Abstract
The design, synthesis, and metal-binding properties of DF3, a new de novo designed di-iron protein model are described (“DF” represents due ferri, Italian for “two iron,” “di-iron”). DF3 is the latest member of the DF family of synthetic proteins. They consist of helix–loop–helix hairpins, designed to dimerize and form an antiparallel four-helix bundle that encompasses a metal-binding site similar to those of non-heme carboxylate-bridged di-iron proteins. Unlike previous DF proteins, DF3 is highly soluble in water (up to 3 mM) and forms stable complexes with several metal ions (Zn, Co, and Mn), with the desired secondary structure and the expected stoichiometry of two ions per protein. UV–vis studies of Co(II) and Fe(III) complexes confirm a metal-binding environment similar to previous di-Co(II)- and di-Fe(III)-DF proteins, including the presence of a µ-oxo-di-Fe(III) unit. Interestingly, UV–vis, EPR, and resonance Raman studies suggest the interaction of a tyro-sine adjacent to the di-Fe(III) center. The design of DF3 was aimed at increasing the accessibility of small molecules to the active site of the four-helix bundle. Indeed, binding of azide to the di-Fe(III) site demonstrates a more accessible metal site compared with previous DFs. In fact, fitting of the binding curve to the Hill equation allows us to quantify a 150% accessibility enhancement, with respect to DF2. All these results represent a significant step towards the development of a functional synthetic DF metalloprotein.
Keywords: Di-iron-oxo proteins, Four-helix bundle, Metalloprotein models, Thermodynamic stability, Spectroscopic characterization
Introduction
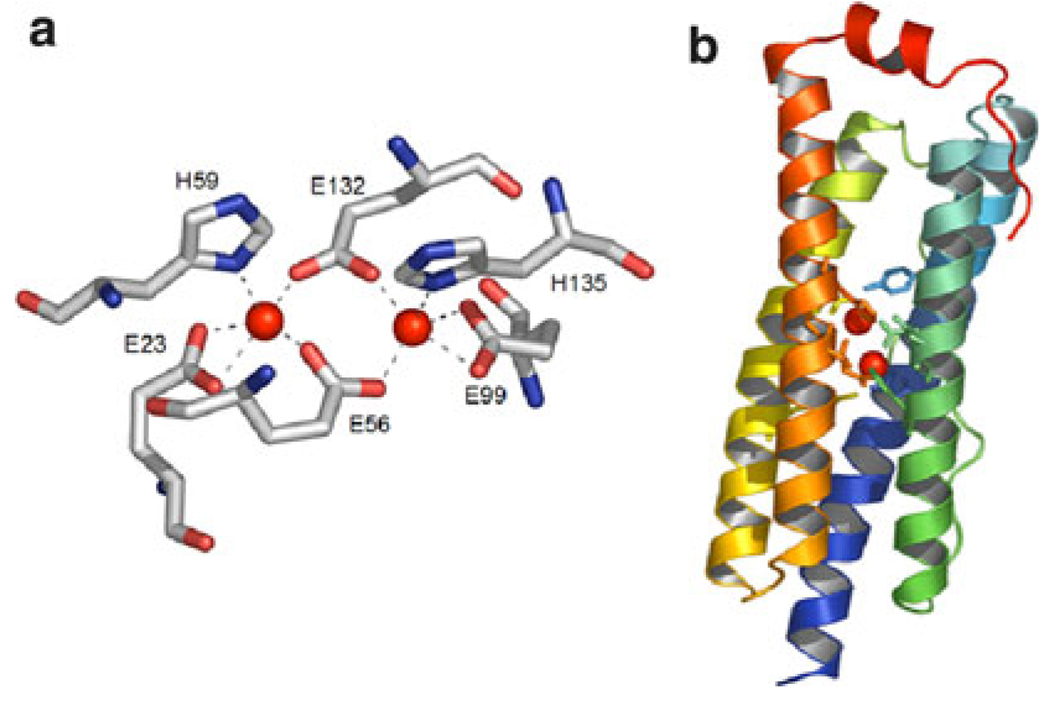
Carboxylate-bridged di-iron proteins belong to a super-family of proteins that catalyze a wide range of important dioxygen-dependent biological reactions and processes. Examples of the diverse functions performed by these enzymes include the selective oxidation of methane to methanol by soluble methane monooxygenase [1], the generation of a catalytically essential tyrosyl radical for DNA biosynthesis by ribonucleotide reductase R2 subunit [2], and the desaturation of fatty acids by stearoyl-ACP Δ9-desaturase [3]. Interestingly, despite this functional diversity, the active sites of all these enzymes are remarkably similar, and share two structural motifs: (1) a dioxygen-reactive di-iron site consisting of two iron ions coordinated by two His residues and four carboxylate side chains (Fig. 1a) and (2) a four-helix bundle motif that houses the di-iron center (Fig. 1b) [4]. Therefore, it seems clear that small differences in the structures of these proteins must be responsible for tuning the reactivity of the di-iron site towards their different functions.
Fig. 1.
An example of a di-iron protein: the structure of bacterioferritin, as derived from X-ray crystallography [Protein Data Bank (PDB) code 1NF4] [6]. a Details of the di-iron(II) site in bacterioferritin. b The four-helix bundle motif housing the di-iron(II) site; first and second coordination shell residues are depicted as sticks; the red spheres indicate the metal ions. The images were generated using PyMOL [7]
A complete understanding of how these differences affect functionalities has yet to be achieved. There have been, however, major steps forward in the field, such as the recent evidence of a remarkable switch of a di-iron site reactivity from saturation to oxidation by a single mutation in the four-helix bundle of a Δ9-desaturase [5].
The highly conserved di-iron metal center and the simple secondary structure that supports it provide a formidable modeling challenge for chemists, who aim to understand their mechanisms and to develop bioinspired proteins and catalysts with improved and new functions. We have undertaken these tasks using a de novo protein design approach with the due ferri (DF) family of synthetic proteins [8–25]. These are simplified models of di-iron proteins and were designed after an analysis of the structures of several natural di-iron enzymes. The homodimeric subset of the DF family consists of helix–loop–helix hairpins that dimerize to form an antiparallel four-helix bundle. It provides a four Glu, two His site in the correct geometry for binding a dinuclear metal center, as well as proper second-shell interactions, which are known to be crucial for structural stability and function. Our first model, DF1, was designed for maximal stability [8]. Thus, the interior of the protein was efficiently packed with a large number of hydrophobic side chains, resulting in a high degree of conformational stability. The structural characterization showed DF1 to be an excellent structural model of di-iron proteins. It adopts a folded, nativelike conformation in the apo and metal-bound forms and was able to bind metal ions such as Zn(II), Co(II), and Mn(II) in the correct geometry and stoichiometry [8, 9]. Unfortunately, DF1 was not able to support any function, because access to its active site was hampered by the compact hydrophobic core around the metal center. To improve the functional properties of the initial model, subsequent work focused on opening the cleft for binding small ligands to the dimetal center. DF1 was thus subjected to several changes in the sequence, as well as in the loop structure (DF1 and DF2 subsets) [10–12, 14–16]. In particular, substitution of Leu13 and Leu13′ in DF1 with smaller side chain amino acids, such as Ala and Gly, afforded a cavity large enough to allow access of small molecules to the metal center. The L13A-DF1 [10] and L13G-DF1 [11] variants, as well as the DF2 subset [12, 14–16], bind exogenous ligands, such as phenol and acetate, and display ferroxidase activity. However, optimization of the catalytic activity required further changes in the second-shell ligands. Modeling suggested that residues at positions 13 and 9 play a critical role for the accessibility to the metal site. Combinatorial studies on the DFtet subset (a heterotetrameric system consisting of four disconnected helices that came together by noncovalent self-assembly) resulted in the selection of G4-DFtet (a variant with Gly residues at positions 9 and 13) as the best candidate for function [17–19]. Unfortunately, structural data were not obtainable for the DFtet subset, and it was not possible to correlate active-site structure and activity. We ultimately focused on the much easier to characterize symmetric helix–loop–helix dimer. DF3 encompasses all the main features thought to be crucial for function, in particular (1) Gly residues at positions 9 and 13 of the sequence, (2) a redesigned “Rose-like” αL–β interhelical loop. The new protein shows improved solubility and active-site accessibility, while retaining the unique nativelike structure, as assessed by NMR structural characterization [26]. Most importantly, DF3, with its well-defined active site, displays ferroxidase and oxidase activity. Both iron binding and catalytic activity of DF3 were reported in a previous communication [26]. In this paper, we describe the spectroscopic, thermodynamic, and binding properties of DF3. Different spectroscopic techniques were used to fully characterize DF3 and demonstrate its capability to form stable complexes with different metal ions. Binding of an exogenous ligand (azide) was also analyzed to prove the enhanced accessibility to the di-metal site in DF3, with respect to previously characterized DFs. All the spectroscopic and structural features reported herein help to correlate structure with function in de novo designed metalloproteins.
Materials and methods
Peptide synthesis and purification
DF3 was chemically synthesized by automated solid-phase synthesis using an ABI 433A peptide synthesizer (Applied Biosystems, Foster City, CA, USA). The N- and C-termini were acetylated and amidated, respectively. The instrumental software 9-fluorenylmethoxycarbonyl protocols were modified to provide an improved synthetic outcome. Benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate/hydroxybenzotriazole activation was used instead of the instrumental O-benzotriazole-N,N,N′,N′-tetramethyluronium hexafluorophosphate activation. The synthesis was carried out on a 0.1-mmol scale using PAL-PEG-PS resin from Applied Biosystems (substitution level 0.22 mmol g−1).
The crude product (416 mg) was analyzed to homogeneity by analytical reverse-phase high-performance liquid chromatography (RP-HPLC), performed with a Shimadzu LC-10ADvp equipped with an SPDM10Avp diode-array detector. A Vydac C18 column (4.6 mm × 150 mm; 5 µm), eluted with an H2O/0.1% trifluoroacetic acid (TFA) (solvent A) and CH3CN/0.1% TFA (solvent B) linear gradient, from 10 to 80% (solvent B) over 35 min, at 1 mL min−1 flow rate, was used in all analyses. Purification was accomplished by preparative RP-HPLC (22 mm × 250 mm; 10 µm) Vydac C18 column, at a flow rate of 22 mL min−1, with a gradient of acetonitrile in 0.1% aqueous TFA, from 10 to 80% over 58.1 min. Pure DF3 was obtained in 18% yield from the crude product and its purity was ascertained by analytical RP-HPLC and matrix-assisted laser desorption ionization time of flight mass spectrometry.
UV–vis analysis
UV–vis spectra were recorded with a Cary 5000 UV–vis–near IR spectrophotometer equipped with a thermostatted cell compartment (Varian, Palo Alto, CA, USA) using quartz cuvettes with 1- or 0.2-cm path lengths. Wavelength scans were performed at 25 °C from 200 to 800 nm, with a 60 nm min−1 scan speed. Extinction coefficients are expressed per dimetal site unless noted otherwise.
Preparation of the metal complexes and azide binding studies
Both protein and metal stock solutions were freshly prepared. The initial DF3 concentration was determined spectrophotometrically using ε280 = 19,060 cm−1 M−1 (per dimer) [27]. Metal ion concentrations in the pure DF3 complex and in the metal stock solutions were measured by a Varian Spectra AA 220 atomic absorption spectrometer, equipped with an MK7 burner. The Co(II)–DF3 complex was prepared by adding 1.05 equiv (with respect to the monomer) of CoCl2 (from an aqueous stock solution) to the DF3 solution (at a concentration in the range 70–100 µM) in a 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (50 mM HEPES, 100 mM NaCl, pH 7.0).
In the case of the di-Fe(III)–DF3 complex, 1.05 equiv of Mohr’s salt (in 0.1% H2SO4 water solution) was added dropwise to an aqueous solution of DF3 (0.25 mM pH 3.0). The pH was then raised to 7.0 by addition of buffer (200 mM HEPES, 200 mM NaCl, pH 7.0) and the mixture was incubated for 1 h at room temperature under atmospheric oxygen. The protein solution became pink during the incubation period, and a small quantity of precipitates, presumably iron oxides and aggregated protein, appeared. The solution was centrifuged to remove these precipitates and any excess of residual free metal salts in the supernatant was removed using a Vivaspin filter (5,000 molecular weight cutoff) (Vivascience, Edgewood, NY, USA). Pure complexes were obtained after three cycles of washing with the appropriate buffer solution.
For the azide titration studies, aliquots of 3 × 10−3 g of NaN3 were added to a 200 µL solution of freshly prepared di-Fe(III)-DF3 (78 µM) in 50 mM HEPES, 0.1 M NaCl, pH 7. Following each addition, the mixture was mixed for 5 min, and its UV–vis spectrum was acquired at 25 °C. This process was repeated until the concentration of NaN3 in the sample was 2.4 M.
Circular dichroism analysis
Circular dichroism (CD) measurements were performed at 25 °C, using a J-750 spectropolarimeter equipped with a thermostated cell holder (JASCO, Easton, MD, USA). Cells of 0.5-cm path length were used in all measurements.
Mean residue ellipticities [θ] were calculated using the equation [θ] = θobs/(10 × l × C × n), in which θobs is the ellipticity measured in millidegrees, l is the path length of the cell in centimeters, C is the concentration in moles per liter, and n is the number of residues in the protein.
Metal ion titrations were carried out by adding small aliquots of freshly prepared aqueous stock solutions of MCl2 (M is Co, Mn, or Zn) to the protein solution (protein concentrations in the range 12–16 µM per monomer, in 10 mM phosphate buffer at pH 7). The variations in protein concentration and pH were negligible. Samples were left to equilibrate for 30 min before measurement. Metal-binding data were subjected to a nonlinear least-squares analysis, using KaleidaGraph (Synergy Software, Reading, PA, USA).
Chemical denaturation studies were performed on samples containing apo-DF3 at 8–10 µM concentration per monomer, in 10 mM phosphate buffer at pH 7.2, 10 mM NaCl and increasing amounts of guanidine hydrochloride (GdnHCl) (0–6 M, with approximately 0.3 M increments). For the denaturation studies in the presence of metal ions, the solutions contained an approximately 100-fold excess of metal ions. Samples were left to equilibrate for 12 h before measurement. GdnHCl denaturation curves were obtained by monitoring the CD signal at 222 nm upon GdnHCl addition, with a 60-s averaging time. Data were normalized as the fraction unfolded (f u), calculated as f u = (θobs − θ0)/(θ∞ − θ0) in which θobs, θ0 and θ∞ represent the ellipticity at the actual GdnHCl concentration, at zero GdnHCl concentration, and of the unfolded protein, respectively. The data curves were fit to the equation for dimerization-linked folding [9, 28–30], using KaleidaGraph:
in which Df is the folded dimer in equilibrium with the unfolded monomers, Mu.
The data were analyzed by the linear extrapolation method using the following equation:
in which , a u, and b f are the molar ellipticities and the slopes of the baselines for the dimeric and monomeric protein, respectively; ΔG° is the free energy of dimerization and folding (1 M standard state) extrapolated to 0 M GdnHCl, and m is the linear change in ΔG° with respect to the GdnHCl concentration.
Resonance Raman studies
Resonance Raman spectra were collected with an Acton AM-506 spectrometer using a Princeton Instruments liquid-N2-cooled CCD detector (LN-1100 PB) with 4-cm−1 spectral resolution. Laser excitation (514.5 nm) was provided by a Spectra Physics 2065-75 argon ion laser. Spectra were obtained at 80–100-mW power in a 90° backscattering geometry using spinning flat-bottomed NMR tubes. Spectra were typically collected using 64 accumulations of 60 s each. Thus, laser exposure in each experiment totaled at least 1 h. Samples were somewhat sensitive to photodecomposition under these conditions, based on the decrease of the absorbance at 490 nm following laser exposure. Raman shifts were referenced to indene and are accurate to approximately 1 cm−1. Baseline correction and curve fitting to determine peak positions were carried out using Grams/AI Spectral Notebook, version 7.02 (Thermo Galactic).
Electron paramagnetic resonance studies
Electron paramagnetic resonance (EPR) spectra were measured in perpendicular mode at −269 °C (liquid helium temperature) with a Bruker ELEXSYS E500 spectrometer equipped with an Oxford Instruments ESR-910 cryostat.
All EPR spectra were collected at 9.64 GHz using a power of 2.0 mW, a modulation amplitude of 10 G, and a modulation frequency of 100 kHz. EPR experiments were performed on samples containing di-Fe(III)–DF3 at 2.2 mM concentration. A high-spin Fe(III) EPR spin standard was examined under identical conditions to compare the intensity of the g = 4.3 signal of this spin standard with that of di-Fe(III)–DF3.
Results and discussion
Design and synthesis
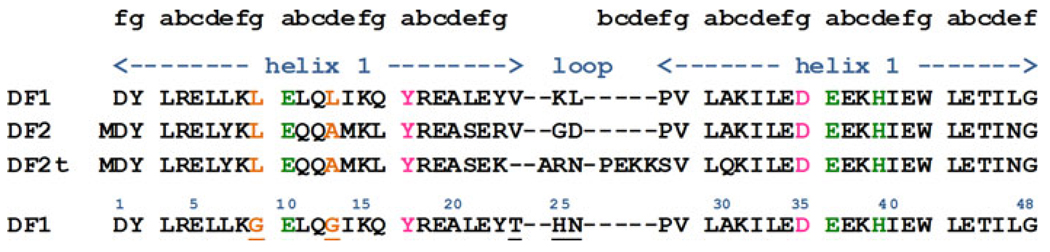
DF3 was designed to integrate all the results derived from previous studies of the DF family of artificial metalloproteins. Like the previous DF proteins, the dimeric di-iron protein DF1 formed the framework for the design of DF3. Changes in the DF1 sequence were first aimed at improving active-site access. To compensate for the thermodynamic cost of carving an active-site access channel into the protein, it was necessary to optimize the interhelical loop conformation (Fig. 2).
Fig. 2.
Sequence alignment of DF family proteins with respect to the prototype DF1. The coordinating residues are highlighted in green, second-shell ligands in pink, and residues involved in active site access in orange. The new substitutions made in the DF3 sequence are underlined
As reported in detail in a previous communication [26], we introduced (1) Gly residues at positions 9 and 13 with the aim of opening up the active site, and creating a cavity large enough to accommodate exogenous ligands, and (2) a new interhelical loop, derived from our previous analysis of natural interhelical turns [14]. These changes were also aimed at increasing water solubility, while retaining the desired folded form and sufficient thermodynamic stability [26].
The 48 amino acid peptide DF3 was synthesized using solid-phase methods with 9-fluorenylmethoxycarbonyl protocols. The crude peptide obtained was more than 90% pure, as assessed by analytical RP-HPLC, and it was further purified to homogeneity by preparative RP-HPLC to a single, symmetric peak. The collected fractions were analyzed by analytical RP-HPLC, mixed, lyophilized, and stored at −20 °C. The identity of the peptide was further ascertained by matrix-assisted laser desorption ionization time of flight measurements, which confirmed the expected molecular mass (DF3, 5,779 amu calculated, 5,776 ± 5 amu observed).
The new DF3 protein showed improved solubility. In particular apo-DF3 proved to be extremely soluble in water (up to 3 mM), compared with other DF members (10 µM, DF1; 0.5 mM, DF2). This feature represents a significant improvement in the properties of the DF family of artificial di-iron proteins. For DF1 and DF2 members in the apo state, addition of organic solvents, such as dimethyl sulf-oxide and acetonitrile, was often required to obtain aqueous solutions at millimolar concentrations. These coordinating solvents should be avoided because they may bind to the di-metal site and affect its potential functionality. Indeed, in the X-ray structure of di-Mn–L13A-DF1 a bridging dimethyl sulfoxide molecule, derived from the crystallization buffer, was found in the metal site [10]. Thus, it is desirable to use water as the sole solvent. The remarkably high water solubility of DF3 allowed us to perform all the studies described in this paper in aqueous solutions.
CD spectroscopy and metal ion binding to DF3
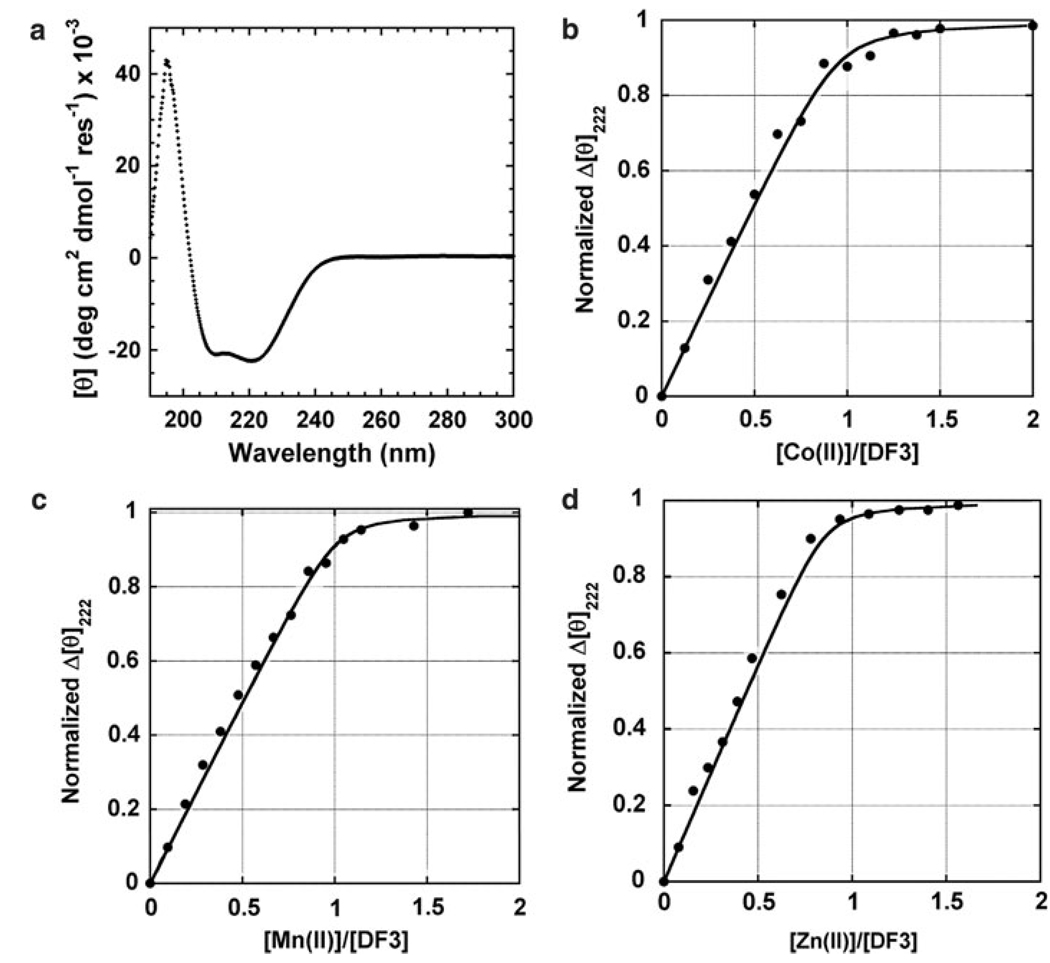
CD spectroscopy was used to analyze the secondary structure of DF3. The far-UV CD spectrum of apo-DF3 in 10 mM phosphate buffer, pH 7.0, clearly indicates the protein to be in a helical conformation, as designed (Fig. 3a). The spectrum shows a maximum at 195 nm ([θ]195 = 42,271 deg cm2 dmol−1 res−1) and minima at 208 nm ([θ]208 = −20,725 deg cm2 dmol−1 res−1) and 222 nm ([θ]222 = −22,093 deg cm2 dmol−1 res−1). All these values are consistent with those observed for previous members of the DF family [8]. Thus, the changes made in the sequence did not affect the secondary structure of DF3.
Fig. 3.
Circular dichroism (CD) studies of DF3. a The far-UV CD spectrum of apo-DF3 in 10 mM phosphate buffer at pH 7. b–d M(II) titrations [M(II) is Co, Mn, and Zn] following the change in the CD signal at 222 nm upon increasing the amount of M(II). The curves were generated from a nonlinear least-squares fit of the data to a 1:1 binding isotherm
DF3 binds divalent metal ions, while retaining its folded helical conformation. CD studies allowed us to assess both the effect of metal binding on the DF3 secondary structure and the binding stoichiometry. Addition of M(II) ions (M is Mn, Co, and Zn) to a solution of apo-DF3 at pH 7 resulted in a small, but measurable, decrease of the band at 222 nm ([θ]222 decreases to −22,915 deg cm2 dmol−1 res−1), consistent with an overall increase of α-helicity. This finding can be ascribed to the stabilization of a metal-bound four-helix bundle [di-M(II)–DF3]. In fact, titration with Na+ ions (which are not expected to bind into the four-helix bundle) or with Co(II) under noncoordinating conditions (acidic pH) does not cause any change in the CD signal.
Titration experiments demonstrated that the binding of metal ions to apo-DF3 followed in all cases a 1:1 stoichiometry, with one metal ion binding one DF3 monomer (two ions per DF3 dimer) (Fig. 3b–d). The dissociation constants (K diss) for divalent metal ion binding were calculated at pH 7 from the titration data using a 1:1 (metal ion to monomer) binding model. Nonlinear least-squares analysis gave values in the micromolar range [0.24 ± 0.02 µM for Co(II), 0.23 ± 0.04 µM for Mn(II), and 0.11 ± 0.01 µM for Zn(II)], and in agreement with the Irving–Williams series [31]. A comparison with previous metal-binding data for DF proteins and bacterioferritin indicates similar overall binding affinities, with DF3 showing the lowest metal selectivity among the other DFs (Table 1). In particular, DF3 has a comparable affinity for Zn(II), with respect to other DFs and bacterioferritin, whereas the affinities of DF3 for Mn(II) and Co(II) are the highest among all the DF proteins. This result may be ascribed to a more flexible metal-binding site in DF3. From a coordination geometry point of view, Zn(II) is well documented to strongly prefer to bind protein ligands in a tetrahedral geometry, whereas Co(II) and Mn(II) prefer to bind in higher coordination number geometries, such as octahedral and trigonal bipyramidal [32].
Table 1.
pKmet for dinuclear metalloproteins
For example, an analysis of crystal structures of metalloproteins present in the Protein Data Bank (PDB), with resolutions of 1.6 Å or lower, revealed that coordination numbers equal to or higher than 5 represent only 25% of all the crystallographically characterized Zn(II) metalloproteins [33]. In the case of Fe(II/III)- and Mn(II)-containing proteins, 88 and 100% of them have coordination numbers equal to or higher than 5, respectively [33]. Furthermore, on the basis of hard and soft acid/base theory, the DF proteins should prefer coordination with hard Lewis acids such as Mn(II) and Fe(III), owing to the high number of carboxylate oxygen donors (hard Lewis bases) present in the metal-binding site [34]. All the DF proteins synthesized to date, however, have been shown to have higher affinity for Zn(II), a softer Lewis acid (Table 1). Thus, since the nature of the coordinating amino acid residues at the metal site in DF3 is the same as in previous DF proteins, the higher affinity of DF3 for binding Mn(II) and Co(II) could be the result of a more structurally flexible metal-binding site that imposes fewer geometric restrictions on the metal ions. As a consequence, metals that prefer coordination numbers equal to or higher than 5, such as Mn(II), Co(II), and Fe(II/III), would be favored to bind DF3.
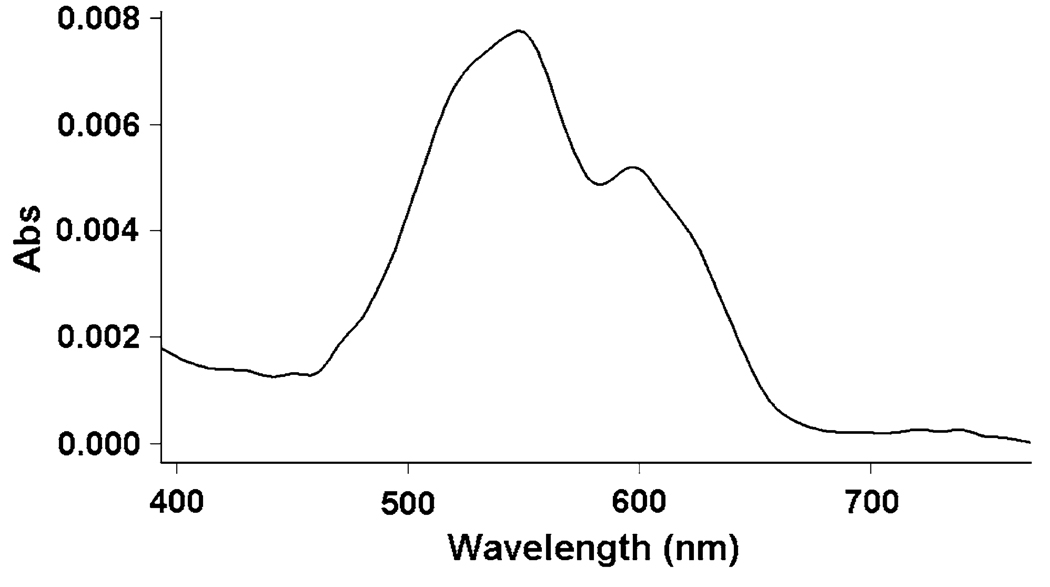
UV–vis spectroscopy was used to obtain more detailed information regarding the metal-binding site in DF3. The paramagnetic Co(II) complex is particularly useful in this respect, as the molar extinction coefficient of Co(II) complexes increases as the coordination number decreases [10–20 M−1 cm−1 (coordination number 6); 100–150 M−1 cm−1 (coordination number 5); 400–600 M−1 cm−1 (coordination number 4)] [36]. The shape, position, and intensity of the absorption bands in the visible spectrum of di-Co(II)–DF3 suggest that the metal ions are in a pentacoordinated geometry [36], as intended in the design (Fig. 4, Table 2). Furthermore, the spectrum is remarkably similar to the spectra of previous di-Co(II)–DF proteins [8, 12, 17, 21], and to the spectrum of di-Co(II)– bacterioferritin, which contains an identical four Glu, two His binding site (Table 2) [37, 38]. However, di-Co(II)–DF3 exhibits lower molar extinction coefficients per Co(II) ion compared with the other di-Co(II) proteins discussed here. This may represent a tendency for the metal site to adopt a less strained geometry.
Fig. 4.
The visible spectrum of di-Co(II)–DF3 in 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 0.1 M NaCl, pH 7. The concentration of DF3 was 78 µM. The extinction coefficient at 548 nm was 98 M−1 cm−1 per Co(II) ion. The spectrum was recorded in a 1 cm path length quartz cuvette
Table 2.
Spectroscopic data for Co(II)-substituted DF derivatives, compared with Co(II)–bacterioferritin
Thus, the binding stoichiometry and the coordinating environment of the active site are both consistent with the intended design of DF3 and with the crystal structures of di-Zn(II)–DF1 (PDB code 1EC5) [8], di-Zn(II)–DF2t (PDB code 1MFT) [14], and di-Co(II)–DF1-L13A (PDB codes 1OVU and 1OVV) [20].
Thermodynamic stability of DF3
DF3 is thermodynamically stable in water and at room temperature and pH 7. CD spectroscopic studies have shown that the peptide exists in equilibrium between a folded helical dimer and an unfolded monomer. As expected for a monomer–dimer equilibrium, its secondary structure is lost in a single transition when chemical denaturation (GdnHCl) is induced. Further, the GdnHCl-induced unfolding of DF3 (in the apo and metal-bound forms) was found to be reversible; a full recovery of the ellipticity of the native state was observed upon suitable dilution of completely unfolded samples.
The free energies of dimerization of the DF3 proteins were determined by globally fitting the baseline and ther-modynamic parameters to an equilibrium between folded dimers and unfolded monomers [9]. Thus, the apparent free energy of dimerization extrapolated to zero denaturant concentration of apo-DF3 was calculated to be 8.1 ± 0.3 kcal mol−1 (33.9 kJ mol−1) at pH 7.
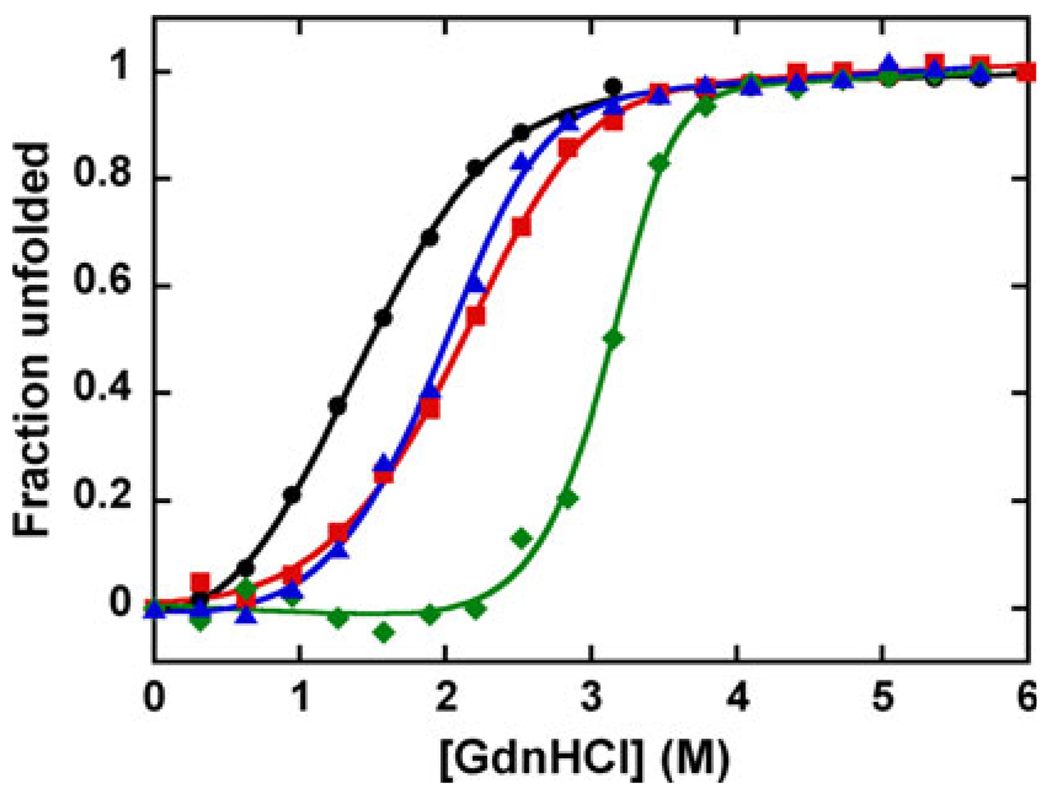
The presence of divalent metals significantly increases the thermodynamic stability of DF3 at pH 7 (Fig. 5, Table 3). The folded form of DF3 was stabilized by 10.4 kcal mol−1 (43.5 kJ mol−1) in the case of Zn(II), and only by 2.3 and 2.9 kcal mol−1 (9.6 and 12.1 kJ mol−1) for Co(II) and Mn(II), respectively. The extent of thermodynamic stabilization agrees well with the strength of metal binding (Zn > Mn ≈ Co), as previously discussed.
Fig. 5.
The effect of metals on the thermodynamic stability of DF3. Guanidine hydrochloride (GdnHCl) denaturation curves of apo-DF3 + 1 mM EDTA (circles), apo-DF3 + 1 mM Co(II) (squares), apo-DF3 + 1 mM Mn(II) (triangles), and apo-DF3 + 1 mM Zn(II) (diamonds). [θ]222 was monitored as a function of the concentration of added denaturant in 10 mM phosphate buffer and 0.1 M NaCl (pH 7). The smooth curves were generated by globally fitting the free energy of dimerization in the absence of GdnHCl, m , and the baseline parameters to the data as described in [9]
Table 3.
Thermodynamic parameters derived from the fit of the guanidine hydrochloride induced unfolding curves for apo-DF3 and di-M(II)–DF3 (M is Co, Mn, Zn) at pH 7
| Protein | (kcal mol−1) | [GdnHCl]1/2 (M) | m (kcal mol−1 M−1) |
|---|---|---|---|
| apo-DF3 | 8.1 ± 0.3 | 1.48 | 1.4 ± 0.1 |
| di-Co(II)–DF3 | 10.4 ± 0.3 | 2.11 | 1.8 ± 0.1 |
| di-Mn(II)–DF3 | 11.0 ± 0.6 | 2.01 | 2.2 ± 0.2 |
| di-Zn(II)–DF3 | 18.5 ± 1.3 | 3.15 | 3.7 ± 0.4 |
1 kcal = 4.184 kJ
The m values derived from the denaturation curve fitting also increase with the strength of metal binding (Table 3). The m value is typically interpreted as being proportional to the difference in solvent-accessible surface area between the unfolded and the folded state of a protein [41]. Consequently, in di-Zn(II)–DF3 the maximum decrease of solvent-accessible surface area is observed upon folding. Hence, the observed increase of the m value when divalent ions are present could be related to an increase in the compactness of the four-helix bundle due to strong metal ion binding.
Spectroscopic properties of di-Fe(III)–DF3
DF3 is able to bind Fe(II) ions and to catalyze their oxidation to the ferric state under aerobic conditions (ferrox-idase activity), forming a stable µ-oxo-di-Fe(III) complex.
The UV–vis spectrum of the pure di-Fe(III)–DF3 complex exhibits a strong band at λmax = 350 nm (ε = 5,270 M−1 cm−1), consistent with an oxo-to-Fe(III) charge transfer transition in a µ-oxo-di-Fe(III) unit (Fig. 6).
Fig. 6.
The UV–vis spectrum of di-Fe(III)–DF3 in 100 mM HEPES, 0.1 M NaCl, pH 7. The concentration of DF3 was 1.5 mM. The extinction coefficients at 350 and 500 nm were 5,270 and 1,200 M−1 cm−1, respectively. The inset shows the magnified spectrum between 400 and 800 nm. The spectrum was recorded in a 0.2 cm path length quartz cuvette
Proteins and model complexes containing oxo-bridged di-iron clusters typically exhibit intense absorption bands in the UV region (λmax = 300–370 nm, ε = 4,000–9,000 M−1 cm−1 per di-iron site) [42–47]. Furthermore, similar ligand-to-metal charge transfer (LMCT) bands were described in previous studies with di-Fe(III)–DF proteins [10, 12, 19, 22]. The assignment has recently been confirmed for di-Fe(III)–DF2t, as the µ-oxo-di-Fe(III) unit was structurally characterized by X-ray crystallography [16].
A moderately intense broad band between 400 and 700 nm (ε500 nm = 1,200 M−1 cm−1) was observed in the UV–vis spectrum of di-Fe(III)–DF3 (Fig. 6, inset). We assigned this absorption to a O(pπ) → Fe(dπ*) tyrosinate-to-Fe(III) LMCT transition [48, 49], on the basis of resonance Raman data (vide infra), as well as on its similarity with well-characterized iron proteins and small-molecule model complexes with phenolate/tyrosinate–Fe(III) interactions. Examples include the iron carrier transferrins (λmax = 464 nm; ε = 2,500 M−1 cm−1) [48, 50, 51], protocatechuate-3,4-dioxygenase (λmax = 450 nm; ε = 2,600 M−1 cm−1) [52–54], and mammalian binuclear purple acid phosphatases (λmax = 510 nm; ε = 4,000 M−1 cm−1) [55–58]. The position and the intensity of the LMCT transitions in both tyrosinate–iron enzymes and model complexes have been shown to depend on the ligand environment [59–63]. In particular, the molar extinction coefficient depends on the number of phenolate groups coordinated to the metal, with each phenolate bound to the Fe(III) ion contributing approximately 1,000–2,000 M−1 cm−1 to the intensity of the absorption [64]. Mammalian binuclear purple acid phosphatase, with only one tyrosinate ligand bound to the Fe(III) site, represents an interesting and as yet unexplained exception to this relationship [65]. On the basis of these data, the extinction coefficient at 500 nm for di-Fe(III)–DF3 is consistent with the coordination of one Tyr residue to the Fe(III) ion.
To better characterize the di-iron center in DF3, we carried out EPR and resonance Raman spectroscopic studies. EPR studies showed that di-Fe(III)–DF3 is EPR-silent, consistent with an antiferromagnetically coupled di-Fe(III) site.
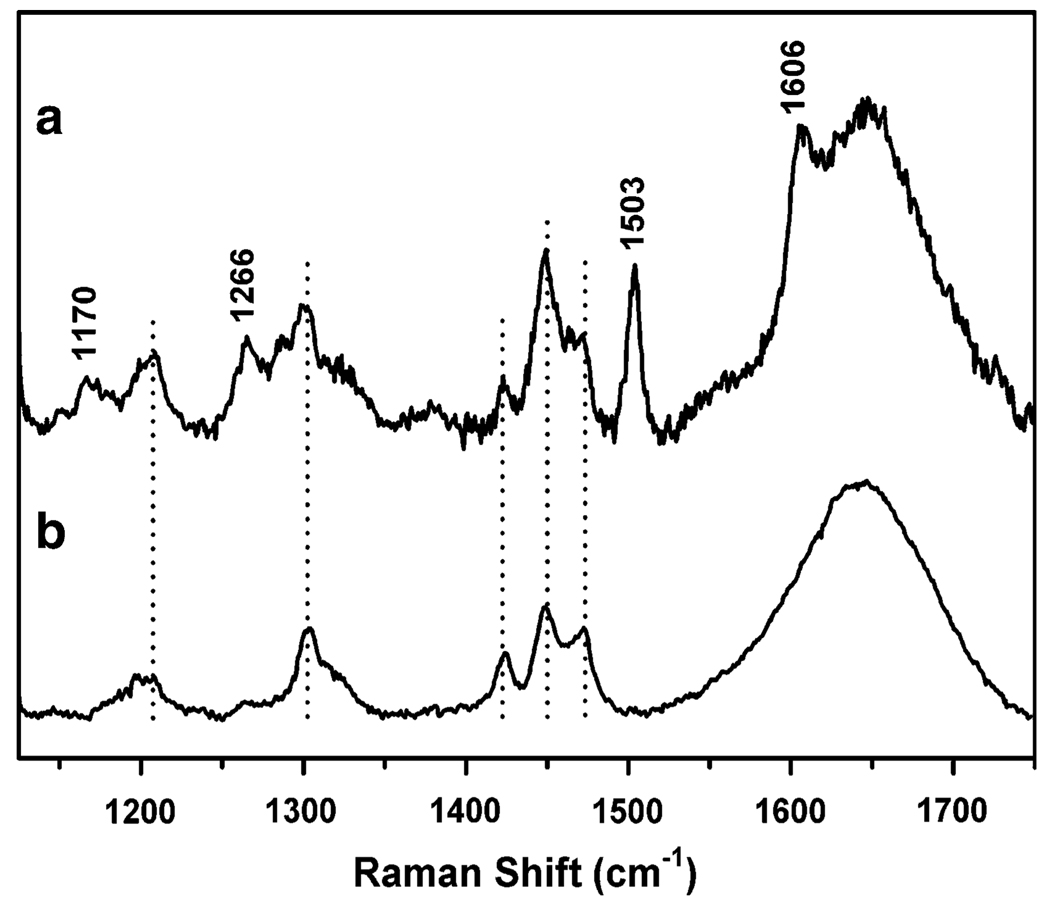
The resonance Raman spectrum, obtained with laser excitation at 514.5 nm, showed four bands at 1,170, 1,266, 1,503, and 1,606 cm−1 (Fig. 7a). The intensity pattern and frequencies of these bands correlate well with the ring deformation modes of a tyrosinate bound to Fe(III), as observed in other Fe(III)–tyrosinate proteins and related model complexes [23, 49, 61, 66–68]. Unfortunately, attempts to observe the Fe–O vibrations (500–600 cm−1) and the Fermi doublet (800–900 cm−1) characteristic of Fe(III)–phenolates were unsuccessful. These vibrations, however, are often less resonance-enhanced than the ring modes and would be difficult to observe owing to the low signal-to-noise ratio in the present resonance Raman studies.
Fig. 7.
Resonance Raman spectra (λex = 514.5 nm) of a di-Fe(III)–DF3 in 100 mM pH 7.0 HEPES (0.1 M NaCl, 0.1 M KI) and b buffer solution alone (100 mM pH 7.0 HEPES, 0.1 M NaCl, 0.1 M KI). Drop lines indicate features associated with the buffer and background, whereas features associated with Fe(III)–tyrosinate ring modes are indicated with their Raman shifts in units of per centimeter
Recently, the coordination of a Tyr residue to a di-Fe(III) center has been spectroscopically characterized in two variants of the monomeric single-chain derivative of DF, namely, DFsc and DFscE11D [23, 25]. A combination of resonance Raman, near-IR CD, magnetic CD, variable-temperature, variable-field magnetic CD, and X-ray absorption spectroscopies was needed to fully characterize the di-Fe(II) and di-Fe(III) sites [23, 25]. On the basis of our experimental data and in analogy with this recent spectroscopic characterization, we can assign the 500-nm chromophore to a tyrosinate-to-Fe(III) LMCT transition. We suggest that the reaction of DF3 with Fe(II) ions under aerobic conditions results in the formation of a µ-oxo-di-Fe(III) unit with one Tyr residue coordinated to the dimetal site (Fig. 8).
Fig. 8.
The proposed coordination site of di-Fe(III)–DF3. One Tyr residue (either Y17 or Y17′) may coordinate the Fe(III) ion
The amino acid sequence of DF3 contains two Tyr residues (Tyr17/Tyr17′), which were intended in the design to form strong second-shell hydrogen bonds with the primary Glu 10′/Glu10 ligands of the neighboring monomer. The structural characterization of di-Zn(II)–DF3 [26], as well as of previously characterized DF proteins [8, 10, 11, 15, 16, 20], showed that these two Tyr residues are in close proximity of the dimetal site. Thus, one Tyr could terminally coordinate to the di-iron center, as already observed for di-Fe(III)–DFsc [23, 25]. We have recently obtained di-Fe(III)–DF3 crystals suitable for X-ray diffraction analysis. These studies will allow us to definitively characterize the di-Fe(III) site in DF3.
Accessibility to the metal site
The substitution of Leu at positions 9 and 13 (which blocked access to the active site) with the small Gly residue was effective in improving the active-site access. The first experimental evidence of this finding was that different M(II) ions were able to spontaneously enter into the hydrophobic core of the folded apo-DF3 dimer and coordinate to the binding site. This observation is remarkably different from that for DF1, which had to be denatured first and then refolded in the presence of metals to form the desired dimetal complexes.
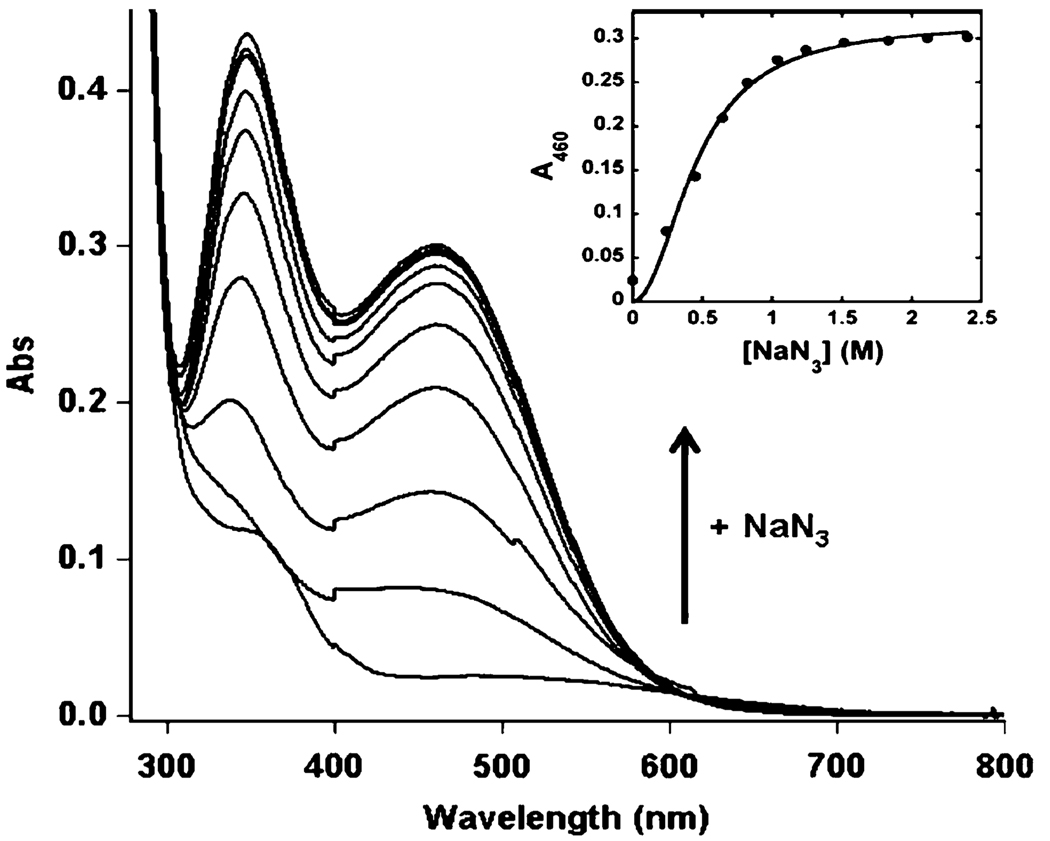
In addition, the diferric form of DF3 shows enhanced accessibility to the metal site compared with previous di-Fe(III)–DF complexes. Addition of azide to a solution of di-Fe(III)–DF3 results in the immediate appearance of two intense charge transfer bands at λmax = 346 nm (ε346 = 1.20 × 104 M−1 cm−1) and λmax = 460 nm (ε460 = 7.70 × 103 M−1 cm−1), characteristic of N3−-to-Fe(III) transitions (Fig. 9).
Fig. 9.
Titration of di-Fe(III)–DF3 (78 µM) with NaN3 in 50 mM HEPES, 0.1 M NaCl, pH 7 in a 1 cm path length quartz cuvette. UV– vis spectrum of di-Fe(III)–DF3 upon addition of increasing amounts of NaN3. The inset shows the increase in absorbance at 460 nm as a function of ligand concentration. The curve was obtained from a fit of the data to the Hill equation (see the text)
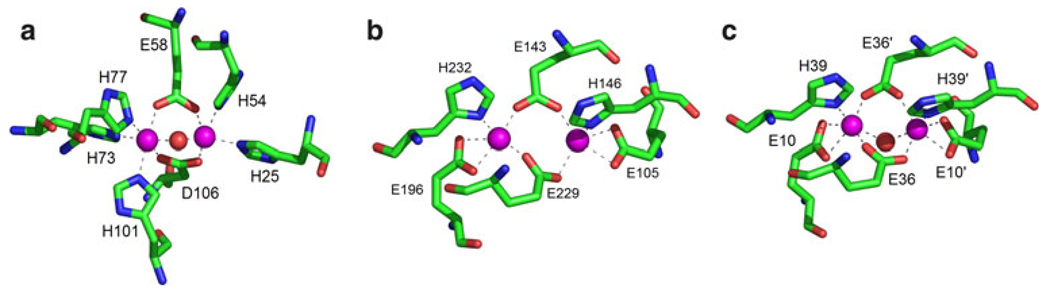
The spectrum of N3−-bound di-Fe(III)–DF3 is almost identical to that of the N3−-bound di-Fe(III)–stearoyl-ACP Δ9-desaturase complex (λmax of 345 and 456 nm, extinction coefficients not reported) and to that of the previously characterized azido-di-Fe(III)–DF2 spectrum (λmax = 340 nm, ε340 = 1.28 × 104 M−1 cm−1; λmax = 460 nm, ε460 = 8.70 × 103 M−1 cm−1) [10, 69]. In addition, the spectrum also resembles that of the azidomethemerythrin complex (λmax = 326 nm, ε326 = 6.75 − 103 M−1 cm−1; λmax = 446 nm, ε446 = 3.70 × 103 M−1 cm−1) [42, 70]. The extinction coefficients of the azide complex of di-Fe(III)–DF3 are approximately 2 times greater than those of azidomethemerythrin, which is known to bind just one azide anion per di-iron site in a nonbridging mode [71, 72]. The small differences in the positions of the absorption bands can be related to the first coordination sphere of the metal ions. Figure 10 compares the active-site structures of hemerythrin (Fig. 10a), stearoyl-ACP Δ9-desaturase (Fig. 10b), and the DF proteins (Fig. 10c).
Fig. 10.
Comparison of the active-site structure in different di-iron proteins and model compounds, as derived from X-ray crystallography. a Deoxyhemerythrin (PDB code 1HMD) [73]. b Stearoyl-ACP Δ9-desaturase (PDB code 1AFR) [74]. c L13G-DF1 (PDB code 1LT1) [11]. The images were generated using PyMOL [7]
A titration of di-Fe(III)-DF3 with sodium azide (Fig. 9, inset) allowed us to obtain a binding curve that was fitted to the Hill equation ([NaN3]N/(K + [NaN3]N) [75]. Comparison of the values obtained from the fit (N = 2.04 ± 0.15, K = 0.20 ± 0.02 M, and R = 0.99) with those obtained previously for di-Fe(III)–DF2 (N = 1.5 ± 0.1, K = 0.56 M, and R = 0.99) demonstrates that the accessibility to the di-iron site of exogenous molecules has been greatly improved in di-Fe(III)–DF3. The accessibility enhancement could be quantified to be close to 150%, as the K values indicate. In addition, the Hill coefficient (N) and the high extinction coefficient of the azido-to-Fe(III) absorption bands suggest that two azide molecules may bind to the di-Fe(III) site of DF3. Thus, these results are in agreement with the previous suggestion (vide supra) of an accessible and coordinatively flexible metal-binding site in di-Fe(III)–DF3, which is capable of accommodating small molecules.
Conclusions
This work describes the metal-binding properties and stability of DF3, a new de novo designed di-iron protein. DF3 exhibits several enhanced properties with respect to previous members of the DF family of synthetic metalloproteins. DF3 folds into a homodimeric four-helix bundle and binds metal ions in the desired stoichiometry. Our studies indicate that, compared with all DF proteins reported thus far, DF3 has the highest affinity for redox-active metals such as Co(II) and Mn(II). DF3 is able to bind Fe(II), forming, upon dioxygen exposure, a µ-oxo-di-Fe(III) center, commonly found in natural di-Fe(III) enzymes. UV–vis and resonance Raman studies indicated that a Tyr residue may terminally coordinate the di-Fe(III) site, similarly to the recently characterized di-Fe(III)–DFsc [23, 26]. Notably, the di-Fe(III)–DF3 complex exhibits a long lifetime, being stable under atmospheric dioxygen for at least 5 months at 4 °C. Only minor decomposition was observed for longer times. This is an important observation, since it was recently reported that the activity of small-molecule non-heme iron complexes in the oxidation of alkanes is related to their stabilities during the oxidizing reaction conditions [76].
DF3 shows an increased accessibility to the active site, as expected. It can easily be reconstituted with different metal ions, and its di-iron site is able to bind exogenous small molecules, such as dioxygen and azide.
All the spectroscopic and structural features reported herein demonstrate the ability of a designed protein to finely tune the active-site structure to accommodate different metal ions and exogenous ligands. Further, the complete analysis of the di-iron complex supports the ability of di-Fe(III)–DF3 to catalyze oxidation reactions, as we recently reported [26].
In conclusion, DF3 is a promising candidate for further adjustments in the structure aimed at developing functional models of dinuclear metalloproteins housing redox-active metal ions.
Acknowledgments
The authors wish to thank Marco Trifuoggi for metal content analysis. This work was supported by the European Commission (Marie Curie Fellowship HPMD-GH-01-00113-03 to R.T.M.R.), the US National Institutes of Health (Grant GM-38767 to L.Q. and graduate traineeship GM-08700 to E.F.), and the Italian MIUR (PRIN 2007, prot. 2007KAWXCL).
Abbreviations
- CD
Circular dichroism
- DF
Due ferri
- EPR
Electron paramagnetic resonance
- GdnHCl
Guanidine hydrochloride
- HEPES
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
- LMCT
Ligand-to-metal charge transfer
- PDB
Protein Data Bank
- RP-HPLC
Reverse-phase high-performance liquid chromatography
- TFA
Trifluoroacetic acid
Contributor Information
Rafael Torres Martin de Rosales, Department of Chemistry, University of Naples “Federico II”, Via Cintia, 80126 Naples, Italy.
Marina Faiella, Department of Chemistry, University of Naples “Federico II”, Via Cintia, 80126 Naples, Italy.
Erik Farquhar, Department of Chemistry, Centre for Metals in Biocatalysis, University of Minnesota, Minneapolis, MN 55455, USA.
Lawrence Que, Jr, Department of Chemistry, Centre for Metals in Biocatalysis, University of Minnesota, Minneapolis, MN 55455, USA.
Concetta Andreozzi, Department of Chemistry, University of Naples “Federico II”, Via Cintia, 80126 Naples, Italy.
Vincenzo Pavone, Department of Chemistry, University of Naples “Federico II”, Via Cintia, 80126 Naples, Italy.
Ornella Maglio, Department of Chemistry, University of Naples “Federico II”, Via Cintia, 80126 Naples, Italy; IBB, CNR, Via Mezzocannone 16, 80134 Naples, Italy.
Flavia Nastri, Department of Chemistry, University of Naples “Federico II”, Via Cintia, 80126 Naples, Italy.
Angela Lombardi, Department of Chemistry, University of Naples “Federico II”, Via Cintia, 80126 Naples, Italy, alombard@unina.it.
References
- 1.Merkx M, Kopp DA, Sazinsky MH, Blazyk JL, Müller J, Lippard SJ. Angew Chem Int Ed. 2001;40:2782–2807. doi: 10.1002/1521-3773(20010803)40:15<2782::AID-ANIE2782>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 2.Kolberg M, Strand KR, Graff P, Kristoffer Andersson K. Biochim Biophys Acta. 2004;1699:1–34. doi: 10.1016/j.bbapap.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Fox BG, Lyle KS, Rogge CE. Acc Chem Res. 2004;37:421–429. doi: 10.1021/ar030186h. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz DM., Jr J Biol Inorg Chem. 1997;2:159–167. [Google Scholar]
- 5.Guy JE, Abreu IA, Moche M, Lindqvist Y, Whittle E, Shanklin J. Proc Natl Acad Sci USA. 2006;103:17220–17224. doi: 10.1073/pnas.0607165103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macedo S, Romao CV, Mitchell E, Matias PM, Liu MY, Xavier AV, LeGall J, Teixeira M, Lindley P, Carrondo MA. Nat Struct Mol Biol. 2003;10:285–290. doi: 10.1038/nsb909. [DOI] [PubMed] [Google Scholar]
- 7.DeLano WL. PyMOL. Palo Alto: DeLano Scientific; 2002. [Google Scholar]
- 8.Lombardi A, Summa CM, Geremia S, Randaccio L, Pavone V, DeGrado WF. Proc Natl Acad Sci USA. 2000;97:6298–6305. doi: 10.1073/pnas.97.12.6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maglio O, Nastri F, Pavone V, Lombardi A, DeGrado WF. Proc Natl Acad Sci USA. 2003;100:3772–3777. doi: 10.1073/pnas.0730771100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Costanzo L, Wade H, Geremia S, Randaccio L, Pavone V, DeGrado WF, Lombardi A. J Am Chem Soc. 2001;123:12749–12757. doi: 10.1021/ja010506x. [DOI] [PubMed] [Google Scholar]
- 11.DeGrado WF, Costanzo LD, Geremia S, Lombardi A, Pavone V, Randaccio L. Angew Chem Int Ed Eng. 2003;42:417–420. doi: 10.1002/anie.200390127. [DOI] [PubMed] [Google Scholar]
- 12.Pasternak A, Kaplan S, Lear JD, DeGrado WF. Protein Sci. 2001;10:958–969. doi: 10.1110/ps.52101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calhoun JR, Nastri F, Maglio O, Pavone V, Lombardi A, DeGrado WF. Biopolymers. 2005;80:264–278. doi: 10.1002/bip.20230. [DOI] [PubMed] [Google Scholar]
- 14.Lahr SJ, Engel DE, Stayrook SE, Maglio O, North B, Geremia S, Lombardi A, DeGrado WF. J Mol Biol. 2005;346:1441–1454. doi: 10.1016/j.jmb.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Maglio O, Nastri F, Calhoun JR, Lahr S, Wade H, Pavone V, DeGrado WF, Lombardi A. J Biol Inorg Chem. 2005;10:539–549. doi: 10.1007/s00775-005-0002-8. [DOI] [PubMed] [Google Scholar]
- 16.Wade H, Stayrook SE, DeGrado WF. Angew Chem Int Ed Eng. 2006;45:4951–4954. doi: 10.1002/anie.200600042. [DOI] [PubMed] [Google Scholar]
- 17.Summa CM, Rosenblatt MM, Hong JK, Lear JD, DeGrado WF. J Mol Biol. 2002;321:923–938. doi: 10.1016/s0022-2836(02)00589-2. [DOI] [PubMed] [Google Scholar]
- 18.Marsh ENG, DeGrado WF. Proc Natl Acad Sci USA. 2002;99:5150–5154. doi: 10.1073/pnas.052023199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan J, DeGrado WF. Proc Natl Acad Sci USA. 2004;101:11566–11570. doi: 10.1073/pnas.0404387101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geremia S, Di Costanzo L, Randaccio L, Engel DE, Lombardi A, Nastri F, DeGrado WF. J Am Chem Soc. 2005;127:17266–17276. doi: 10.1021/ja054199x. [DOI] [PubMed] [Google Scholar]
- 21.Calhoun JR, Kono H, Lahr S, Wang W, DeGrado WF, Saven JG. J Mol Biol. 2003;334:1101–1115. doi: 10.1016/j.jmb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Wei PP, Skulan AJ, Wade H, DeGrado WF, Solomon EI. J Am Chem Soc. 2005;127:16098–16106. doi: 10.1021/ja053661a. [DOI] [PubMed] [Google Scholar]
- 23.Calhoun JR, Bell CB, Smith TJ, Thamann TJ, DeGrado WF, Solomon EI. J Am Chem Soc. 2008;130:9188–9189. doi: 10.1021/ja801657y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maglio O, Nastri F, Torres Martin de Rosales R, Faiella M, Pavone V, DeGrado WF, Lombardi A. C R Chimie. 2007;10:703–720. [Google Scholar]
- 25.Bell CB, Calhoun JR, Bobyr E, Wei P-p, Hedman B, Hodgson KO, DeGrado WF, Solomon EI. Biochemistry. 2009;48:59–73. doi: 10.1021/bi8016087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faiella M, Andreozzi C, Torres Martin de Rosales R, Pavone V, Maglio O, Flavia Nastri F, DeGrado WF, Lombardi A. Nat Chem Biol. 2009;5:882–884. doi: 10.1038/nchembio.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill SC, vonHippel PH. Anal Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 28.Bolen DW, Santoro MM. Biochemistry. 1988;27:8069–8074. doi: 10.1021/bi00421a015. [DOI] [PubMed] [Google Scholar]
- 29.Santoro MM, Bolen DW. Biochemistry. 1988;27:8063–8068. doi: 10.1021/bi00421a014. [DOI] [PubMed] [Google Scholar]
- 30.Mann CJ, Matthews CR. Biochemistry. 1993;32:5282–5290. doi: 10.1021/bi00071a002. [DOI] [PubMed] [Google Scholar]
- 31.Da Silva JJRF, Williams RJP. The biological chemistry of the elements. Oxford: Oxford University Press; 1991. [Google Scholar]
- 32.Dudev T, Lim C. Chem Rev. 2003;103:773–787. doi: 10.1021/cr020467n. [DOI] [PubMed] [Google Scholar]
- 33.Harding MM. Acta Crystallogr D. 2001;57:401–411. doi: 10.1107/s0907444900019168. [DOI] [PubMed] [Google Scholar]
- 34.Pearson RG. J Am Chem Soc. 1963;85:3533–3539. [Google Scholar]
- 35.LeBrun NE, Keech AM, Mauk MR, Mauk AG, Andrews SC, Thomson AJ, Moore GR. FEBS Lett. 1996;397:159–163. doi: 10.1016/s0014-5793(96)01172-6. [DOI] [PubMed] [Google Scholar]
- 36.Bertini I, Luchinat C. Adv Inorg Biochem. 1984;6:71–111. [PubMed] [Google Scholar]
- 37.Frolow F, Kalb AJ, Yariv J. Nat Struct Biol. 1994;1:453–460. doi: 10.1038/nsb0794-453. [DOI] [PubMed] [Google Scholar]
- 38.Keech AM, LeBrun NE, Wilson MT, Andrews SC, Moore GR, Thomson AJ. J Biol Chem. 1997;272:422–429. doi: 10.1074/jbc.272.1.422. [DOI] [PubMed] [Google Scholar]
- 39.Bryson JW, Betz SF, Lu HS, Suich DJ, Zhou HXX, Oneil KT, DeGrado WF. Science. 1995;270:935–941. doi: 10.1126/science.270.5238.935. [DOI] [PubMed] [Google Scholar]
- 40.Myers JK, Pace CN, Scholtz JM. Biochemistry. 1997;36:10923–10929. doi: 10.1021/bi9707180. [DOI] [PubMed] [Google Scholar]
- 41.Schellman JA. Annu Rev Biophys Biophys Chem. 1987;16:115–137. doi: 10.1146/annurev.bb.16.060187.000555. [DOI] [PubMed] [Google Scholar]
- 42.Reem RC, McCormick JM, Richardson DE, Devlin FJ, Stephens PJ, Musselman RL, Solomon EI. J Am Chem Soc. 1989;111:4688–4704. [Google Scholar]
- 43.Petersson L, Graslund A, Ehrenberg A, Sjoberg BM, Reichard P. J Biol Chem. 1980;255:6706–6712. [PubMed] [Google Scholar]
- 44.Yang XO, Le Brun NE, Thomson AJ, Moore CR, Chasteen ND. Biochemistry. 2000;39:4915–4923. doi: 10.1021/bi992631f. [DOI] [PubMed] [Google Scholar]
- 45.Yang XK, Chen-Barrett Y, Arosio P, Chasteen ND. Biochemistry. 1998;37:9743–9750. doi: 10.1021/bi973128a. [DOI] [PubMed] [Google Scholar]
- 46.Fox BG, Shanklin J, Ai JY, Loehr TM, Sanders-Loehr J. Biochemistry. 1994;33:12776–12786. doi: 10.1021/bi00209a008. [DOI] [PubMed] [Google Scholar]
- 47.Bollinger JM, Edmondson DE, Huynh BH, Filley J, Norton JR, Stubbe J. Science. 1991;253:292–298. doi: 10.1126/science.1650033. [DOI] [PubMed] [Google Scholar]
- 48.Gaber BP, Miskowski V, Spiro TG. J Am Chem Soc. 1974;96:6868–6873. doi: 10.1021/ja00829a010. [DOI] [PubMed] [Google Scholar]
- 49.Que L., Jr . Resonance Raman spectra of heme and metalloproteins. vol 3. New York: Wiley; 1998. Biological applications of Raman spectroscopy; pp. 491–521. [Google Scholar]
- 50.Aasa R, Malmstron BG, Saltman P, Vanngard T. Biochim Biophys Acta. 1963;75:203–222. doi: 10.1016/0006-3002(63)90599-7. [DOI] [PubMed] [Google Scholar]
- 51.MacGillivray RTA, Moore SA, Chen J, Anderson BF, Baker H, Luo Y, Bewley M, Smith CA, Murphy MEP, Wang Y, Mason AB, Woodworth RC, Brayer GD, Baker EN. Biochemistry. 1998;37:7919–7928. doi: 10.1021/bi980355j. [DOI] [PubMed] [Google Scholar]
- 52.Tatsuno Y, Saeki Y, Iwaki M, Yagi T, Nozaki M, Kitagawa T, Otsuka S. J Am Chem Soc. 1978;100:4614–4615. [Google Scholar]
- 53.Ohlendorf DH, Lipscomb JD, Weber PC. Nature. 1988;336:403–405. doi: 10.1038/336403a0. [DOI] [PubMed] [Google Scholar]
- 54.Davis MI, Orville AM, Neese F, Zaleski JM, Lipscomb JD, Solomon EI. J Am Chem Soc. 2002;124:602–614. doi: 10.1021/ja011945z. [DOI] [PubMed] [Google Scholar]
- 55.Sträter N, Klabunde T, Tucker P, Witzel H, Krebs B. Science. 1995;268:1489–1492. doi: 10.1126/science.7770774. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Ming LJ, Que L, Vincent JB, Crowder MW, Averill BA. Biochemistry. 1992;31:5263–5268. doi: 10.1021/bi00138a004. [DOI] [PubMed] [Google Scholar]
- 57.Guddat LW, McAlpine AS, Hume D, Hamilton S, de Jersey J, Martin JL. Structure. 1999;7:757–767. doi: 10.1016/s0969-2126(99)80100-2. [DOI] [PubMed] [Google Scholar]
- 58.Gaber BP, Sheridan JP, Bazer FW, Roberts RM. J Biol Chem. 1979;254:8340–8342. [PubMed] [Google Scholar]
- 59.Que L., Jr Coord Chem Rev. 1983;50:73–108. [Google Scholar]
- 60.Casella L, Gullotti M, Pintar A, Messori L, Rockenbauer A, Györ M. Inorg Chem. 1987;26:1031–1038. [Google Scholar]
- 61.Pyrz JW, Roe AL, Stern LJ, Que L., Jr J Am Chem Soc. 1985;107:614–620. [Google Scholar]
- 62.He Q-Y, Mason AB, Woodworth RC, Tam BM, MacGillivray RTA, Grady JK, Chasteen ND. Biochemistry. 1997;36:14853–14860. doi: 10.1021/bi9719556. [DOI] [PubMed] [Google Scholar]
- 63.Bull C, Ballou DP. J Biol Chem. 1981;256:12673–12680. [PubMed] [Google Scholar]
- 64.Ainscough EW, Brodie AM, Plowman JE, Brown KL, Addison AW, Gainsford AR. Inorg Chem. 1980;19:3655–3663. [Google Scholar]
- 65.Lauffer RB, Antanaitis BC, Aisen P, Que L., Jr J Biol Chem. 1983;258:14212–14218. [PubMed] [Google Scholar]
- 66.Sharma KD, Andersson LA, Loehr TM, Terner J, Goff HM. J Biol Chem. 1989;264:12772–12779. [PubMed] [Google Scholar]
- 67.Salama S, Stong JD, Neilands JB, Spiro TG. Biochemistry. 1978;17:3781–3785. doi: 10.1021/bi00611a017. [DOI] [PubMed] [Google Scholar]
- 68.Meckelenburg SL, Mason AB, Woodworth RC, Donohoe RJ. Biospectroscopy. 1997;3:435–444. [Google Scholar]
- 69.Fox BG, Shanklin J, Somerville C, Munck E. Proc Natl Acad Sci USA. 1993;90:2486–2490. doi: 10.1073/pnas.90.6.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garbett K, Darnall DW, Klotz IM, Williams RJP. Arch Biochem Biophys. 1969;135:419–434. doi: 10.1016/0003-9861(69)90559-1. [DOI] [PubMed] [Google Scholar]
- 71.Stenkamp RE, Sieker LC, Jensen LH. J Mol Biol. 1978;126:457–466. doi: 10.1016/0022-2836(78)90052-9. [DOI] [PubMed] [Google Scholar]
- 72.Holmes MA, Stenkamp RE. J Mol Biol. 1991;220:723–737. doi: 10.1016/0022-2836(91)90113-k. [DOI] [PubMed] [Google Scholar]
- 73.Holmes MA, Le Trong I, Turley S, Sieker LC, Stenkamp RE. J Mol Biol. 1991;218:583–593. doi: 10.1016/0022-2836(91)90703-9. [DOI] [PubMed] [Google Scholar]
- 74.Lindqvist Y, Huang W, Schneider G, Shanklin J. EMBO J. 1996;15:4081–4092. [PMC free article] [PubMed] [Google Scholar]
- 75.Hill AV. J Physiol (Lond) 1910;40:iv–vii. [Google Scholar]
- 76.England J, Davies CR, Banaru M, White AJP, Britovsek GJP. Adv Synth Catal. 2008;350:883–897. [Google Scholar]