Abstract
Purpose
To estimate the reproducibility of BOLD MRI measurements in the evaluation of intrarenal oxygenation levels.
Materials and Methods
In this study, the reproducibility of semiquantitative BOLD MRI measurements performed on a 1.5 T scanner with a multiple gradient-echo sequence in the renal medulla and cortex, and their response to furosemide and waterload, were assessed in eight healthy young subjects (25.6 ± 4.1 years). Each subject underwent an identical experimental procedure on two separate days.
Results
Renal measurements were shown to be reproducible within ~12% from day to day based on a coefficient of variance (CV) analysis. The changes in following administration of furosemide were statistically significant, as shown by ANOVA and a paired Student’s t-test, and were deemed reliable based on the reliable change index (RCI). However, values following waterload were not statistically significant, and were not deemed reliable.
Conclusion
measurements were reproducible over 270 days within 12%. Furosemide produced a significant and reliable change (~30%), and the magnitude of change (5.7 s−1) was reproducible and consistent with our previous data. The response to waterload, however, did not reach statistical significance, and the magnitude did not reach the level that we had previously reported.
Keywords: Kidney, BOLD MRI, reproducibility, oxygenation, furosemide, water diuresis
It is now well recognized that the renal medulla operates at a low oxygenation level (renal medullary hypoxia) (1), which makes it highly susceptible to even mild reductions in blood flow. Renal ischemia accounts for almost 50% of the observed cases of acute renal failure (2). Over the years, in several in vitro and in vivo animal studies, it has been shown that this type of acute renal failure usually involves hypoxic injury to the renal medullary tubules (3). Renal dysfunction may also play a role in the development of all forms of hypertension in humans and laboratory animals (4). Many experiments have shown that medullary blood flow (and presumably medullary oxygenation status) is reduced in hypertension, and, more importantly, that reduced medullary blood flow is sufficient to produce hypertension (4,5). All of these studies were performed with the use of invasive microelectrodes or Doppler flow probes in rat kidneys. The availability of a noninvasive technique to monitor renal medullary blood flow in humans under normal conditions and during physiological and pharmacological stresses may allow for an extension of these observations to humans.
Blood oxygenation level-dependent (BOLD) MRI has been used extensively in various organs, such as the brain (6,7). The BOLD MRI technique exploits the fact that the magnetic properties of hemoglobin vary depending on whether it is in the oxygenated or deoxygenated form. This affects the relaxation time of the neighboring water molecules, and in turn influences the MRI signal on - weighted images. The rate of spin dephasing is closely related to the tissue content of deoxyhemoglobin. Since the oxygen tension (pO2) of capillary blood is thought to be in equilibrium with the surrounding tissue, changes estimated by BOLD MRI can be interpreted as changes in tissue pO2 (8). A strong correspondence has been demonstrated between BOLD MRI measurements in humans and earlier animal data obtained with the use of invasive microelectrodes (9).
In previous studies, we used BOLD MRI to measure the response of the renal medulla to furosemide in healthy volunteers (8,10). Furosemide is one of the so-called “loop” diuretics. These diuretics have been reported to improve medullary oxygenation, presumably because they selectively decrease oxygen use by blocking active transport in the thick ascending limbs (1,9). One of the main findings in previous studies was that the increased average level of medullary oxygenation in young healthy subjects following pharmacological maneuvers can be monitored noninvasively with BOLD MRI (8,11).
Water diuresis is the result of reduced osmotic reabsorption of water, which leads to increased solute- free water clearance and hypoosmotic urine. Water diuresis can potentially “washes out” obstructing cellular debris and casts, and thus may protect against acute renal failure (12). Water diuresis can be induced by drinking large amounts of hypotonic fluid. During water diuresis, the amount of water reabsorbed in the proximal tubules is normal, and the maximal urine flow that can be produced is about 16 ml/min. Our previous studies showed that water diuresis decreased intrarenal in young healthy human subjects evaluated by BOLD MRI (10,13,14).
Since BOLD MRI is primarily used to assess changes following a physiological or pharmacological maneuver, it is essential that we understand the level of reproducibility of baseline measurements (i.e., how close are the measurements obtained in the same subject under similar conditions on two different days or times?). This is usually estimated by means of a test-retest analysis. Another significant measurement is the response to a maneuver ( ). In this case, it is important to know how the magnitude of response compares to variations in baseline , and how much this magnitude varies from day to day. The reproducibility of baseline and would depend not only on the variability associated with the measurement system, but also on the physiological status of the subject at these two different time points (especially in a water diuresis study). To minimize the effects of ingested substances on the BOLD test, an overnight fast is required to ensure that the volunteer is in the basal state. However, the pre- fast ingestion of a salty, sweet, or fatty food, or alcohol or coffee may still induce some physiological variations. Exercise and emotional or physical stress may also contribute to physiological variations.
In this study, we investigated the degree of reproducibility of BOLD measurements in the renal medulla and cortex in the same group of healthy individuals before and after administration of furosemide and induction of water diuresis on different days.
MATERIALS AND METHODS
Subjects
Eight healthy young subjects (four females and four males, mean age 25.6 ± 4.1 years) participated in this study. None of the subjects had any known history of renal disease. After the study was explained to them, each volunteer gave informed consent according to a protocol approved by the institutional review board.
The subjects took part in the study after they abstained from food and water for about 12 hours overnight. A coronal plane passing through the center of both kidneys was prescribed for the scout images, and baseline BOLD MRI images were acquired along several axial planes. For the furosemide studies, 20 mg of furosemide were injected intravenously. Post-furosemide images were acquired starting about 5 minutes following administration to measure the BOLD response. For the waterload studies, the subjects were taken out of the magnet after the baseline measurements were performed, and were asked to drink 20 mL of water per kilogram of body weight within 15 minutes in order to induce water diuresis. Urinary output was measured every 15 minutes. After it exceeded 5 mL per minute, the subject returned to the magnet and another set of BOLD MRI measurements was obtained to measure the BOLD response to water diuresis. Each volunteer came to the MRI facility four times on different days. The first time was for the waterload study, the second time for the furosemide study, the third time for a repeated furosemide study, and the fourth time for a repeated waterload study. These four studies gave us four base-line BOLD data ( ) sets for each individual, and two sets of data each for the response ( ) to furosemide and waterload, respectively.
MRI Technique
We performed all of the studies on a 1.5 T scanner (CV/i; General Electric Medical Systems, Milwaukee, WI) using a multiple gradient-echo (mGRE) sequence (TR/TE/flip angle = 63 msec/7− 49.1 msec/30°) to acquire 16 -weighted images within a single breath-hold of about 12 seconds. The choice of the range of TEs was consistent with our previous estimate of in the renal medulla of about 50 ms (8). We constructed maps on the scanner platform using inbuilt FUNC-TOOL by fitting a single exponential function to the signal intensity vs. TE data. A multicoil array was used for signal reception.
Data Analysis
All data were acquired by L.P.L and analyzed by P.P. Regions of interest (ROIs) covering at least 20 pixels were drawn on the anatomic template. Typically, 10–20 ROIs for each cortex and medulla were obtained from several slices and both kidneys. We combined the data to obtain a single representative mean value of per subject per time point.
Statistical Methods
We analyzed the data using repeated measures analysis of variance (ANOVA) models. These models are flexible and can accommodate the multiple within-subject measurements of acquired before and after a maneuver, in different renal regions (e.g., the medulla and cortex), and replicated a different number of times.
Measurements
To determine the reproducibility of , we examined the repeated baseline measures (prior to experimental intervention) across the four separate assessments. We also compared the baseline values on four different days using the coefficient of variance (CV) obtained by calculating the overall standard deviation (SD) divided by the mean for different days and/or different subjects on each day.
Measurements
Because of the nature of BOLD MRI contrast, the absolute magnitude of the change in is less important than relative changes and consistency from day to day. With this in mind, we considered two types of statistical analysis. For the following analyses, the BOLD response is defined as the difference in signal response between baseline and the experimental intervention (furosemide and waterload). We analyzed the data with repeated-measures ANOVA to examine the BOLD response to furosemide and waterload at the two separate assessments for effects of region (medulla vs. cortex) and time (time 1 vs. time 2). The first was a group analysis, and an ANOVA test was used to compare measurements performed on two different days. When the ANOVA test showed significant differences, a Student’s t-test was used to determine which mean was significantly different.
The other analysis was based on each individual subject. For this analysis, we used the reliable change index (RCI) to evaluate the BOLD response ( ) to furosemide and waterload for each individual. The RCI (14) is defined as
| (1) |
Sdiff is the standard error of difference between two test scores, and SEM is the mean within-subject SD. The advantage of this index is that it takes into account information about inter- and intrasubject variability. The RCI is widely used to assess clinical significance after treatment. Whereas the ANOVA and t-test only determine whether the average variation between two groups is “significant,” the RCI helps to determine whether a significant change observed in each individual is reliable (i.e., it is not just a fluctuation caused by an imprecise measuring instrument or by chance (15)). If the RCI is >1.96, the difference is deemed reliable (i.e., the difference observed is unlikely to be due to chance).
RESULTS
Baseline Reproducibility of Measurements
The shortest interval was 81 ± 35 and the longest interval was 272 ± 17 days (Table 1) over the four baseline measurements. The data in Tables 2 and 3 show the baseline BOLD measurements and statistical results. There were no significant differences between the four assessments in the baseline measures obtained for either the medulla or the cortex (P ≥ 0.05 by ANOVA test). The average of the intraindividual CV over 4 days was 12 ± 0.07% (mean ± SD) in the medulla (Table 2), and 12 ± 0.05% (mean ± SD) in the cortex (Table 3). The average of the interindividual CV over eight volunteers was 13 ± 0.03% (mean ± SD) in the medulla (Table 2), and 12 ± 0.01% (mean ± SD) in the cortex (Table 3).
Table 1.
Time Intervals Between Individual Baseline (Pre-maneuver) Measurements
| Subject | Baseline interval between two measurements |
|||||
|---|---|---|---|---|---|---|
| Day 2-Day 1 | Day 3-Day 2 | Day 4-Day 3 | Day 3-Day 1 | Day 4-Day 2 | Day 4-Day 1 | |
| 1 | 96 | 64 | 105 | 160 | 169 | 265 |
| 2 | 148 | 36 | 88 | 184 | 124 | 272 |
| 3 | 148 | 115 | 47 | 233 | 162 | 310 |
| 4 | 103 | 129 | 46 | 232 | 175 | 278 |
| 5 | 90 | 58 | 105 | 148 | 163 | 253 |
| 6 | 93 | 61 | 114 | 154 | 175 | 268 |
| 7 | 84 | 65 | 116 | 149 | 181 | 265 |
| 8 | 103 | 132 | 30 | 235 | 162 | 265 |
| Mean | 108 | 83 | 81 | 187 | 164 | 272 |
| SD | 25 | 37 | 35 | 40 | 18 | 17 |
Table 2.
Baseline (Pre-maneuver) Values in Renal Medulla
| Subject |
-baseline in medulla |
CV (intra) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Mean | SD | Min | Max | ||
| 1 | 19.42 | 21.32 | 22.56 | 16.75 | 20.01 | 2.53 | 16.75 | 22.56 | 0.13 |
| 2 | 18.18 | 17.62 | 17.16 | 16.86 | 17.46 | 0.58 | 16.86 | 18.18 | 0.03 |
| 3 | 19.14 | 16.07 | 16.33 | 22.83 | 18.59 | 3.15 | 16.07 | 22.83 | 0.17 |
| 4 | 20.22 | 16.92 | 17.13 | 19.11 | 18.35 | 1.59 | 16.92 | 20.22 | 0.09 |
| 5 | 16.90 | 24.74 | 20.42 | 18.12 | 20.05 | 3.45 | 16.90 | 24.74 | 0.17 |
| 6 | 15.24 | 21.70 | 19.22 | 25.30 | 20.36 | 4.23 | 15.24 | 25.30 | 0.21 |
| 7 | 18.19 | 17.88 | 17.76 | 18.60 | 18.11 | 0.38 | 17.76 | 18.60 | 0.02 |
| 8 | 21.57 | 18.85 | 17.27 | 23.93 | 20.41 | 2.94 | 17.27 | 23.93 | 0.14 |
| Mean | 18.61 | 19.39 | 18.48 | 20.19 | 0.12 | ||||
| SD | 1.96 | 2.94 | 2.11 | 3.34 | 0.07 | ||||
| Min | 15.24 | 16.07 | 16.33 | 16.75 | |||||
| Max | 21.57 | 24.74 | 22.56 | 25.30 | |||||
| CV (inter)a | 0.11 | 0.15 | 0.11 | 0.17 | |||||
Inter-subject CV (mean ± SD): 0.13 ± 0.03.
Table 3.
Baseline (Pre-maneuver) Values in Renal Cortex
| Subject |
baseline in cortex |
CV (intra) | Max | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Mean | SD | Min | |||
| 1 | 13.26 | 16.08 | 16.02 | 11.61 | 14.24 | 2.19 | 11.61 | 16.08 | 0.15 |
| 2 | 13.79 | 14.50 | 13.23 | 13.00 | 13.63 | 0.67 | 13.00 | 14.50 | 0.05 |
| 3 | 14.06 | 14.23 | 14.37 | 16.06 | 14.68 | 0.93 | 14.06 | 16.06 | 0.06 |
| 4 | 15.63 | 13.16 | 12.77 | 15.90 | 14.37 | 1.63 | 12.77 | 15.90 | 0.11 |
| 5 | 14.06 | 16.54 | 14.17 | 12.57 | 14.33 | 1.64 | 12.57 | 16.54 | 0.11 |
| 6 | 10.53 | 15.84 | 14.35 | 16.14 | 14.21 | 2.58 | 10.53 | 16.14 | 0.18 |
| 7 | 15.17 | 13.31 | 13.06 | 14.58 | 14.03 | 1.01 | 13.06 | 15.17 | 0.07 |
| 8 | 16.37 | 11.43 | 11.48 | 15.52 | 13.70 | 2.62 | 11.43 | 16.37 | 0.19 |
| Mean | 14.11 | 14.39 | 13.68 | 14.42 | 0.12 | ||||
| SD | 1.78 | 1.73 | 1.36 | 1.79 | 0.05 | ||||
| Min | 10.53 | 11.43 | 11.48 | 11.61 | |||||
| Max | 16.37 | 16.54 | 16.02 | 16.14 | |||||
| CV (inter)a | 0.13 | 0.12 | 0.10 | 0.12 | |||||
Inter-subject CV (mean ± SD): 0.12 ± 0.01.
BOLD Response ( ) to Furosemide
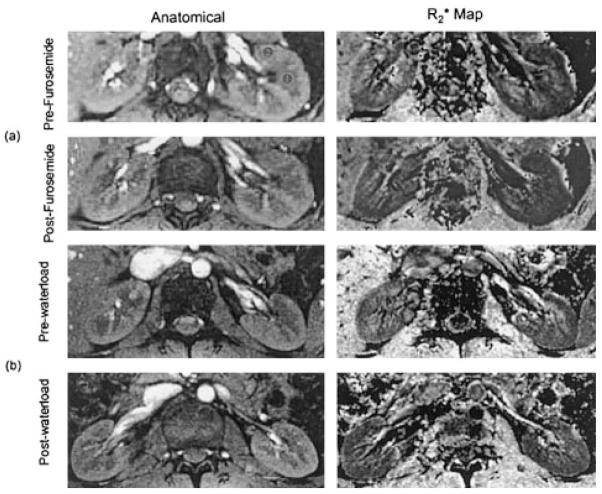
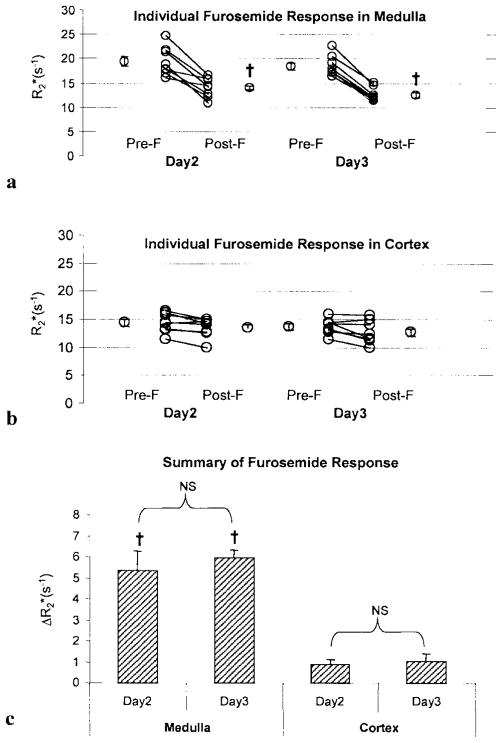
The average interval between the two furosemide studies was 83 ± 37 days. Typical anatomical images and maps are shown in Fig. 1a. (Note that the windowing is identical on both the pre- and post- maps.) On the pre-furosemide map, the medulla is relatively bright, implying lower tissue oxygenation. On the post-furosemide map of the same slice, the medulla shows reduced brightness, indicating increased tissue oxygenation. The individual and mean values (averaged over all subjects) pre- and post-furosemide are shown in Fig. 2a and b. A statistically significant decrease in medullary post-furosemide was observed on both days, but the change in the cortex did not reach statistical significance on either day by a paired two-tailed Student’s t-test. The BOLD response to furosemide was more pronounced in the medulla than in the cortex, and no significant difference was found in the BOLD responses obtained at day 2 vs. day 3 for either region. Figure 2c summarizes the BOLD response ( ) following furosemide administration from studies on both occasions. There was a statistically significant change in at both time points in the medulla, but not in the cortex, according to the paired two-tailed Student’s t-test. The magnitude of the change in the medulla (5.7 s−1) was similar to previous findings (8, 11). Individual measurements for baseline and furosemide conditions obtained on different days were further examined for evidence of reliable change in each subject. These findings indicated that all of the studies showed a reliable response in the medulla at both time points (Table 4), except for two subjects on day 2 (shown in bold). The changes in the cortex were not deemed reliable (Table 4). The average response on day 2 vs. day 3 was not statistically different in the medulla or the cortex, as shown in Fig. 2. The average normalized BOLD MRI response to furosemide (i.e., ) was 27 ± 11.2% on day 2, and 32 ± 3.6% on day 3. This is significantly higher than the 12% variation in the baseline measurements.
Figure 1.
Representative images obtained with an mGRE sequence in a healthy subject. On the left is the anatomic image of the kidney (first image of the series of 16 GRE images). On the right is the map. The windowing is held constant across all maps. Image group a shows representative pre- and post-furosemide maps. Image group b shows representative pre- and post-waterload maps. Note that the medulla looks darker in the post- maps. Marked on the pre-furosemide anatomical image are typical ROIs used in the medulla and cortex.
Figure 2.
Illustration of individual changes in medullary (a) and cortical (b) post-furosemide in eight healthy young volunteers. The average of multiple ROIs from images obtained at multiple locations starting 5 minutes after furosemide administration was used as post-furosemide . Mean values (pre–post) furosemide were averaged over all subjects. (c) Summary of changes in in response to furosemide in eight young subjects. Columns are means ± SE (standard error). †Implies p < 0.05 by paired two tailed Student’s t-test. Pre-F: pre-furosemide and Post-F: post-furosemide. NS implies no significant difference between measurements on day 2 vs day 3 by ANOVA.
Table 4.
Individual Response to Furosemide on Day 2 and Day 3 Based on RCI Analysis*
| Subject | Medulla |
Cortex |
||
|---|---|---|---|---|
| Day 2 | Day 3 | Day 2 | Day 3 | |
| 1 | 3.88 | 4.18 | 1.02 | 0.14 |
| 2 | 0.93 | 2.76 | 0.25 | 1.26 |
| 3 | 0.96 | 2.46 | −0.17 | 1.85 |
| 4 | 2.58 | 3.23 | 0.17 | 0.22 |
| 5 | 4.40 | 2.79 | 0.79 | 0.12 |
| 6 | 3.31 | 3.50 | 0.46 | −0.36 |
| 7 | 2.81 | 2.89 | 0.45 | 1.03 |
| 8 | 4.30 | 3.08 | 0.79 | 0.98 |
RCI > 1.96 indicates a reliable change.
BOLD Response ( ) to Waterload
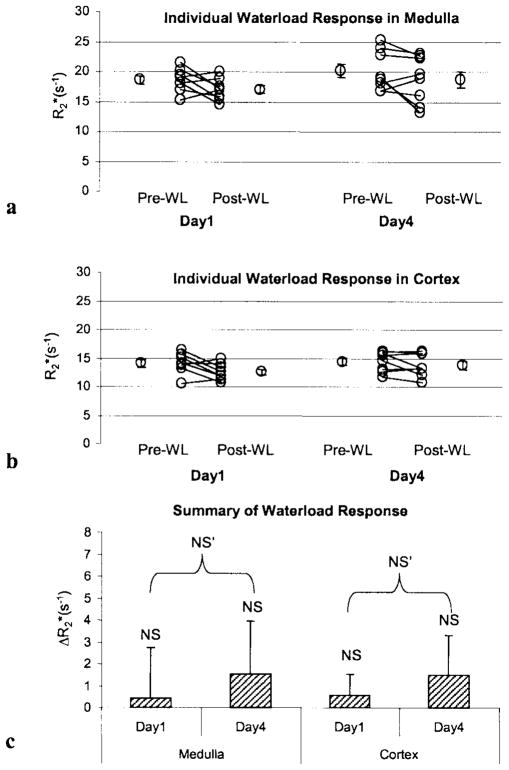
The average interval between the two waterload studies was 272 ± 17 days. Typical anatomical images and maps are shown in Fig. 1b. Note that on the pre-waterload map, the medulla is slightly brighter than on the post-waterload map, implying lower tissue oxygenation. On the post-waterload map of the same slice, the medulla shows slightly reduced brightness, indicating increased tissue oxygenation. The individual and mean values (averaged over all subjects) pre- and post-waterload are shown in Fig. 3a and b. The data were analyzed for the BOLD response to waterload at the two different times (day 1 vs. day 4). The results indicated no significant differences between the two different times (i.e., the BOLD response to waterload did not differ significantly between day 1 and day 4). Figure 3c summarizes the mean over all subjects pre- and post-waterload on two different days. Note the absence of significant change in both the medulla and the cortex on both days. Based on the RCI, none of the subjects (Table 5) showed a significant response to waterload on either day, except for one at one time point (shown in bold). Also note that the sign of RCI is variable, indicating that in some subjects at either time point the actually increased post-waterload.
Figure 3.
Illustration of individual changes in medullary (a) and cortical (b) post- waterload from eight healthy young volunteers. The average of multiple ROIs from images obtained at multiple locations after the urine flow reached 5 mL/minute following water ingestion was used as the post-waterload . Mean values (pre-post) waterload were averaged over all subjects. Pre-WL: Pre-water load. Post-WL: Post-water load. c: Comparison of changes in in response to waterload in eight young subjects. Columns are means ± SE. NS implies no significant difference by paired two-tailed Student’s t-test. NS′ implies no significant difference between measurements on day 1 vs. day 4 by ANOVA.
Table 5.
Individual Response to Waterload on Day 1 and Day 4 Based on RCI analysis*
| Subject | Medulla |
Cortex |
||
|---|---|---|---|---|
| Day 1 | Day 4 | Day 1 | Day 4 | |
| 1 | 1.51 | −0.76 | 1.22 | 0.39 |
| 2 | −0.26 | 0.32 | −0.57 | −0.08 |
| 3 | −0.31 | 0.19 | 0.07 | −0.08 |
| 4 | 1.00 | 2.18 | 1.42 | 1.29 |
| 5 | 0.39 | −0.53 | 1.05 | −0.28 |
| 6 | −0.66 | 1.01 | −0.35 | 0.15 |
| 7 | 1.40 | 1.73 | 1.57 | 1.27 |
| 8 | 1.68 | 0.32 | 1.46 | −0.14 |
RCI > 1.96 indicates a reliable change.
DISCUSSION
Before we discuss specific observations, a few remarks must be made. While it is our objective to use the BOLD MRI technique to follow changes in tissue oxygenation, the relaxation rate is only indirectly related to tissue oxygenation, and there is no simple quantitative relationship between the two. Hence, the absolute magnitude of the observed change in each individual is of less relevance than the relative changes and their statistical significance. For example, it is not so important if the magnitude of the change observed following furosemide in a single individual was 50% different on two different days, as long as the change was deemed reliable on each occasion in this long-term reproducibility study. The group analysis on the other hand, is useful in terms of summarizing the individual data, and allowing comparisons to be made at different time points or from different sites. In this regard, in addition to statistical measures, average magnitude changes could be useful (e.g., for comparing age-related changes). We also need to keep in mind that both and can be affected by both measurement uncertainty and physiological differences between each individual subject and at different times. Future studies may have to control for such physiological differences, probably by acquiring additional information. However, it is not clear at this time what parameters would be most useful.
The test-retest reproducibility of renal BOLD measurements has not previously been reported. The average of baseline values from all ROIs in each individual showed no significant difference over 4 days (Tables 2 and 3) in the renal medulla or cortex as evaluated by the ANOVA test. The magnitude of the baseline value in the medulla and cortex was similar to that in our previous reports (8,10,11,13,14). The intrasubject CV as a measure of baseline reproducibility (~12%) is acceptable considering that several factors may potentially influence baseline variation, such as individual’s biological and physiological variations, difficulty in prescribing exactly the same imaging slice in a given subject between days, relatively long intervals between repeated studies (from 30 days to 310 days), drift in the scanner, performance, etc. We also observed that the inter- and intrasubject CVs were approximately equal (Tables 2 and 3).
The changes in the medulla following furosemide administration (Fig. 2a) were consistent among all individuals and between the two days. All subjects showed a positive response, with the pre- to post-furosemide difference in reaching statistical significance in the medulla. The magnitude of the change was comparable to our previous reported values (8,11). Furthermore, 88% of the measurements had an RCI of >1.96. This result suggests that the change with furosemide as evaluated by BOLD MRI was highly reliable.
The renal cortex did not show significant changes in after furosemide injection (see Fig. 2). The RCI was <1.96 for 94% of the measurements. This result agrees with our previous observation that furosemide improves oxygenation only in the renal medulla (8,11). It also suggests that is an effective indicator of relative intrarenal oxygenation status.
The waterload response did not show significant changes in by t-test on either occasion (Fig. 3). The fact that the RCI was <1.96 for 97% of the individual measurements suggests that waterload did not produce a reliable change in the medulla or the cortex as evaluated by BOLD MRI. There was considerable temporal and intersubject variability in the BOLD MRI response to waterload ( ), as shown in Table 5 (note, for example, the change in the sign of at the two time points for certain individuals).
The waterload response results do not agree with our previous data obtained at a different site with a different scanner (10,13). However, the lack of response to waterload was also apparent in a subsequent study at the same site and on the same scanner, but with a different set of subjects (F. H. Epstein, personal communication). It is not yet clear why furosemide produces a reliable and reproducible change in while waterload does not. However, a possible hypothesis is that furosemide inhibits water reabsorption in the ascending loop of Henle. This reduced reabsorption means there is reduced oxygen demand, which in turn results in an increase in medullary oxygenation. This can be considered as a direct pharmacological effect, and may explain the degree of consistency and the relative magnitude of the BOLD MRI response in each subject following furosemide administration. On the other hand, we believe that the change in medullary oxygenation during waterload is probably related to the stimulation of endogenous prostaglandins. This can be considered as an indirect, physiological effect. It is therefore possible that there is a greater degree of variability in intrasubject (performed at two different times) and intersubject measurements. The apparent endothelial dependence makes waterload an attractive physiological paradigm (10). However, the lack of a significant and reliable response following the water diuresis must be taken into consideration for experiments designed to be used with the waterload paradigm. Direct stimulation/inhibition of the prostaglandin system may be a more robust paradigm, similar to our recent finding that nitric oxide inhibition has a reduced response in hypertensive kidneys (16). A recent report from Zuo et al (17) illustrated the spatial heterogeneity in response to waterload within an individual kidney. In that study, a qualitative visual index was used, and the ROIs represented only the “borderline” region (between the cortex and medulla). Statistical significance was observed post-waterload; however, the magnitude of change reported was still considerably smaller than that in our previous study and that of furosemide.
In conclusion, the major findings of this study were that could be measured in a reproducible way (within ~12% over four measurements carried out over a period of 270 days) in the renal medulla and cortex, and that changes in following furosemide administration were on the order of 30% and were not significantly different over two measurements performed about 83 days apart. While there was considerable variation in individual response in terms of absolute magnitude, the changes were deemed reliable based on RCI analysis. Waterload, on the other hand, did not produce any significant change (group analysis) on either occasion, nor were the changes deemed reliable at either time point.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (DK 53221) to P.V.P. We thank Dr. Ann Ragin and Ms. Linda Odom for their help with the statistical analyses. We thank Dr. Belinda Li of GE Medical Systems (Milwaukee, WI) for her editorial help during the preparation of this manuscript.
References
- 1.Brezis M, Rosen S. Hypoxia of the renal medulla—its implications for disease. N Engl J Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 2.Lindner A, Sherrard DJ. Acute renal failure. N Engl J Med. 1996;335:1320–1321. Author reply 1321–1322. [PubMed] [Google Scholar]
- 3.Heyman SN, Brezis ML, Reubinoff CA. Acute renal failure with selective medullary injury in the rat. J Clin Invest. 1988;82:401–412. doi: 10.1172/JCI113612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowley AW., Jr Long-term control of arterial blood pressure. Physiol Rev. 1992;72:231–300. doi: 10.1152/physrev.1992.72.1.231. [DOI] [PubMed] [Google Scholar]
- 5.Mattson DL, Roman RJ, Cowley AW., Jr Role of nitric oxide in renal papillary blood flow and sodium excretion. Hypertension. 1992;19(Pt 2):766–769. doi: 10.1161/01.hyp.19.6.766. [DOI] [PubMed] [Google Scholar]
- 6.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wacker CM, Wacker CM, Hartlep AW, et al. Susceptibility-sensitive magnetic resonance imaging detects human myocardium supplied by a stenotic coronary artery without a contrast agent. J Am Coll Cardiol. 2003;41:834–840. doi: 10.1016/s0735-1097(02)02931-5. [DOI] [PubMed] [Google Scholar]
- 8.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94:3271–3275. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 9.Brezis M, Agmon Y, Epstein FH. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol. 1994;267(Pt 2):F1059–F1062. doi: 10.1152/ajprenal.1994.267.6.F1059. [DOI] [PubMed] [Google Scholar]
- 10.Prasad PV, Epstein FH. Changes in renal medullary pO2 during water diuresis as evaluated by blood oxygenation level-dependent magnetic resonance imaging: effects of aging and cyclooxygenase inhibition. Kidney Int. 1999;55:294–298. doi: 10.1046/j.1523-1755.1999.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein FH, Prasad PV. Effects of furosemide on medullary oxygenation in younger and older subjects. Kidney Int. 2000;57:2080–2083. doi: 10.1046/j.1523-1755.2000.00057.x. [DOI] [PubMed] [Google Scholar]
- 12.Garber SL, Garber SL, Salmassi J, Arruda JA, Dunea G. Xanthopterin-induced renal dysfunction: a reversible model of crystal nephropathy. Nephron. 1995;69:71–78. doi: 10.1159/000188363. [DOI] [PubMed] [Google Scholar]
- 13.Epstein FH, Veves A, Prasad PV. Effect of diabetes on renal medullary oxygenation during water diuresis. Diabetes Care. 2002;25:575–578. doi: 10.2337/diacare.25.3.575. [DOI] [PubMed] [Google Scholar]
- 14.Prasad PV, Chen Q, Goldfarb JW, Epstein FH, Edelman RR. Breath-hold R2* mapping with a multiple gradient-recalled echo sequence: application to the evaluation of intrarenal oxygenation. J Magn Reson Imaging. 1997;7:1163–1165. doi: 10.1002/jmri.1880070633. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Storey P, Kim D, Li W, Prasad PV. Kidneys in hypertensive rats show reduced response to nitric oxide synthase inhibition as evaluated by BOLD MRI. J Magn Reson Imaging. 2003;17:671–675. doi: 10.1002/jmri.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo CS, Rofsky NM, Mahallati H, et al. Visualization and quantification of renal R2* changes during water diuresis. J Magn Reson Imaging. 2003;17:676–682. doi: 10.1002/jmri.10314. [DOI] [PubMed] [Google Scholar]