Abstract
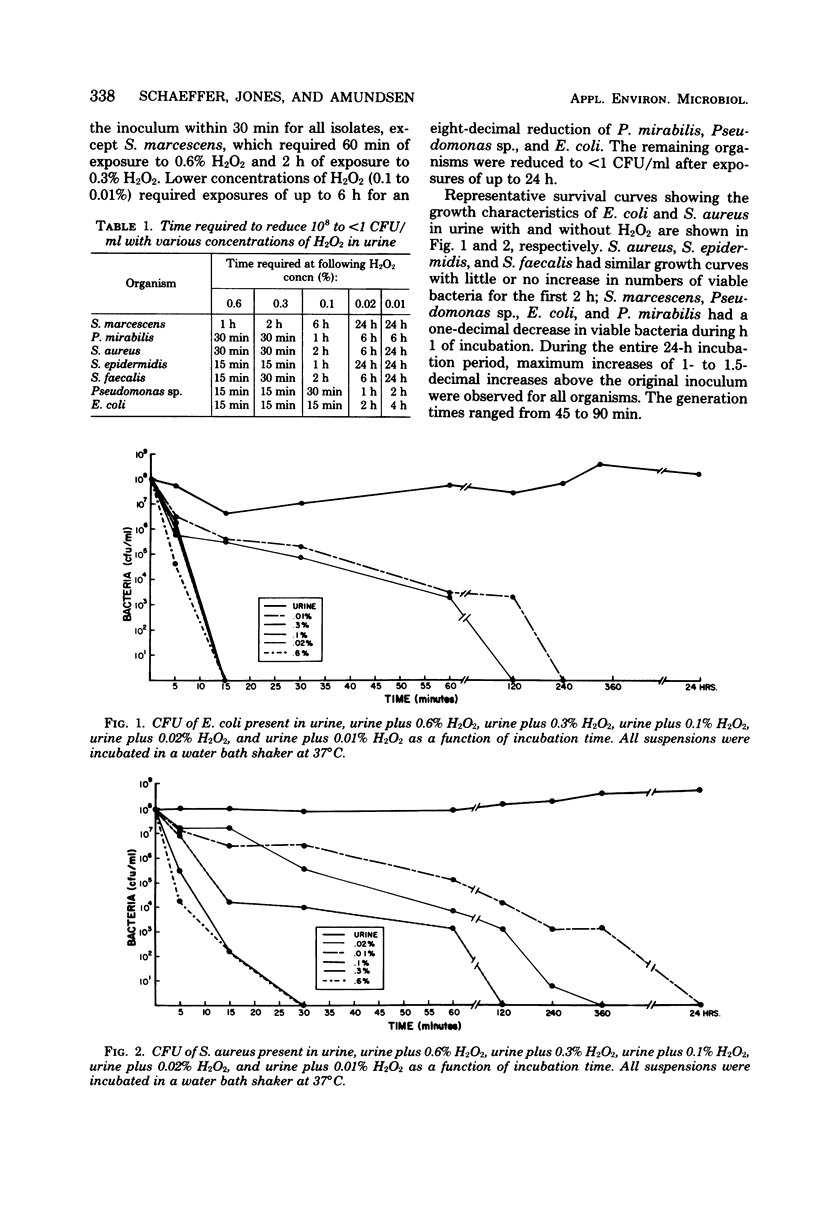
Bacterial contamination of urinary drainage bags is a frequent source of bladder bacteriuria in patients with indwelling catheters. Previous work demonstrated that the addition of 30 ml of 3% H2O2 prevented bacterial contamination of urinary drainage bags for up to 8 h in patients with urinary infections (greater than 10(5) colony-forming units per ml). Survival curves of a variety of organisms in filter-sterilized urine with various concentrations of H2O2 (0.6 to 0.01%) were constructed. Organisms with high cellular catalase activity (Staphylococcus aureus, Serratia marcescens, and Proteus mirabilis) required 30 to 60 min of exposure to 0.6% H2O2 for a reduction of 10(8) to less than 1 colony-forming unit per ml, whereas Escherichia coli, Streptococcus sp., and Pseudomonas sp. required only 15 min of exposure. The efficacy of H2O2 in urine was maintained despite exposure to room temperature for 5 days and reinoculation with bacterial suspensions. H2O2 is inexpensive and relatively nontoxic, and these data suggest that periodic instillation of H2O2 into urinary drainage bags may eliminate a source of bladder bacteriuria and environmental contamination.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin V. M., Olson N. F. Influence of catalase activity on resistance of coagulase-positive staphylococci to hydrogen peroxide. Appl Microbiol. 1968 Feb;16(2):267–270. doi: 10.1128/am.16.2.267-270.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. E., Dimmick R. L. Effect of 3 percent hydrogen peroxide on the viability of Serratia marcescens. J Bacteriol. 1966 Mar;91(3):925–929. doi: 10.1128/jb.91.3.925-929.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOT J. S., SHARP R. F., LEWIS L. Urinary pH. J Urol. 1959 Feb;81(2):339–343. doi: 10.1016/S0022-5347(17)66022-1. [DOI] [PubMed] [Google Scholar]
- Eisenstark A. Mutagenic and lethal effects of visible and near-ultraviolet light on bacterial cells. Adv Genet. 1971;16:167–198. [PubMed] [Google Scholar]
- Nagano H., Fujimoto T. [Studies on the mechanisms of bactericidal action of hydrogen peroxide (author's transl)]. Yakugaku Zasshi. 1975 Sep;95(9):1108–1113. doi: 10.1248/yakushi1947.95.9_1108. [DOI] [PubMed] [Google Scholar]
- Taylor W. I., Achanzar D. Catalase test as an aid to the identification of Enterobacteriaceae. Appl Microbiol. 1972 Jul;24(1):58–61. doi: 10.1128/am.24.1.58-61.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoakum G. H. Tryptophan photoproduct(s): sensitized induction of strand breaks (or alkali-labile bonds) in bacterial deoxyribonucleic acid during near-ultraviolet irradiation. J Bacteriol. 1975 Apr;122(1):199–205. doi: 10.1128/jb.122.1.199-205.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoakum G., Ferron W., Eisenstark A., Webb R. B. Inhibition of replication gap closure in Escherichia coli by near-ultraviolet light photoproducts of L-tryptophan. J Bacteriol. 1974 Jul;119(1):62–69. doi: 10.1128/jb.119.1.62-69.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshpe-Purer Y., Henis Y. Factors affecting catalase level and sensitivity to hydrogen peroxide in Escherichia coli. Appl Environ Microbiol. 1976 Oct;32(4):465–469. doi: 10.1128/aem.32.4.465-469.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


