Abstract
We have used limited trypsin digestion and reactivity with PEG-maleimides (MPEG) to study Ca2+-induced conformational changes of IP3Rs in their native membrane environment. We found that Ca2+ decreased the formation of the 95-kDa C-terminal tryptic fragment when detected by an Ab directed at a C-terminal epitope (CT-1) but not with an Ab recognizing a protected intraluminal epitope. This suggests that Ca2+ induces a conformational change in the IP3R that allows trypsin to cleave the C-terminal epitope. Half-maximal effects of Ca2+ were observed at ∼0.5 μm and was sensitive to inhibition by IP3. Ca2+ also stimulated the reaction of MPEG-5 with an endogenous thiol in the 95-kDa fragment. This effect was eliminated when six closely spaced cysteine residues proximal to the transmembrane domains were mutated (C2000S, C2008S, C2010S, C2043S, C2047S, and C2053S) or when the N-terminal suppressor domain (amino acids 1–225) was deleted. A cysteine substitution mutant introduced at the C-terminal residue (A2749C) was freely accessible to MPEG-5 or MPEG-20 in the absence of Ca2+. However, cysteine substitution mutants in the interior of the tail were poorly reactive with MPEG-5, although reactivity was enhanced by Ca2+. We conclude the following: a) that large conformational changes induced by Ca2+ can be detected in IP3Rs in situ; b) these changes may be driven by Ca2+ binding to the N-terminal suppressor domain and expose a group of closely spaced endogenous thiols in the channel domain; and c) that the C-terminal cytosol-exposed tail of the IP3R may be relatively inaccessible to regulatory proteins unless Ca2+ is present.
Keywords: Calcium, Calcium Intracellular Release, Calcium Transport, Inositol Phosphates, Inositol Phospholipid
Introduction
Inositol 1,4,5-trisphosphate receptors (IP3Rs)3 are large tetrameric intracellular Ca2+-release channels that mediate the release of Ca2+ from endoplasmic reticulum stores into the cytosol in response to IP3 (1–5). Three different isoforms exist, which can form homo- and heteroligomers. All three isoforms consist of an IP3 binding domain in the N-terminal region, a channel domain containing six transmembrane segments in the C-terminal region and an intervening regulatory domain. Apart from IP3, the principal regulator of the channel is Ca2+, which exerts a biphasic effect on channel function with activation at low concentrations and inhibition at high concentrations. The structural organization of this complex protein and the mechanism by which binding of IP3 and Ca2+ are linked to channel gating are active areas of investigation.
An important feature of the mechanism of the channel involves conformational changes in the protein. In particular, the gating mechanism is thought to involve conformational changes initiated by IP3 binding that are propagated to various regions of the channel domain (6, 7). The IP3 binding domain of the receptor is organized into a “core” segment that binds IP3 with high affinity (amino acids 226–604) and a “suppressor domain” that inhibits IP3 binding to the core (amino acids 1–225). Crystal structures for the suppressor domain and the IP3-liganded core domain have been obtained (8, 9). Biophysical measurements using fusion proteins encoding the ligand binding domain have suggested that IP3 favors the formation of compact conformations, whereas Ca2+ promotes the formation of more extended structures (10). Conformational changes induced by IP3 also have been observed by gel-filtration analysis (11) and have been utilized to design probes for the measurement of IP3 in cells (12, 13). Conformational changes promoted by Ca2+ have been observed in electron microscopy analysis of detergent purified IP3Rs from mouse cerebellum. In this system, Ca2+ transforms receptors from a square to a windmill shape (14, 15). The relationship of the conformational changes detected in fusion proteins or isolated purified receptors to the conformational dynamics of full-length receptors embedded in native membranes remains to be established.
In the present study, we have utilized two indirect biochemical approaches to measure the Ca2+-induced conformational changes in native IP3Rs in situ. Our experiments are focused on the 95kDa C-terminal trypsin fragment containing the channel domain and the cytosol-exposed C-terminal tail. The results provide insights into the structural changes of the receptor and suggest that accessibility of critical regions of the receptor to regulatory proteins also could be altered by Ca2+-induced conformational changes.
EXPERIMENTAL PROCEDURES
Materials
Methoxy-polyethylene glycol maleimide (MPEG) (molecular weight of 20,000) and (molecular weight of 5,000) were from NEKTAR Therapeutics (San Carlos, CA). Rat brains were obtained from Pel Freeze Biologicals (Rogers, AR). All other chemicals were purchased from Sigma-Aldrich.
Antibodies
The Ab recognizing the C-terminal epitope (amino acids 2733–2749; designated CT-1 Ab) of type I IP3R present in trypsin fragment V has been characterized previously (16). An alternative Ab (designated IL-3) recognizing an epitope within the intraluminal loop (amino acids 2499–2516) between transmembrane domains 5 and 6 also has been described previously (17). Both Abs were raised in rabbits by Cocalico Biologicals (Reamstown, PA) and were affinity-purified using the antigenic peptide coupled to Ultralink beads by procedures described by the manufacturer (Pierce, Rockford, IL).
Expression Constructs
The wild-type cDNA encoding the rat type I IP3R SI(-)/SII(+)/SIII(+) splice variant in pCMV3 was a kind gift of Dr. Thomas Sudhof (University of Texas, Southwestern Medical Center) (18). The point mutants introducing cysteine substitutions into the C-terminal tail (S2681C, S2703C, S2716C, and A2749C) were made using the QuikChange mutagenesis kit (Stratagene, CA) utilizing a cassette encompassing the BstBI/XbaI fragment of the type I IP3R in pBluescript (Invitrogen). Mutants were confirmed by sequencing, and the BstBI/XbaI digested inserts were subcloned into the full wild-type IP3R cDNA in pCMV3. The D442N mutant has been described previously (19). The E2100D Ca2+ regulation mutant (20) was a kind gift of Dr. Kevin Foskett. The 1–225 suppressor domain deletion mutant was a kind gift of Drs. Humberdt deSmedt and Jan Parys.
Cell Culture and Transfection
COS-7 cells were grown on 100- or 150-mm plates (Sarstedt) in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% fetal bovine serum, 0.1 mg/ml streptomycin, and 100 international units/ml penicillin (all from Invitrogen) until 70–80% confluent. Transfections typically were done overnight in Dulbecco's modified Eagle's medium without serum. LT-1 (Mirus) and NovaFECTOR (VennNova, Inc.) were used together during transfections, and each was added at a cationic lipid to DNA ratio of 1:1. Transfections typically involved 5 μg and 20 μg of DNA for 100- and 150-mm plates, respectively. After 24 h, serum containing Dulbecco's modified Eagle's medium was added, and cells were used 48 h after transfection.
Preparation of Microsomes
At 48-h post-transfection, confluent COS-7 cells were washed twice with ice-cold phosphate-buffered saline and scraped into isolation buffer containing 320 mm sucrose, 0.5 mm EGTA, and 10 mm Tris (pH 7.8). 50 ml of this buffer was supplemented with one protease inhibitor mixture tablet (Roche Applied Science). Lysates were prepared from the cells by passing them five times through a 26.5-gauge needle. Cell debris was removed by centrifuging at 500 × g for 5 min, and the supernatant was spun for an additional 50 min at 100,000 × g. The microsome pellet was resuspended in isolation buffer. Cerebellum microsomes were prepared by first excising the cerebellum from frozen rat brains. The tissue was homogenized 10 times in isolation buffer using a Dounce homogenizer with a tight fitting pestle. The homogenate was spun at 500 × g for 15 min, and the supernatant was removed and spun for an additional 50 min at 100,000 × g. The microsome pellet was resuspended in isolation buffer. All microsomes were either used fresh or stored frozen in liquid nitrogen.
Reaction with MPEG and Trypsin Digestion
Microsome preparations were incubated at a final protein concentration of 0.5 mg/ml in a buffer containing 120 mm NaCl, 20 mm Tris-HCl (pH 7.2) and 0.5 mm HEDTA in the presence or absence of MPEG-5 or MPEG-20. Incubations were carried out at room temperature for the indicated times. The reaction was stopped by the addition of 20 mm dithiothreitol. Trypsin digestion was carried out subsequent to MPEG reaction at a final concentration of 4 μg/ml for 5 min at room temperature. Titrations with different concentrations of Ca2+ were carried out by addition of 10× stock solutions of Ca2+ buffers containing (final concentration) 5 mm TrisHepes (pH 7.5), 0.5 mm HEDTA, and different concentrations of CaCl2 to achieve the indicated concentrations of free Ca2+ as determined by calibration with the fluorescent dyes Calcium orange and Calcium green-5N (Invitrogen). The Kd values used in these calculations were 0.22 and 14 μm, respectively. Where Sr2+ and Ba2+ were used, the free divalent cation concentrations were calculated using the program MAXC (Chris Patton, Stanford University).
Electrophoresis and Immunoblotting
7% gels were transferred to nitrocellulose membranes (Bio-Rad) and blocked in a 10% milk solution in Tris-buffered saline containing 0.1% Tween 20. Blots were developed with chemiluminescent substrates (Pierce). In cases where a blot was probed sequentially with more than one antibody, the nitrocellulose was stripped at 60 °C for 30 min in stripping buffer (2% SDS, 100 mm β-mercaptoethanol, and 62.5 mm Tris-HCl (pH 6.8)) before probing with the next antibody.
Akt Kinase Phosphorylation of IP3Rs
Cerebellum microsomes were first stripped of any endogenous associated kinases by treatment for 20 min in 0.5 m KCl, 50 mm Tris-HCl (pH 7.2), and 0.25 mm EDTA. The membranes were reisolated by centrifugation (30 min, 100,000 × g) and incubated at 1 mg protein/ml in the presence or absence of 2.2 μm free Ca2+ and 0.1 mm MgATP, 10 nm okadaic acid, 20 μCi [γ-32P]ATP, and 160 ng of recombinant activated human Akt kinase-1 (Biomol). The membranes were incubated for 2 h at 30 °C and lysed in 500 μl of a buffer containing 150 mm NaCl, 50 mm TrisHepes (pH 7.8), 1% Triton X-100, 1 mm EDTA, 0.5 mm phenylmethylsulfonyl fluoride, and a protease inhibitor mixture (Roche Applied Science). IP3R was immunoprecipitated overnight with CT-1 Ab and processed on a 5% gel. The gel was transferred to nitrocellulose, and radioactive IP3R was detected and quantitated by phosphorimaging.
RESULTS
Ca2+ Alters Accessibility of the C-terminal Tail to Trypsin
Trypsin cleaves the type I IP3R into five well defined fragments of which the ∼95-kDa trypsin fragment V contains the channel domains and the cytosol-exposed C-terminal tail of 160 amino acids (21). The formation of fragment V in cerebellum microsomes after a 5-min trypsin treatment is shown in Fig. 1A, lane 2. Fragment V was detected by immunoblotting with a C-terminal Ab (CT-1) recognizing the last 18 amino acids located in the C-tail. The inclusion of 0.9 μm free Ca2+ for 5 min prior to trypsin digestion led to a marked inhibition of the formation of fragment V (Fig. 1A, lane 4). This partially was prevented by the inclusion of 10 μm IP3 together with Ca2+ (Fig. 1A, lane 5), although IP3 added alone was without effect (lane 3). The data from multiple experiments are quantitated in Fig. 1C. The apparent loss of fragment V in the presence of Ca2+ was not due to altered proteolysis because the same blots probed for fragment V with an Ab that recognized a protected endoplasmic reticulum intraluminal epitope (IL-3 Ab) showed no changes in the levels of fragment V (Fig. 1B). Our interpretation of this data is that Ca2+ induces a conformational change in the receptor that allows trypsin access to the C-terminal tail, resulting in cleavage and loss of the CT-1 Ab epitope.
FIGURE 1.
The effect of Ca2+ and IP3 on the formation of trypsin fragment V. Cerebellum microsomes were incubated for 5 min with 0.9 μm free Ca2+ and/or 10 μm IP3 as indicated (A). The membranes were then digested with trypsin (4 μg/ml) for a further 5 min, and the samples were then analyzed by SDS-PAGE and immunoblotting with an Ab directed at the terminal 17 amino acids of the C terminus (CT-1 Ab) or an Ab raised to a peptide sequence in the intraluminal loop between TM -5 and -6 (IL-3 Ab) (B). Densitometric quantitation of blots in A from three separate experiments are shown (mean ± S.E.) (C). Quantitation was carried out using NIH ImageJ software. WB, Western blot.
Additional experiments using the loss of the CT-1 epitope as a probe for the Ca2+-dependent conformational change are shown in Fig. 2. The effects of Ca2+ could be reversed by the addition of EGTA 30 s prior to addition of trypsin (Fig. 2A, lane 4). The addition of Ca2+ after trypsin digestion had been inactivated produced no effect on the detection of fragment V. The effects of Ca2+ were not mimicked or inhibited by Mg2+, suggesting a high degree of specificity consistent with the Ca2+ regulatory sites identified previously in IP3Rs (22). Sr2+ mimicked the effect of Ca2+, albeit at higher concentrations, whereas Ba2+ was relatively ineffective (Fig. 2B). This divalent cation specificity is similar to that reported for an activatory modulatory site on rat liver IP3Rs (23). The concentration dependence on Ca2+ is shown in Fig. 2C. Half-maximal inhibition of the CT-1 detectable fragment V occurred at ∼0.5 μm Ca2+. The shape of the titration curve was particularly steep in the range 0.35–0.6 μm. The presence of IP3 made the titration curve shallower and decreased the sensitivity to Ca2+. The effects of IP3 were not observed with the inactive isomer Ins-(1,3,4)-P3 (data not shown).
FIGURE 2.
Reversibility, divalent cation dependence and concentration dependence of the effects of Ca2+. A, cerebellum microsomes were incubated for 5 min in the absence (lane 1) or presence (lane 2) of 2.2 μm free Ca2+ for 5 min, followed by a 5-min digestion with trypsin (4 μg/ml). 1 mm EGTA was added 1 min or 30 s prior to trypsin digestion in lanes 3 and 4, respectively. In lane 5, the trypsin digestion was carried out first for 5 min in the absence of Ca2+ (0.5 mm HEDTA). The digestion was stopped by the addition of 10 μg/ml soybean trypsin inhibitor, and 0.4 mm CaCl2 was added to give a final free concentration 2.2 μm. B, microsomes were incubated for 5 min with 1 mm Mg2+, 2.2 μm Ca2+, or the indicated free concentrations (μm) of Sr2+ or Ba2+ (as described under “Experimental Procedures”). The membranes were then digested for 5 min with trypsin. C, cerebellum microsomes were incubated for 5 min with the indicated concentrations of buffered free Ca2+ in the presence or absence of 10 μm IP3. The samples were digested for 5 min with trypsin and immunoblotted with CT-1 Ab. The top panel shows a representative immunoblot of the 95-kDa fragment V, and the lower panel is a compilation of the data from three separate experiments (mean ± S.E.).
Ca2+ Alters MPEG Reactivity with Endogenous and Mutant Cysteines in the C-terminal Domain
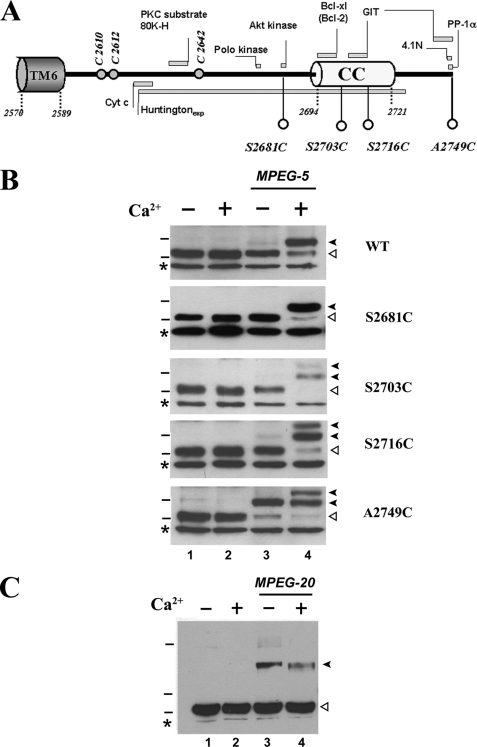
To examine accessibility and conformational changes of domains, we used the alternative strategy of measuring reactivity of endogenous and mutant cysteines with MPEG using gel-shift assays (24, 25). There are 13 potentially MPEG-accessible endogenous cysteines in fragment V of which three are located in the C-terminal tail, which is also the site of interaction with several important regulatory proteins (Fig. 3A). To assess accessibility we introduced four additional cysteines into the C-terminal tail (S2681C, S2703C, S2716C, and A2749C). All of these substitution mutants retained channel function as measured with a 45Ca2+ flux assay (26) (data not shown). In agreement with our previous observations (25), none of the endogenous cysteines in the wild-type receptor fragment V was reactive with MPEG-5 in the absence of Ca2+ based on the lack of a gel shift of fragment V detected with IL-3 Ab (Fig. 3B, top panel, lane 3). However, in the presence of Ca2+, a single shifted band was observed (Fig. 3B, top panel, lane 4), indicating that Ca2+ led to the exposure of at least one endogenous reactive cysteine. The cysteine substitution mutant placed at the extreme C terminus of the protein (A2749C) was highly accessible, as indicated by reaction with MPEG-5 even in the absence of Ca2+ (Fig. 3B, bottom panel, lane 3). However, the three cysteine substitution mutants placed further into the tail did not react with MPEG-5 over the 5-min incubation period. When Ca2+ was present the A2749C mutant producing a doublet of shifted bands, presumably corresponding to the combined reaction of the endogenous and mutant cysteine sites (Fig. 3B, bottom panel, lane 4). The same doublet also was seen for the S2716C and S2703C mutant. Because neither of these mutants reacted appreciably in the absence of Ca2+, this suggests that Ca2+ may enhance the accessibility of this portion of the C-tail. Only the reaction with the endogenous cysteine was seen in the S2681C mutant incubated in the presence of Ca2+. The degree of accessibility of the C-terminal A2749C residue was assessed by examining reactivity with the larger 20-kDa MPEG derivative (Fig. 3C). This mutant reacted with MPEG-20 in the absence of Ca2+, but no additional bands were observed in the presence of Ca2+. These results are compatible with a restricted environment around the endogenous cysteine that would be unfavorable for reaction with the larger MPEG-20.
FIGURE 3.
The effect of calcium on the MPEG reactivity of cysteine substitution mutants in the C-terminal tail. A, schematic of the C-terminal tail of the IP3R with the location of three endogenous cysteines and four cysteine substitution mutants indicated by open circles. The location of the transmembrane domain 6 (S6) and the coiled-coil domain (CC) as well as several sites for interaction with key proteins are indicated (for additional details, see text). GIT; G-protein-coupled receptor kinase-interacting protein, PP1-α; protein phosphatase 1-α, 4.1N; neuron-specific isoform of erythrocyte protein band 4.1; Cyt c, cytochrome c; PKC, protein kinase C. B, microsomal membranes were prepared from COS cells transfected with wild-type IP3R (WT) or the indicated cysteine substitution mutants and then reacted with 0.5 mm MPEG-5 for 5 min in the presence or absence of a free [Ca2+] of 2.2 μm. The samples were then digested with trypsin. Cleaved fragments from the C-terminal domain were detected on immunoblots with affinity-purified IL-3 Ab. The open arrow indicates the position of the 95-kDa fragment V, and the closed arrows indicate its shift to higher molecular weights. The asterisk identifies a nonspecific IL3-reactive band that is not shifted by MPEG-5. C, experiments with MPEG-20 were performed with membranes expressing the A2749C mutant as described in B.
Ser2681 is the site proposed to be phosphorylated by Akt kinase (27, 28). It is therefore somewhat surprising that a cysteine mutant introduced at this site was unreactive with MPEG-5. However, reactivity to MPEG was monitored over a short period, whereas incubations with kinases are carried out for longer times, and the accessibility criteria for a protein kinase and an MPEG molecule could be very different. The physiological significance of an increased access of the C-tail in the presence of Ca2+ was examined by measuring the phosphorylation of cerebellar IP3Rs by Akt kinase in vitro in the presence or absence of Ca2+ (Fig. 4). The incubation of isolated membranes for 2 h with activated Akt kinase led to the phosphorylation of the receptor, which was stimulated 2.5-fold by incubation in the presence of 2.2 μm free Ca2+. Other substrate proteins in the membranes did not show a similar enhancement (data not shown), indicating that the effect of Ca2+ is not due to a general enhancement of the catalytic activity of the recombinant Akt kinase. The data are consistent with the hypothesis that a Ca2+-induced conformation change in the IP3R causes an increased accessibility of the C-tail to interacting proteins. We attempted to test this further by examining the effects of Ca2+ on the interaction of added cytochrome c or endogenous Bcl-2, but we were not able to co-immunoprecipitate these proteins with IP3Rs under our experimental conditions (data not shown). The presence of Ca2+ did not alter the co-immunoprecipitation of IP3Rs with PP1-α (data not shown), as anticipated for a protein that interacts with the last 12 C-terminal residues (Fig. 3A) (29).
FIGURE 4.
Phosphorylation of IP3Rs in cerebellum microsomes by Akt kinase in the presence and absence of Ca2+. Cerebellum microsomes were phosphorylated with [γ-32P]ATP and recombinant Akt kinase in the presence or absence of 2.2 μm Ca2+ as described under “Experimental Procedures.” After 2 h at 30 °C, the membranes were lysed and immunoprecipitated with CT-1 Ab. The immunoprecipitates were analyzed for radioactivity by phosphorimaging and for IP3R levels by immunoblotting. The fold increase in radioactivity was quantitated and is shown as mean ± S.E. for three experiments.
Mutagenesis Studies to Identify Endogenous Reactive Cysteines and Ca2+ Modulatory Sites
To locate the endogenous cysteine(s) reacting with MPEG-5 in the presence of Ca2+, we have utilized a mutagenesis approach (Fig. 5A). Initially, we examined the effect of removing the three endogenous cysteine residues in the C-tail by making progressive deletions from the C terminus (Fig. 5B). These “tail-less” mutants were tagged at the C terminus with the HA epitope, and as a control, we examined the behavior of the wild-type receptor tagged with HA. Fig. 5B shows that Ca2+ treatment caused the loss of HA but not IL-3 immunoreactivity in fragment V, confirming that the native cerebellum and recombinant receptor behave similarly. Deletion of 24 amino acids from the C-tail (TL1 mutant) completely prevented the loss of HA signal induced by Ca2+. This suggests that the trypsin cleavage site must be located within the terminal 24 amino acids, and several candidate residues are underlined in the sequence shown in Fig. 5C. The cleavage at sequences in the distal portion of the C-tail also is consistent with the lack of a noticeable decrease of molecular weight of the IL-3 reactive fragment V band. Interestingly, the TL1 deletion mutant showed some reactivity with MPEG-5 even in the absence of Ca2+. The deletion of 60 (TL2 mutant) or 141 amino acids (TL5 mutant) showed maximum reactivity with MPEG-5 in the complete absence of Ca2+. These data suggest that the endogenous cysteine exposed by Ca2+ in wild-type receptors already is exposed when significant portions of the C-tail are deleted. Because an MPEG-5 shift is observed with the TL-5 mutant, we conclude that the endogenous reactive cysteine probably does not involve the three residues located in the C-tail.
FIGURE 5.
MPEG-5 reactivity with cysteine mutants. A, a schematic of trypsin fragment V with the cytosolic-facing 13 endogenous cysteines potentially accessible to MPEG-5 (orange) and seven inaccessible endogenous cysteines (purple). The central panel of blots (B) shows the effect of deleting segments of the C-terminal tail corresponding to the removal of 24 (TL-1), 60 (TL-2), and 141 (TL-5) amino acids. The latter construct removes the three endogenous cysteines in the C-tail. Each of the tail-less constructs contained a C-terminal HA-tag for detection. Microsomal membranes were prepared and incubated in the presence or absence of 2.2 μm free [Ca2+] for 5 min followed by a 5-min incubation with 0.5 mm MPEG-5 (lanes 3 and 4). All samples were processed on 7% SDS-PAGE. Unshifted and shifted bands (open and closed triangles, respectively) were detected by immunoblotting with IL-3 or HA antibodies. The sequences in C are derived from the C-terminal region of the receptor and indicate potential trypsin cleavage sites (underlined italic) preceding the CT-1 Ab epitope (pink line). The blots in D show the effect of mutating each of the 10 endogenous cysteines in the segment prior to the transmembrane domains. The highlighted panel shows the elimination of the MPEG shift upon mutating a cluster of six closely spaced cysteines. The experimental conditions are as described for B except IL-3 Ab was used for detection of all blots. ER, endoplasmic reticulum.
We addressed the role of the 10 cysteine residues located proximal to the transmembrane domains by making mutations individually or in groups (Fig. 5, A and D). The data show that each of the mutations retained a Ca2+-dependent MPEG-5 shift and, in some cases, even showed enhanced multiple shifts (e.g. C2008S/C2010S). It is possible that MPEG-5 may react with multiple cysteines in a closely spaced cluster. The reaction with any one cysteine may prevent reaction at adjacent cysteines because of steric hindrance due to the large MPEG molecule and the relatively restricted space available at the reactive site. In accord with this hypothesis, the mutation of six cysteines (C2000S, C2008S, C2010S, C2043S, C2047S, and C2053S) in two closely spaced clusters was necessary to eliminate the Ca2+-dependent MPEG-5 shift. It is possible that the absence of MPEG-5 reactivity could be secondary to a major structural defect in the six-cysteine mutant. This seems unlikely because the mutant was cut by trypsin to form the normal fragment V (Fig. 5D) and retained 43 ± 7% (n = 3) of the channel activity of the wild-type receptor measured with 10 μm IP3 using 45Ca2+ flux assays (data not shown).
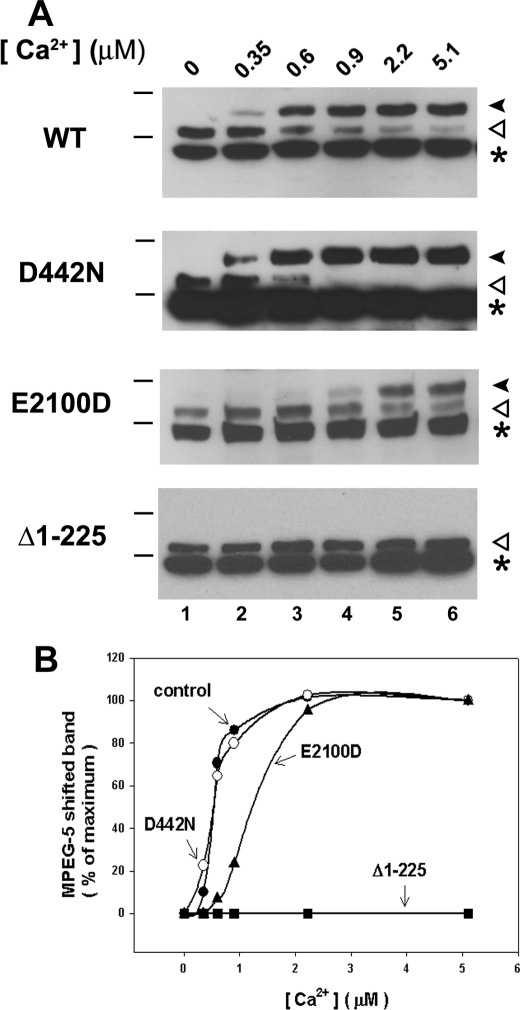
In Fig. 6, we have used mutations that are known to modify the Ca2+ sensitivity of channel function. The D442N mutant previously has been shown to have a decreased sensitivity to inhibition of the channel by high concentrations of Ca2+ (19). The E2100D mutation has been shown to result in shifts of both the activation and inhibition of channel function to higher Ca2+ concentrations (20). The D442N mutation did not have any effect on the Ca2+-induced MPEG shift of the 95-kDa fragment. However, the E2100D mutant did decrease the sensitivity of the Ca2+ effect (Fig. 6B). We also tested a construct in which the entire N-terminal suppressor domain was deleted (Δ1–225). A putative Ca2+ binding site is thought to be present in this domain (30). This construct was cleaved normally by trypsin to generate the C-terminal 95-kDa fragment, but no reactivity with MPEG-5 was observed in the presence of Ca2+. The Δ1–225 mutant also failed to show a Ca2+-induced trypsin cleavage of the CT-1 epitope (data not shown).
FIGURE 6.
Ca2+ sensitivity of MPEG-5 reactivity in selected IP3R mutants. A, MPEG-5 reaction of the endogenous cysteine in the 95-kDa C-terminal trypsin fragment of microsomes prepared from COS cells transfected with the indicated mutants was measured at different buffered free [Ca2+] by immunoblotting with the intraluminal IL-3 Ab. The position of the 95-kDa unshifted and shifted bands are shown by the open and closed arrows, respectively. The asterisk indicates the presence of a variable intensity, nonspecific band detected by the Ab. B, the magnitude of the shifted band was estimated densitometrically using low exposure blots and is expressed as a percentage of the maximum shift. The data are the mean of two experiments on independent microsome preparations. WT, wild-type.
DISCUSSION
In the present study, we have used indirect biochemical approaches to observe conformational changes in full-length IP3Rs in their native membrane environment. To do this, we measured changes in accessibility of the protein to trypsin and changes of MPEG reactivity with endogenous and mutant thiols. The experiments focused on the 95-kDa C-terminal trypsin fragment containing the channel domain and C-terminal tail. The results reveal large changes in accessibility induced by Ca2+ in this segment of the receptor. It is possible that this reflects direct effects of Ca2+ on the conformation of the 95-kDa fragment. However, large conformation transitions in the density corresponding to the channel domain were not observed previously in EM studies (15). Hence, we favor the view that the changes in accessibility arise indirectly because of Ca2+-induced conformational changes occurring elsewhere in the protein. The structural and physiological implications of the results are discussed below.
Although homology models of the channel domain have been constructed (31), a detailed molecular structure of the entire 95-kDa C-terminal segment is not available. However, several groups have obtained low resolution structures of purified IP3Rs from electron microscopy studies (32–34). Although there are considerable differences in these reports, particularly in the assignment of the density corresponding to the ligand binding domain, all the structures visualized are ∼200Å3 and show overall 4-fold symmetry. The receptor has been described variously as resembling a flower (32), a hot air balloon (33), or a pinwheel (34). A model consistent with our observations is shown in Fig. 7 and is based on the EM structure reported by da Fonseca et al. (32). The model shows the presence of a small vestibule/space on the cytosolic side between the channel domain (Fig. 7, shown in blue) and the rest of the receptor, which is narrow at the center of the receptor (∼15 Å) and widens at the periphery (∼30 Å) (32). A similarly located cavity also has been noted in the other EM models (33, 34). We suggest that in the presence of Ca2+, a large conformational transition of the receptor widens this space sufficiently to allow MPEG-5 (hydrated diameter of ∼50 Å (35)) to access a cluster of reactive cysteines located in this compartment. The reactivity of more than one cysteine is inferred from mutagenesis experiments, which indicate that a group of six closely spaced cysteines must be mutated to remove the MPEG-5 reactivity. The available space around the reactive cysteines must be relatively restricted because no reactivity is observed with the much larger MPEG-20 (hydrated diameter of ∼130 Å (36)). In Fig. 7, the Ca2+-dependent conformational transition is shown as a large outward movement of the densities that have been assigned to the regulatory domain of the receptor (Fig. 7, shown in gray) (32). This Ca2+-induced change could be the basis of the previously documented “square” to “windmill” conformational transition seen in EM studies of the purified cerebellar IP3 receptor (15). This model predicts that Ca2+ also should promote large changes in the MPEG accessibility of cysteines in the ligand binding domain (Fig. 7, shown in red). This has been confirmed experimentally (37). It should be noted that the Ca2+-dependent change in MPEG accessibility of fragment V also was observed when the MPEG reaction was carried out after the receptor had been first cleaved with trypsin (data not shown). This is in agreement with previous findings that the receptor retains function even when partially digested with trypsin (38).
FIGURE 7.
Model to account for Ca2+ effects on MPEG-5 reactivity and trypsin cleavage of the C-terminal tail. A, the model shows a representation of the IP3R based on the EM structure reported by da Fonseca et al. (32) in which the densities corresponding to the ligand binding (pink), regulatory (gray), and channel domains (blue) are as shown. A cross-sectional side view with just two subunits is shown for clarity. The C-terminal tail is shown as projecting perpendicularly from the membrane and running in a central channel formed by the ligand binding domain. The model draws attention to a small vestibule above the channel domain, which is proposed to enlarge as a result of a Ca2+-driven conformational change arising from the outward movement of ligand binding and regulatory domains. This results in increased accessibility of an endogenous cluster of cysteines located in the vestibule to MPEG-5. The opening of a crevice in the ligand binding domain also allows increased accessibility of trypsin to a cleavage site in the tail. For additional details, see text.
Our experiments also provide information on the accessibility of residues in the C-terminal tail. A cysteine substitution introduced at the last amino acid of the tail (A2749C) was readily accessible to both MPEG-5 and MPEG-20 in the absence of Ca2+ (Fig. 3). By contrast, none of other three cysteine substitution mutants in the C-tail (S2681C, S2703C, and S2716C) or the three endogenous cysteines (Cys2610, Cys2613, and Cys2642) were reactive with MPEG-5. The S2703C and S2716C sites are located in a coiled-coil domain present in the C-tail (Fig. 3A), and it is therefore possible that these sites are occluded because of oligomerization of this domain (7). However, in the presence of Ca2+, there was an enhanced reactivity of the S2716C site and to a lesser extent the S2703C site. We also have shown that trypsin (22 kDa) gains access to a cleavage site within the last 24 amino acids of the C-tail only in the presence of Ca2+. Interestingly, Yoshikawa et al. (21) also observed that the tip of the C-terminal tail was labile to trypsin, although in their study, this occurred only with higher concentrations of trypsin, and Ca2+ was not included in the incubations. We conclude from our data that only the most terminal amino acids of the C-tail are solvent-exposed in the absence of Ca2+ and that the conformational changes induced by Ca2+ cause an increase in accessibility of the tail which is most marked (based on the MPEG gel-shift assay) for the ∼35 C-terminal amino acids.
Our interpretation of these observations is based on the hypothesis that the 160 amino acid C-tail projects perpendicularly from the membrane and runs in a central channel formed by the ligand binding domain (Fig. 7). This arrangement is consistent with the limited access of endogenous or substituted cysteine residues in the C-tail in the absence of Ca2+. In this model, the terminal residues of the C-tail are proposed to project from the central density into the solvent space. This orientation is analogous to the C-tail of the full-length KcsA channel, whose recently published crystal structure shows a long perpendicular helix with a small bulge helix near the membrane (39). This model also is consistent with the reported ability of recombinant domains from the IP3R C-tail to form dimers (40) or tetramers (7). When Ca2+ is present, we suggest that a conformational transition initiated in the ligand binding domain and transmitted to the connected side arms, results in the opening of a crevice in the central density that would account for the enhanced accessibility of the C-tail under these conditions (Fig. 7B). Deletion mutants of the C-tail appear to enhance reactivity of the endogenous cysteines in trypsin fragment V even without added Ca2+ (Fig. 5B). The reason for this is not clear, but we speculate that the absence of a correctly folded C-tail may increase the MPEG-5 accessible space around the reactive cysteines.
A number of proteins of different sizes have been proposed to interact with the C-tail at various locations (Fig. 3A). These include 4.1N, 98 kDa (41); BclXl, 26 kDa (42); PP1-α, 37 kDa (29); cytochrome c, 12 kDa (43), 80K-H protein kinase C substrate, 80 kDa (44), G-protein-coupled receptor kinase-interacting protein, 95 kDa (45); and Huntington-associated protein 1A, 70 kDa (46). In addition, Ser2618 is the phosphorylation site for Akt kinase (56 kDa (27, 28)), and Thr2656 is the phosphorylation site for Polo kinase (68 kDa (47)). Because of these interactions, it has generally been assumed that the C-tail is freely accessible along its entire length in the native receptor. The present data using MPEG reactivity and trypsin cleavage suggest that this may not be the case. In those instances where the protein interacts with the C-terminal 14 amino acids (e.g. PP1-α and 4.1N) accessibility should not be an issue. However, proteins interacting further into the tail would be expected to require Ca2+-induced conformational changes or other structural alterations of the receptor to facilitate accessibility to their binding sites. We have shown that the phosphorylation of the receptor by Akt kinase is indeed enhanced in the presence of Ca2+ (Fig. 4). Interestingly, the functional effect of cytochrome c is observed only in the presence of Ca2+ (48), and the interaction with G-protein-coupled receptor kinase-interacting protein also is stimulated by cytosolic Ca2+ elevation (45). Based on the available data, we would suggest that Ca2+ could be an important factor regulating protein-protein interactions with the IP3R and that even a transient elevation of Ca2+ might be sufficient to allow association of interacting proteins with the C-tail.
Ca2+ activates channel function at low concentrations and has an inhibitory effect at higher concentrations. What, then, is the functional significance of the Ca2+-induced conformational changes observed in the present study? Whether these changes accompany activation or inhibition of the channel is difficult to assess from the present data. The half-maximal concentration over which the Ca2+ effects are observed are ∼0.5 μm. These concentrations are larger than required to activate the channel in 45Ca2+ flux assays on COS cell microsomes (26, 31). A key feature of the inhibitory site is that the affinity for Ca2+ is decreased by IP3 (5). The sensitivity of the Ca2+ effect on trypsin cleavage was decreased by IP3, although IP3 had no effects on the Ca2+ stimulation of MPEG reactivity (data not shown). This does not necessarily mean that Ca2+ mediates two different effects at two distinct sites. In the model shown in Fig. 7, conformational changes induced by IP3 in the central domain could interfere with the access of trypsin without necessarily affecting MPEG access. In our experiments Sr2+, rather than Ba2+, facilitated the conformational changes in the receptor. This is the specificity reported for the activatory binding site in the liver (23). The location and function of divalent cation binding sites in the IP3R has not been established firmly, and multiple Ca2+ binding sites could function to both activate and inhibit the channel (5). A “Ca2+ sensor” mutation (E2100D) has been described in which the Ca2+ sensitivity for activation and inhibition of the channel are decreased (20). This mutant also showed a decreased sensitivity for the Ca2+ effect on MPEG reactivity. In addition, the removal of the N-terminal suppressor domain was sufficient to prevent the Ca2+ effect entirely. These data support the view that the suppressor domain may be critically important for driving Ca2+-dependent conformational changes in the receptor. Further mutagenesis studies are required to identify distinct Ca2+ binding sites in the receptor and to understand the relationship of conformational changes in the protein to channel function.
Acknowledgments
We are grateful to Drs. Kevin Foskett, Jan Parys, Humbert de Smedt, and David Yule for supplying plasmids used in this study.
This work was supported by National Institutes of Health Grant DK34804 (to S. K. J.) and Training Grant T32-AA-7463 (to G. A.).
- IP3R
- inositol 1,4,5-trisphosphate receptor
- CT
- C-terminal epitope
- C-tail
- C-terminal tail
- HA
- hemagglutinin
- IP3
- myo-inositol 1,4,5-trisphosphate
- Ab
- antibody
- MPEG
- methoxy-polyethylene glycol maleimide
- HEDTA
- N-(2-hydroxyethyl)ethylenediaminetriacetic acid.
REFERENCES
- 1.Patel S., Joseph S. K., Thomas A. P. (1999) Cell Calcium 25, 247–264 [DOI] [PubMed] [Google Scholar]
- 2.Berridge M. J., Bootman M. D., Roderick H. L. (2003) Nat. Rev. Mol. Cell. Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 3.Taylor C. W., da Fonseca P. C., Morris E. P. (2004) Trends Biochem. Sci. 29, 210–219 [DOI] [PubMed] [Google Scholar]
- 4.Mikoshiba K. (2007) J. Neurochem. 102, 1426–1446 [DOI] [PubMed] [Google Scholar]
- 5.Foskett J. K., White C., Cheung K. H., Mak D. O. (2007) Physiol. Rev. 87, 593–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchida K., Miyauchi H., Furuichi T., Michikawa T., Mikoshiba K. (2003) J. Biol. Chem. 278, 16551–16560 [DOI] [PubMed] [Google Scholar]
- 7.Schug Z. T., Joseph S. K. (2006) J. Biol. Chem. 281, 24431–24440 [DOI] [PubMed] [Google Scholar]
- 8.Bosanac I., Alattia J. R., Mal T. K., Chan J., Talarico S., Tong F. K., Tong K. I., Yoshikawa F., Furuichi T., Iwai M., Michikawa T., Mikoshiba K., Ikura M. (2002) Nature 420, 696–700 [DOI] [PubMed] [Google Scholar]
- 9.Bosanac I., Yamazaki H., Matsu-Ura T., Michikawa T., Mikoshiba K., Ikura M. (2005) Mol. Cell 17, 193–203 [DOI] [PubMed] [Google Scholar]
- 10.Chan J., Whitten A. E., Jeffries C. M., Bosanac I., Mal T. K., Ito J., Porumb H., Michikawa T., Mikoshiba K., Trewhella J., Ikura M. (2007) J. Mol. Biol. 373, 1269–1280 [DOI] [PubMed] [Google Scholar]
- 11.Mignery G. A., Südhof T. C. (1990) EMBO J. 9, 3893–3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remus T. P., Zima A. V., Bossuyt J., Bare D. J., Martin J. L., Blatter L. A., Bers D. M., Mignery G. A. (2006) J. Biol. Chem. 281, 608–616 [DOI] [PubMed] [Google Scholar]
- 13.Shirakawa H., Ito M., Sato M., Umezawa Y., Miyazaki S. (2006) Biochem. Biophys. Res. Commun. 345, 781–788 [DOI] [PubMed] [Google Scholar]
- 14.Hamada K., Miyata T., Mayanagi K., Hirota J., Mikoshiba K. (2002) J. Biol. Chem. 277, 21115–21118 [DOI] [PubMed] [Google Scholar]
- 15.Hamada K., Terauchi A., Mikoshiba K. (2003) J. Biol. Chem. 278, 52881–52889 [DOI] [PubMed] [Google Scholar]
- 16.Joseph S. K., Samanta S. (1993) J. Biol. Chem. 268, 6477–6486 [PubMed] [Google Scholar]
- 17.Khan M. T., Joseph S. K. (2003) Biochem. J. 375, 603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mignery G. A., Newton C. L., Archer B. T., 3rd, Südhof T. C. (1990) J. Biol. Chem. 265, 12679–12685 [PubMed] [Google Scholar]
- 19.Joseph S. K., Brownell S., Khan M. T. (2005) Cell Calcium 38, 539–546 [DOI] [PubMed] [Google Scholar]
- 20.Tu H., Nosyreva E., Miyakawa T., Wang Z., Mizushima A., Iino M., Bezprozvanny I. (2003) Biophys. J. 85, 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshikawa F., Iwasaki H., Michikawa T., Furuichi T., Mikoshiba K. (1999) J. Biol. Chem. 274, 316–327 [DOI] [PubMed] [Google Scholar]
- 22.Taylor C. W., Laude A. J. (2002) Cell Calcium 32, 321–334 [DOI] [PubMed] [Google Scholar]
- 23.Marshall I. C., Taylor C. W. (1994) Biochem. J. 301, 591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu J., Deutsch C. (2001) Biochemistry 40, 13288–13301 [DOI] [PubMed] [Google Scholar]
- 25.Joseph S. K., Nakao S. K., Sukumvanich S. (2006) Biochem. J. 393, 575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehning D., Joseph S. K. (2000) J. Biol. Chem. 275, 21492–21499 [DOI] [PubMed] [Google Scholar]
- 27.Khan M. T., Wagner L., 2nd, Yule D. I., Bhanumathy C., Joseph S. K. (2006) J. Biol. Chem. 281, 3731–3737 [DOI] [PubMed] [Google Scholar]
- 28.Szado T., Vanderheyden V., Parys J. B., De Smedt H., Rietdorf K., Kotelevets L., Chastre E., Khan F., Landegren U., Söderberg O., Bootman M. D., Roderick H. L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 2427–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang T. S., Tu H., Wang Z., Bezprozvanny I. (2003) J. Neurosci. 23, 403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sienaert I., Nadif, Kasri N., Vanlingen S., Parys J. B., Callewaert G., Missiaen L., de Smedt H. (2002) Biochem. J. 365, 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schug Z. T., da Fonseca P. C., Bhanumathy C. D., Wagner L., 2nd, Zhang X., Bailey B., Morris E. P., Yule D. I., Joseph S. K. (2008) J. Biol. Chem. 283, 2939–2948 [DOI] [PubMed] [Google Scholar]
- 32.da Fonseca P. C., Morris S. A., Nerou E. P., Taylor C. W., Morris E. P. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 3936–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato C., Hamada K., Ogura T., Miyazawa A., Iwasaki K., Hiroaki Y., Tani K., Terauchi A., Fujiyoshi Y., Mikoshiba K. (2004) J. Mol. Biol. 336, 155–164 [DOI] [PubMed] [Google Scholar]
- 34.Serysheva I. I., Bare D. J., Ludtke S. J., Kettlun C. S., Chiu W., Mignery G. A. (2003) J. Biol. Chem. 278, 21319–21322 [DOI] [PubMed] [Google Scholar]
- 35.Howorka S., Movileanu L., Lu X., Magnon M., Cheley S., Braha O., Bayley H. (2000) J. Am. Chem. Soc. 122, 2411–2416 [Google Scholar]
- 36.Lin J. K., Ladisch M. R., Patterson J. A., Noller C. H. (1987) Biotechnol. Bioeng. 22, 976–981 [DOI] [PubMed] [Google Scholar]
- 37.Anyatonwu G., Joseph S. K. (2009) J. Biol. Chem. 284, 8093–8102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshikawa F., Iwasaki H., Michikawa T., Furuichi T., Mikoshiba K. (1999) J. Biol. Chem. 274, 328–334 [DOI] [PubMed] [Google Scholar]
- 39.Uysal S., Vásquez V., Tereshko V., Esaki K., Fellouse F. A., Sidhu S. S., Koide S., Perozo E., Kossiakoff A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 6644–6649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galvan D. L., Mignery G. A. (2002) J. Biol. Chem. 277, 48248–48260 [DOI] [PubMed] [Google Scholar]
- 41.Fukatsu K., Bannai H., Inoue T., Mikoshiba K. (2006) Biochem. Biophys. Res. Commun. 342, 573–576 [DOI] [PubMed] [Google Scholar]
- 42.White C., Li C., Yang J., Petrenko N. B., Madesh M., Thompson C. B., Foskett J. K. (2005) Nat. Cell Biol. 7, 1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boehning D., van Rossum D. B., Patterson R. L., Snyder S. H. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 1466–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawaai K., Hisatsune C., Kuroda Y., Mizutani A., Tashiro T., Mikoshiba K. (2009) J. Biol. Chem. 284, 372–380 [DOI] [PubMed] [Google Scholar]
- 45.Zhang S., Hisatsune C., Matsu-Ura T., Mikoshiba K. (2009) J. Biol. Chem. 284, 29158–29169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang T. S., Tu H., Chan E. Y., Maximov A., Wang Z., Wellington C. L., Hayden M. R., Bezprozvanny I. (2003) Neuron 39, 227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanderheyden V., Wakai T., Bultynck G., De Smedt H., Parys J. B., Fissore R. A. (2009) Cell Calcium 46, 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boehning D., Patterson R. L., Sedaghat L., Glebova N. O., Kurosaki T., Snyder S. H. (2003) Nat. Cell Biol. 5, 1051–1061 [DOI] [PubMed] [Google Scholar]