Abstract
While many are the examples of DNA damaging treatments that induce p21 accumulation, the conception of p21 upregulation as the universal response to genotoxic stress has come to an end. Compelling evidences have demonstrated the existence of converging signals that negatively regulate p21 bellow basal levels when replication forks are blocked. Moreover, conclusive reports identified the E3-ligase CRL4CDT2 (CUL4-DDB1-CDT2) as the enzymatic complex that promotes p21 proteolysis when treatments such as UV irradiation trigger replication fork stress. A pre-requisite for CRL4CDT2-driven proteolysis is the interaction of p21 with PCNA. Interestingly as well, CRL4CDT2-dependent proteolysis is not limited to p21 and affects other PCNA partners, including the specialized DNA polymerase η(pol eta). These recent discoveries are particularly intriguing since the UV-induced degradation of p21 has been shown to be required for efficient pol η recruitment to DNA lesions. Herein we review the findings that lead to the identification of the molecular mechanism that triggers damage-induced PCNA-coupled protein proteolysis. We propose a novel model in which CRL4CDT2-dependent protein degradation facilitates a sequential and dynamic exchange between PIP box-bearing proteins at stall forks during Translesion DNA synthesis (TLS). Moreover, given the tight spatiotemporal control that CRL4CDT2-driven proteolysis is able to confer to PCNA-regulated processes, we discuss the impact that this degradation mechanism might have in other molecular switches associated with the repair of damaged DNA.
Keywords: p21, PCNA, Pol η, CRL4CDT2 (CUL4-DDB1-CDT2), Translesion DNA synthesis, UV irradiation, replication fork
1. p21 levels after DNA damage: what to expect?
Few topics in cell biology have received as much attention as the p53 activation pathway after genotoxic stress. Since p21Cip1/WAF1 discovery [1,2], and during the “golden p53 age” in the 90’s, a strong dogma stating that DNA damage activates p53 to trigger accumulation of p21 and subsequent cell cycle arrest was established. The knowledge of this emerging pathway spread widely in the scientific community and transcended to such a degree that nowadays it can be found in most cell biology books. The universality of this pathway was therefore not questioned for about 15 years and is still deeply seated in many scientists’ minds.
During the last few years, a significant number of reports provided increasing evidence of exceptions in the induction of p21 after genotoxic stress, both at mRNA and protein level [3-12]. However, a somehow expected skepticism caused that these findings went unnoticed. This is particularly noteworthy in the case of UV irradiation. Several reports demonstrated that p21 is not induced after UV, but on the contrary it is actively downregulated [6-10,12]. This notion is conceptually important not only because it provides an obvious exception to the p53/p21 dogma, but also because it suggests the existence of a novel signaling pathway that promotes p21 degradation in certain conditions of genotoxic stress. From a more practical point of view, being aware of this exception is beneficial since a significant number of research groups still attempt to detect the “expected” p21 upregulation after UV irradiation, troubleshooting the experiments endlessly when they do not observe an increase in p21 levels.
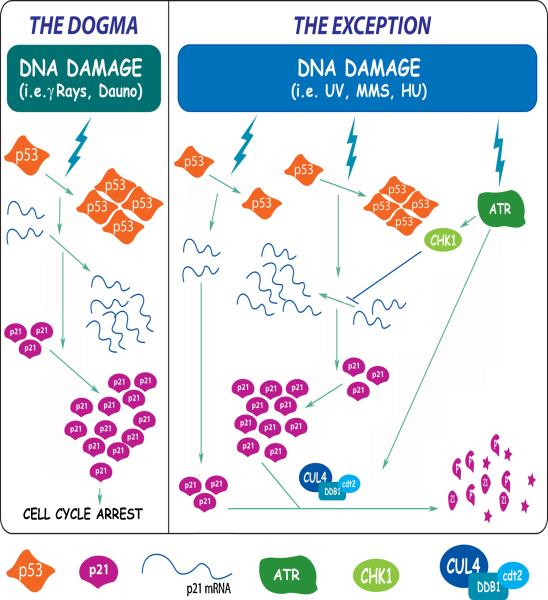
The conception of p21 as a stress-induced protein is therefore certainly restricted by these findings, which mandates the re-evaluation of the physiological relevance of p21 levels both before and after stress. It is well established that high p21 levels induced by p53 accumulation after some genotoxic agents (i.e. gamma irradiation) are involved in cell cycle arrest. However, basal p21 levels, that were historically considered insufficient to affect any cellular process, have recently gained the attention of the field due to the discovery of the ability of the cell to downregulate p21 “below the basal levels” in certain conditions (i.e. after UV)(Figure 1).
Fig 1. Molecular mechanisms of p53-dependent p21 upregulation and p53-independent p21 downregulation after DNA damage.
Diagram depicting the opposite pathways that modulate p21 levels after different types of DNA damage. The left panel summarizes the main events in the classical p53-dependent p21 upregulation pathway that relies on p53 transcriptional activation and leads to cell cycle arrest. The DNA damage-induced p21 proteolysis pathway is represented on the right panel. When this pathway is activated, the final p21 levels are similarly low regardless of the efficient accumulation of a transcriptionally active p53. The latter process depends on the E3 ligase CRL4CDT2 (CUL4-DDB1-CDT2). Different experimental models (see main text) have shown that ATR activation is required both for the impairment of p21 mRNA transcriptional elongation (with a mechanism that involves CHK1 activation) and for the efficient p21 proteolysis (with a yet unclear mechanism).
2. Keeping p21 levels low: redundancy or specificity?
Among the multiple roles reported for p21 [13], the most studied ones are related to its CDK and PCNA binding domains. It is well established that low p21 levels are required to allow efficient CDK activity during S-phase. In fact, it should be kept in mind that basal p21 levels are amounts of protein compatible with the progression of the cell cycle and therefore not sufficient to inhibit CDK activity [14]. Much more difficult to predict is the effect that low levels of p21 might have on the fraction of PCNA bound to replication forks. In this respect, by comparing p21+/+ cells with p21 knock-out derivatives, our group showed that basal p21 levels in proliferant cells selectively impair the interaction of certain polymerases with chromatin-bound PCNA [15] (for review see [16]). These data therefore suggest that the mechanism that downregulates basal p21 levels is more likely to regulate a function of p21 at replication forks.
Although upregulating p21 levels is mechanistically rather simple (achieved by p53-induced transcriptional activation), the pathways in charge of its downregulation are much more complexly controlled. Multiple cellular mechanisms were reported to keep p21 levels low, acting in cooperation during transcription, basal protein turnover and DNA damage-induced protein degradation.
2.1 Keeping p21 low at the mRNA level
The first evidence of an exception to the p53/p21 dogma was observed when RKO cells were treated with drugs that block DNA replication, as hidroxyurea (HU) or aphidicolin (APH). Similarly to other agents as γ rays, HU and APH induce high levels of p53, but are not followed by the expected increase in p21 mRNA levels [4]. Although the level of impairment in p21 mRNA accumulation varies among different cell lines, these observations suggested that p21 transcription is controlled at some step during replication blockage. Subsequentially, chromatin inmunoprecipitation experiments scanning the p21 promoter and coding sequences revealed that upon HU treatment, p53 efficiently accumulate, binds to p21 promoter, and recruits components of the transcription machinery, but RNA Pol II-dependent elongation is impaired [5].
Failure to upregulate p21 mRNA was also observed after UV irradiation in other p53wt cell lines [7].Thus, it seems that different types of DNA damage that converge on the blockage of replication forks might potentially impair p21 mRNA transcription despite the efficient activation of p53. This is in line with recent findings showing that HU-dependent block of p21 mRNA elongation depends on ATR/CHK1 activity [17], a pathway that is known to be activated after replication fork stress [18].
2 Keeping p21 low at the protein level
Various pathways control p21 protein turnover. Some of these pathways operate to maintain the basal levels of p21 [19-22] while others are in charge of the increased proteolysis of p21 after DNA damage [6-12].
The control of basal p21 turnover is a subject of controversy, being the ubiquitin-dependent or independent nature of its proteolysis the major point of disagreement. While there is consensus on the fact that p21 can be ubiquitinated in vivo, different groups have reported that p21 ubiquitination is not a pre-requisite to allows its proteosomal degradation [20,22]. In fact, it was shown that p21 C-terminus is able to directly interact with the 20s subunit of the proteosome, bypassing the necessity of ubiquitination [20]. Other groups support the opposite model, in which ubiquitination of p21 is required to trigger its proteolysis [21,23,24]. Two E3-ligases were implicated in ubiquitin-dependent p21 basal turnover: SCFskp2 controlling p21 levels during G1/S [24] and APC/Ccdc20 triggering p21 degradation during G2/prometafase [23].
It was not until recently that evidence of a DNA-damage inducible mechanism for p21 degradation was reported. An initial report stated that p21 proteolysis is induced by low but not high UV doses [12]. However, subsequent papers have shown that p21 degradation after UV increases in a dose and time-dependent manner [6-8,11]. In fact, p21 degradation is only clearly observed in most cellular systems at high UV doses (>10J/m2), while lower doses seem to keep p21 levels rather unchanged (see figure 2 and following sections for further discussion).
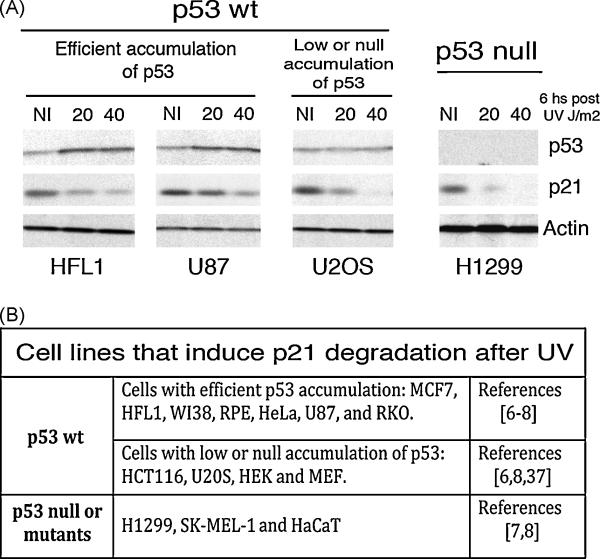
Fig 2. p21 downregulation after UV is independent of p53 activation.
A) p21 proteolysis is actively induced by UV light in p53+/+ cells (regardless of the efficient, low or undetectable p53 activation) and p53 null cells. Normal human fibroblasts (HFL1), glioma cells (U87), osteosarcoma cells (U2OS) and lung carcinoma cells (H1299) were subjected to 20 or 40 J/m2 of UVC. 6 hours later total protein extracts were prepared and used in Western Blot experiments revealed with p53 (DO1), p21 (C19) and actin specific antibodies. B) Table summarizing the different cell lines that were previously reported to trigger UV-induced p21 proteolysis. The p53 status and the level of induction are specified in each case within the respective reference.
Other genotoxic agents are also able to efficiently trigger p21 degradation, such as MMS [8], HU [3,8] and Cisplatin [11]. Not surprisingly, all these agents cause replication fork stalling, thus suggesting that the signals that trigger p21 proteolysis might not result from the simple accumulation of DNA damage, but more likely, from the accumulation of damage-processing intermediates. This is in line with the fact that ATR is essential for p21 degradation ([7,12] and our unpublished results). However, it is not yet clear whether p21 degradation requires also CHK1 or results from independent ATR downstream effectors. A report in line with the latter possibility indicates that p21 phosphorylation by the GSK-3β serine/threonine kinase is required for its proteosomal degradation [7].
It is important to highlight at this point that p53 accumulation is not observed in all the cellular systems used after UV irradiation. For example, p53 accumulation is efficient in normal HFL1 fibroblast and U87 glioma cells, but not in U20S and HCT116 cells. Nonetheless, p21 degradation is similarly detected in these cell lines regardless of p53 levels (figure 2). Moreover, even in p53 null or mutant cells lines, the residual amounts of p21 detected are also efficiently degraded after UV (figure 2). Remarkably in line, the DNA damage-induced p21 degradation pathway is dominant over others signal capable of upregulating p53. In fact, if cells are treated with agents that efficiently activate p53 and markedly increase p21 levels (i.e. γ rays and Daunorubicin), the subsequent treatment with UV or HU triggers efficient p21 proteolysis, regardless of the continuous presence of the p53-activating agent throughout the whole length of the experiment [4,8]. These data brings into light a counterintuitive concept: the reduction and not the upregulation of p21 levels is a priority for the cell after some types of genotoxic stress.
The E3-ligase involved in the UV-induced p21 degradation was initially reported to be SCFskp2, the same complex working under unstressed conditions[12]. However, several subsequent reports demonstrate that UV-induced p21 degradation occurs with identical efficiency in cells with impaired or null expression of the SCFskp2 complex [6,7,9,10]. Moreover, DNA damage-induced p21 ubiquitination have some particular features, which suggested that a different E3-ligase would be involved. First, the agents able to increase p21 proteolysis are characterized for triggering replication stress, thus suggesting that the E3-ligase involved might be activated at stalled replication forks. Second, p21 undergo a non-canonical type of ubquitination at its N-terminus [21], which was shown to be required for the UV-induced p21 degradation pathway [8]. Recent reports by three different groups had identified CRL4CDT2 as the E3-ligase involved in the UV-induced degradation of p21[9,10,25]. As predicted, this complex is associated to replication forks, is recruited to DNA after genotoxic stress and is able to ubiquitinate target proteins at their N-terminus ([9,10,26], see next section for further discussion about the mechanism).
In summary, if we consider the various versatile pathways presented in this section, it seems that when it comes to the regulation of the p21 levels, the most accurate answer to the original question inquiring whether these pathways provide “redundancy or specificity” is simply: both.
3. Emergence of a PCNA-coupled degradation pathway
Protein degradation coupled to PCNA interaction is a mechanism that was originally described for the CDK inhibitor Xic1 [27] and the licensing factor of DNA replication, CDT1 [28]. Since PCNA is a master coordinator of the replication process [29], coupling proteolysis to PCNA confers the unique attribute to inactivate proteins at the replication fork. Notably, these replication-coupled inactivation mechanisms are not restricted to metazoans, since similar processes were described in S. pombe [30] and even in E. coli [31]. More intriguingly, PCNA-coupled degradation of CDT1 was also shown to be activated after DNA damage [26] and to depend on the CRL4CDT2 E3 ligase complex [32]. Several subsequent reports revealed that other PCNA partners such as p21, Pol η, CKI 1 and E2F follow similar degradation patterns [9,10,33,34]. These findings are exciting, since they suggest the existence of an undefined degradation pathway that could depend on the recruitment of target proteins to replication forks.
3.1 Coupling protein ubiquitination to PCNA: How does it work?
Recent findings revealed that proteins engaged by the PCNA-coupled degradation pathway share two common features. First, they all are able to interact with PCNA directly. This interaction requires a special motif known as PCNA interaction protein, or PIP box. In fact, for all the proteins reported so far, mutations of critical residues on the PIP boxes inhibit protein degradation [9,10,26-28,33,34]. Second, all the identified proteins that follow the PCNA-coupled pathway (CDT1, p21, Pol η, E2F and CKI 1) are triggered to proteosomal degradation by the same E3-ligase complex: CRL4CDT2.
CRL4CDT2, also known as the CUL4-DDB1-CDT2 complex, is member of the culling-RING finger ligases (CRLs). Cullin 4 and the adaptor protein DDB1 constitute the conserved core of the complex. The ubiquitination specificity is conferred by a third protein, known as DCAF (for DDB / CUL4 associated factor)[35]. Although the interest of this review is focused on CDT2, the CUL4-DDB1 core associates with others DCAFs, like DDB2 and CSA, which were previously shown to trigger the degradation of the DNA repair proteins XPC and CSB respectively [36,37]. Outstandingly, more than 50 novel DCAFs have been recently identified, opening an entire new field for investigating the stability-control of proteins involved in the DNA metabolism [35].
Mutational analysis of central proteins in this pathway suggested that the mechanism of recruitment of the CRL4CDT2 complex is hierarchic, involving an initial interaction of the PCNA partner with PCNA, which is followed by a subsequent recruitment of the E3 complex. The ubiquitination step takes place in situ, and seems crucial to allow the sequential recruitment to other/s PIP box bearing proteins to PCNA. Other significant feature of this pathway is that the degradation of PCNA partners is apparently linked only to chromatin bound PCNA and not to free PCNA [28,38]. This latter feature is particularly important since it might explain why CRL4CDT2-coupled degradation occurs in S-phase or after DNA damage, two circumstances where PCNA is bound to chromatin.
The data discussed above therefore indicate that the number of processes controlled by PCNA-coupled degradation is difficult to predict. In theory, any event that involves recruitment and tight interaction of PCNA partners with the DNA could represent a potential trigger for its CRL4cdt-coupled degradation. This possibility is particularly intriguing for the various DNA repair processes that require PCNA, since CRL4CDT2-coupled degradation might represent a central component for the inactivation and/or sequential recruitment of repair proteins (see section 4 for further discussion)
3.2 Specialized PIP boxes drive PCNA-coupled degradation
Perhaps the most puzzling aspect of this emerging pathway is why some PIP box bearing proteins such as p21 and CDT1 are degraded by the PCNA-coupled mechanism while many others PIP box proteins seems to be stable. Experiments using small peptides containing the PIP box of p21 demonstrate that the E3 complex interacts directly with the PIP box [38]. This observation suggests that the PIP box sequence itself, might be responsible for both the docking onto PCNA and the activation of the ubiquitination step. Thus, it is possible to hypothesize that a small difference between the PIP boxes might determine whether or not a PCNA partner is subjected to PCNA-driven proteolysis.
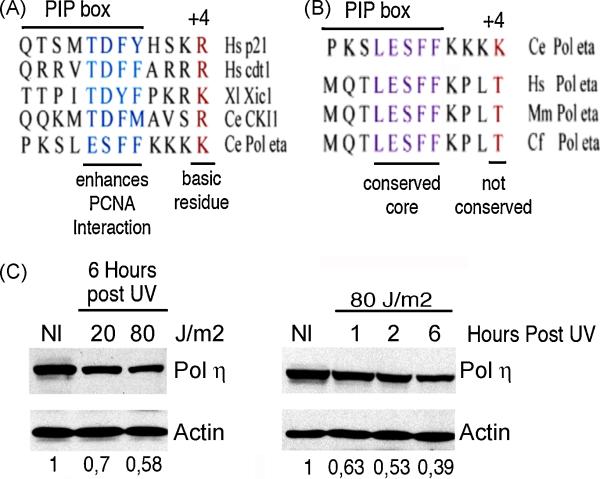
Initial mutagenesis analyses suggested that, in addition to the PIP box, basic aminoacidic clusters flanking the PIP box are important to allow ubiquitination [10]. More recently, a paper by Havens and collaborators reported the intriguing finding that the PIP boxes of known CRL4CDT2 targets like p21 and CDT1 share two functional features [38]. The first one is a high affinity for PCNA. This condition was fulfilled in most of the PCNA partners examined by a TD motif in positions 5 and 6 of the PIP box. The second is a basic aminoacid (K or R) in position +4 from the PIP box. Without exception, all the PIP boxes analyzed in that study share this last feature (figure 3A). Notably, these specialized PIP boxes were shown to be “portable”, and sufficient to trigger the degradation of stable PCNA partners such as FEN1. This was proved utilizing a site-direct mutagenesis approach to alter the PIP box of FEN1 in a way that mimicked critical residues of a specialized PIP box [38].These findings provide additional and categorical evidence supporting the existence of a precisely controlled PCNA-coupled, S phase-driven and DNA damaged-induced pathway that promotes degradation of proteins such as p21.
Fig 3. Several PCNA partners are triggered to degradation by the same PCNA-coupled mechanism.
A) Sequence alignment of proteins reported to undergo PCNA-coupled degradation. The conserved features of their PIP boxes are: a PIP box core that allows highly efficient interaction with PCNA and a basic residue in position +4 from the PIP box. Important: a TD domain is present in the PIP box core of all PCNA-partners, except in Pol η. B) Sequence alignment of Pol η from C. elegans and Pol η from differents mammals (Hs: Homo sapiens, Mm: Mus musculus and Cf: Canis familiaris). All the variants share a well-conserved PIP box core but the mammalian variants do not share the basic residue in position +4. C) Exogenously expressed human GFP-Pol η is actively degraded after UV irradiation. In the left panel, samples were collected 6 hours after irradiation with the indicated increasing UV doses. A time course using a fixed UV dose was also performed and is presented in the right panel. Western blots were revealed with specific antibodies for GFP (to detect GFP- Pol eta) and for actin (used as a loading control). The numbers at the bottom of each lane indicate the relative amount of Pol η in respect to unirradiated controls. These values were calculated using densitometric analysis and normalized with respect to actin levels for each sample.
While more work is required to establish whether these conserved PIP boxes are common to every CRL4CDT2 target, it is likely that there will be at least some exceptions. For example, Polymerase η from C.elegans, a PCNA partner that was recently shown to be degraded by this pathway [34], carry a conserved specialized PIP box (figure 3A) [38]. However, despite a very conserved PIP box core, the flaking residues of Ce Pol η are not conserved in mammalian variants of Pol η, including human Pol η(figure 3B). This sequence divergence suggests that Pol η in mammals might not be sensitive to the stress induced degradation pathway. However, we were able to detect UV-induced degradation of human Pol η in a dose and time dependent fashion (Figure 3C), thus indicating that CRL4CDT2 might be able to recognize other types of PIP boxes. Given the high number of known PCNA partners, it is likely that these findings will prompt the identification of novel targets of the CRL4CDT2 shortly.
4. PCNA-coupled degradation: a master switch for TLS?
During DNA replication, synthesis across damaged DNA is achieved by specialized DNA polymerases in a process known as TLS (Translesion DNA synthesis)[39,40]. The initial recruitment of TLS polymerases to the replication fork is stimulated by mono-ubiquitination of PCNA [41]. TLS polymerases can act either alone or in pairs depending on the type of damage (in the latter case there is a sequential recruitment of two polymerases, that carry out an initial and an extension step respectively). In any case, DNA synthesis must be resumed later by a replicative Polymerase, which is reloaded to replication forks with a poorly understood mechanism [42].
TLS polymerases must be tightly controlled since they are prone to induce mutagenesis due to their permissive active site and their lack of proofreading activity. One protein that was reported to be critical in the control of TLS is p21. In fact, UV-induced p21 proteolysis was shown to facilitate at least two independent TLS-associated events. First, p21 degradation after UV is required for efficient PCNA mono-ubiquitination, indicating that p21 impairs PCNA ubiquitination [8]. Second, p21/PCNA interaction strongly impairs Pol η focal organization at DNA lesions [15]. This effect is linked to the ability of p21 to selectively impairs Pol η interaction with chromatin-bound PCNA without altering the loading/activity of the replicative polymerase δ [15]. These studies revealed that, while very high levels of p21 might impair PCNA-driven DNA replication, the much lower p21 levels reached during basal expression are sufficient to selectively block PCNA interaction with TLS polymerases. Thus, these data collectively suggest that p21 degradation after UV represent a biological relevant step critical for the success of TLS [16].
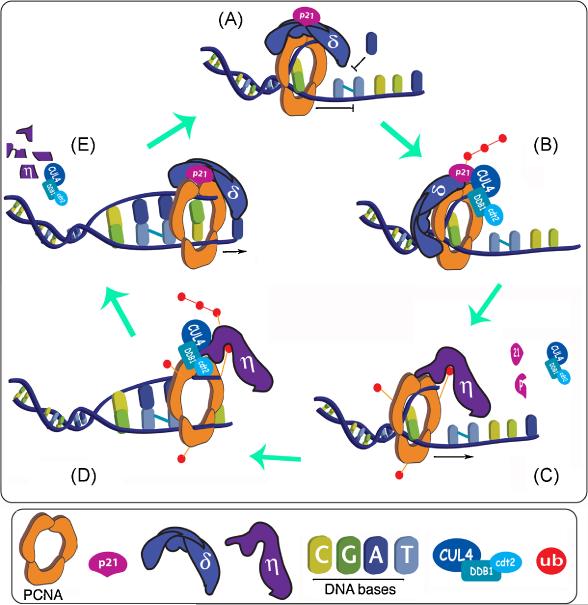
In this context it is appealing to imagine that PCNA-coupled p21 degradation might represent the perfect switch to shut down “in situ” the negative effect of p21 on TLS when required. The model we propose envisions replication fork stalling at DNA lesions as the initial signal that triggers p21 degradation. This event promotes PCNA ubiquitination, which in combination with the increase in free PCNA binding sites that results from p21 degradation, allows the recruitment of Pol η and promote TLS (Figure 4). Moreover, findings by the group of Matthew Michael suggest that the rationale of this model could be extended to other sequential steps of TLS. Using a C. elegans model, they discovered that Pol η follow an identical degradation mechanism as p21 after UV (coupled to PCNA and dependent on CRL4CDT2 ). They hypothesize that Pol η degradation might be required for Pol η unload after TLS, an event with a rather puzzling and yet obscure mechanism [34]. Therefore, it is tempting to speculate that the PCNA-coupled degradation pathway might control the sequential exchange of PIP box bearing proteins at the PCNA clamp during TLS. This extended model would therefore includes the initial step of TLS Polymerase recruitment facilitated by p21 degradation, the TLS Polymerase unload step (to allow the re-load of the replicative Polymerase), and even possibly the switch to the second polymerase in the two-TLS-polymerases pathway (Figure 4).
Fig 4. A dynamic model for the regulation of Translesion DNA synthesis (TLS) by PCNA-coupled degradation.
Our view of the potential sequence of events that promote the progression of replication forks at DNA lesions: A) A replication fork is stalled at a timidine dimer due to the incapacity of a replicative DNA polymerase to synthesize across DNA lesions. Until that point, both p21 and the replicative DNA polymerase can coexist at the replication fork (see main text) B) The CRLCDT2 complex is recruited to the fork and ubiquitinates p21. C) p21 degradation allows PCNA mono-ubiquitination and facilitates the subsequential recruitment of Pol η to the same binding site that was occupied by p21. The PCNA-Pol η complex is then able to replicate across the damage. D) The CRLCDT2 complex recruited to the fork ubiquitinates Pol η. E) Pol η proteolysis is followed by the reload of a replicative DNA polymerase. This processive PCNA complex takes over DNA synthesis until another lesion in the DNA blocks the replication fork and this cycle restart. The sequential loading and disassembly of CRLCDT2 complexes to replication forks has not been tested and is represented like that only for simplification purposes.
A final point that still needs to be addressed is the fact that both p21 and Pol η levels are kept rather constant when cells are treated with low doses of UV and MMS. While it is still difficult to reach a consensus regarding which is the right dose of UV that should be used in these type of studies, it was reported that the amount of DNA damage required for the detection of p21 or Pol η degradation is incompatible with long term cell survival [34,43]. At first glance, these data seem to suggest that our model cannot apply to a physiological scenario. However, the significance of unaltered total protein levels when analyzing processes restricted to single replication forks dynamics should not be overestimated. A low frequency of DNA lesions reduces the total number of replication forks that will encounter DNA lesions and trigger CRL4CDT2 activation. Thus, unaffected p21 and Pol η total levels after low amounts of DNA damage do not imply lack of activation of the degradation pathway at a limited number of stalled forks. Our model propose that while severe downregulation of p21/ Pol η levels might be observed only with the high doses used for experimentation, “physiological” amounts of DNA damage might trigger rates of PCNA-coupled degradation that could be balanced with opposite rates of protein synthesis. Thus, the degradation of PIP box bearing proteins could still take place in situ at stalled forks without noticeably affecting the total levels of targeted proteins. More work will be required to corroborate this dynamic model and to check if it is valid for the various proteins that follow the PCNA-coupled degradation pathway.
5. Future perspectives
In addition to the implications of the PCNA-coupled degradation model discussed above, there are some other potential consequences for the degradation of p21 and other PCNA-partners. A few recent reports support this possibility. First, p21 degradation [8,11], PCNA ubiquitination [8] and Pol η recruitment to damage sites [44,45] are observed in G1/G2. These intriguing data suggest that the PCNA-coupled degradation pathway could also be associated with the DNA damage response outside S-phase. In line with this, recent evidence suggests that p21 might be involved in the control of the base excision repair pathway (BER). On the one hand, certain types of oxidative DNA damage typically repaired by BER, like the ones induced by hydrogen peroxide and potasium bromate, were shown to induce p21 degradation [11]. On the other hand, the TLS polymerase Ι (Pol iota) was implicated in BER [46]. Since at least one BER sub-pathway (long patch BER) is dependent on PCNA, the resemblance of these observations with the ones regarding TLS and p21 suggest that p21-degradation might be relevant also for the efficient activation of BER.
Many others questions still wait for an answer, not only regarding the DNA damage-induced p21 degradation, but also related to the processes that might be affected by the expanding PCNA-coupled degradation pathway. One thing is certain: the findings discussed in this review will serve as foundations to shed light into the contribution that PCNA-coupled degradation might have on the plethora of mechanisms orchestrated by PCNA.
Acknowledgments
We thank Dr. P. Cassano for helpful suggestions and careful reading of the manuscript. The work in our laboratory is supported by Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and the National Institute of Health (RO3-TW007440). VG is a researcher from the National Council of Sciences in Argentina (CONICET) and GS is supported by a fellowship from CONICET.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest None.
References
- [1].Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- [2].el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein WAF1 B. a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- [3].Gottifredi V, McKinney K, Poyurovsky MV, Prives C. Decreased p21 levels are required for efficient restart of DNA synthesis after S phase block. J Biol Chem. 2003 doi: 10.1074/jbc.M310373200. [DOI] [PubMed] [Google Scholar]
- [4].Gottifredi V, Shieh S, Taya Y, Prives C. p53 accumulates but is functionally impaired when DNA synthesis is blocked. Proc Natl Acad Sci U S A. 2001;98:1036–1041. doi: 10.1073/pnas.021282898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mattia M, Gottifredi V, McKinney K, Prives C. p53-Dependent p21 mRNA elongation is impaired when DNA replication is stalled. Mol Cell Biol. 2007;27:1309–1320. doi: 10.1128/MCB.01520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee H, Zeng SX, Lu H. UV Induces p21 rapid turnover independently of ubiquitin and Skp2. J Biol Chem. 2006;281:26876–26883. doi: 10.1074/jbc.M605366200. [DOI] [PubMed] [Google Scholar]
- [7].Lee JY, Yu SJ, Park YG, Kim J, Sohn J. Glycogen synthase kinase 3beta phosphorylates p21WAF1/CIP1 for proteasomal degradation after UV irradiation. Mol Cell Biol. 2007;27:3187–3198. doi: 10.1128/MCB.01461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Soria G, Podhajcer O, Prives C, Gottifredi V. P21Cip1/WAF1 downregulation is required for efficient PCNA ubiquitination after UV irradiation. Oncogene. 2006;25:2829–2838. doi: 10.1038/sj.onc.1209315. [DOI] [PubMed] [Google Scholar]
- [9].Abbas T, Sivaprasad U, Terai K, Amador V, Pagano M, Dutta A. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev. 2008;22:2496–2506. doi: 10.1101/gad.1676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nishitani H, Shiomi Y, Iida H, Michishita M, Takami T, Tsurimoto T. CDK inhibitor p21 is degraded by a PCNA coupled Cul4-DDB1Cdt2 pathway during S phase and after UV irradiation. J Biol Chem. 2008 doi: 10.1074/jbc.M806045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Savio M, Coppa T, Cazzalini O, Perucca P, Necchi D, Nardo T, Stivala LA, Prosperi E. Degradation of p21CDKN1A after DNA damage is independent of type of lesion, and is not required for DNA repair. DNA Repair (Amst) 2009;8:778–785. doi: 10.1016/j.dnarep.2009.02.005. [DOI] [PubMed] [Google Scholar]
- [12].Bendjennat M BJ, T Jascur, H Brickner, V Barbier, A Sarasin, A Fotedar. Fotedar R UV Irradiation Triggers ubiquitin-dependent degradation of p21WAF1 to promote DNA repair. Cell. 2003;114:599–610. doi: 10.1016/j.cell.2003.08.001. [DOI] [PubMed] [Google Scholar]
- [13].Dotto GP. p21(WAF1/Cip1): more than a break to the cell cycle? Biochim Biophys Acta. 2000;1471:M43–56. doi: 10.1016/s0304-419x(00)00019-6. [DOI] [PubMed] [Google Scholar]
- [14].Cai K, Dynlacht BD. Activity and nature of p21(WAF1) complexes during the cell cycle. Proc Natl Acad Sci U S A. 1998;95:12254–12259. doi: 10.1073/pnas.95.21.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Soria G, Speroni J, Podhajcer OL, Prives C, Gottifredi V. p21 differentially regulates DNA replication and DNA-repair-associated processes after UV irradiation. J Cell Sci. 2008;121:3271–3282. doi: 10.1242/jcs.027730. [DOI] [PubMed] [Google Scholar]
- [16].Prives C, Gottifredi V. The p21 and PCNA partnership: a new twist for an old plot. Cell Cycle. 2008;7:3840–3846. doi: 10.4161/cc.7.24.7243. [DOI] [PubMed] [Google Scholar]
- [17].Beckerman R, Donner AJ, Mattia M, Peart MJ, Manley JL, Espinosa JM, Prives C. A role for Chk1 in blocking transcriptional elongation of p21 RNA during the S-phase checkpoint. Genes Dev. 2009;23:1364–1377. doi: 10.1101/gad.1795709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gottifredi V, Prives C. The S phase checkpoint: when the crowd meets at the fork. Semin Cell Dev Biol. 2005;16:355–368. doi: 10.1016/j.semcdb.2005.02.011. [DOI] [PubMed] [Google Scholar]
- [19].Coleman ML, Marshall CJ, Olson MF. Ras promotes p21(Waf1/Cip1) protein stability via a cyclin D1-imposed block in proteasome-mediated degradation. Embo J. 2003;22:2036–2046. doi: 10.1093/emboj/cdg189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Touitou R, Richardson J, Bose S, Nakanishi M, Rivett J, Allday MJ. A degradation signal located in the C-terminus of p21WAF1/CIP1 is a binding site for the C8 alpha-subunit of the 20S proteasome. Embo J. 2001;20:2367–2375. doi: 10.1093/emboj/20.10.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bloom J, Amador V, Bartolini F, DeMartino G, Pagano M. Proteasome-Mediated Degradation of p21 via N-Terminal Ubiquitinylation. Cell. 2003;115:71–82. doi: 10.1016/s0092-8674(03)00755-4. [DOI] [PubMed] [Google Scholar]
- [22].Sheaff RJ, Singer JD, Swanger J, Smitherman M, Roberts JM, Clurman BE. Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol Cell. 2000;5:403–410. doi: 10.1016/s1097-2765(00)80435-9. [DOI] [PubMed] [Google Scholar]
- [23].Amador V, Ge S, Santamaria PG, Guardavaccaro D, Pagano M. APC/C(Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol Cell. 2007;27:462–473. doi: 10.1016/j.molcel.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. Role of SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 during S-phase. J Biol Chem. 2003 doi: 10.1074/jbc.M301774200. [DOI] [PubMed] [Google Scholar]
- [25].Kim Y, Starostina NG, Kipreos ET. The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev. 2008;22:2507–2519. doi: 10.1101/gad.1703708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Senga T, Sivaprasad U, Zhu W, Park JH, Arias EE, Walter JC, Dutta A. PCNA is a cofactor for Cdt1 degradation by CUL4/DDB1-mediated N-terminal ubiquitination. J Biol Chem. 2006;281:6246–6252. doi: 10.1074/jbc.M512705200. [DOI] [PubMed] [Google Scholar]
- [27].Chuang LC, Yew PR. PCNA recruits CDK inhibitor Xic1 to DNA and couples its proteolysis to DNA polymerase switching. J Biol Chem. 2005 doi: 10.1074/jbc.M506429200. [DOI] [PubMed] [Google Scholar]
- [28].Arias EE, Walter JC. PCNA functions as a molecular platform to trigger Cdt1 destruction and prevent re-replication. Nat Cell Biol. 2006;8:84–90. doi: 10.1038/ncb1346. [DOI] [PubMed] [Google Scholar]
- [29].Moldovan GL, Pfander B, Jentsch PCNA S. the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- [30].Ralph E, Boye E, Kearsey SE. DNA damage induces Cdt1 proteolysis in fission yeast through a pathway dependent on Cdt2 and Ddb1. EMBO Rep. 2006;7:1134–1139. doi: 10.1038/sj.embor.7400827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Su’etsugu M, Takata M, Kubota T, Matsuda Y, Katayama T. Molecular mechanism of DNA replication-coupled inactivation of the initiator protein in Escherichia coli: interaction of DnaA with the sliding clamp-loaded DNA and the sliding clamp-Hda complex. Genes Cells. 2004;9:509–522. doi: 10.1111/j.1356-9597.2004.00741.x. [DOI] [PubMed] [Google Scholar]
- [32].Jin J, Arias EE, Chen J, Harper JW, Walter JC. A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol Cell. 2006;23:709–721. doi: 10.1016/j.molcel.2006.08.010. [DOI] [PubMed] [Google Scholar]
- [33].Shibutani ST, de la Cruz AF, Tran V, Turbyfill WJ, 3rd, Reis T, Edgar BA, Duronio RJ. Intrinsic negative cell cycle regulation provided by PIP box- and Cul4Cdt2-mediated destruction of E2f1 during S phase. Dev Cell. 2008;15:890–900. doi: 10.1016/j.devcel.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kim SH, Michael WM. Regulated proteolysis of DNA polymerase eta during the DNA-damage response in C. elegans. Mol Cell. 2008;32:757–766. doi: 10.1016/j.molcel.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lee J, Zhou P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol Cell. 2007;26:775–780. doi: 10.1016/j.molcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- [36].Groisman R, Kuraoka I, Chevallier O, Gaye N, Magnaldo T, Tanaka K, Kisselev AF, Harel-Bellan A, Nakatani Y. CSA-dependent degradation of CSB by the ubiquitin-proteasome pathway establishes a link between complementation factors of the Cockayne syndrome. Genes Dev. 2006;20:1429–1434. doi: 10.1101/gad.378206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sugasawa K, Okuda Y, Saijo M, Nishi R, Matsuda N, Chu G, Mori T, Iwai S, Tanaka K, Tanaka K, Hanaoka F. UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell. 2005;121:387–400. doi: 10.1016/j.cell.2005.02.035. [DOI] [PubMed] [Google Scholar]
- [38].Havens CG, Walter JC. Docking of a specialized PIP Box onto chromatin-bound PCNA creates a degron for the ubiquitin ligase CRL4Cdt2. Mol Cell. 2009;35:93–104. doi: 10.1016/j.molcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Guo C, Kosarek-Stancel JN, Tang TS, Friedberg EC. Y-family DNA polymerases in mammalian cells. Cell Mol Life Sci. 2009;66:2363–2381. doi: 10.1007/s00018-009-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Waters LS, Minesinger BK, Wiltrout ME, D’Souza S, Woodruff RV, Walker GC. Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiol Mol Biol Rev. 2009;73:134–154. doi: 10.1128/MMBR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lehmann AR, Niimi A, Ogi T, Brown S, Sabbioneda S, Wing JF, Kannouche PL, Green CM. Translesion synthesis: Y-family polymerases and the polymerase switch. DNA Repair (Amst) 2007;6:891–899. doi: 10.1016/j.dnarep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- [42].Friedberg EC, Lehmann AR, Fuchs RP. Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol Cell. 2005;18:499–505. doi: 10.1016/j.molcel.2005.03.032. [DOI] [PubMed] [Google Scholar]
- [43].Itoh T, Linn S. The fate of p21CDKN1A in cells surviving UV-irradiation. DNA Repair (Amst) 2005;4:1457–1462. doi: 10.1016/j.dnarep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- [44].Soria G, Belluscio L, van Cappellen WA, Kanaar R, Essers J, Gottifredi V. DNA damage induced Pol eta recruitment takes place independently of the cell cycle phase. Cell Cycle. 2009;8:3340–3348. doi: 10.4161/cc.8.20.9836. [DOI] [PubMed] [Google Scholar]
- [45].Akagi JI, Masutani C, Kataoka Y, Kan T, Ohashi E, Mori T, Ohmori H, Hanaoka F. Interaction with DNA polymerase eta is required for nuclear accumulation of REV1 and suppression of spontaneous mutations in human cells. DNA Repair (Amst) 2009;8:585–599. doi: 10.1016/j.dnarep.2008.12.006. [DOI] [PubMed] [Google Scholar]
- [46].Petta TB, Nakajima S, Zlatanou A, Despras E, Couve-Privat S, Ishchenko A, Sarasin A, Yasui A, Kannouche P. Human DNA polymerase iota protects cells against oxidative stress. Embo J. 2008;27:2883–2895. doi: 10.1038/emboj.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]