. 2003;10(4):299–313. doi: 10.1097/01.GME.0000056039.51813.21. [DOI] [PubMed] [Google Scholar]
- 5.Walji R. Black cohosh (Cimicifuga racemosa <L.>Nutt.): safety and efficacy for cancer patients. Support Care Cancer. 2007;15:913–921. doi: 10.1007/s00520-007-0286-z. [DOI] [PubMed] [Google Scholar]
- 6.Warnecke G. Influencing menopausal symptoms with a phytotherapeutic agent: successful therapy with Cimicifuga mono-extract. Med Welt. 1985;36:871–74. [Google Scholar]
- 7.Stoll W. Phytopharmacon influences atrophic vaginal epithelium: double-blind study--Cimicifuga vs. estrogenic substances. Therapeutikon. 1987;1:23–31. [Google Scholar]
- 8.Jacobson JS, et al. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol. 2001;19(10):2739–45. doi: 10.1200/JCO.2001.19.10.2739. [DOI] [PubMed] [Google Scholar]
- 9.Liske E, et al. Physiological investigation of a unique extract of black cohosh (Cimicifugae racemosae rhizoma): a 6-month clinical study demonstrates no systemic estrogenic effect. J Womens Health Gend Based Med. 2002;11(2):163–74. doi: 10.1089/152460902753645308. [DOI] [PubMed] [Google Scholar]
- 10.Munoz G. Hernandez, Pluchino S. Cimicifuga racemosa for the treatment of hot flushes in women surviving breast cancer. Maturitas. 2003;44(Suppl 1):S59–65. doi: 10.1016/s0378-5122(02)00349-3. [DOI] [PubMed] [Google Scholar]
- 11.Osmers R, et al. Efficacy and safety of isopropanolic black cohosh extract for climacteric symptoms. Obstet Gynecol. 2005;105(5 Pt 1):1074–83. doi: 10.1097/01.AOG.0000158865.98070.89. [DOI] [PubMed] [Google Scholar]
- 12.Newton KM, et al. Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals, soy, hormone therapy, or placebo: a randomized trial. Ann Intern Med. 2006;145(12):869–79. doi: 10.7326/0003-4819-145-12-200612190-00003. [DOI] [PubMed] [Google Scholar]
- 13.Johnson BM, van Breemen RB. In vitro formation of quinoid metabolites of the dietary supplement Cimicifuga racemosa (black cohosh) Chem Res Toxicol. 2003;16(7):838–46. doi: 10.1021/tx020108n. [DOI] [PubMed] [Google Scholar]
- 14.Kennelly EJ, et al. Analysis of thirteen populations of black cohosh for formononetin. Phytomedicine. 2002;9(5):461–7. doi: 10.1078/09447110260571733. [DOI] [PubMed] [Google Scholar]
- 15.Burdette JE, et al. Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J Agric Food Chem. 2003;51(19):5661–70. doi: 10.1021/jf034264r. [DOI] [PubMed] [Google Scholar]
- 16.Einer-Jensen N, et al. Cimicifuga and Melbrosia lack oestrogenic effects in mice and rats. Maturitas. 1996;25(2):149–53. doi: 10.1016/0378-5122(96)01052-3. [DOI] [PubMed] [Google Scholar]
- 17.Freudenstein J, Dasenbrock C, Nisslein T. Lack of promotion of estrogen-dependent mammary gland tumors in vivo by an isopropanolic Cimicifuga racemosa extract. Cancer Res. 2002;62(12):3448–52. [PubMed] [Google Scholar]
- 18.Beck V, et al. Comparison of hormonal activity (estrogen, androgen and progestin) of standardized plant extracts for large scale use in hormone replacement therapy. J Steroid Biochem Mol Biol. 2003;84(2-3):259–68. doi: 10.1016/s0960-0760(03)00034-7. [DOI] [PubMed] [Google Scholar]
- 19.Bodinet C, Freudenstein J. Influence of marketed herbal menopause preparations on MCF-7 cell proliferation. Menopause. 2004;11(3):281–289. doi: 10.1097/01.gme.0000094209.15096.2b. [DOI] [PubMed] [Google Scholar]
- 20.Raus K, et al. First-time proof of endometrial safety of the special black cohosh extract (Actaea or Cimicifuga racemosa extract) CR BNO 1055. Menopause. 2006;13(4):678–91. doi: 10.1097/01.gme.0000196813.34247.e2. [DOI] [PubMed] [Google Scholar]
- 21.Onorato J, Henion JD. Evaluation of triterpene glycoside estrogenic activity using LC/MS and immunoaffinity extraction. Anal Chem. 2001;73(19):4704–10. doi: 10.1021/ac010409m. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, et al. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J Agric Food Chem. 2001;49(5):2472–9. doi: 10.1021/jf0014157. [DOI] [PubMed] [Google Scholar]
- 23.Lupu R, et al. Black cohosh, a menopausal remedy, does not have estrogenic activity and does not promote breast cancer cell growth. Int J Oncol. 2003;23(5):1407–12. doi: 10.3892/ijo.23.5.1407. [DOI] [PubMed] [Google Scholar]
- 24.Klein K. Oerter, et al. Estrogen bioactivity in fo-ti and other herbs used for their estrogen-like effects as determined by a recombinant cell bioassay. J Clin Endocrinol Metab. 2003;88(9):4077–9. doi: 10.1210/jc.2003-030349. [DOI] [PubMed] [Google Scholar]
- 25.Dixon-Shanies D, Shaikh N. Growth inhibition of human breast cancer cells by herbs and phytoestrogens. Oncol Rep. 1999;6(6):1383–7. doi: 10.3892/or.6.6.1383. [DOI] [PubMed] [Google Scholar]
- 26.Bodinet C, Freudenstein J. Influence of Cimicifuga racemosa on the proliferation of estrogen receptor-positive human breast cancer cells. Breast Cancer Res Treat. 2002;76(1):1–10. doi: 10.1023/a:1020241509382. [DOI] [PubMed] [Google Scholar]
- 27.Wuttke W, et al. Phytoestrogens: endocrine disrupters or replacement for hormone replacement therapy? Maturitas. 2003;44(Suppl 1):S9–20. doi: 10.1016/s0378-5122(02)00344-4. [DOI] [PubMed] [Google Scholar]
- 28.Jarry H, et al. In vitro effects of the Cimicifuga racemosa extract BNO 1055. Maturitas. 2003;44(Suppl 1):S31–8. doi: 10.1016/s0378-5122(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 29.Seidlova-Wuttke D, et al. Silymarin is a selective estrogen receptor beta (ERbeta) agonist and has estrogenic effects in the metaphysis of the femur but no or antiestrogenic effects in the uterus of ovariectomized (ovx) rats. J Steroid Biochem Mol Biol. 2003;86(2):179–88. doi: 10.1016/s0960-0760(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 30.Jarry H, Gorkow C, Wuttke W. Treatment of menopausal symptoms with extracts of Cimicifuga racemosa: in vivo and in vitro evidence for estrogenic activity. Phytopharmaka Forsch. 1995:99–112. [Google Scholar]
- 31.Borrelli F, Ernst E. Cimicifuga racemosa: a systematic review of its clinical efficacy. Eur J Clin Pharmacol. 2002;58(4):235–41. doi: 10.1007/s00228-002-0457-2. [DOI] [PubMed] [Google Scholar]
- 32.Rhyu MR, et al. Black cohosh (Actaea racemosa, Cimicifuga racemosa) behaves as a mixed competitive ligand and partial agonist at the human mu opiate receptor. J Agric Food Chem. 2006;54(26):9852–7. doi: 10.1021/jf062808u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brück K, Zeisberger E. Adaptive changes in thermoregulation and their neuropharmacological basis. In: Schönbaum E, Lomax P, editors. Thermoregulation: Physiology and biochemistry. Pergamon; New York: 1990. pp. 255–307. [Google Scholar]
- 34.Zubieta JK, et al. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med. 1996;2(11):1225–9. doi: 10.1038/nm1196-1225. [DOI] [PubMed] [Google Scholar]
- 35.Jewett DM. A simple synthesis of [11C]carfentanil using an extraction disk instead of HPLC. Nucl Med Biol. 2001;28(6):733–4. doi: 10.1016/s0969-8051(01)00226-8. [DOI] [PubMed] [Google Scholar]
- 36.Rogers JL. Recruitment of women research participants: the Women’s Health Registry at the University of Michigan. J Womens Health (Larchmt) 2007;16:721–8. doi: 10.1089/jwh.2006.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith Y. Perceptions of clinical research participation among African American Woman. J of Women ’s Health. 2007;16:423–8. doi: 10.1089/jwh.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reame N. Neuroendocrinology of the Perimenopause. In: Lobo R, editor. Treatment of the Perimenopausal Woman. Lippincott, Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- 39.Reame N. A positron emission tomography (PET) study of opioid binding activity and LH secretion as a model for testing herbal estrogen-like mimics on CNS function. Fertil Steril. 2005;84:S228. [Google Scholar]
- 40.Cole RJ, et al. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 41.Regestein Q. Self-reported sleep in postmenopausal women. Menopause. 2004;11:198–207. doi: 10.1097/01.gme.0000097741.18446.3e. [DOI] [PubMed] [Google Scholar]
- 42.Duker EM, et al. Effects of extracts from Cimicifuga racemosa on gonadotropin release in menopausal women and ovariectomized rats. Planta Med. 1991;57(5):420–4. doi: 10.1055/s-2006-960139. [DOI] [PubMed] [Google Scholar]
- 43.Khoury SA, et al. Diurnal patterns of pulsatile luteinizing hormone secretion in hypothalamic amenorrhea: reproducibility and responses to opiate blockade and an alpha 2-adrenergic agonist. J Clin Endocrinol Metab. 1987;64(4):755–62. doi: 10.1210/jcem-64-4-755. [DOI] [PubMed] [Google Scholar]
- 44.Minoshima S, et al. Automated detection of the intercommissural line for stereotactic localization of functional brain images. J Nucl Med. 1993;34(2):322–9. [PubMed] [Google Scholar]
- 45.Logan J. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Mintun MA, et al. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15(3):217–27. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- 47.Meyer CR, et al. Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Med Image Anal. 1997;1(3):195–206. doi: 10.1016/s1361-8415(97)85010-4. [DOI] [PubMed] [Google Scholar]
- 48.Worsley KJ, et al. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12(6):900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 49.Reame N.e.a. Differential effects of aging on activin A and its Binding protein, follistatin, across the menopause transition. Fertil Steril. 2007;88:1003–05. doi: 10.1016/j.fertnstert.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250(4 Pt 1):E486–93. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 51.Pockaj B, Gallagher J. Phase III Double-Blind, Randomized, Placebo-Controlled Crossover Trial of Black Cohosh in the Management of Hot Flashes: NCCTG Trial N01CC. J Clin Oncol. 2006;24:2836–2841. doi: 10.1200/JCO.2005.05.4296. [DOI] [PubMed] [Google Scholar]
- 52.Zubieta JK, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25(34):7754–62. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott D. Opposite dopamine and opioid responses define placebo and nocebo effects. Arch Gen Psychiatry. 2008 doi: 10.1001/archgenpsychiatry.2007.34. in press. [DOI] [PubMed] [Google Scholar]
- 54.DeFazio J, et al. The effects of naloxone on hot flashes and gonadotropin secretion in postmenopausal women. J Clin Endocrinol Metab. 1984;58(3):578–81. doi: 10.1210/jcem-58-3-578. [DOI] [PubMed] [Google Scholar]
- 55.Lightman SL, et al. Climacteric flushing: clinical and endocrine response to infusion of naloxone. Br J Obstet Gynaecol. 1981;88(9):919–24. doi: 10.1111/j.1471-0528.1981.tb02229.x. [DOI] [PubMed] [Google Scholar]
- 56.Ropert JF, Quigley ME, Yen SS. Endogenous opiates modulate pulsatile luteinizing hormone release in humans. J Clin Endocrinol Metab. 1981;52(3):583–5. doi: 10.1210/jcem-52-3-583. [DOI] [PubMed] [Google Scholar]
- 57.Reid RL, Quigley ME, Yen SS. The disappearance of opioidergic regulation of gonadotropin secretion in postmenopausal women. J Clin Endocrinol Metab. 1983;57(6):1107–10. doi: 10.1210/jcem-57-6-1107. [DOI] [PubMed] [Google Scholar]
- 58.Popovic V. Effect of sodium valproate on luteinizing hormone secretion in pre- and postmenopausal women and its modulation by naloxone infusion. J Clin Endocrinol Metab. 1996;81:2520–2524. doi: 10.1210/jcem.81.7.8675571. [DOI] [PubMed] [Google Scholar]
- 59.Johnson MD. Acute effects of estradiol on circulating luteinizing hormone and prolactin concentrations and on serotonin turnover in individual brain nuclei. Endocrinology. 1983;113:1935–41. doi: 10.1210/endo-113-6-1935. [DOI] [PubMed] [Google Scholar]
- 60.Johnson MD. Effects of opiate antagonists on serotonin turnover and on luteinizing hormone and prolactin secretion in estrogen- or morphine-treated rats. Neuroendocrinology. 1984;38:322–7. doi: 10.1159/000123911. [DOI] [PubMed] [Google Scholar]
- 61.Johnson MD. Role of central serotonin systems in the stimulatory effects of ovarian hormones and naloxone on luteinizing hormone release in female rats. Endocrinology. 1986;118:1180–86. doi: 10.1210/endo-118-3-1180. [DOI] [PubMed] [Google Scholar]
- 62.Gore A, Terasawa E. Neural circuits regulating pulsatile luteinizing hormone release in the female guinea-pig: opioid, adrenergic and serotonergic interactions. J Neuroendocrinol. 2001;13:239–248. doi: 10.1046/j.1365-2826.2001.00618.x. [DOI] [PubMed] [Google Scholar]
- 63.Arendash G. Serotonin involvement in the inhibition of episodic luteinizing hormone release during electrical stimulation. Endocrinology. 1978;102:1199–1206. doi: 10.1210/endo-102-4-1199. [DOI] [PubMed] [Google Scholar]
- 64.Foresta C. Naloxone reduces the fenfluramine-induced prolactin release in man. Clin Endocrinol. 1985;22:539–43. doi: 10.1111/j.1365-2265.1985.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 65.Urban R, Veldhuis JD. Effects of short-term stimulation of serotoninergic pathways on the pulsatile secretion of luteinizing hormone in the absence and presence of acute opiate-receptor blockage. J Androl. 1990;11:227–32. [PubMed] [Google Scholar]
- 66.Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. discussion 123-33. [DOI] [PubMed] [Google Scholar]
- 67.Lukacs JL, et al. Midlife women’s responses to a hospital sleep challenge: aging and menopause effects on sleep architecture. Journal of Women’s Health. 2004;13(3):333–40. doi: 10.1089/154099904323016491. [DOI] [PubMed] [Google Scholar]
- 68.Smith YR, et al. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci. 2006;26(21):5777–85. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Minoshima S, et al. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 70.Wagner A, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 71.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386:824-827. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 72.Mayberg H. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 73.Drevets WC, Raichie M. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition and Emotion. 1998;12:353–385. [Google Scholar]
- 74.Zubieta JK, et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry. 2003;60(11):1145–53. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- 75.Kennedy S, et al. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63:1129–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- 76.Zubieta JK, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293(5528):311–5. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 77.Rainville P. Pain Affect Encoded in Human Anterior Cingulate But Not Somatosensory Cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 78.Phan K. Functional Neuroanatomy of Emotion: A Meta-Analysis of Emotion Activation Studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 79.Petrovic P, et al. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295(5560):1737–40. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 80.Lieberman M. The neural correlates of placebo effects: a disruption account. Neuroimage. 2004;22:447–455. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 81.Wager TD. Placebo-Induced Changes in fMRI in the Anticipation and Experience of Pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 82.Wager T, DJ S, Zubieta J. Placebo effects on human μ-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104:11056–61. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Freedman RR. Physiology of hot flashes. Am J Human Biol. 2001;13(4):453–64. doi: 10.1002/ajhb.1077. [DOI] [PubMed] [Google Scholar]
- 84.Craig A. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 85.Freedman RR. Cortical activation during menopausal hot flashes. Fertil Steril. 2006;85:674–678. doi: 10.1016/j.fertnstert.2005.08.026. [DOI] [PubMed] [Google Scholar]