Abstract
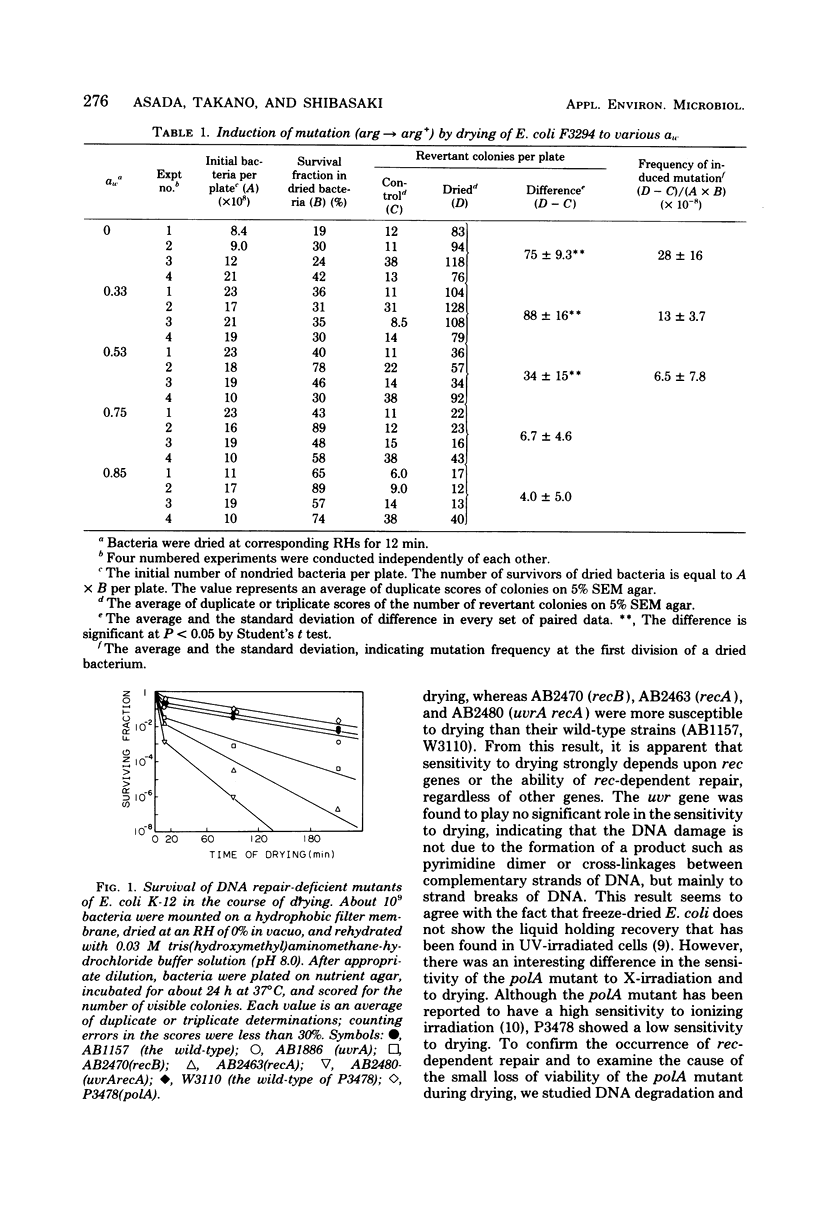
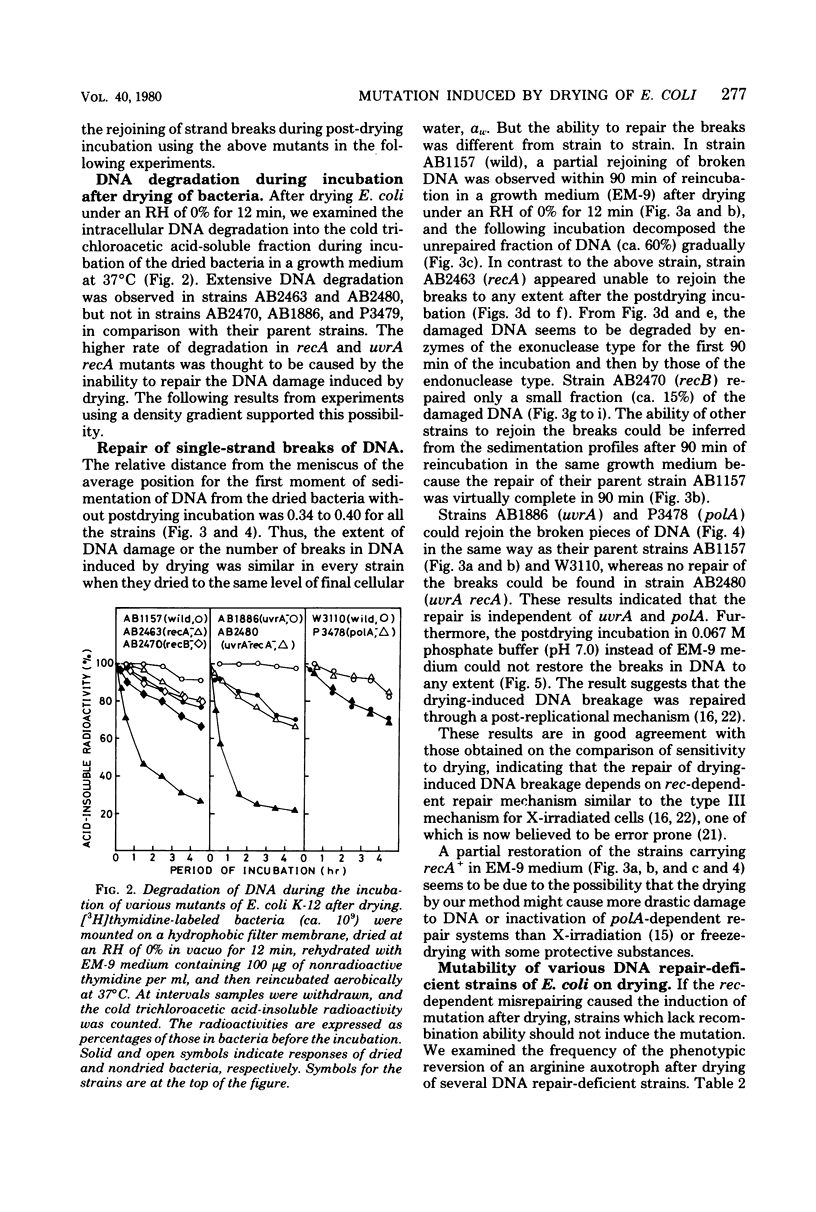
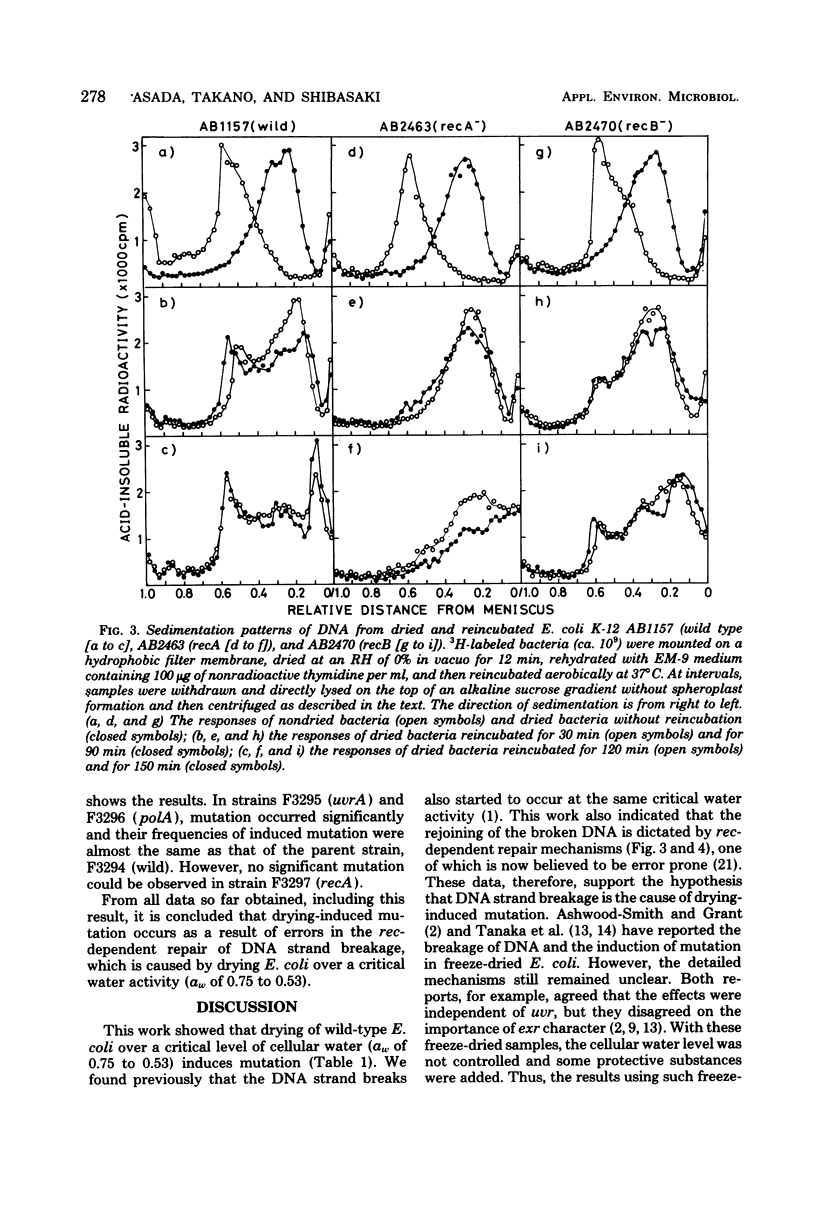
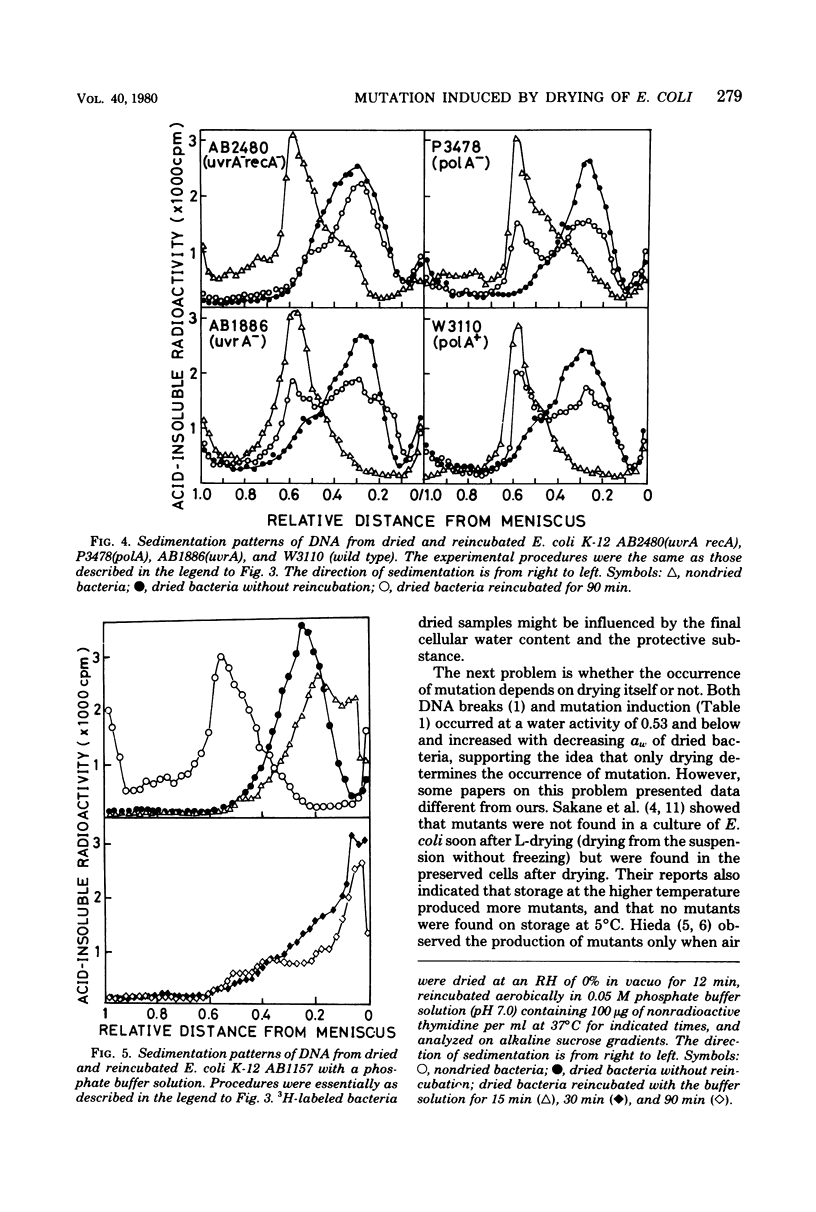
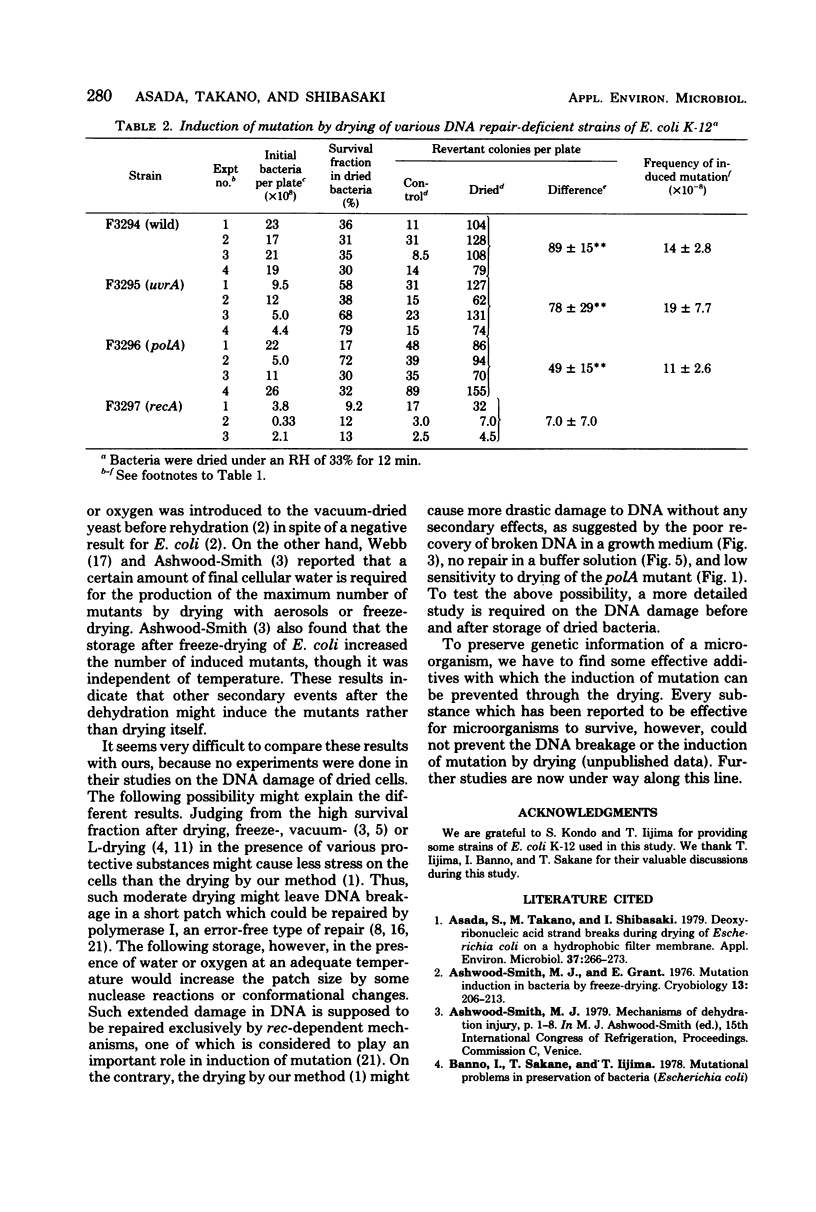
Drying of Escherichia coli to a required cellular water level was conducted on a hydrophobic membrane at the corresponding relative humidity. Mutation from an arginine auxotroph to the prototroph was induced by drying to a water activity (aw) of 0.53 and below, but not to an aw of 0.75 and above. The critical aw below which mutation occurred in the course of drying was similar to that for induction of deoxyribonucleic acid (DNA) strand breakage in the bacteria. Some ultraviolet or gamma-irradiation-sensitive strains, e.g., strains of carrying recA, recB, and uvrA recA were more sensitive to drying than the wild-type strains or strains carrying uvrA and polA. The DNA strand breakage of every strain was observed to be to a similar extent after drying to an aw of less than 0.53. The drying-resistant strains repaired the damaged DNA partially during postdrying incubation in a growth medium but not in phosphate buffer solution, while the drying-sensitive strains could not at all. Significant mutation on drying occurred in the wild-type strains, strains carrying uvrA and polA, but not in strains carrying recA. It is, therefore, concluded that the mutation is caused by errors in rec-dependent repair of the drying-induced breakage in DNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asada S., Takano M., Shibasaki I. Deoxyribonucleic acid strand breaks during drying of Escherichia coli on a hydorohobic filter membrane. Appl Environ Microbiol. 1979 Feb;37(2):266–273. doi: 10.1128/aem.37.2.266-273.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood-Smith M. J., Grant E. Mutation induction in bacteria by freeze-drying. Cryobiology. 1976 Apr;13(2):206–213. doi: 10.1016/0011-2240(76)90134-6. [DOI] [PubMed] [Google Scholar]
- Kondo S., Ichikawa H., Iwo K., Kato T. Base-change mutagenesis and prophage induction in strains of Escherichia coli with different DNA repair capacities. Genetics. 1970 Oct;66(2):187–217. doi: 10.1093/genetics/66.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T., Tanaka Y., Yoh M., Takeda Y., Miwatani T. Deoxyribonucleic acid strand breaks during freeze-drying and their repair in Escherichia coli. J Bacteriol. 1977 Jun;130(3):1393–1396. doi: 10.1128/jb.130.3.1393-1396.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson M. C., Boyle J. M., Setlow R. B. Ultraviolet- and X-ray-induced responses of a deoxyribonucleic acid polymerase-deficient mutant of Escherichia coli. J Bacteriol. 1971 Jul;107(1):61–67. doi: 10.1128/jb.107.1.61-67.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servin-Massieu M. Effects of freeze-drying and sporulation on microbial variation. Curr Top Microbiol Immunol. 1971;54:119–150. doi: 10.1007/978-3-642-65123-6_5. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Ohnishi T., Takeda Y., Miwatani T. Lethal effect of freeze-drying on radiation-sensitive mutants of Escherichia coli. Biken J. 1975 Dec;18(4):267–269. [PubMed] [Google Scholar]
- Tanaka Y., Yoh M., Takeda Y., Miwatani T. Induction of mutation in Escherichia coli by freeze-drying. Appl Environ Microbiol. 1979 Mar;37(3):369–372. doi: 10.1128/aem.37.3.369-372.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. DNA polymerase required for rapid repair of x-ray--induced DNA strand breaks in vivo. Science. 1971 May 21;172(3985):851–854. doi: 10.1126/science.172.3985.851. [DOI] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. The rapair of DNA single-strand breaks in E. coli K-12 x-irradiated in the presence or absence of oxygen; the influence of repair on cell survival. Radiat Res. 1973 Aug;55(2):334–345. [PubMed] [Google Scholar]
- WEBB S. J. BOUND WATER, METABOLITES AND GENETIC CONTINUITY. Nature. 1964 Jul 25;203:374–377. doi: 10.1038/203374a0. [DOI] [PubMed] [Google Scholar]
- WITKIN E. M. PHOTOREVERSAL AND "DARK REPAIR" OF MUTATIONS TO PROTOTROPHY INDUCED BY ULTRAVIOLET LIGHT IN PHOTOREACTIVABLE AND NON-PHOTOREACTIVABLE STRAINS OF ESCHERICHIA COLI. Mutat Res. 1964 May;106:22–36. doi: 10.1016/0027-5107(64)90049-1. [DOI] [PubMed] [Google Scholar]
- Webb S. J. Mutation of bacterial cells by controlled desiccation. Nature. 1967 Mar 18;213(5081):1137–1139. doi: 10.1038/2131137b0. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet-induced mutation and DNA repair. Annu Rev Microbiol. 1969;23:487–514. doi: 10.1146/annurev.mi.23.100169.002415. [DOI] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. X-ray sensitivity and repair capacity of a polA1 exrA strain of Escherichia coli K-12. J Bacteriol. 1973 Apr;114(1):121–127. doi: 10.1128/jb.114.1.121-127.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



