Abstract
Recent findings have established a role for the ST6Gal-1 sialyltransferase in modulating inflammatory cell production during Th1 and Th2 responses. ST6Gal-1 synthesizes the Sia(α2,6) to Gal(β1,4)GlcNAc linkage on glycoproteins on cell surfaces and in systemic circulation. Engagement of P1, one of six promoter/regulatory regions driving murine ST6Gal-1 gene expression, generates the ST6Gal-1 for myelopoietic regulation. P1 utilization, however, is restricted to the liver and silent in hematopoietic cells. We considered the possibility that myelopoiesis is responsive to the sialylation of liver-derived circulatory glycoproteins, such that reduced α2,6-sialylation results in elevated myelopoiesis. However, 2-dimensional differential in gel electrophoresis (2D-DIGE) analysis disclosed only minimal alterations in the sialylation of sera glycoproteins of ST6Gal-1-deficient mice when compared with wild-type controls, either at baseline or during an acute phase response when the demand for sialylation is greatest. Furthermore, sera from ST6Gal-1-deficient animals did not enhance myelopoietic activity in ex vivo colony formation assays. Whereas there was only minimal consequence to the α2,6-sialylation of circulatory glycoproteins, ablation of the P1 promoter did result in strikingly depressed levels of ST6Gal-1 released into systemic circulation. Therefore, we considered the alternative possibility that myelopoiesis may be regulated not by the hepatic sialyl glycoproteins, but by the ST6Gal-1 that was released directly into circulation. Supporting this, ex vivo colony formation was notably attenuated upon introduction of physiologic levels of ST6Gal-1 into the culture medium. Our data support the idea that circulatory ST6Gal-1, mostly of hepatic origin, limits myelopoiesis by a mechanism independent of hepatic sialylation of serum glycoproteins.
Keywords: Blood, Gene Expression, Glycosylation, Liver, Serum, Acute Phase Response, Myelopoiesis, Sialyltransferase
Introduction
α2,6-Sialic acid modification is a common feature of glycoproteins in systemic circulation, particularly those glycoproteins originating from the liver. The α2,6 attachment of sialic acid to Gal(β1,4)GlcNAc termini of glycoproteins is mediated by the sialyltransferase ST6Gal-1. Mutant mice unable to express ST6Gal-1 are essentially devoid of α2,6-sialyl modifications, as evidenced by the lack of binding to the Sambucus nigra lectin (SNA)2 that specifically recognizes these structures (1). Hepatic expression of ST6Gal-1 is principally driven by P1 (2, 3), one of six independently operative promoter regions regulating transcription of the ST6Gal-1 gene (4). Another promoter, P3, is responsible for low-level ST6Gal-1 expression in the liver (5). Increased expression of liver ST6Gal-1, mediated by the P1 promoter, has long been recognized to be an integral part of the acute phase response (APR) (6–8), and it was generally believed that elevation of ST6Gal-1 was necessary to address the increased demand for sialylation of the acute phase proteins. The Siat1ΔP1 mouse, with a specific inactivation of P1 without compromising ST6Gal-1 gene expression from the remaining promoters, was generated to disclose the physiologic necessity for the pool of ST6Gal-1 generated as a result of P1 transcriptional activity (5).
It has long been known that a soluble form of ST6Gal-1, as well as a number of other sialyltransferases, is also present in systemic circulation (9, 10). The principal source of soluble ST6Gal-1 is the liver, where the ST6Gal-1 catalytic domain is liberated from its membrane anchor by the proteolytic action of BACE1 (11–13). There have been reports suggesting that the untethered ST6Gal-1 glycosylates a different subset of hepatic glycoproteins than the tethered form (14, 15). Proteolytically untethered ST6Gal-1 is subsequently released into systemic circulation, where it has been generally regarded as a by-product of metabolic inefficiency without significant physiologic function, although circulatory ST6Gal-1 activity also increases during the acute phase response (6–8, 16). Despite a lack of understanding for the physiologic function of the circulatory sialyltransferase, there have been numerous reports strongly correlating serum ST6Gal-1 with pathologic conditions, such as cancer (17). Elevated circulatory ST6Gal-1 activity has been associated with disease severity and poorer prognosis in oral and breast cancers (18, 19). Increased circulatory ST6Gal-1 in pretransplant is also a predictor for delayed graft function and rejection in kidney transplant recipients (20). A 2-fold increase in circulating sialyltransferase activity was also observed in atherosclerosis patients when compared with healthy donors (21).
Previous findings from this laboratory established a role for the P1-mediated pool of ST6Gal-1 in the regulation of myelopoiesis, as mice with a specific ablation to the P1 region had excessive neutrophilic and eosinophilic inflammatory responses (22, 23). Siat1ΔP1 animals had the identical degree of excessive inflammation as Siat1-null animals that were completely devoid of ST6Gal-1, indicating only the P1-mediated pool of ST6Gal-1 was important for myelopoietic regulation. Because P1 transcriptional activity was not detected in the bone marrow (22), the data implicated the control of myelopoiesis by ST6Gal-1 produced by an extramedullary source, presumably the liver. To establish the link between hepatically expressed ST6Gal-1 and the myelopoietic events occurring in the bone marrow, we re-examined the biochemical consequence of ST6Gal-1 deficiency in the liver, particularly in relation to its role in the proper sialylation of serum glycoproteins. We considered that aberrantly sialylated glycoproteins in sera of Siat1ΔP1 and Siat1-null animals have myelopoietic enhancing activities. However, we showed that the sialylation of liver-derived serum glycoproteins was only minimally impacted upon inactivation of P1, and the only major biochemical consequence was a diminished pool of ST6Gal-1 in systemic circulation. Furthermore, in vitro colony formation assays did not disclose the presence of myelopoietic enhancing activity in Siat1ΔP1 serum. On the other hand, recombinant ST6Gal-1, when added at physiological levels found in systemic circulation, significantly repressed ex vivo colony formation. This observation is consistent with a novel idea that myelopoietic activity can be regulated by extrinsically produced ST6Gal-1, resulting in enhanced myelopoiesis in animals with decreased circulatory ST6Gal-1 levels.
MATERIALS AND METHODS
2D-DIGE and Analysis
Serum was collected from sacrificed animals at the following points: at rest (before treatment), 48 h, or 72 h after turpentine treatment. Serum samples were depleted of albumin with a Murine Serum Albumin Depletion kit (Qiagen), and proteins were precipitated and quantified with using a 2D Clean-Up Kit and 2D Quant Kit (Amersham Biosciences). 50 μg of each test sample was labeled with 250 pmol of Cy2, Cy5, or Cy3 (Siat1-null, Siat1ΔP1, and wild-type, respectively) according to the manufacturer's protocols. After quenching the reaction, the labeled samples were combined and mixed with unlabeled proteins for the poststaining with Deep Purple (GE Healthcare). For α2,6-sialic acid glycoprotein enrichment combined labeled samples were dialyzed into phosphate-buffered saline with a Slide-A-Lyzer 10K MWCO (Pierce). α2,6-Sialic acid containing glycoprotein separation was performed by SNA-agarose (Vector Laboratories) affinity chromatography. After separation, enriched proteins were dialyzed into water, dried in a SpeedVac Concentrator (Thermo Scientific) and reconstituted in DeStreak Rehydration solution (GE Healthcare) containing 1% IPG buffer (GE Healthcare).
The combined labeled and unlabeled samples were focused on IPG strips (24 cm, pH 3–11 NL, GE Healthcare) followed by reduction of the proteins with DTT (Sigma) and alkylation with iodoacetamide (Sigma). For the second SDS-PAGE dimension, strips were run on 10% acrylamide gels. Gels were scanned with a Typhoon 9410 Scanner (GE Healthcare) at the following laser wavelengths: Cy3, 532 nm; Cy5, 633 nm; and Cy2, 488 nm and then stained with Deep Purple (GE Healthcare). Images from total protein stains were matched with CyDye images, and pick lists were created using the BVA (biological variation analysis) module of the DyCyder software (GE Healthcare). The ANOVA was performed and spots with a p value ≤0.05 were selected for excision from the gel with the Ettan Spot Picker (GE Healthcare), and the gel plugs were placed into a 96-microwell plate.
Mass spectrometric identification of the protein spots was performed as follows. Tryptic in-gel digestion of the gel plugs was performed with an Ettan Digester (GE Healthcare), and the digests were analyzed by MALDI-MS (micro MX, Waters) in the reflectron, positive-ion mode using the matrix α-cyano-4-hydroxycinnamic acid. All spectra were processed and transformed to the PKL file format and the PKL files were used to search the Rodentia subset of the Swiss-Prot data base (release 55.2, containing 23,827 sequences) using MASCOT (Matrix Science, v 2.2.0). The search parameters were as follows: trypsin as the proteolytic enzyme with 0 possible missed cleavages, carboxyamidomethylation of cysteine as a fixed modification, oxidation of methionine as a variable modification, the allowable mass error was 100 ppm for peptides and the instrument was set to MALDI-ToF. The Mascot default significance threshold of p < 0.05 for assignments was used in the searches and a minimum of two unique peptides were used as a criteria for a match. In some instances, the tryptic digests were further analyzed by LC nanoelectrospray-tandem mass spectrometry (LC-ESI-MS/MS) using a nanoACQUITY UPLC (Waters) coupled to a Q-ToF Premier mass spectrometer (Waters/Micromass). Ions with +2 to +4 charges only were selected for tandem MS (MS/MS) using the preset data dependent acquisition collision energy parameters.
MS/MS spectra were transformed to the PKL file format and the PKL files were used to search the Rodentia subset of the Swiss-Prot data base (release 55.2, containing 23,827 sequences) using MASCOT (Matrix Science, v 2.2.0). The search parameters were as follows: trypsin as the proteolytic enzyme with 2 possible missed cleavages, carboxyamidomethylation of cysteine as a fixed modification, oxidation of methionine and phosphorylation of STY as a variable modification, the allowable mass error was 100 ppm for peptides and 100 mDa for fragment ions. The Mascot default significance threshold of p < 0.05 for assignments was used in the searches and a minimum of two unique peptides were used as a criteria for a match.
Ex Vivo CFU Assays
A total of 30,000 bone marrow-nucleated cells from wild-type or Siat1ΔP1 in a volume of 0.1 ml were plated in 0.9 ml of methylcellulose medium (MethoCult 3234 or 3534; Stem Cell Technologies). All cultures were placed in a humidified incubator with 5% CO2 at 37 °C. For the clonogenic activity assays, 30,000 nucleated bone marrow wild-type or Siat1ΔP1 cells were grown in MethoCult 3234 in the presence of 20 ng/ml rmG-CSF (Chemicon International) and 2 ng/ml rmIL-3 (Chemicon International) for 4 days to assess granulocyte and monocyte colony formation or to assess eosinophil colony formation, 750,000 nucleated bone marrow cells were grown for 7 days in MethoCult 3534 supplemented with 20 ng/ml rmIL-5 (R&D Systems). Cell aggregates with >50 cells were scored as colonies. Plasma harvested from resting animals of wild-type, Siat1ΔP1, and Siat1-null backgrounds by terminal heart puncture after sacrifice was added to assays to a final concentration of 10% (v/v). Colonies with more than 50 cells were counted 6 days after incubation. For ST6Gal-1 supplementation studies, wild-type cells were grown in the presence of commercially available recombinant soluble ST6Gal-1 (Calbiochem) or recombinant ST6Gal-1 generated from transfected CHO cell media at wild-type APR levels (11.25 fmol/h × μl). Inactivation of ST6Gal-1 was achieved by incubation at 100 °C for 10 min.
Sialyltransferase Assays
Sialyltransferase assays were carried out under incubation conditions as described previously (5, 24). For the measurement of ST6Gal-1 activity, 2.5 μl of serum was incubated for 4 h at 37 °C with CMP-[3H]NeuNAc and monitored for the transfer of [3H]NeuNAc to an exogenously supplied acceptor compound (Toronto Research Chemicals or gift from Dr. K. Matta): Galβ4GlcNAcα-o-Bn (Acceptor A), Galβ3GlcNAcα-o-Bn (Acceptor B), or Galβ4GalNAcα-o-Bn (Acceptor C), each having a different affinity for subsets of gal-sialyltransferases (25–27). Reactions with Acceptor C utilized 1.5 μl of serum incubated for 2 h at 37 °C. [3H]NeuNAc enzymatically transferred to supplied acceptors was measured after recovery and separation from unreacted CMP-[3H]NeuNAc by C18-reverse phase chromatography. Separation of reacted Acceptor A into α2,6- and α2,3-sialic acid fractions was performed with SNA-agarose (Vector Laboratories) affinity chromatography. [3H] was quantified by scintillation counting.
Liver sialyltransferase mRNA was determined by real-time RT-PCR. Total RNA was isolated using TRIzol (Invitrogen), and cDNA was synthesized from 1 μg RNA using the iScript reverse transcriptase kit (Bio-Rad) according to the manufacturer's instructions. Real-time PCR reactions, using iQ SYBR-green Supermix (Bio-Rad), were performed on the My iQ Single-Color Real-Time PCR Detection System (Bio-Rad). Primer pairs for each mRNA, available on request, were designed based on sequence information deposited in GenBankTM. Relative mRNA levels are derived from ΔCt, the difference of the threshold cycle value of the target mRNA and the Ct value for RPL32, a ribosomal protein mRNA used as a reference standard.
Animals and General Methodology
The Siat1ΔP1 mouse was generated by gene-targeted deletion of the P1 promoter of Siat1 as described previously (5). Siat1ΔP1 mice were backcrossed 11 successive generations into the C57BL/6 background. Siat1-null animals (28) were originally obtained from the Consortium for Functional Glycomics, and they have been backcrossed >6 generations into C57BL/6. For all experiments reported here, age- and sex-matched (typically 55–70-day-old) C57BL/6 animals were used as wild-type controls. All animals in this study are of C57BL/6J background.
For the turpentine elicitation of the acute phase response, animals were injected subcutaneously with 100 μl of turpentine at the scruff of the neck. The mice were sacrificed and serum collected via terminal heart puncture at the following points: before injection, 48 h, or 72 h after turpentine treatment. Liver samples were also collected at these time points and snap-frozen in liquid nitrogen. All animals studies presented here have been approved by the Institute Animal Care and Use Committee of Roswell Park Cancer Institute.
For Western blot analysis, serum aliquots, 0.1 μl of serum in 10 μl of buffer corresponding to 5–10 μg protein, were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a PVDF membrane (Millipore). Blots were blocked in TBST containing 5% bovine serum albumin for 1 h at room temperature or 4 °C overnight. Blots were probed with SNA-biotin (Vector Laboratories) at a working concentration of 0.08 μg/ml for the detection of α2,6-sialic acids or MAA-biotin (Vector Laboratories) at a working concentration of 50 μg/ml for the detection of α2,3-sialic acids. Both were subsequently incubated with streptavidin-alkaline phosphatase (Vector Laboratories) at a 1:50,000 dilution or streptavidin-Cy5 at 1:1,000 (GE). AKP-blots were visualized with AttoPhos AP (Promega) on a Storm (GE), fluorescent blots were visualized on a Typhoon Trio (GE), both types were quantified with ImageQuant.
RESULTS
Essentially Normal Serum Sialylation Profiles in Mice with Varying Degrees of ST6Gal-1 Deficiency
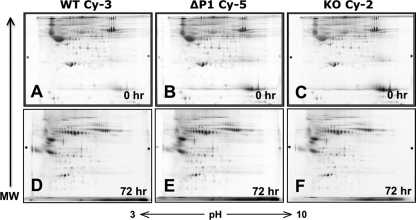
Earlier published data from low resolution 2-dimensional gel electrophoresis was unable to reveal changes in the serum sialylation profile of the Siat1ΔP1 mouse, despite disrupting the major sialyltransferase responsible for hepatic sialylation of circulatory glycoproteins (5). In view of the myelopoietic phenotype associated with liver ST6Gal-1 deficiency, we used a variation of the differential in gel electrophoresis method (2D-DIGE) to probe for more subtle changes in serum sialylation. We differentially labeled de-albuminized serum glycoproteins from wild-type (WT), Siat1ΔP1 (ΔP1), and Siat1-null (KO) animals with CyDyes (Cy-3, Cy-5, and Cy-2, respectively), and the combined sera were subjected to 2-dimensional gel electrophoresis. Changes in the Cy-5, Cy-2, to Cy-3 signal ratios for each of the spots lying along the isoelectric (horizontal) dimension should reveal genotype-related differences in the extent of sialic acid modifications for each glycoprotein species, with each sialic acid conferring an additional negative charge. A signal ratio of 1:1:1, therefore, would indicate no genotype-related differences in the degree of sialylation. While the combined sera was separated on a single gel, Fig. 1, A–C shows the separate dye channels for wild-type, Siat1ΔP1, and Siat1-null sera, respectively. The results showed a surprising complete absence of genotype-related signal shift along the isoelectric dimension, indicating that the presence or absence of hepatic ST6Gal-1 had no consequence on the extent of serum glycoproteins sialylation while the animals are at rest. In view of no observable sialylation shift at baseline, we reasoned that diminished sialylation in ST6Gal-1-deficient animals may be observable only when there is heightened demand for sialylation, such as during an acute phase response (APR) when a dramatic increase in production of many liver-derived sera sialyl-glycoproteins takes place. APR was elicited by subcutaneous injection of the sterile irritant, turpentine. Sera collected at 72 h from turpentine-treated wild-type, Siat1ΔP1, and Siat1-null mice were subjected to 2-dimensional gel analysis (Fig. 1, D–F, respectively). Despite the induction of heavily sialylated acute phase proteins, such as α1-acid glycoprotein, haptoglobin, and C-reactive protein, the extent of sialylation for each of these glycoproteins remain unchanged in the Siat1ΔP1 or in the Siat1-null mouse. Similar results were seen in sera collected 48 h after turpentine induction (data not shown). Thus, the presence of hepatic ST6Gal-1 was not required to fully sialylate hepatic glycoproteins. In the absence of ST6Gal-1, the α2,3-sialyltransferases, such as ST3Gal-3 and ST3Gal-4 that were abundantly expressed in the liver, can compensate to effect full sialylation (see Fig. 4).
FIGURE 1.
Serum glycoproteins of ST6Gal-1-deficient animals are not undersialylated. Serum pooled from 5 animals from each of the three genotypes, WT, Siat1ΔP1 (ΔP1), or Siat1-null (KO), were collected, albumin-depleted, and separately labeled with CyDyes Cy-3, Cy-5, and Cy-2, respectively, before combining together for two-dimensional polyacrylamide gel electrophoresis as detailed in “Materials and Methods.” The analysis was performed on sera collected from animals at rest (0 h) or during an APR 72 h after subcutaneous injection of turpentine (72 h). Shown are the 2D-PAGE separation signal for each of the three dye channels, with the molecular weight and isoelectric directions represented as vertical and horizontal dimensions, respectively.
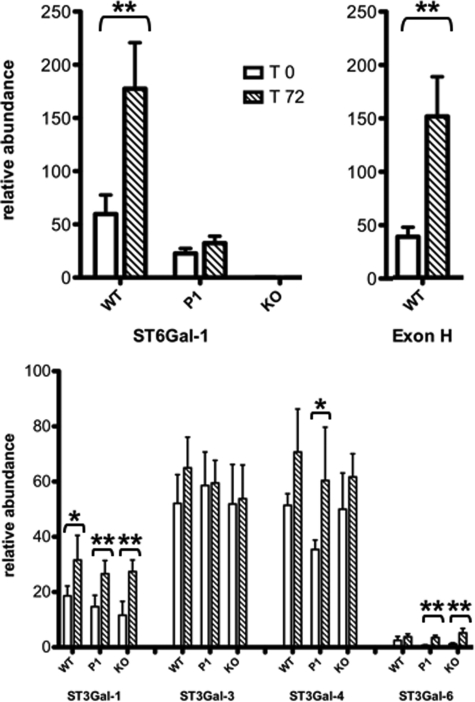
FIGURE 4.
RT-PCR analysis of selected hepatic sialyltransferase mRNAs. Expression of (top left) total ST6Gal-1 mRNA, (top right) P1-transcribedST6Gal-1 mRNA that contains the unique Exon H sequence (2), and (bottom) selected hepatic sialyltransferases were measured by real-time PCR relative to the expression of RPL32, a ribosomal protein, as a reference standard. RT-PCR analysis was performed on liver samples from WT, Siat1ΔP1 (P1) or Siat1-null (KO) strains during either rest (T0) or 72 h after turpentine elicited APR (T72). The determinations were based on 5 animals from each of the strains for each of the time points, where (*) denotes p < 0.05 by Student's t test.
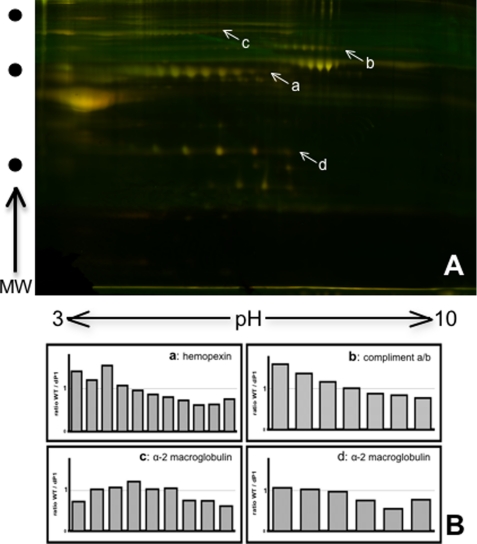
In lieu of any observable change to the extent of serum glycoprotein sialylation, we reasoned that hepatic ST6Gal-1 deficiency may result in a qualitative shift of serum glycoproteins that are normally extensively modified with α2,6-linked to α2,3-linked sialic acids. Serum glycoproteins containing α2,6-sialyl structures from wild-type, Siat1ΔP1, and Siat1-null mice were identified on Western blots probed with the SNA lectin. As expected, there were no α2,6-sialic acids on serum glycoproteins from Siat1-null mice, because these animals are completely unable to express ST6Gal-1. However, we also expected that inactivation of P1, the major promoter driving hepatic ST6Gal-1 expression, would dramatically reduce α2,6-sialylation. Contrary to expectation, sera glycoproteins revealed little to no qualitative differences in α2,6-sialylation between wild-type and Siat1ΔP1 animals, whether the animals were at rest or at 48 h or 72 h after elicitation of APR by turpentine (Fig. 2A). There was a subtle reduction in the overall degree of α2,6-sialylation associated with the ΔP1 animals, which curiously was more pronounced while the animals were at rest (32% reduction compared with wild-type) than when the animals were undergoing APR (20% reduction at 72 h after turpentine elicitation) (Fig. 2B). There was also a concomitant but subtle reduction in the overall degree of sera α2,3-sialylation, as assessed by reactivity to the Maackia amurensis lectin (MAA), associated with Siat1ΔP1 and Siat1-null relative to wild-type animals. The reduction in overall sera MAA reactivity was noticeable in mice at rest and at 48 h but not 72 h after elicitation for APR (Fig. 2C). To assess if the decrease in α2,6-sialylation was more pronounced in some glycoproteins than in others, 2D-DIGE was performed on a combined mixture of SNA-purified glycoproteins of wild-type (Cy-3) and Siat1ΔP1 (Cy-5) (Fig. 3A). The isoelectric distribution for a number of serum glycoprotein species, including hemopexin, complement a/b, and α2-microglobulin, indicated a general shift of the Siat1ΔP1 glycoproteins toward the anode, as indicated by the declining Cy-3/Cy-5 ratios (Fig. 3B). Together, the data suggest that inactivation of the major promoter driving hepatic ST6Gal-1 expression resulted in a general but subtle decrease in the level of α2,6-sialylation of all major glycoproteins, without preference toward any particular major glycoprotein.
FIGURE 2.
SiatΔP1 serum glycoproteins are positive for α2,6-sialic acids. A, protein polyacrylamide gel blot of WT, Siat1ΔP1 (ΔP1) and Siat1-null (KO) serum proteins (0.05 μl from a pooled serum of 5 animals) at rest (0), at 48 h (48), or at 72 h (72) after turpentine elicited APR were separated on SDS-polyacrylamide gels were probed with SNA to visualize α2,6-linked sialic acid-containing glycoproteins. Quantification of (A) total SNA signal and (B) total MAA signal from Western blots, with relative signals reference to the wild-type signal of 1. Sera from 5 individual animals were used for each determination utilizing ImageQuant software, where (*) denotes p < 0.05 based on Student's t test.
FIGURE 3.
2D-DIGE comparative analysis of α2,6-sialic acid-containing wild-type and Siat1ΔP1 serum glycoproteins. A, sera pools from 5 animals of WT and from Siat1ΔP1 (ΔP1) mice at rest were de-albuminized and minimally labeled with CyDyes (wild-type, green; Siat1ΔP1, red; overlap, yellow). The combined labeled wild-type and Siat1ΔP1 pools were subjected to SNA-agarose purification for the α2,6-sialic acid containing glycoprotein fraction, and subjected to 2D-DIGE on a single 2D gel. B, differential quantitation of spots was performed utilizing DeCyder software to illuminate protein entities with genotype-dependent shift in isoelectric distribution (horizontal dimension). Shifts in the isoelectric distribution in the α2,6-sialyl glycoproteins imply differences in the overall levels of sialic acid modifications, where each sialic acid moiety confers an additional negative charge. Some α2,6-sialic acid-containing glycoproteins with reduced overall sialylation in Siat1ΔP1 animals are hemopexin (a), complement a/b (b), α2-macroglobulin (c and d), with identities determined by mass spectrometric identification as outlined in “Materials and Methods.” Shifts in the isoelectric distribution of the above mentioned glycoproteins, where each data point represents one corresponding spot on panel A, are displayed in panel B as signal strength ratios of wild-type versus Siat1ΔP1 along the isoelectric dimension.
Liver and Serum Sialyltransferase Profiles in Mice with Varying Degrees of ST6Gal-1 Deficiency
The observation that serum glycoprotein sialylation profiles were essentially normal despite the inactivation of ST6Gal-1 warranted a more detailed examination of hepatic expression of not only ST6Gal-1, but also the α2,3-sialyltransferases that may also contribute to sialylation of serum glycoproteins. Quantitative real-time RT-PCR analysis of liver mRNA confirmed previous observations that in wild-type animals, acute phase response is accompanied by a 3-fold elevation of ST6Gal-1 mRNA (Fig. 4A). The P1 promoter of Siat1 is liver specific and uniquely generates transcripts containing Exon H sequence at its 5′UTR (2). The elevation of hepatic ST6Gal-1 expression was due to increased activity from the P1 promoter, resulting in a similar fold elevation of Exon H-containing ST6Gal-1 mRNA (Fig. 4B). ST3Gal-3, ST3Gal-4, and ST3Gal-6 have the theoretical potential to sialylate the lactosamine termini in lieu of ST6Gal-1. ST3Gal-3 and ST3Gal-4 were also abundantly expressed in the liver. The ST3Gal-4, but not ST3Gal-3, expression appeared to be marginally activated in all 3 animal strains. However, with the exception of Siat1ΔP1, statistical significance was not reached. Hepatic ST3Gal-6 mRNA was also elevated in APR, but the overall level of hepatic ST3Gal-6 mRNA was minimal compared with the other α2,3-sialyltransferases. Among the α2,3-sialyltransferases examined, only ST3Gal-1 mRNA in liver was minimally up-regulated during the acute phase response (less than 2-fold over baseline) (Fig. 4C). However, ST3Gal-1 modifies only Gal(β1,3)GalNAc termini on O-linked glycans, and does not compete for the lactosamine termini present on N-linked glycans used by ST6Gal-1. Importantly, the pattern of α2,3-sialyltransferase gene expression was unchanged in the liver of Siat1ΔP1 and Siat1-null animals, both while the animals were at rest and during the acute phase response (T72), confirming that only the level of ST6Gal-1 expression was altered in both mutants when compared with wild-type animals.
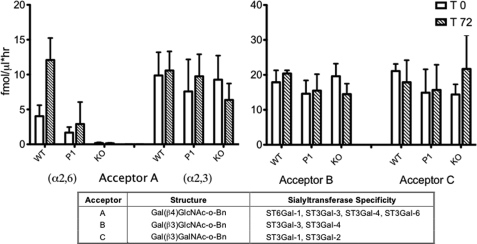
The intact catalytic domain of sialyltransferases can be proteolytically liberated from the membrane anchor, and released into systemic circulation as serum sialyltransferases. In the absence of striking changes in serum sialylation profiles in the Siat1ΔP1 and Siat1-null animals, we examined the consequence of hepatic ST6Gal-1 deficiency to circulating sialyltransferases activities. First we used the synthetic acceptor, Gal(β1,4)GlcNAc-o-Bn (Acceptor A, Fig. 5), which should serve as substrate to ST6Gal-1, ST3Gal-3, ST3Gal-4, and ST3Gal-6. The α2,6-sialylated product, a consequence only of ST6Gal-1 activity, could be isolated by SNA lectin chromatographic separation of the reaction products from the α2,3-sialyl products constructed by ST3Gal-3, -4, and -6. As clearly indicated in Fig. 5, circulatory ST6Gal-1 activity was severely diminished in the Siat1ΔP1 mouse and absent in the Siat1-null animal. Moreover, APR-associated induction of circulatory ST6Gal-1 activity, as seen in the wild-type animal, was abolished upon ablation of the P1 promoter. Significantly, circulatory α2,3-sialylation activity remained unchanged upon inactivation of ST6Gal-1, either while the animals were at rest or during an acute phase response. To confirm this latter observation, we used Acceptor B, Gal(β1,3)GlcNAc-o-Bn, which could serve as acceptor substrate only for ST3Gal-3 and ST3Gal-4, but not for ST6Gal-1. Circulatory sialyltransferases activities toward Acceptor B was unaffected by ST6Gal-1 deficiency while the animals were at rest and during APR. Acceptor C, Gal(β1,3)GalNAc-o-Bn, was used to specifically monitor ST3Gal-1 in systemic circulation. Curiously, while liver mRNA data showed that APR was accompanied by a 2-fold elevation in hepatic ST3Gal-1 gene expression, this induction was not reflected in circulatory ST3Gal-1 activity. Also importantly, α2,3-sialyltransferase activities present in systemic circulation remained unchanged in both Siat1ΔP1 and Siat1-null animals.
FIGURE 5.
Serum enzymatic activity of selected gal-sialyltranferases during an APR. Serum was harvested from WT, Siat1ΔP1 (P1), or Siat1ΔP1 (KO) strains at baseline (T0) or 72 h after turpentine-elicited APR (T72). Sera from 4 animals of each strain were pooled and assessed for sialyltranferase activity by CMP-[3H]NeuNAc and an artificial acceptor substrate as described in Materials and methods. Acceptor A enzymatic product was further separated by SNA-agarose to distinguish the α2,6- and α2,3-sialylated products. The chart indicates the chemical structure of each acceptor and the sialyltransferase preferences for each of these acceptor substrates.
Extrinsic ST6Gal-1 Rather Than Aberrantly Sialylated Serum Factors Controls Myelopoiesis
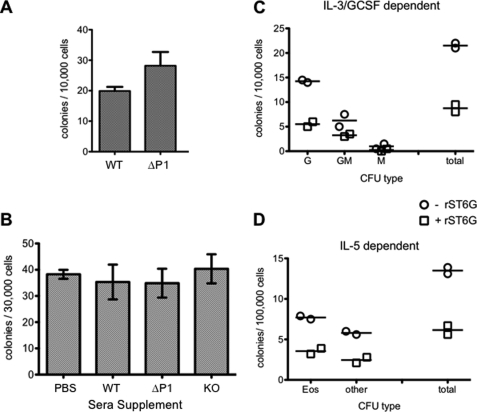
The Siat1ΔP1 bone marrow is endowed with greater myelopoietic activity than the wild-type marrow, which can be recapitulated in an ex vivo semi-solid culture system assessing myeloid colony forming activity in the presence of G-CSF and IL-3 (Fig. 6A). To examine the possibility that an aberrantly sialylated glycoprotein present in the plasma of ST6Gal-1-deficient animals is acting as a myelopoiesis enhancing factor, we assessed the ability of plasma from ST6Gal-1-deficient animals to enhance ex vivo colony formation. Bone marrow cells were seeded in semi-solid media containing either 10% (v/v) Siat1ΔP1 or Siat1-null plasma, and colony formation activity was compared with control wells supplemented with wild-type plasma, also at 10% (v/v). As clearly shown in Fig. 6B, myeloid colony formation was not enhanced upon supplementation with either Siat1ΔP1 or Siat1-null plasma, relative to wild-type plasma. Next, we considered the alternative possibility that extrinsic ST6Gal-1, present in wild-type animals but severely diminished in Siat1ΔP1 and altogether absent in Siat1-null animals, is a myelosuppressive agent. When the semi-solid media was supplemented with recombinant ST6Gal-1, to an activity level roughly equivalent to that found in wild-type animals during APR (11 fmol/h/μl), there was dramatically suppressed total clonogenic activity, which was due mostly to reduced granulocyte (G) and eosinophil (Eos) colony formation (Fig. 6, C and D). Heat-inactivated recombinant ST6Gal-1 did not suppress clonogenic activity, suggesting a requirement for catalytically active enzyme (data not shown).
FIGURE 6.
Attenuation of ex vivo colony formation by ST6Gal-1. Bone marrow cells were extracted from resting animals, placed into MethoCult (semi-solid media), incubated for 4–7 days. Colonies were assessed visually. Panel A, CFU of WT and Siat1ΔP1 (ΔP1) bone marrow cells, with the difference yielding a statistical significance of p < 0.05 by Student's t test. Panel B, wild-type bone marrow cell cultures were supplemented with 10% (v/v) of PBS, or plasma from WT, Siat1ΔP1 (ΔP1), or Siat1-null (KO) animals at rest. Panels C and D, wild-type bone marrow cells were cultured in the absence or presence of recombinant ST6Gal-1 (rST6G). rST6G was added to yield a final sialyltransferase activity of 11 fmol/h/μl, which was roughly equivalent to level present in plasma of wild-type animals during APR. Each point was performed in duplicate (D) or triplicate (C) as shown. Colony classification as follows: G, granulocyte; GM, granulocyte/monocyte; M, monocyte; Eos, eosinophil; Other, non-eosinophil. The rST6G supplementation study has been repeated >3 times, and a representative experiment is presented. Heat inactivated rST6G resulted in no suppression of clonogenic activity (data not shown). Cultures in panels A–C were supplemented with IL-3, and G-CSF; cultures in panel D were supplemented with IL-5.
DISCUSSION
It is presumed that multiple, independent ST6Gal-1 promoters exist to differentially address the need for ST6Gal-1 in different physiologic functions. The humoral response (28–30), maintenance of myeloid homeostatic balance (22), modulation of dendritic cell activity (31), T cell functionality (32), and integrin signaling (33–35) are but a few physiologic processes with implicated ST6Gal-1 participation. The idea of ST6Gal-1 pleiotropy was validated by the Siat1ΔP1 mouse, with an inactivation to P1, the major promoter driving liver ST6Gal-1 expression, without compromising the ability to utilize the remaining promoters. The Siat1ΔP1 mouse did not have the compromised B cell response of the Siat1-null mouse, suggesting that P1 did not generate the ST6Gal-1 involved in humoral immunity (5).
The Siat1ΔP1 mouse was created specifically to disclose the biologic role for the P1-mediated pool of ST6Gal-1. At baseline, the Siat1ΔP1 mouse was normal in all respects, with nominal alterations to circulatory blood counts, normal clotting times, cytokines, and major inflammatory proteins (see supplemental data). However, upon challenge with pro-inflammatory stimuli, the Siat1ΔP1 mouse responded with overly robust neutrophilic and eosinophilic inflammatory responses that had been attributed to hyperactive myelopoiesis (22, 23). Direct P1-mediated transcriptional activity in the bone marrow could not be detected, and its utilization appears to be highly restricted to the liver. To explain how inactivation of the major transcriptional promoter for liver ST6Gal-1 expression could result in a bone marrow consequence, we had hypothesized that myelopoietic events were effected by aberrantly sialylated Siat1ΔP1 serum glycoproteins. Although early evidence from our laboratory has already indicated the extent of serum glycoprotein sialylation was essentially unaltered in Siat1ΔP1 animals (5), the idea that P1 inactivation could impact the sialylation of a small subset of glycoproteins prompted the present investigation. 2D-DIGE procedures designed to reveal small alterations still failed to identify even subtle differences to the extent of sialylation despite ablation of the major hepatic promoter, or for that matter, a complete inactivation of the ST6Gal-1 gene. Therefore, ST6Gal-1 is completely unnecessary for fully sialylated serum glycoproteins, despite the fact that normal serum has an abundance of α2,6-sialyl structures constructed by ST6Gal-1. Compensatory mechanisms, such the α2,3-sialyltransferases to sialylate exposed galactose termini, or the hepatic asialo-glycoprotein receptor to remove undersialylated serum components, may contribute to maintain the fully sialylated state of the circulatory glycoproteins in the ST6Gal-1-deficient animals. Consistent with the latter view is the slight decrease in total sera SNA and MAA lectin binding activity from Siat1ΔP1 and Siat1-null mice, indicating a reduction in both α2,6- and α2,3-sialyl structures when only ST6Gal-1 was deficient (Fig. 2, B and C). Because many sera glycoproteins are concomitantly decorated with both α2,3- and α2,6-sialic acids, the exposed galactose termini from ST6Gal-1 insufficiency might have caused the removal of serum components that also had α2,3-sialyl substitutions.
It was unexpected that destruction of the major mechanism to express liver ST6Gal-1 resulted only in nominal under-representation of α2,6-sialic acid modifications in systemic circulation. This finding implies that the P1-mediated pool of ST6Gal-1 was non-essential for much of the α2,6-sialylation requirements of serum glycoproteins, even during periods of high demand such as during an acute phase response. The residual ST6Gal-1 after inactivation of the P1 promoter appeared sufficient to address these needs.
Based only on the nominal reduction in α2,6-sialylation of circulatory glycoproteins, the idea that an aberrantly sialylated serum component in Siat1ΔP1 mice with biologic activity as a myelopoietic enhancer could not be discounted. To interrogate for the presence of a myelopoietic enhancing factor, in vitro myelopoietic colony forming assays supplemented with 10% v/v plasma from ST6Gal-1 deficient animals, which should have the enhancer, were compared with assays supplemented with plasma from wild-type animals, which should not have the enhancing factor. The inability of Siat1ΔP1 or Siat1-null plasma, relative to wild-type plasma, to stimulate colony formation in vitro, together with the lack of prominent alterations to serum sialylation, strongly argue against an aberrantly sialylated serum component in the ST6Gal-1-deficient animals acting as a myelopoietic enhancing factor.
A number of sialyltransferase activities are normally present in systemic circulation, including ST3Gal-1 acting on Galβ1,3GalNAc termini common on O-linked glycans, and α2,3-sialyl activities toward both type 1 and type 2 lactosamine glycans on both N- and O- linked glycans. Turpentine-elicited APR was accompanied by the induction of not only ST6Gal-1 but also ST3Gal-1 mRNA in the liver (Fig. 4C), which was also reported by Yasukawa et al. (36). However, the increased ST3Gal-1 mRNA expression did not result in increased ST3Gal-1 activity in systemic circulation (Fig. 5). It is of note that the liver response to inflammatory insult, e.g. the APR, resulted in the elevation of only the ST6Gal-1 activity in the blood. P1 inactivation resulted in dramatic reduction of only the ST6Gal-1 activity in systemic circulation, which appeared to be the only overt biochemical consequence of P1 ablation. Therefore, we pursued the unconventional idea that circulatory ST6Gal-1 may serve as an attenuator of myelopoiesis, such that diminished circulatory ST6Gal-1 levels would promote release from myelopoietic repression. This possibility is supported by ex vivo myeloid colony formation assays, showing that presence of recombinant ST6Gal-1 resulted in a dramatic attenuation of myeloid colony formation activity, including the formation of granulocyte-, granulocyte/monocyte-, and eosinophil colonies. The amount of recombinant ST6Gal-1 used was equivalent to the physiologic level in sera of wild-type mice during APR, roughly 11–12 fmol/h/μl. Moreover, suppression of ex vivo myelopoietic activity could be reproduced using recombinant ST6Gal-1 from a number of sources, including those produced within our laboratory by transfection of CHO cells with rat-derived sequences, to commercially available recombinant human ST6Gal-1 generated in insect cells (data not shown). It should also be noted that colony forming assays with plasma supplementation (Fig. 6B) also resulted in a mild though statistically insignificant repression of colony forming activity in the presence of wild-type plasma, which contained ST6Gal-1, relative to Siat1ΔP1 or Siat1-null plasma or the saline control. The mildness of the repression was likely due to the fact that plasma was only present at 10% v/v; thus the contribution of ST6Gal-1 from the wild-type plasma was anticipated to be only 1/10th of physiologic baseline levels, or 0.3–0.5 fmol/h/μl. Together, these observations strongly implicate a role for extrinsic ST6Gal-1, e.g. the circulatory ST6Gal-1, in the negative regulation of bone marrow myelopoiesis. Indeed, this idea has further support in our recent observations that circulatory ST6Gal-1 activity was strikingly suppressed during acute pulmonary eosinophilic inflammation in mice models that mimics aspects of human acute asthma, and that both Siat1ΔP1 and Siat1-null mice had more severe acute pulmonary Th2 response than their wild-type counterparts (23). Suppressed circulatory ST6Gal-1 also accompanies other conditions that require elevated production of inflammatory cells, such as during bacterial and thioglycollate-induced peritonitis (5, 22), or during recovery from cyclophosphamide-induced myelosuppression.3
Taken together, the data strongly indicate a role for extrinsically produced ST6Gal-1, e.g. those originating from the liver, in the regulation of bone marrow hematopoietic events. The mechanistic basis of this novel regulatory axis is under current investigation, but it does predict that ST6Gal-1 originating from extramedullar sources is recruited by bone marrow cells in the construction of α2,6-sialyl structures that are important for myeloid homeostatic regulation. Preliminary data indicate that the presence of extrinsic ST6Gal-1 results in α2,6-sialyl structures of hematopoietic cells both in vivo and in ex vivo situations.4 Further, the hypothesis predicts that the presence of extrinsic ST6Gal-1 will have a suppressive effect in myelopoiesis, and this prediction has been verified in vitro in the present work demonstrating the inhibition of in vitro colony forming activity by addition of roughly physiological levels of ST6Gal-1 to the medium. In vivo recapitulation of this process as well as elucidation of the mechanism of ST6Gal-1 action in hematopoiesis are currently in progress and will be the subject of a separate report.
Supplementary Material
Acknowledgments
This research utilized core facilities supported in part by RPCI NCI-funded Cancer Center Support Grant CA16056.
This work was supported, in whole or in part, by National Institutes of Health Grant AI056082 (to J. T. Y. L.) and National Institutes of Health Grant AI078429 (to J. T. Y. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
M. Nasirikenari and J. T. Y. Lau, unpublished observations.
M. B. Jones, M. Nasirikenari, and J. T. Y. Lau, unpublished observations.
- SNA
- Sambucus nigra lectin
- 2D-DIGE
- two-dimensional differential in gel electrophoresis
- MAA
- Maackia amurensis lectin
- APR
- acute phase response
- CFU
- colony-forming units
- WT
- wild type
- MS
- mass spectrometry.
REFERENCES
- 1.Martin L. T., Marth J. D., Varki A., Varki N. M. (2002) J. Biol. Chem. 277, 32930–32938 [DOI] [PubMed] [Google Scholar]
- 2.Hu Y. P., Dalziel M., Lau J. T. Y. (1997) Glycoconj. J. 14, 407–411 [DOI] [PubMed] [Google Scholar]
- 3.Dalziel M., Lemaire S., Ewing J., Kobayashi L., Lau J. T. Y. (1999) Glycobiology 9, 1003–1008 [DOI] [PubMed] [Google Scholar]
- 4.Wuensch S. A., Huang R. Y., Ewing J., Liang X., Lau J. T. (2000) Glycobiology 10, 67–75 [DOI] [PubMed] [Google Scholar]
- 5.Appenheimer M. M., Huang R. Y., Chandrasekaran E. V., Dalziel M., Hu Y. P., Soloway P. D., Wuensch S. A., Matta K. L., Lau J. T. (2003) Glycobiology 13, 591–600 [DOI] [PubMed] [Google Scholar]
- 6.Jamieson J. C., Lammers G., Janzen R., Woloski B. M. (1987) Comp. Biochem. Physiol. 87, 11–15 [DOI] [PubMed] [Google Scholar]
- 7.Lammers G., Jamieson J. C. (1986) Comp. Biochem. Physiol.-B: Comp. Biochem. 84, 181–187 [DOI] [PubMed] [Google Scholar]
- 8.Woloski B. M., Gospodarek E., Jamieson J. C. (1985) Biochem. Biophys. Res. Commun. 130, 30–36 [DOI] [PubMed] [Google Scholar]
- 9.Evans I. M., Hilf R., Murphy M., Bosmann H. B. (1980) Cancer Res. 40, 3103–3111 [PubMed] [Google Scholar]
- 10.Maguire T. M., Coughlan C. M., Seckl J. R., Breen K. C. (1998) Biochim. Biophys. Acta 1379, 23–28 [DOI] [PubMed] [Google Scholar]
- 11.Kitazume S., Suzuki M., Saido T. C., Hashimoto Y. (2004) Glycoconj. J. 21, 25–29 [DOI] [PubMed] [Google Scholar]
- 12.Kitazume S., Saido T. C., Hashimoto Y. (2004) Glycoconj. J. 20, 59–62 [DOI] [PubMed] [Google Scholar]
- 13.Kitazume S., Nakagawa K., Oka R., Tachida Y., Ogawa K., Luo Y., Citron M., Shitara H., Taya C., Yonekawa H., Paulson J. C., Miyoshi E., Taniguchi N., Hashimoto Y. (2005) J. Biol. Chem. 280, 8589–8595 [DOI] [PubMed] [Google Scholar]
- 14.Kitazume S., Oka R., Ogawa K., Futakawa S., Hagiwara Y., Takigawa H., Kato M., Kasahara A., Miyoshi E., Taniguchi N., Hashimoto Y. (2009) Glycobiology 19, 479–487 [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto I., Futakawa S., Oka R., Ogawa K., Marth J. D., Miyoshi E., Taniguchi N., Hashimoto Y., Kitazume S. (2007) J. Biol. Chem. 282, 34896–34903 [DOI] [PubMed] [Google Scholar]
- 16.Kaplan H. A., Woloski B. M., Hellman M., Jamieson J. C. (1983) J. Biol. Chem. 258, 11505–11509 [PubMed] [Google Scholar]
- 17.Dall'Olio F., Chiricolo M. (2001) Glycoconj. J. 18, 841–850 [DOI] [PubMed] [Google Scholar]
- 18.Ip C., Dao T. (1978) Cancer Res. 38, 723–728 [PubMed] [Google Scholar]
- 19.Shah M. H., Telang S. D., Shah P. M., Patel P. S. (2008) Glycoconj. J. 25, 279–290 [DOI] [PubMed] [Google Scholar]
- 20.Thorne-Tjomsland G., Hosfield T., Jamieson J. C., Liu B., Nickerson P., Gough J. C., Rush D. N., Jeffery J. R., McKenna R. M. (2000) Transplantation 69, 806–808 [DOI] [PubMed] [Google Scholar]
- 21.Gracheva E. V., Golovanova N. K., Ezhov M. V., Malyshev P. P., Kukharchuk V. V., Prokazova N. V. (1999) Biochemistry 64, 1315–1319 [PubMed] [Google Scholar]
- 22.Nasirikenari M., Segal B. H., Ostberg J. R., Urbasic A., Lau J. T. (2006) Blood 108, 3397–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasirikenari M., Chandrasekaran E. V., Matta K. L., Segal B. H., Bogner P. N., Lugade A. A., Thanavala Y., Lee J. J., Lau J. T. (2010) J. Leukoc. Biol. 87, 457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandrasekaran E. V., Jain R. K., Larsen R. D., Wlasichuk K., Matta K. L. (1995) Biochemistry 34, 2925–2936 [DOI] [PubMed] [Google Scholar]
- 25.Weinstein J., de Souza e Silva U., Paulson J. C. (1982) J. Biol. Chem. 257, 13845–13853 [PubMed] [Google Scholar]
- 26.Kono M., Ohyama Y., Lee Y. C., Hamamoto T., Kojima N., Tsuji S. (1997) Glycobiology 7, 469–479 [DOI] [PubMed] [Google Scholar]
- 27.Okajima T., Fukumoto S., Miyazaki H., Ishida H., Kiso M., Furukawa K., Urano T. (1999) J. Biol. Chem. 274, 11479–11486 [DOI] [PubMed] [Google Scholar]
- 28.Hennet T., Chui D., Paulson J. C., Marth J. D. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 4504–4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh S., Bandulet C., Nitschke L. (2006) Int. Immunol. 18, 603–611 [DOI] [PubMed] [Google Scholar]
- 30.Collins B. E., Smith B. A., Bengtson P., Paulson J. C. (2006) Nat. Immunol. 7, 199–206 [DOI] [PubMed] [Google Scholar]
- 31.Crespo H. J., Cabral M. G., Teixeira A. V., Lau J. T., Trindade H., Videira P. A. (2009) Immunology 128, e621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amano M., Galvan M., He J., Baum L. G. (2003) J. Biol. Chem. 278, 7469–7475 [DOI] [PubMed] [Google Scholar]
- 33.Woodard-Grice A. V., McBrayer A. C., Wakefield J. K., Zhuo Y., Bellis S. L. (2008) J. Biol. Chem. 283, 26364–26373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaikh F. M., Seales E. C., Clem W. C., Hennessy K. M., Zhuo Y., Bellis S. L. (2008) Exp. Cell Res. 314, 2941–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seales E. C., Shaikh F. M., Woodard-Grice A. V., Aggarwal P., McBrayer A. C., Hennessy K. M., Bellis S. L. (2005) J. Biol. Chem. 280, 37610–37615 [DOI] [PubMed] [Google Scholar]
- 36.Yasukawa Z., Sato C., Kitajima K. (2005) Glycobiology 15, 827–837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.