Abstract
12/15-lipoxygenase (12/15-LOX) contributes to acute neuronal injury and edema formation in mouse models of middle cerebral artery occlusion (MCAO). The apoptosis-inducing factor (AIF) is implicated in caspase-independent forms of apoptosis, and has been linked to ischemic neuronal cell death. We show here that increased AIF in the peri-ischemic cortex of mouse colocalizes with 12/15-LOX after 2 h of MCAO. The 12/15-LOX inhibitor baicalein prevents the increase and nuclear localization of AIF, suggesting this pathway may be partially responsible for the neuroprotective qualities of baicalein. Using an established cell line model of neuronal oxidative stress, we show that 12/15-LOX activated after glutathione depletion leads to AIF translocation to the nucleus, which is abrogated by the 12/15-LOX inhibitor baicalein (control: 19.3%±6.8% versus Glutamate: 64.0%±8.2% versus glutamate plus baicalein: 11.4%±2.2%). Concomitantly, resident proteins of the ER are dispersed throughout the cell (control: 31.0%±8.4% versus glutamate: 70.0%±5.5% versus glutamate plus baicalein: 8.0%±2.7%), suggesting cell death through organelle damage. Taken together, these findings show that 12/15-LOX and AIF are sequential actors in a common cell death pathway that may contribute to stroke-induced brain damage.
Keywords: apoptosis, apoptosis-inducing factor, lipoxygenase, mitochondria, oxidative stress, stroke
Introduction
A variety of pathways contribute to brain damage after transient focal ischemia. Among the proteins mediating oxidative stress, 12/15-lipoxygenase (12/15-LOX) is an important contributor to neuronal cell death, and inhibition of 12/15-LOX is neuroprotective in vitro (Li et al, 1997; Pallast et al, 2009; van Leyen et al, 2008) and in vivo (Khanna et al, 2005; Lapchak et al, 2007; van Leyen et al, 2006). Likewise, edema formation and leakage of the blood–brain barrier are reduced by either pharmacological inhibition or gene knockout of 12/15-LOX (Jin et al, 2008). Immunohistochemistry shows increased expression of 12/15-LOX in the peri-infarct region both 4 and 24 h after transient focal ischemia (Jin et al, 2008; van Leyen et al, 2006). It has to date been unclear which mode of cell death is initiated by 12/15-LOX in ischemic brain. Early studies have shown that 12/15-LOX is capable of damaging mitochondria (van Leyen et al, 1998) and releasing cytochrome c (Maccarrone et al, 2001), but these experiments were conducted in vitro, not in whole cells. We have previously used an established model of neuronal oxidative stress by glutathione depletion in HT22 cells, where cell death is dependent on 12/15-LOX (Li et al, 1997), but independent of excitotoxic effects (Murphy et al, 1990). Because cell death in this model did not involve activation of caspase-3 (van Leyen et al, 2005), but did involve mitochondrial damage (Pallast et al, 2009), we decided to investigate caspase-independent pathways of cell death. Here we used a mouse model of transient focal ischemia to show colocalization of increased 12/15-LOX and apoptosis-inducing factor (AIF) in the peri-infarct area. Using the glutathione depletion model in HT22 cells, we found that 12/15-LOX aggregates in a perinuclear compartment upon glutamate challenge, leading to 12/15-LOX-dependent dispersal of proteins that normally reside in the endoplasmic reticulum (ER), and to nuclear translocation of AIF. These findings suggest an organelle damage pathway in which 12/15-LOX-dependent translocation of AIF to the nucleus may contribute to brain injury after transient focal ischemia.
Materials and methods
Mouse Model of Middle Cerebral Artery Occlusion
CD1 mice were subjected to 2 h of transient focal ischemia and 22 h of reperfusion, as described by van Leyen et al (2006). Briefly, general anesthesia was maintained with 1% to 1.5% isoflurane through a facemask. Laser Doppler flowmetry was used to measure cerebral cortical microperfusion (3 mm lateral to the bregma). Body temperature was monitored and maintained at 36.5°C to 37.5°C with a feed-back heating pad. After midline skin incision, the right external carotid artery was exposed, and its branches were electrocoagulated. A 7.0 nylon monofilament coated with silicon was introduced into internal carotid artery through the external carotid artery to occlude the origin of the middle cerebral artery. Baicalein (300 mg/kg) or vehicle was injected intraperitoneally just before induction of middle cerebral artery occlusion (MCAO), according to (van Leyen et al, 2006). To allow reperfusion, blood flow was restored by withdrawal of the nylon suture. All animals were assessed with laser Doppler flowmetry to confirm adequate induction of focal ischemia and successful reperfusion between experimental groups. All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Immunohistochemistry
To assess the distribution and expression of 12/15-LOX and AIF after transient focal ischemia, anesthetized CD1 mice were perfused transcardially with ice-cold phosphate-buffered saline (PBS, pH 7.4), followed with ice-cold 4% paraformaldehyde in PBS (pH 7.4). The brains were removed, immersed in the same fixative overnight at 4°C, and cryoprotected in 15% and 30% sucrose solutions in PBS at 4°C. Frozen coronal sections (20 μm thick) were prepared using a cryostat. After blocking with PBS containing 0.2% Triton X-100 and 3% bovine serum albumin, the sections were incubated overnight at 4°C with primary antibodies directed against AIF (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:100 dilution), and LOX (affinity-purified rabbit polyclonal antibody directed against the C-terminus of mouse and human 12/15-LOX; 1:100 dilution) in PBS/0.2% Triton X-100/2% BSA. The sections were washed with PBS and incubated with secondary antibodies (Invitrogen, Carlsbad, CA, USA, 1:1,000) and 1 μmol/L To-Pro-3 iodide (Invitrogen) for 30 mins. Brain sections were imaged using a scanning confocal microscope (Zeiss LSM 5 Pascal) in combination with the LSM 5 Pascal Confocal Microscopy Software 3.2.
Immunocytochemistry
HT22 cells were cultured in DMEM containing 10% fetal bovine serum and penicillin/streptomycin (all media from Invitrogen), and treated as indicated. For image acquisition using an inverted fluorescence microscope (Nikon Eclipse TE2000-S) in combination with the PictureFrame software, HT22 cells grown in 24-well plates (Corning, Corning, NY, USA) were treated for 10 to 12 h (LOX staining) or 14 to 18 h (AIF and KDEL staining) as indicated, and then washed with PBS and fixed with 1 ml 4% paraformaldehyde in PBS for 20 mins. The cells were washed with PBS and then permeabilized with 1 ml of 0.2% Triton X-100 in PBS containing 10% FBS for 20 mins. After another washing step the cells were incubated for 45 mins with primary antibodies directed against AIF (1:200 dilution), LOX (1:100 dilution), and KDEL (Stressgen, Ann Arbor, MI, USA, 1:400 dilution), followed by 45 mins of incubation with the appropriate secondary antibodies (Invitrogen, 1:1,000) and 1 μg/ml 4,6-diamidino-2-phenylindole (Sigma, St Louis, MO, USA). For image acquisition using a scanning confocal microscope, HT22 cells were grown on Lab-Tek four-well chamber slides (Electron Microscopy Sciences, Hatfield, PA, USA) and treated as described above. The cells were stained as above, except that the primary AIF antibody was diluted 1:100 and instead of DAPI, 1 μmol/L To-Pro-3 iodide (Invitrogen) was added. The staining intensity of the confocal images was imaged using the three-dimensional Interactive Plugin in NIH ImageJ.
Cell Fractionation and Western Blotting
To analyze AIF localization by Western blotting, HT22 cells were grown in 75-cm2 flasks (Corning) and treated with or without 5 mmol/L glutamate when 50% confluent. After 14 h of incubation, the cells were trypsinized, collected, and washed with PBS. The cells were then resuspended in 200 μl of a lysis buffer containing 20 mmol/L HEPES–KOH (pH 7.4), 10 mmol/L NaCl, 1.5 mmol/L MgCl2, 1 mmol/L EDTA, 1 mmol/L EGTA, 250 mmol/L sucrose and freshly added 1 mmol/L DTT, 2 μg/ml aprotinin, and 0.1 mmol/L phenylmethylsulfonyl fluoride. To disrupt the plasma membrane, the cells were resuspended 25 times with a yellow pipette tip, followed by grinding eight times with a Disposable Pellet Pestle (Kontes Glass Company, Vineland, NJ, USA). Unbroken cells were removed by low-speed centrifugation for 15 mins at 500 r.p.m. (30 g), and then nuclei were pelleted by 15 mins of centrifugation at 3,100 r.p.m. (1,025 g), followed by 15 mins of centrifugation at 1,0000 r.p.m. (10,000 g) to pellet mitochondria. The post-mitochondrial supernatant was further centrifuged for 60 mins at 40,000 r.p.m. in a Beckman TL-100 ultracentrifuge with a TLA-45 rotor to remove residual membranes. The supernatant, containing the cytosolic fraction, was precipitated with 5% tricarboxylic acid and all fractions were resuspended with reducing LDS sample buffer (Invitrogen). The nuclear, cytosolic, and mitochondrial fractions, representing 20 μg protein per lane, were separated on 4% to 12% NuPAGE gels or 4% to 20% Tris–glycine gels (all from Invitrogen), blotted to nitrocellulose membranes, and probed with antibodies to either AIF (1:1,000) or subunit-IV of mitochondrial cytochrome oxidase (Molecular Probes/Invitrogen, Carlsbad, CA, USA; 1:500). Other antibodies used included protein disulphide isomerase (PDI) (Assay Designs, Ann Arbor, MI, USA; 1:500); VDAC (1:1,000), and 12-LOX (Cayman Chemicals, Ann Arbor, MI, USA). Density scanning using NIH ImageJ was used to determine the percentage of AIF signal in the nuclear fraction for each sample.
Statistical Analysis
To determine the percentage of cells featuring either leakage of ER-resident proteins (3H), or nuclear localization of AIF (4F), a series of 30 fluorescence micrographs was scored by a researcher masked to the treatment conditions. Statistical significance was determined by analysis of variance followed by Tukey's test, with P<0.05 considered statistically significant.
Results
Increased 12/15-LOX Expression Coincides with Nuclear AIF in Ischemic Brain
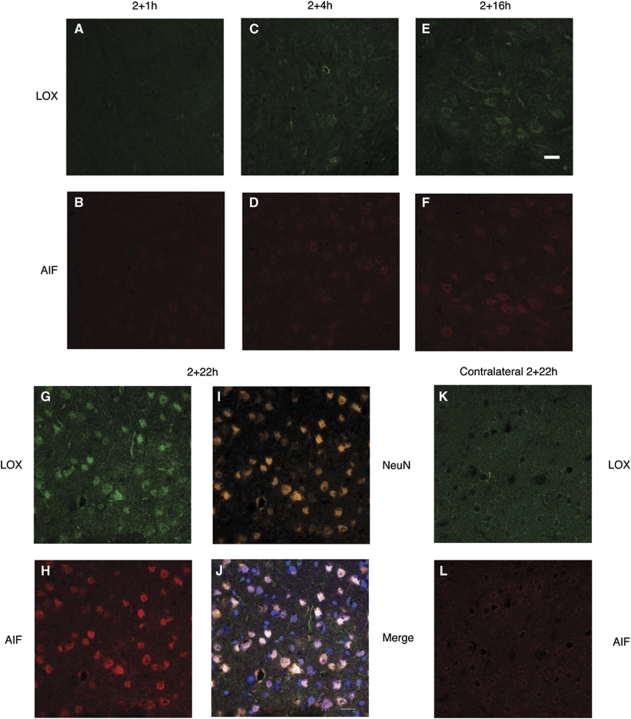
We used a mouse model of transient focal ischemia to investigate a possible correlation between the expression of LOX and AIF in ischemic brain. Analyzed by immunohistochemistry followed by confocal microscopy, LOX expression was greatly increased in a time-dependent manner on the ipsilateral as compared with that on the contralateral side after 2 h of ischemia and varying times of reperfusion (see also Figures 1A, 1C, 1E, 1G, and 1K). This staining pattern confirmed our previous findings (Jin et al, 2008; van Leyen et al, 2006). Remarkably, most of the cells showing increased LOX staining at 2+22 h were also positive for AIF immunoreactivity (Figure 1H). The time course showed that increases in LOX immunoreactivity preceded the increase in AIF staining (see also Figures 1A–1H). Colocalization with the neuronal marker NeuN (Figure 1I) suggested that most of the LOX- and AIF-positive cells were neurons. Furthermore, overlay with the DNA-binding dye To-Pro-3 (Figure 1J) showed that several of the marked cells had a nuclear localization for AIF at 2+22 h, consistent with the known function of AIF in promoting cell death. The contralateral side (Figures 1K and 1L) showed only low levels of expression for both LOX and AIF, confirming previous studies (Jin et al, 2008; Zhao et al, 2004).
Figure 1.
LOX colocalizes with AIF in ischemic brain. (A, C, E, G) After 2 h of ischemia, there is gradual increase in LOX immunoreactivity on the ischemic (ipsilateral) side of the brain. (B, D, F, H) AIF also increases, but with some time delay. (I and J) Co-staining with the neuronal marker NeuN and an overlay of AIF, LOX, and NeuN with the DNA dye To-Pro-3 indicates nuclear localization for both AIF and LOX in some cells. (K and L) The contralateral side shows only little expression of both LOX and AIF. Scale bar, 20 μm.
The 12/15-LOX Inhibitor Baicalein Prevents the AIF Increase in Ischemic Brain
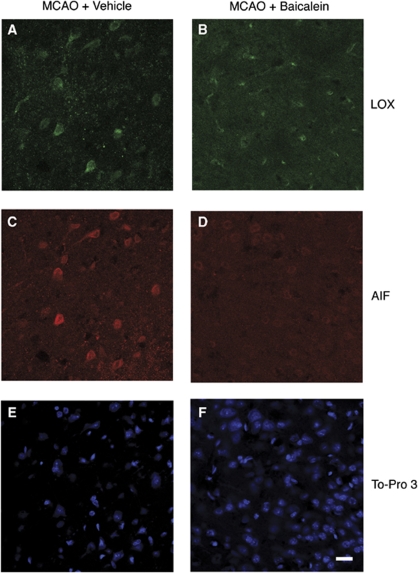
If increased LOX expression indeed precedes that of AIF in ischemic brain, one might expect that inhibiting 12/15-LOX could prevent the increase and nuclear localization of AIF. From our previous studies, we knew that the 12/15-LOX inhibitor baicalein is robustly neuroprotective, decreasing both infarct size and edema formation (Jin et al, 2008; van Leyen et al, 2006). To determine whether baicalein can prevent increased AIF expression in neuronal nuclei, we injected mice with either baicalein or vehicle, immediately before induction of MCAO. Staining brain sections taken after 2 h of ischemia and 22 h of reperfusion, both LOX and AIF were increased in the peri-infarct area of vehicle-treated mice, as expected (Figures 2A and 2C). Baicalein-treated mice also showed increased LOX expression, albeit at a somewhat reduced level as compared with that after vehicle treatment (see also Figures 2A and 2B). Remarkably, AIF staining was drastically reduced in baicalein-treated mice (Figure 2D), suggesting that the 12/15-LOX inhibitor can indeed prevent the increase in nuclear AIF in the infarcted brain. The nuclear DNA dye To-Pro 3 confirms the presence of a large number of cells in the fields shown in Figures 2E and 2F.
Figure 2.
Treatment with the 12/15-LOX inhibitor baicalein prevents increased nuclear AIF after MCAO. (A and C) Vehicle-treated mice express both LOX and AIF at 22 h following 2 h of ischemia. (B and D) Baicalein treatment partially reduces LOX expression, but completely prevents the increase in AIF expression in ischemic brain.
LOX Aggregates Around the Nucleus after Oxidative Challenge
To investigate the significance of LOX colocalization with AIF, we turned to an established model of neuronal oxidative stress, oxidative glutamate toxicity, in which cell death is known to depend on 12/15-LOX activity. Independent of excitotoxic effects, exogenous glutamate in neuronal HT22 cells leads to glutathione depletion and cell death within 24 h. Previous studies suggested mitochondria as targets for the normally cytosolic 12/15-LOX, prompting us to determine the localization of 12/15-LOX after glutamate administration.
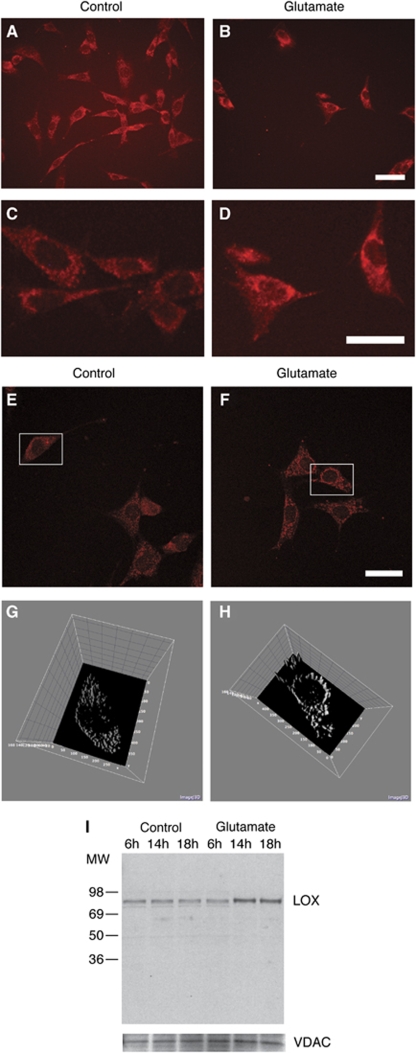
Control-treated cells were stained throughout the cell, with the nucleus excluded, suggesting cytosolic localization (Figures 3A and 3C). In contrast, after treatment with 5 mmol/L glutamate for 12 h, LOX staining intensified and the protein aggregated in a perinuclear compartment (Figures 3B and 3D). To confirm this result, we used confocal microscopy and three-dimensional processing using NIH ImageJ (Figures 3E–3H).
Figure 3.
Localization of LOX in glutamate-treated cells. HT22 cells were treated or not with 5 mmol/L glutamate for 12 h. LOX immunostaining shows a diffuse staining pattern in control cells (A, higher magnification, C), suggesting cytosolic localization of the enzyme. In glutamate-treated cells, the staining shifts to the perinuclear compartment of the cells (B and D). Scale bar, 50 μm. Confocal microscopy (E, F) in conjunction with three-dimensional graphing of the staining intensity using NIH ImageJ (G and H) confirmed the localization change after glutamate treatment. (I) Blotting mitochondrial fractions from HT22 cells treated for the indicated time periods shows that LOX associates with mitochondria after glutamate treatment. The mitochondrial VDAC channel was used as loading control.
Because of the localization change of 12/15-LOX and its known propensity for binding to organelle membranes, we decided to test whether LOX directly associates with mitochondria in glutamate-treated HT22 cells. Blotting for 12/15-LOX in the mitochondrial fraction of either glutamate- or control-treated cells, we found increase of the normally cytosolic 12/15-LOX in the mitochondria after prolonged glutamate treatment (Figure 3I).
Leakage of ER-Resident Proteins after 12/15-LOX Activation
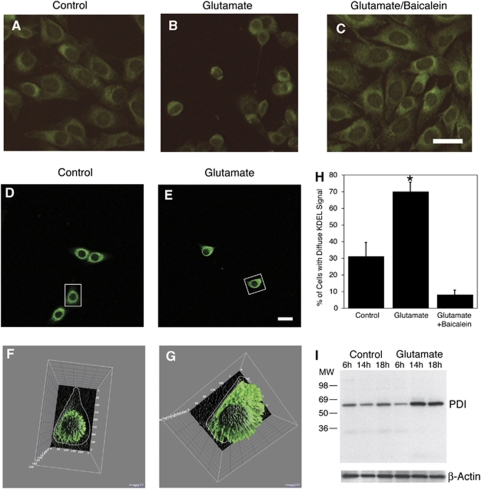
The perinuclear space comprises two of the major organelles of the cell, the ER and most of the cell's mitochondria. Previous in vitro studies have documented that 12/15-LOX can bind to and permeabilize both ER and mitochondrial membranes (van Leyen et al, 1998). This prompted us to investigate if perinuclear localization of 12/15-LOX has similar consequences. We therefore decided to study the intracellular distribution of the normally luminal proteins of the ER, which feature a characteristic lysine–aspartate–glutamate–leucine (KDEL) sequence at their C-terminus. An antibody directed against this KDEL sequence shows the punctate staining typical of ER localization in control-treated HT22 cells (Figure 4A). After 14 h of glutamate treatment, KDEL staining becomes more diffuse, spreading out over the cell (Figure 4B). Importantly, this is not seen in cells co-incubated with the 12/15-LOX inhibitor baicalein (Figure 4C, used at 10-μmol/L concentration). Analysis by confocal microscopy (Figures 4D and 4E) and three-dimensional imaging of the staining intensity using NIH ImageJ (Figures 4F and 4G) confirmed the change in distribution, while a masked evaluation underscored the statistical significance of these findings (Figure 4H).
Figure 4.
Damage to the ER through 12/15-LOX. (A) An antibody directed against the KDEL sequence of soluble ER proteins shows the punctate staining typical of ER localization in control-treated HT22 cells. (B) After 14 h of glutamate treatment, KDEL staining becomes more diffuse, spreading out over the cell. This is not seen in cells co-incubated with the 12/15-LOX inhibitor baicalein (C, 10 μmol/L concentration). Analysis by confocal microscopy (D and E) and three-dimensional imaging of the staining intensity using NIH ImageJ (F and G) confirmed these findings. Scale bar, 20 μm. (H) Statistical analysis showed significant differences in staining pattern (*P<0.05 compared with control or glutamate/baicalein-treated cells). (I) A cytosolic fraction of HT22 cells treated with glutamate contains increased amounts of the KDEL protein PDI, indicating leakage from ER.
To more directly address the question of whether or not leakage of soluble ER-resident proteins occurs, we isolated a cytosolic fraction from HT22 cells at different time points after glutamate addition. PDI is one of the most prominent KDEL proteins, and normally resides in the lumen of the ER. Correspondingly, we found little PDI in the cytosol of control cells, as well as after 6 h of glutamate treatment (Figure 4I). After 14 and 18 h however, increasing amounts of PDI were detected in the cytosolic fraction, indicating that PDI is indeed released to the cytosol in glutamate-treated cells. Taken together, these results suggest that ER membranes are damaged in HT22 cells.
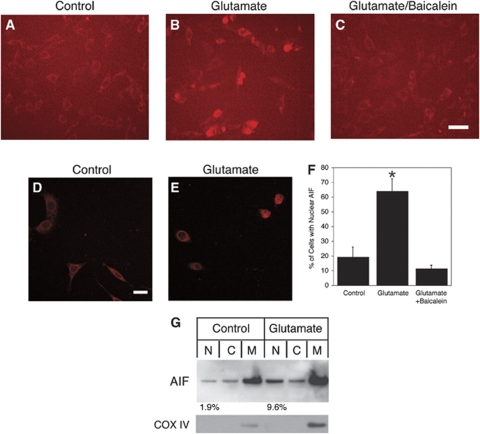
Translocation of AIF to the Nucleus in HT22 Cells
After observing the changes in AIF staining intensity and localization in ischemic brain, we decided to study whether AIF is also involved in the 12/15-LOX-dependent death of HT22 cells. After incubation with glutamate for longer periods of time (16 to 18 h), nuclear AIF was frequently detected (Figure 5B). This was typically not seen in cells co-treated with baicalein (Figure 5C). Examination by confocal microscopy confirmed these observations (see also Figures 5D and 5E). Both increased nuclear localization of AIF in glutamate-treated cells, and its inhibition in baicalein-treated cells, were statistically significant (Figure 5F). As an alternate approach, we fractionated HT22 cells after 14 h of incubation. Increased levels of AIF were detected by Western blotting in the nuclear fraction of glutamate-treated cells (Figure 5G).
Figure 5.
Nuclear translocation of AIF in glutamate-treated cells. Compared with control-treated cells (A), after 16 to 18 h of glutamate treatment nuclear localization of the apoptotic factor AIF was observed (B). This effect was reversed by co-treatment with baicalein (C). Scale bar, 50 μm. (D and E) Confocal microscopy confirmed this result (scale bar, 20 μm). (F) Statistical analysis showed significantly increased nuclear AIF staining in glutamate-treated cells compared to both control treated and glutamate + baicalein treated cells (*P<0.05). (G) Cell fractionation after 14 h of glutamate treatment, followed by Western blotting, showed increase in nuclear AIF in the presence of glutamate (9.6% of the total AIF signal, compared with 1.9% for the control-treated cells). The mitochondrial membrane protein cytochrome oxidase subunit-IV (COX IV) as control shows absence of mitochondrial contamination from the nuclear and cytosolic fractions.
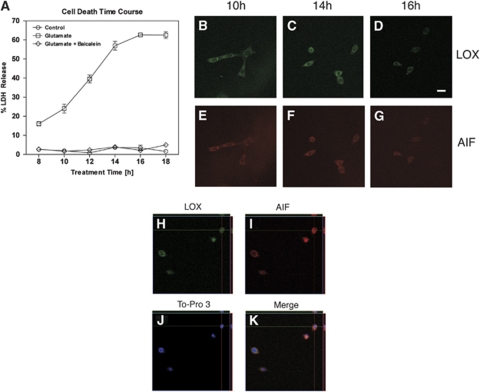
To get a better sense of the time course of cell damage through oxidative glutamate toxicity, we worked up HT22 cells after different times of glutamate treatment. Determining lactate dehydrogenase (LDH) release as a measure of cell death, we found significant increases in glutamate toxicity already at 12 h of glutamate treatment, and this was completely prevented by co-incubation with baicalein (Figure 6A). Although from the data presented here it may look as though LDH release plateaus at 16 h, we know from previous studies that cell death can continue to increase, up to approximately 90% cell death at 24 h (Li et al, 1997; Pallast et al, 2009; van Leyen et al, 2005). Co-staining HT22 cells at different time points for LOX and AIF, we found that even at 10 h of treatment, there was some focal accumulation of LOX signal in the perinuclear area (Figure 6B), while AIF was still largely confined outside of the nucleus (Figure 6E). With increasing time of glutamate exposure, some nuclear translocation of AIF could be detected (see Figures 6F and 6G). After 18 h, the remaining cells had a shrunken appearance, and both AIF and LOX were found in the nucleus in some cases (Figures 6H–6K), similar to what was observed in the brain after transient focal ischemia (see Figure 1).
Figure 6.
The time course for AIF translocation to the nucleus in HT22 cells. (A) Cell death increases after approximately 10 to 12 h of glutamate treatment, and this is prevented by co-administration of 10 μmol/L baicalein. (B–G) LOX starts accumulating in a perinuclear compartment already after 10 h, while AIF appears in the nucleus at later time points. Scale bar, 20 μm. (H–K) After 18 h of incubation in the presence of glutamate, some of the LOX immunoreactivity in HT22 cells localizes to the nucleus (top left), similar to AIF (top right, images generated from a confocal Z-stack using the Ortho setting in the LSM 5 Pascal software). An overlay with the nuclear stain To-Pro-3 confirms that some, but not all the LOX and AIF present coincide with nuclear DNA (bottom right).
Discussion
Our findings reported in this study suggest a possible mechanism for neuronal cell death in ischemic brain through 12/15-LOX and the proapoptotic protein AIF. AIF translocation from mitochondria to the nucleus is frequently implicated in caspase-independent forms of cell death (Boujrad et al, 2007; Cao et al, 2003; Modjtahedi et al, 2006). AIF has previously been shown to be upregulated after transient focal ischemia (Plesnila et al, 2004), permanent MCAO (Zhao et al, 2004), and transient global ischemia (Cao et al, 2003), and this is supported by our findings using the filament model of transient focal ischemia. This study shows that the level of 12/15-LOX increases in a time-dependent manner on the ischemic side of the brain, and that of AIF increases, mostly in the same neurons, somewhat later (see Figure 1). Furthermore, inhibition of 12/15-LOX with baicalein in vivo prevents the increase in AIF (Figure 2). Baicalein is both an antioxidant and a direct inhibitor of 12/15-LOX (Deschamps et al, 2006). Using 12/15-LOX-knockout mice we have previously shown that the neuroprotective properties of baicalein are mediated by its inhibition of 12/15-LOX (van Leyen et al, 2006). This study suggests that this protection may in part be caused by baicalein preventing the proapoptotic effects of AIF. Because of difficulties in deriving mechanistic information from in vivo studies, we have turned to an oxidative glutamate toxicity model in neuronal HT22 cells. This is a cell line with mostly neuron-like characteristics originally derived from the murine hippocampus. When HT22 cells are treated with exogenous glutamate, the glutathione levels reduce and 12/15-LOX is activated, eventually causing the cells to die (Li et al, 1997). We have shown here that cell death in this model starts at around 10 to 12 h of treatment, and increases with time. 12/15-LOX-dependent neuronal cell death in HT22 cells supports a sequence of events where LOX aggregation in a perinuclear compartment precedes leakage of ER-resident proteins and nuclear AIF translocation. Some additional points should be made: (1) the consequences of ER permeabilization in HT22 cells remain unclear. While damage to ER fits with the known characteristics of 12/15-LOX in causing organelle damage (van Leyen et al, 1998), we do not know whether this directly or indirectly contributes to the cell death process. This appears plausible, however, considering that both ER stress and several specific ER-associated proteins have been linked to various forms of apoptosis (Paschen and Mengesdorf, 2005). (2) We do not know if additional proteins and other factors are required to cause AIF translocation to the nucleus. Major roles for calpain proteases (Cao et al, 2007), poly(ADP-ribose) polymerase-1 (Yu et al, 2002), and truncated Bid (Culmsee et al, 2005) have been shown in various models of neuronal injury. Proteolytic processing of membrane-bound AIF has been seen as a requirement, although this has recently been called into question (Wang et al, 2009). While our Western blot results do not show a clear difference in apparent molecular weights for the nuclear and mitochondrial populations of AIF, the protein is known to show unusual migration behavior on sodium dodecyl sulfate–polyacrylamide gel electrophoresis. What is clear from our experimental results, though, is that LOX activity is required for nuclear translocation of AIF in this oxidative stress model. (3) While the overall evidence for a role of AIF in apoptotic cell death is strong, it remains unclear how AIF translocation to the nucleus leads to cell death. Considering the concomitant release of ER-resident proteins, there may be several possible modes of cell death once 12/15-LOX has been activated. Future studies will have to show whether inhibiting AIF translocation alone is sufficient to restore viability to the HT22 cells, but at present this seems unlikely. Nevertheless, there are indications that reducing AIF translocation is neuroprotective in vivo; both Harlequin mutant mice (Culmsee et al, 2005), which have lower levels of AIF, and PARP-1-knockout mice (Eliasson et al, 1997) show significantly reduced infarct volumes. It remains to be seen how the current proposed mechanism of 12/15-LOX-related AIF translocation fits into the picture. (4) A recent report suggested release of AIF into the cytosol as part of a possible cell death mechanism in HT22 cells (Fukui et al, 2009). We detected only low levels of AIF in the cytosol in Western blots; furthermore, cytosolic localization of AIF is hard to reconcile with the proapoptotic function of AIF in the nucleus, suggesting this may be at most an intermediate stage. Another recent report implicated glutathione peroxidase-4 in controlling the cytotoxic effects of 12/15-LOX (Seiler et al, 2008). It will be interesting to see if this antioxidant protein is relevant to oxidative glutamate toxicity in HT22 cells, and to ischemic brain damage in vivo. (5) Finally, there are some important differences between the cell death described here in HT22 cells and neuronal cell death after ischemia in vivo. One striking difference is the increased expression of both 12/15-LOX and AIF in ischemic neurons (see Figure 1). In HT22 cells, we do not see such clear upregulation, although it appears to be the case in some of our experiments (e.g., compare Figures 5A and 5B). Furthermore, the environment surrounding ischemic neurons in the brain may differ drastically from the mostly supportive growth medium of cultured HT22 cells. One trigger may be common to both systems, though: a reduction in glutathione levels is characteristic both of the brain after ischemia, and of the initial steps of oxidative glutamate toxicity. Further studies are clearly needed to validate the mechanisms described here in primary cells and in vivo.
In summary, we show an oxidative stress-related cell death mechanism in HT22 cells that is characterized by sequentially timed aggregation of 12/15-LOX in proximity to the nucleus, followed by damage to the ER and nuclear translocation of mitochondrial AIF. This mechanism, which reflects the role of 12/15-LOX in causing organelle degradation, may similarly apply to ischemic brain, where it provides a rationale for the coordinate upregulation of 12/15-LOX and AIF in the neurons of the peri-infarct area. Prevention of organelle damage might be an important part of the therapeutic mechanisms of LOX inhibitors. Future studies will be needed to determine whether additional cell death mechanisms are activated by 12/15-LOX in ischemic brain.
Acknowledgments
We thank I Bagayev for help with confocal microscopy and H-Y Kim for ischemic brain sections. Support through grants from the NIH (R01NS049430 to KvL, R01NS53560 and P01NS555104 to EHL), and a Scientist Development grant from the American Heart Association (to KvL.), is gratefully acknowledged.
The authors declare no conflict of interest.
References
- Boujrad H, Gubkina O, Robert N, Krantic S, Susin SA. AIF-mediated programmed necrosis: a highly regulated way to die. Cell Cycle. 2007;6:2612–2619. doi: 10.4161/cc.6.21.4842. [DOI] [PubMed] [Google Scholar]
- Cao G, Clark RS, Pei W, Yin W, Zhang F, Sun FY, Graham SH, Chen J. Translocation of apoptosis-inducing factor in vulnerable neurons after transient cerebral ischemia and in neuronal cultures after oxygen–glucose deprivation. J Cereb Blood Flow Metab. 2003;23:1137–1150. doi: 10.1097/01.WCB.0000087090.01171.E7. [DOI] [PubMed] [Google Scholar]
- Cao G, Xing J, Xiao X, Liou AK, Gao Y, Yin XM, Clark RS, Graham SH, Chen J. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, Blomgren K, Plesnila N. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen–glucose deprivation and focal cerebral ischemia. J Neurosci. 2005;25:10262–10272. doi: 10.1523/JNEUROSCI.2818-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps JD, Kenyon VA, Holman TR. Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases. Bioorg Med Chem. 2006;14:4295–4301. doi: 10.1016/j.bmc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Fukui M, Song JH, Choi JY, Choi HJ, Zhu BT. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur J Pharmacol. 2009;617:1–11. doi: 10.1016/j.ejphar.2009.06.059. [DOI] [PubMed] [Google Scholar]
- Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, van Leyen K. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation following transient focal ischemia. Stroke. 2008;39:2538–2543. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Roy S, Slivka A, Craft TK, Chaki S, Rink C, Notestine MA, DeVries AC, Parinandi NL, Sen CK. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke. 2005;36:2258–2264. doi: 10.1161/01.STR.0000181082.70763.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Maher P, Schubert D, Zivin JA. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007;150:585–591. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Melino G, Finazzi-Agro A. Lipoxygenases and their involvement in programmed cell death. Cell Death Differ. 2001;8:776–784. doi: 10.1038/sj.cdd.4400908. [DOI] [PubMed] [Google Scholar]
- Modjtahedi N, Giordanetto F, Madeo F, Kroemer G. Apoptosis-inducing factor: vital and lethal. Trends Cell Biol. 2006;16:264–272. doi: 10.1016/j.tcb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Schnaar RL, Coyle JT. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. FASEB J. 1990;4:1624–1633. [PubMed] [Google Scholar]
- Pallast S, Arai K, Wang X, Lo EH, van Leyen K. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. J Neurochem. 2009;111:882–889. doi: 10.1111/j.1471-4159.2009.06379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W, Mengesdorf T. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium. 2005;38:409–415. doi: 10.1016/j.ceca.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Plesnila N, Zhu C, Culmsee C, Groger M, Moskowitz MA, Blomgren K. Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J Cereb Blood Flow Metab. 2004;24:458–466. doi: 10.1097/00004647-200404000-00011. [DOI] [PubMed] [Google Scholar]
- Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Radmark O, Wurst W, Bornkamm GW, Schweizer U, Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- van Leyen K, Arai K, Jin G, Kenyon V, Gerstner B, Rosenberg PA, Holman TR, Lo EH. Novel lipoxygenase inhibitors as neuroprotective reagents. J Neurosci Res. 2008;86:904–909. doi: 10.1002/jnr.21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leyen K, Duvoisin RM, Engelhardt H, Wiedmann M. A function for lipoxygenase in programmed organelle degradation. Nature. 1998;395:392–395. doi: 10.1038/26500. [DOI] [PubMed] [Google Scholar]
- van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37:3014–3018. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- van Leyen K, Siddiq A, Ratan RR, Lo EH. Proteasome inhibition protects HT22 neuronal cells from oxidative glutamate toxicity. J Neurochem. 2005;92:824–830. doi: 10.1111/j.1471-4159.2004.02915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kim NS, Li X, Greer PA, Koehler RC, Dawson VL, Dawson TM. Calpain activation is not required for AIF translocation in PARP-1-dependent cell death (parthanatos) J Neurochem. 2009;110:687–696. doi: 10.1111/j.1471-4159.2009.06167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Cheng D, Barreto-Chang OL, Sapolsky RM, Steinberg GK. Bcl-2 transfection via herpes simplex virus blocks apoptosis-inducing factor translocation after focal ischemia in the rat. J Cereb Blood Flow Metab. 2004;24:681–692. doi: 10.1097/01.WCB.0000127161.89708.A5. [DOI] [PubMed] [Google Scholar]