Abstract
Mechanical overloading of cartilage producing hydrostatic stress, tensile strain, and fluid flow can adversely affect chondrocyte function and precipitate osteoarthritis (OA). Application of high fluid shear stress to chondrocytes recapitulates the earmarks of OA, as evidenced by the release of pro-inflammatory mediators, matrix degradation, and chondrocyte apoptosis. Elevated levels of cyclooxygenase-2 (COX-2), prostaglandin (PG) E2, and interleukin (IL)-6 have been reported in OA cartilage in vivo, and in shear-activated chondrocytes in vitro. Although PGE2 positively regulates IL-6 synthesis in chondrocytes, the underlying signaling pathway of shear-induced IL-6 expression remains unknown. Using the human T/C-28a2 chondrocyte cell line as a model system, we demonstrate that COX-2-derived PGE2 signals via up-regulation of E prostanoid (EP) 2 and down-regulation of EP3 receptors to raise intracellular cAMP, and activate protein kinase A (PKA) and phosphatidylinositol 3-kinase (PI3-K)/Akt pathways. PKA and PI3-K/Akt transactivate the NF-κB p65 subunit via phosphorylation at Ser-276 and Ser-536, respectively. Binding of p65 to the IL-6 promoter elicits IL-6 synthesis in sheared chondrocytes. Selective knockdown of EP2 or ectopic expression of EP3 blocks PKA- and PI3-K/Akt-dependent p65 activation and markedly diminishes shear-induced IL-6 expression. Similar inhibitory effects on IL-6 synthesis were observed by inhibiting PKA, PI3-K, or NF-κB using pharmacological and/or genetic interventions. Reconstructing the signaling network regulating shear-induced IL-6 expression in chondrocytes may provide insights for developing therapeutic strategies for arthritic disorders and for culturing artificial cartilage in bioreactors.
Keywords: CREB, Cyclooxygenase (COX) Pathway, G Protein-coupled Receptors (GPCR), Interleukin, NF-κB, PI 3-Kinase, Prostaglandins, Protein Kinase A (PKA), Chondrocytes, Shear
Introduction
Excessive chronic or repetitive mechanical loading of articular cartilage producing hydrostatic stress, tensile strain, and fluid flow (1) leads to irreversible cartilage erosion and osteoarthritic (OA)2 disease (2). Numerous in vitro studies support the concept that low fluid shear (<10 dyn/cm2) is chondroprotective (3), whereas high shear stress (>10 dyn/cm2) elicits the release of pro-inflammatory cytokines such as interleukin-6 (IL-6) (4), and mediates matrix degradation (3) and chondrocyte cell death (5, 6). Although OA is classified as a non-inflammatory joint disease, prostaglandins and cytokines are believed to play a role in the pathogenesis and progression of the disease. Prior work has shown that OA cartilage spontaneously releases prostaglandin (PG)E2 at 50-fold higher levels than in normal cartilage (7). The superinduction of PGE2 is mediated by cyclooxygenase-2 (COX-2) protein, whose levels are markedly up-regulated in OA-affected cartilage (7). The clinical correlates of OA are joint pain, dysfunction, and restricted motion. IL-6 has been implicated in pain signaling (8). Moreover, IL-6 mRNA expression occurs in human chondrocytes from OA but not normal cartilage (4). Catabolic and pro-inflammatory mediators such as PGE2 and IL-6 alter matrix homeostasis and participate in the destruction of articular cartilage, thereby contributing to OA.
Accumulating evidence suggests that IL-6 synthesis is positively regulated by either endogenous COX-2-derived or exogenously added PGE2 in many different cells including macrophages (9), osteoblasts (10), synovial (11), and gingival (12) fibroblasts. PGE2 exerts its biological functions via binding to four distinct transmembrane G-protein-coupled receptors (GPCRs) termed E prostanoid (EP) 1, EP2, EP3, and EP4. Following PGE2 binding, the EP receptors activate distinct intracellular signaling pathways, which may account for the pleiotropic effects of this prostaglandin. EP1 couples to Gq protein and raises intracellular calcium (13). EP2 and EP4 elevate intracellular cAMP levels by activating adenylate cyclase via stimulatory G (Gs) proteins (13). The major signaling pathway of the EP3 receptor is the inhibition of adenylate cyclase, and thus reduction of intracellular cAMP, via inhibitory G (Gi) proteins (13). Human chondrocytes primed with exogenous PGE2 synthesize IL-6 via an unknown signaling pathway (14).
Although high fluid shear (16 dyn/cm2) has been reported to induce IL-6 mRNA and protein expression in human chondrocytes (4), the underlying mechanism of this process has yet to be elucidated. Because OA is often a consequence of excessive mechanical forces (2) and given that application of high fluid shear to chondrocytes recapitulates the earmarks of OA (3, 5, 6), we here delineate the signaling pathway of IL-6 induction in shear-activated human T/C-28a2 chondrocytes. Specifically, we demonstrate that COX-2-derived PGE2 signals via EP2 and EP3 receptors to regulate intracellular cAMP levels in chondrocytes, which in turn stimulate protein kinase A (PKA) and phosphatidylinositol 3-kinase (PI3-K)/Akt pathways. PKA and PI3-K/Akt transactivate the NF-κB p65 subunit via phosphorylation at Ser-276 and Ser-536, respectively, which in turn binds to the IL-6 promoter and mediates IL-6 mRNA synthesis in shear-stimulated chondrocytes.
EXPERIMENTAL PROCEDURES
Reagents
The COX-2 selective inhibitor NS398, the EP and DP receptor antagonist AH6809, the selective agonist for EP3 receptor sulprostone, PGE2, forskolin, the PKA inhibitor H89, and the NF-κB inhibitor 6-amino-4-(4-phenoxyphenylethylamino) quinazoline (QNZ) were obtained from Enzo Life Sciences International Inc (Plymouth Meeting, PA). The EP3 and p65 cDNA plasmids were supplied from Origene Technologies (Rockville, MD) and subcloned to the pCMV6-XL vector. The IL-6 promoter reporter constructs pIL6-luc651 (-651/+1) and pIL6-luc651 ΔNF-κB (NF-κB site mutation) were gifts from Dr. Eickelberg (15). The PI3-K inhibitors, LY294002 and wortmannin, were from Sigma-Aldrich. p65 siRNA and antibodies specific for β-actin, COX-2, Akt, p-Akt (Ser-473), NF-κB p65, p-p65 (Ser-276), p-p65 (Ser-536), CREB, and p-CREB (Ser-133) were purchased from Cell Signaling Technology, Inc. (Danvers, MA). CREB1 and ATF4 siRNAs as well as monoclonal antibodies specific for p65 and IL-6 were obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). The cAMP enzyme immunoassay kit and antibodies specific for EP2 and EP3 receptors were from Cayman Chemical. All reagents for qRT-PCR and SDS-PAGE experiments were purchased from Bio-Rad Laboratories. Reagents for electrophoretic mobility shift assays (EMSA) were obtained from Pierce Chemical Company. The Dual-Luciferase Reporter Assay kit was purchased from Promega (Madison, WI). All other reagents were from Invitrogen (Carlsbad, CA), unless otherwise specified.
Cell Culture and Shear Stress Exposure
Human primary articular chondrocytes (Cell Applications) or T/C-28a2 chondrocytic cells were grown (37 °C in 5% CO2) on glass slides in F12/Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (5, 16–18). Before shear exposure, cells were incubated for 18 h in serum-free medium supplemented with 1% Nutridoma-SP (Roche), a low serum replacement that maintains chondrocyte phenotype and establishes quiescence in the monolayer (19, 20). Cells were then subjected to a shear stress level of 20 dyn/cm2 for prescribed periods of time in medium containing 1% Nutridoma-SP, using a streamer gold flow device (Flexcell International, Hillsborough, NC). In select experiments, the pharmacological agents were added to the medium at the indicated concentrations just before the onset of shear exposure. It is well established that transmission of the shear stress signal throughout the cell involves a complex interplay between cytoskeletal and biochemical constituents, and results in changes in structure, metabolism, and gene expression (21).
Transient Transfection and Reporter Gene Assays
For ectopic expression of EP3 receptor or p65, T/C-28a2 chondrocytes were transfected with 1.6 μg/slide of plasmid containing the EP3 or p65 cDNA by using Lipofectamine 2000. In control experiments, cells were transfected with 1.6 μg/slide of the empty vector pCMV6-XL (OriGene Technologies). In select experiments, T/C-28a2 cells were transfected with 1.6 μg/slide of the IL-6 promoter reporter construct pIL-6-luc651 or pIL6-luc651 ΔNF-κB. In RNA interference assays, T/C-28a2 cells were transfected with 100 nm siRNA oligonucleotide sequence specific for CREB1, ATF4, or p65. In control experiments, cells were transfected with 100 nm control siRNA. For EP2 shRNA experiments, T/C-28a2 chondrocytes were transfected with 1.6 μg/slide of plasmid containing the EP2 shRNA or scramble shRNA control. The following oligonucleotide sequences were used to specifically target the EP2 mRNA (underlined, sense and antisense sequences; boldface italicized, loop with linker): top strand, 5′-GATCCCCGATCCAGCTGCCTATTGATTTCAAGAGAATCAATAGGCAGCTGGATCTTTTTC-3′; bottom strand, 5′-TCGAGAAAAAGATCCAGCTGCCTATTGATTCTCTTGAAATCAATAGGCAGCTGGATCGGG-3′. Top and bottom strands were annealed, subcloned into the XhoI/BglII sites of pSuper, and the resulting construct sequence was verified. Transfected cells were allowed to recover for at least 12 h in growth medium and then incubated overnight in medium containing 1% Nutridoma-SP before their exposure to shear or static conditions. In promoter activity experiments, luciferase activities were measured by using the Dual-Luciferase Reporter Assay kit (Promega), as previously described (5, 17).
Quantitative Real-Time PCR (qRT-PCR)
qRT-PCR assays were performed on the iCycler iQ detection system (Bio-Rad) using total RNA, the iScript one-step RT-PCR kit with SYBR green (Bio-Rad) and primers. The GenBankTM accession numbers and forward (F-) and reverse (R-) primers are as follows: Cox-2 (NM_000963), F-TGAGCATCTACGGTTTGCTG, R-AACTGCTCATCACCCCATTC; EP1 (NM_000955), F-TGGGCCTCTGGTTGTGCTTA, R-TTCGGCCTCCACCTTCTTTG; EP2 (NM_000956), F-CGTGCACCTACTTCGCTTTC, R-GAGGTCCCATTTTTCCTTTC; EP3 (NM_198712), F-TTCTGCACCCGCCTCAACCA, R-AGGAGAGCCCGAAAACAGTCAT; EP4 (NM_000958), F-TCGCGCAAGGAGCAGAAGGAGACG, R-GGACGGTGGCGAGAATGAGGAAGG; IL-6 (NM_000600), F-ATGAACTCCTTCTCCACAAGCGC, R-GAAGAGCCCTCAGGCTGGACT; CREB1 (NM_134442), F-CCAGGTATCTATGCCAGCAG, R-TCTGTGTTCCGGAGAAAAGTC; ATF4 (NM_182810), F-CATTCCTCGATTCCAGCAAAGCAC, R-TTCTCCAACATCCAATCTGTCCCG; p65 (NM_001145138), F-CTGCAGTTTGATGATGAAGA, R-TAGGCGAGTTATAGCCTCAG; GAPDH (NM_002046), F-CCACCCATGGCAAATTCCATGGCA, R-TCTAGACGGCAGGTCAGGTCCACC.
GAPDH was used as internal control. Reaction mixtures were incubated at 50 °C for 15 min followed by 95 °C for 5 min, and then 35 PCR cycles were performed with the following temperature profile: 95 °C 15 s, 58 °C 30 s, 68 °C 1 min, 77 °C 20 s. Data were collected at the (77 °C 20 s) step to remove possible fluorescent contribution from dimer primers (22). Gene expression values were normalized to GAPDH.
Western Blot Analysis
T/C-28a2 cells, from sheared and matched static control specimens, were lysed in radioimmune precipitation assay buffer (25 mm Tris·HCl, pH 7.6, 150 mm NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS) containing a mixture of proteinase inhibitors (Pierce). The protein content of the cell lysates was determined using bicinchoninic acid (BCA) protein assay reagent (Pierce). Total cell lysates (4 μg) were subjected to SDS-PAGE, transferred to a membrane, and probed with a panel of specific antibodies. Each membrane was only probed using one antibody. β-Actin was used as loading control. All Western hybridizations were performed at least in triplicate using a different cell preparation each time.
Preparation of Cytosolic and Nuclear Extracts
Cytosolic and nuclear extracts were isolated using the NE-PER nuclear and cytoplasmic extraction kit (Pierce) following the manufacturer's instructions as previously described (17).
EMSA and Supershift Assay
A 5′-biotinylated oligonucleotide probe (5′-GGGATTTTCC-3′) was synthesized containing the NF-κB cis-element present on the IL-6 promoter. EMSAs were performed with a commercially available nonradioisotopic EMSA kit (LightShift Chemiluminescence EMSA kit; Pierce). Briefly, nuclear extracts (1–2 μg) were incubated in 10× binding buffer (supplemented with 50 ng of poly(dI-dC), 2.5% glycerol, 0.05% Nonidet P-40, 5 mm MgCl2, and 0.25 mg of bovine serum albumin), containing 20 fmol of biotinylated, double-stranded probe for NF-κB for 30 min on ice. For competition binding, a 200-fold excess of unlabeled (cold) probe was incubated with nuclear extracts before the inclusion of the biotinylated one. For supershift assays, the nuclear extracts were preincubated for 30 min on ice with an anti-p65 antibody. The biotinylated oligonucleotide probe specific for NF-κB was then added to the reaction mixture and incubated for another 30 min on ice. To exclude the possibility of nonspecific binding, a 5′-biotinylated random probe (5′-TCATTTTGTC-3′) designed using a random sequence generator was used in shift and supershift assays (supplemental Fig. S1). The protein-DNA complexes were resolved on a native 6% polyacrylamide retardation gel in 0.5× Tris borate-EDTA running buffer at 10 mA for 1 h, transferred to a nylon membrane (Pierce), visualized using the LightShift Chemiluminescence kit (Pierce) and exposed to Kodak x-ray film (Pierce).
Measurement of Intracellular cAMP Concentration
cAMP levels were determined by cyclic AMP enzyme immunoassay kit following the manufacturer's instructions (Cayman Chemical). The protein concentration of total cell lysate was used as loading control, and the results were expressed as pmol of cAMP/μg of total protein.
RESULTS
Shear Stress Induces IL-6 Expression in Human Chondrocytes via a COX-2-dependent Pathway
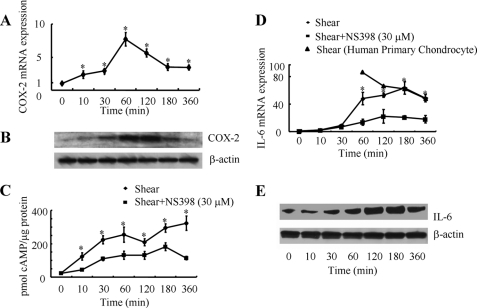
IL-6 expression has been detected in chondrocytes from OA but not normal cartilage (4). Prior work has shown that high fluid shear stress (16 dyn/cm2) induces IL-6 production in human articular chondrocytes in vitro (4). However, the signaling pathway of IL-6 induction in human chondrocytes in response to elevated levels of fluid shear remains unknown. In light of accumulating evidence suggesting that OA is often a consequence of abnormal mechanical forces (23), we here aimed to delineate the mechanism by which shear stress induces IL-6 expression in human chondrocytes. The human T/C-28a2 chondrocyte cell line was chosen as a model system, because T/C-28a2 cells have been shown to behave much like primary human chondrocytes when cultured under appropriate conditions (19, 20). In view of previous observations showing a positive correlation between endogenous COX-2-mediated PGE2 production and IL-6 synthesis (24, 25), we first evaluated the effects of shear stress on COX-2 expression and formation of cAMP, because PGE2 is a well-known activator of the cAMP signaling pathway. In accord with prior data (17), high shear stress (20 dyn/cm2) rapidly induces COX-2 mRNA (Fig. 1A) and protein (Fig. 1B) expression in human T/C-28a2 chondrocytes and cAMP production (Fig. 1C). IL-6 mRNA and protein up-regulation follows later, after 30–60 min of cell stimulation with fluid shear (Fig. 1, D and E). Treatment of T/C-28a2 chondrocytes with the selective COX-2 inhibitor NS398 (30 μm) markedly suppresses both intracellular cAMP accumulation (Fig. 1C) and IL-6 mRNA expression (Fig. 1D), suggesting the potential involvement of cAMP in shear-induced IL-6 synthesis in human chondrocytes. To validate previously published observations suggesting that T/C-28a2 cells represent an appropriate model for studying chondrocyte function in vitro (19, 20), we examined the responses of human primary articular chondrocytes to shear stress (20 dyn/cm2). Our data revealing significant similarities between primary articular chondrocytes and T/C-28a2 cells in the induction of IL-6 mRNA synthesis in response to shear stress (Fig. 1D) reinforce the aforementioned notion.
FIGURE 1.
Shear stress induces COX-2 and IL-6 synthesis and cAMP production in human chondrocytes. T/C-28a2 chondrocytes were exposed to either fluid shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for the indicated time intervals in the absence (♦) or presence (■) of the selective COX-2 inhibitor NS398 (30 μm). COX-2 (A) and IL-6 (D) mRNA synthesis was determined by qRT-PCR. GAPDH served as internal control. COX-2 (B) and IL-6 (E) protein expression was determined by Western blotting. cAMP formation was detected by a cAMP enzyme immunoassay kit (C). Data represent the mean ± S.D. of three independent experiments. *, p < 0.05 with respect to static controls (A) or the NS398-treated samples (C, D). In select experiments, human primary articular chondrocytes were subjected to either shear stress (20 dyn/cm2) or static conditions for the indicated time intervals (D).
Shear-induced IL-6 mRNA Synthesis Proceeds via an EP2-/EP3-dependent Mechanism
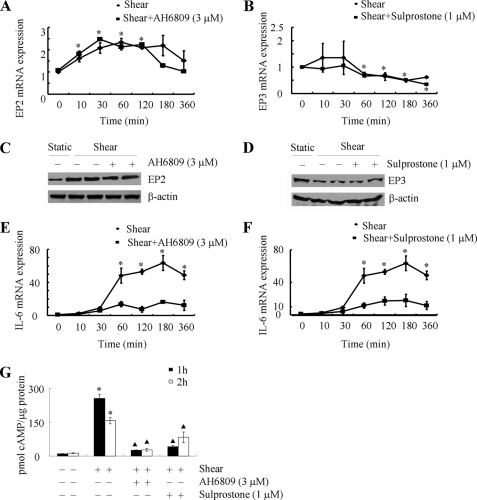
The effects of PGE2 are mediated via four different transmembrane GPCRs, namely EP1–4, which are involved in the activation of phospholipase C (EP1) and activation (EP2, EP4) or inhibition (EP3) of adenyl cyclase (13). In agreement with previously published data from primary human chondrocytes (26), T/C-28a2 cells express EP2, EP3 and very low levels of EP4, but lack EP1, receptors (supplemental Fig. S2A). As a next step, we assessed the influence of shear stress on EP receptor expression. Application of high fluid shear up-regulates EP2 (Fig. 2, A and C) while it down-regulates EP3 (Fig. 2, B and D) receptor expression at both the mRNA and protein levels in T/C-28a2 chondrocytes. No significant changes are noted in the mRNA expression levels of EP1 and EP4 receptors in sheared relative to static control T/C-28a2 chondrocytes (supplemental Fig. S2B). The divergent effects of shear stress on EP2 and EP3 receptor expression are in line with the enhanced cAMP accumulation observed in shear-activated chondrocytes (Fig. 1C). Thus, we next investigated the effects of an EP2 receptor antagonist, AH6809 (9, 27), and an EP3 receptor agonist, sulprostone (14), on shear-induced cAMP formation and IL-6 synthesis. Incubation of T/C-28a2 chondrocytes with AH6809 (3 μm) does not alter the mRNA, and protein expression levels of EP2 receptor in shear-stimulated cells (Fig. 2, A and C). However, this pharmacological intervention dramatically attenuates shear-induced cAMP production (Fig. 2G) and IL-6 mRNA expression (Fig. 2E) in T/C-28a2 chondrocytes. Similarly, sulprostone (1 μm), although it does not impair the EP3 receptor transcript and protein levels (Fig. 2, B and D), inhibits the accumulation of cAMP (Fig. 2G) and IL-6 synthesis (Fig. 2F) in sheared chondrocytes.
FIGURE 2.
Shear stress differentially modulates the expression of EP2 and EP3 receptors, which in turn regulate cAMP production and IL-6 mRNA synthesis in human chondrocytes. T/C-28a2 cells were subjected to fluid shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for the indicated time intervals in the absence (♦) or presence (■) of the EP2 receptor antagonist AH6809 (10 μm) (A, C) or the EP3 receptor agonist sulprostone (1 μm) (B, D). EP2 (A) and EP3 (B) receptor mRNA expression was determined by qRT-PCR. GAPDH served as internal control. Data represent the mean ± S.E. of at least three independent experiments. *, p < 0.05 with respect to static control. EP2 (C) and EP3 (D) receptor protein expression in sheared (20 dyn/cm2 for 1 or 2 h) and static control T/C-28a2 chondrocytes in the absence or presence of the aforementioned pharmacological agents is shown by immunoblotting using specific Abs. β-Actin was probed as loading control. The Western blot assays are representative of three independent experiments, all revealing similar results. IL-6 mRNA synthesis (E, F) and cAMP formation (G) were determined by qRT-PCR and a cAMP enzyme immunoassay kit, respectively. Data represent the mean ± S.D. of three independent experiments. *, ▴, p < 0.05 with respect to static control and sheared specimens, respectively.
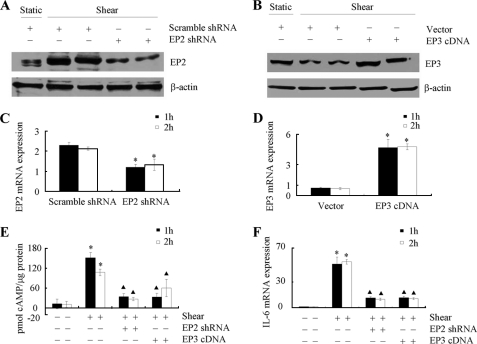
To verify the involvement of EP2 receptor in the signaling pathway of IL-6 induction in shear-stimulated chondrocytes, and given the rather non-selective nature of AH6809 (28), experiments were carried out by transfecting T/C-28a2 cells with an EP2 shRNA-containing plasmid. This genetic intervention effectively knocks down EP2 protein (Fig. 3A) and mRNA (Fig. 3C) expression in sheared T/C-28a2 chondrocytes relative to cells transfected with a scramble shRNA control. EP2 receptor knockdown also inhibits the shear-induced accumulation of intracellular cAMP (Fig. 3E) and IL-6 mRNA synthesis (Fig. 3F). Similarly, to confirm the role of EP3 receptor in this signaling pathway, and in view of its shear-induced down-regulation, T/C-28a2 chondrocytes were transfected with a plasmid containing the cDNA of EP3 receptor. The efficacy of EP3 receptor overexpression is demonstrated at both the translational (Fig. 3B) and transcriptional (Fig. 3D) levels relative to cells transfected with an empty vector. Moreover, this genetic intervention results in a drastic reduction of cAMP formation (Fig. 3E) and IL-6 synthesis (Fig. 3F) in sheared T/C-28a2 chondrocytes. Taken together, our data reveal that shear stress up-regulates and down-regulates EP2 and EP3 receptor expression, respectively, thereby elevating intracellular cAMP levels, which in turn play a key role in the induction of IL-6.
FIGURE 3.
EP2 knockdown and EP3 overexpression reverse shear-induced cAMP formation and IL-6 mRNA synthesis in human chondrocytes. T/C-28a2 chondrocytes were subjected to shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for the indicated periods of time. In select experiments, T/C-28a2 chondrocytes were transfected with an EP2 shRNA plasmid or scramble shRNA control before their exposure to fluid shear. In separate experiments, cells were transfected with either a plasmid containing the cDNA of EP3 receptor or the empty vector prior to shear exposure. EP2 (A) and EP3 (B) receptor protein expression in sheared (20 dyn/cm2 for 1or 2 h) and static control T/C-28a2 chondrocytes is shown by immunoblotting using specific Abs. β-actin was probed as loading control. The Western blot assays are representative of three independent experiments, all revealing similar results. EP2 receptor knockdown (C) and EP3 receptor overexpression (D) were also demonstrated at the transcriptional level by qRT-PCR. Data represent the mean ± S.E. of at least three independent experiments. *, p < 0.05 with respect to scramble shRNA control or empty vector control. cAMP formation (E) and IL-6 mRNA synthesis (F) were determined by a cAMP enzyme immunoassay kit and qRT-PCR, respectively. Data represent the mean ± S.D. of three independent experiments. *, ▴, p < 0.05 with respect to static control and sheared specimens, respectively.
Shear Stress Induces PI3-K and PKA Activation in Human Chondrocytes and Mediates IL-6 Synthesis via a COX-2/EP2/EP3-dependent Pathway
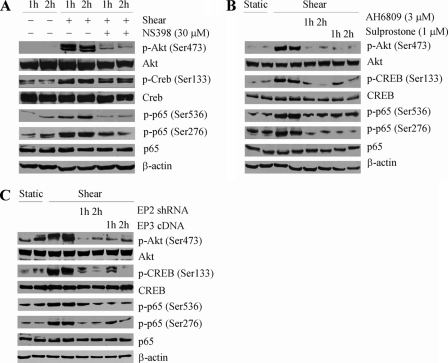
We next aimed to delineate the signaling mechanism of IL-6 up-regulation in sheared chondrocytes. In view of our observations showing that shear-induced cAMP formation precedes IL-6 mRNA expression in human chondrocytes (Fig. 1), we examined the potential contribution of downstream effectors of cAMP activity to this signaling cascade, which include PI3-K and PKA (29, 30). Exposure of T/C-28a2 chondrocytes to high shear stress increases the phosphorylation levels of Akt at Ser-473 and CREB at Ser-133 without affecting total Akt and CREB levels (Fig. 4). The shear-induced Akt and CREB phosphorylation are significantly suppressed by treating T/C-28a2 cells with the selective COX-2 inhibitor NS398 (Fig. 4A), the EP2 receptor antagonist AH6809 or the EP3 receptor agonist sulprostone (Fig. 4B). Along these lines, selective EP2 receptor knockdown or EP3 receptor overexpression (Fig. 4C) reverses shear-induced Akt and CREB phosphorylation to near background levels in T/C-28a2 chondrocytes.
FIGURE 4.
Shear stress induces phosphorylation of Akt, CREB, and p65 in human chondrocytes via a COX-2/EP2/EP3-dependent pathway. T/C-28a2 cells were subjected to shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for 1 or 2 h in the absence or presence of the selective COX-2 inhibitor NS398 (30 μm) (A), the EP2 receptor antagonist AH6809 (3 μm) or the EP3 receptor agonist sulprostone (1 μm) (B). In select experiments, T/C-28a2 chondrocytes were transfected with an EP2 shRNA plasmid or scramble shRNA control before their exposure to fluid shear (C). In separate experiments, cells were transfected with either a plasmid containing the cDNA of EP3 receptor or the empty vector prior to shear exposure (C). Phosphorylated Akt (Ser-473), CREB (Ser-133), and p65 (Ser-536 and Ser-276) are showing by immunoblotting using specific Abs. Equal loading in each lane is ensured by the similar intensities of total Akt, CREB, p65, and β-actin. These Western blots are representative of three independent experiments, all revealing similar results.
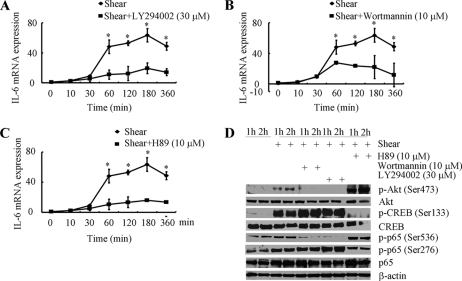
To evaluate the involvement of PI3-K in the regulation of shear-induced IL-6 mRNA synthesis in human chondrocytes, T/C-28a2 cells were treated with the selective PI3-K inhibitors, LY294002 (30 μm) or wortmannin (10 μm). These pharmacological inhibitors abrogate the phosphorylation of Akt at Ser-473 without affecting total Akt levels (Fig. 5D), and markedly attenuate shear-induced IL-6 mRNA synthesis (Fig. 5, A and B). Furthermore, treatment of T/C-28a2 chondrocytes with the PKA inhibitor H89 (10 μm) drastically diminishes the induction of IL-6 mRNA expression in response to high fluid shear stimulation (Fig. 5C). This pharmacological intervention is also effective in suppressing CREB phosphorylation at Ser-133, while it appears to increase Akt phosphorylation at Ser-473 (Fig. 5D), which is in line with a potential crosstalk between PI3-K and PKA (29). Cumulatively, our data suggest that shear stress induces IL-6 mRNA synthesis in human chondrocytes via PKA- and PI3-K/Akt-dependent pathways, which are in turn regulated by a COX-2/EP2/EP3-dependent mechanism.
FIGURE 5.
Involvement of PI3-K and cAMP/PKA signaling pathways in IL-6 mRNA synthesis in shear-activated human chondrocytes. T/C-28a2 cells were subjected to fluid shear stress (20 dyn/cm2) for prescribed periods of time in the absence of presence of the PI3-K inhibitors (LY294002 (30 μm) or wortmannin (10 μm)) or the PKA inhibitor H89 (10 μm). IL-6 mRNA synthesis was determined by qRT-PCR (A–C). GAPDH served as internal control. Data represent the mean ± S.D. of at least three independent experiments. *, p < 0.05 with respect to all pharmacological treatments. D, Western blots showing the effects of the PI3-K inhibitors (LY294002; wortmannin) and PKA inhibitor H89 on shear-mediated phosphorylation of Akt (Ser-473), CREB (Ser-133), and p65 (Ser-536, Ser-276) using specific Abs. Equal loading in each lane is ensured by the similar intensities of total Akt, CREB, p65, and β-actin. These Western blots are representative of three independent experiments, all revealing similar results.
NF-κB p65 Subunit, but Not CREB, Is Involved in Shear-induced IL-6 mRNA Synthesis
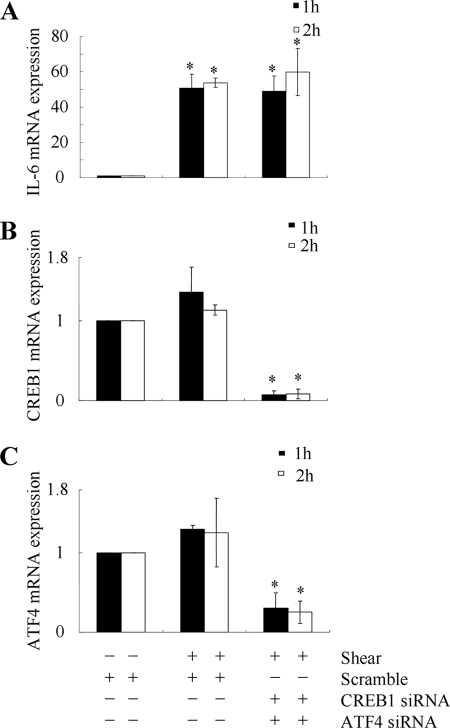
The promoter of the IL-6 gene contains several cis-elements including a cAMP response element (CRE) motif, NF-κB, and AP-1 sites, which have been implicated in the induction of IL-6 in different cell types (9, 31). In light of our observations showing that fluid shear induces CREB phosphorylation (Fig. 4), we assessed the potential contributions of CREB1 and CREB2 (also called ATF4) to IL-6 induction using T/C-28a2 cells transfected with either an siRNA oligonucleotide sequence specific for CREB1 or ATF4, or control siRNA. The efficacy of CREB1 and ATF4 knockdown is documented at the transcript level, whereas the control siRNA fails to alter CREB1 and ATF4 mRNA expression levels (Fig. 6, B and C). Simultaneous knockdown of CREB1 and ATF4 does not impair the extent of shear-induced IL-6 mRNA synthesis (Fig. 6A), suggesting the absence of their functional role in this process.
FIGURE 6.
CREB1 and ATF4 are not involved in shear-induced IL-6 synthesis in human T/C-28a2 chondrocytes. T/C-28a2 cells were transfected with an siRNA oligonucleotide sequence specific for CREB1 or ATF4, or a control siRNA, before being subjected to shear stress (20 dyn/cm2) for the indicated periods of time. (A) IL-6, (B) CREB1, and (C) ATF4 mRNA levels were determined by qRT-PCR. GAPDH served as internal control. Data represent the mean ± S.D. of three independent experiments.
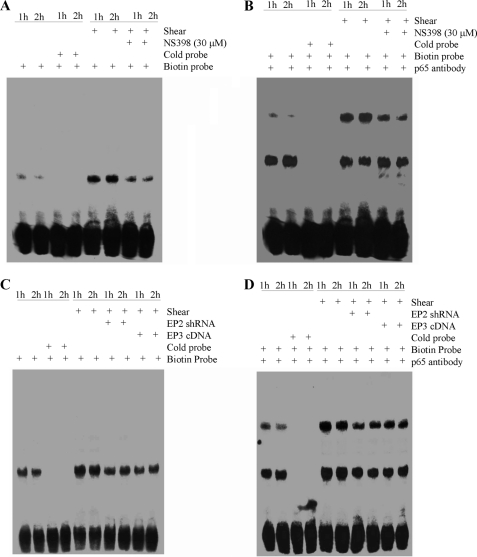
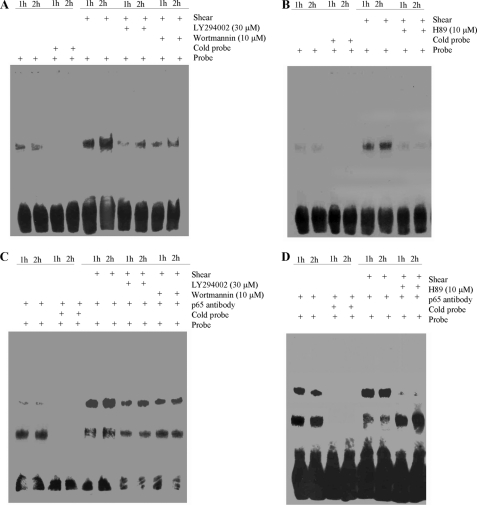
The potential involvement of NF-κB in the induction of IL-6 in shear-activated chondrocytes was first disclosed by gel and supershift assays. Incubation of nuclear extracts from sheared versus untreated T/C-28a2 chondrocytes with the biotinylated NF-κB probe leads to the formation of the NF-κB specific DNA-protein complex (Fig. 7A). Furthermore, incubating nuclear extracts from shear-activated T/C-28a2 cells with an anti-p65 antibody prior to the addition of the biotinylated NF-κB probe results in a marked supershift of the complex (Fig. 7B). Both the formation of the NF-κB-specific DNA-protein complex and the supershift are inhibited by treating T/C-28a2 chondrocytes with the selective COX-2 inhibitor NS398 (Fig. 7, A and B). Similarly, EP2 receptor knockdown or EP3 receptor overexpression are effective in antagonizing the NF-κB-specific gel shift and supershift (Fig. 7, C and D). Moreover, the PI3-K inhibitors LY294002 or wortmannin (Fig. 8, A and C) or the PKA inhibitor H89 (Fig. 8, B and D) exert similar inhibitory effects.
FIGURE 7.
Fluid shear induces NF-kB activation in human chondrocytes via a COX-2/EP2/EP3-dependent pathway. T/C-28a2 cells were subjected to shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for 1 or 2 h in the absence or presence of the COX-2 inhibitor NS398 (30 μm) (A and B). In select experiments, T/C-28a2 chondrocytes were transfected with either an EP2 shRNA plasmid or a plasmid containing the cDNA of EP3 receptor (C and D). Nuclear extracts were then isolated, and NF-κB-specific DNA-protein complex formation was determined by EMSA (A, C). Supershift (B, D) assays using an anti-p65 Ab were carried out as outlined under “Experimental Procedures.” Results of a competition experiment using 50-fold unlabeled NF-κB oligonucleotide (cold probe) are shown. These gels are representative of three independent experiments, all revealing similar results.
FIGURE 8.
Fluid shear differentially modulates the expression of EP2 and EP3 receptors, which in turn activate PKA and PI3-K signaling pathways that mediate NF-κB activation in human chondrocytes. T/C-28a2 cells were subjected to shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for 1 or 2 h in the absence or presence of the PI3-K inhibitors (LY294002 (30 μm) or wortmannin (10 μm)) or the PKA inhibitor H89 (10 μm). Nuclear extracts were prepared for the determination of NF-κB-specific DNA-protein complex formation by EMSA (A, B). Supershift (C, D) assays using an anti-p65 Ab were carried out as outlined under “Experimental Procedures.” Results of a competition experiment using 50-fold unlabeled NF-κB oligonucleotide (cold probe) are shown. These gels are representative of three independent experiments, all revealing similar results.
Application of fluid shear to T/C-28a2 chondrocytes induces p65 phosphorylation at both Ser-536 and Ser-276 without affecting total p65 protein levels (Fig. 4). Treatment of cells with the selective COX-2 inhibitor NS398 (30 μm) diminishes shear-induced p65 phosphorylation at both sites down to basal levels (Fig. 4A). Akin inhibitory effects are noted in sheared T/C-28a2 cells pretreated with either the EP2 receptor antagonist AH6809 or the EP3 receptor agonist sulprostone (Fig. 4B). Similarly, EP2 receptor knockdown or EP3 receptor overexpression inhibits the shear-induced phosphorylation of p65 at both sites. The PI3-K inhibitors, LY294002 (30 μm) or wortmannin (10 μm), nearly abrogate p65 phosphorylation at Ser-536 while leaving intact the phosphorylation at Ser-276 in shear-activated T/C-28a2 chondrocytes (Fig. 5D). It is noteworthy that the PKA inhibitor H89 (10 μm) has the reverse effects on p65 phosphorylation (Fig. 5D).
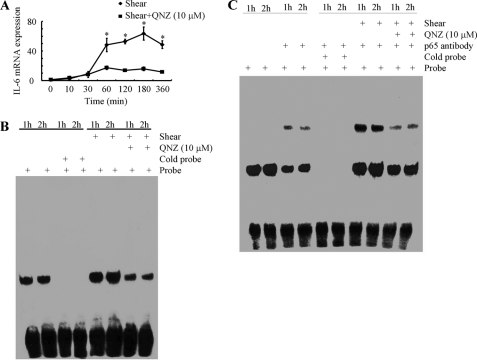
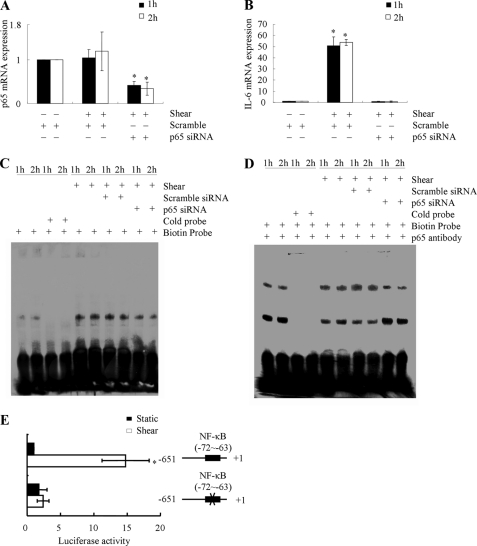
To demonstrate the functional contribution of NF-κB to the shear-induced IL-6 mRNA synthesis in T/C-28a2 chondrocytes, cells were incubated with the NF-κB inhibitor QNZ (10 μm). This treatment significantly diminishes the induction of IL-6 mRNA in shear-activated T/C-28a2 chondrocytes (Fig. 9A), and markedly attenuates the NF-κB-specific gel shift and supershift (Fig. 9, B and C). To confirm the key role of the NF-κB p65 subunit in the regulation of shear-induced IL-6 mRNA expression, experiments were performed using human T/C-28a2 chondrocytes transfected with either an siRNA oligonucleotide sequence specific for p65. This genetic intervention effectively knocks down p65 mRNA expression in sheared T/C-28a2 chondrocytes relative to cells transfected with a scramble siRNA control (Fig. 10A), and abogrates shear-induced IL-6 mRNA synthesis (Fig. 10B). Moreover, p65 knockdown inhibits the formation of the NF-κB specific DNA-protein complex and its supershift (Fig. 10, C and D).
FIGURE 9.
Effect of NF-κB inhibition on shear-induced IL-6 mRNA synthesis in human chondrocytes. T/C-28a2 chondrocytes were subjected to shear stress (20 dyn/cm2) or static conditions (0 dyn/cm2) for indicated time intervals in the absence or presence of the NF-κB specific inhibitor QNZ (10 μm). IL-6 mRNA expression was determined by qRT-PCR (A). GAPDH served as internal control. Data represent the mean ± S.D. of three independent experiments. *, p < 0.05 with respect to QNZ treatment and no treatment control. Gel-shift (B) and supershift (C) experiments using an anti-p65 Ab were carried out as described under “Experimental Procedures.” These gels are representative of three independent experiments, all revealing similar results.
FIGURE 10.
Effect of p65 knockdown on IL-6 mRNA synthesis and promoter activity in shear-activated human chondrocytes. T/C-28a2 chondrocytes were transfected with an siRNA oligonucleotide sequence specific for p65 or an siRNA control, before being sheared (20 dyn/cm2) for 1 or 2 h (A–D). p65 (A) and IL-6 (B) mRNA expression was determined by qRT-PCR. GAPDH served as internal control. Data represent the mean ± S.D. of three independent experiments. *, p < 0.05 with respect to p65 knockdown and controls. Gel-shift (C) and supershift (D) experiments using an anti-p65 Ab were carried out as described under “Experimental Procedures.” These experiments are representative of three independent experiments, all revealing similar results. In select experiments, T/C-28a2 chondrocytes were transfected with the IL-6 promoter reporter construct pIL-6-luc651 or pIL6-luc651 ΔNF-κB before being sheared (20 dyn/cm2) for 1 h (E). Luciferase activities were measured by using the Dual-Luciferase Reporter Assay kit. Data represent the mean ± S.D. of three independent experiments. *, p < 0.05 with respect to pIL6-luc651 ΔNF-κB and vector control.
We next examined the effects of shear stress on IL-6 promoter activity in T/C-28a2 chondrocytes transiently transfected with a construct encompassing the 5′-flanking region of the human IL-6 gene from −651 to +1 bp (−651/+1) cloned into a promoterless luciferase expression vector (15). As shown in Fig. 10E, application of shear stress (20 dyn/cm2 for 60 min) to T/C-28a2 chondrocytes increases the IL-6 promoter activity relative to static controls. Introduction of a mutation into the NF-κB binding site (−72/−63) nearly abrogates the shear-induced luciferase activity relative to the wild-type reporter (Fig. 10E), thereby providing additional evidence for the key role of NF-κB in the regulation of shear-induced IL-6 expression in human T/C-28a2 chondrocytes.
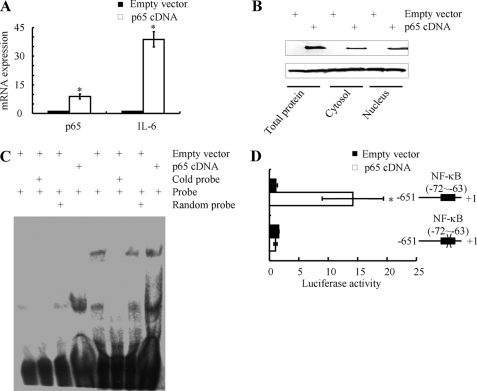
Given the critical role of the NF-κB p65 subunit in induction of IL-6 in shear-activated chondrocytes, we next investigated whether ectopic expression of p65 is sufficient to induce IL-6 mRNA synthesis in statically incubated T/C-28a2 cells. Transfection of T/C-28a2 cells with a plasmid containing the p65 cDNA markedly increases the p65 mRNA (Fig. 11A) and protein levels (Fig. 11B) relative to cells transfected with a vector control. Ectopic expression of p65 also triggers the translocation of p65 from the cytosol to the nucleus in human T/C-28a2 chondrocytes. Moreover, this genetic intervention induces the IL-6 mRNA synthesis (Fig. 11A), the formation of the NF-kB-specific DNA-protein complex and its supershift (Fig. 11C) as well as promoter activity (Fig. 11D).
FIGURE 11.
Effect of p65 ectopic expression on IL-6 mRNA synthesis and promoter activity in statically-incubated human chondrocytes. T/C-28a2 cells were transfected with either a plasmid containing the p65 cDNA or the empty vector. p65 and IL-6 mRNA synthesis was determined by qRT-PCR (A). p65 expression in whole cell lysates, cytosolic and nuclear extracts was measured by Western blotting (B). Gel-shift and supershift (C) experiments using an anti-p65 Ab were carried out as described under “Experimental Procedures.” These experiments are representative of three independent experiments, all revealing similar results. In select experiments, T/C-28a2 chondrocytes were also transfected with the IL-6 promoter reporter construct pIL-6-luc651 or pIL6-luc651 ΔNF-κB (D). Luciferase activities were measured by using the Dual-Luciferase Reporter Assay kit. Data represent the mean ± S.D. of three independent experiments. *, p < 0.05 with respect to pIL6-luc651 ΔNF-κB and vector control.
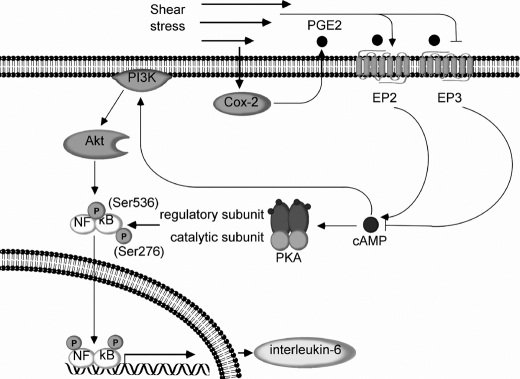
Taken together, these data reveal that high shear stress induces PI3-K and PKA activation in human chondrocytes via COX-2-dependent cAMP production mediated via EP2 receptor up-regulation and EP3 receptor down-regulation (Fig. 12). PI3-K and PKA in turn transactivate the NF-kB p65 subunit via phosphorylation at Ser-536 and Ser-276, respectively (Fig. 12). Binding of p65 to the IL-6 promoter elicits shear-induced IL-6 mRNA synthesis in sheared T/C-28a2 chondrocytes (Fig. 12).
FIGURE 12.
Proposed cascade of signaling events regulating IL-6 synthesis in human chondrocytes stimulated with high shear stress. Elevated levels of fluid shear (20 dyn/cm2) induce COX-2 expression and PGE2 release in human chondrocytes. Shear-induced COX-2 up-regulates EP2 receptor expression and concomitantly diminishes EP3 receptor expression. Shear-induced COX-2-derived PGE2 signals via EP2 and EP3 to stimulate cAMP formation, which in turn up-regulates PI3-K/Akt and PKA activities. PI3-K/Akt and PKA phosphorylate the NF-κB p65 subunit at Ser-536 and Ser-276, respectively, leading to NF-κB activation. Binding of NF-κB p65 subunit to IL-6 promoter induces IL-6 synthesis in human T/C28a2 chondrocytes.
DISCUSSION
Excessive mechanical loading can directly damage the articular cartilage, adversely affect chondrocyte function, and precipitate OA (2). IL-6 expression (4) as well as superinduction of COX-2 accompanied by markedly increased levels of PGE2 release (7) have been detected in OA-affected cartilage and in shear-activated human chondrocytes in vitro (4, 5). In view of the involvement of IL-6 in pain signaling associated with arthritic disorders (8) and the role of mechanical forces in the pathogenesis and progression of OA, we here delineated the signaling pathway of its induction in mechanically stimulated human T/C-28a2 chondrocytes. We demonstrate that shear-induced COX-2-derived PGE2 signals via the EP2 and EP3 receptors to mediate intracellular cAMP accumulation, which in turn regulates PKA- and PI3-K/Akt-dependent NF-κB activation and IL-6 mRNA and protein expression in human T/C-28a2 chondrocytes (Fig. 12).
PGE2 exerts its biological actions by binding to a group of four GPCRs. Li et al. (14) reported that EP2 and EP4 and to a lesser extent EP3 are expressed in human adult articular cartilage. In contrast, Aoyama et al. concluded that EP2 and EP3 are abundantly present in human articular chondrocytes isolated from four individuals primarily of young ages (26). Li et al. (14, 26) attributed their differences to age variations between the cartilage specimens used in these two studies, and speculated that EP3 may be more prevalent in cartilage of a younger age. Our data reveal that human T/C-28a2 chondrocytes express EP2, EP3, and very low levels of EP4, but lack EP1, receptors.
High fluid shear induces EP2 up-regulation and EP3 down-regulation at both mRNA and protein levels. Shear stress does not significantly modulate the expression of EP1 and EP4 receptors in human T/C-28a2 chondrocytes. The differential regulation of EP2 and EP3 receptors mediates the increase in intracellular cAMP levels observed in sheared chondrocytes. Indeed, use of the EP2 receptor antagonist AH6809 or selective EP2 receptor knockdown significantly inhibits cell cAMP accumulation and IL-6 synthesis in human chondrocytes. Use of the EP3 receptor agonist sulprostone or ectopic expression of EP3 leads to similar inhibitory effects on intracellular cAMP and IL-6 expression. Our data further reveal that the EP2/EP3-dependent up-regulation of cell cAMP promotes PKA- and PI3-K/Akt activation, as evidenced by the increased phosphorylated levels of CREB and Akt, respectively. Prior work has suggested that EP4 rather EP2 stimulation preferentially results in the activation of the PI3-K-dependent pathway (30). However, the results of this study disclose that selective EP2 knockdown or EP3 overexpression dramatically attenuates the phosphorylation levels of Akt in sheared chondrocytes. Our data along with previously published results (30) suggest that all EP receptors that are capable of effectively modulating intracellular cAMP levels have the ability to regulate the PI3-K pathway. Their specific contribution to this process may be related to the modulation of their relative expression levels and activity by the application of an external signal.
Numerous studies suggest that PGE2 exerts catabolic effects in articular cartilage (14, 27, 32), as evidenced by the decreased aggrecan synthesis and total proteoglycan accumulation. Attur et al. (27) concluded that the PGE2-mediated proteoglycan degradation and up-regulation of cartilage-degrading enzymes, such as matrix metalloproteinase-13, in human OA chondrocytes occurs via an EP4-dependent/EP2-independent signaling pathway. In contrast, Li et al. (14) reported that EP2 is the major mediator of PGE2-induced suppression of proteoglycan accumulation in articular chondrocytes, which is not accompanied by any significant modulation of MMP activity. Their data (14) are in accord with our results, which suggest that inhibition of the EP receptor signaling may be beneficial to antagonize the catabolic activity of PGE2 in articular cartilage and the IL-6-dependent pain symptoms associated with OA in human joints. In agreement with these observations, Chen et al. (9) recently reported that an EP2 agonist induces IL-6 production in RAW 264.7 macrophages. The dominant role of EP2 has also been documented in the regulation of tumor necrosis factor (TNF)-α-induced IL-6 production in human rheumatoid arthritis activated synovial fibroblasts (RASFs) (33). Although blockade of EP2 activity reduces TNF-α-mediated IL-6 synthesis in human RASFs (33), an EP2 agonist attenuates IL-6 production in IL-1-activated rat synovial cells (34). However, all the aforementioned previously-published results regarding the involvement of distinct EP receptors in the regulation of IL-6 synthesis and matrix degradation need to be interpreted with caution, since they were acquired using rather non-selective pharmacological agents. To validate our findings, we also used genetic interventions to knock down the EP2 receptor via RNAi or overexpress the EP3 receptor. These interventions interfere with intracellular cAMP accumulation, and PKA-/Akt-dependent NF-κB activation and IL-6 synthesis in sheared chondrocytes.
The promoter of the human IL-6 gene contains several cis-elements that can bind diverse trans-acting factors such as CREB and NF-κB, which have been implicated in the induction of IL-6 in other cell types (9, 31). Even though fluid shear up-regulates CREB phosphorylation in T/C-28a2 chondrocytes, knockdown of both CREB1 and CREB2 (ATF4) fails to interfere with the induction of IL-6 synthesis, suggesting the lack of their functional contribution to this process. Shear activation of human T/C-28a2 chondrocytes enhances the formation of NF-κB-specific DNA-protein complex. Furthermore, incubation of nuclear extracts from shear-stimulated T/C-28a2 chondrocytes with an anti-p65 antibody results in a marked supershift of the complex. Use of the selective COX-2 inhibitor significantly reduces the shear-induced NF-κB activation in both gel shift and supershift assays. EP2 receptor knockdown or EP3 receptor overexpression leads to similar inhibitory effects. Akin results are obtained by inhibiting the downstream targets of cAMP, PKA, and PI3-K. Through the use of RNAi, we demonstrated the functional role of the NF-κB p65 subunit in shear-induced IL-6 mRNA synthesis in human T/C-28a2 chondrocytes. In agreement with our previously published results (17), p65 knockdown did not affect the extent of shear-induced COX-2 expression in chondrocytes (data not shown), thereby suggesting that COX-2 is upstream of NF-κB and eliminating the possibility of a feedback mechanism from NF-κB to COX-2. The key role of NF-κB in shear-induced IL-6 transcriptional activity is further demonstrated by the use of wild-type and NF-κB mutated IL-6 promoter-reporter constructs. Interestingly, ectopic expression of p65 in human chondrocytes stimulates IL-6 mRNA synthesis.
The NF-κB p65 subunit is phosphorylated at both Ser-276 and Ser-536 in shear-activated human chondrocytes. Similar observations were made for T/C-28a2 chondrocytes stimulated with exogenously added PGE2 or forskolin (supplemental Fig. S3). The selective COX-2 inhibitor NS398 as well as EP2 knockdown or EP3 overexpression inhibit shear-induced p65 phosphorylation at both sites, suggesting that p65 phosphorylation occurs downstream of COX-2 generated PGE2. Inhibition of PKA by H89 and PI3-K by wortmannin and LY294002 block p65 phosphorylation at Ser-276 and Ser-576, respectively. Each pharmacological inhibitor alone diminishes shear-induced IL-6 synthesis, thereby suggesting that p65 phosphorylation at both Ser-276 and Ser-536 is essential for maximal induction of IL-6 in shear-activated human chondrocytes.
A wide array of pro-inflammatory mediators, including IL-6, IL-1β, and TNF-α, are up-regulated in OA cartilage relative to healthy controls (35). IL-1β and TNF-α are thought to play a pivotal role in cartilage degradation in OA patients, by stimulating chondrocytes and synoviocytes to produce matrix proteases and prostaglandins, among others (35). Accumulating evidence suggests that nearly all pro-inflammatory mediators involved in pathogenesis and progression of OA and rheumatoid arthritis are modulated by NF-κB (36). Thus, NF-κB is an important therapeutic target for arthritic diseases. Indeed, inhibition of NF-κB represses the progression of the early experimental OA (37) and joint swelling in mice with collagen-induced arthritis (38). Our data showing the key role of NF-κB in the induction of IL-6 in shear-activated chondrocytes further underscore its involvement in pain signaling associated with OA.
In conclusion, we demonstrate that high fluid shear regulates IL-6 synthesis in human chondrocytes via COX-2-derived PGE2 signaling that proceeds through an EP2-/EP3-dependent mechanism to upregulate cAMP and mediate PKA- and PI3-K-dependent p65 binding to the IL-6 promoter. Reconstructing the signaling pathway regulating the catabolic responses of chondrocytes induced by excessive shear stress may identify additional potential therapeutic targets for controlling OA pathogenesis and/or progression and may be useful in the design of bioreactors for cartilage tissue engineering applications.
Supplementary Material
This work was supported, in whole or in part, by the National Institutes of Health NIAMS Grant R01 AR053358.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- OA
- osteoarthritis
- COX-2
- cyclooxygenase-2
- PGE2
- prostaglandin E2
- EP
- E prostanoid
- IL-6
- interleukin-6
- PKA
- protein kinase A
- PI3-K
- phosphatidylinositol 3-kinase
- GPCR
- G-protein-coupled receptor
- Gi
- inhibitory G
- Gs
- stimulatory G
- RASFs
- rheumatoid arthritis activated synovial fibroblasts
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- Ab
- antibody.
REFERENCES
- 1.Carter D. R., Beaupré G. S., Wong M., Smith R. L., Andriacchi T. P., Schurman D. J. (2004) Clin. Orthop. Relat. Res. 427, (suppl.), S69–S77 [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter J. A., Martin J. A., Brown T. D. (2006) Biorheology 43, 603–609 [PubMed] [Google Scholar]
- 3.Yokota H., Goldring M. B., Sun H. B. (2003) J. Biol. Chem. 278, 47275–47280 [DOI] [PubMed] [Google Scholar]
- 4.Mohtai M., Gupta M. K., Donlon B., Ellison B., Cooke J., Gibbons G., Schurman D. J., Smith R. L. (1996) J. Ortho. Res. 14, 67–73 [DOI] [PubMed] [Google Scholar]
- 5.Healy Z. R., Lee N. H., Gao X., Goldring M. B., Talalay P., Kensler T. W., Konstantopoulos K. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 14010–14015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu F., Wang P., Kontrogianni-Konstantopoulos A., Konstantopoulos K. (2010) Cell Death Differ. in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin A. R., Attur M., Patel R. N., Thakker G. D., Marshall P. J., Rediske J., Stuchin S. A., Patel I. R., Abramson S. B. (1997) J. Clin. Invest. 99, 1231–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenn D., Richter F., Schaible H. G. (2007) Arthritis Rheum. 56, 351–359 [DOI] [PubMed] [Google Scholar]
- 9.Chen B. C., Liao C. C., Hsu M. J., Liao Y. T., Lin C. C., Sheu J. R., Lin C. H. (2006) J. Immunol. 177, 681–693 [DOI] [PubMed] [Google Scholar]
- 10.Takaoka Y., Niwa S., Nagai H. (1999) J. Biochem. 126, 553–558 [DOI] [PubMed] [Google Scholar]
- 11.Inoue H., Takamori M., Shimoyama Y., Ishibashi H., Yamamoto S., Koshihara Y. (2002) Br. J. Pharmacol. 136, 287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noguchi K., Shitashige M., Endo H., Kondo H., Ishikawa I. (2002) J. Periodontal Res. 37, 29–36 [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto Y., Narumiya S. (2007) J. Biol. Chem. 282, 11613–11617 [DOI] [PubMed] [Google Scholar]
- 14.Li X., Ellman M., Muddasani P., Wang J. H., Cs-Szabo G., van Wijnen A. J., Im H. J. (2009) Arthritis Rheum. 60, 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eickelberg O., Pansky A., Mussmann R., Bihl M., Tamm M., Hildebrand P., Perruchoud A. P., Roth M. (1999) J. Biol. Chem. 274, 12933–12938 [DOI] [PubMed] [Google Scholar]
- 16.Abulencia J. P., Gaspard R., Healy Z. R., Gaarde W. A., Quackenbush J., Konstantopoulos K. (2003) J. Biol. Chem. 278, 28388–28394 [DOI] [PubMed] [Google Scholar]
- 17.Healy Z. R., Zhu F., Stull J. D., Konstantopoulos K. (2008) Am. J. Physiol. Cell Physiol 294, C1146–C1157 [DOI] [PubMed] [Google Scholar]
- 18.Wang P., Zhu F., Konstantopoulos K. (2010) Am. J. Physiol. Cell Physiol. 298, C1445–C1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldring M. B., Birkhead J. R., Suen L. F., Yamin R., Mizuno S., Glowacki J., Arbiser J. L., Apperley J. F. (1994) J. Clin. Invest. 94, 2307–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldring M. B. (2004) Methods Mol. Med. 100, 37–52 [DOI] [PubMed] [Google Scholar]
- 21.Papadaki M., Eskin S. G. (1997) Biotechnol. Prog. 13, 209–221 [DOI] [PubMed] [Google Scholar]
- 22.Zhu F., Massana R., Not F., Marie D., Vaulot D. (2005) FEMS Microbiol. Ecol. 52, 79–92 [DOI] [PubMed] [Google Scholar]
- 23.Martin J. A., Buckwalter J. A. (2006) Biorheology 43, 517–521 [PubMed] [Google Scholar]
- 24.Anderson G. D., Hauser S. D., McGarity K. L., Bremer M. E., Isakson P. C., Gregory S. A. (1996) J. Clin. Invest. 97, 2672–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinson R. M., Williams J. A., Shacter E. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 4885–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoyama T., Liang B., Okamoto T., Matsusaki T., Nishijo K., Ishibe T., Yasura K., Nagayama S., Nakayama T., Nakamura T., Toguchida J. (2005) J. Bone Miner Res. 20, 377–389 [DOI] [PubMed] [Google Scholar]
- 27.Attur M., Al-Mussawir H. E., Patel J., Kitay A., Dave M., Palmer G., Pillinger M. H., Abramson S. B. (2008) J. Immunol. 181, 5082–5088 [DOI] [PubMed] [Google Scholar]
- 28.Chan C. L., Jones R. L., Lau H. Y. (2000) Br. J. Pharmacol. 129, 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciullo I., Diez-Roux G., Di Domenico M., Migliaccio A., Avvedimento E. V. (2001) Oncogene 20, 1186–1192 [DOI] [PubMed] [Google Scholar]
- 30.Fujino H., West K. A., Regan J. W. (2002) J. Biol. Chem. 277, 2614–2619 [DOI] [PubMed] [Google Scholar]
- 31.Grassl C., Luckow B., Schlöndorff D., Dendorfer U. (1999) J. Am. Soc. Nephrol. 10, 1466–1477 [DOI] [PubMed] [Google Scholar]
- 32.Notoya K., Jovanovic D. V., Reboul P., Martel-Pelletier J., Mineau F., Pelletier J. P. (2000) J. Immunol. 165, 3402–3410 [DOI] [PubMed] [Google Scholar]
- 33.Kunisch E., Jansen A., Kojima F., Löffler I., Kapoor M., Kawai S., Rubio I., Crofford L. J., Kinne R. W. (2009) J. Immunol. 183, 1328–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurihara Y., Endo H., Akahoshi T., Kondo H. (2001) Clin. Exp. Immunol. 123, 323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martel-Pelletier J. (2004) Osteoarthritis Cartilage 12, Suppl. A, S31–S33 [DOI] [PubMed] [Google Scholar]
- 36.Tak P. P., Firestein G. S. (2001) J. Clin. Invest. 107, 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L. X., Lin L., Wang H. J., Wei X. L., Fu X., Zhang J. Y., Yu C. L. (2008) Osteoarthritis Cartilage 16, 174–184 [DOI] [PubMed] [Google Scholar]
- 38.Gerlag D. M., Ransone L., Tak P. P., Han Z., Palanki M., Barbosa M. S., Boyle D., Manning A. M., Firestein G. S. (2000) J. Immunol. 165, 1652–1658 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.