Abstract
Tissue inhibitor of metalloproteinases 4 (TIMP4) is expressed highly in heart and found dysregulated in human cardiovascular diseases. It controls extracellular matrix remodeling by inhibiting matrix metalloproteinases (MMPs) and is implicated in processes including cell proliferation, apoptosis, and angiogenesis. Timp4-deficient mice (Timp4−/−) were generated to assess TIMP4 function in normal development and in models of heart disease. We deleted exons 1–3 of the Timp4 gene by homologous recombination. Timp4−/− mice are born healthy, develop normally, and produce litters of normal size and gender distribution. These mice show no compensation by overexpression of Timp1, Timp2, or Timp3 in the heart. Following cardiac pressure overload by aortic banding, Timp4−/− mice have comparable survival rate, cardiac histology, and cardiac function to controls. In this case, Timp4 deficiency is compensated by increased cardiac Timp2 expression. Strikingly, the induction of myocardial infarction (MI) leads to significantly increased mortality in Timp4−/− mice primarily due to left ventricular rupture. The post-MI mortality of Timp4−/− mice is reduced by administration of a synthetic MMP inhibitor. Furthermore, combining the genetic deletion of Mmp2 also rescues the higher post-MI mortality of Timp4−/− mice. Finally, Timp4−/− mice suffer reduced cardiac function at 20 months of age. Timp4 is not essential for murine development, although its loss moderately compromises cardiac function with aging. Timp4−/− mice are more susceptible to MI but not to pressure overload, and TIMP4 functions in its capacity as a metalloproteinase inhibitor after myocardial infarction.
Keywords: Cardiac Hypertrophy, Extracellular Matrix, Gene Knockout, Heart, Matrix Metalloproteinase, Tissue Inhibitors Metalloprotease (TIMPS), MMPi, Myocardial Infarction, Pressure Overload
Introduction
Tissue inhibitors of metalloproteinases (TIMPs)5 comprise a family of four endogenous inhibitors. Classically, matrix metalloproteinases (MMPs) and TIMPs are known as important regulators of extracellular matrix turnover during physiologic and numerous pathologic processes. Several other functions also have been ascribed to MMPs, many of which extend to their inhibitors (1). TIMPs also exhibit functions that appear to be independent of their metalloproteinase inhibitory capacity (2).
TIMP4 is the most recently discovered member of the TIMP family. It inhibits several soluble MMPs (types 1, 2, 3, 7, 8, 9, 12, 13, 19, and 26) and membrane-type MMPs (MT1, MT2, and MT3) (3–7). TIMP4 also inhibits a disintegrin and metalloproteinase (ADAM) 28 and ADAM33 (7) but not ADAM10 (which is inhibited by TIMP1 and TIMP3) or ADAM17 (which is inhibited only by TIMP3) (2, 7, 8). Inhibition of the ADAMTS (ADAM with thrombospondin motifs) family by TIMP4 has not yet been reported. Although Timp genes 1, 2, and 3 are widely expressed, the Timp4 gene exhibits a restricted tissue expression pattern, with the highest expression in the heart, followed by brain, ovary, and skeletal muscle, and TIMP4 protein is detectable in the serum (9–11). Several lines of evidence suggest a specific role in cardiovascular pathology: Timp4 is induced following endothelial injury of rat carotid artery (12); experimental ischemia-reperfusion injury results in the immediate release of TIMP4 from the myocardium (13); and down-regulation of Timp4 is a common finding in animal models of heart failure (14–16) or myocardial infarction (17, 18). Similar observations have been observed in patients with ischemic cardiomyopathy (19).
To understand the functions of TIMP4 in the whole organism, we generated Timp4 knock-out mice. Mice lacking other Timp genes develop normally at the gross level and can reproduce, Timp1 knock-outs (20) exhibit impaired fertility (21), unfavorable changes in heart geometry (22), and resistance to bacterial infections (23, 24); Timp2 knock-outs (25) or mice producing truncated TIMP2 (26) demonstrate reduced pro-MMP2 activation. Behavioral and motor phenotypes subsequently were characterized in Timp2-deficient mice (27, 28). Timp3 knock-out mice have accelerated post-lactation mammary gland involution (29) and develop spontaneous air space enlargement in the lungs (30). In addition, these mice exhibit abnormal processing of tumor necrosis factor α (31), compromised heart function with aging (32), and increased susceptibility to aortic banding (AB) (33). Here, we show that Timp4-deficient mice develop and reproduce normally but are prone specifically to fatal ventricular wall rupture after myocardial infarction (MI), a pathology blocked by a synthetic MMP inhibitor or by the deletion of Mmp2.
EXPERIMENTAL PROCEDURES
Generation of Knock-out Mice
A knock-out construct with neomycin resistance and thymidine kinase selection cassettes was designed to delete a 2.4-kb genomic fragment containing exons 1–3 of the Timp4 gene (9). This deletes translation initiation codon (atg) and is predicted to eliminate transcription initiation.
Phenotypic Analyses
For analyses of aged mice, Timp4−/− mice were kept on outbred C57BL/6 × 129SvEv background. RNA analyses were performed using Northern blotting and real-time Taqman® RT-PCR. Cardiac hydroxyproline was determined by a modified Woessner's protocol.
Functional Studies of the Cardiovascular System
The systolic blood pressures and heart rates of 5-month-old, unanesthetized, male Timp4+/+ (n = 9) and Timp4−/− (n = 5) mice were measured using a tail-cuff method (TSE Blood Pressure Measuring System 209000-9002-1, TSE Systems GmbH, Bad Homburg, Germany) on a temperature-controlled platform. Before measurements, restrained mice were trained to inflating and deflating tail cuff. Blood flow in caudal arteries was monitored by a pulse transducer, whereas arteries were occluded gradually by an inflating pressure cuff. Systolic arterial pressure equals the pressure in tail cuff when blood flow became undetectable by sensor. Heart rate was obtained by continuous monitoring of pulse signal. For each mouse, at least 30 consecutive measurements were performed. Unreliable measurements, containing for example movement artifacts, were discarded and the average of remaining measurements (20 to 25 per mouse) was used for statistical analyses.
For in vivo echocardiographic measurements, 20-month-old male Timp4−/− mice (n = 6) and control littermates (n = 4) were anesthetized with diazepam (10 mg/kg) and ketamine (60 mg/kg) and studied by transthoracic Doppler echocardiography using Acuson Sequoia 512 (Siemens Acuson, Mountain View, CA) with a 15-MHz linear transducer. Left ventricular dimensions and mass and fractional shortening were measured in short axis M mode images of the left ventricle (34). Myocardial performance index, a sensitive Doppler-based measure of global left ventricular function was calculated from time intervals obtained from transmitral inflow and aortic outflow (35). To assess diastolic function, ratio of transmitral flow velocity during early and late diastole (E/A ratio) was calculated. Coronary microvascular function was assessed by measuring coronary flow reserve in the middle part of descending left coronary artery. Coronary flow reserve was calculated as the ratio of peak diastolic flow velocity during maximal vasodilatation induced by adenosine (0.32 mg/kg/min) to resting flow velocity (36). Cardiac function measurements following pressure overload or myocardial infarction were performed under light isoflurane anesthesia (0.75%) by echocardiography and in vivo hemodynamic measurements (33).
Mice (Timp4+/+ and Timp4−/−) used in pressure overload and myocardial infarction studies were of C57Bl background, derived by six back-crossings into this strain. Pressure overload was subjected to male Timp4−/− and control mice at the age of 8 weeks by performing constriction of descending aorta as described earlier (33). Briefly, mice were anesthetized with ketamine-xylazine, intubated, and ventilated with rodent ventilator. During surgery, descending aorta was identified, and a ligature was tied around descending aorta, and a 27-gauge needle was placed parallel to it. Next, the needle was removed, but the ligature was left, causing constant and permanent constriction of aorta. The thorax was closed, and mice were monitored for up to 12 weeks. Sham-operated mice underwent similar operation except that the ligation of aorta was not tightened, and it was removed before closure of thorax. At 1, 3, 6, and 12 weeks after aortic-banding, cardiac function was monitored by echocardiography as described (33).
10–12-week-old Timp4+/+ and Timp4−/− male mice were subjected to myocardial infarction by left anterior descending coronary artery (LAD) ligation (37) or to sham operation. Briefly, mice were anesthetized with ketamine-xylazine, intubated, and ventilated with rodent ventilator. Thorax and pericardium were opened to expose LAD, which was ligated. Then, chest was closed, and mice were monitored up to 7 days. Heart function was evaluated on surviving mice by echocardiography and in vivo hemodynamics before sacrifice for tissue collection (33). The synthetic MMP inhibitor PD166793 (Pfizer, Inc.) was administered daily (25 mg/kg) by gavage, starting 1 week before MI until the mice were euthanized.
Generation of the knock-out mice and phenotypic analyses of aged mice were performed under protocols approved by the Institutional Committee for Animal Welfare (University of Turku, Turku, Finland). The AB and MI protocols were approved by the Ontario Cancer Institute Animal Care Committee (Toronto, Canada) in accordance with guidelines of the Canadian Council for Animal Care.
Second Harmonic Generation Imaging
For visualization of fibrillar collagen in vivo, second harmonic and autofluorescence was captured on a Zeiss LSM 510 META NLO microscope, using a C-Apo 40×/1.2 NA objective lens. Intact hearts were isolated, immersed in phosphate-buffered saline, and imaged through a glass coverslip. Excitation was provided by a Chameleon femtosecond pulsed laser tuned to 840 nm, and second harmonic generation signal was collected via epidetection through a 425/35 bandpass filter, with the autofluorescence signal collected simultaneously through a 525/25 bandpass filter. The nature of the second harmonic signal was confirmed by spectral analysis using a META detector, showing a narrow peak corresponding to half the wavelength of the input excitation. Image volumes were collected in infarct and peri-infarct regions, and maximum intensity projections of regions of interest were generated using NIH ImageJ software. Additional methods can be found in the supplemental material.
RESULTS
Generation of Timp4 Knock-out Mice
Electroporation of the targeting construct (Fig. 1A) into embryonic stem cells resulted in 216 clones, which were screened by Southern blotting. One clone harboring the correct targeting event was identified by the presence of a 4.9-kb mutant band in addition to the wild-type band (Fig. 1B). Injection of targeted embryonic stem cells into blastocysts gave rise to four high percentage chimeric males, of which two frequently produced heterozygous pups. To confirm that the mutant Timp4 allele did not produce any Timp4 mRNA, total heart RNA from animals of the three genotypes was analyzed by Northern blotting for Timp4 mRNA expression (supplemental Fig. 1). Heterozygous mice had 50.7% (p < 0.001; n = 9; Fig. 1C) of the mRNA levels seen in control littermates for the major 1.1-kb Timp4 transcript. No Timp4 mRNA was detected in Timp4−/− (n = 5) mice. These observations were confirmed by RT-PCR (data not shown). Breeding of the Timp4-deficient mice resulted in litters of normal size and gender distribution (supplemental Table 1).
FIGURE 1.
Targeting the Timp4 gene. A, the targeting construct was produced by cloning genomic BglII-HindIII and EcoRI-Acc65I fragments of the murine Timp4 gene into pKO Scrambler 921, which contains neomycin (neo) and thymidine kinase (tk) cassettes. The mutant allele lacks the first three exons. B, Southern blot analysis of genomic DNA after EcoRI digestion, hybridized with a 5′-probe, showing the 5.7-kb wild-type and the 4.9-kb mutant fragments. C, the histogram shows quantification of Northern analysis (see supplemental Fig. 1) of adult cardiac tissue revealing a lack of Timp4 mRNA in homozygous mice, and 50.7% reduction of Timp4 mRNA in heterozygous mice (mean ± S.E.; *, p < 0.001 versus Timp4+/+). D–F, Taqman RT-PCR analysis of RNA for Timp1, Timp2, and Timp3 in hearts of Timp4+/+ and Timp4−/− mice. Values were normalized to 18 S rRNA and are expressed as mean ± S.E.; n = 4–6 for each group.
Characterization of the Cardiac Structure and Function in Timp4−/− Mice
As the literature suggests a role for TIMP4 in heart, cardiac tissue of Timp4−/− mice was investigated at the age of 5 months. Measurement of body weights and heart-to-body weight ratios in Timp4−/− and Timp4+/+ mice revealed no differences (supplemental Table 2). Neither were any statistically significant differences observed in blood pressure or heart rate in 5-month-old knock-out and control mice (supplemental Table 2). Furthermore, histologic evaluation of the heart at 5 months of age showed no differences in the cardiomyocyte size (supplemental Table 2) or the amount of collagen (data not shown) as indicated by trichrome staining. Determination of the hydroxyproline content in cardiac collagen showed essentially identical levels between Timp4−/− (n = 8) and control mice (n = 4; 1.80 ± 0.05 versus 1.81 ± 0.08 μg/mg) at the age of 5 months. Taqman RT-PCR analyses showed that the cardiac mRNA levels of Timp genes 1, 2, and 3 remained comparable between Timp4+/+ and Timp4−/− mice (Fig. 1, D–F).
Timp4 Deficiency Does Not Affect Survival or Heart Dysfunction Following Pressure Overload
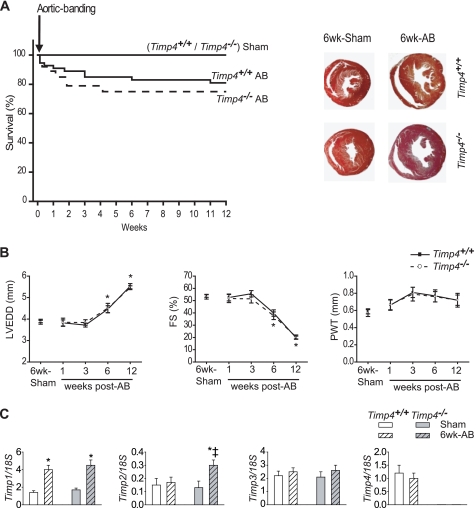
To investigate the effects of a Timp4 deficiency on chronic pressure overload, Timp4−/− and control mice were subjected to aortic banding at the age of 8 weeks. The survival rates were similar over the next 12 weeks (Fig. 2A). Echocardiography at 1, 3, 6, and 12 weeks post-AB, showed comparable cardiac function in Timp4-deficient and control mice (Fig. 2B). Histological evaluation of cardiac hypertrophy and fibrosis at 6 weeks did not reveal any differences (data not shown). Moreover, the expression levels of the natriuretic peptides (atrial natriuretic peptide, ANP, and natriuretic peptide B, BNP), as well as collagen types I and III, were comparable in Timp4+/+ and Timp4−/− hearts (data not shown). We then determined whether the lack of post-AB phenotype might be due to compensatory mechanisms involving other Timp genes. In contrast to control mice, we observed a 1.6-fold (p < 0.05) increase in cardiac Timp2 expression in Timp4-deficient mice at 6 weeks post-AB (Fig. 2C), whereas the expression levels of Timp1 and Timp3 remained comparable between genotypes.
FIGURE 2.
The loss of TIMP4 did not impact the cardiac response to pressure overload. A, survival of AB- or sham-operated Timp4+/+ and Timp4−/− mice (n = 20/group), and representative macroscopic heart cross-sections at 6-weeks post-AB. B, left ventricular end-diastolic dimension (LVEDD), fractional shortening (FS) and posterior wall thickness (PWT) are equivalent in these mice up to 12-weeks post-AB. Data are mean ± S.E.; n = 3, sham groups; n = 6, AB groups; *, p < 0.05 versus corresponding sham group. C, Taqman RT-PCR analysis of RNA for Timp1, Timp2, Timp3, and Timp4 in hearts of Timp4+/+ and Timp4−/− mice at 6 weeks after sham operation or aortic banding. Values were normalized to 18 S rRNA and are expressed as mean ± S.E.; n = 4–6 for each group. *, p < 0.05 versus corresponding sham; ‡, p < 0.05 versus Timp4+/+ AB.
Timp4 Loss Increases Mortality after Myocardial Infarction
To further characterize the significance of TIMP4 in heart disease, Timp4 knock-out mice were subjected to experimental myocardial infarction induced by LAD ligation. This is known to result in severe morbidity by large myocardial infarct and progressive left ventricular (LV) dilation (Fig. 3B). Timp4−/− mice showed significantly increased mortality in comparison with Timp4+/+ mice after MI between days 3 to 7 (Fig. 3A; supplemental Fig. 2). In autopsy, a LV wall rupture was identified as the cause of death in the majority of cases in both genotypes (Fig. 4A). By echocardiography and in vivo hemodynamics, we observed a decrease in fractional shortening, an increase in LV end-diastolic diameter and pressure, and suppressed LV peak rates of pressure-rise and pressure-fall in mice at 7 days after MI (Fig. 3C). These studies indicated severely deteriorated LV function post-MI, but the extent of this dysfunction was comparable between genotypes. Thus, Timp4−/− mice exhibit greater mortality, but those that recover have deterioration of heart function similar to controls.
FIGURE 3.
Timp4−/− mice experience significantly higher mortality following MI. A, rupture-related survival of Timp4+/+ and Timp4−/− mice following MI or sham operation; n = 27 for Timp4−/− MI and n = 20 for Timp4+/+ MI; *, p < 0.05 versus sham; ‡, p < 0.05 versus Timp4+/+ MI. B, representative macroscopic cross-sectional images of Timp4+/+ and Timp4−/− hearts at indicated times after sham operation or MI. C, functional and structural analyses showing fractional shortening, LV end-diastolic dimension (LVEDD), LV end-diastolic pressure (LVEDP), and LV peak rates of pressure-rise and pressure-fall (±dP/dt) in mice at 1-week post-sham or post-MI. Data represent mean ± S.E.; n = 6/group; *, p < 0.05 versus corresponding sham group.
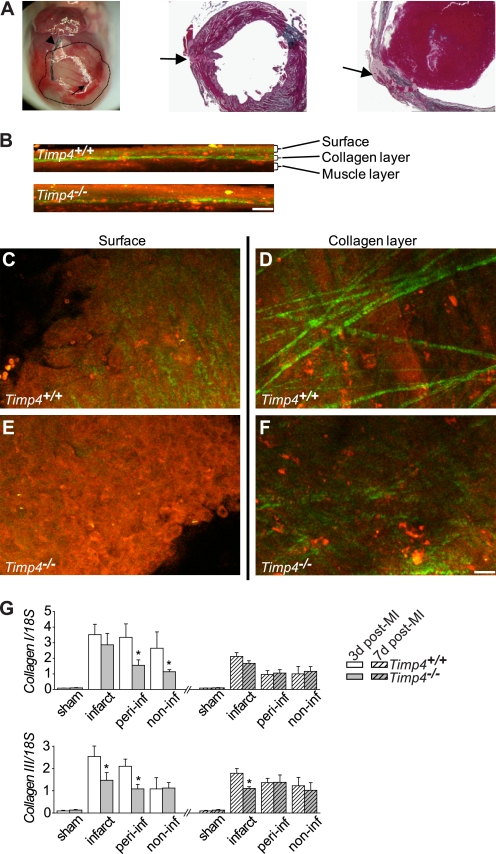
FIGURE 4.
Cardiac rupture and decreased collagen synthesis and disruption of collagen network in Timp4−/− hearts after MI. A, a representative picture of a ruptured heart following MI and trichrome-stained fixed LV tissue sections showing the rupture area (arrow) and LAD ligation (arrowhead). The infarct area is marked with a dotted line. B–F, second harmonic generation (green pseudocolor) and multiphoton fluorescence (red pseudocolor) images indicating compromised fibrillar collagen and altered cellularity in Timp4-deficient hearts post-MI. B, the depth profile extending below the heart surface. C–F, 10-μm-thick maximum intensity projections of the surface and collagen layers of Timp4+/+ and Timp4−/− heart infarct regions. Scale bar, 20 μm (B–F). G, Taqman RT-PCR analysis of RNA for collagen I and III in hearts of Timp4+/+ and Timp4−/− mice at 3 and 7 (dashed bars) days after sham operation or myocardial infarction. Values were normalized to 18 S rRNA and are expressed as mean ± S.E.; n = 4–6 for each group. *, p < 0.05 versus Timp4+/+ MI. peri-inf, peri-infarct; non-inf, noninfarct.
Cardiac Collagen Network Is Compromised in Timp4-deficient Mice Post-MI
The structure of the cardiac collagen network was evaluated as a possible cause for increased rupture in Timp4−/− mice post-MI. The fibrillar collegen network was visualized alongside cellular autofluorescence using combined second harmonic generation and multiphoton fluorescence microscopy. In Timp4-deficient mice, the fibrillar collagen network is compromised as indicated by reduced collagen fiber intensity (Fig, 4, B–F). Expression analysis of major cardiac collagens (types I and III) revealed reduced collagen synthesis at 3 and 7 days post-MI (Fig, 4G). Further, synthesis of Timp genes and major gelatinases and collagenases were analyzed at 7 days post-MI. Expression of Timp1–3 genes were comparable in Timp4-deficient and control mice (data not shown). In zymography, gelatinase activity of the cardiac tissue was comparable between genotypes at 3 days post-MI (supplemental Fig. 3).
Neutrophil Accumulation Is Increased in Timp4−/− Hearts Post-MI
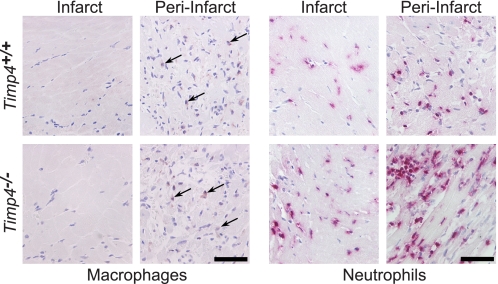
After MI, a controlled inflammatory process takes place to replace necrotic areas with scar tissue. Multiphoton fluorescence microscopy suggested increased cellularity in Timp4−/− cardiac tissue (Fig. 4, B, C, and E). Possibly altered inflammatory cell accumulation was investigated by immunohistochemistry in Timp4-deficient and control hearts at 3 days post-MI. Staining of macrophages indicated a slight increase in macrophage numbers in Timp4−/− hearts, but quantification was not feasible because of low intensity of staining (Fig. 5). In Timp4-deficient hearts, accumulation of neutrophils was significantly increased in infarct, peri-infarct, and noninfarct areas when compared with Timp4+/+ MI hearts (Fig. 5). In contrast, angiogenesis seemed to be similar in Timp4−/− and Timp4+/+ mice as indicated by CD31 staining (supplemental Fig. 4).
FIGURE 5.
Increased neutrophil accumulation in Timp4−/− hearts at 3 days post-MI. Immunodetection of macrophages (arrow) and neutrophils in infarct and peri-infarct areas of Timp4+/+ and Timp4−/− hearts. Scale bar, 50 μm.
MMPi Treatment or the Genetic Ablation of Mmp2 Reduces the Post-MI Mortality of Timp4−/− Mice
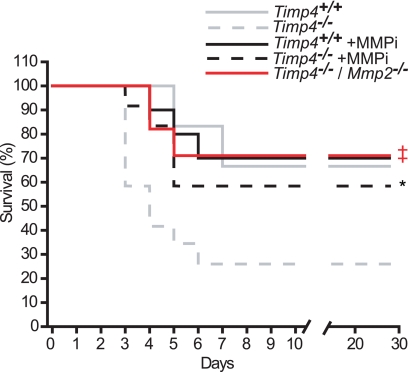
To test whether the poor post-MI survival was related to the loss of MMP inhibitory function in the Timp4−/− heart, a broad spectrum MMP-specific inhibitor (PD166793; MMPi) was orally administered to Timp4-deficient and control MI-mice as described previously (33). MMPi treatment did not affect the post-MI survival rate of Timp4+/+ mice; however, the excessive lethality seen in Timp4−/− mice was rescued by MMPi treatment, making the survival rate of MMPi-treated Timp4−/− mice comparable with Timp4+/+ mice (Fig. 6). Earlier studies have shown a reduction in post-MI rupture rates in Mmp2 knock-out mice (38, 39). An Mmp2 deficiency was crossed into the model and the double-deficient Timp4−/−/Mmp2−/− mice were observed to have a post-MI survival rate (Fig. 6) similar to controls. Moreover, the accumulation of neutrophils in Timp4−/−/Mmp2−/− hearts post-MI was now comparable with control mice (Fig. 7, A and B).
FIGURE 6.
Improved post-MI survival in Timp4−/− mice following MMPi treatment or deletion of Mmp2. The graph shows the rupture-related post-MI survival of Timp4+/+ (solid line) and Timp4−/− (dashed line) mice that did not receive additional treatment (gray; data from Fig. 3) or mice treated with MMPi (black; n = 9 for Timp4+/+ +MMPi; n = 11 for Timp4−/− +MMPi; *, p < 0.05 versus untreated Timp4−/− mice). A red solid line represents Timp4/Mmp2 double knock-out mice (n = 10, ‡, p < 0.05 versus Timp4−/− mice without MMPi treatment).
FIGURE 7.
Increased cardiac neutrophil accumulation of Timp4-deficient mice post-MI is normalized by ablation of Mmp2. A, neutrophil staining of Timp4+/+, Timp4−/−, and double knock-out (Timp4−/−/Mmp2−/−) cardiac tissue at 3 days following MI. Scale bar, 50 μm. B, histomorphometric quantification of neutrophils in cardiac tissue post-MI. Values expressed as mean ± S.E., *, p < 0.05 versus wild-type control in same group; ‡, p < 0.05 versus double knock-out in same group.
Cardiac Function Is Moderately Compromised in Aged Timp4−/− Mice
To determine the effect of aging on heart structure and function of Timp4−/− and Timp4+/+ mice, in vivo echocardiography was performed at the age of 20 months. Impaired heart function in Timp4 knock-out mice was evident by a poor myocardial performance index (supplemental Fig. 5), whereas the fractional shortening (41.9 ± 0.9 in Timp4+/+ versus 45.8 ± 2.1% in Timp4−/−) and E/A ratio (data not shown) were comparable between genotypes. Moreover, coronary flow reserve, a measurement of coronary microvascular function, was decreased in Timp4−/− mice (supplemental Fig. 5). Also, echocardiography demonstrated increased septal and posterior wall thickness (data not shown) accompanied by increased LV mass in Timp4-deficient mice (supplemental Fig. 5). Next, we asked whether this moderate dysfunction of Timp4−/− hearts was a reflection of aberrant cardiac fibrosis or myocardial hypertrophy. Myocyte cross-sectional area, assessed by histomorphometry, showed no differences between the genotypes (supplemental Fig. 5), and although increased fibrosis could be seen using Masson's trichrome staining in both genotypes as a function of age up to 20 months, the quantification of collagen by hydroxyproline assay did not reveal differences between control and Timp4−/− mice (supplemental Fig. 5).
DISCUSSION
High expression of Timp4 mRNA in the heart (9, 10), animal models of heart disease (14, 15, 18), and in patients with cardiovascular pathology (11, 19) together suggest a specific role for TIMP4 in the development, physiology, and pathology of the cardiovascular system. Mice with a targeted deletion of Timp4 exhibited normal development, we were able to observe a cardiac phenotype only in 20-month-old Timp4 knock-out mice. In models of cardiac stress, our studies demonstrated that TIMP4 is essential during the healing response to myocardial infarction. This was not the case following aortic banding, where the induction of TIMP2 appears to compensate for the lack of TIMP4.
Cardiac TIMPs serve important functions, as gleaned from the use of Timp-null mice. Timp1−/− mice display altered LV geometry by 4 months of age (22), and Timp3−/− mice exhibit features of human dilated cardiomyopathy at 21 months but not earlier (32). In response to pressure overload, Timp3 knock-outs develop heart failure, which is prevented by combining genetic deletion of tumor necrosis factor α with inhibition of MMPs (33). After MI, Timp1 knock-outs show accelerated, MMP-dependent LV remodeling, without any effect on survival or LV rupture (40, 41). Timp3−/− knockouts have increased mortality and LV rupture within the first week of MI, as well as a greater cardiac dysfunction at 1–4 weeks post-MI (42). Here, we establish that Timp4−/− mice are specifically predisposed to LV rupture after MI, which is prevented by MMPi-treatment or the loss of Mmp2. A decrease in TIMP4 levels has been reported in animal models of MI (17, 18) and in cardiac patients (19, 43). The present study highlights TIMP4 as a critical regulator of MMP activity during the healing process following myocardial infarction.
Supplementary Material
Acknowledgments
We thank Merja Lakkisto and Tuula Oivanen for expert technical help. The Umeå Transgene Core Facility (Umeå University, Umeå, Sweden) is acknowledged for performing the blastocyst injections.
This work was supported by research grants from the Academy of Finland, the Canadian Institutes of Health Research, and the Heart and Stroke Foundation of Canada.

The on-line version of this article (available at http://www.jbc.org) contains supplemental methods, Tables 1 and 2, Figs. 1–5, and additional references.
- TIMP
- tissue inhibitor of metalloproteinases
- MMP
- matrix metalloproteinase
- AB
- aortic banding
- ADAM
- a distintegrin and metalloproteinase
- ADAMTS
- a disintegrin and metalloproteinase with thrombospondin motifs
- LAD
- left anterior descending coronary artery
- LV
- left ventricular
- MI
- myocardial infarction
- MMPi
- matrix metalloproteinase inhibitor
- RT
- reverse transcription
- ANP
- atrial natriuretic peptide
- BNP
- natriuretic peptide B.
REFERENCES
- 1.Murphy G., Nagase H. (2008) Mol. Aspects Med. 29, 290–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambert E., Dassé E., Haye B., Petitfrère E. (2004) Crit. Rev. Oncol. Hematol. 49, 187–198 [DOI] [PubMed] [Google Scholar]
- 3.Morrison C. J., Butler G. S., Bigg H. F., Roberts C. R., Soloway P. D., Overall C. M. (2001) J. Biol. Chem. 276, 47402–47410 [DOI] [PubMed] [Google Scholar]
- 4.Zhao H., Bernardo M. M., Osenkowski P., Sohail A., Pei D., Nagase H., Kashiwagi M., Soloway P. D., DeClerck Y. A., Fridman R. (2004) J. Biol. Chem. 279, 8592–8601 [DOI] [PubMed] [Google Scholar]
- 5.Stratmann B., Farr M., Tschesche H. (2001) Biol. Chem. 382, 987–991 [DOI] [PubMed] [Google Scholar]
- 6.Stracke J. O., Hutton M., Stewart M., Pendás A. M., Smith B., López-Otin C., Murphy G., Knäuper V. (2000) J. Biol. Chem. 275, 14809–14816 [DOI] [PubMed] [Google Scholar]
- 7.Melendez-Zajgla J., Del Pozo L., Ceballos G., Maldonado V. (2008) Mol. Cancer 7, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amour A., Knight C. G., Webster A., Slocombe P. M., Stephens P. E., Knäuper V., Docherty A. J., Murphy G. (2000) FEBS Lett. 473, 275–279 [DOI] [PubMed] [Google Scholar]
- 9.Rahkonen O. P., Koskivirta I. M., Oksjoki S. M., Jokinen E., Vuorio E. I. (2002) Biochim. Biophys. Acta 1577, 45–52 [DOI] [PubMed] [Google Scholar]
- 10.Leco K. J., Apte S. S., Taniguchi G. T., Hawkes S. P., Khokha R., Schultz G. A., Edwards D. R. (1997) FEBS Lett. 401, 213–217 [DOI] [PubMed] [Google Scholar]
- 11.Koskivirta I., Rahkonen O., Mäyränpää M., Pakkanen S., Husheem M., Sainio A., Hakovirta H., Laine J., Jokinen E., Vuorio E., Kovanen P., Järveläinen H. (2006) Histochem Cell Biol. 126, 335–342 [DOI] [PubMed] [Google Scholar]
- 12.Dollery C. M., McEwan J. R., Wang M., Sang Q. A., Liu Y. E., Shi Y. E. (1999) Circ. Res. 84, 498–504 [DOI] [PubMed] [Google Scholar]
- 13.Schulze C. J., Wang W., Suarez-Pinzon W. L., Sawicka J., Sawicki G., Schulz R. (2003) Circulation 107, 2487–2492 [DOI] [PubMed] [Google Scholar]
- 14.Hoit B. D., Takeishi Y., Cox M. J., Gabel M., Kirkpatrick D., Walsh R. A., Tyagi S. C. (2002) Mol. Cell Biochem. 238, 145–150 [DOI] [PubMed] [Google Scholar]
- 15.Li H., Simon H., Bocan T. M., Peterson J. T. (2000) Cardiovasc. Res. 46, 298–306 [DOI] [PubMed] [Google Scholar]
- 16.Seeland U., Kouchi I., Zolk O., Itter G., Linz W., Böhm M. (2002) J. Mol. Cell Cardiol. 34, 151–163 [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee R., Brinsa T. A., Dowdy K. B., Scott A. A., Baskin J. M., Deschamps A. M., Lowry A. S., Escobar G. P., Lucas D. G., Yarbrough W. M., Zile M. R., Spinale F. G. (2003) Circulation 107, 618–625 [DOI] [PubMed] [Google Scholar]
- 18.Wilson E. M., Moainie S. L., Baskin J. M., Lowry A. S., Deschamps A. M., Mukherjee R., Guy T. S., St John-Sutton M. G., Gorman J. H., 3rd, Edmunds L. H., Jr., Gorman R. C., Spinale F. G. (2003) Circulation 107, 2857–2863 [DOI] [PubMed] [Google Scholar]
- 19.Li Y. Y., Feldman A. M., Sun Y., McTiernan C. F. (1998) Circulation 98, 1728–1734 [DOI] [PubMed] [Google Scholar]
- 20.Soloway P. D., Alexander C. M., Werb Z., Jaenisch R. (1996) Oncogene 13, 2307–2314 [PubMed] [Google Scholar]
- 21.Nothnick W. B. (2001) Reproduction 122, 923–927 [DOI] [PubMed] [Google Scholar]
- 22.Roten L., Nemoto S., Simsic J., Coker M. L., Rao V., Baicu S., Defreyte G., Soloway P. J., Zile M. R., Spinale F. G. (2000) J. Mol. Cell Cardiol. 32, 109–120 [DOI] [PubMed] [Google Scholar]
- 23.Lee M. M., Yoon B. J., Osiewicz K., Preston M., Bundy B., van Heeckeren A. M., Werb Z., Soloway P. D. (2005) Infect. Immun. 73, 661–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osiewicz K., McGarry M., Soloway P. D. (1999) Ann. N.Y. Acad. Sci. 878, 494–496 [DOI] [PubMed] [Google Scholar]
- 25.Wang Z., Juttermann R., Soloway P. D. (2000) J. Biol. Chem. 275, 26411–26415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caterina J. J., Yamada S., Caterina N. C., Longenecker G., Holmbäck K., Shi J., Yermovsky A. E., Engler J. A., Birkedal-Hansen H. (2000) J. Biol. Chem. 275, 26416–26422 [DOI] [PubMed] [Google Scholar]
- 27.Jaworski D. M., Boone J., Caterina J., Soloway P., Falls W. A. (2005) Brain Res. 1051, 81–89 [DOI] [PubMed] [Google Scholar]
- 28.Jaworski D. M., Soloway P., Caterina J., Falls W. A. (2006) J. Neurobiol. 66, 82–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fata J. E., Leco K. J., Voura E. B., Yu H. Y., Waterhouse P., Murphy G., Moorehead R. A., Khokha R. (2001) J. Clin. Invest. 108, 831–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leco K. J., Waterhouse P., Sanchez O. H., Gowing K. L., Poole A. R., Wakeham A., Mak T. W., Khokha R. (2001) J. Clin. Invest. 108, 817–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammed F. F., Smookler D. S., Taylor S. E., Fingleton B., Kassiri Z., Sanchez O. H., English J. L., Matrisian L. M., Au B., Yeh W. C., Khokha R. (2004) Nat. Genet. 36, 969–977 [DOI] [PubMed] [Google Scholar]
- 32.Fedak P. W., Smookler D. S., Kassiri Z., Ohno N., Leco K. J., Verma S., Mickle D. A., Watson K. L., Hojilla C. V., Cruz W., Weisel R. D., Li R. K., Khokha R. (2004) Circulation 110, 2401–2409 [DOI] [PubMed] [Google Scholar]
- 33.Kassiri Z., Oudit G. Y., Sanchez O., Dawood F., Mohammed F. F., Nuttall R. K., Edwards D. R., Liu P. P., Backx P. H., Khokha R. (2005) Circ. Res. 97, 380–390 [DOI] [PubMed] [Google Scholar]
- 34.Tanaka N., Dalton N., Mao L., Rockman H. A., Peterson K. L., Gottshall K. R., Hunter J. J., Chien K. R., Ross J., Jr. (1996) Circulation 94, 1109–1117 [DOI] [PubMed] [Google Scholar]
- 35.Broberg C. S., Pantely G. A., Barber B. J., Mack G. K., Lee K., Thigpen T., Davis L. E., Sahn D., Hohimer A. R. (2003) J. Am. Soc. Echocardiogr. 16, 814–823 [DOI] [PubMed] [Google Scholar]
- 36.Saraste A., Kytö V., Saraste M., Vuorinen T., Hartiala J., Saukko P. (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H871–875 [DOI] [PubMed] [Google Scholar]
- 37.Ohta K., Nakajima T., Cheah A. Y., Zaidi S. H., Kaviani N., Dawood F., You X. M., Liu P., Husain M., Rabinovitch M. (2004) Am. J. Physiol. Heart Circ. Physiol. 287, H286–292 [DOI] [PubMed] [Google Scholar]
- 38.Hayashidani S., Tsutsui H., Ikeuchi M., Shiomi T., Matsusaka H., Kubota T., Imanaka-Yoshida K., Itoh T., Takeshita A. (2003) Am. J. Physiol. Heart Circ. Physiol. 285, H1229–1235 [DOI] [PubMed] [Google Scholar]
- 39.Matsumura S., Iwanaga S., Mochizuki S., Okamoto H., Ogawa S., Okada Y. (2005) J. Clin. Invest. 115, 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creemers E. E., Davis J. N., Parkhurst A. M., Leenders P., Dowdy K. B., Hapke E., Hauet A. M., Escobar P. G., Cleutjens J. P., Smits J. F., Daemen M. J., Zile M. R., Spinale F. G. (2003) Am. J. Physiol. Heart Circ. Physiol. 284, H364–371 [DOI] [PubMed] [Google Scholar]
- 41.Ikonomidis J. S., Hendrick J. W., Parkhurst A. M., Herron A. R., Escobar P. G., Dowdy K. B., Stroud R. E., Hapke E., Zile M. R., Spinale F. G. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H149–158 [DOI] [PubMed] [Google Scholar]
- 42.Tian H., Cimini M., Fedak P. W., Altamentova S., Fazel S., Huang M. L., Weisel R. D., Li R. K. (2007) J. Mol. Cell Cardiol. 43, 733–743 [DOI] [PubMed] [Google Scholar]
- 43.Kassiri Z., Khokha R. (2005) Thromb. Haemost. 93, 212–219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.