Abstract
Agonists such as those acting at muscarinic receptors are thought to induce contraction of smooth muscle primarily through inositol 1,4,5-trisphosphate production and release of Ca2+ from sarcoplasmic reticulum. However, the additional Ca2+-mobilizing messengers cyclic adenosine diphosphate ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) may also be involved in this process, the former acting on the sarcoplasmic reticulum, the latter acting on lysosome-related organelles. In this study, we provide the first systematic analysis of the capacity of inositol 1,4,5-trisphosphate, cADPR, and NAADP to cause contraction in smooth muscle. Using permeabilized guinea pig detrusor and taenia caecum, we show that all three Ca2+-mobilizing messengers cause contractions in both types of smooth muscle. We demonstrate that cADPR and NAADP play differential roles in mediating contraction in response to muscarinic receptor activation, with a sizeable role for NAADP and acidic calcium stores in detrusor muscle but not in taenia caecum, underscoring the heterogeneity of smooth muscle signal transduction systems. Two-pore channel proteins (TPCs) have recently been shown to be key components of the NAADP receptor. We show that contractile responses to NAADP were completely abolished, and agonist-evoked contractions were reduced and now became independent of acidic calcium stores in Tpcn2−/− mouse detrusor smooth muscle. Our findings provide the first evidence that TPC proteins mediate a key NAADP-regulated tissue response brought about by agonist activation of a cell surface receptor.
Keywords: Calcium Intracellular Release, Inositol Phosphates, Lysosomes, Ryanodine, Smooth Muscle, NAADP, Cyclic ADP-ribose
Introduction
Studies of pharmacomechanical coupling in smooth muscle have generally emphasized the role of inositol 1,4,5-trisphosphate (IP3)2 production and release of Ca2+ from the sarcoplasmic reticulum (1, 2). However, recent studies suggest that the two other major intracellular Ca2+-mobilizing messengers cyclic adenosine diphosphate ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) may also play a role in smooth muscle contraction (3–13). Although the former acts on the sarcoplasmic reticulum (SR), likely by activating ryanodine receptors (RyRs) (5, 14), the latter appears to target Ca2+ storing lysosome-related organelles (15). Agonists stimulating NAADP production in smooth muscle cells include the potent vasoconstrictor, endothelin-1 (7, 9, 10, 12, 16), and the hormone of parturition, oxytocin (13). The nature of the Ca2+ release mechanism activated by NAADP has recently been the subject of much debate (17, 18). However, a novel class of endolysosomal channels, the two-pore channels (TPCs), have recently been proposed as major candidates for a family of NAADP-regulated Ca2+ release channels (19–23). Whether these channels play a role in Ca2+ signal transduction pathways for receptors coupled to NAADP production remains an important question (24). In this study, we have carried out the first systematic comparison of IP3, cADPR, and NAADP as mediators of contractile responses in guinea pig bladder detrusor and taenia caecum smooth muscles permeabilized by β-escin. In detrusor muscle, we report that activation of muscarinic receptors, the major physiological mechanism for emptying the urinary bladder, evokes contractile responses through release of Ca2+ from both the SR and the acidic stores, with an increasing dependence of the contractile response on acidic stores with increasing carbachol concentrations. In addition, for the first time, we have studied the role played by TPC proteins in smooth muscle contraction using muscles derived from Tpcn2−/− mice. We show that NAADP-evoked contractions are abolished in permeabilized detrusor muscle from Tpcn2−/− mice, and in the absence of TPC2 expression, muscarinic receptors no longer couple to Ca2+ release from acidic stores.
MATERIALS AND METHODS
Tissue Preparation
Male guinea pigs (350–400 g) and mice (wild-type and Tpcn2−/−) were used. Tpcn2−/− mice were developed from an embryonic stem cell line (YHD437) containing a gene trap mutation in the Tpcn2 gene (19). Animals were killed by dislocation of the neck. The bladder and taenia caecum from guinea pigs and the bladder from mice were isolated. Tissues were placed in Hepes-buffered modified Krebs solution. The mucosa and connective tissues were removed from the bladders under a dissecting microscope. Small strips (150–250 μm in diameter, 3–4 mm in length) of smooth muscle were dissected from bladder detrusor and taenia caecum. A small hook was tied to one end of a strip to attach it to the transducer, and a snare of 5/0 surgical silk captured the other end and was used to mount the strip in a fixed position in a 500-μl chamber in one of a series of small chambers in a Perspex block. The chamber was filled with Hepes-buffered modified Krebs solution at room temperature, and the strips were equilibrated for 30 min under a resting tension of 100 mg. Solution changes were made by moving the Perspex block. After stable responses had been achieved to 80 mm K+ and 50 μm carbachol in intact strips, they were moved into relaxing solution (see below) and incubated for a few minutes. Then, the tissues were permeabilized with β-escin (80 μm for guinea pig and 100 μm for mouse) in relaxing solution for 30 min at pH 6.8. This was followed by a 4-min wash in relaxing solution before beginning an experiment (25). Muscle fibers were accepted as permeabilized if the maximum tension obtainable by 10−4 m Ca2+ after permeabilization was found to be greater than the tension produced by 80 mm K+ applied in the same strip before permeabilization (26). The contractile force was measured by a sensitive force transducer (ADInstruments) connected to an Apple Macintosh PowerBook 1400c computer using Chart software and MacLab 8 hardware.
Drugs and Solutions
All drugs and solutions were prepared by using 18 megaohms/cm of deionized water (PURELAB UHQ, USF ELGA). Normal tissue Hepes-buffered modified Krebs solution contained (in mm) 126 NaCl; 6 KCl; 2 CaCl2; 1.2 MgCl2; 14 glucose, and 10.5 HEPES. The pH of this Krebs solution was adjusted to 7.2 with NaOH. 80 mm K+ Krebs solution was prepared by replacing NaCl with an equivalent amount of KCl. Permeabilized tissues relaxing solution contained (in mm) 130 potassium propionate; 4 MgCl2; 4 Na2-ATP; 20 Tris-maleate; 10 creatine phosphate; 4 EGTA with creatine phosphokinase (3.3 units/ml) and protease inhibitor leupeptin (1 μm). The pH of this solution was adjusted to 6.8 with KOH. Activating solutions were the same as relaxing solution except that EGTA was lowered to 0.05 mm, free Ca2+ concentration was adjusted to the desired value, and 1 μm calmodulin added as specified to compensate loss through β-escin permeabilization and is required for enhanced Ca2+ uptake into the SR and as a regulator of contractile activity. GTP (100 μm) was also added when carbachol was used as a stimulus as this is a necessary component for the G protein-coupled transduction pathway downstream of carbachol binding to its receptor. In experiments with cumulative Ca2+-response curves, EGTA in the activating solution was kept at 0.05 mm. The free Ca2+ concentration was calculated using a computer program (“Bound and Determined”) (27) and confirmed by measuring the fluorescence of fluo-4FF (Invitrogen) in the solution using the equation [Ca2+]free = Kd ((F − Fmin)/(Fmax − F)) and following the manufacturer's parameters. The free Ca2+ concentration is expressed as the negative logarithm (pCa). When Ca2+-response curves were elicited in the presence of an agonist or an antagonist, the substance was added to the Ca2+ solution first, and then the pH was adjusted to 6.8. When drugs were added to an organ chamber, they were made up in relaxing solution containing 0.05 mm EGTA, and the concentration given is the estimated final concentration. In the experiments with intracellular Ca2+ inhibitors, the strips were incubated with these substances for 15 min in relaxing solution, and then contraction was elicited. The exception to this was the high concentration of NAADP (100 μm) used to desensitize the receptors, which was applied to the bath for only 3 min before eliciting a response by a contractile agent. Thapsigargin and ryanodine were dissolved in ethanol, and their vehicle (0.5% of bathing solution) was checked to show that it did not affect the contractions when tested alone.
Drugs used were β-escin (aescin), cADPR, NAADP, IP3, carbamylcholine chloride (carbachol), creatine phosphokinase, leupeptin, EGTA, Na2-ATP, thapsigargin, ryanodine, heparin sodium salt, GTP, calmodulin from Sigma; creatine phosphate disodium salt and bafilomycin A1 from Calbiochem (Nottingham, UK); and 8-NH2-cADPR from Molecular Probes.
IP3-, cADPR-, and NAADP-induced Contractions
After permeabilization, the preparations were relaxed by exposure to the relaxing solution for 4 min. Intracellular Ca2+ stores were loaded by exposure to activating solution at pCa ∼6 for 10 min. Then, a contractile response was elicited by IP3 (50 μm), cADPR (50 μm), NAADP (1 μm), or cADPR + NAADP. Contractile responses to IP3, cADPR, and NAADP were also obtained in the presence of various inhibitors such as IP3 inhibitor heparin (1 mg/ml), SR depletors, thapsigargin (5 μm) + ryanodine (100 μm), cADPR inhibitor 8-NH2-cADPR (30 μm), NAADP inhibitors bafilomycin (100 nm), and a high concentration of NAADP (100 μm).
cADPR and NAADP Concentration-dependent Contractions
After permeabilization, the preparations were relaxed by exposure to the relaxing solution for 4 min. Intracellular Ca2+ stores were loaded by exposure to activating solution at pCa ∼6 for 10 min. Then, concentration-response curves were elicited by either cADPR (25–100 μm) or NAADP (200 nm-5 μm), each concentration being applied in another tissue.
Carbachol-induced Contractions
After permeabilization, the preparations were relaxed by exposure to the relaxing solution for 4 min. Intracellular Ca2+ stores were loaded by exposure to activating solution at pCa ∼6 for 10 min. Then, a contractile response was elicited by carbachol (50 μm) in the presence of GTP (100 μm). Carbachol responses were then obtained in the presence of thapsigargin (5 μm) + ryanodine (100 μm), thapsigargin (5 μm) + ryanodine (100 μm) + bafilomycin (100 nm), bafilomycin (100 nm) alone, and a high concentration of NAADP (100 μm).
Gene Expression Studies
Total RNA was extracted from bladders of wild-type and Tpcn2−/− mice following an RNeasy QiaRNA extraction procedure including an on-column DNase digestion step (Qiagen). Reverse transcription-PCR was performed in a reaction containing RNA, the SuperScript II one-step reverse transcription-PCR system with Platinum Taq (Invitrogen), and the following gene-specific primers: for Tpcn1, exon 16–23 amplicon (sense: 5′-TACTTGGTGGTGGCTGTCAA-3′; antisense: 5′-GACAACGGTGAGCTCAAACA-3′), and for Tpcn2, exon 9–14 amplicon (sense: 5′-TATACTCAGAACCGGGCCTTT-3′; antisense: 5′-AGACAAGAGGTTTCCCAGAGC-3′) following the manufacturer's instructions. The following parameters were used for reverse transcription: 50 °C (30 min), 94 °C (2 min), and for PCR: 30 cycles of 94 °C (15 s), 57 °C Tpcn1, 59 °C Tpcn2 (30 s), and 68 °C (1 min) followed by a final extension at 68 °C (10 min).
Data Analysis
Contractions are expressed as the percentage of the response to 80 mm K+ elicited in intact tissues before permeabilization, that is 243.0 ± 16.6 mg (n = 77) in guinea pig taenia caecum; 147.2 ± 9.2 mg (n = 101) in guinea pig detrusor; 110.1 ± 6.4 mg (n = 54) in wild-type mice detrusor; and 112.0 ± 8.4 mg (n = 45) in Tpcn2−/− mice detrusor. Data were given as mean ± S.E. of n experiments. Statistical analysis was performed by using one-way analysis of variance followed by a Bonferroni test for comparing multiple groups. A Student's t test was used for comparing two groups. p < 0.05 was accepted as statistically significant.
RESULTS
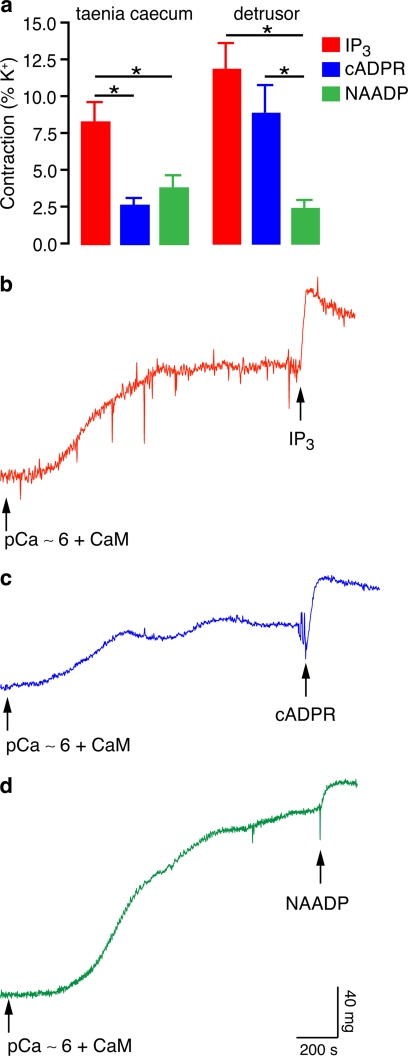
To carry out a systematic comparison of the effects of the SR Ca2+-mobilizing messengers, IP3 and cADPR, with the new messenger, NAADP, upon contraction in guinea pig detrusor and taenia caecum smooth muscles, we used tissues permeabilized with β-escin (28). After intracellular stores were loaded with pCa ∼6 in the presence of calmodulin, we tested the effect of the three Ca2+-mobilizing messengers. We first tried to compare the three Ca2+-mobilizing agents at a constant concentration of 50 μm, but we observed that this concentration of NAADP causes self-inhibition (data not shown), in line with previous observations (3, 29, 30). Therefore, we used 1 μm NAADP, which is a submaximal concentration, and 50 μm cADPR to compare these responses with 50 μm IP3. IP3 (50 μm), cADPR (50 μm), and NAADP (1 μm) were added, and all were found to induce a contraction in both tissues (Fig. 1). IP3 induced the largest contractions in both tissues, whereas the cADPR contractile response was greater than that to NAADP in detrusor but not in taenia caecum. All of these effects were abolished in the presence of EGTA (4 mm).
FIGURE 1.
IP3, cADPR, and NAADP all mediate smooth muscle contraction in the guinea pig taenia caecum and detrusor. a, IP3 (50 μm), cADPR (50 μm), and NAADP (1 μm) induced contraction in both tissues after the strips were loaded with pCa ∼6 (n = 7–13). Error bars indicate S.E. b–d, original traces showing the increase in tension by IP3 (50 μm) in detrusor (b), by cADPR (50 μm) in detrusor (c), and by NAADP (1 μm) in taenia caecum (d). *, p < 0.05. CaM, calmodulin.
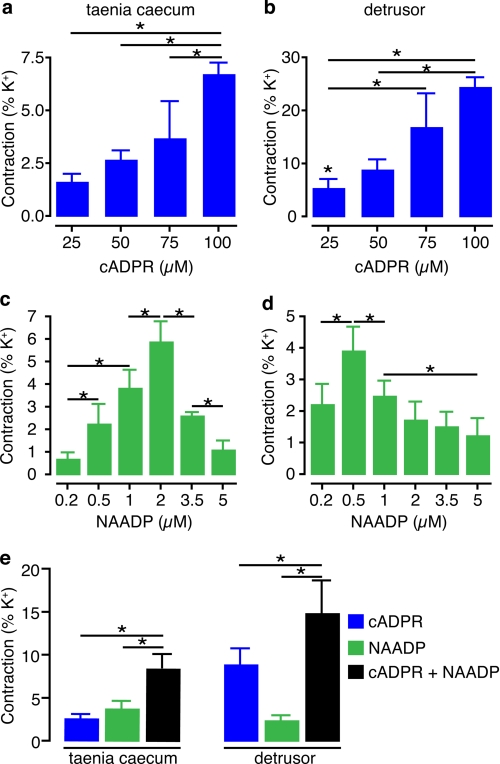
We next determined concentration-response curves for cADPR and NAADP using this system. When the strips were loaded with pCa ∼6, cADPR (25–100 μm) and NAADP (0.2–5 μm) induced concentration-dependent contraction both in taenia caecum and in detrusor (Fig. 2, a–d). The maximum cADPR contractions obtained at 100 μm were 6.8 ± 0.5% of that induced by K+ (80 mm) in the taenia caecum muscle strips prior to permeabilization, and 24.6 ± 1.6% in detrusor strips. NAADP evoked a maximum response at 2 μm (5.9 ± 0.8%) in taenia caecum and at 500 nm (3.9 ± 0.7%) in detrusor, showing the hallmark bell-shaped concentration-response relationship for this messenger. We also studied the effect of adding cADPR and NAADP together. When cADPR (50 μm) and NAADP (1 μm) were applied together to strips loaded with pCa ∼6, they induced a contraction (Fig. 2e) (p < 0.05) that was broadly additive.
FIGURE 2.
Concentration-dependent contractions evoked by cADPR and NAADP in guinea pig taenia caecum and detrusor. a–d, concentration-dependent contractions obtained by increasing concentrations of cADPR (25–100 μm) in taenia caecum (a) and in detrusor (b) and of NAADP (200 nm–5 μm) in taenia caecum (c) and in detrusor (d) after the permeabilized strips were loaded with calcium (pCa ∼6) (n = 4–13). e, effects of cADPR (50 μm) and NAADP (1 μm) applied together after permeabilized strips were loaded with pCa6 (n = 7–13). *, p < 0.05. Error bars indicate S.E
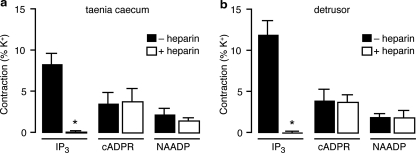
We also investigated the pharmacology of the responses to the three different Ca2+-mobilizing messengers in guinea pig detrusor. After intracellular stores were loaded with pCa ∼6, we found that IP3 inhibitor heparin (1 mg/ml) blocked contractions by IP3 (50 μm) but not by cADPR (50 μm) or NAADP (1 μm), both in taenia caecum and in detrusor (Fig. 3). Depleting sarcoplasmic reticulum Ca2+ stores with thapsigargin (5 μm) and ryanodine (100 μm) abolished contraction responses to both cADPR (50 μm) and IP3 (50 μm), but not to NAADP (1 μm), in both tissues (supplementa Table 1). In contrast, NAADP (1–2 μm) induced-contractions were reduced by high concentrations of NAADP (100 μm), which alone evoked no contractile responses, and bafilomycin A1 (100 nm), a vacuolar H+-ATPase inhibitor, which abrogates Ca2+ storage in acidic stores, in both tissues (supplemental Table 2).
FIGURE 3.
Heparin selectively blocked IP3 but not cADPR- or NAADP-evoked contractions in guinea pig taenia caecum and detrusor. IP3 inhibitor heparin (1 mg/ml) blocked contractions by IP3 (50 μm) but not by cADPR (50 μm) or NAADP (1 μm) in taenia caecum (a) and in detrusor (b) (n = 4–13). *, p < 0.05 when compared with its corresponding control response in the absence of heparin. Error bars indicate S.E.
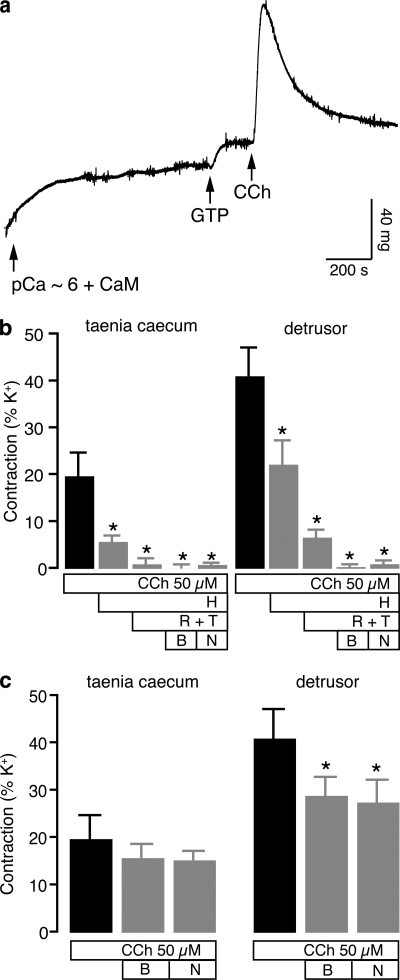
We next investigated the effect of these inhibitors on contractions induced by the muscarinic agonist, carbachol. We found that carbachol (50 μm) in the presence of GTP (100 μm) induced a contraction in both tissues after loading permeabilized strips with pCa ∼6 (Fig. 4a). This response was reduced only partially by heparin (1 mg/ml) in both tissues. The addition of sarcoplasmic reticulum depletors thapsigargin (5 μm) and ryanodine (100 μm) completely abolished the carbachol response in taenia (Fig. 4b). However, bafilomycin (100 nm) or NAADP (100 μm) in the presence of heparin (1 mg/ml), thapsigargin (5 μm), and ryanodine (100 μm) were necessary to completely abolish the carbachol contraction in detrusor (Fig. 4b). Furthermore, bafilomycin (100 nm) or desensitizing concentrations of NAADP (100 μm) applied alone decreased carbachol contractions in bladder detrusor (p < 0.05) but not in taenia caecum (p > 0.05; Fig. 4c).
FIGURE 4.
Carbachol-evoked contractions in guinea pig taenia caecum and detrusor. a, an original trace showing the increase in tension by pCa ∼6 and then by carbachol (CCh; 50 μm) in the presence of GTP (100 μm) in taenia caecum. b, the additive effects of inhibitors heparin (H; 1 mg/ml), thapsigargin (T; 5 μm), ryanodine (R; 100 μm), bafilomycin (B; 100 nm), and NAADP (N; 100 μm) on CCh (50 μm) contractions in taenia caecum and in detrusor. c, the effects of bafilomycin (100 nm) and NAADP (100 μm) alone on CCh (50 μm) contractions in both tissues. *, p < 0.05 when compared with carbachol control response (n = 4–10). Error bars indicate S.E.
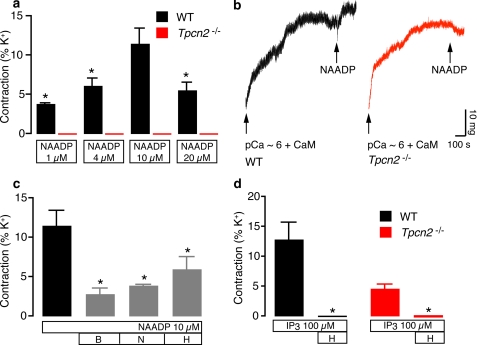
Recently evidence has emerged that the TPC proteins are endolysosomal Ca2+ channels with key roles in the NAADP response (20). We showed that expression of recombinant mammalian TPC1 and TPC2 led to an enhanced NAADP-induced Ca2+ response (19, 20), a finding subsequently confirmed by two other studies (21, 22), and that the Ca2+ response to NAADP was abolished in pancreatic beta cells from Tpcn2−/− mice (19). However, it still remains to be shown whether physiological responses in response to an agonist are abnormal in such knock-out mice. In this study, we investigated whether smooth muscle contraction in the bladder detrusor was affected in Tpcn2−/− mice. PCR analysis shows that mRNA for both TPC1 and TPC2 is expressed in wild-type detrusor muscle, whereas only TPC1 is expressed in detrusor tissue from Tpcn2−/− mice (supplemental Fig. 1). Because receptor-coupled Ca2+ mobilization from acidic stores occurs only in guinea pig detrusor muscle (Fig. 4c), we focused on this preparation in mouse. After intracellular stores were loaded with pCa ∼6, NAADP induced concentration-dependent contractions in wild-type mice with a maximum at 10 μm, but none of these concentrations caused contractions of Tpcn2−/− detrusor (Fig. 5, a and b). NAADP- (10 μm) induced contraction in wild-type mice detrusor was inhibited by bafilomycin (100 nm) and NAADP (100 μm) in different ratios but also reduced by heparin (1 mg/ml) (p < 0.05, Fig. 5c). Contractions evoked by IP3 (100 μm) were completely blocked by heparin (1 mg/ml) but were significantly smaller in Tpcn2−/− detrusor muscle when compared with wild-type (Fig. 5d).
FIGURE 5.
Abolition of NAADP-evoked responses in Tpcn2−/− mouse detrusor muscle. a, NAADP induced a concentration dependent contraction in wild-type (WT) but not in Tpcn2−/− mouse detrusor (n = 4–11,*, p < 0.05 when compared with 10 μm NAADP). b, original tracings showing NAADP (10 μm) response in wild-type and Tpcn2−/− mice detrusor. c, the inhibition in NAADP- (10 μm) induced contraction (control) by heparin (H; 1 mg/ml), bafilomycin (B; 100 nm) and NAADP (N; 100 μm) in wild-type mice detrusor. *, p < 0.05 when compared with NAADP control response (n = 4–11). d, IP3 (100 μm) induced a contraction (control) in both wild-type and Tpcn2−/− mouse detrusor that was abolished by heparin (1 mg/ml). *, p < 0.05 when compared with IP3 control response (n = 3–13). Error bars in a, c, and d indicate S.E.
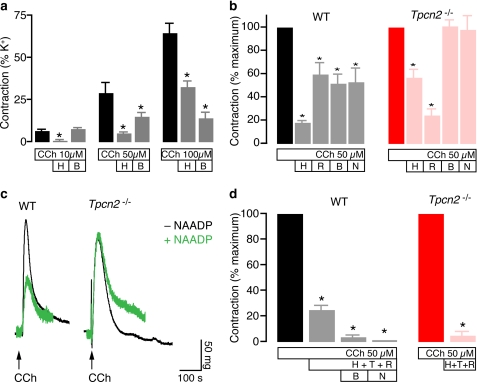
We also studied the response to the agonist carbachol in wild-type and knock-out animals. Carbachol (10–100 μm) in the presence of GTP (100 μm) induced robust contractions in wild-type mice detrusor after loading permeabilized strips with pCa ∼6 (Fig. 6a). The IP3 inhibitor heparin (1 mg/ml) abolished a 10 μm carbachol contraction but only partially reduced the 50 and 100 μm carbachol responses. However, bafilomycin (100 nm) did not affect 10 μm carbachol contraction but progressively inhibited a greater proportion of the contractile responses as agonist concentrations were raised (Fig. 6a). Indeed, a sizeable fraction of the contractile response, around 50%, was dependent on functional acidic stores with carbachol concentrations of 50 μm and above (Fig. 6, a and b).
FIGURE 6.
Carbachol-evoked contractions in Tpcn2−/− mouse detrusor muscle. a, the effects of heparin (H; 1 mg/ml) and bafilomycin (B; 100 nm) on contractions induced by increasing concentrations of CCh (10/50/100 μm) in wild-type mice detrusor. *, p < 0.05 when compared with matching control response in each concentration group (n = 4–14). b, the effects of heparin (1 mg/ml), ryanodine (R; 100 μm), bafilomycin (100 nm), and NAADP (N; 100 μm) on CCh- (50 μm) induced contractions in both wild-type (WT) and Tpcn2−/− mouse detrusor. Values were normalized against control carbachol-induced contractions in each group. *, p < 0.05 when compared with matching control response in each group (n = 5–14). c, original tracings showing the effect of NAADP (100 μm) on CCh (100 μm) response in detrusor strips from wild-type and Tpcn2−/− mice. d, the additive effects of inhibitors heparin (1 mg/ml), thapsigargin (T; 5 μm), ryanodine (100 μm), bafilomycin (100 nm), and NAADP (100 μm) on CCh (50 μm) contractions in both wild-type and Tpcn2−/− detrusor (c). *, p < 0.05 when compared with carbachol control response (n = 4–14). Error bars in a–d indicate S.E.
Carbachol (50 μm)-evoked contractions in both wild-type and Tpcn2−/− mice were reduced by both heparin (1 mg/ml) and ryanodine (100 μm) (p < 0.05, Fig. 6b). However, bafilomycin (100 nm) or a desensitizing NAADP concentration (100 μm) decreased carbachol contractions in wild-type mice detrusor (p < 0.05) but not in Tpcn2−/− mice (p > 0.05; Fig. 6, b and c). When sarcoplasmic reticulum depletors thapsigargin (5 μm) and ryanodine (100 μm) and IP3 inhibitor heparin (1 mg/ml) were applied together, a residual carbachol response in wild-type mice detrusor could be observed (p < 0.05), whereas the responses in Tpcn2−/− mice were abolished ((p < 0.05, Fig. 6d). The addition of bafilomycin (100 nm) or NAADP (100 μm) in the presence of heparin (1 mg/ml), thapsigargin (5 μm), and ryanodine was necessary to completely abolish the carbachol contraction in wild-type mice detrusor (Fig. 6d). In detrusor muscle from Tpcn2−/− mice, ryanodine inhibited a greater proportion of the contractile response evoked by carbachol (Fig. 6b), possibly indicating a compensatory increase in RyR expression or sensitivity when TPC2 channels are ablated.
DISCUSSION
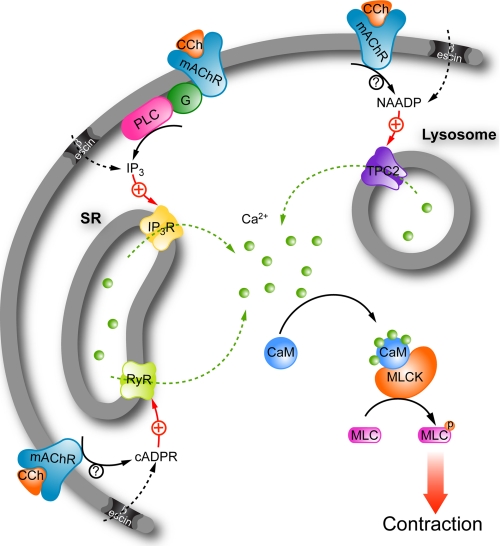
In this report, we have carried out the first systematic study of the role of the three Ca2+-mobilizing messengers, IP3, cADPR, and NAADP, in smooth muscle. We show for the first time that cADPR and NAADP have differential roles in Ca2+ signaling in guinea pig and mouse taenia caecum and detrusor (Fig. 7). Although IP3 and cADPR are important in carbachol-induced contractions in taenia caecum, NAADP is involved only in detrusor receptor mechanisms; thus, key differences in Ca2+-signaling mechanisms are apparent between two different smooth muscle types and may be important in determining heterogeneity of function of various types of smooth muscle.
FIGURE 7.
Schematic diagram of receptor-mediated Ca2+-signaling pathways in mouse detrusor muscle. CCh activation of muscarinic acetylcholine receptors (mAChRs) stimulates contraction of detrusor muscle by evoking Ca2+ release from both SR and acidic (lysosomal) stores. Ca2+ binds to calmodulin (CaM), which then activates myosin-light chain kinase (MLCK), which phosphorylates myosin light chains (MLCs), promoting contraction. The three principal Ca2+-mobilizing messengers, IP3, cADPR, and NAADP may all stimulate contractile responses in β-escin permeabilized myocytes, with IP3 and cADPR mobilizing Ca2+ from the SR, by activating IP3 receptors and RyRs, respectively, and NAADP from acidic stores by activating TPC2 channels. IP3 production is stimulated by mAChR via G proteins (G) coupling to phospholipase C (PLC), whereas cADPR and NAADP synthesis may be controlled by ADP-ribosyl cyclases such as CD38. mAChRs require the expression of TPC2 and action of NAADP for coupling to Ca2+ release from acidic stores (lysosomes). Greater mAChR stimulation was required to effect coupling to Ca2+ release from acidic stores when compared with the SR, raising the possibility of weaker coupling to NAADP synthesis or selective coupling of a lower affinity mAChR subtype to NAADP synthases.
That NAADP was significantly more potent than either IP3 or cADPR and that NAADP contractile responses resembled a bell-shaped curve in both tissues is in line with previous observations in mammalian cells such as pancreatic cells and human T-lymphocytes (29–32). The fact that the effect of adding both cADPR and NAADP is broadly additive suggests mobilization of Ca2+ from distinct stores.
Our findings regarding the effects of adding pharmacological agents that block either the endoplasmic reticulum or acidic Ca2+ stores support the two-pool model, which suggests that localized NAADP-induced signals persist in the presence of IP3 and ryanodine antagonists (33–35). In addition, our finding that NAADP-induced contractions were abolished by the vacuolar H+ pump inhibitor bafilomycin in both tissues supports the idea that the origin of the NAADP response is an acidic store (7, 15, 35). The contractile response obtained by carbachol in the presence of GTP was reduced only partially by the IP3 inhibitor heparin in both tissues, suggesting that carbachol contractions are not entirely mediated by IP3. Thapsigargin and ryanodine added to the bath medium were able to abolish carbachol contractions in taenia caecum, suggesting a lack of role for NAADP or acidic stores in mediating muscarinic responses in this tissue as reported for rabbit airway muscle (36). However, in detrusor smooth muscle, the combination of sarcoplasmic reticulum depletors with bafilomycin was necessary to completely abolish the carbachol contraction. This finding shows for the first time that endoplasmic reticulum Ca2+ stores regulated by IP3 and cADPR and acidic Ca2+ stores regulated by NAADP may play differential roles in mediating physiological responses in two smooth muscle tissues of the same organ in response to agonist stimulation with a particular role for NAADP-sensitive acidic stores in detrusor muscle.
A major finding of this study is that using mice defective in expression of the two-pore channel TPC2, we show that TPC2 is required for NAADP-mediated contractility and also mediates a proportion of the response to the agonist carbachol. Our finding that contraction of permeabilized wild-type mouse detrusor was reduced by heparin, as well as inhibited by bafilomycin and NAADP, indicates that cross-talk between NAADP- and IP3-sensitive mechanisms also operate here as in other cell types (19, 29). Moreover, the fact that contractions evoked by IP3 were completely blocked by heparin but were significantly smaller in Tpcn2−/− detrusor muscle when compared with wild-type suggests a reduction of sensitivity in tissues from Tpcn2−/− animals.
As regards the response to carbachol, our finding that contractions elicited by increasing concentrations of carbachol in wild-type mice detrusor showed different sensitivity to heparin and bafilomycin suggests that NAADP involvement in carbachol responses occurs predominantly at higher concentrations of carbachol. Lower carbachol concentrations may selectively couple to IP3 signals, whereas as the concentration of carbachol increases, so NAADP-signaling mechanisms are recruited. Whether different subclasses of muscarinic receptors selectively couple to different messengers or whether greater occupancy of receptors are required to stimulate NAADP production are both possibilities. This contrasts with the situation in pancreatic acinar cells where high affinity cholecystokinin receptors couple to NAADP production, whereas low affinity receptors couple to IP3 formation (24, 29, 37). These findings are interesting in light of previous studies showing cross-talk between IP3-, cADPR-, and NAADP-induced Ca2+ release (3, 34).
The fact that in detrusor muscle from Tpcn2−/− mice, ryanodine inhibited a greater proportion of the contractile response evoked by carbachol may indicate a compensatory increase in RyR expression or sensitivity when TPC2 channels are ablated. Thus, our findings provide further support for the hypothesis that TPC proteins constitute key elements of the NAADP receptor. The sensitivity of the contractile response to ryanodine is also apparent in Tpcn2−/− tissues (Fig. 6b), indicating that functional RyRs are present. The lack of response to NAADP in Tpcn2−/− detrusor muscle (Fig. 6c) makes RyRs unlikely direct targets for NAADP, as proposed by others (7, 8), in this physiological system. Moreover, our findings provide the first evidence that disrupting the expression of TPC proteins alters a key tissue response that is mediated by NAADP in response to a physiological agonist.
Supplementary Material
This work was supported by a Wellcome Trust Programme Grant (to A. G. and J. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1 and Tables 1 and 2.
- IP3
- inositol 1,4,5-trisphosphate
- NAADP
- nicotinic acid adenine dinucleotide phosphate
- cADPR
- cyclic adenosine diphosphate ribose
- TPC
- two-pore channel protein
- RyR
- ryanodine receptor
- SR
- sarcoplasmic reticulum
- CCh
- carbachol
- mAChR
- muscarinic acetylcholine receptor.
REFERENCES
- 1.Iacovou J. W., Hill S. J., Birmingham A. T. (1990) J. Urol. 144, 775–779 [DOI] [PubMed] [Google Scholar]
- 2.Wray S., Burdyga T. (2010) Physiol. Rev. 90, 113–178 [DOI] [PubMed] [Google Scholar]
- 3.Boittin F. X., Galione A., Evans A. M. (2002) Circ. Res. 91, 1168–1175 [DOI] [PubMed] [Google Scholar]
- 4.Yusufi A. N., Cheng J., Thompson M. A., Burnett J. C., Grande J. P. (2002) Exp. Biol. Med. (Maywood) 227, 36–44 [DOI] [PubMed] [Google Scholar]
- 5.Boittin F. X., Dipp M., Kinnear N. P., Galione A., Evans A. M. (2003) J. Biol. Chem. 278, 9602–9608 [DOI] [PubMed] [Google Scholar]
- 6.Li P. L., Lee H. C., Nelson M. T., Meininger G. A., Van Breemen C. (2003) Acta Physiol. Scand. 179, 339–352 [DOI] [PubMed] [Google Scholar]
- 7.Kinnear N. P., Boittin F. X., Thomas J. M., Galione A., Evans A. M. (2004) J. Biol. Chem. 279, 54319–54326 [DOI] [PubMed] [Google Scholar]
- 8.Evans A. M., Wyatt C. N., Kinnear N. P., Clark J. H., Blanco E. A. (2005) Pharmacol. Ther. 107, 286–313 [DOI] [PubMed] [Google Scholar]
- 9.Zhang F., Zhang G., Zhang A. Y., Koeberl M. J., Wallander E., Li P. L. (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H274–H282 [DOI] [PubMed] [Google Scholar]
- 10.Gambara G., Billington R. A., Debidda M., D'Alessio A., Palombi F., Ziparo E., Genazzani A. A., Filippini A. (2008) J. Cell Physiol. 216, 396–404 [DOI] [PubMed] [Google Scholar]
- 11.Kinnear N. P., Wyatt C. N., Clark J. H., Calcraft P. J., Fleischer S., Jeyakumar L. H., Nixon G. F., Evans A. M. (2008) Cell Calcium 44, 190–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thai T. L., Churchill G. C., Arendshorst W. J. (2009) Am. J. Physiol. Renal Physiol 297, F510–F516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aley P. K., Noh H. J., Gao X., Tica A. A., Brailoiu E., Churchill G. C. (2010) J. Pharmacol. Exp. Ther. 333, 726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jude J. A., Wylam M. E., Walseth T. F., Kannan M. S. (2008) Proc. Am. Thorac. Soc. 5, 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Churchill G. C., Okada Y., Thomas J. M., Genazzani A. A., Patel S., Galione A. (2002) Cell 111, 703–708 [DOI] [PubMed] [Google Scholar]
- 16.Barone F., Genazzani A. A., Conti A., Churchill G. C., Palombi F., Ziparo E., Sorrentino V., Galione A., Filippini A. (2002) FASEB J. 16, 697–705 [DOI] [PubMed] [Google Scholar]
- 17.Galione A., Petersen O. H. (2005) Mol. Interv. 5, 73–79 [DOI] [PubMed] [Google Scholar]
- 18.Guse A. H., Lee H. C. (2008) Sci. Signal. 1, re10. [DOI] [PubMed] [Google Scholar]
- 19.Calcraft P. J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K. T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., Wyatt C. N., Parrington J., Ma J., Evans A. M., Galione A., Zhu M. X. (2009) Nature 459, 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galione A., Evans A. M., Ma J., Parrington J., Arredouani A., Cheng X., Zhu M. X. (2009) Pflügers Arch. 458, 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zong X., Schieder M., Cuny H., Fenske S., Gruner C., Rötzer K., Griesbeck O., Harz H., Biel M., Wahl-Schott C. (2009) Pflügers Arch. 458, 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brailoiu E., Churamani D., Cai X., Schrlau M. G., Brailoiu G. C., Gao X., Hooper R., Boulware M. J., Dun N. J., Marchant J. S., Patel S. (2009) J. Cell Biol. 186, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruas M., Rietdorf K., Arredouani A., Davis L. C., Lloyd-Evans E., Koegel H., Funnell T. M., Morgan A. J., Ward J. A., Watanabe K., Cheng X., Churchill G. C., Zhu M. X., Platt F. M., Wessel G. M., Parrington J., Galione A. (2010) Curr. Biol. 20, 703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galione A. (2006) Biochem. Soc. Trans. 34, 922–926 [DOI] [PubMed] [Google Scholar]
- 25.Tugba Durlu-Kandilci N., Brading A. F. (2007) Eur. J. Pharmacol. 566, 172–180 [DOI] [PubMed] [Google Scholar]
- 26.Endo M., Yagi S., Iino M., Kakuta Y. (1977) in Excitation-Contraction Coupling in Smooth Muscle (Casteels R., Godfraind T., Rüegg J. C. eds) pp. 199–209, Elsevier Science Publishers B.V., Amsterdam [Google Scholar]
- 27.Brooks S. P., Storey K. B. (1992) Anal. Biochem. 201, 119–126 [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi S., Kitazawa T., Somlyo A. V., Somlyo A. P. (1989) J. Biol. Chem. 264, 17997–18004 [PubMed] [Google Scholar]
- 29.Cancela J. M., Churchill G. C., Galione A. (1999) Nature 398, 74–76 [DOI] [PubMed] [Google Scholar]
- 30.Masgrau R., Churchill G. C., Morgan A. J., Ashcroft S. J., Galione A. (2003) Curr. Biol. 13, 247–251 [DOI] [PubMed] [Google Scholar]
- 31.Berg I., Potter B. V., Mayr G. W., Guse A. H. (2000) J. Cell Biol. 150, 581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson J. D., Misler S. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 14566–14571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genazzani A. A., Galione A. (1996) Biochem. J. 315, 721–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Churchill G. C., Galione A. (2001) EMBO J. 20, 2666–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamasaki M., Masgrau R., Morgan A. J., Churchill G. C., Patel S., Ashcroft S. J., Galione A. (2004) J. Biol. Chem. 279, 7234–7240 [DOI] [PubMed] [Google Scholar]
- 36.Iizuka K., Yoshii A., Dobashi K., Horie T., Mori M., Nakazawa T. (1998) J. Physiol. 511, 915–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamasaki M., Thomas J. M., Churchill G. C., Garnham C., Lewis A. M., Cancela J. M., Patel S., Galione A. (2005) Curr. Biol. 15, 874–878 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.