Abstract
The mammalian target of rapamycin (mTOR) kinase is an important component of PTEN/PI3K/Akt signaling pathway, which is frequently deregulated in prostate cancer (CaP). Recent studies suggest that targeting PTEN/PI3K/Akt and mTOR signaling pathway could be an effective strategy for the treatment of hormone refractory CaP. Here, we show that the treatment of androgen-independent and PTEN-negative human CaP PC3 cells with fisetin, a dietary flavonoid, resulted in inhibition of mTOR kinase signaling pathway. Treatment of cells with fisetin inhibited mTOR activity and downregulated Raptor, Rictor, PRAS40 and GβL that resulted in loss of mTOR complexes (mTORC)1/2 formation. Fisetin also activated the mTOR repressor TSC2 through inhibition of Akt and activation of AMPK. Fisetin-mediated inhibition of mTOR resulted in hypophosphorylation of 4EBP1 and suppression of Cap-dependent translation. We also found that fisetin treatment leads to induction of autophagic-programmed cell death rather than cytoprotective autophagy as shown by small interfering RNA Beclin1-knockdown and autophagy inhibitor. Taken together, we provide evidence that fisetin functions as a dual inhibitor of mTORC1/2 signaling leading to inhibition of Cap-dependent translation and induction of autophagic cell death in PC3 cells. These results suggest that fisetin could be a useful chemotherapeutic agent in treatment of hormone refractory CaP.
Introduction
In the USA and in many western countries, prostate cancer (CaP) is the most commonly diagnosed cancer and second leading cause of cancer-related death in men (1). Treatments such as hormone therapy including antiandrogen therapy and orchiectomy have contributed to reducing fatality of CaP. Despite the initial efficacy of androgen deprivation therapy, most CaP relapses and become hormone refractory that becomes resistant to hormone manipulation. Currently, there is no curative therapy for hormone refractory CaP rendering this subtype of disease a significant public health burden (2). The exact molecular mechanism of the onset of hormone independence has not been elucidated. However, recent studies have shown that it is associated with phosphatase tensin homolog (PTEN)/phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway (3). The mammalian target of rapamycin (mTOR) is an important component of PTEN/PI3K/Akt signaling pathway, which is frequently dysregulated in various cancers including CaP (4). Recent studies suggest that targeting mTOR signaling pathway could be an effective strategy for the treatment of cancer (5). Moreover, mTOR signaling is involved in CaP progression especially in transition to hormone refractory disease (6). The mTOR kinase forms two distinct multiprotein complexes called mTORC1 and mTORC2 where rapamycin-insensitive companion of mammalian target of rapamycin (Rictor)-associated mTORC2 mediates Akt activation, which in turn stimulates and activates regulatory-associated protein of mammalian target of rapamycin (Raptor)-associated mTORC1. The activated mTORC1 regulates cell growth through controlling numerous processes including Cap-dependent protein translation and autophagy (7). This indicates that inhibition of not only mTORC1 but also mTORC2 is necessary to block the progression of advanced CaP efficiently.
Fisetin (3,3′,4′,7-tetrahydroxyflavone) (Figure 1A) is a naturally occurring flavonoid found in fruits and vegetables such as strawberry and onion (8). Fisetin is known to possess antioxidative (9) and anti-inflammatory (10) effects. We recently showed that fisetin induces apoptosis and cell cycle arrest in LNCaP human CaP cells (11) and inhibits androgen receptor signaling and tumor growth in athymic nude mice (12). We hypothesized that fisetin may provide chemotherapeutic effects against hormone-independent subtype of CaP. In this study, we determined the effect of fisetin on PTEN-negative hormone refractory PC3 CaP cells. Here, we provide evidence that fisetin can inhibit both mTORC1 and mTORC2, which results in inhibition of Cap-dependent protein translation and induction of autophagic cell death in PC3 cells. These results suggest that fisetin could be a useful chemotherapeutic agent in treatment of hormone refractory CaP.
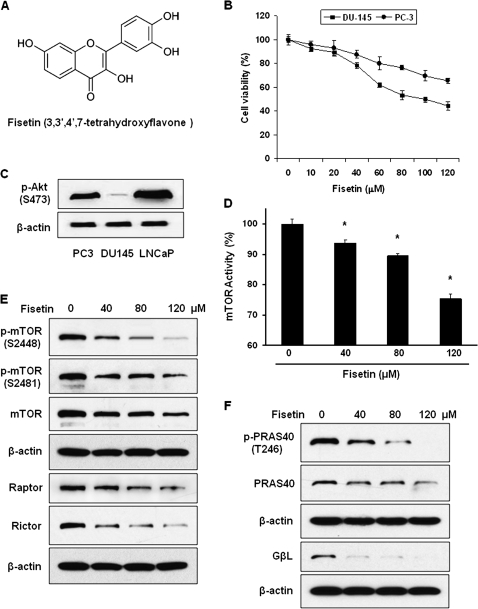
Fig. 1.
Effect of fisetin on viability of PC3 CaP cells. (A) The molecular structure of fisetin. (B) MTT assay of PC3 and DU145 CaP cells. Cells were treated with fisetin up to 120 μM for 48 h. The absorbance was measured at 540 nm. The assay was performed in triplicate and the data are presented as mean ± standard error of the mean (SEM). (C) Expression of p-Akt (S473) in PC3, DU145 and LNCaP cells shown by western blot. (D) Effect of fisetin on mTOR kinase activity. To measure the effect of fisetin on the activity of mTOR kinase, the capability of the cell lysate to phosphorylate the mTOR substrate S6K70 was measured. The data represent average of three independent experiments and shown as mean ± SEM; *P < 0.05 versus control. (E) Effect of fisetin on mTOR, Raptor and Rictor shown by western blot. (F) Effect of fisetin on PRAS40 and G protein β-like protein (GβL) shown by western blot. Anti-β-actin antibody was used to verify equal loading in western blots.
Materials and methods
Reagents and cell culture
Human CaP cell lines PC3, DU145 and LNCaP cells were purchased from American Tissue Type Culture Collection (Manassas, VA). LNCaP cells were cultured in Dulbecco's modified Eagle's medium supplemented with 5% heat-inactivated fetal bovine serum (FBS) and antibiotics (Cambrex, Walkersville, MD). PC3 and DU-145 cells were grown in RPMI-1640 and minimum essential medium, respectively, supplemented with 10% FBS (HyClone, Logan, UT) and antibiotics. Fisetin, 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT), VP16, chloroquine (CQ) and monodansylcadaverine (MDC) were purchased from Sigma (St Louis, MO). Z-VAD was obtained from R&D systems (Minneapolis, MN).
Cell viability assay
Cells were seeded and cultured with different doses of fisetin (10–120 μM) for 24, 48, 72 and 96 h. Culture media were replaced with 0.05% MTT solution and incubated at 37°C for 2 h. Dimethyl sulfoxide was added after removal of the MTT solution. After brief incubation, the absorbance of the solution was measured in a microplate reader.
Plasmids, siRNA and transfection
The bicistronic luciferase reporter construct was used to measure Cap-dependent translation. To determine autophagy, pGFP-LC3 plasmid was utilized. The LC3 complementary DNA was subcloned into pGFP vector (Clontech, Mountain View, CA). Plasmids were amplified and purified using HiSpeed plasmid maxi kit (Qiagen, Valencia, CA). The non-targeting- and Beclin1-specific small interfering RNAs (siRNAs) were purchased from Dharmacon (Lafayette, CO) and cells were transfected using the nucleofection kit V specific for PC-3 transfection from Amaxa Biosystems (Gaithersburg, MD).
Detection of apoptosis
To measure apoptosis, APO-DIRECT kit (Phoenix Flow System, San Diego, CA) was utilized as per manufacturer's manual. Apoptosis was also detected using Hoechst 33258. Briefly, cells were fixed with 4% paraformaldehyde for 10 min followed by incubation with 10 μM of Hoechst 33258 for 30 min in the dark. After brief rinse with phosphate-buffered saline (PBS), apoptosis was detected by fluorescence microscopy.
Detection of autophagy
Cells were seeded and cultured in tissue culture-treated chamber glass slides (BD Falcon, Franklin Lakes, NJ). After 24 h of transfection of pGFP-LC3 plasmid, media were replaced with fresh media with or without fisetin and cultured for another 24 h. Fluorescent imaging was performed to detect punctuate pattern of green fluorescent protein (GFP)-LC3, which is indicative of autophagy. Autophagy was also detected through visualizing autophagic vacuoles with MDC. Cells were incubated with 50 μM MDC for 1 h at 37°C. Cells were then washed with PBS followed by fixation with 4% paraformaldehyde for 15 min at room temperature. After brief rinse with PBS, MDC that was incorporated in autophagosome was analyzed by fluorescence microscopy.
Luciferase assay
Cells grown in six-well plates were transiently transfected with 1 μg of bicistronic plasmid that contains both Photinus pyralis (firefly) and Renilla reniformis (sea pansy) luciferase reporters. After 24 h, cells were treated with fisetin for another 24 h. The luciferase activities of Renilla and firefly were determined using Dual Luciferase Reporter Assay System (Promega, Madison, WI) according to the manufacturer’s protocol. Briefly, Passive Lysis Buffer was used to lyse the cells. The lysate was then mixed with Luciferase Assay Reagent II to measure the luminescence from firefly luminescence reporter. After quantifying the firefly luminescence, Stop & Glow Reagent was added to quench this reaction and to measure luminescence from Renilla. Luminescence was measured in triplicates using 2020n single tube luminometer from Turner Biosystems (Promega).
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and western blotting
Cells were harvested at specified time points after treatment and protein lysates were prepared for western blot analysis. Cell lysates were prepared using RIPA buffer [25 mM Tris–HCl, pH 7.6, 150 mM NaCl, 1% NP40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS)] supplemented with protease and phosphatase inhibitors. Xcell SureLock system (Invitrogen, Carlsbad, CA) was used for gel electrophoresis and western blotting. For determination of LC3 by western blot, we used 1% SDS sample buffer to lyse cells and quantified protein with Bio-Rad DC protein assay kit (Hercules, CA). All the primary antibodies, except β-actin antibody (Sigma), were purchased from Cell Signaling Technology (Danvers, MA) and secondary antibodies were purchase from GE Healthcare (Piscataway, NJ).
Fluorescence microscopy
Briefly, slides were rinsed with PBS and cells were fixed with 2% paraformaldehyde and permeabilized in methanol. After washing with PBS, slides were blocked with 2% donkey serum. Primary and secondary antibodies were incubated in 5% donkey serum. ProLong Gold Antifade-DAPI (Invitrogen) was used as mounting media. For analysis, Bio-Rad Radiance 2100 MP Rainbow system in the W.M. Keck Laboratory for Biological Imaging at the University of Wisconsin–Madison was used.
mTOR kinase assay.
The activity of mTOR was measured with colorimetric K-LISA mTOR activity assay kit (Calbiochem, San Diego, CA). Briefly, mTOR proteins in the cells are immunoprecipitated with mTOR antibody and protein A/G-agarose. The resulting immunocomplex and adenosine triphosphate (ATP) are added to the S6K70-coated wells. After phosphorylation of S6K70 at T389 by the active mTOR in the sample, the phosphorylated substrate can be detected with anti-S6K70-T389 antibody. The mTOR activity can be measured in terms of absorbance in microplate reader.
Phospho-Akt ELISA
To quantify the endogenous levels of p-Akt (S473) in cells, PathScan Phospho-Akt (S473) ELISA assay (Cell Signaling Technology) was performed as per manufacturer’s manual. Briefly, phospho-Akt (S473) proteins in cell lysate were captured by the corresponding antibody that was coated in the microplate. After adding the horseradish peroxidase-linked secondary antibody and chemiluminescent substrate, the magnitude light emission, which is proportional to the quantity of phospho-Akt (S473) protein, was measured.
7-Methyl-guanosine cap binding assay
Total of 700 μg of cellular proteins in lysis buffer [20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1 mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4 and 1 μg/ml leupeptin] was mixed with 50 μl of 7-methyl-GTP-Sepharose-4B bead suspension (GE Healthcare) and incubated overnight. After washing the pellet, the affinity complex was recovered and boiled with SDS sample buffer. The supernatant was recovered after centrifugation and subjected to SDS–polyacrylamide gel electrophoresis and western blotting.
Immunoprecipitation
Total of 500 μg of cellular proteins was used for immunoprecipitation. The lysate was precleared by incubating with 20 μl of protein A/G-agarose and 1 μg of rabbit IgG for 1 h.
Measurement of ATP
The levels of ATP in cells were measured using ATP bioluminescence assay kit CLS II from Roche (Indianapolis, IN) according to the manufacturer’s protocol. Briefly, equal number of cells were collected and boiled in 9 vol of 100 mM Tris and 4 mM ethylenediaminetetraacetic acid, pH 7.75 for 2 min to extract ATP. After brief spin, 100 μl of supernatant fraction was mixed with equal volume of luciferase reagent and the luminescence was measured.
Colony formation assay
Briefly, cells were seeded in top agar containing 0.3% agar with RPMI-1640 and 10% FBS. Bottom agar is consisted of 0.5% agar, RPMI-1640 and 10% FBS. Media with dimethyl sulfoxide or indicated doses of fisetin were added and replaced every 3 days.
Statistical analysis of the data
Microsoft Excel software was used to calculate the mean and the standard error of the mean. The t-test was used to compare the means of groups and P values <0.05 were considered significant.
Results
Fisetin induces growth inhibition of PC3 CaP cells and decreases the activity of mTOR kinase
In the present study, we evaluated the effect of fisetin on hormone-independent CaP cell lines DU145 and PC3. In our cell viability assay, PC3 cells showed less sensitivity to fisetin than DU145 (Figure 1B). This might be due to the fact that PC3 cells possess PTEN-null mutation (13), which results in constitutive activation of Akt (Figure 1C), a kinase that is known to be correlated with progression of CaP (14). Since phosphorylation of S473 of Akt is a positive regulator of mTOR signaling pathway, we further tested whether fisetin may provide chemotherapeutic effects against hormone-independent CaP through inhibiting mTOR pathway using PC3 cell line as a model system. We first measured the activity of mTOR kinase in PC3 cells treated with fisetin using the enzyme-linked immunosorbent assay-based assay. Treatment of cells with 40, 80 and 120 μM of fisetin for 48 h inhibited the activity of mTOR by 6, 11 and 25%, respectively (Figure 1D). For further experiments, cells were treated with 40, 80 and 120 μM of fisetin for 48 h unless otherwise stated in the text.
Fisetin inhibits phosphorylation of mTOR and expression of the mTORC1 and mTORC2 constituents
Since the activity of mTOR kinase is decreased upon fisetin treatment, we performed western blot of proteins that consist mTORCs. Data in Figure 1E show that not only basal expression of mTOR but also autophosphorylation of mTOR at S2481 and Akt-mediated phosphorylation at S2448, which correlates with mTOR activity (15), are repressed by fisetin. Our data also indicate that the level of Raptor and Rictor, the companion proteins of mTORC1 and mTORC2, respectively, are also downregulated by fisetin (Figure 1E). The level of both total and phosphorylated proline-rich Akt substrate PRAS40, which binds Raptor to form mTORC1, is also decreased upon fisetin treatment (Figure 1F). This indicates that fisetin represses phosphorylation of PRAS40 that leads to inhibition of the substrate binding to mTORC1. Finally, the level of G protein β-like protein (GβL), which constitutes a part of both mTORCs, has been significantly downregulated by fisetin treatment (Figure 1F).
Fisetin inhibits formation of mTORC1/2 and activation of Akt
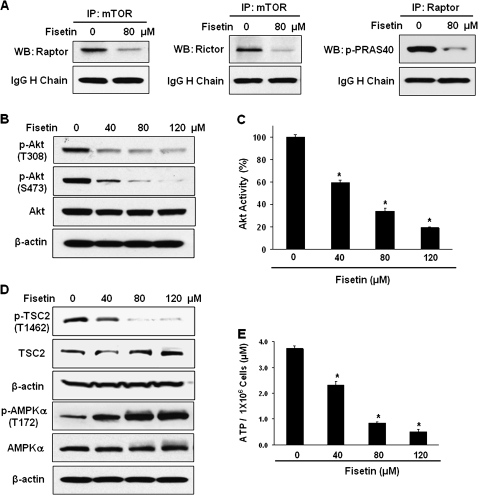
To determine whether the downregulation of mTORC constituents leads to less mTORC formation, immunoprecipitation was performed. As expected, there were less Raptor and Rictor isolated with mTOR antibody after fisetin treatment (Figure 2A). Also, less p-PRAS40 (T246) was identified in the immunocomplex purified with Raptor antibody after treating cells with fisetin (Figure 2A). These results indicate that fisetin inhibits mTORC1 and mTORC2 formation, which may lead to the inhibition of mTOR signaling pathway.
Fig. 2.
(A) Effect of fisetin on mTOR signaling complex formation. Cells were treated with fisetin and the lysate was immunoprecipitated using mTOR and Raptor antibodies. The immunoprecipitate was analyzed by western blot with Raptor, Rictor and p-PRAS40 (T246) antibodies. The bands of the heavy (H) chain of IgG are shown to verify equal loading. (B) Effect of fisetin on the activation of Akt. Western blot experiment of Akt was performed with the lysates of cells after treated with fisetin. (C) The phosphorylation of Akt was measured with PathScan p-Akt (S473) sandwich ELISA kit. The data shown are representative results of three independent experiments and are presented as mean ± SEM; *P < 0.05 versus control. (D) Effect of fisetin on the activation of TSC2 and AMPK. Western blots were performed with antibodies against TSC2, p-TSC2, AMPKα and p-AMPKα. In western blots, anti-β-actin antibody was used to verify equal loading. (E) Effect of fisetin on cellular energy status. ATP levels of cells grown with or without fisetin were measured with ATP bioluminescence assay kit CLS II. The data represent average of three independent experiments and shown as mean ± SEM; *P < 0.05 versus control.
Since we observed downregulation of mTORC2, we measured the activity of Akt after fisetin treatment. Akt has been shown to be upregulated in CaP and its expression is correlated with progression of CaP (14). Akt is direct target of mTORC2 and its phosphorylation at S473 by mTORC2 contributes to its full activation (16). We found that fisetin effectively decreased the phosphorylation of Akt at both T308 and S473 (Figure 2B). The effect of fisetin on the phosphorylation of Akt was confirmed by p-Akt ELISA assay. Treating cells with fisetin decreased the level of p-Akt to 59, 34 and 19% at 40, 80 and 120 μM, respectively (Figure 2C).
Fisetin activates mTOR inhibitor tuberous sclerosis complex 2 through regulating Akt and adenosine monophosphate-activated protein kinase
In the mTOR signaling pathway, ras homolog enriched in brain (Rheb) guanosine triphosphatase plays a critical role in regulation of mTORC1 activity. The guanosine triphosphatase activating protein activity of tuberous sclerosis complex (TSC) is modulated by phosphorylation through kinases, positively by adenosine monophosphate-activated protein kinase (AMPK) and negatively via Akt (17). Since fisetin inactivated Akt, we measured the level of p-TSC2 (T1462) that is mediated by Akt (18). As shown in Figure 2D, the level of mTOR antagonizer TSC2 increased whereas the level of p-TSC2 (T1462) decreased dose dependently upon fisetin treatment. Since p-AMPKα (T172) activates TSC complex, we measured the level of phosphorylation of catalytic α subunit of AMPK. As expected, fisetin dose dependently increased phosphorylation of AMPK (Figure 2D).
We also quantified the endogenous level of energy in terms of ATP after fisetin treatment because it is well known that the increase in the intracellular adenosine monophosphate:ATP ratio activates AMPK (19). We observed that the ATP levels decreased from 3.7 to 2.3, 0.8 and 0.5 μM per 1 × 106 cells at 40, 80 and 120 μM of fisetin treatment, respectively (Figure 2E). As compared with control group, there was 38, 78 and 87% decrease in ATP levels at 40, 80 and 120 μM of fisetin, respectively (Figure 2E). Overall, these results indicate that fisetin suppresses mTOR through inactivation of Akt and activation of AMPK, which result in the activation of TSC complex.
Fisetin dephosphorylates and inactivates S6K70 and inhibits its target proteins ribosomal protein S6 and eukaryotic translation initiation factor 4B
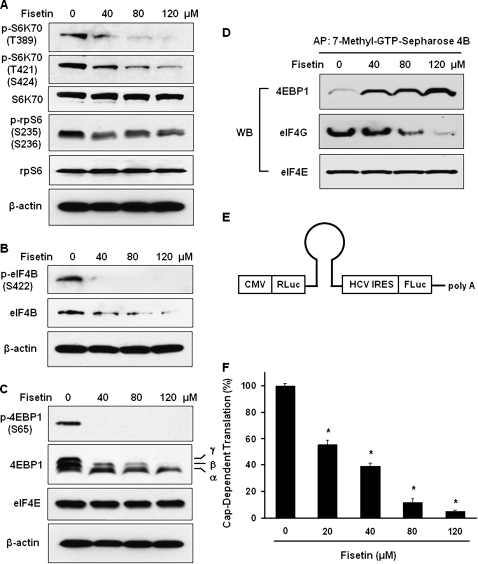
We next assessed the effect of fisetin on downstream targets of mTOR. It is well established that activated mTOR transmits its signal to p70-S6 kinase (S6K70) to activate it and conducts negative signal to eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1), which binds and inhibits eukaryotic translation initiation factor (eIF) 4E (7). Among the several phosphorylation sites of S6K70, phosphorylation at T421/S424 is necessary for its activation and phosphorylation at T389 is known to be required for full kinase function (20). The activation of S6K70 has significantly decreased after fisetin treatment (Figure 3A). Subsequently, we assessed the level of S6K70 target proteins, ribosomal protein S6 (rpS6) and eIF4B. The activated S6K70 phosphorylates rpS6 at sites including S235 and S236 that leads to increase in translation of messenger RNAs that encode proteins involved in translational apparatus (21). Our data show that inactivated S6K70 resulted in decreased phosphorylation of rpS6 that may lead to decrease in translational apparatus formation (Figure 3A). Phosphorylation of eIF4B at S422 is critical for its function (22) and we observed that fisetin decreases both total and phosphorylated level of eIF4B in PC3 cells (Figure 3B).
Fig. 3.
Effect of fisetin on mTOR target proteins. (A) Fisetin-mediated changes in mTOR target protein S6K70 and its substrates rpS6 and (B) eIF4B. (C) Western blot results of 4EBP1 and eIF4E are shown. Three different forms of 4EBP1 can be resolved by electrophoresis, the hyperphosphorylated γ form, the intermediately phosphorylated β form and the most mobile and non-phosphorylated α form. In western blots, anti-β-actin antibody was used to verify equal loading. Effect of fisetin on the formation of translation initiation complex by 7-methyl-GTP-Sepharose chromatography. (D) Cells were treated with 40, 80 and 120 μM of fisetin followed by affinity precipitation (AP) of the lysate with 7-methyl-GTP-Sepharose. The resulting affinity complex was washed and subjected to western blot using antibodies against 4EBP1, eIF4G and eIF4E. Effect of fisetin on Cap-dependent protein translation. (E) The structure of the bicistronic luciferase reporter construct is presented. The cassette is transcriptionally regulated by cytomegalovirus (CMV) promoter. The expression of Renilla luciferase (RLuc) is directed by Cap-dependent translation, whereas the firefly luciferase (FLuc) expression is driven by hepatitis C virus (HCV) internal ribosomal entry site (IRES)-dependent translation, i.e. Cap-independent translation. A stem-loop structure is inserted into the bicistronic structure to prevent the leaky scanning of the ribosome. (F) Cells grown in six-well plates were transfected with 1 μg of bicistronic plasmid per well and treated with fisetin 24 h after transfection. After 24 h of treatment, the luciferase activities of Renilla and firefly were determined using Dual Luciferase Reporter Assay System. The result is presented as relative Cap-dependent translation (%) compared with control that is calculated by (Renilla luciferase activity/Firefly luciferase activity) × 100. The data shown are representative results of three independent experiments and are presented as mean ± SEM; *P < 0.05 versus control.
Fisetin dephosphorylates and activates 4EBP1, the inhibitor of Cap-dependent translation
Translational control is primarily performed at initiation step (22) where eIF4F binds to the 7-methyl-guanosine Cap structure at the 5′ end of messenger RNA to recruit 40S ribosomal subunit (23). The eIF4F complex consists of an RNA helicase eIF4A, a Cap-binding protein eIF4E and a scaffolding protein eIF4G (22). However, eIF4A alone possesses minimum helicase activity that can be augmented by cofactor eIF4B (22). The eIF4G binds to messenger RNA through binding to eIF4E that can be prevented by 4EBP1 binding to eIF4E (24). We next evaluated the effect of fisetin on 4EBP1 as it is another target of mTOR. Our data show that fisetin converts 4EBP1 from hyperphosphorylated γ form to the hypo- or non-phosphorylated α form (Figure 3C), which permits 4EBP1 to sequester eIF4E resulting in reduced level of Cap-dependent translation (25). The translational repressor 4EBP1 undergoes sequential phosphorylation and final phosphorylation at S65 results in release of 4EBP1 from eIF4E (25). Our data clearly show that fisetin abrogates the phosphorylation of 4EBP1 (S65) even at 40 μM (Figure 3C).
Fisetin inhibits the assembly of protein translation initiation complex and effectively suppresses Cap-dependent translation
To determine whether the effect of fisetin on translation initiation factors correlate well with the actual formation of translation initiation complex, we performed 7-methyl-GTP-Sepharose chromatography. We observed the increase in 4EBP1 bound to eIF4E and concurrent reduction in eIF4G binding to eIF4E (Figure 3D), indicating that fisetin deactivates the translational apparatus. Since fisetin inhibited the formation of initiation complex, we tested whether this actually results in decreased Cap-dependent translation. In our bicistronic luciferase reporter assay (Figure 3E), we observed that fisetin treatment (20, 40, 80 and 120 μM) resulted in decreased Cap-dependent translation by 45, 61, 89 and 95%, respectively, when compared with control (Figure 3F).
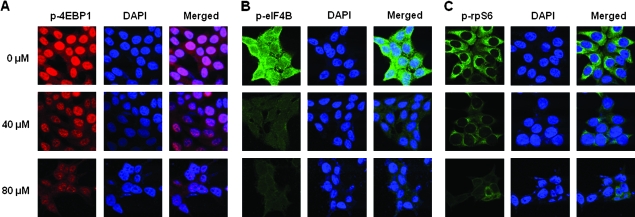
To further confirm that fisetin inhibits mTOR target proteins, we performed fluorescent imaging of fisetin-treated cells with p-4EBP1-, p-eIF4B- and p-rpS6-specific antibodies (Figure 4A–C, respectively). As expected, the level of these target proteins are downregulated by fisetin as shown by diminished fluorescence in the antibody and merged panel when compared with the corresponding panels of control.
Fig. 4.
Effect of fisetin on mTOR target proteins. Immunofluorescent imaging of mTOR target proteins upon fisetin treatment is shown. Cells were seeded and cultured for 24 h in two-chamber glass slides followed by treatment with indicated doses of fisetin for 48 h before fixing and labeling with antibodies. For labeling, anti-rabbit antibodies against (A) p-4EBP1 (S65), (B) p-eIF4B (S422) and (C) p-rpS6 (S235/S236) (all from Cell Signaling Technology) were used. Alexa Fluor goat and anti-rabbit IgGs (Invitrogen) were used as secondary antibodies. 4′,6-Diamidino-2-phenylindole (DAPI) was used to counter stain the nucleus; ×600.
Fisetin induces minimum apoptosis but effectively inhibits colony formation
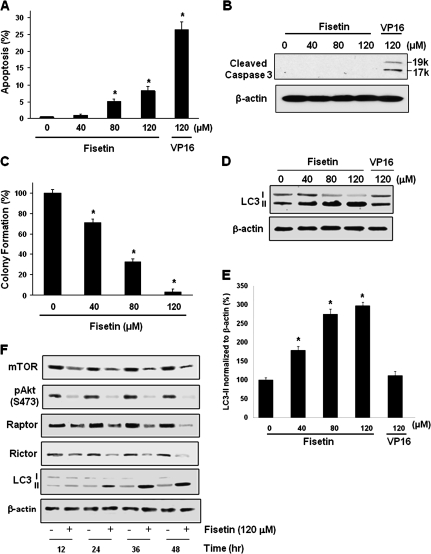
As shown earlier in MTT assay (Figure 1B), fisetin-treated cells exhibited significant decrease in viability. To elucidate the type of cell death mediated by fisetin, we performed apoptosis assay that resulted in 0.6, 1.1, 5.0 and 8.3% of apoptosis in cells treated with 0, 40, 80 and 120 μM of fisetin, respectively, whereas etoposide (VP16)-treated cells, a known inducer of apoptosis in PC3 cells (26), resulted in 27% of apoptosis, indicating that there is minimal apoptosis in fisetin-treated cells (Figure 5A). This was confirmed by western blot analysis where cleaved caspase-3 was detected only in VP16-treated cells (Figure 5B).
Fig. 5.
Effect of fisetin on apoptosis of PC3 cells. (A) Apoptosis was measured in cells treated with indicated doses of fisetin or VP16 for 48 h. APO-DIRECT system was utilized for quantification of apoptosis. (B) Western blot with cleaved caspase-3 antibody was performed to detect active caspase-3. (C) Effect of fisetin on colony formation. Colony formation assay was performed in six-well plates in triplicate with or without fisetin. Initially, 5000 cells were seeded in top agar and grown for 4 weeks. Colonies were counted from three different fields and compared with that of control. The data are presented as mean ± SEM; *P < 0.05 versus control. (D) Effect of fisetin on LC3-II levels. Western blot analysis with LC3 I/II-specific antibody is presented. (E) Relative density of LC3-II protein normalized to β-actin as percent compared with control is plotted. Western blot of β-actin was used to verify equal loading. (F) Time-dependent effect of fisetin (120 μM) on mTOR pathway and LC3-II levels in PC3 cells. Western blot of β-actin was used to verify equal loading.
In colony formation assay, the clonogenicity of the fisetin-treated group showed significant dose-dependent decrease where 120 μM of fisetin-treated group exhibited only 3% clonogenicity compared with control (Figure 5C). Since our MTT assay showed that 120 μM of fisetin decreased cell viability by 55% (Figure 1B), our data indicate that there might be another type of cell death other than apoptosis in fisetin-treated cells. Apoptosis is the most well recognized form of programmed cell death and recently, considerable evidence suggests that autophagy may be another type of programmed cell death (27).
Fisetin induces LC3 II protein expression
In fact, it is well known that the active mTOR not only activates protein translation initiation but also inhibits autophagy (28,29). To detect autophagy, we performed western blot using light chain (LC) 3 antibody. Western blot analysis and relative density of the LC3 II bands normalized to β-actin showed that fisetin-treated cells (40, 80 and 120 μM) resulted in 79, 175 and 198% increase in LC3-II levels, respectively, when compared with control; whereas 120 μM of VP16-treated cells revealed only 12% increase (Figure 5D and E). We next determined kinetic analysis of reduction in mTOR pathway and LC3-II protein in fisetin-treated PC3 cells. We found that reduction in phosphorylation of Akt after fisetin treatment is an early event that occurred at 12 h posttreatment (Figure 5F). However, reduction in mTOR, Raptor and Rictor occurred at 24 h after treatment followed by increased expression of LC3-II protein expression (Figure 5F). These results suggest that inhibition in pAkt, mTOR, Rictor and Raptor precede increased expression of LC3-II protein expression after fisetin treatment.
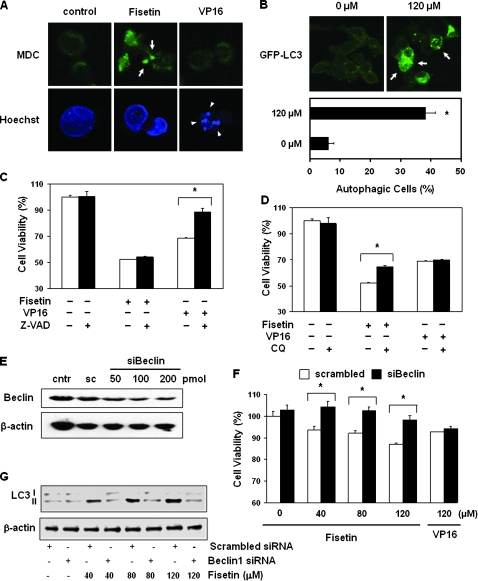
Fisetin induces autophagy and CQ attenuates the effect of fisetin in PC3 cells
To confirm the induction of autophagy, we stained the cells with autophagy-specific dye MDC and apoptosis-specific dye Hoechst 33258. MDC is known to accumulate in autophagosomes and is clearly seen in cells treated with fisetin but not in VP16-treated cells (Figure 6A, top panel). On the contrary, only VP16-treated cells showed Hoechst 33258 staining that represents fragmented chromatin, the hallmark of apoptosis (Figure 6A, bottom panel). We also detected autophagy by utilizing GFP-LC3 plasmid (30). GFP-LC3 serves as a very good marker for autophagy since it exhibits bright punctuate pattern in autophagic cells, which indicates formation of autophagic isolation membranes and autophagosomes (31). In this experiment, the localization of GFP-LC3 has changed from diffused pattern in control cells to the punctuate pattern in fisetin-treated cells (Figure 6B, top panel). On average, 38% of fisetin-treated cells exhibited dotted pattern of GFP-LC3, whereas control cells showed only 6% (Figure 6B, bottom panel).
Fig. 6.
Effect of fisetin on induction of autophagy. (A) Detection of autophagy by MDC. Cells were treated with either 120 μM of fisetin or VP16 for 48 h followed by detection of autophagy and apoptosis by MDC and Hoechst 33258, respectively. Autophagosome formation is indicated by arrows and fragmented chromatin is indicated by arrow heads; ×600. (B) Detection of autophagy by GFP-LC3. PC3 cells were transfected with GFP-LC3 and treated with or without 120 μM of fisetin for 24 h. Fluorescent microscope was used to detect the punctuate pattern (indicated by arrows) of GFP-LC3 (top panel). Also the number of cells with bright GFP-LC3 dots of total number of cells from five different fields was counted and compared with that of control (bottom panel). The data are presented as mean ± SEM; *P < 0.05 versus control, ×600. Effect of (C) Z-VAD and (D) CQ on fisetin and VP16-treated cells. Cells were treated with combination of fisetin (120 μM), VP16 (120 μM), Z-VAD (100 μM) and CQ (10 μM) as indicated. After 48 h of treatment, cells were subjected to viability assay. The data shown represent three independent experiments and are presented as mean ± SEM; *P < 0.05 versus paired control. (E) Effect of Beclin1-knockdown in PC3 cells. Western blot of Beclin1 is shown. Cells were transiently transfected with scrambled siRNA (sc) or indicated doses of Beclin1 siRNA (siBeclin1). Anti-β-actin antibody was used as loading control. (F) Viability assay of Beclin1-knockdown PC3 cells treated with fisetin or VP16. Equal number of cells was seeded in six-well plates and transfected with 100 pmol of scrambled siRNA (scrambled) or Beclin1 siRNA (siBeclin1). After 24 h of transfection, cells were treated with indicated doses of fisetin or VP16 for another 24 h followed by MTT assay. The average viability of scrambled siRNA-transfected cells was set to 100%. The data represent average of six independent experiments and are shown as mean ± SEM; *P < 0.05 versus control. (G) Effect of fisetin treatment on LC3-II protein expression in Beclin1-knockdown PC3 cells. Cells were transfected with 100 pmol of scrambled siRNA (scrambled) or Beclin1 siRNA (siBeclin1). After 24 h of transfection, cells were treated with indicated doses of fisetin or VP16 for another 24 h followed by western blot analysis. Western blot of β-actin was used to verify equal loading.
Autophagy is not only recognized as a type of programmed cell death but also serves as a mechanism in sustaining cell survival through partial autodigestion thereby recycling long-lived proteins and organelles (32). To elucidate the role of fisetin-induced autophagy in PC3 cells, we treated cells either with fisetin or VP16 alone or together with autophagy inhibitor CQ or general caspase inhibitor Z-VAD. As expected, treatment of cells with fisetin or VP16 alone decreased viability significantly, whereas cotreating cells with VP16 and Z-VAD resulted in increased viability (89%) compared with the group treated with VP16 alone that showed viability of 69% (Figure 6C, right panel). On the contrary, the effect of Z-VAD was minimal in cells cotreated with fisetin and Z-VAD (Figure 6C, middle panel). But when the cells were treated with fisetin together with CQ, the viability augmented from 52% (fisetin-treated group) to 64% (Figure 6D, middle panel). Cotreatment with VP16 and CQ had no effect when compared with VP16-treated group (Figure 6D, right panel). These data indicate that fisetin induces autophagic cell death in PC3 cells.
Beclin1-knockdown PC3 cells are less sensitive to fisetin treatment
To confirm our result, we performed viability assay of Beclin1-knockdown cells after fisetin treatment. Beclin1 is involved in initial step of autophagosome formation and thus essential for autophagy (33). We performed western blot with cells transfected with scrambled or Beclin1 siRNA in various doses and selected 100 pmol as optimal dose for further experiment (Figure 6E). We compared the viability of scrambled and siBeclin1-transfected cells at each dose of fisetin or VP16 (Figure 6F). In non-treated control group, scrambled and siBeclin1-transfected cells resulted in negligible difference in viability (100 versus 103%, respectively), whereas 40 μM of fisetin showed 94% (scrambled) versus 104% (siBeclin1) in viability after 24 h. Similarly, 80 μM of fisetin rescued the viability of siBeclin1-transfected cells by 10% when compared with its paired control. Treatment of siBeclin1-tranfected cells with 120 μM of fisetin showed a significant increase in cell viability when compared to scrambled-transfected cells. Finally, in VP16-treated group, Beclin1-knockdown cells resulted in only marginal increase in viability when compared with that of its paired control (93 versus 94%, respectively) (Figure 6F). To verify that the observed decrease in viability upon fisetin treatment is due to the induction of autophagic cell death, we performed a western blot using LC3 antibody with or without knocking down the Beclin1 gene. As expected, scrambled siRNA group showed increase in LC3-II levels when treated with fisetin (Figure 6G). However, fisetin-treated Beclin1-knockdown cells had no effect on LC3-II levels when compared with control indicating that sustained viability in this group is indeed due to the inhibition of autophagic cell death (Figure 6G). These data, together with the previous results, indicate that fisetin induces autophagic programmed cell death rather than cytoprotective autophagy in PC3 cells suggesting a possible role of mTOR inhibitors as chemotherapeutic agent in advanced CaP.
Discussion
In this study, we have shown that fisetin induces inhibition of both mTORC1 and mTORC2 that leads to the repression of Cap-dependent protein translation and induction of autophagy in hormone-independent PC3 CaP cells. The rapamycin sensitive mTORC1 signaling regulates various cellular pathways including Cap-dependent translation and autophagy through coordinating molecules including S6K70 and 4EBP1. On the other hand, Akt is currently the only known direct target of mTORC2, which is rapamycin insensitive. These two mTOR complexes are linked through Akt since mTORC2-activated Akt activates mTORC1 through phosphorylation of TSC2 and PRAS40. Therefore, the early clinical trials with mTOR inhibitors as chemotherapeutic agents, which suppress only mTORC1, demonstrated unpromising results (7). Hence, it is suggested that dual inhibition of mTOR and other signaling pathways such as PTEN/PI3K/Akt would be an effective strategy in targeting cancer (34). Here, we report that natural food constituent fisetin decreases the phosphorylation of mTOR kinase and represses the expression of Raptor, Rictor, PRAS40 and GβL (Figure 1E and F) that resulted in less formation of both mTORC1 and mTORC2 in PC3 cells (Figure 2A).
The long-term androgen ablation therapy for CaP is shown to reinforce the PI3K/Akt pathway that contributes to the increased resistance of cancer cells to induction of apoptosis (5). In advanced CaP, PTEN is often mutated resulting in hyperactive Akt that leads to constitutively active mTOR signaling. As a result, targeting Akt remains an attractive therapeutic approach in CaP. The full activation of Akt requires dual phosphorylation on T308 by PI3K/pyruvate dehydrogenase [lipoamide] kinase isozyme 1 and on S473 by mTORC2. This indicates that blocking the phosphorylation on both sites is necessary for efficient inhibition of advanced CaP. It has been shown that the treatment of PC3 cells with rapamycin failed to inhibit the activation of Akt (35). Our data is quite promising in which we found that fisetin targets not only the mTOR signaling pathway but also the phosphorylation of AKT (Figure 2B).
Moreover, the ability of fisetin to target both Akt and mTOR signaling pathway possesses another benefit. The mechanism of resistance to mTORC1 inhibitors may also due to the existence of negative feedback loop from S6K to Akt signaling through receptor tyrosine kinase/PI3K pathway (7). This indicates that loosing feedback inhibition of Akt by using mTORC1 inhibitors may attenuate their therapeutic effects and promote chemoresistance. In this regard, an agent that is capable of dual inhibition of mTORC1 and Akt, such as fisetin, would be beneficial. It is important to note that Akt has multiple downstream targets including MDM2, p53 and BAD (36). This indicates that fisetin may target not only mTOR signaling pathway but also other signaling pathways that are involved in cell proliferation and survival, which is an advantage in targeting cancer.
We observed that fisetin inhibited mTOR targets including rpS6, eIF4B and 4EBP1 in PC3 cells (Figures 3 and 4). Recently, the expression of phosphorylated 4EBP1 has been shown to be associated with malignant progression and an adverse prognosis regardless of the upstream oncogenic alterations in cancers including breast, ovary and CaP (37). Therefore, our data suggest that fisetin could be a useful chemotherapeutic agent against CaP and variety of other cancers.
Rapamycin and its derivatives such as CCI-779 (Wyeth Pharmaceuticals), AP23573 (ARIAD Pharmaceuticals) and RAD001 (Novartis) are mTORC1 inhibitors (38). Rapamycins elicit their action by first binding to the immunophilin FK506-binding protein 12 and the resulting complex then binds mTORC1 thereby inhibiting its function (39). On the contrary, it is known that mTORC2 does not bind to rapamycin–FK506-binding protein 12 complex (40). Recent studies have shown that alkylphospholipid perifosine inhibited mTOR signaling and induced autophagy in human lung cancer cell lines (41). Perifosine inhibited mTOR signaling pathway through a different mechanism to that of rapamycin by facilitating the degradation of major components in the mTOR axis (41). Clinical trials with mTORC1 inhibitors as cancer chemotherapeutic agents has been successful only in specific tumors including renal cell carcinoma, whereas the trials of using such agents for other cancers has been less successful (5). Therefore, it is tempting to speculate that dual treatment of tumor with rapamycin and yet undeveloped mTORC2-specific inhibitor would be beneficial (42).
Autophagy is a catabolic process involving physiological turnover of long-lived proteins and damaged organelles in autophagosome thereby promoting cell survival. On the other hand, excessive ‘self-eating’ beyond a certain threshold will result in ‘self-cannibalization’, i.e. autophagic cell death, which is caspase-independent (43). Autophagy is emerging as new target for cancer therapy (44) but, however, whether autophagy causes survival or death in cancer cells is complicated and depends on specific context (45). Treatment of ovarian cancer cells (46) and glioma cells (47) with resveratrol and curcumin, respectively, induced autophagic cell death. We observed that fisetin induces autophagy in PC3 cells leading to autophagic cell death rather than cytoprotective autophagy as confirmed by the use of CQ and Beclin1-knockdown cells (Figures 5 and 6).
It is known that cancer cells at the center of tumor mass are poorly vascularized and the induction of autophagy may allow the stressed cancer cells to survive in such low-nutrient and low-oxygen (hypoxic) environment (43). This might explain the existence of LC3 II band in non-treated cells (Figure 5D), which indicates that PC3 cells possess basal level of autophagy. It could be hypothesized that in PC3 cells, fisetin triggers the cells to undergo maximum autophagy beyond the threshold, which will result in destructive autophagic cell death. Recently, hypoxia is emerging as a common characteristic of poor prognosis-associated CaP and may also be involved in transition to androgen independency (48). Therefore, the observed role of fisetin in PC3 cells is quite interesting.
Our data that fisetin suppresses Cap-dependent translation and induces autophagic cell death through inhibition of both mTORC1 and mTORC2 complexes provide a good rationale for further comprehensive research and development of fisetin as a dual mTORC1/2 inhibitor against hormone refractory CaP. Moreover, our previous research showed that fisetin had minimum effect in normal prostate epithelial cells, whereas it induced apoptotic cell death in hormone-dependent CaP cells (11). Therefore, fisetin may be beneficial to patients with mixed population of CaP. Taken together, we suggest that fisetin could be a useful chemotherapeutic agent against CaP.
Funding
United States Public Health Service (R01CA 120451). Clinical and Translational Science Award program of the National Center for Research Resources, National Institutes of Health (1UL1RR025011 to J.J.J).
Acknowledgments
We thank Dr Noboru Mizushima (Tokyo Medical and Dental University, Japan) for LC3 cDNA and Dr Mei-Ru Chen (National Taiwan University, Taiwan) for bicistronic luciferase reporter construct.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AMPK
adenosine monophosphate-activated protein kinase
- ATP
adenosine triphosphate
- CaP
prostate cancer
- CQ
chloroquine
- MTT
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide
- eIF
eukaryotic translation initiation factor
- 4EBP1
eukaryotic translation initiation factor 4E-binding protein 1
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- mTOR
mammalian target of rapamycin
- MDC
monodansylcadaverine
- PBS
phosphate-buffered saline
- PI3K
phosphatidylinositol-3-kinase
- PTEN
phosphatase tensin homolog
- Raptor
regulatory-associated protein of mammalian target of rapamycin
- Rictor
rapamycin-insensitive companion of mammalian target of rapamycin
- rpS6
ribosomal protein S6
- siRNA
small interfering RNA
- SDS
sodium dodecyl sulfate
- TSC
tuberous sclerosis complex
References
- 1.Jemal A, et al. Cancer statistics, 2009. CA Cancer J. Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Mike S, et al. Chemotherapy for hormone-refractory prostate cancer. Cochrane Database Syst. Rev. 2006 doi: 10.1002/14651858.CD005247.pub2. CD005247. [DOI] [PubMed] [Google Scholar]
- 3.Jiao J, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67:6083–6091. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
- 4.Easton JB, et al. mTOR and cancer therapy. Oncogene. 2006;25:6436–6446. doi: 10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- 5.Faivre S, et al. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 6.Kinkade CW, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J. Clin. Invest. 2008;118:3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guertin DA, et al. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Arai Y, et al. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 9.Hanneken A, et al. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Invest. Ophthalmol. Vis. Sci. 2006;47:3164–3177. doi: 10.1167/iovs.04-1369. [DOI] [PubMed] [Google Scholar]
- 10.Higa S, et al. Fisetin, a flavonol, inhibits TH2-type cytokine production by activated human basophils. J. Allergy Clin. Immunol. 2003;111:1299–1306. doi: 10.1067/mai.2003.1456. [DOI] [PubMed] [Google Scholar]
- 11.Khan N, et al. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis. 2008;29:1049–1056. doi: 10.1093/carcin/bgn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan N, et al. A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer Res. 2008;68:8555–8563. doi: 10.1158/0008-5472.CAN-08-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persad S, et al. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc. Natl Acad. Sci. USA. 2000;97:3207–3212. doi: 10.1073/pnas.060579697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Y, et al. Increase of AKT/PKB expression correlates with gleason pattern in human prostate cancer. Int. J. Cancer. 2003;107:676–680. doi: 10.1002/ijc.11471. [DOI] [PubMed] [Google Scholar]
- 15.Inoki K, et al. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 16.Sarbassov DD, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 17.Kimball SR. Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med. Sci. Sports Exerc. 2006;38:1958–1964. doi: 10.1249/01.mss.0000233796.16411.13. [DOI] [PubMed] [Google Scholar]
- 18.Manning BD, et al. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 19.Xu ZX, et al. A plant triterpenoid, avicin D, induces autophagy by activation of AMP-activated protein kinase. Cell Death Differ. 2007;14:1948–1957. doi: 10.1038/sj.cdd.4402207. [DOI] [PubMed] [Google Scholar]
- 20.Pullen N, et al. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 21.Jefferies HB, et al. Rapamycin suppresses 5'TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raught B, et al. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J. 2004;23:1761–1769. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svitkin YV, et al. Eukaryotic translation initiation factor 4E availability controls the switch between cap-dependent and internal ribosomal entry site-mediated translation. Mol. Cell. Biol. 2005;25:10556–10565. doi: 10.1128/MCB.25.23.10556-10565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mader S, et al. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol. Cell. Biol. 1995;15:4990–4997. doi: 10.1128/mcb.15.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hay N, et al. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 26.Salido M, et al. X-ray microanalysis of etoposide-induced apoptosis in the PC-3 prostatic cancer cell line. Cell Biol. Int. 2001;25:499–508. doi: 10.1006/cbir.2000.0763. [DOI] [PubMed] [Google Scholar]
- 27.Tsujimoto Y, et al. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12(suppl. 2):1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 28.Pattingre S, et al. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90:313–323. doi: 10.1016/j.biochi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Puissant A, et al. AMPK- and p62/SQSTM1-dependent autophagy mediate resveratrol-induced cell death in chronic myelogenous leukemia. Autophagy. 2010;6:655–657. doi: 10.4161/auto.6.5.12126. [DOI] [PubMed] [Google Scholar]
- 30.Kabeya Y, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizushima N. Methods for monitoring autophagy. Int. J. Biochem. Cell Biol. 2004;36:2491–2502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Mathew R, et al. Role of autophagy in cancer. Nat. Rev. Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy. 2008;4:947–948. doi: 10.4161/auto.6787. [DOI] [PubMed] [Google Scholar]
- 34.LoPiccolo J, et al. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist. Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verheul HM, et al. Combination strategy targeting the hypoxia inducible factor-1 alpha with mammalian target of rapamycin and histone deacetylase inhibitors. Clin. Cancer Res. 2008;14:3589–3597. doi: 10.1158/1078-0432.CCR-07-4306. [DOI] [PubMed] [Google Scholar]
- 36.Hennessy BT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 37.Armengol G, et al. 4E-binding protein 1: a key molecular “funnel factor” in human cancer with clinical implications. Cancer Res. 2007;67:7551–7555. doi: 10.1158/0008-5472.CAN-07-0881. [DOI] [PubMed] [Google Scholar]
- 38.Carracedo A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawyers CL. Will mTOR inhibitors make it as cancer drugs? Cancer Cell. 2003;4:343–348. doi: 10.1016/s1535-6108(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 40.Sarbassov DD, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 41.Fu L, et al. Perifosine inhibits mammalian target of rapamycin signaling through facilitating degradation of major components in the mTOR axis and induces autophagy. Cancer Res. 2009;69:8967–8976. doi: 10.1158/0008-5472.CAN-09-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geissler EK, et al. mTOR, cancer and transplantation. Am. J. Transplant. 2008;8:2212–2218. doi: 10.1111/j.1600-6143.2008.02391.x. [DOI] [PubMed] [Google Scholar]
- 43.Kondo Y, et al. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 44.Hoyer-Hansen M, et al. Autophagy: an emerging target for cancer therapy. Autophagy. 2008;4:574–580. doi: 10.4161/auto.5921. [DOI] [PubMed] [Google Scholar]
- 45.Kondo Y, et al. Autophagy and cancer therapy. Autophagy. 2006;2:85–90. doi: 10.4161/auto.2.2.2463. [DOI] [PubMed] [Google Scholar]
- 46.Opipari AW, et al. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004;64:696–703. doi: 10.1158/0008-5472.can-03-2404. [DOI] [PubMed] [Google Scholar]
- 47.Aoki H, et al. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol. Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 48.Marignol L, et al. Hypoxia in prostate cancer: a powerful shield against tumour destruction? Cancer Treat. Rev. 2008;34:313–327. doi: 10.1016/j.ctrv.2008.01.006. [DOI] [PubMed] [Google Scholar]