Abstract
Low molecular weight hyaluronan enhances or induces inflammation through toll-like receptor 4 (TLR-4).However, the effects of high molecular weight hyaluronan (HA900) on TLR-4 are unknown. In this study, HA900 (900 kDa) was administered orally to MRL-lpr/lpr mice, a Th-1-type autoimmune disease model. Lymphoaccumulation of double-negative T cells, which is enhanced by proinflammatory cytokines, was suppressed by HA900 treatment. Cytokine array analysis showed that HA900 treatment enhanced production of interleukin-10, an anti-inflammatory cytokine, and down-regulated chemokine production. HA900 colocalized with TLR-4 on the luminal surface of epithelial cells in the large intestine. These cells are parts of the immune system and express cytokines. DNA array analysis of the tissue from the large intestine showed that HA900 treatment up-regulated suppressor of cytokine signaling 3 (SOCS3) expression and down-regulated pleiotrophin expression. Treatment of cultured double-negative T cells from MRL-lpr/lpr mice with pleiotrophin rescued these cells. SOCS3, which is known to suppress inflammation, was enhanced by HA900 treatment. In TLR-4 knockdown HT29 cells (a cell line derived from large intestinal cells), HA900 did not bind to HT29 cells and did not up-regulate SOCS3 expression. Our results suggest that oral administration of HA900 modulates Th-1-type autoimmune disease and inflammation by up-regulating SOCS3 expression and down-regulating pleiotrophin expression via TLR-4 in intestinal epithelial cells.
Keywords: Cell Death, Hyaluronate, Immunology, Inflammation, Toll-like Receptors (TLR), Pleiotrophin, Suppressor of Cytokine Signaling 3
Introduction
Many reports have shown that low molecular weight hyaluronan (HA)2 enhances or induces inflammation via toll-like receptor 4 (TLR-4) (1, 2). It is well known that the functions of HA are molecular weight-dependent (3, 4). Therefore, we hypothesized that the actions of HA via TLR-4 depend on its molecular weight. MRL-lpr/lpr mice (3) are characterized by lymphoaccumulation of double-negative T cells (DNT cells), i.e. lymph node enlargement in their lymph nodes and spleens, and proliferation of these cells is enhanced by proinflammatory cytokines (5, 6).
TLR-4 is a type of HA receptor found on the surface of large intestinal epithelial cells, which form part of the immune system and express cytokines. SOCS3 expression suppresses inflammation and arthritis (7–9).
In this study, we showed that oral administration of high molecular weight HA (HA900) up-regulated suppressor of cytokine signaling 3 (SOCS3). In large intestinal epithelial cells, HA900 bound to TLR-4 on the cell surface and was not systemically absorbed.
Furthermore, HA900 suppressed pleiotrophin expression, which is implicated in inflammation, in intestinal tissue and in arthritis. Pleiotrophin mRNA in the spinal cord is significantly up-regulated after induction of experimental autoimmune encephalomyelitis (10, 11), and pleiotrophin is expressed in adults with inflammatory diseases, particularly in rheumatoid arthritis (12). These findings suggest that HA900 controls the immune system through up-regulation of SOCS3 and down-regulation of pleiotrophin. This is the first study to show a relationship between TLR-4 and high molecular weight HA.
EXPERIMENTAL PROCEDURES
Materials
HA900 and HA oligosaccharides (HA oligos prepared by HCl degradation; Mr 3200–15,000) were obtained from Q.P. Corp. (Tokyo, Japan).
Mice
Four-week-old female MRL-lpr/lpr mice were purchased from Charles River Japan (Yokohama, Japan) and cared for at our animal facility. All animal protocols were approved by the Animal Experiment Committee at Hyaluronan Research Institute, Inc.
Animal Experiments
Female MRL-lpr/lpr mice were randomly placed into three groups. One group received water (control group); one received HA900 (200 mg/kg/day), and one received HA oligos (200 mg/kg/day). The substances were administered orally in water bottles to mice between 14 and 18 weeks of age. The volume of water ingested by each mouse (about 7 ml/day) was used to calculate the dose; each mouse received 29 mg/ml HA900 or HA oligos. One day after the final administration, all animals were sacrificed by exsanguination under general anesthesia. Their lymph nodes were weighed to assess the effects of treatment on DNT cells.
Quantification of Serum HA Concentration
HA polymer (average Mr of 757,000; Calbiochem) was dissolved in 50 mm phosphate/citrate buffer (pH 5.0) at 0.01 mg/ml as a stock coating solution, and 50 μl of this solution was poured into each well of a 96-well amine-conjugated microtiter plate (Sumitomo Bakelite, Japan). A volume of 10 μl of sodium cyanoborohydrate solution (10 mg/ml) was added to each well and incubated for 1 h at 25 °C. After washing three times with 10 mm phosphate-buffered saline (pH 6.8) containing 0.05% Tween 20 (PBS/Tween), 100 μl of diluted Block Ace® (DS Pharma Biomedical, Japan) solution (diluted 1:4 with pure water) was poured into each well and incubated for 1 h at 37 °C. After washing with PBS/Tween, 50 μl of different dilutions of standard HA solutions as well as diluted serum samples were poured into the wells followed by 50 μl of biotinylated HA-binding protein (Hokudo, Japan) solution (0.1 mg/ml) in diluted Block Ace® solution (diluted 1:10 with pure water). After incubation for 1 h at 37 °C followed by washing with PBS/Tween, 100 μl of horseradish peroxidase (HRP)-conjugated streptavidin (Avidin-PO, Calbiochem) solution (0.05 mg/ml) in diluted Block Ace® solution (1:10 dilution) was poured into each well, and the plates were incubated for 1 h at 37 °C. Finally, after washing with PBS/Tween, 100 μl of TMB peroxidase substrate was poured into each well and incubated for 20 min at room temperature before 100 μl of 0.1 n HCl was added. Absorbance at 450:630 nm was measured using an MTP-300 Microplate Reader (Corona Electric, Japan).
Estimation of Molecular Weight of Serum HA
The molecular weight of serum HA was estimated by agarose gel electrophoresis. In brief, samples were diluted to give HA concentrations of 30–35 μg/ml and mixed with an equal volume of 40% glycerol. A 0.75% agarose gel (10 × 10 × 0.2 cm) was prepared in 40 mm Tris-HCl buffer (pH 8.0) containing 50 mm sodium acetate and 9 mm EDTA (TAE buffer). Samples (10 μl) were loaded on the agarose gel, and electrophoresis was performed at a constant voltage of 25 V for 5 h at room temperature. After electrophoresis, the gel was soaked in TAE buffer containing 2 mm l-ascorbic acid and 0.1 mm iron sulfate for 30 min before being washed twice with TAE buffer for 15 min. Hyaluronan in the gel was transferred to a positively charged nylon membrane (10 × 10 cm) (HybondTM-N+, GE Healthcare) at a constant electric current of 100 mA for 2 h in a semi-dry transfer unit (Hoefer® SemiPhorTM, GE Healthcare). After the transfer, the membrane was soaked in 10% skimmed milk in 50 mm Tris-HCl (pH 7.5) containing 0.1 m sodium chloride (TBS) overnight and washed twice with TBS. The membrane was then soaked in Block Ace® solution (diluted 1:10) containing 0.1 mg/ml biotinylated HA-binding protein and incubated for 1 h at room temperature. After washing three times with PBS/Tween, the membrane was soaked in Block Ace® solution (diluted 1:10) containing 0.05 mg/ml Avidin-PO. After washing three times with PBS/Tween, the membrane was rinsed with PBS and pure water to remove detergent and reacted with a chemical luminescent reagent mixture (SuperSignal, Pierce) for 5 min. The results were recorded on x-ray film (RX-U; Fuji Photo Film, Japan).
DNA Array Analysis
Large intestinal tissues from control and HA900 groups were used in DNA array analyses (Takara Bio, Japan). To confirm the results, mRNA transcripts for SOCS3 were detected by RT-PCR, and quantification was performed using three samples. The intensities observed were typical, and clear statistical differences were found.
RT-PCR
Total RNA was isolated from large intestinal tissues of mice and HT29 cells using TRIzol reagents (Invitrogen); 1 μg of total RNA was used for the reverse transcript reactions. PCR amplification was performed for 35 cycles.
For RT-PCR of the intestinal tissues, the SOCS3 primer sequences used in the study were as follows: forward, 5′-GCGAGAAGATTCCGCGGTA-3′; reverse, 5′-CCGTTGACAGTCTTCCGACAA-3′. For RT-PCR of the HT29 cells, the SOCS3 primer sequences used in the study were as follows: forward, 5′-CTTCAGCTCCAAGAGCGAGTA-3′; reverse, 5′-GAGCTGTCGCGGATCAGAAAG-3′. Glyceraldehyde-3-phosphate dehydrogenase primer sequences used were as follows: forward, 5′-ACCACAGTCCATCAC-3′; reverse, 5′-TCCACCACCCTGTTGCTGTA-3′.
Histopathology
Frozen sections of lymph nodes taken from mice were treated with water or HA900 and immunostained with an antibody to pleiotrophin (Acris) followed by HRP-labeled rabbit IgG (Lab Vision Corp.). Frozen sections of large intestinal tissues and submandibular lymph nodes were stained with biotinylated HA-binding protein (Hokudo, Japan) followed by streptavidin-HRP (Lab Vision Corp.) and diaminobenzidine. Streptomyces hyaluronidase digestion of tissue sections was used as a control. The frozen sections of large intestinal tissues were also used for double staining with biotinylated HA-binding protein and anti-TLR-4 antibody (Abnova Corp.). Frozen sections of submandibular lymph nodes were analyzed for apoptosis using the terminal dUTP nick end-labeling method.
Western Blotting for SOCS3 in HT29 Cells
Confluent HT29 cells were left untreated or were treated with HA oligos or with HA900 for 24 h. The cell lysates were obtained using a lysate kit (Funakoshi, Japan). The lysates were electrophoresed using Tris acetate gel. The samples were then transferred to a polyvinylidene difluoride membrane. The membrane was immunostained for SOCS3 (first antibody, anti-human SOCS3 (Cell Signaling Technology, Japan); second antibody, HRP-anti-mouse IgG (Abnova)). Signals were detected using FCL solution (GE Healthcare) and recorded on x-ray film.
In Vitro Study of DNT Cells
DNT cells were obtained from lymph nodes of MRL-lpr/lpr mice and treated (4 × 105 cells/well) with pleiotrophin (0, 1, 10, and 100 ng/ml) or phorbol 12-myristate 13-acetate (PMA; 100 ng/ml) for 6 h. A methanethiosulfonate assay was then performed using incubation with Cell Count Reagent SF (Nacalai Tesque, Kyoto, Japan) for 1 h. Data are shown as means ± S.E. of triplicate values. Each experiment was repeated at least three times.
Binding of HA900 or HA Oligos to Large Intestinal Cells or HT29 Cells
Confluent HT29 cells were incubated with 1 μg/ml HA900 or HA oligos for 1 h at room temperature. Large intestinal tissues from MRL-lpr/lpr mice treated orally with HA900 or HA oligos were frozen and sectioned. HT29 and large intestinal cells were then treated with biotinylated HA-binding protein. HT29 cells were then treated with anti-TLR-4 antibody followed by Texas Red anti-mouse IgG (GenWay Biotech Inc.). The same experiments were performed using TLR-4 knockdown HT29 cells.
SOCS3 Expression in HT29 Cells
HT29 cells were left untreated or were treated with 1 μg/ml HA900 or HA oligos for 24 h at 37 °C. The samples were then used in Western blotting for SOCS3 antibody. Immunostaining for SOCS3 was performed in HT29 cells left untreated or treated with 1 μg of HA oligos or 0.01, 0.1, and 1 μg of HA900. The cells were then treated with a secondary antibody labeled with HRP (Abnova) followed by diaminobenzidine. The intensity of staining was analyzed by NIH Image.
TLR-4 Knockdown
Lipofectamine (Invitrogen) was mixed with three types of TLR-4 RNA (CAGACTTGCGGGTTCTACATCAAAT, CGGATGGCAACATTTAGAATTAGTT, and GATCTCAGTAGAAATGGCTTGAGTT). The solution was added to HT29 cells in 24 wells of a 96-well plate. The plate was then incubated for 48 h at 37 °C. Then HA900 or HA oligos were added, and cells were incubated for 24 h. The knockdown and wild-type HT29 cells were immunostained for TLR-4, HA, and SOCS3.
Array Analysis of Cytokines and Chemokines
Serum (10 μl) was taken from each mouse, and sera from each group were pooled. Sera taken from mice who received water as well as those that received HA900 were used for array analyses of cytokines and chemokines (enzyme-linked immunosorbent assay, Raybio; n = 2). This experiment was repeated twice (n = 4).
RESULTS
Treatment with HA900 (200 mg/kg/day) suppressed weight increases in submandibular (Fig. 1, a and b), mesenteric, inguinal, and axillary lymph nodes compared with controls, whereas treatment with HA oligos showed a trend toward lower weights but did not reach statistical significance (Fig. 1c). Cytokine and chemokine arrays showed up-regulation of IL-10 and down-regulation of MCP-5, MIP-2, regulated on activation normal T cell expressed and secreted, P-selectin, stem cell factor (SCF), and VEGF (Fig. 2). In the serum of C57 BL/6 mice (C57), the levels of MCP-5, SCF, and VEGF were not detected, because the C57 BL/6 mice were normal.
FIGURE 1.
a and b, HA900 suppressed submandibular lymph node enlargement. c, total weight of lymph nodes in the HA900 group was less than that in the control group. n = 6; *, p < 0.05; Tukey's multiple comparison test. HAO, hyaluronan oligosaccharides.
FIGURE 2.
Results of cytokine and chemokine array analysis of serum. Most of the humoral factors in serum or MRL-lpr/lpr mice, except for IL-10, were decreased by the treatment with HA900. The y axis indicates relative intensity of the expression. C57, serum from normal C57 BL/6 mice. n = 6; *, p < 0.05; Tukey's multiple comparison test. RANTES, regulated on activation normal T cell expressed and secreted.
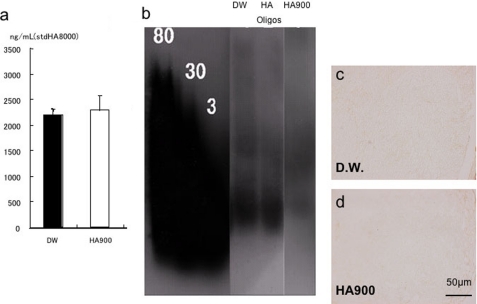
Treatment with HA900 did not elicit any changes in the concentration or molecular weight of HA in plasma (Fig. 3, a and b). Furthermore, HA was not detected in lymph nodes of control or HA900 groups (Fig. 3, c and d).
FIGURE 3.
a, HA concentration in serum. HA900 treatment did not change HA concentration. Standard HA, 8 kDa. b, molecular weight of HA in serum. HA900 treatment did not change the molecular weight of HA. Standard HA, 80, 800 kDa; 30, 300 kDa; 3, 30 kDa. c and d, HA staining in submandibular lymph nodes was not detected.
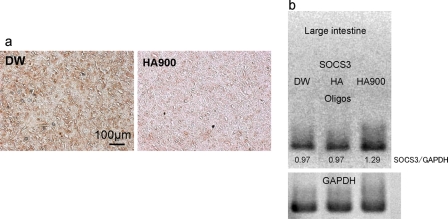
DNA array analysis of large intestinal tissues showed that HA900 treatment up-regulated SOCS3 (ratio of HA900/DW, 2.0) and down-regulated pleiotrophin (ratio of HA900/DW, 0.5). Immunostaining for pleiotrophin in lymph nodes was stronger in the control group than in the HA900 group (Fig. 4a). RT-PCR analysis showed that SOCS3 mRNA expression in large intestines was stronger in the HA900 group than in the control group (Fig. 4b).
FIGURE 4.
Immunostaining for pleiotrophin in lymph nodes (a) and RT-PCR of SOCS3 in large intestinal tissues (b). Pleiotrophin expression was diminished by the treatment with HA900 (a). Ratio of the intensity (SOCS3/glyceraldehyde-3-phosphate dehydrogenase (GAPDH)) showed that HA900 treatment up-regulated mRNA expression of SOCS3 (b).
DNT cells were obtained from lymph nodes of MRL-lpr/lpr mice by mincing the lymph nodes. It is well known that more than 90% of the lymphocytes of lymph nodes are DNT cells (5, 13).
A large amount of cell debris (indicative of cell death of DNT cells) was observed among untreated controls (Fig. 5a). In contrast, only a small amount of debris was observed in control lymphocytes after treatment with pleiotrophin (Fig. 5a) or PMA (data not shown). PMA was used because PMA generally activates lymphocytes. Furthermore, a methanethiosulfonate assay also showed that treatment of DNT cells from MRL-lpr/lpr mice with pleiotrophin suppressed cell death as did treatment with PMA (Fig. 5b). Apoptosis in lymph node tissues from MRL-lpr/lpr mice increased after treatment with HA900 as indicated by the brown-stained cells (Fig. 5d), but this did not occur in the control group treated with distilled water (Fig. 5c).
FIGURE 5.
a, morphology of cell death in submandibular lymph node tissues. Fragmented cell debris (arrows) was seen in untreated cultures, and a small amount of debris was observed in the presence of pleiotrophin. b, appearance of fragmented cell debris and methanethiosulfonate assay show that treatment with pleiotrophin suppressed cell death in DNT cells. On the other hand, terminal dUTP nick end-labeling method shows that HA900 treatment induced apoptosis in lymph nodes (c and d). Data are shown as mean ± S.E. *, p < 0.05, Tukey's multiple comparison test.
HA was detected in the lamina propria in intestinal tissues of control and HA900 groups (Fig. 6, a–c) but was also selectively detected on the luminal surface and intracellular space of intestinal epithelial cells in the HA900 group (Fig. 6, b and c) where it was often colocalized with TLR-4 (Fig. 6, f and g). The colocalization was restricted to this area. Most of the endogenous subepithelial HA was not colocalized with TLR-4 (Fig. 6, e–g). Treatment with S. hyaluronidase, specific for HA, abolished HA (Fig. 6d).
FIGURE 6.
HA staining in tissues from the large intestine (a–d). HA was detected on the luminal surface of intestinal epithelial cells (arrows) in the HA900 group but not in the control group. d, pretreatment with Streptomyces hyaluronidase abolished HA. Vertical section of large intestinal tissue was stained with biotinylated HA-binding protein (green, e) and anti-TLR-4 (red, g). f, in the merge, yellow and orange indicate the colocalization of HA900 and TLR-4. Yellow arrows indicate the apical surface of the intestine. White arrows indicate endogenous HA in the villi connective tissue. * indicates lamina propria.
Immunostaining for SOCS3 showed that HA900 treatment up-regulated SOCS3 expression (Fig. 7b) compared with control and HA oligo groups (Fig. 7a). Western blotting and RT-PCR showed increased SOCS3 expression in HT29 cells after treatment with HA900 but not when treated with HA oligos (Fig. 7, c and d).
FIGURE 7.
SOCS3 immunostaining (a and b), Western blotting for SOCS3 (c), and RT-PCR for SOCS3 (d) in HT29 cells. In any case, HA900 up-regulated the SOCS3 expression. N, nontreatment control; HAO, hyaluronan oligosaccharide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Wild-type HT29 cells expressed TLR-4 that was colocalized with HA900 (Fig. 8) or HA oligos (data not shown). On the other hand, neither HA900 (Fig. 9) nor HA oligos (data not shown) bound TLR-4 knockdown HT29 cells. Furthermore, HA900 did not change SOCS3 expression in the knockdown HT29 cells (Fig. 9).
FIGURE 8.
In wild-type HT29 cells treated with HA900, HA (green) was colocalized with TLR-4 (red). In the merge, yellow also indicated the colocalization.
FIGURE 9.
Effects of TLR-4 knockdown in HT29 cells. Neither immunostaining of TLR-4 (red) nor HA staining (green) was detected. SOCS3 expression was not changed by the treatment with HA900.
DISCUSSION
Fig. 10 shows our model based on the studies in this report. TLR-4 receptors respond to bacterial endotoxins by up-regulation of pleiotrophin and pro-inflammatory cytokines. This increases inflammatory responses, including DNT lymphoaccumulation. Our studies support the model in which HA900 interacts with TLR-4 by a mechanism that blocks expression of pleiotrophin and up-regulates SOCS3. This would be consistent with the study showing that HA injections in LPS-treated mice promoted survival (14). The mechanism may be related to HA interaction with CD44 capturing the kinase required for LPS-TLR-4 receptor activation. Otherwise, our model would have to find a way to distinguish between TLR-4 interaction with bacterial endotoxin and HA. Thus, cooperation from another receptor (CD44) may be crucial.
FIGURE 10.
a, intestinal bacteria stimulate TLR-4 to induce inflammation and lymphoaccumulation via pleiotrophin. b, HA900 down-regulates pleiotrophin expression in intestinal epithelial cells, leading to suppression of inflammation, arthritis, and lymphoaccumulation. c, serum IL-10 concentration was reduced by HA900 treatment. It is speculated that HA900 up-regulates IL-10 expression, which induces SOCS3 expression in intestinal epithelial cells, leading to suppression of inflammation and arthritis. White arrows, up-regulation or down-regulation.
Lymphoaccumulation reflects inflammation by Th-1-type cytokines in MRL-lpr/lpr mice (5, 6). Therefore, it is adequate and convenient to observe the lymphoaccumulation to examine the effect of substances on the immune system.
Treatment with HA900 did not elicit any changes in the molecular weight or concentration of HA in serum. Furthermore, staining for HA in lymph nodes was not changed by treatment with HA900, which suggests that HA900 can modulate the function of the intestinal epithelium as a part of the immune system. Cultured DNT cells from MRL-lpt/lpr mice exhibited apoptotic programmed cell death without stimulation (3, 15); whereas treatment of DNT cells with pleiotrophin suppressed cell death. Furthermore, HA900 treatment induced apoptosis in the lymph node tissues. These results suggest that HA900 induces cell death of DNT cells by down-regulating pleiotrophin expression. Many reports have shown that SOCS3 suppresses inflammation, that IL-10 inhibits lipopolysaccharide-induced CD40 gene expression through induction of SOCS3 (16), and that induction of the SOCS3-CIS3 complex is a strategy for treating inflammatory arthritis (17). Our studies show that HA900 treatment of MRL-lpt/lpr mice increased SOCS3 mRNA and IL-10 consistent with its ability to suppress inflammatory responses. Intra-articular injection of HA900 suppresses inflammation in osteoarthritis (18).
In this study, oral administration of HA900 reduced lymphoaccumulation in MRL-lpr/lpr mice, which are a well established model for autoimmune diseases. These mice develop lymphoaccumulation (lymphoadenopathy), hypergammaglobulinemia, serum autoantibodies, and generalized autoimmune disease, including glomerulonephritis and arthritis, and have also been used as a model for systemic lupus erythematosus (3). This lymphoaccumulation disorder involves a marked increase in DNT cells (CD4−CD8−) in lpr mice and is explained by defects in Fas, which mediates apoptosis (19). Lymphoaccumulation also occurs due to hyperproduction of proinflammatory cytokines (5, 6).
Most endogenous HA does not bind to TLR-4. Exogenous HA was found in apical epithelial cells of the upper villi of the large intestine, and colocalization of HA900 and TLR-4 was observed in these cells. HA900 may be taken up by intestinal epithelial cells via TLR-4. These results suggest that exogenous HA affects intestinal epithelial cells.
We had speculated that oral administration of HA900 would stimulate intestinal epithelial cells, which produce cytokines as protection against infection following exposure to bacteria. When administered, HA900 colocalized with TLR-4 on intestinal epithelial cells, but HA900 did not bind TLR-4 knockdown HT29 cells, suggesting that HA900 is bound to TLR-4. TLR-4 is known to be expressed on the surface of intestinal epithelial cells (20, 21) and is an HA receptor (1). Coexpression of TLR4 and MD-2 is necessary and sufficient for LPS responsiveness in intestinal epithelial cells (20), and expression of MD-2 is blocked by the STAT inhibitor SOCS3 (20). HA900 may prevent MD-2 binding to TLR-4 in commensal pathogen-associated molecular patterns such as LPS, or HA900-induced SOCS3 may reduce lymphoaccumulation because SOCS3 is known to suppress production of proinflammatory cytokines and improve arthritis (10).
The biological activity of HA is dependent on its molecular weight (3). Low molecular weight HA enhances inflammation via TLR-4 (3), whereas the present results suggest that HA900 may suppress inflammation involving chemokines, leukocyte and lymphocyte adhesion, P-selectin, and SCF expression via TLR-4. Overexpression of proinflammatory cytokines causes autoimmune disease and inflammation. MRL-lpr/lpr mice do not exhibit these conditions in a clean environment because of diminished levels of intestinal bacterial flora (22). Array analysis of cytokines (a total of 37) and chemokines showed that HA900 treatment up-regulated IL-10, and suppressed MCP-5, MIP-2, regulated on activation normal T cell expressed and secreted, P-selectin, SCF, and VEGF. IL-10 suppresses chemokine production (23, 24) and enhances SOCS3 expression (25). Furthermore, down-regulation of P-selectin suppresses the infiltration of lymphocytes and leukocytes (26, 27). The absolute suppression of SCF indicates that arthritis may be suppressed because SCF is implicated in processes in the arthritic synovium (28). The suppression of VEGF also suggests potential improvement of arthritis (29). Low molecular weight HA activates the innate immune response via TLR-2 in an MyD88-, IL-1R-associated kinase-, tumor necrosis factor receptor-associated factor-6-, protein kinase Cζ-, and NF-κB-dependent pathway. Furthermore, intact high molecular weight HA can inhibit TLR-2 signaling (30).
Arthritis did not occur in MRL-lpr/lpr mice in this study because these mice were too young. However, up-regulation of SOCS3 and suppression of pleiotrophin by HA900 treatment should suppress arthritis. Immunostaining, Western blotting, and RT-PCR for SOCS3 in HT29 cells showed that SOCS3 expression was enhanced by treatment with HA900 but not with HA oligos.
HA900, which was added to HT29 cells, showed colocalization with TLR-4 in these cells. During treatment with TLR-4 small interfering RNA, TLR-4 protein expression was diminished. HA900 neither bound to TLR-4 knockdown HT29 cells nor up-regulated SOCS3 in these cells compared with wild-type HT29 cells treated with HA900. We conclude that oral administration of HA900 suppresses inflammation and Th-1-type autoimmune disease by up-regulating SOCS3 and down-regulating pleiotrophin via TLR-4 in intestinal epithelial cells.
Acknowledgments
We are indebted to Q.P. Corp. for providing HA900 (Hyabest (J)) and HA oligos (Hyalo Oligo). We thank Dr. Watanabe, Dr. Oonuki, H. Yamanokuchi, and Dr. Podyma-Inoue for their technical assistance. We thank Dr. Hascall and Dr. Yanagishita for reviewing our manuscript.
Footnotes
- HA
- hyaluronan
- PBS
- phosphate-buffered saline
- HRP
- horseradish peroxidase
- TLR-4
- toll-like receptor 4
- RT
- reverse transcription
- oligo
- oligonucleotide
- DNT
- double-negative T cell
- PMA
- phorbol 12-myristate 13-acetate
- IL
- interleukin
- LPS
- lipopolysaccharide
- SCF
- stem cell factor
- VEGF
- vascular endothelial growth factor
- DW
- distilled water.
REFERENCES
- 1.Termeer C., Benedix F., Sleeman J., Fieber C., Voith U., Ahrens T., Miyake K., Freudenberg M., Galanos C., Simon J. C. (2002) J. Exp. Med. 195, 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang D., Liang J., Fan J., Yu S., Chen S., Luo Y., Prestwich G. D., Mascarenhas M. M., Garg H. G., Quinn D. A., Homer R. J., Goldstein D. R., Bucala R., Lee P. J., Medzhitov R., Noble P. W. (2005) Nat. Med. 11, 1173–1179 [DOI] [PubMed] [Google Scholar]
- 3.Stern R., Asari A. A., Sugahara K. N. (2006) Eur. J. Cell Biol. 85, 699–715 [DOI] [PubMed] [Google Scholar]
- 4.Xu H., Ito T., Tawada A., Maeda H., Yamanokuchi H., Isahara K., Yoshida K., Uchiyama Y., Asari A. (2002) J. Biol. Chem. 277, 17308–17314 [DOI] [PubMed] [Google Scholar]
- 5.Xu H., Kurihara H., Ito T., Nakajima S., Hagiwara E., Yamanokuchi H., Asari A. (2001) J. Autoimmun. 16, 87–95 [DOI] [PubMed] [Google Scholar]
- 6.Kikawada E., Lenda D. M., Kelley V. R. (2003) J. Immunol. 170, 3915–3925 [DOI] [PubMed] [Google Scholar]
- 7.Sivakumar P. V., Westrich G. M., Kanaly S., Garka K., Born T. L., Derry J. M., Viney J. L. (2002) Gut 50, 812–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuerer-Maly C. C., Eckmann L., Kagnoff M. F., Falco M. T., Maly F. E. (1994) Immunology 81, 85–91 [PMC free article] [PubMed] [Google Scholar]
- 9.Jo D., Liu D., Yao S., Collins R. D., Hawiger J. (2005) Nat. Med. 11, 892–898 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki A., Hanada T., Mitsuyama K., Yoshida T., Kamizono S., Hoshino T., Kubo M., Yamashita A., Okabe M., Takeda K., Akira S., Matsumoto S., Toyonaga A., Sata M., Yoshimura A. (2001) J. Exp. Med. 193, 471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X., Mashour G. A., Webster H. F., Kurtz A. (1998) Glia 24, 390–397 [DOI] [PubMed] [Google Scholar]
- 12.Pufe T., Bartscher M., Petersen W., Tillmann B., Mentlein R. (2003) Arthritis Rheum. 48, 660–667 [DOI] [PubMed] [Google Scholar]
- 13.Xu H., Kurihara H., Ito T., Kikuchi H., Yoshida K., Yamanokuchi H., Asari A. (2005) J. Biol. Chem. 280, 20879–20886 [DOI] [PubMed] [Google Scholar]
- 14.Muto J., Yamasaki K., Taylor K. R., Gallo R. L.(2009) Mol. Immunol. 47, 449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Houten N., Budd R. C. (1992) J. Immunol. 149, 2513–2517 [PubMed] [Google Scholar]
- 16.Lee H. G., Cowman M. K. (1994) Anal. Biochem. 219, 278–287 [DOI] [PubMed] [Google Scholar]
- 17.Böcker U., Yezerskyy O., Feick P., Manigold T., Panja A., Kalina U., Herweck F., Rossol S., Singer M. V. (2003) Int. J. Colorectal Dis. 18, 25–32 [DOI] [PubMed] [Google Scholar]
- 18.Qin H., Wilson C. A., Roberts K. L., Baker B. J., Zhao X., Benveniste E. N. (2006) J. Immunol. 177, 7761–7771 [DOI] [PubMed] [Google Scholar]
- 19.Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. (1992) Nature 356, 314–317 [DOI] [PubMed] [Google Scholar]
- 20.Abreu M. T., Arnold E. T., Thomas L. S., Gonsky R., Zhou Y., Hu B., Arditi M. (2002) J. Biol. Chem. 277, 20431–20437 [DOI] [PubMed] [Google Scholar]
- 21.Abreu M. T., Thomas L. S., Arnold E. T., Lukasek K., Michelsen K. S., Arditi M. (2003) J. Endotoxin Res. 9, 322–330 [DOI] [PubMed] [Google Scholar]
- 22.Maldonado M. A., Kakkanaiah V., MacDonald G. C., Chen F., Reap E. A., Balish E., Farkas W. R., Jennette J. C., Madaio M. P., Kotzin B. L., Cohen P. L., Eisenberg R. A. (1999) J. Immunol. 162, 6322–6330 [PubMed] [Google Scholar]
- 23.Szczepanik A. M., Ringheim G. E. (2003) J. Alzheimers Dis. 5, 105–117 [DOI] [PubMed] [Google Scholar]
- 24.Petit-Bertron A. F., Fitting C., Cavaillon J. M., Adib-Conquy M. (2003) J. Leukocyte Biol. 73, 145–154 [DOI] [PubMed] [Google Scholar]
- 25.Mihaescu A., Thornberg C., Mattsson S., Wang Y., Jeppsson B., Thorlacius H. (2007) Dis. Colon Rectum 50, 2194–2202 [DOI] [PubMed] [Google Scholar]
- 26.Bonder C. S., Norman M. U., Macrae T., Mangan P. R., Weaver C. T., Bullard D. C., McCafferty D. M., Kubes P. (2005) Am. J. Pathol. 167, 1647–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassatella M. A., Gasperini S., Bovolenta C., Calzetti F., Vollebregt M., Scapini P., Marchi M., Suzuki R., Suzuki A., Yoshimura A. (1999) Blood 94, 2880–2889 [PubMed] [Google Scholar]
- 28.Ceponis A., Konttinen Y. T., Takagi M., Xu J. W., Sorsa T., Matucci-Cerinic M., Santavirta S., Bankl H. C., Valent P. (1998) J. Rheumatol. 25, 2304–2314 [PubMed] [Google Scholar]
- 29.Clavel G., Bessis N., Lemeiter D., Fardellone P., Mejjad O., Ménard J. F., Pouplin S., Boumier P., Vittecoq O., Le Loët X., Boissier M. C. (2007) Clin. Immunol. 124, 158–164 [DOI] [PubMed] [Google Scholar]
- 30.Scheibner K. A., Lutz M. A., Boodoo S., Fenton M. J., Powell J. D., Horton M. R. (2006) J. Immunol. 177, 1272–1281 [DOI] [PubMed] [Google Scholar]