Abstract
Despite the worldwide extent of methamphetamine dependence, no medication has been shown to effectively treat afflicted individuals. One relatively unexplored approach is modulation of cholinergic system function. Animal research suggests that enhancement of central cholinergic activity, possibly at nicotinic acetylcholine receptors (nAChRs), can reduce methamphetamine-related behaviors. Further, preliminary findings indicate that rivastigmine, a cholinesterase inhibitor, may reduce craving for methamphetamine after administration of the drug in human subjects. We therefore performed a double-blind, placebo-controlled, crossover pilot study of the safety and tolerability of varenicline in eight methamphetamine-dependent research subjects. Varenicline is used clinically to aid smoking cessation, and acts as a partial agonist at α4b2 nAChRs with full agonist properties at α7 nAChRs. Oral varenicline dose was titrated over one week to reach 1 mg twice daily, and then was co-administered with 30 mg methamphetamine, delivered in 10 intravenous (iv) infusions of 3 mg each. Varenicline was found to be safe in combination with iv methamphetamine, producing no cardiac rhythm disturbances or alterations in vital sign parameters. No adverse neuropsychiatric sequelae were detected either during varenicline titration or following administration of methamphetamine. The results suggest that varenicline warrants further investigation as a potential treatment for methamphetamine dependence.
Keywords: varenicline, methamphetamine, treatment, safety, nicotinic, acetylcholine
Introduction
Methamphetamine (MA) abuse is a global public health problem with a large public health impact.1 In 2008, it was estimated that there were 100,000–200,000 MA-dependent individuals in the United States.2 Nonetheless, no medications are approved for the treatment of MA dependence.3
Studies in animal models suggest that enhancement of central cholinergic activity, possibly at nicotinic acetylcholine receptors (nAChRs), can reduce MA-related behaviors. For example, administration of either nicotine or donepezil, a cholinesterase inhibitor, reduced reinstatement induced by exposure to MA-related cues and by administration of priming doses of MA rats.4 In addition, administration of the alkaloid lobeline, which has high affinity for nicotinic acetylcholine receptors (nAChRs) and also inhibits the function of vesicular monoamine and dopamine transporters, can reduce behavioral and biochemical markers of addiction in rodent models of stimulant dependence.5–7 Lastly, preliminary findings indicate that rivastigmine, a cholinesterase inhibitor, may reduce positive subjective effects of MA in human subjects.8
On the basis of these findings, we hypothesized that varenicline, which is used clinically as a treatment for smoking cessation and is a partial agonist at α4b2 nAChRs and a full agonist at α7 nAChRs,9 may be a useful treatment for MA dependence. The purpose of this pilot study was to evaluate the safety and tolerability of varenicline when co-administered with MA.
Methods
Subjects
Twelve nontreatment-seeking MA-dependent research participants were enrolled in this research study after receiving a detailed explanation of the study and giving written informed consent as approved by the UCLA Institutional Review Board.
Participants were excluded if they had major medical illness as determined by medical history, physical examination, screening electrocardiogram (EKG), and laboratory assay. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) was administered to establish MA dependence (inclusion criterion) and to exclude those participants who met criteria for other current Axis I diagnoses (including dependence on other drugs of abuse or alcohol) other than nicotine or marijuana dependence. Any history of recent psychotropic medication use was exclusionary. The participants selected were active tobacco smokers and had recently used MA (as assessed via urine test) at screening. Participants resided at the UCLA General Clinical Research Center (GCRC) for the duration of the study (18 days divided into two phases; see below), and were monitored via daily urine and alcohol breath testing to confirm abstinence from alcohol, MA, opiates, cannabinoids, PCP, and cocaine. Participants were asked to abstain from MA for at least four days before admission to inpatient phases of the study, and were required to have an MA-free urine drug screen prior to both admissions. All participants were compensated for their participation.
Study design
The study was conducted on an inpatient basis in two crossover phases of varenicline or placebo administration, separated by a two- to four-week washout period. In phase I, qualified participants were tested with 30 mg intravenous (iv) MA (over 2.5 hours) on day 1 prior to initiating study medication, to exclude any who might have an exaggerated response to iv MA alone. Given tolerability of IV MA administration, on day 3 in the evening, participants were given the first dose of the first test compound condition (0.5 mg varenicline or placebo). They received this treatment once in the evening for two days, then twice daily for two days, then a double dose (ie, 1.0 mg varenicline or placebo) twice daily for three days, and then a final dose (1.0 mg varenicline or placebo) on the morning of the last day of the first phase (day 10). Participants were given a self-report questionnaire in the morning on each inpatient day to monitor depressive symptoms (Beck Depression Inventory [BDI]).10 Participants were discharged from the hospital after all study procedures were complete on day 10.
After at least 14 days, participants returned to complete phase II of inpatient procedures, which were identical to those on days 3–10 of phase I of the study, but with the opposite test compound condition (varenicline or placebo), and were discharged again after all procedures on day 8.
Participants returned for assessment of any delayed adverse events on a follow-up visit at least 14 days after discharge from phase II of the study, after which time they completed the study.
MA challenge sessions
Participants received double-blinded challenge sessions with iv infusions of either MA or saline in the mornings and afternoon of days 1 and 9 of phase I, and day 7 of phase II. All infusion procedures were performed and monitored by study physicians (TZ and KM). Via a patient-controlled analgesia (PCA) pump, either 3 mg of MA or saline was infused intravenously over 2 minutes, every 15 minutes, for a total of 10 infusions (30 mg MA or saline over 2.5 hours). In the afternoons of the same study days, the challenge sessions were repeated with the opposite infusion condition (10 infusions of 3 mg MA or saline). Participants received a total of 30 mg of iv MA during each challenge day. During challenge sessions, participants had their blood pressure and heart rate monitored frequently, and the EKG was evaluated via continuous cardiac monitoring.
Data analysis
All data were statistically analyzed as raw scores (Number Cruncher Statistical Systems, Kaysville, UT; SPSS Inc., Chicago, IL). The data obtained during challenge sessions included cardiovascular measures (heart rate and blood pressure), which were analyzed by three-factor, repeated measures analysis of variance (ANOVA) with Test Compound (varenicline, placebo), Infusion (MA, saline) and Time (−15, 5, 20, 35, 50, 65, 80, 95, 110, 125, and 140 minutes) as factors. The data were also assessed across the study duration on cardiovascular parameters (measured once daily) and BDI using a two-factor, repeated measures ANOVA with Test Compound and Day (1–8) as factors. Following each ANOVA, Tukey–Kramer post hoc tests were conducted if a significant main effect or interaction was detected. The data from the BDI were also analyzed across study conditions by independent-samples t-test. The data from adverse events frequencies were analyzed by one-sided Fisher’s exact test. Effects were considered significant at P < 0.05.
Results
Demographics
Of the 12 enrolled participants, eight subjects completed both phases of the study (six males, two females; see Table 1). Three subjects voluntarily withdrew from the study due to personal reasons, and one was excluded before initiating study procedures due to positive urine drug testing after admission. Six of the completing subjects were Caucasian, one was Hispanic, and one was African–American.
Table 1.
Demographic characteristics
| Mean (SD) | |
|---|---|
| Age (years) | 39.3 (5.8) |
| Education (years) | 12.5 (2.0) |
| Body mass index | 24.6 (3.4) |
| Cigarettes smoked | |
| Per day | 13.4 (8.3) |
| Number of years | 16.6 (10.2) |
| Alcoholic drinks consumed | |
| Per week | 1.2 (1.4) |
| Number of years | 13.7 (6.9) |
| MA use | |
| Number of days in the past 30 days | 22.9 (9.7) |
| Number of years | 15.3 (8.8) |
| Marijuana use | |
| Number of days in the past 30 days | 5.3 (11.2) |
| Number of years | 10.8 (13.5) |
| Cocaine use | |
| Number of days in the past 30 days | 0.0 (0.0) |
| Number of years | 1.9 (3.4) |
| Opioids use | |
| Number of days in the past 30 days | 0.0 (0.0) |
| Number of years | 2.4 (6.0) |
| Hallucinogen use | |
| Number of days in the past 30 days | 0.0 (0.0) |
| Number of years | 1.8 (1.8) |
| Sedatives use | |
| Number of days in the past 30 days | 0.0 (0.0) |
| Number of years | 0.6 (0.9) |
Notes: The demographic data are expressed as mean values for eight participants. The bracketed values indicate standard deviation.
Adverse events (AEs)
No serious adverse events were reported during the study. Six participants reported at least one mild AE when they received placebo, and seven participants reported at least one mild AE when they received varenicline (see Table 2 for AEs). Of the 12 enrolled participants, 11 took part in at least one Test Compound condition. Of the three subjects who discontinued the study for personal reasons, two were receiving placebo and one was receiving varenicline. The most commonly reported AE was insomnia: six participants sporadically reported insomnia during varenicline maintenance, and six participants sporadically reported insomnia during placebo maintenance. The BDI score of only one participant exceeded 15 during varenicline dose titration. Other infrequently reported AEs were headache, nausea, diarrhea and constipation. Fisher’s exact test, performed to compare the frequencies of reported study condition-related adverse events between the varenicline and placebo conditions, revealed no overall differences.
Table 2.
Summary of adverse events
| Adverse events | Varenicline | Placebo | p |
|---|---|---|---|
| Insomnia | 67% | 55% | 0.47 |
| Headache | 33% | 9% | 0.22 |
| Nausea | 11% | 9% | 0.71 |
| Depressed mood; | 11% | 0% | 0.45 |
| BDI > 15 | |||
| Constipation | 0% | 18% | 0.29 |
Cardiovascular measures
The three-factor, repeated-measures ANOVA revealed a significant main effect of Time and interaction of Time × Infusions on systolic pressure (Table 3). Post hoc analyses indicated that MA infusions significantly increased systolic pressure at several time points (35, 65, 80, 95, 110, 125, and 140 minutes) relative to that at −15 minutes, regardless of varenicline or placebo administration. Saline infusions did not significantly alter systolic pressure across time, regardless of varenicline or placebo administration (Figure 1, top panel).
Table 3.
Summary of F statistics
| Test compound (df1,7) | Infusion (df1,7) | Time (df10,70) | Test compound × Infusion(df1,7) | Test Compound × Time (df10,70) | Infusion × Time (df10,70) | Test compound×) Infusion × Time (df10,70) | |
|---|---|---|---|---|---|---|---|
| Cardiovascular measures | |||||||
| Systolic pressure | 0.2 | 3.23 | 2.06 | 1.1 | 0.54 | 11.39 | 1.46 |
| Diastolic pressure | 1.25 | 0.3 | 2.83 | 2.5 | 0.82 | 5.34 | 3.39 |
| Heart rate | 0.27 | 0.85 | 5.07 | 7.56 | 1.67 | 7.09 | 0.28 |
| Test compound (df1,7) | Day (df7,49) | Test compound× Day (df7,49) | |||||
| Beck depression inventory (BDI) | 2.10 | 1.35 | 1.00 | ||||
Figure 1.
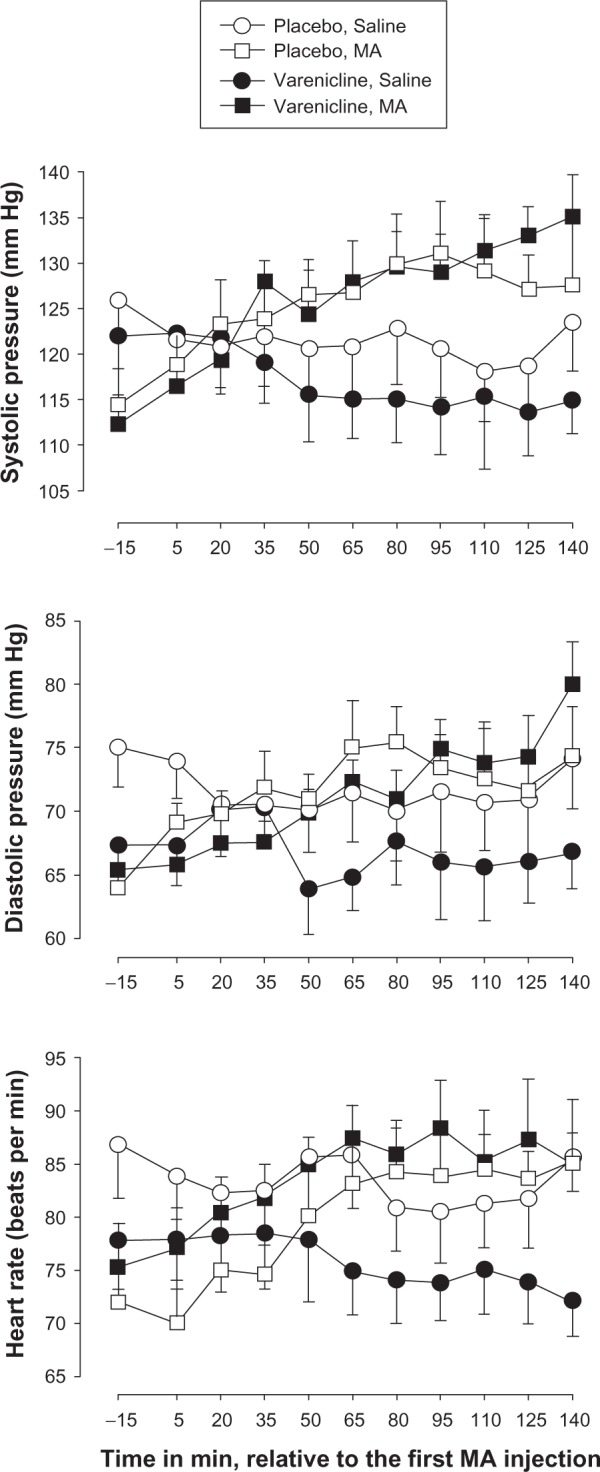
Time-course of effects of methamphetamine (MA) and saline varenicline or placebo maintenance on systolic pressure (top panel), diastolic pressure (middle panel) and heart rate (bottom panel).
Notes: The data collected during infusion sessions in phase I (day 9) and phase II (day 7) are shown. X-axis: Time in min, relative to the first MA infusion. Data are expressed as mean of eight participants. Unidirectional brackets indicate one standard error of mean.
For diastolic pressure, the three-factor repeated-measure ANOVA revealed a significant main effect of Time, interaction of Time × Infusions and interaction of Time × Infusions × Test Compound (Table 3). Post hoc analyses indicated that MA significantly increased diastolic pressure at several time points (65, 80, 95, 110, 125, and 140 minutes) relative to that at −15 minutes. Saline infusions did not significantly alter diastolic pressure across time. Varenicline did not significantly modify MA-induced enhancement in diastolic pressure across time. Although there was a trend for varenicline to reduce baseline diastolic pressure across time relative to that observed after placebo (see Figure 1, middle panel, open and closed circles), the trend did not achieve statistical significance.
For heart rate, the three-factor, repeated measures ANOVA revealed a significant main effect of Time, interaction of Time × Infusions and interaction of Infusions × Study Drug (Table 3). Post hoc analyses indicated that MA significantly increased heart rate at several time points (65, 80, 95, 110, 125, and 140 minutes) relative to that at −15 minutes, regardless of varenicline or placebo administration. Saline infusions did not significantly alter heart rate across time, regardless of varenicline or placebo administration. Varenicline did not significantly modify MA-induced enhancement in heart rate across time. Although there was a trend for varenicline to reduce baseline heart rate relative to placebo (Figure 1, bottom panel, open and closed circles), the trend did not achieve statistical significance.
Considering changes in cardiovascular effects over the course of the study, the two-factor repeated-measure ANOVA did not reveal significant effects of Day, Test Compound, or interaction on systolic pressure, diastolic pressure, and heart rate (Table 3). The daily administration of varenicline or placebo did not affect cardiovascular parameters across days.
Depressive symptoms
The two-factor, repeated measures ANOVA did not reveal a significant main effect of Test Compound, Day and interaction of Test Compound × Day (Table 3) on BDI scores. Because varenicline did not significantly affect depressive symptoms across days, the data were combined across days, and presented as a function of Test Compound in Figure 2. There was no significant difference in the average BDI reported by participants across study conditions (Figure 2). No suicidal thoughts were recorded by any participant at any time during the study.
Figure 2.
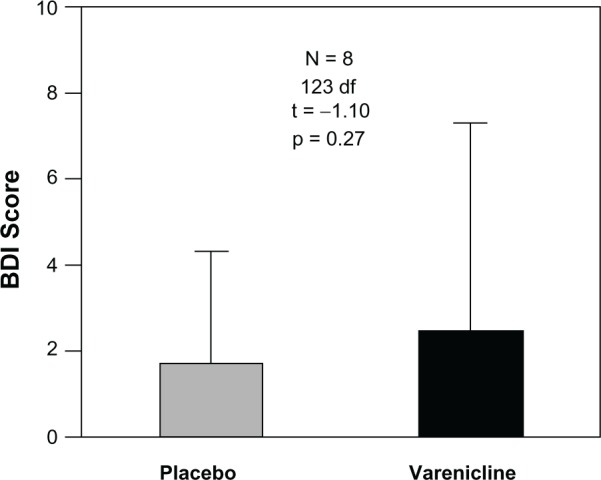
Average BDI scores across study conditions during test compound administration (varenicline and placebo). BDI scores were recorded in the mornings after each dose of both varenicline and placebo study conditions, and were averaged across both study conditions and participants.
Notes: Bars represent mean of eight participants and 64 measurements for placebo, 63 for varenicline. Unidirectional brackets indicate one standard deviation.
Abbreviation: BDI, Beck’s Depression Inventory.
Discussion
Varenicline (2 mg daily dose) was well tolerated in the MA-dependent participants who also were cigarette smokers, and had been pre-screened to exclude individuals with substantial medical or psychiatric co-morbidities. No significant differences were observed between the effects of varenicline and placebo in terms of overall adverse events, depressive symptoms, or cardiovascular parameters. Moreover, there was no association between the varenicline treatment and propensity to discontinue the study. Therefore, the present data are consistent with the previously documented safety and tolerability of varenicline treatment in healthy tobacco-dependent subjects with no pre-existing psychiatric or medical conditions.9,11–13
While randomized controlled trials have shown varenicline to be safe and well-tolerated in healthy participants, many case reports of neuropsychiatric disturbances coincident with varenicline treatment have been published recently.14 Post-marketing surveillance by the US Food and Drug Administration has led to the issuance of a “black box warning” about the potential for “serious neuropsychiatric symptoms” for varenicline in July of 2009.15 These concerns have also been addressed by two recent retrospective studies.16,17 In a cohort study of 2700 patients taking varenicline, two suicide attempts were noted in patients with pre-existing during psychiatric illness.16 In a retrospective review of 50 patients followed through 12 weeks of varenicline therapy, each of to the four patients who discontinued varenicline use due to mood problems had pre-existing psychiatric conditions.17 Taken together, these early reports provide preliminary evidence that varenicline treatment may be associated with a worsening of psychiatric symptoms in a minority of individuals with a history of psychiatric illness. However, the present results, combined with the findings from randomized controlled trials,9,11–13 suggest that varenicline is not likely to be associated with neuropsychiatric sequelae in individuals without a history of psychiatric disease.
To our knowledge, our study is the first to address the safety or tolerability of varenicline when co-administered with a stimulant in humans. Varenicline is well-absorbed orally irrespective of dose or presence of food, is primarily excreted in the urine unmetabolized, and has no documented drug–drug interactions.18 Therefore, there is no a priori reason that it should interact clinically with MA, which is primarily hepatically metabolized by the cytochrome p450 system.19 Along with the lack of known metabolic interactions for varenicline, our data should provide reassurance to clinicians who may be concerned that patients taking varenicline may be illicitly abusing stimulants, as there is not likely to be additional risk via a potentially harmful interaction between varenicline and MA.
There are several limitations of this pilot study, including the small sample size although there is not even a trend for untoward interactions between varenicline and MA at the doses given. In addition, the repeated dosing with a low dose (3 mg) of iv MA may not allow generalization of these data to clinical populations of MA-dependent patients, who may take substantially greater doses of MA. Lastly, as varenicline was administered over a relatively short period (slightly longer than one week) the present results may not be representative of findings in clinical populations taking varenicline for longer periods of time.
Given the lack of neuropsychiatric adverse events seen in otherwise healthy, MA-dependent participants while taking varenicline, along with the safety and tolerability of varenicline when co-administered with iv MA, we believe that varenicline should continue to be tested as a possible candidate medication for the treatment of MA dependence. In this vein, other investigators have also proposed that varenicline may have utility for the treatment of addictive disorders beyond nicotine dependence.20 Overall, the present data suggest that varenicline warrants further investigation as a potential treatment for MA dependence.
Footnotes
Disclosure
This work was supported by NIH grants P50DA018185 (SS), P20DA022539 (EDL) and MOI RR00865 (UCLA GCRC), an endowment from the Thomas P and Katherine K Pike Chair in Addiction Studies, and by a generous gift from the Marjorie M Greene Trust. The authors report no conflicts of interest in this work.
References
- 1.Buxton JA, Dove NA. The burden and management of crystal meth use. CMAJ. 2008;178(12):1537–1539. doi: 10.1503/cmaj.071234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration, Office of Applied Studies . The DASIS Report – Primary Methamphetamine/Amphetamine Admissions to Substance Abuse Treatment: 2005. Rockville, MD: Substance Abuse and Mental Health Services Administration; Feb 7, 2008. [Google Scholar]
- 3.Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J. Pharmacotherapy of methamphetamine addiction: an update. Subst Abus. 2008;29(3):31–49. doi: 10.1080/08897070802218554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci U S A. 2006;103(22):8523–8527. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002;63(2):89–98. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- 6.Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT. Lobeline attenuates d-methamphetamine self-administration in rats. J Pharmacol Exp Ther. 2001;298(1):172–179. [PubMed] [Google Scholar]
- 7.Neugebauer NM, Harrod SB, Stairs DJ, Crooks PA, Dwoskin LP, Bardo MT. Lobelane decreases methamphetamine self-administration in rats. Eur J Pharmacol. 2007;571(1):33–38. doi: 10.1016/j.ejphar.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De La Garza R, 2nd, Mahoney JJ, 3rd, Culbertson C, Shoptaw S, Newton TF. The acetylcholinesterase inhibitor rivastigmine does not alter total choices for methamphetamine, but may reduce positive subjective effects, in a laboratory model of intravenous self-administration in human volunteers. Pharmacol Biochem Behav. 2008;89(2):200–208. doi: 10.1016/j.pbb.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 10.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 11.Nides M, Glover ED, Reus VI, et al. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. Am J Health Behav. 2008;32(6):664–675. doi: 10.5555/ajhb.2008.32.6.664. [DOI] [PubMed] [Google Scholar]
- 12.Faessel H, Ravva P, Williams K. Pharmacokinetics, safety, and tolerability of varenicline in healthy adolescent smokers: a multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2009;31(1):177–189. doi: 10.1016/j.clinthera.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Garrison GD, Dugan SE. Varenicline: a first-line treatment option for smoking cessation. Clin Ther. 2009;31(3):463–491. doi: 10.1016/j.clinthera.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Pirmoradi P, Roshan S, Nadeem SS. Neuropsychiatric disturbance after initiation of varenicline in a patient with a history of alcohol abuse and major depression. Am J Health Syst Pharm. 2008;65(17):1624–1626. doi: 10.2146/ajhp070641. [DOI] [PubMed] [Google Scholar]
- 15.US Food Drug Administration Chantix and Zyban to Get Boxed Warning on Serious Mental Health Events. Jul 2, 2009. [Accessed on November 1, 2009]. Available from: http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm170356.htm.
- 16.Kasliwal R, Wilton LV, Shakir SA. Safety and drug utilization profile of varenicline as used in general practice in England: interim results from a prescription-event monitoring study. Drug Saf. 2009;32(6):499–507. doi: 10.2165/00002018-200932060-00006. [DOI] [PubMed] [Google Scholar]
- 17.Purvis TL, Mambourg SE, Balvanz TM, Magallon HE, Pham RH. Safety and effectiveness of varenicline in a veteran population with a high prevalence of mental illness. Ann Pharmacother. 2009;43(5):862–867. doi: 10.1345/aph.1L661. [DOI] [PubMed] [Google Scholar]
- 18.Jiménez-Ruiz C, Berlin I, Hering T. Varenicline: a novel pharmacotherapy for smoking cessation. Drugs. 2009;69(10):1319–1338. doi: 10.2165/00003495-200969100-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kraemer T, Maurer HH. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277–289. doi: 10.1097/00007691-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 20.McKee SA, Harrison EL, O’Malley SS, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66(2):185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


