Abstract
Background
Cigarette smoking is an established risk factor for colorectal cancer. Because colorectal carcinogenesis is a heterogeneous process, we investigated whether cigarette smoking is differentially associated with molecularly defined subtypes of colorectal cancer.
Methods
We evaluated associations between smoking and incident colorectal cancer, overall and by microsatellite instability (MSI) phenotype (MSI-high vs MSI-low or microsatellite stable), CpG island methylator phenotype (CIMP positive or CIMP negative), and BRAF mutation status (BRAF mutation positive or BRAF mutation negative), among 37 399 participants in a population-based cohort study (the Iowa Women’s Health Study). Cigarette smoking (and other exposures) was assessed by self-report at baseline in 1986, including smoking status (never and ever [former or current]), age at initiation, total duration, average number of cigarettes smoked per day, cumulative pack-years, and induction period. Vital status and state of residence were determined by mailed follow-up questionnaires in 1987, 1989, 1992, and 1997 and by linkage to Iowa death certificate records. Nonrespondents were checked via the National Death Index to identify descendants. Participants with newly diagnosed (ie, incident) colorectal cancer were identified through annual linkage with the Iowa Cancer Registry. Archived paraffin-embedded tumor tissue specimens were obtained for 555 patients with colorectal cancer who were diagnosed from January 1, 1986, through December 31, 2002, and MSI status, CIMP status, and BRAF status were determined. Multivariable Cox regression models were fit to estimate relative risks (RRs) and 95% confidence intervals (CIs).
Results
Ever-smokers were at moderately increased risk for incident colorectal cancer (RR = 1.19, 95% CI = 1.05 to 1.35) compared with never-smokers. Higher risk estimates were observed for current smokers with MSI-high tumors (RR = 1.99, 95% CI = 1.26 to 3.14), CIMP-positive tumors (RR = 1.88, 95% CI = 1.22 to 2.90), and BRAF mutation–positive tumors (RR = 1.92, 95% CI = 1.22 to 3.02). Other smoking-related variables (ie, age at initiation, total duration, average number of cigarettes smoked per day, cumulative pack-years, and induction period) were also associated with MSI-high, CIMP-positive, and BRAF mutation–positive tumor subtypes. Conversely, cigarette smoking status (ever vs never) was not associated with the MSI-low or microsatellite stable (RR = 1.00, 95% CI = 0.79 to 1.25), CIMP-negative (RR = 1.02, 95% CI = 0.81 to 1.30), or BRAF mutation–negative subtypes (RR = 1.00, 95% CI = 0.65 to 1.27).
Conclusions
In this prospective study of older women, cigarette smoking was associated with the MSI-high, CIMP-positive, and BRAF mutation–positive colorectal cancer subtypes, which indicates that epigenetic modification may be functionally involved in smoking-related colorectal carcinogenesis.
CONTEXT AND CAVEATS
Prior knowledge
Cigarette smoking is a risk factor for colorectal cancer.
Study design
This is a prospective study that evaluated associations between smoking and newly diagnosed colorectal cancer overall and by microsatellite instability (MSI) phenotype (MSI-high vs MSI-low or microsatellite stable), CpG island methylator phenotype (CIMP positive or CIMP negative), and BRAF mutation status (positive or negative) in a population-based cohort study, the Iowa Women's Health Study.
Contribution
Self-reported ever-smokers were at a moderately higher risk for incident colorectal cancer than never-smokers. Higher risk estimates were observed for current smokers with MSI-high tumors, CIMP-positive tumors, and BRAF mutation–positive tumor subtypes, as were other smoking-related variables (ie, age at initiation, total duration, average number of cigarettes smoked per day, cumulative pack-years, and induction period). However, cigarette smoking status (ever vs never) was not statistically significantly associated with the MSI-low or microsatellite stable, CIMP-negative, or BRAF mutation–negative subtypes.
Implications
Epigenetic modification may be functionally involved in smoking-related colorectal carcinogenesis.
Limitations
Data on biomarkers were obtained for only 555 of the 1255 eligible patients with colorectal cancer. The study population was made up of older women who were predominately white and so findings may not be generalizable to other demographic subgroups.
From the Editors
Worldwide, colorectal cancer accounts for more than 600 000 deaths per year (1). In the United States, approximately 50 000 fatalities are attributed to and 800 000 person-years of life are lost from this disease annually (2,3). Recent data from the Surveillance, Epidemiology, and End Results (SEER) program show encouraging declines in annual mortality rates from colorectal cancer (4). However, current trends are not sufficient to achieve the colorectal cancer mortality targets set by organizations, such as the American Cancer Society and Healthy People 2010 (5,6). Computer simulation models have indicated that greater attention to lifestyle modification could have a comparable, or perhaps even greater, impact on colorectal cancer mortality rates than increased chemotherapy use during the next 10–20 years (7).
Cigarette smoking represents a potentially modifiable, yet arguably underappreciated, risk factor for colorectal cancer. In a recent meta-analysis, Botteri et al. (8) estimated that ever-smokers were 18% more likely to develop colorectal cancer than never-smokers on the basis of data from 106 observational studies with 39 779 patients with newly diagnosed (ie, incident) colorectal cancer (pooled relative risk [RR] = 1.18, 95% confidence interval [CI] = 1.11 to 1.25). Smoking-related colorectal cancer risks were also higher for proximal than distal colorectal cancers (although the point estimates were not statistically significantly different), prompting speculation that tobacco exposure might have differential effects on heterogeneous pathways of colorectal carcinogenesis. Unfortunately, existing data were not sufficient to assess associations between cigarette smoking and molecularly defined colorectal cancer risks in the pooled data analyses.
To address this knowledge gap, we evaluated overall and subtype-specific colorectal cancer risks that were associated with cigarette smoking among participants enrolled in the prospective population-based Iowa Women's Health Study (IWHS). We have also previously reported (9) increased proximal, but not distal, colorectal cancer risk among cigarette smokers in the IWHS cohort. Therefore, we chose to focus our molecular analyses on tumor characteristics that have been associated with colorectal cancer anatomical location, including microsatellite instability (MSI) phenotype, CpG island methylator phenotype (CIMP), and BRAF mutation status (10–14). Because most MSI-high tumors among older women result from acquired deficiencies in the DNA mismatch repair system (15,16), we further analyzed MLH1, MSH2, MSH6, and PMS2 protein expression and MLH1 promoter methylation to provide additional mechanistic information about subtype-specific associations. The overall goal of this molecular epidemiology investigation was to evaluate a more precise pathogenic model for smoking-related colorectal carcinogenesis with potential implications for colorectal cancer prevention at several levels, including behavioral modification, risk stratification, and early detection.
Participants and Methods
Study Population
All aspects of this study were reviewed and approved by the appropriate institutional review board(s) at the University of Iowa, University of Minnesota, and Mayo Clinic. The investigators and the institutional review boards judged that return of a questionnaire implied consent to take part in this research. Methods used for IWHS subject recruitment and enrollment have been previously described (17). In brief, a 16-page baseline questionnaire was mailed in January 1986 to randomly selected women who were aged 55–69 years, who resided in Iowa, and who held a valid driver's license. Among 98 029 women who received the questionnaires, 41 836 (43%) responded; colorectal cancer incidence rates have been shown to be similar between the responders and nonresponders (18). For this study, exclusion criteria (not mutually exclusive) were history of malignancy other than skin cancer (n = 3830), unable to be followed longitudinally for at least 1 day (n = 10), or incomplete smoking data (n = 660), leaving 37 399 women in the final analytic cohort.
Risk Factor Assessment
Cigarette smoking patterns among IWHS participants were comprehensively ascertained at baseline in 1986, including smoking status (never, ever, former, or current), age at smoking initiation (years), smoking duration (years), average number of cigarettes smoked per day, cumulative pack-years, and induction period (difference between the date of baseline smoking assessment and age at onset of cigarette smoking). Smoking-related variables without specified categories were treated as continuous variables. Potential confounding variables were also acquired from the baseline questionnaire, and these included body mass index; waist to hip ratio; physical activity level; alcohol consumption; exogenous estrogen use; and daily intake of total calories, fat, sucrose, red meat, calcium, folate, vitamin E, and methionine. Family history of colorectal cancer and nonsteroidal anti-inflammatory drug use were not systematically recorded at baseline and were not included in the current data analyses.
Ascertainment of Patients With Incident Colorectal Cancer
Vital status and state of residence were determined by mailed follow-up questionnaires in 1987, 1989, 1992, and 1997 and by linkage to Iowa death certificate records. Nonrespondents were checked via the National Death Index to identify descendants. Patients with incident colorectal cancer were identified through the Iowa Cancer Registry, which participates in the National Cancer Institute's SEER program (19). Annual matching between a computer-generated list of all cohort members and the records of Iowans with incident cancer in the SEER program registry was performed by use of combinations of first, last, and maiden names; zip code; birth date; and social security number. Colorectal cancer diagnoses were identified by use of International Classification for Diseases in Oncology (ICD-O) codes of 18.0, 18.2–18.9, 19.9, and 20.9. Cancers located in the cecum, ascending colon, hepatic flexure, transverse colon, and splenic flexure were categorized as proximal colorectal cancer in accordance with established convention (20,21). Cancers located throughout the remaining colorectum were categorized as distal colorectal cancer, including tumors in the descending colon, sigmoid colon, rectosigmoid junction, and rectum. Beginning in 2006, archived paraffin-embedded tissue specimens were requested for all patients with incident colorectal cancer who were diagnosed from January 1, 1986, through December 31, 2002. Tissue specimens were retrieved for a total of 732 (58%) of the 1255 patients with colorectal cancer. For this study, 22 patients were excluded because of incomplete smoking data. To assess the possibility of selection bias, general demographics, smoking patterns, and tumor characteristics (size and stage) were compared between patients whose tissue specimens could be retrieved and those whose specimens could not be retrieved; no statistically significant differences were observed for any comparison (data not shown). All incident colorectal cancer diagnoses were confirmed by a single gastrointestinal pathologist, and tissue processing (including DNA extraction) was completed with high-quality usable samples obtained from 555 patients with colorectal cancer.
Tissue Selection and DNA Extraction
Paraffin blocks were serially cut into sections that were 5 or 10 μm thick. One slide was stained with hematoxylin and eosin, and areas of neoplastic (ie, >50% dysplastic cells in field of view) and normal tissue were identified. Tumor tissue and normal tissue were scraped from unstained slides and placed into separate tubes for DNA extraction by use of the QIAamp tissue kit (QIAgen, Valencia, CA) according to the manufacturer's instructions.
Analysis of MSI
MSI testing (16) was performed on paired tumor and normal DNA samples with 10 established DNA microsatellite markers: four mononucleotide repeats (BAT25, BAT26, BAT40, and BAT34C4), five dinucleotide repeats (ACTC, D5S346, D18S55, D17S250, and D10197), and one complex marker (MYCL). Polymerase chain reaction (PCR) for the various microsatellite markers was carried out on matched tumor and normal DNA for each of the patients studied. Standard PCR conditions (95°C for 12 minutes followed by 38 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds, with a final extension for 10 minutes at 72°C) were used with a master mix that included 10× buffer type II, Taq gold, and all four deoxyribonucleotide triphosphates. Primers were custom ordered with various fluorescent dyes from Applied Biosystems (Foster City, CA). PCR product was analyzed on an ABI 3100 (Applied Biosystems). Tumors were classified as MSI-high if at least 30% of the markers demonstrated instability and as MSI-low or microsatellite stable if less than 30% of the markers demonstrated instability (10,22). MSI status could be determined for 540 (97%) of the 555 patients with colorectal cancer.
CpG Island Methylation
Tumor DNA was treated with sodium bisulfite (Zymo Research, Orange, CA) and subsequently analyzed by use of automated real-time PCR-based MethyLight (PCR primers and reaction components were obtained from Applied Biosystems and from Biosearch Technologies, Novato, CA) to amplify methylated CpG sites in the promoter regions of an established five-gene panel for CIMP (CACNA1G, IGF2, NEUROG1, RUNX3, and SOCS1) and in the promoter region of MLH1. MethyLight quantitatively measures the levels of methylated DNA in a specific region of the genome. These levels are then compared with a methylated reference sample (M.SssI-treated DNA) to calculate the percentage of methylated reference. CpG island methylation status was reported as previously described (14). Tumors were defined as CIMP positive if promoter hypermethylation was found in three or more genes of the five-gene panel and as CIMP negative if promoter hypermethylation was found in zero to two genes of the five-gene panel. CIMP status could be determined for 527 (95%) of the 555 patients with colorectal cancer.
Detection of BRAF V600E Mutations
Tumor DNA was analyzed by use of a fluorescent allele–specific PCR to detect the V600E point mutation in exon 15 of the BRAF gene. Briefly, a multiplex PCR containing forward primers for the wild-type sequence and for the V600E alteration, along with a common reverse primer, was carried out on tumor DNA for each of the patients studied. Thermocycler conditions were 95°C for 10 minutes followed by 35 cycles of 94°C for 30 seconds, 56°C for 40 seconds, and 72°C for 30 seconds. There was a final extension for 10 minutes at 72°C.
Primers were custom ordered with fluorescent dyes from Applied Biosystems, and the PCR product was analyzed on an ABI 3100 (Applied Biosystems). The BRAF mutation status of tumors was defined as positive if the V600E point mutation was detected and negative if the V600E point mutation was not detected. BRAF mutation status could be determined for 537 (97%) of the 555 patients with colorectal cancer.
Expression of DNA Mismatch Repair Proteins
Immunohistochemical analyses of the protein expression of the four DNA mismatch repair proteins (ie, MLH1, MSH2, MSH6, and PMS2) were performed and graded as previously described (23). Briefly, 5-μm tissue sections from formalin-fixed paraffin-embedded tissue were stained with antibody against hMLH1 (clone G168 15; 1 mg/mL; Biocare Medical, Concord, CA), hMSH2 (clone FE11; 0.5mg/mL; Biocare Medical), hMSH6 (clone BC/44; 0.5 μg/mL; Biocare Medical), and PMS2 (clone A16-4; BD Biosciences/Pharmingen, San Jose, CA). Tumor cells that showed an absence of nuclear staining in the presence of normal positive staining in surrounding cells were interpreted as having an absence of expression of these proteins. The DNA mismatch repair status was defined as deficient if one or more proteins above were not detected and proficient if all four DNA mismatch repair proteins were detected. DNA mismatch repair status could be determined for 547 (99%) of the 555 patients with colorectal cancer.
Statistical Analysis
Measures of agreement across molecularly defined tumor subtypes of colorectal cancer were examined by use of kappa coefficients. Follow-up for incident events was calculated as the time from completion of the baseline questionnaire in 1986 until the age at first colorectal cancer diagnosis, date of move from Iowa, or date of death. If none of these events occurred, a woman was assumed to be alive, cancer-free, and living in Iowa through December 31, 2002. Cox proportional hazards regression analysis was used to estimate relative risks and 95% confidence intervals for associations between cigarette smoking and incident colorectal cancer. All eligible IWHS participants were included in these Cox regression analyses, regardless of eventual cancer status. Incidence was modeled as a function of age because age is a better predictor of cancer risk in our cohort than follow-up time (24). We assessed the effects of smoking status (never, ever, former, or current), age in years at smoking initiation (categorized as >30 or ≤30 years), total smoking duration (categorized as 1–19, 20–39, or ≥40 years), average number of cigarettes smoked per day (categorized as 1–19, 20, or >20 cigarettes smoked per day), cumulative cigarette pack-years (categorized as 1–19, 20–39, or ≥40 pack-years), and smoking induction period (defined as difference between the date of baseline smoking assessment and age at onset of cigarette smoking; categorized as <35, 35–39, 40–44, or ≥45 years). For all such analyses, never-smokers were modeled as the reference group. Tests for trend were carried out for each smoking variable by ordering the categorized values from lowest to highest category and including the resulting variable as a linear term with 1 df in a Cox proportional hazards model. The Cox regression proportionality assumption was formally evaluated by fitting and testing a smoking-by-time interaction term. We first assessed smoking associations with overall colorectal cancer risk. Subsequent analyses examined associations with risk of colorectal cancer as defined by subsets according to anatomical subsite (proximal or distal), MSI phenotype (MSI-high vs microsatellite stable or MSI-low), CIMP status (CIMP positive or CIMP negative), BRAF mutation status (BRAF mutation positive or BRAF mutation negative), MLH1 promoter hypermethylation (yes or no), and DNA mismatch repair protein expression (deficient or proficient). As with the analyses of overall colorectal cancer risk, all eligible IWHS participants were included in the subtype-specific Cox regression analyses. For these analyses, the outcome variable was incident colorectal cancer with marker of interest, and all other types of colorectal cancer (including those with missing or unknown values for the marker of interest) were considered censored observations at the date of diagnosis. We also examined associations of smoking with colorectal cancer risk by subset as defined by tissue availability (available vs not available) by using the same multi-outcome analytic approach as described above to determine whether incomplete tissue access introduced any association biases. Two sets of Cox regression models were fit, one accounting for age and one adjusting for age and other potential confounding variables: body mass index (quartiles); waist to hip ratio (quartiles); physical activity level (low, moderate, or high); alcohol consumption (0, 0–3.4, or >3.4 g/d); exogenous estrogen use (never or ever); and daily intake (quartiles) of total calories (kcal/d), fat (g/d), sucrose (g/d), red meat (g/d), calcium (mg/d), folate (μg/d), vitamin E (mg/d), and methionine (g/d). All statistical tests were two-sided, and all analyses were carried out with the SAS (SAS Institute, Inc, Cary, NC) and S-Plus (Insightful, Inc, Seattle, WA) software systems (SAS[r] proprietary software, release 8.2 [TS2MO]; Splus, version 8.0.1 for Sun SPARC; and Sun OS, version 5.8, 32-bit:2006).
Results
We used self-reported information at study baseline in 1986 to identify 12 761 (34%) of the 37 399 women as ever-smokers and 24 638 (66%) as never-smokers. The mean age for ever-smokers (61.7 years; standard deviation [SD] = 4.2 years) was slightly lower than that for never-smokers (62.4 years; SD = 4.2 years). Compared with never-smokers, ever-smokers also reported higher alcohol consumption and slightly more frequent use of exogenous estrogens, along with lower intakes of total calories, fat, red meat, calcium, folate, methionine, vitamin E, and sucrose (Table 1). Furthermore, both body mass index and physical activity level were lower among ever-smokers, whereas waist to hip ratio was similar between both ever- and never-smokers. Among the 12 761 ever-smokers, 5553 (44%) were categorized as current smokers and 7208 (56%) were categorized as former smokers. Mean values for other smoking-related variables are provided in Table 1.
Table 1.
Characteristics of participants by smoking status*
| Characteristic | Never-smokers (n = 24 638) | Ever-smokers (n = 12 761) |
| Age at enrollment, y (SD) | 62.4 (4.2) | 61.7 (4.2) |
| Body mass index, kg/m2 (SD) | 27.3 (5.0) | 26.4 (5.2) |
| Waist to hip ratio (SD) | 0.837 (0.084) | 0.841 (0.090) |
| Physical activity index, No. (%) | ||
| Low | 10 874 (45.1) | 6506 (51.7) |
| Medium | 6957 (28.9) | 3172 (25.2) |
| High | 6279 (26.0) | 2910 (23.1) |
| Estrogen use, No. (%) | ||
| Never | 15 521 (63.7) | 7392 (58.5) |
| Ever | 8848 (36.3) | 5241 (41.5) |
| Alcohol consumption, g/d (SD) | 2.1 (5.7) | 6.7 (12.5) |
| Total calories, kcal/d (SD) | 1807.8 (712.4) | 1761.9 (762.5) |
| Total fat, g/d (SD) | 68.4 (31.1) | 67.6 (33.5) |
| Red meat, g/d (SD) | 91.6 (73.8) | 86.7 (75.2) |
| Calcium, mg/d† (SD) | 1101.7 (561.6) | 1072.0 (589.9) |
| Folate, μg/d† (SD) | 432.7 (259.9) | 421.0 (270.6) |
| Methionine, g/d (SD) | 1.9 (0.9) | 1.8 (0.9) |
| Vitamin E, mg/d† (SD) | 67.7 (149.7) | 65.7 (149.6) |
| Sucrose, g/d (SD) | 42.70 (23.5) | 38.6 (26.2) |
| Age at smoking initiation, y (SD) | — | 21.9 (7.0) |
| Duration smoked, y (SD) | — | 31.2 (13.0) |
| Average No. of cigarettes per day (SD) | — | 16.8 (9.8) |
| Cumulative pack-years (SD) | — | 27.8 (20.8) |
| Induction period, y (SD) | — | 39.8 (7.6) |
| Time since smoking cessation, y (SD) | — | 15.7 (12.1) |
Results are presented as the mean unless otherwise indicated. — = characteristic not relevant for never-smokers; SD = standard deviation.
Including supplements.
Among the 555 patients with incident colorectal cancer for whom molecular marker data were obtained, subtype distributions were 393 (71%) MSI-low or microsatellite stable and 147 (26%) MSI-high, 363 (65%) CIMP negative and 164 (30%) CIMP positive, 385 (69%) BRAF mutation negative and 152 (27%) BRAF mutation positive, 143 (26%) with deficient DNA mismatch repair protein expression (including 138 with MLH1 and/or PMS2 absent, two with MSH6 absent, and three with PMS2 absent) and 404 (73%) with proficient DNA mismatch repair protein expression, and 130 (23%) with and 401 (72%) without MLH1 promoter hypermethylation. Overall, the MSI-high, CIMP-positive, and BRAF mutation–positive subtypes were strongly correlated (kappa coefficient ≥ 0.65 and P < .001 for each pairwise comparison) with each other. Nearly all colorectal cancer patients (96%) with the MSH-high phenotype also had a deficient DNA mismatch repair protein expression status, and 100 (77%) patients with MLH1 hypermethylation also had a BRAF mutation–positive status.
In age-adjusted risk models, ever-smokers were found to be at a moderately higher overall colorectal cancer risk than never-smokers (RR = 1.20, 95% CI = 1.07 to 1.35). Adjustment for other potential confounding factors did not appreciably alter the overall colorectal cancer risk estimate (RR = 1.19, 95% CI = 1.05 to 1.35). Results of analyses that defined subjects as only those with available tissue (RR = 1.15, 95% CI = 0.95 to 1.39) were similar to those of overall analyses, indicating that there were no substantive tissue-related ascertainment biases. Associations between other smoking-related variables (age at initiation, total duration, average number of cigarettes per day, cumulative pack-years, and induction period) and incident colorectal cancer were highly consistent and suggested a positive dose–response relationship (Table 2). Analyses that were based on anatomical subsite further revealed that each smoking variable was associated with moderately elevated risks for proximal colorectal cancer (Table 2). Smoking more than 20 cigarettes per day (RR = 1.71, 95% CI = 1.24 to 2.36) or having 40 cumulative pack-years of smoking or more (RR = 1.58, 95% CI = 1.21 to 2.08) were associated with the highest risks of proximal colorectal cancer. Conversely, only total smoking duration and induction period were marginally associated with distal colorectal cancer risk.
Table 2.
Associations between cigarette smoking and incident colorectal cancer (CRC), overall and by anatomical subsite*
| Smoking variable | Person-years | Overall CRC (n = 1233) | Proximal CRC (n = 621) | Distal CRC (n = 585) | |||
| RR (95% CI)† | RR (95% CI)‡ | RR (95% CI)† | RR (95% CI)‡ | RR (95% CI)† | RR (95% CI)‡ | ||
| Never smoking | 375 486 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Ever smoking | 180 409 | 1.20 (1.07 to 1.35) | 1.19 (1.05 to 1.35) | 1.36 (1.15 to 1.60) | 1.33 (1.11 to 1.58) | 1.08 (0.91 to 1.28) | 1.08 (0.90 to 1.29) |
| Former | 104 111 | 1.18 (1.02 to 1.36) | 1.16 (1.00 to 1.35) | 1.34 (1.10 to 1.62) | 1.32 (1.07 to 1.62) | 1.06 (0.86 to 1.31) | 1.04 (0.84 to 1.30) |
| Current | 76 297 | 1.22 (1.04 to 1.44) | 1.23 (1.03 to 1.46) | 1.39 (1.11 to 1.73) | 1.34 (1.06 to 1.70) | 1.10 (0.87 to 1.39) | 1.13 (0.88 to 1.45) |
| Ptrend | .003 | .007 | <.001 | .003 | .38 | .34 | |
| Age at smoking initiation, y | |||||||
| >30 | 17 795 | 1.12 (0.82 to 1.52) | 1.12 (0.82 to 1.53) | 1.27 (0.84 to 1.92) | 1.28 (0.85 to 1.94) | 1.03 (0.65 to 1.63) | 1.01 (0.63 to 1.62) |
| ≤30 | 161 711 | 1.21 (1.07 to 1.36) | 1.19 (1.05 to 1.36) | 1.36 (1.15 to 1.61) | 1.33 (1.11 to 1.59) | 1.08 (0.91 to 1.29) | 1.08 (0.90 to 1.31) |
| Ptrend | .003 | .007 | <.001 | .002 | .38 | .41 | |
| Total smoking duration, y | |||||||
| 1–19 | 40 381 | 1.18 (0.95 to 1.46) | 1.17 (0.94 to 1.46) | 1.38 (1.03 to 1.85) | 1.41 (1.05 to 1.90) | 1.05 (0.77 to 1.44) | 1.00 (0.71 to 1.39) |
| 20–39 | 87 073 | 1.04 (0.88 to 1.23) | 1.05 (0.88 to 1.24) | 1.25 (1.00 to 1.57) | 1.24 (0.98 to 1.56) | 0.84 (0.65 to 1.08) | 0.86 (0.66 to 1.11) |
| ≥40 | 50 848 | 1.42 (1.20 to 1.69) | 1.40 (1.17 to 1.68) | 1.45 (1.14 to 1.85) | 1.36 (1.05 to 1.76) | 1.46 (1.15 to 1.87) | 1.50 (1.16 to 1.93) |
| Ptrend | <.001 | .002 | <.001 | .006 | .11 | .085 | |
| Average No. of cigarettes per day | |||||||
| 1–19 | 95 965 | 1.10 (0.95 to 1.28) | 1.10 (0.94 to 1.29) | 1.22 (0.99 to 1.50) | 1.20 (0.97 to 1.50) | 1.03 (0.83 to 1.28) | 1.04 (0.83 to 1.31) |
| 20 | 54 007 | 1.30 (1.08 to 1.56) | 1.28 (1.06 to 1.55) | 1.42 (1.11 to 1.84) | 1.38 (1.06 to 1.79) | 1.21 (0.93 to 1.57) | 1.22 (0.93 to 1.60) |
| >20 | 28 561 | 1.39 (1.09 to 1.76) | 1.32 (1.04 to 1.69) | 1.80 (1.32 to 2.46) | 1.71 (1.24 to 2.36) | 1.02 (0.70 to 1.49) | 0.97 (0.66 to 1.44) |
| Ptrend | <.001 | .002 | <.001 | <.001 | .32 | .44 | |
| Cumulative pack-years | |||||||
| 1–19 | 74 225 | 1.12 (0.95 to 1.32) | 1.13 (0.95 to 1.34) | 1.31 (1.04 to 1.64) | 1.33 (1.05 to 1.68) | 0.97 (0.75 to 1.24) | 0.96 (0.74 to 1.25) |
| 20–39 | 59 187 | 1.13 (0.94 to 1.36) | 1.11 (0.92 to 1.34) | 1.14 (0.88 to 1.49) | 1.08 (0.81 to 1.43) | 1.16 (0.89 to 1.49) | 1.18 (0.90 to 1.53) |
| ≥40 | 42 566 | 1.42 (1.18 to 1.72) | 1.39 (1.14 to 1.70) | 1.67 (1.29 to 2.16) | 1.58 (1.21 to 2.08) | 1.20 (0.90 to 1.60) | 1.20 (0.89 to 1.62) |
| Ptrend | <.001 | .002 | <.001 | .003 | .15 | .15 | |
| Smoking induction period§, y | |||||||
| <35 | 34 086 | 1.06 (0.83 to 1.35) | 1.07 (0.83 to 1.37) | 1.15 (0.81 to 1.63) | 1.16 (0.81 to 1.66) | 1.03 (0.73 to 1.46) | 1.03 (0.72 to 1.47) |
| 35–40 | 46 825 | 1.08 (0.87 to 1.35) | 1.04 (0.83 to 1.32) | 1.33 (0.98 to 1.81) | 1.31 (0.95 to 1.80) | 0.86 (0.62 to 1.20) | 0.80 (0.56 to 1.14) |
| 40–44 | 52 266 | 1.17 (0.97 to 1.42) | 1.19 (0.98 to 1.45) | 1.55 (1.21 to 2.00) | 1.56 (1.20 to 2.02) | 0.88 (0.65 to 1.19) | 0.90 (0.66 to 1.22) |
| ≥45 | 46 331 | 1.38 (1.16 to 1.64) | 1.35 (1.12 to 1.62) | 1.31 (1.02 to 1.68) | 1.23 (0.95 to 1.60) | 1.50 (1.17 to 1.93) | 1.53 (1.18 to 1.98) |
| Ptrend | <.001 | .001 | <.001 | .003 | .085 | .070 | |
P values were based on test for trend in ordinally scaled smoking variables. All statistical tests were two-sided. CI = confidence interval; ref = referent; RR = relative risk.
Adjusted for age.
Adjusted for age; body mass index; waist to hip ratio; physical activity level; alcohol consumption; exogenous estrogen use; and daily intake of total calories, fat, sucrose, red meat, calcium, folate, vitamin E, and methionine.
Induction period was defined as the difference between baseline age and age at onset of cigarette smoking.
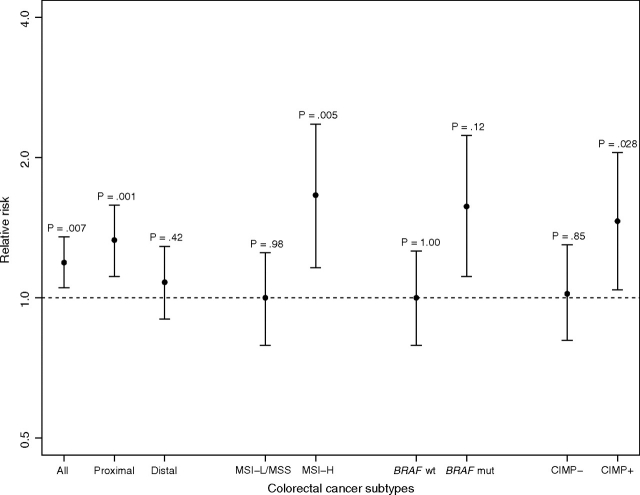
Molecularly defined colorectal cancer subtypes were also differentially associated with cigarette smoking. Specifically, independent risk estimates for MSI-high, CIMP-positive, or BRAF mutation–positive status were strongly associated with each smoking variable (smoking status, age at initiation, total duration, average number of cigarettes smoked per day, cumulative pack-years and induction period) (Tables 3–5 and Figure 1). Compared with never-smokers, ever-smokers had statistically significant increased risks for tumors with MSI-high (RR = 1.66, 95% CI = 1.16 to 2.36), CIMP-positive (RR = 1.46, 95% CI = 1.04 to 2.05), or BRAF mutation–positive (RR = 1.57, 95% CI = 1.11 to 2.23) colorectal cancer subtypes. Conversely, cigarette smoking status (ever vs never) was not associated with the MSI-low or microsatellite stable (RR = 1.00, 95% CI = 0.79 to 1.25), CIMP-negative (RR = 1.02, 95% CI = 0.81 to 1.30), or BRAF mutation–negative (RR = 1.00, 95% CI = 0.65 to 1.27) colorectal cancer subtypes. When ever-smokers were stratified by current or former smoking status, current smokers were found to be at the highest risks for MSI-high (RR = 1.99, 95% CI = 1.26 to 3.14), CIMP-positive (RR = 1.88, 95% CI = 1.22 to 2.90), and BRAF mutation–positive (RR = 1.92, 95% CI = 1.22 to 3.02) colorectal cancer subtypes, whereas former smokers were found to be at moderately elevated subtype-specific risks for MSI-high (RR = 1.46, 95% CI = 0.95 to 2.23), CIMP positive (RR = 1.21, 95% CI = 0.79 to 1.84), and BRAF mutation positive (RR = 1.36, 95% CI = 0.89 to 2.08). Tests for trend across current, former, and never smoking status demonstrated that the observed differences in risks for MSI-high, CIMP-positive, and BRAF mutation–positive tumors were statistically significant (Ptrend = .002, .006, and .004, respectively).
Table 3.
Associations between cigarette smoking and incident colorectal cancer by microsatellite instability (MSI) status*
| Smoking variable | Person-years | Microsatellite stable/MSI-low (n = 393) | MSI-high (n = 147) | ||||
| No. of patients | Median time to diagnosis, y | RR (95% CI)† | No. of patients | Median time to diagnosis, y | RR (95% CI)† | ||
| Never smoking | 375 486 | 270 | 11.80 | 1.00 (ref) | 87 | 12.95 | 1.00 (ref) |
| Ever smoking | 180 409 | 123 | 10.06 | 1.00 (0.79 to 1.25) | 60 | 12.07 | 1.66 (1.16 to 2.36) |
| Former | 104 111 | 76 | 10.06 | 1.03 (0.79 to 1.35) | 32 | 12.27 | 1.46 (0.95 to 2.23) |
| Current | 76 297 | 47 | 10.04 | 0.94 (0.68 to 1.31) | 28 | 11.88 | 1.99 (1.26 to 3.14) |
| Ptrend | .83 | .002 | |||||
| Age at smoking initiation, y | |||||||
| >30 | 17 795 | 7 | 12.71 | 0.47 (0.21 to 1.05) | 5 | 10.36 | 1.23 (0.50 to 3.05) |
| ≤30 | 161 711 | 114 | 9.88 | 1.06 (0.84 to 1.34) | 54 | 12.07 | 1.69 (1.17 to 2.44) |
| Ptrend | .76 | .005 | |||||
| Total smoking duration, y | |||||||
| 1–19 | 40 381 | 27 | 10.54 | 0.98 (0.65 to 1.48) | 13 | 11.65 | 1.75 (0.97 to 3.16) |
| 20–39 | 87 073 | 50 | 9.31 | 0.90 (0.66 to 1.24) | 25 | 12.61 | 1.54 (0.96 to 2.46) |
| ≥40 | 50 848 | 42 | 9.92 | 1.06 (0.75 to 1.49) | 21 | 11.12 | 1.72 (1.04 to 2.85) |
| Ptrend | .95 | .012 | |||||
| Average No. of cigarettes per day | |||||||
| 1–19 | 95 965 | 60 | 10.05 | 0.90 (0.68 to 1.21) | 32 | 11.34 | 1.61 (1.06 to 2.46) |
| 20 | 54 007 | 37 | 10.06 | 1.00 (0.70 to 1.44) | 20 | 13.58 | 1.84 (1.10 to 3.08) |
| >20 | 28 561 | 24 | 9.71 | 1.27 (0.82 to 1.96) | 8 | 12.69 | 1.61 (0.77 to 3.38) |
| Ptrend | .57 | .010 | |||||
| Cumulative pack-years | |||||||
| 1–19 | 74 225 | 46 | 10.18 | 0.90 (0.65 to 1.25) | 24 | 11.47 | 1.65 (1.03 to 2.63) |
| 20–39 | 59 187 | 40 | 9.92 | 1.00 (0.71 to 1.41) | 18 | 10.87 | 1.46 (0.85 to 2.50) |
| ≥40 | 42 566 | 32 | 9.22 | 1.06 (0.72 to 1.55) | 16 | 14.09 | 1.86 (1.06 to 3.24) |
| Ptrend | .90 | .012 | |||||
| Smoking induction period‡, y | |||||||
| <35 | 34 086 | 14 | 11.47 | 0.64 (0.36 to 1.12) | 6 | 11.21 | 1.04 (0.45 to 2.41) |
| 35–40 | 46 825 | 31 | 6.89 | 1.25 (0.85 to 1.85) | 12 | 11.88 | 1.78 (0.93 to 3.41) |
| 40–44 | 52 266 | 37 | 11.99 | 1.09 (0.76 to 1.55) | 23 | 12.48 | 2.48 (1.53 to 4.02) |
| ≥45 | 46 331 | 39 | 9.30 | 0.94 (0.66 to 1.33) | 18 | 12.46 | 1.28 (0.76 to 2.17) |
| Ptrend | .89 | .011 | |||||
CI = confidence interval; RR = relative risk.
Adjusted for age; body mass index; waist to hip ratio; physical activity level; alcohol consumption; exogenous estrogen use; and daily intake of total calories, fat, sucrose, red meat, calcium, folate, vitamin E, and methionine. P values were based on test for trend in ordinally scaled smoking variables. All statistical tests were two-sided.
Induction period was defined as the difference between baseline age and age at onset of cigarette smoking.
Table 5.
Associations between cigarette smoking and incident colorectal cancer by BRAF mutation status*
| Smoking variable | Person-years | BRAF mutation negative (n = 385) | BRAF mutation positive (n = 152) | ||||
| No. of patients | Median time to diagnosis, y | RR (95% CI)† | No. of patients | Median time to diagnosis, y | RR (95% CI)† | ||
| Never smoking | 375 486 | 262 | 11.71 | 1.00 (ref) | 93 | 12.90 | 1.00 (ref) |
| Ever smoking | 180 409 | 123 | 10.04 | 1.00 (0.79 to 1.26) | 59 | 12.06 | 1.57 (1.11 to 2.23) |
| Former | 104 111 | 78 | 9.97 | 1.06 (0.81 to 1.38) | 31 | 11.81 | 1.36 (0.89 to 2.08) |
| Current | 76 297 | 45 | 10.14 | 0.91 (0.65 to 1.27) | 28 | 12.10 | 1.92 (1.22 to 3.02) |
| Ptrend | .75 | .004 | |||||
| Age at smoking initiation, y | |||||||
| >30 | 17 795 | 8 | 11.58 | 0.55 (0.26 to 1.16) | 4 | 9.32 | 0.92 (0.34 to 2.51) |
| ≤30 | 161 711 | 113 | 9.88 | 1.05 (0.83 to 1.33) | 54 | 11.93 | 1.64 (1.14 to 2.35) |
| Ptrend | .80 | .008 | |||||
| Total smoking duration, y | |||||||
| 1–19 | 40 381 | 29 | 9.97 | 1.07 (0.71 to 1.59) | 11 | 12.21 | 1.43 (0.76 to 2.69) |
| 20–39 | 87 073 | 47 | 9.48 | 0.86 (0.62 to 1.18) | 27 | 11.81 | 1.61 (1.02 to 2.53) |
| ≥40 | 50 848 | 43 | 10.04 | 1.07 (0.76 to 1.50) | 20 | 11.25 | 1.58 (0.95 to 2.62) |
| Ptrend | .88 | .018 | |||||
| Average No. of cigarettes per day | |||||||
| 1–19 | 95 965 | 59 | 10.04 | 0.88 (0.65 to 1.18) | 31 | 11.39 | 1.52 (1.00 to 2.32) |
| 20 | 54 007 | 42 | 10.21 | 1.16 (0.83 to 1.63) | 16 | 13.58 | 1.38 (0.79 to 2.42) |
| >20 | 28 561 | 20 | 9.22 | 1.07 (0.66 to 1.72) | 12 | 12.10 | 2.27 (1.22 to 4.21) |
| Ptrend | .64 | .006 | |||||
| Cumulative pack-years | |||||||
| 1–19 | 74 225 | 45 | 9.97 | 0.89 (0.64 to 1.23) | 24 | 11.73 | 1.60 (1.01 to 2.54) |
| 20–39 | 59 187 | 42 | 10.25 | 1.03 (0.73 to 1.46) | 16 | 10.39 | 1.24 (0.71 to 2.18) |
| ≥40 | 42 566 | 31 | 9.30 | 1.04 (0.71 to 1.53) | 17 | 12.89 | 1.87 (1.09 to 3.21) |
| Ptrend | .88 | .021 | |||||
| Smoking induction period‡, y | |||||||
| <35 | 34 086 | 16 | 10.41 | 0.74 (0.44 to 1.26) | 5 | 12.06 | 0.83 (0.34 to 2.05) |
| 35–40 | 46 825 | 27 | 6.89 | 1.09 (0.72 to 1.65) | 15 | 11.81 | 2.22 (1.24 to 3.98) |
| 40–44 | 52 266 | 36 | 12.23 | 1.06 (0.74 to 1.53) | 21 | 11.39 | 2.16 (1.32 to 3.55) |
| ≥45 | 46 331 | 42 | 9.30 | 1.00 (0.71 to 1.40) | 17 | 12.89 | 1.18 (0.69 to 2.01) |
| Ptrend | .83 | .030 | |||||
CI = confidence interval; RR = relative risk.
Adjusted for age; body mass index; waist to hip ratio; physical activity level; alcohol consumption; exogenous estrogen use; and daily intake of total calories, fat, sucrose, red meat, calcium, folate, vitamin E, and methionine. P values were based on test for trend in ordinally scaled smoking variables. All statistical tests were two-sided.
Induction period was defined as the difference between baseline age and age at onset of cigarette smoking.
Figure 1.
Cigarette smoking and subtype-specific colorectal cancer risks. Relative risks (solid circles) and 95% confidence intervals (error bars) were for associations between ever smoking and incident colorectal cancer that were based on multivariable Cox regression models; never smoking was the reference group for all analyses. P values were based on the Wald test assessing whether or not the relative risk differed from unity. All statistical tests were two-sided. BRAF mut = BRAF mutation positive; BRAF wt = BRAF mutation negative; CIMP– = CpG island methylator phenotype negative; CIMP+ = CpG island methylator phenotype positive; MSI-H = microsatellite instability-high; MSI-L/MSS = microsatellite instability-low and/or microsatellite stable.
Table 4.
Associations between cigarette smoking and incident colorectal cancer by CpG island methylator phenotype (CIMP) status*
| Smoking variable | Person-years | CIMP negative (n = 363) | CIMP positive (n = 164) | ||||
| No. of patients | Median time to diagnosis, y | RR (95% CI)† | No. of patients | Median time to diagnosis, y | RR (95% CI)† | ||
| Never smoking | 375 486 | 247 | 11.81 | 1.00 (ref) | 102 | 12.96 | 1.00 (ref) |
| Ever smoking | 180 409 | 116 | 10.22 | 1.02 (0.81 to 1.30) | 62 | 11.88 | 1.46 (1.04 to 2.05) |
| Former | 104 111 | 75 | 10.06 | 1.10 (0.83 to 1.44) | 31 | 12.48 | 1.21 (0.79 to 1.84) |
| Current | 76 297 | 41 | 11.71 | 0.91 (0.64 to 1.29) | 31 | 11.68 | 1.88 (1.22 to 2.90) |
| Ptrend | .83 | .006 | |||||
| Age at smoking initiation, y | |||||||
| >30 | 17 795 | 8 | 11.58 | 0.59 (0.28 to 1.25) | 3 | 6.43 | 0.61 (0.19 to 1.94) |
| ≤30 | 161 711 | 107 | 10.06 | 1.08 (0.85 to 1.38) | 57 | 11.68 | 1.53 (1.08 to 2.17) |
| Ptrend | .61 | .021 | |||||
| Total smoking duration, y | |||||||
| 1–19 | 40 381 | 28 | 10.01 | 1.11 (0.74 to 1.67) | 11 | 11.65 | 1.25 (0.67 to 2.35) |
| 20–39 | 87 073 | 48 | 10.24 | 0.95 (0.69 to 1.31) | 25 | 12.09 | 1.32 (0.83 to 2.09) |
| ≥40 | 50 848 | 37 | 10.14 | 1.00 (0.70 to 1.45) | 24 | 11.25 | 1.69 (1.05 to 2.70) |
| Ptrend | .92 | .024 | |||||
| Average No. of cigarettes per day | |||||||
| 1–19 | 95 965 | 57 | 10.30 | 0.92 (0.68 to 1.24) | 31 | 11.39 | 1.34 (0.88 to 2.03) |
| 20 | 54 007 | 37 | 10.36 | 1.12 (0.78 to 1.60) | 20 | 13.08 | 1.54 (0.93 to 2.56) |
| >20 | 28 561 | 21 | 10.12 | 1.23 (0.77 to 1.96) | 10 | 11.31 | 1.71 (0.88 to 3.33) |
| Ptrend | .44 | .025 | |||||
| Cumulative pack-years | |||||||
| 1–19 | 74 225 | 44 | 10.18 | 0.94 (0.67 to 1.31) | 23 | 11.65 | 1.34 (0.84 to 2.14) |
| 20–39 | 59 187 | 38 | 10.25 | 1.01 (0.71 to 1.45) | 18 | 9.96 | 1.25 (0.74 to 2.13) |
| ≥40 | 42 566 | 30 | 10.09 | 1.11 (0.75 to 1.64) | 18 | 12.50 | 1.77 (1.05 to 2.99) |
| Ptrend | .72 | .032 | |||||
| Smoking induction period‡, y | |||||||
| <35 | 34 086 | 16 | 10.59 | 0.81 (0.48 to 1.37) | 3 | 12.21 | 0.44 (0.14 to 1.38) |
| 35–40 | 46 825 | 28 | 9.49 | 1.23 (0.82 to 1.86) | 14 | 11.66 | 1.79 (0.98 to 3.25) |
| 40–44 | 52 266 | 32 | 12.77 | 1.03 (0.70 to 1.52) | 24 | 11.75 | 2.18 (1.37 to 3.47) |
| ≥45 | 46 331 | 39 | 9.38 | 1.00 (0.71 to 1.43) | 19 | 11.29 | 1.18 (0.71 to 1.96) |
| Ptrend | .78 | .027 | |||||
CI = confidence interval; RR = relative risk.
Adjusted for age; body mass index; waist to hip ratio; physical activity level; alcohol consumption; exogenous estrogen use; and daily intake of total calories, fat, sucrose, red meat, calcium, folate, vitamin E and methionine. P values were based on test for trend in ordinally scaled smoking variables. All statistical tests were two-sided.
Induction period was defined as the difference between baseline age and age at onset of cigarette smoking.
To further characterize potential underlying mechanisms for the observed colorectal cancer subtype-specific risk associations, smoking status was analyzed with respect to DNA mismatch repair protein expression, MLH1 protein expression, and MLH1 promoter hypermethylation status. The observed colorectal cancer risks for ever-smokers were highly consistent with associations that were based on the MSI-high, CIMP-positive, and BRAF mutation–positive subtypes (RR = 1.78, 95% CI = 1.25 to 2.55, for deficient DNA mismatch repair protein expression; RR = 1.75, 95% CI = 1.19 to 2.57, for deficient MLH1 protein expression; RR = 1.71, 95% CI = 1.17 to 2.49, for MLH1 promoter hypermethylation; and RR = 1.82, 95% CI = 1.19 to 2.80, for coexistent MLH1 promoter methylation and BRAF mutation–positive status).
When examining the Cox regression proportional hazards assumption, we found deviation from proportionality for the former smoker hazard ratios with many of the CRC subtypes. In each of these instances, the deviation seemed to lie in the fact that the hazard ratio was slightly higher in the earlier ages of follow-up compared with the later ages (data not shown). Although nonproportionality was detected for former smokers, the reported hazard ratios can be interpreted as average risk estimates across the follow-up spectrum for each relevant subtype-specific association.
Discussion
In this large prospective study of older women, we found that cigarette smoking was strongly associated with the MSI-high, CIMP-positive, and BRAF mutation–positive colorectal cancer subtypes. Conversely, cigarette smoking was only modestly associated with incident colorectal cancer overall, with an observed risk estimate for ever-smokers (RR = 1.19, 95% CI = 1.05 to 1.35) that was nearly identical to the pooled relative risk (RR = 1.18, 95% CI = 1.11 to 1.25) that has been reported from a recent large meta-analysis by Botteri et al. (8). As previously reported (25), we again noted a stronger association between smoking-related variables and proximal vs distal colorectal cancer among IWHS participants, which is consistent with existing data regarding the subsite distribution of MSI-high, CIMP-positive, or BRAF mutation–positive tumors (10–14). Thus, these findings indicate that epigenetic modification may be functionally involved in smoking-related colorectal carcinogenesis.
Although existing data for associations between tobacco exposure and molecularly defined colorectal cancer subtypes are limited, some previous observational studies (13,26) have reported higher odds ratios (ORs) for MSI-high tumors than for MSI-low or microsatellite stable tumors among cigarette smokers. To our knowledge, only two other case–control studies, one including patients with colon cancer (27) and the other including patients with rectal cancer (28), have examined smoking-related colorectal cancer risks by CIMP and/or BRAF mutation status. Samowitz et al. (27) reported that, compared with never-smokers, heavy smokers (ie, >20 cigarettes per day) were at increased risk for CIMP-positive (OR = 2.06, 95% CI = 1.43 to 2.97) or BRAF mutation–positive (OR = 3.16, 95% CI = 1.80 to 5.54) colon cancers. Lower risk estimates were observed among subjects who smoked 20 or fewer cigarettes per day (OR = 1.36, 95% CI = 1.03 to 1.81, for CIMP-positive tumors; and OR = 1.99, 95% CI = 1.26 to 3.13, for BRAF mutation–positive tumors). More recently, Curtin et al. (28) found that smokers with a history of more than 20 pack-years of smoking were more likely to develop either CIMP-positive (OR = 1.5, 95% CI = 0.8 to 2.8) or BRAF mutation–positive (OR = 4.2, 95% CI = 1.3 to 14.2) rectal cancers, although the former point estimate was not statistically significant. It should be noted that these studies (27,28) had modest subject participation rates of 75.6% and 65.2% for case patients and 63.7% and 65.3% for control subjects, respectively, and that cigarette smoking was based on retrospective exposure assessment. Also, the markers (hypermethylation of two or more gene loci: CDKN2A, MLH1, MINT1, MINT2, and MINT31) that were used to define CIMP-positive tumors were different from our study, reflecting a current lack of consensus regarding optimal definition of the methylator phenotype. Nonetheless, the previously reported case–control data are in strong agreement with our prospective cohort data, supporting the proposed molecularly defined subtype-specific colorectal cancer risks associated with cigarette smoking.
Rates for MSI-high and CIMP-positive colorectal cancers have ranged from 15% to 25% and 20% to 30%, respectively, with slightly higher rates reported among older women, as was observed in the IWHS cohort (13,22,29–32). Outside of heritable syndromes (such as Lynch syndrome), MSI-high tumors result primarily from epigenetic silencing of DNA mismatch repair protein expression, which is caused by MLH1 promoter hypermethylation in approximately 95% of all sporadic colorectal cancers (15,16). BRAF mutation status can be used as an additional highly specific marker for differentiating sporadic (BRAF mutation-positive) from syndromic (BRAF mutation-negative) MSI-high tumors (11,33,34). Although the temporal order of molecular events associated with MSI-high and/or CIMP-positive colorectal cancers remains incompletely defined, BRAF mutation–positive tumors appear to be strongly associated with these epigenetically mediated phenotypes (35). Tobacco exposure has been shown to stimulate DNA methyltransferase (36), and cigarette smoking has been associated with CpG island methylation in lung cancer (37–39), bladder cancer (40), and head and neck cancer (41). Thus, the subtype-specific risks for MSI-high, CIMP-positive, or BRAF mutation–positive tumors (as well as DNA mismatch repair–deficient, MLH1-deficient, or MLH1-hypermethylated tumors) that we observed in this study could be plausibly explained by epigenetic modification(s) induced by cigarette smoking, although further studies are needed to clarify the extent to which these molecular events might influence genetic alterations in BRAF or other growth-regulating genes. Additional investigation of smoking variables associated with intracellular events that are upstream and/or downstream of DNA methylation may also be informative. However, at present, molecular markers for assessing DNA methylation pathways remain largely undefined, which prevented us from further clarifying the role of epigenetic modifications in smoking-related colorectal carcinogenesis.
Several features of the IWHS cohort were well suited to our evaluation of cigarette smoking and the molecularly defined subtype-specific colorectal cancer risks of interest. First, as noted above, older women are known to more frequently have MSI-high and CIMP-positive colorectal cancers, which provided an enriched sample set for these subtype-specific associations. Second, the availability of detailed exposure data and prolonged follow-up time allowed us to estimate long-term risks associated with multiple smoking-related variables, including the induction period, which is ostensibly a key indicator of colorectal cancer risk that is often difficult to assess among female smokers (42). Comprehensive exposure data provided the ability to adjust for multiple potentially relevant confounding factors in our multivariable regression models as well, although the possibility of residual confounding remains.
Limitations to our study should also be acknowledged. First, the reported findings cannot be directly extrapolated to other demographic subgroups (eg, younger women, men, and nonwhite subjects), which will require further investigation in more diverse subject populations. Second, although linkage to the Iowa Cancer Registry afforded comprehensive colorectal cancer case ascertainment and ready access to well-annotated tissue specimens, we were not able to retrieve adequate tissue specimens from all IWHS subjects with incident colorectal cancer for the described molecular analyses. However, as noted above, tissue-related ascertainment biases did not appear to influence the observed smoking-related risk estimates for colorectal cancer.
In summary, the results from our prospective population-based cohort study have provided additional support that cigarette smoking is a risk factor for colorectal cancer but further indicate that the smoking-related risk may pertain to specific molecularly defined colorectal cancer subtypes that develop through epigenetically mediated carcinogenic pathways. These data appear to have several relevant clinical and/or research implications. For example, more aggressive colorectal cancer screening recommendations may be warranted for cigarette smokers. Indeed, recently updated practice guidelines from the American College of Gastroenterology have supported initiating colorectal cancer screening at a younger age among heavy smokers (ie, at age 45 years instead of 50 years) (43). Also, emerging colorectal cancer screening technologies, such as stool assays that are based on methylation markers (44,45), might be particularly informative for patients with a history of prolonged tobacco use. Because MSI-high, CIMP-positive, and/or BRAF mutation–positive tumors are thought to arise from serrated polyps (46), further efforts to characterize the natural history of these putatively premalignant lesions among chronic smokers could also yield important mechanistic information. Moreover, evaluation of demethylating compounds to reduce the risk for colorectal cancer and/or other smoking-related cancers may represent a promising chemoprevention strategy for individuals who are exposed to tobacco smoke.
Funding
National Cancer Institute at the National Institutes of Health (R01 CA107333 to P.J.L.).
Footnotes
The funding source had no role in the study design, analyses, drafting of the manuscript, or decision to publish.
The authors thank Tiffany I. Long at the University of Southern California Epigenome Center for her valuable assistance with the DNA methylation assays. Preliminary data from the current study were presented at the American Gastroenterological Association/American Society of Clinical Oncology Gastrointestinal Cancer Symposium in San Francisco, CA, on January 17, 2009. General associations between cigarette smoking and colorectal cancer risk among participants in the Iowa Women's Health Study were previously reported by Limburg et al. (25).
References
- 1.Garcia M, Jemal A, Ward EM, et al. Global Cancer Facts and Figures 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 2.Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100(23):1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yabroff KR, Bradley CJ, Mariotto AB, et al. Estimates and projections of value of life lost from cancer deaths in the United States. J Natl Cancer Inst. 2008;100(24):1755–1762. doi: 10.1093/jnci/djn383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horner MJ, Ries LA, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2006. Bethesda, MD: National Cancer Institute; 2009. http://seer.cancer.gov/csr/1975_2006/. Accessed June 16, 2009. [Google Scholar]
- 5.Byers T, Barrera E, Fontham ET, et al. A midpoint assessment of the American Cancer Society challenge goal to halve the U.S. cancer mortality rates between the years 1990 and 2015. Cancer. 2006;107(2):396–405. doi: 10.1002/cncr.21990. [DOI] [PubMed] [Google Scholar]
- 6.Services USDoHaH. Healthy People 2010: With Understanding and Improving Health and Objectives for Improving Health. 2nd ed. Washington, DC: U.S. Government Printing Office; 2000. [Google Scholar]
- 7.Vogelaar I, van Ballegooijen M, Schrag D, et al. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer. 2006;107(7):1624–1633. doi: 10.1002/cncr.22115. [DOI] [PubMed] [Google Scholar]
- 8.Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 9.Limburg PJ, Vierkant RA, Cerhan JR, et al. Cigarette smoking and colorectal cancer: long-term, subsite-specific risks in a cohort study of postmenopausal women. Clin Gastroenterol Hepatol. 2003;1(3):202–210. doi: 10.1053/cgh.2003.50030. [DOI] [PubMed] [Google Scholar]
- 10.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248–5257. [PubMed] [Google Scholar]
- 11.Kambara T, Simms LA, Whitehall VL, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53(8):1137–1144. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nosho K, Irahara N, Shima K, et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One. 2008;3(11):e3698. doi: 10.1371/journal.pone.0003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slattery ML, Curtin K, Anderson K, et al. Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J Natl Cancer Inst. 2000;92(22):1831–1836. doi: 10.1093/jnci/92.22.1831. [DOI] [PubMed] [Google Scholar]
- 14.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38(7):787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham JM, Christensen ER, Tester DJ, et al. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res. 1998;58(15):3455–3460. [PubMed] [Google Scholar]
- 16.Cunningham JM, Kim CY, Christensen ER, et al. The frequency of hereditary defective mismatch repair in a prospective series of unselected colorectal carcinomas. Am J Hum Genet. 2001;69(4):780–790. doi: 10.1086/323658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folsom AR, Kaye SA, Prineas RJ, et al. Increased incidence of carcinoma of the breast associated with abdominal adiposity in postmenopausal women. Am J Epidemiol. 1990;131(5):794–803. doi: 10.1093/oxfordjournals.aje.a115570. [DOI] [PubMed] [Google Scholar]
- 18.Bisgard KM, Folsom AR, Hong CP, et al. Mortality and cancer rates in nonrespondents to a prospective study of older women: 5-year follow-up. Am J Epidemiol. 1994;139(10):990–1000. doi: 10.1093/oxfordjournals.aje.a116948. [DOI] [PubMed] [Google Scholar]
- 19.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1117–1121. [PubMed] [Google Scholar]
- 20.Cheng X, Chen VW, Steele B, et al. Subsite-specific incidence rate and stage of disease in colorectal cancer by race, gender, and age group in the United States, 1992-1997. Cancer. 2001;92(10):2547–2554. doi: 10.1002/1097-0142(20011115)92:10<2547::aid-cncr1606>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 21.Troisi RJ, Freedman AN, Devesa SS. Incidence of colorectal carcinoma in the U.S.: an update of trends by gender, race, age, subsite, and stage, 1975-1994. Cancer. 1999;85(8):1670–1676. [PubMed] [Google Scholar]
- 22.Thibodeau SN, French AJ, Cunningham JM, et al. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res. 1998;58(8):1713–1718. [PubMed] [Google Scholar]
- 23.Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20(4):1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 24.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145(1):72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 25.Limburg P, Vierkant R, Cerhan J, et al. Cigarette smoking and colorectal cancer risk: a long-term, prospective study among postmenopausal women. Clin Gastroenterol Hepatol. 2003;1(3):202–210. doi: 10.1053/cgh.2003.50030. [DOI] [PubMed] [Google Scholar]
- 26.Yang P, Cunningham JM, Halling KC, et al. Higher risk of mismatch repair-deficient colorectal cancer in alpha(1)-antitrypsin deficiency carriers and cigarette smokers. Mol Genet Metab. 2000;71(4):639–645. doi: 10.1006/mgme.2000.3089. [DOI] [PubMed] [Google Scholar]
- 27.Samowitz WS, Albertsen H, Sweeney C, et al. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst. 2006;98(23):1731–1738. doi: 10.1093/jnci/djj468. [DOI] [PubMed] [Google Scholar]
- 28.Curtin K, Samowitz WS, Wolff RK, et al. Somatic alterations, metabolizing genes and smoking in rectal cancer. Int J Cancer. 2009;125(1):158–164. doi: 10.1002/ijc.24338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao A, Thun MJ, Jacobs EJ, et al. Cigarette smoking and colorectal cancer mortality in the cancer prevention study II. J Natl Cancer Inst. 2000;92(23):1888–1896. doi: 10.1093/jnci/92.23.1888. [DOI] [PubMed] [Google Scholar]
- 30.Kakar S, Burgart LJ, Thibodeau SN, et al. Frequency of loss of hMLH1 expression in colorectal carcinoma increases with advancing age. Cancer. 2003;97(6):1421–1427. doi: 10.1002/cncr.11206. [DOI] [PubMed] [Google Scholar]
- 31.Toyota M, Ahuja N, Ohe-Toyota M, et al. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96(15):8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Rijnsoever M, Grieu F, Elsaleh H, et al. Characterisation of colorectal cancers showing hypermethylation at multiple CpG islands. Gut. 2002;51(6):797–802. doi: 10.1136/gut.51.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bettstetter M, Dechant S, Ruemmele P, et al. Distinction of hereditary nonpolyposis colorectal cancer and sporadic microsatellite-unstable colorectal cancer through quantification of MLH1 methylation by real-time PCR. Clin Cancer Res. 2007;13(11):3221–3228. doi: 10.1158/1078-0432.CCR-06-3064. [DOI] [PubMed] [Google Scholar]
- 34.McGivern A, Wynter CV, Whitehall VL, et al. Promoter hypermethylation frequency and BRAF mutations distinguish hereditary non-polyposis colon cancer from sporadic MSI-H colon cancer. Fam Cancer. 2004;3(2):101–107. doi: 10.1023/B:FAME.0000039861.30651.c8. [DOI] [PubMed] [Google Scholar]
- 35.Suehiro Y, Wong CW, Chirieac LR, et al. Epigenetic-genetic interactions in the APC/WNT, RAS/RAF, and P53 pathways in colorectal carcinoma. Clin Cancer Res. 2008;14(9):2560–2569. doi: 10.1158/1078-0432.CCR-07-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammons GJ, Yan Y, Lopatina NG, et al. Increased expression of hepatic DNA methyltransferase in smokers. Cell Biol Toxicol. 1999;15(6):389–394. doi: 10.1023/a:1007658000971. [DOI] [PubMed] [Google Scholar]
- 37.Issa JP, Baylin SB, Belinsky SA. Methylation of the estrogen receptor CpG island in lung tumors is related to the specific type of carcinogen exposure. Cancer Res. 1996;56(16):3655–3658. [PubMed] [Google Scholar]
- 38.Vaissiere T, Hung RJ, Zaridze D, et al. Quantitative analysis of DNA methylation profiles in lung cancer identifies aberrant DNA methylation of specific genes and its association with gender and cancer risk factors. Cancer Res. 2009;69(1):243–252. doi: 10.1158/0008-5472.CAN-08-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wistuba, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol. 2001;28(2 Suppl 4):3–13. [PubMed] [Google Scholar]
- 40.Malekzadeh K, Sobti RC, Nikbakht M, et al. Methylation patterns of Rb1 and Casp-8 promoters and their impact on their expression in bladder cancer. Cancer Invest. 2009;27(1):70–80. doi: 10.1080/07357900802172085. [DOI] [PubMed] [Google Scholar]
- 41.Marsit CJ, Christensen BC, Houseman EA, et al. Epigenetic profiling reveals etiologically distinct patterns of DNA methylation in head and neck squamous cell carcinoma. Carcinogenesis. 2009;30(3):416–422. doi: 10.1093/carcin/bgp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giovannucci E. An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10(7):725–731. [PubMed] [Google Scholar]
- 43.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol. 2009;104(3):739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 44.Glockner SC, Dhir M, Yi JM, et al. Methylation of TFPI2 in stool DNA: a potential novel biomarker for the detection of colorectal cancer. Cancer Res. 2009;69(11):4691–4699. doi: 10.1158/0008-5472.CAN-08-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou H, Harrington JJ, Shire AM, et al. Highly methylated genes in colorectal neoplasia: implications for screening. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2686–2696. doi: 10.1158/1055-9965.EPI-07-0518. [DOI] [PubMed] [Google Scholar]
- 46.Morimoto LM, Newcomb PA, Ulrich CM, et al. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer Epidemiol Biomarkers Prev. 2002;11(10 pt 1):1012–1018. [PubMed] [Google Scholar]