Abstract
Background
Opponent-process theories of externalizing disorders (ExD) attribute them to some combination of overactive reward processing systems and/or underactive impaired behavior inhibition systems. Reward processing has been indexed by recruitment of incentive-motivational neurocircuitry of the ventral striatum (VS), including nucleus accumbens (NAcc).
Methods
We used functional magnetic resonance imaging (fMRI) with an incentive task to determine whether externalizing symptomatology in adolescence is correlated with an enhanced VS recruitment by cues for rewards, or by deliveries of rewards. Twelve community-recruited adolescents with externalizing disorders (AED) and 12 age/gender-matched controls responded to targets to win or avoid losing $0, $0.20, $1, $5, or an unknown amount (ranging from $0.20–$5).
Results
Cues to respond for rewards activated the NAcc (relative to cues for no incentive), in both subject groups similarly, with greatest NAcc recruitment by cues for the largest reward. Loss-anticipatory NAcc signal increase was detected in a volume-of-interest analysis- but this increase occurred only in trials when subjects hit the target. Relative to controls, AED showed significantly elevated NAcc activation by a linear contrast between reward notification versus notification of failure to win reward. In a post hoc reanalysis, VS and pregenual anterior cingulate activation by the reward versus nonreward outcome contrast also directly correlated with Child Behavior Checklist (CBCL) Externalizing total scores (across all subjects) in lieu of a binary diagnosis. Finally, both groups showed right insula activation by loss notifications (contrasted with avoided losses).
Conclusions
Externalizing behavior, whether assessed dimensionally with a questionnaire, or in the form of a diagnostic categorization, is associated with an exaggerated limbic response to outcomes of reward-directed behavior. This could be a neurobiological signature of the behavioral sensitivity to laboratory reward delivery that is characteristic of children with externalizing symptomatology. Of interest is future research on incentive-motivational processing in more severe, clinically-referred AED.
Keywords: Conduct Disorder, Oppositional Defiant Disorder, Externalizing Disorders, Reward, Ventral Striatum, Nucleus accumbens
Introduction
Studies of twins reveal a latent, heritable cognitive trait of impulsivity that underlies both substance use disorder (SUD), as well as externalizing disorders (ExD) such as attention deficit hyperactivity disorder (ADHD), and especially Oppositional Defiant Disorder (ODD) and Conduct Disorder (CD) (Button et al., 2006; Kendler, Prescott, Myers, & Neale, 2003; Slutske et al., 1998; Young, Stallings, Corley, Krauter, & Hewitt, 2000). Critically, this impulsive trait in childhood confers significant risk for future substance use or abuse (King, Iacono, & McGue, 2004; Myers, Brown, & Mott, 1995). Of interest here is detecting altered functioning of incentive-motivational circuitry in adolescents with externalizing symptomatology, as a potential neurophysiological correlate of this latent endophenotype of impulsivity.
Laboratory studies have suggested that ExD are characterized by some combination of over-sensitivity to reward-associated stimuli, or an under-sensitivity to punishment-associated stimuli (Newman & Wallace, 1993). For example, adolescents with externalizing symptomatology (such as truancy) preferred a response option with sporadic high rewards but disproportionately large potential losses (Lane & Cherek, 2001), and persisted in making risky responses following a single payoff of the risky response option, suggesting a disproportionate motivational impact of reward deliveries. Children with ODD or CD also showed persistent reward-driven perseveration in the face of increasing punishment contingencies (Fonseca & Yule, 1995; Matthys, van Goozen, de Vries, Cohen-Kettenis, & van Engeland, 1998), and boys with early-onset CD chose a risky response more often following receipt of a small gain, whereas small gains suppressed risky choices in controls (Fairchild et al., 2009). Moreover, omission of expected rewards elicited increased frustration responses in hyperactive children (Douglas & Parry, 1994), also suggesting a sensitivity to instrumental behavior outcomes in AED. Finally, adolescent smokers with an ExD (predominantly ODD, and/or CD) smoked more heavily, despite no difference from non-ExD smokers in age of use onset and pattern of acceleration of tobacco use (Aklin, Moolchan, Luckenbaugh, & Ernst, 2009), suggesting differences in susceptibility to the rewarding properties of nicotine in ExD.
Collectively, these behavioral responses suggest that rewards are especially salient in adolescents with externalizing disorders (AED). Might this have a neurophysiological signature? Functional magnetic resonance imaging (fMRI) experiments with incentive tasks have emerged as powerful probes of human motivational neurocircuitry. These experiments have shown that: 1) the nucleus accumbens (NAcc; the anteromesial aspect of the ventral striatum (VS)) is recruited by learned cues for instrumental rewards, and 2) mesial frontal cortex (mFC) and NAcc are recruited by notification of rewards (Bjork, Knutson et al., 2004; Knutson, Adams, Fong, & Hommer, 2001; Knutson, Fong, Adams, Varner, & Hommer, 2001; Knutson, Taylor, Kaufman, Peterson, & Glover, 2005).
Incentive-motivational processing in AED, however, has been virtually unexplored. Rubia et al (Rubia et al., 2009) compared fMRI activation between incentivized versus non-incentivized trials of a continuous performance test, and reported that boys with CD showed a relative underactivation of right orbitofrontal cortex compared to controls. Gatzke-Kopp et al (Gatzke-Kopp et al., 2009) reported that an admixed sample of AED (predominantly ADHD) showed a more consistent striatal recruitment between blocks with and without monetary rewards for successful responses in a simple discrimination task. However, these studies used block designs which are incapable of distinguishing instrumental response anticipation from outcome notification. Using the monetary incentive delay (MID) task with event-related analysis, adolescents (Scheres, Milham, Knutson, & Castellanos, 2007) and adults (Strohle et al., 2008) with ADHD showed blunted reward-anticipatory activation in the VS.
Stimulant (Ricaurte et al., 2005; Rosa-Neto et al., 2005), antipsychotic (Frankle & Laruelle, 2002) and anti-convulsive (Yatham et al., 2002) medications for externalizing symptoms each alter dopaminergic neurocircuitry implicated in motivational processing. We therefore scanned medication-free, community-recruited AED (predominantly CD or ODD) while they performed a variant of the MID task, and compared their activation with age- and gender-matched controls. We hypothesized that AED characterized more by rules violations would show an exaggerated mesolimbic response to reward cues or deliveries.
Method
All recruitment, informed consent, and testing procedures were approved by the Institutional Review Board of the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
Subjects
Each subject was recruited using public advertisements and underwent screening along with a parent, where the parent provided information on the medical and psychiatric symptomatology of the adolescent. Subjects were right-handed, with no evidence of medical problems in medical history interviews, physician examination, or clinical chemistry panel. Axis I disorders were classified using both self-report and parent interviews with the structured Diagnostic Interview for Children and Adolescents (DICA)(Reich, 2000) for DSM-IV. History of psychotic or affective disorders was exclusionary. All subjects produced negative urine drug screens and negative breath-alcohol readings.
AED (n = 12; age 13–17, mean 15.4 ± 1.4; 9 males) each met DSM-IV criteria for either ODD (n = 6), ADHD (n = 1), CD (n = 3), or comorbid ODD+ADHD (n = 2). Age-and gender-matched adolescent controls (n = 12; age 13–17, mean 15.3 ± 1.4; 9 males) had no lifetime history of any psychiatric disorder or psychotropic medication therapy. Eight AED had been seen by a mental health professional for behavior problems in the past. Each of these subjects had also been prescribed psychotropic medication, but were medication-free > 1 month prior to scanning. Each adolescent and his/her informant also completed the computerized Child Behavior Checklist (Achenbach, 1991) (CBCL). To maximize sensitivity, we analyzed the higher of the parent- versus self-reported externalizing and internalizing total scores. Total scores were used because they represented the closest analogue to the raw symptom counts utilized in DSM-IV diagnostic classification.
Monetary Incentive Delay (MID) task
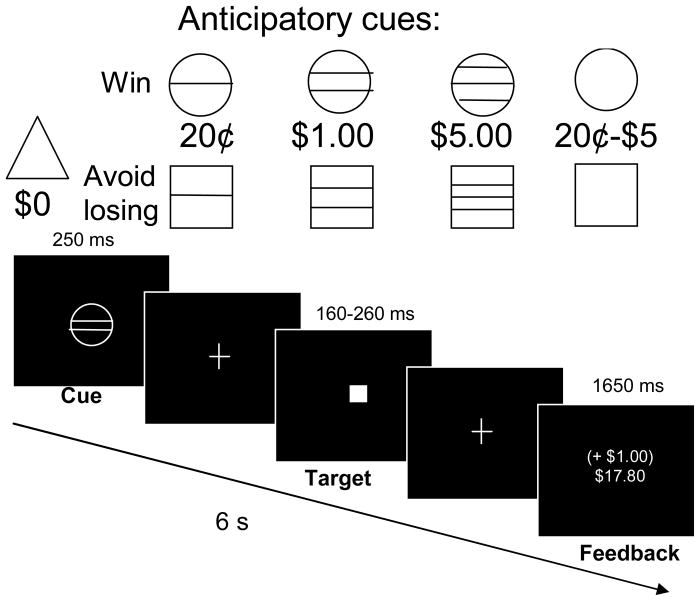
Using a head coil mirror, subjects viewed task stimuli on a screen at the foot of the scanner bed. Trials were contiguous, pseudorandomly-presented, and 6 s in duration. Each trial consisted of: cue presentation, anticipatory delay, target presentation, and success-dependent feedback (Figure 1). First, one of nine cue shapes was presented for 250 msec. Circle-shaped cues signaled that if the subject responded during the subsequent target presentation, he or she would win either 20¢ (18 trials), $1 (18 trials), $5 (18 trials) or a variable amount ranging from 20¢ to $5 (6 trials each with 20¢, $1 or $5 reward) for pressing a button during target presentation. Similarly, square-shaped cues signaled the possibility of losing either 20¢, $1, $5 (18 trials each) or a variable amount from 20¢ to $5 (6 trials each with 20¢, $1 or $5 loss) if the trial target was missed. Nonincentive trials (n = 36; triangle cue) were also presented as a visuomotor control condition, where subjects were instructed to respond to the target, but that trial outcomes would not alter winnings. Each cue was replaced by a crosshair for 2000–2500 msec. Then, a white target square was presented for a varying length of time (180–280 msec). Trials were coded successful if a press was registered during target presentation. The trial then concluded with feedback (1650 msec) of whether money was won or lost during that trial, in tandem with cumulative task earnings.
Figure 1. Monetary incentive delay (MID) task.
Six-second trials were contiguously presented in two, 9.5-minute runs. In each trial, the subject saw one of nine cues indicating the opportunity to either win money (circle series), avoid losing money (square series), or win/lose no money (triangle) by pressing a button while a white square target was presented on the screen a moment later. The subject then saw feedback of whether he or she hit the target, as well as cumulative earnings for that run.
Prior to scanning, subjects were shown an envelope containing the cash they could earn, and were read a script which defined the consequences signaled by each of the nine anticipatory cues. Then, during an offline practice session, reaction times to targets were covertly measured, and a distribution of target presentation durations was set for the scanning task such that each participant would succeed on ~66% of trials. The MID task scan was followed by a structural scan for anatomical colocalization, during which subjects used the response box to rate (scale 1–4) how “excited,” “happy,” “fearful,” and “unhappy” they felt when they saw each of the task cues. Subjects were then paid their task earnings plus $80 standard compensation.
FMRI acquisition
Subjects were scanned with a 3 Tesla MRI scanner (General Electric, Milwaukee, WI) using a quadrature head coil. Functional scans were acquired using a T2*-sensitive echoplanar sequence with a repetition time (TR) = 2000 msec, echo time (TE) =40 msec, flip = 90°. To focus on the NAcc and ventromesial frontal cortex with greater spatial precision, we collected 24 2.0-mm-thick contiguous axial slices. Slices were acquired interleaved, from the base of the mFC superiorly to the apex of the corpus callosum. In-plane resolution was 3.75 × 3.75 mm. Structural scans were acquired using a T1*-weighted sequence (TR, 100 msec; TE, 7 msec; flip, 90°). Each subject’s head was restrained with a Vacu-Fix System deflateable cushion (S&S X-Ray Products, Inc., Houston, TX).
FMRI Analysis
Blood Oxygen-Level Dependent (BOLD) signal was analyzed using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). Briefly, individual time-series datasets were corrected for head motion and preprocessed with bandpass filtration. Datasets were modeled with canonical gammavariate functions time-locked to anticipatory cues and trial outcome notifications. This analysis centered on the four area-under-curve linear contrasts of signal change (hereafter “contrasts”) typically calculated for the MID task: 1) all reward vs nonincentive anticipatory cues, 2) all loss vs nonincentive anticipatory cues, 3) gain vs nongain outcomes in the reward trials, and 4) loss versus nonloss outcomes in loss trials. Groupwise and group-difference maps were calculated in AFNI using recently-developed software to calculate a linear mixed-effects multilevel model for each contrast, using a calculation resistant to the idiosyncratic sampling errors of individual-subject responses. Activations are reported where voxel-wise significance was controlled by the false discovery rate (FDR) to q ≤ .05. Full details are in Supplemental Methods.
Behavior Analysis
We performed mixed-model analyses of variance of affective ratings, hit rates, and reaction times (RT) each as the dependent variable, with incentive magnitude (5 levels: 0, 20¢, $1, and $5, and variable) as the within-subject factor and group (2 levels: AED and control) as the between-subject factor.
Results
Questionnaire measures and task behavior
AED scored significantly higher than controls on both internalizing and externalizing raw scores of the CBCL, with no overlap in score distributions (Table 1). CBCL-externalizing and CBCL-internalizing total scores were also highly correlated within-subject (n= 24 for correlations unless otherwise indicated; Spearman r = .85, p < .0001). Controls and AED performed similarly on the MID task. Subjects showed faster RT to incentivized versus nonincentive targets, resulting in a main effect of incentive magnitude on reaction time (RT) across both reward (F(3,66) = 6.430, p < .001) and loss-avoidance (F(3,66) = 4.672, p < .01) trial types, with no main or interactive effects of subject group. Across both reward and loss-avoidance trials, there were no main or interactive effects of incentive magnitude, incentive valence (gains vs losses) or subject group on target hit rates or on rates of failure to respond to the target.
Table 1.
Psychometric scores and task behavior (standard deviation in parentheses)
| Controls | AED | |t-value| | p value | |
|---|---|---|---|---|
| CBCL internalizing problems total | 5.3 (4.4) | 14.6 (5.5) | 4.568 | <.001 |
| CBCL externalizing problems total | 4.3 (3.1) | 27.2 (11.0) | 6.932 | <.000001 |
| MID task reaction time (ms): | ||||
| Nonincentive | 215 (17) | 211 (19) | 0.679 | ns |
| Win 20¢ | 206 (25) | 213 (18) | 0.710 | ns |
| Win $1 | 206 (30) | 200 (17) | 0.632 | ns |
| Win $5 | 204 (24) | 198 (19) | 0.623 | ns |
| Win variable | 202 (26) | 193 (18) | 0.970 | ns |
| Avoid losing 20¢ | 210 (42) | 206 (19) | 0.430 | ns |
| Avoid losing $1 | 207 (22) | 200 (18) | 0.776 | ns |
| Avoid losing $5 | 206 (22) | 201 (20) | 0.672 | ns |
| Avoid losing variable | 211 (41) | 203 (15) | 0.583 | ns |
| MID task cue affect ratings (1–4 scale): | ||||
| Excitement*: | ||||
| Nonincentive | 1.4 (0.9) | 2.3 (1.3) | 1.923 | <.10 |
| Win 20¢ | 2.3 (1.0) | 2.9 (1.1) | 1.301 | ns |
| Win $1 | 2.8 (1.0) | 3.0 (1.0) | 0.578 | ns |
| Win $5 | 3.3 (1.2) | 3.9 (0.3) | 1.639 | ns |
| Happiness*: | ||||
| Nonincentive | 1.8 (0.9) | 2.4 (1.1) | 1.572 | ns |
| Win 20¢ | 2.2 (0.8) | 2.8 (0.9) | 1.828 | < .10 |
| Win $1 | 2.4 (0.9) | 3.4 (0.7) | 2.814 | < .05 |
| Win $5 | 2.9 (1.1) | 3.3 (1.1) | 0.834 | ns |
| Fearfulness: | ||||
| Nonincentive | 1.0 (0.0) | 1.2 (0.4) | 1.651 | ns |
| Avoid losing 20¢ | 1.8 (1.0) | 2.2 (1.1) | 0.793 | ns |
| Avoid losing $1 | 2.3 (1.1) | 2.4 (1.1) | 0.405 | ns |
| Avoid losing $5 | 2.5 (1.3) | 3.1 (1.0) | 1.186 | ns |
| Unhappiness: | ||||
| Nonincentive | 1.3 (0.9) | 1.5 (0.5) | 0.608 | ns |
| Avoid losing 20¢ | 1.3 (0.5) | 2.3 (1.3) | 2.712 | < .05 |
| Avoid losing $1 | 1.6 (0.8) | 1.8 (0.8) | 0.728 | ns |
| Avoid losing $5 | 2.3 (1.3) | 2.2 (1.5) | 0.046 | ns |
Significant main effect of subject group (AED > controls; p < .05) across all magnitudes
Due to hardware malfunction, computerized mood questionnaire responses were not recorded from three subjects. A main effect of group indicated greater happiness (F(1,19) = 6.627, p < .05) and excitement (F(3,53) = 4.512, p < .05) upon seeing anticipatory cues in AED compared to controls (across all incentive magnitudes; Table 1). There were significant main effects of incentive magnitude on each of the four affective ratings, where participants reported greater happiness (F(3,53) = 12.465, p < .0001) and excitement (F(3,57) = 16.465, p < .0001) as potential reward amounts increased, and also reported greater unhappiness (F(3,57) = 3.711, p < .05) and fearfulness (F(3,57) = 14.437, p < .0001) as potential loss amounts increased, with no interaction effects of group. Motion-correction output indicated that no participant’s head moved more than 1.5 mm between volumes or more than 3 mm overall.
Statistical maps
Anticipatory activation
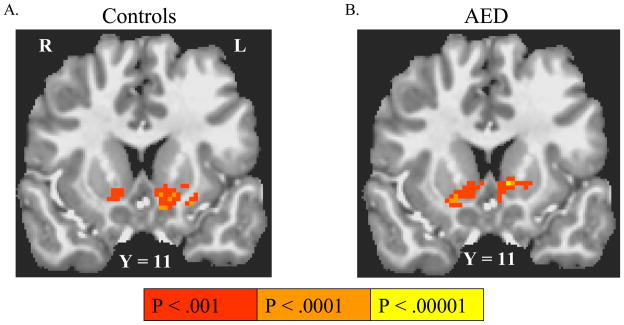
Anticipation of responding for rewards (versus nonincentive) activated NAcc bilaterally in both controls and AED (Table 2; Figure 2). Additional activations by this contrast were found in putamen, thalamus and orbitofrontal cortex in both groups. Anticipation of responding to avoid losses versus anticipation of responding for nonincentive did not activate any brain region in either subject group. There were no significant group differences in anticipatory activation.
Table 2.
Activations by event-related linear contrasts in the MID task*
| Talairach Coordinates: | |t-value|: | uncorrected p | |||
|---|---|---|---|---|---|
| Reward vs nonincentive anticipation | |||||
| Controls | |||||
| L Nucleus accumbens | −9 | 11 | −4 | 4.906 | <.0001 |
| R Nucleus accumbens | 10 | 11 | −5 | 3.914 | <.001 |
| L Putamen | −17 | 3 | −4 | 5.242 | <.0001 |
| L thalamus | −5 | −22 | 5 | 6.659 | <.00001 |
| R thalamus | −3 | −19 | 4 | 5.689 | <.00001 |
| L substantia nigra | −9 | −11 | −7 | 6.675 | <.00001 |
| L orbitofrontal cortex | −19 | 31 | −16 | 4.890 | <.0001 |
| AED | |||||
| L Nucleus accumbens | −8 | 11 | 1 | 5.690 | <.00001 |
| R Nucleus accumbens | 14 | 10 | −6 | 4.640 | <.00001 |
| L dorsolateral thalamus | −10 | −10 | 17 | 4.904 | <.0001 |
| L thalamus | −8 | −14 | 5 | 4.821 | <.0001 |
| Mesial frontal cortex | 2 | 46 | −8 | 6.291 | <.00001 |
| Posterior cingulate gyrus | 3 | −54 | 18 | 6.287 | <.0001 |
| L orbitofrontal cortex | −33 | 40 | −3 | 5.544 | <.0001 |
| Loss-avoidance vs nonincentive anticipation | |||||
| No significant activations in either subject group | |||||
| Reward vs nonreward feedback | |||||
| Controls | |||||
| R Nucleus accumbens | 9 | 8 | −6 | 6.412 | <.00001 |
| L Nucleus accumbens | −9 | 11 | −6 | 5.311 | <.0001 |
| L Putamen | −23 | 3 | 4 | 5.398 | <.0001 |
| R Putamen | 25 | 7 | 9 | 5.171 | <.0001 |
| Mesial orbitofrontal cortex | −4 | 45 | −9 | 5.622 | <.0001 |
| R orbitofrontal cortex | 29 | 39 | −9 | 5.400 | <.0001 |
| AED | |||||
| R Nucleus accumbens | 11 | 13 | −1 | 7.218 | <.000001 |
| L Nucleus accumbens | −10 | 13 | −1 | 9.126 | <.0000001 |
| L Putamen | −21 | 3 | 6 | 5.339 | <.0001 |
| R Putamen | 20 | −1 | −6 | 5.109 | <.0001 |
| Mesial frontal cortex | 3 | 46 | −1 | 6.791 | <.000001 |
| Mesial frontal cortex | 3 | 35 | 4 | 7.570 | <.000001 |
| Loss vs nonloss feedback | |||||
| Controls | |||||
| R Insula | 36 | 13 | −4 | 5.819 | <.00001 |
| AED | |||||
| R Insula | 39 | 12 | −6 | 4.678 | <.001 |
Activation maxima listed here are significant at q < .05 per False Discovery Rate
Figure 2. Activation by anticipation of responding for rewards or to avoid losses.
Coronal and axial images are right-left reversed per radiological convention, with the Talairach coordinate of the image plane indicated. Group-wise activations survive False Discovery Rate correction to q < .05. Anticipation of responding for rewards contrasted with anticipation of non-reward activated the ventral striatum in both controls (A) and in adolescents with externalizing disorders (AED) (B). Anticipation of responding to avoid losses, however, did not activate any brain regions above threshold in either AED or controls.
Outcome-elicited activation
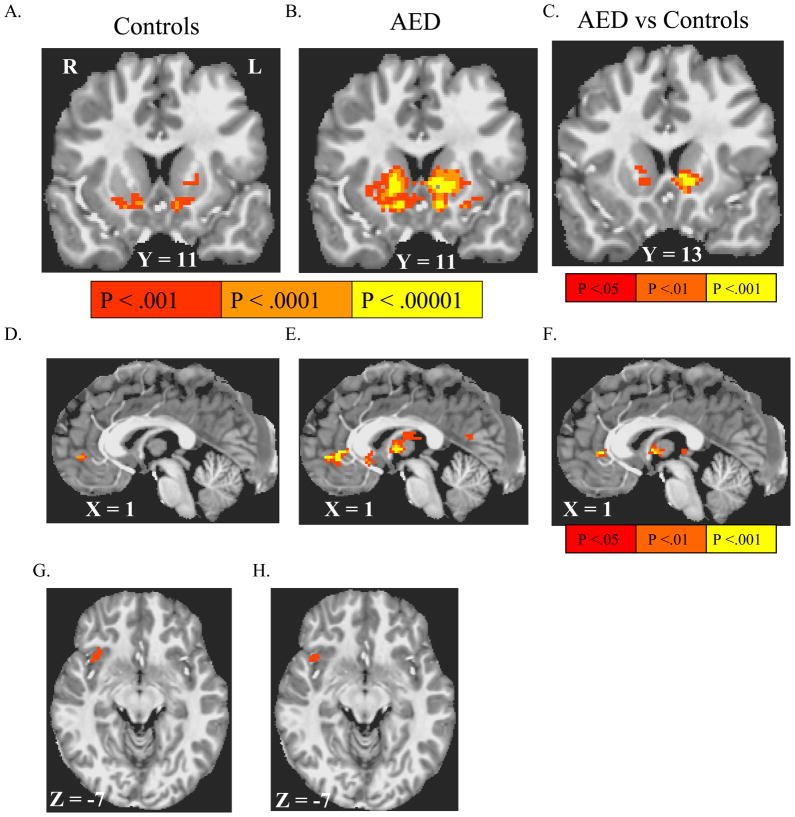
In both controls and in AED, the contrast between notifications of reward versus notifications of failure to win reward in reward trials activated the mFC and VS (including NAcc) bilaterally (Table 2; Figure 3), with significantly greater VS and pregenual anterior cingulate cortex (ACC) activation in AED compared to controls. Notification of losses (contrasted with notification of avoided losses) activated right anterior insula in both controls and in AED (Table 2; Figure 3). To determine whether reward outcome contrast-elicited activation in the VS and ACC also correlated with a dimensional measure of externalizing symptomatology, data were reanalyzed in a mixed-model voxel-wise ANOVA, where CBCL-externalizing total scores were substituted into the model in place of diagnosis, controlling for age. This too indicated a positive partial correlation between CBCL-externalizing scores and activation (Supplemental Figure 1).
Figure 3. Activation by notification of rewards and losses.
Coronal and axial images are right-left reversed per radiological convention, with the Talairach coordinate of the image plane indicated. Group-wise activations survive False Discovery Rate correction to q < .05. Notification of rewards (contrasted with notification of failure to win reward) activated the ventral striatum (VS) in both controls (A) and in adolescents with externalizing disorders (AED) (B), with a significant voxelwise group difference in activation by this contrast depicted in (D). Reward notification also activated mesial frontal cortex in both in controls (D) and in AED (E), with significant voxel-wise group differences depicted in (F). Notification of all losses (versus notification of successful loss avoidance) activated right anterior insula in both controls (G) and in AED (H).
We explored whether the increased reward-outcome contrast-elicited NAcc activation in AED was driven by greater NAcc activation by reward notifications, or a greater NAcc deactivation by nonreward notifications (see supplemental methods). Voxel-wise correlations between externalizing behavior and activation by each of successful and unsuccessful reward trial notifications (considered singly, not in a contrast) indicated a combination of the two. At a relaxed threshold, externalizing symptomatology showed a significant correlation with VS activation by reward notification events as well as a significant correlation with VS deactivation by nonreward notification events. This was true both in dichotomous comparisons between AED and controls (Supplemental Figure 2), and across all subjects, when externalizing symptomatology was measured by CBCL Externalizing scores (Supplemental Figure 3).
Volume-of-interest (VOI) analyses
We further characterized anticipatory BOLD signal change in a volume of interest (VOI) analysis to determine whether individual differences in signal change directly correlated with self-reported mood elicited by the anticipatory cues. Trial-type-averaged hemodynamic responses were assessed in custom-drawn masks that encompassed each of the left and right NAcc. Incentive- anticipatory signal change was calculated as net peak signal increase (6 s lag) relative to signal change in non-incentive trials, and was analyzed as a dependent variable in an omnibus, 4-way mixed-model analysis of variance (ANOVA) across the left and right NAcc masks (see Supplemental Methods).
Anticipatory signal change in nucleus accumbens VOI
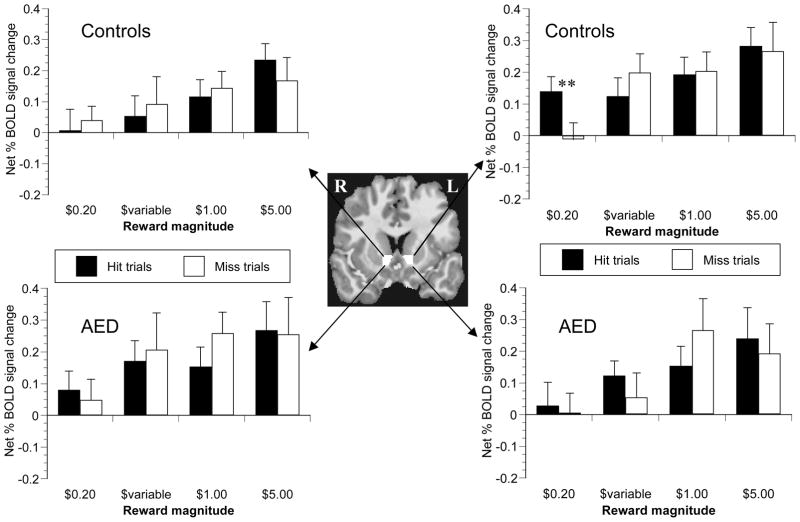
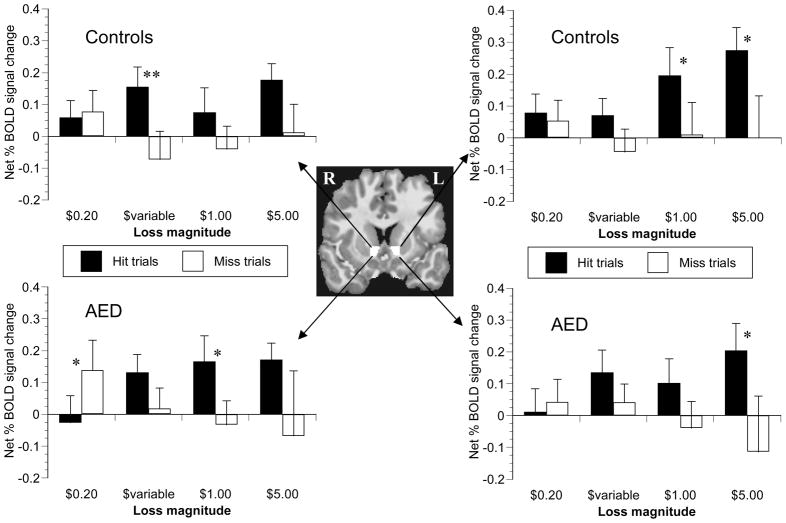
There were no trends for main or interactive effects of subject group or side (left vs right) on NAcc signal change. There were significant main effects of incentive valence (obtain gain or avoid loss) (F(1,22) = 8.823, p < .01) incentive magnitude (F(1,22) = 3.759, p < .05), and trial outcome (success or failure)(F(1,22) = 6.644, p < .05) on the net peak BOLD signal increase (Figures 4 and 5). Anticipatory responses in NAcc were greater during reward trials compared to loss-avoidance trials, in high magnitude incentive trials versus low magnitude trials, and in trials when subjects ultimately hit the target compared to trials with misses. The interaction effect of valence X outcome was significant (F(1,22) = 7.391, p < .05), with a significant effect of outcome in loss-avoidance trials (F(1,22) = 11.393, p < .01), but not in reward trials (F(1,22) = 0.001, p = .97). Analysis of successful loss-avoidance trials only indicated a significant main effect of potential loss magnitude (F(3,66) = 3.955, p < .05), but no main or interaction effects of subject group (p > .70). In sum, anticipation of incentives generally increased NAcc activation relative to nonincentives prior to successful target hits in both groups, but NAcc recruitment was incentive magnitude-sensitive only in gain trials.
Figure 4. Reward cue-elicited peak signal change in nucleus accumbens VOI.
Trial-type-averaged time series data were extracted from a mask custom-drawn for each subject in the nucleus accumbens (NAcc; center). Peak anticipatory signal occurred 6 s after cue presentation. NAcc recruitment increased with incentive magnitude in both controls (upper-most graphs), and in adolescents with externalizing disorders (AED; lower graphs). Analysis of variance across all trial magnitudes indicated that signal change did not significantly differ between trials when the subject did (solid bars) or did not (empty bars) hit the target (ANOVA p = .97). There were no main or interactive effects of subject group on reward-anticipatory NAcc signal change. ** denotes p <.05 per simple-effect two-tailed paired t-test.
Figure 5. Loss-avoidance cue-elicited peak signal change in nucleus accumbens VOI.
Trial-type-averaged time series data were extracted from a mask custom-drawn for each subject in the nucleus accumbens (NAcc; center). Peak anticipatory signal occurred 6 s after cue presentation. NAcc recruitment did not increase with incentive magnitude in either controls (upper-most graphs) or in adolescents with an externalizing disorder (AED; lower graphs). Analysis of variance across all trial magnitudes indicated that signal change was significantly blunted in trials when the subject did not hit the target (empty bars) relative to successful trials (solid bars)(ANOVA p < .01). There were no main or interactive effects of subject group on loss-anticipatory peak NAcc signal change. * denotes p <.10 and ** denotes p < .05 per simple-effect two-tailed paired t-test.
NAcc activation and psychometric measures
In the 21 subjects from whom we collected mood data, we assessed the independent effects of: 1) general externalizing symptomatology, and 2) individual differences in excitement about the task itself (simultaneous independent variables) on how robustly the NAcc responded to cues for rewards (as the dependent variable). This analysis was limited to data extracted from successful trials. In a multiple regression analysis, the NAcc signal change elicited by anticipation of maximum ($5) reward (as a net change relative to that elicited by the nonincentive) showed a significant positive correlation with net reward-related excitement (excitement about the $5 reward cue minus excitement about the nonincentive cue) in left NAcc, with a trend in right NAcc (Figure 6: Left: Beta = .56, p < .01; right: r = .42, p < .10). Bivariate rank-order correlation between net excitement and net NAcc activation showed the same pattern (left NAcc: Spearman r = .49, p < .05; right NAcc: r = .38, p < .10). Conversely, CBCL externalizing scores did not show a significant correlation with net reward-anticipatory signal change-- either in bivariate correlation or in a multiple regression (after controlling for self-reported excitement).
Figure 6. Relationship between NAcc signal change and psychometric measures.
After controlling for Child Behavior Checklist (CBCL) externalizing total scores, individual differences in net reward-related signal change partially correlated with subjects’ self-report ratings of net “excitement” at seeing the cue for maximum reward in the left nucleus accumbens (NAcc) (A), with a trend in the right NAcc (B). Solid circles denote adolescents with externalizing disorders (AED). Hollow squares denote controls.
Discussion
AED did not differ from controls in VS recruitment by reward-anticipatory cues. Rather, AED showed greater VS activation by the contrast between receiving versus not receiving rewards in reward trials. Reward (hit) and nonreward (miss) notifications in reward trials, when analyzed separately, indicted that the group difference in the contrast activation resulted from both greater VS activation by reward notifications in AED as well as greater VS deactivation by nonreward notifications in AED. Substituting CBCL-externalizing scale scores for the dichotomous ExD diagnosis also revealed a direct voxelwise correlation between externalizing symptomatology and reward-outcome-elicited activation. It appears, then, that the motivational neurocircuitry of AED with predominantly ODD/CD symptomatology is more sensitive to instrumental reward trial outcomes, but there were no significant group differences in response-anticipatory activation1. Finally, loss notifications activated right anterior insula in both groups. This is consistent with previous data implicating anterior insula in the mental representation of affective reactions during incentive tasks (Paulus, Rogalsky, Simmons, Feinstein, & Stein, 2003).
As with previous experiments (Bjork, Hommer, Grant, & Danube, 2004; Bjork, Smith, & Hommer, 2008; Knutson, Adams et al., 2001), BOLD signal change in a NAcc VOI correlated with individual differences in self-reported excitement upon seeing the high-reward-predictive cue. Interestingly, the VOI analysis also revealed that anticipatory NAcc activation by prospective losses was evident in successful trials only. Conversely, anticipatory NAcc activation by prospective gains was similar during both successful and unsuccessful trials. This suggests that in adolescents, the NAcc is more consistently recruited across trials by the prospect of winning rewards than by the prospect of avoiding losses.
The greater NAcc and ACC activation by notification of rewards in the AED may be a neurophysiological reflection of how externalizing children show greater behavioral sensitivity to gains in laboratory decision tasks (Lane & Cherek, 2001; Matthys et al., 1998; Matthys, van Goozen, Snoek, & van Engeland, 2004) and is possibly a manifestation or underpinning of a heritable, generalized behavioral risk factor for SUD (Giancola & Moss, 1998) identified in large-scale twin (Iacono, Carlson, Taylor, Elkins, & McGue, 1999; Slutske et al., 1998; Slutske et al., 2002; Young et al., 2000) and longitudinal (Clark, Kirisci, & Moss, 1998) studies. Interestingly, the exaggerated VS activation by the reward versus nonreward outcome contrast in AED seen here is similar to that of adults with SUD (Bjork et al., 2008).
We did not replicate the VS deficit in reward-anticipation activation previously reported in adolescents (Scheres et al., 2007) and adults (Strohle et al., 2008) with ADHD. In contrast, we replicated findings of more prominent mesofrontal cortex activation by reward vs nonreward notifications in adults with ADHD (Strohle et al., 2008). We posit that the key difference was that our subjects primarily had ODD or CD with additional subclinical internalizing symptoms. In contrast, a population selected primarily for “cold-cognitive” attentional decrements may be more vulnerable to reduced VS response to reward anticipation in the MID, because it requires intense vigilance.
This study has several limitations. First, the AED were diagnostically-mixed, reflecting the common co-occurrence of ADHD, ODD and CD, thus compromising syndrome specificity. However, we also included a dimensional approach in addition to binary diagnostic classification. Second, the sample size is modest. However, it nonetheless revealed both a significant group difference in NAcc activation by the reward notification contrast, and no trend toward a group difference in Nacc recruitment by anticipatory cues (to warrant additional subject recruitment). Third, the lack of anticipation-related activation differences may have resulted from our use of a community-recruited sample of AED. Although this mitigated medication confounds, a clinically-referred sample with severe symptomatology might show more activation differences. Fourth, while the scanning range of this study provided fine spatial resolution in the NAcc and the ventral mFC, it missed potential task-elicited activations in superior cerebrum. Finally, although applicants who met lifetime criteria for an affective disorder were excluded, AED also had significantly elevated internalizing symptoms, as is reported in epidemiological study (Boylan, Vaillancourt, Boyle, & Szatmari, 2007). We note, however, that in an exploratory analysis where CBCL internalizing total scores were substituted for externalizing scores, there were no significant voxelwise correlations.
In conclusion, this experiment provides evidence that adolescents characterized by clinically-significant externalizing behavior symptomatology show an exaggerated response of mesolimbic incentive neurocircuitry to reward notifications. Future studies should explore incentive neurocircuitry in more severe cases, and whether deviant mesolimbic incentive processing in early adolescence portends subsequent SUD.
Key Points.
Children with externalizing symptoms have shown heightened behavioral sensitivity to reward delivery in several laboratory tasks
This experiment demonstrates that the motivational neurocircuitry of adolescents with externalizing symptoms is hypersensitive to reward notifications
At a relaxed threshold, externalizing subjects showed more ventral striatum (VS) activation by reward notifications, and more VS deactivation by notifications of failure to win reward
Externalizing symptomatology did not correlate with cue-elicited, reward-anticipatory activation of the VS
Supplementary Material
Acknowledgments
This research was sponsored by intramural research funds of the National Institute on Alcohol Abuse and Alcoholism. Margaret Israel, Cinnamon Danube, and Swati Murthy assisted with subject recruitment and data collection.
Abbreviations
- ADHD
Attention-deficit hyperactivity disorder
- AED
Adolescents with externalizing disorders
- AFNI
Analysis of Functional NeuroImages
- BOLD
Blood oxygen level dependent
- CBCL
Child Behavior Check List
- CD
Conduct disorder
- ExD
Externalizing disorder
- mFC
Mesial frontal cortex
- MID
Monetary incentive delay (task)
- NAcc
Nucleus accumbens
- ODD
Oppositional defiant disorder
- SUD
Substance use disorder
- VS
Ventral striatum
Footnotes
None of the authors has any conflict of interest regarding this research study and its findings, financial or otherwise.
All analyses of this report were repeated after excluding the AED subject who met criteria only for ADHD. In every analysis, his exclusion had a negligible effect on the outcome.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, VT: University of Vermont, Dept. of Psychiatry; 1991. [Google Scholar]
- Aklin WM, Moolchan ET, Luckenbaugh DA, Ernst M. Early tobacco smoking in adolescents with externalizing disorders: inferences for reward function. Nicotine Tob Res. 2009;11(6):750–755. doi: 10.1093/ntr/ntp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34(2–3):133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008;42(4):1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan K, Vaillancourt T, Boyle M, Szatmari P. Comorbidity of internalizing disorders in children with oppositional defiant disorder. Eur Child Adolesc Psychiatry. 2007;16(8):484–494. doi: 10.1007/s00787-007-0624-1. [DOI] [PubMed] [Google Scholar]
- Button TM, Hewitt JK, Rhee SH, Young SE, Corley RP, Stallings MC. Examination of the causes of covariation between conduct disorder symptoms and vulnerability to drug dependence. Twin Res Hum Genet. 2006;9(1):38–45. doi: 10.1375/183242706776402993. [DOI] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Moss HB. Early adolescent gateway drug use in sons of fathers with substance use disorders. Addict Behav. 1998;23(4):561–566. doi: 10.1016/s0306-4603(98)00038-0. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Douglas VI, Parry PA. Effects of reward and nonreward on frustration and attention in attention deficit disorder. J Abnorm Child Psychol. 1994;22(3):281–302. doi: 10.1007/BF02168075. [DOI] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Aitken MR, Savage J, Moore SC, et al. Decision making and executive function in male adolescents with early-onset or adolescence-onset conduct disorder and control subjects. Biol Psychiatry. 2009;66(2):162–168. doi: 10.1016/j.biopsych.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca AC, Yule W. Personality and antisocial behavior in children and adolescents: an enquiry into Eysenck’s and Gray’s theories. J Abnorm Child Psychol. 1995;23(6):767–781. doi: 10.1007/BF01447476. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Laruelle M. Neuroreceptor imaging in psychiatric disorders. Ann Nucl Med. 2002;16(7):437–446. doi: 10.1007/BF02988639. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Beauchaine TP, Shannon KE, Chipman J, Fleming AP, Crowell SE, et al. Neurological correlates of reward responding in adolescents with and without externalizing behavior disorders. J Abnorm Psychol. 2009;118(1):203–213. doi: 10.1037/a0014378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Moss HB. Executive cognitive functioning in alcohol use disorders. Recent Dev Alcohol. 1998;14:227–251. doi: 10.1007/0-306-47148-5_10. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Dev Psychopathol. 1999;11(4):869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction. 2004;99(12):1548–1559. doi: 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25(19):4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR. Risk taking by adolescents with maladaptive behavior histories. Exp Clin Psychopharmacol. 2001;9(1):74–82. doi: 10.1037/1064-1297.9.1.74. [DOI] [PubMed] [Google Scholar]
- Matthys W, van Goozen SH, de Vries H, Cohen-Kettenis PT, van Engeland H. The dominance of behavioural activation over behavioural inhibition in conduct disordered boys with or without attention deficit hyperactivity disorder. J Child Psychol Psychiatry. 1998;39(5):643–651. [PubMed] [Google Scholar]
- Matthys W, van Goozen SH, Snoek H, van Engeland H. Response perseveration and sensitivity to reward and punishment in boys with oppositional defiant disorder. Eur Child Adolesc Psychiatry. 2004;13(6):362–364. doi: 10.1007/s00787-004-0395-x. [DOI] [PubMed] [Google Scholar]
- Myers MG, Brown SA, Mott MA. Preadolescent conduct disorder behaviors predict relapse and progression of addiction for adolescent alcohol and drug abusers. Alcohol Clin Exp Res. 1995;19(6):1528–1536. doi: 10.1111/j.1530-0277.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Newman JP, Wallace JF. Diverse pathways to deficient self-regulation: Implications for disinhibitory psychopathology in children. Clin Psychol Rev. 1993;13:699–720. [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19(4):1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) J Am Acad Child Adolesc Psychiatry. 2000;39(1):59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Mechan AO, Yuan J, Hatzidimitriou G, Xie T, Mayne AH, et al. Amphetamine treatment similar to that used in the treatment of adult attention-deficit/hyperactivity disorder damages dopaminergic nerve endings in the striatum of adult nonhuman primates. J Pharmacol Exp Ther. 2005;315(1):91–98. doi: 10.1124/jpet.105.087916. [DOI] [PubMed] [Google Scholar]
- Rosa-Neto P, Lou HC, Cumming P, Pryds O, Karrebaek H, Lunding J, et al. Methylphenidate-evoked changes in striatal dopamine correlate with inattention and impulsivity in adolescents with attention deficit hyperactivity disorder. Neuroimage. 2005;25(3):868–876. doi: 10.1016/j.neuroimage.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry. 2009;166(1):83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(5):720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PA, Bucholz KK, Dunne MP, et al. Common genetic risk factors for conduct disorder and alcohol dependence. J Abnorm Psychol. 1998;107(3):363–374. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Madden PA, Bucholz KK, Statham DJ, Martin NG. Personality and the genetic risk for alcohol dependence. J Abnorm Psychol. 2002;111(1):124–133. [PubMed] [Google Scholar]
- Strohle A, Stoy M, Wrase J, Schwarzer S, Schlagenhauf F, Huss M, et al. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage. 2008;39(3):966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Shiah IS, Lam RW, Ngan E, Scarrow G, et al. PET study of [(18)F]6-fluoro-L-dopa uptake in neuroleptic- and mood-stabilizer-naive first-episode nonpsychotic mania: effects of treatment with divalproex sodium. Am J Psychiatry. 2002;159(5):768–774. doi: 10.1176/appi.ajp.159.5.768. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics. 2000;96:684–695. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.