Abstract
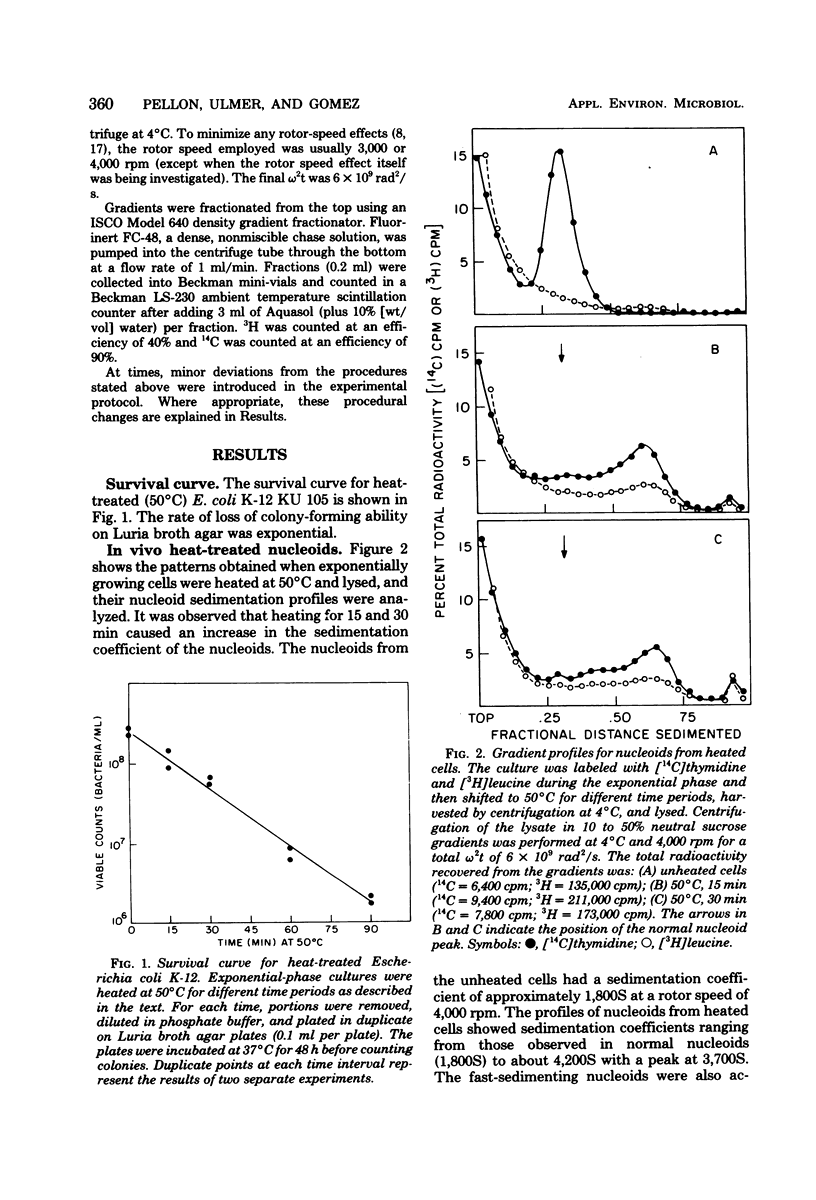
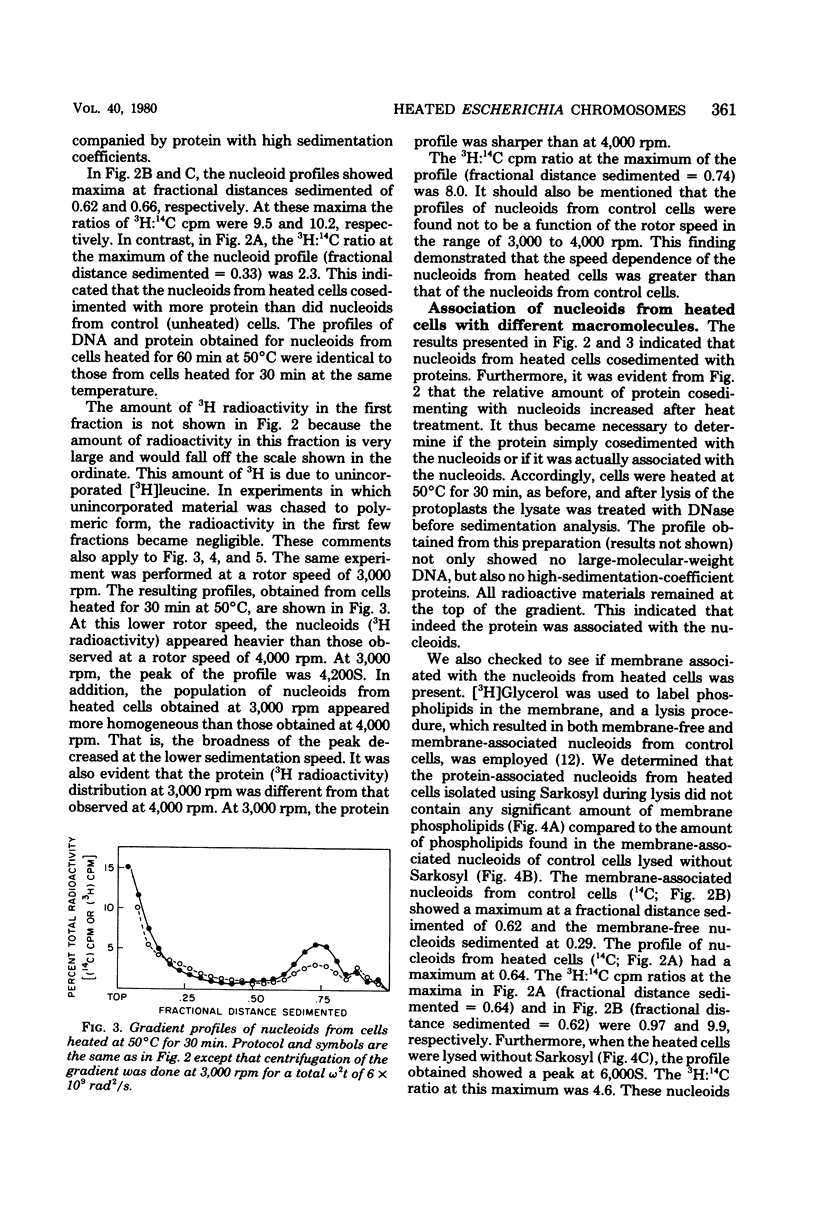
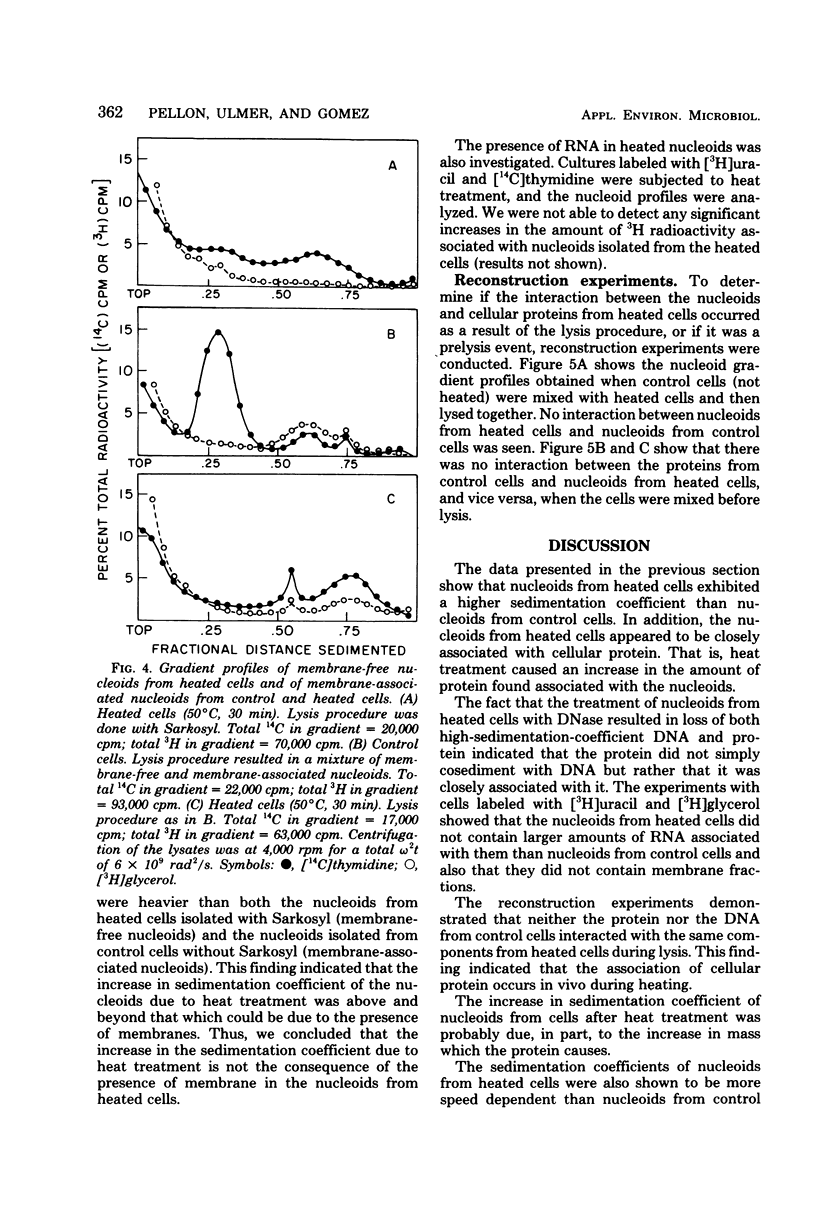
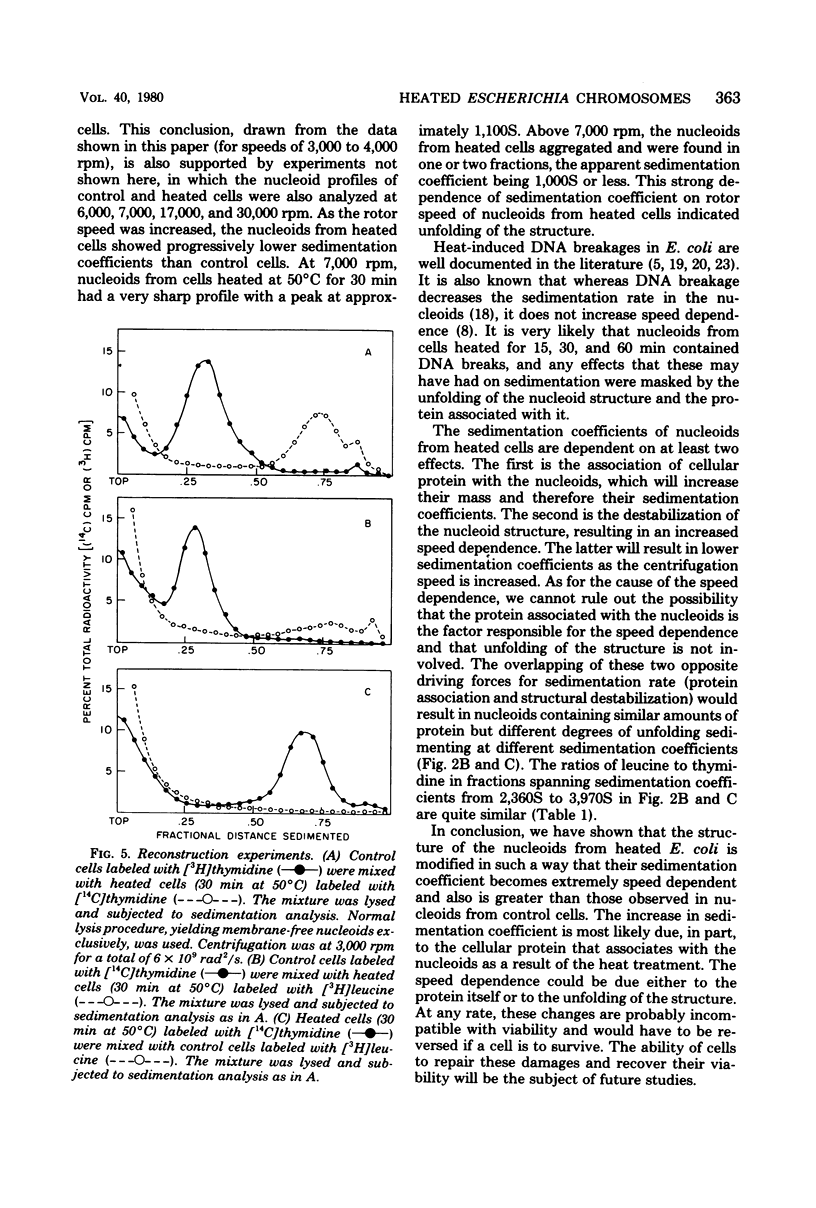
The folded chromosome or nucleoid of Escherichia coli was analyzed by low-speed sedimentation in neutral sucrose gradients after in vivo heat treatment. Heat treatment of cultures at 50 degree C for 15, 30, and 60 min resulted in in vivo association of the nucleoids with cellular protein. Structural changes, determined by the increase in speed dependence of the nucleoids from heated cells, also occurred. These changes were most likely due to the unfolding of the typical compact nucleoid structure. The nucleoids from heated cells also had notably higher sedimentation coefficients (3,000 to 4,500S) than nucleoids from control cells (1,800S). These nucleoids did not contain greater than normal amounts of membrane phospholipids or ribonucleic acid. We propose that the protein associated with the nucleoids from heated cells causes the observed sedimentation coefficient increases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beuchat L. R. Injury and repair of gram-negative bacteria, with special consideration of the involvement of the cytoplasmic membrane. Adv Appl Microbiol. 1978;23:219–243. doi: 10.1016/s0065-2164(08)70071-6. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Ashwood-Smith M. J., Munson R. J. Correlation of bacterial sensitivities to ionizing radiation and mild heating. J Gen Microbiol. 1969 Sep;58(1):115–124. doi: 10.1099/00221287-58-1-115. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Ashwood-Smith M. J., Munson R. J. Susceptibility of mild thermal and of ionizing radiation damage to the same recovery mechanisms in Escherichia coli. Biochem Biophys Res Commun. 1969 Apr 29;35(2):193–196. doi: 10.1016/0006-291x(69)90266-6. [DOI] [PubMed] [Google Scholar]
- CUMMINGS D. J. SEDIMENTATION AND BIOLOGICAL PROPERTIES OF T-PHAGES OF ESCHERICHIA COLI. Virology. 1964 Jul;23:408–418. doi: 10.1016/0042-6822(64)90264-8. [DOI] [PubMed] [Google Scholar]
- Grecz N., Bhatarakamol S. Apurinic acid endonuclease implicated in DNA breakage in Escherichia coli subjected to mild heat. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1183–1188. doi: 10.1016/s0006-291x(77)80104-6. [DOI] [PubMed] [Google Scholar]
- Hecht R. M., Pettijohn D. E. Studies of DNA bound RNA molecules isolated from nucleoids of Escherichia coli. Nucleic Acids Res. 1976 Mar;3(3):767–788. doi: 10.1093/nar/3.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht R. M., Stimpson D., Pettijohn D. Sedimentation properties of the bacterial chromosome as an isolated nucleoid and as an unfolded DNA fiber. Chromosomal DNA folding measured by rotor speed effects. J Mol Biol. 1977 Apr 15;111(3):257–277. doi: 10.1016/s0022-2836(77)80051-x. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A. Bacterial injury: a review. Can J Microbiol. 1977 Aug;23(8):935–944. doi: 10.1139/m77-139. [DOI] [PubMed] [Google Scholar]
- Materman E. C., Van Gool A. P. Compact Escherichia coli nucleoids in a highly supercoiled conformation. J Bacteriol. 1978 Aug;135(2):703–706. doi: 10.1128/jb.135.2.703-706.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materman E. C., Van Gool A. P. Nucleoid release from Escherichia coli cells. J Bacteriol. 1978 Feb;133(2):878–883. doi: 10.1128/jb.133.2.878-883.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn D. E. Prokaryotic DNA in nucleoid structure. CRC Crit Rev Biochem. 1976 Nov;4(2):175–202. doi: 10.3109/10409237609105458. [DOI] [PubMed] [Google Scholar]
- Pettijohn D., Hecht R. M., Stimpson D., van Scoyk S. An explanation for rotor speed effects observed during sedimentation of large folded DNA molecules. J Mol Biol. 1978 Feb 25;119(2):353–359. doi: 10.1016/0022-2836(78)90441-2. [DOI] [PubMed] [Google Scholar]
- Pierson M. D., Gomez R. F., Martin S. E. The involvement of nucleic acids in bacterial injury. Adv Appl Microbiol. 1978;23:263–285. doi: 10.1016/s0065-2164(08)70073-x. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G., Bridges B. A. Evidence for indirect production of DNA strand scissions during mild heating of Escherichia coli. J Gen Microbiol. 1972 Jun;71(1):191–193. doi: 10.1099/00221287-71-1-191. [DOI] [PubMed] [Google Scholar]
- Ulmer K. M., Gomez R. F., Sinskey A. J. Ionizing radiation damage to the folded chromosome of Escherichia coli K-12: repair of double-strand breaks in deoxyribonucleic acid. J Bacteriol. 1979 May;138(2):486–491. doi: 10.1128/jb.138.2.486-491.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer K. M., Gomez R. F., Sinskey A. J. Ionizing radiation damage to the folded chromosome of Escherichia coli K-12: sedimentation properties of irradiated nucleoids and chromosomal deoxyribonucleic acid. J Bacteriol. 1979 May;138(2):475–485. doi: 10.1128/jb.138.2.475-485.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock E., Grigg G. W. Repair of thermally induced DNA breakage in Escherichia coli. Nat New Biol. 1972 May 17;237(72):76–79. doi: 10.1038/newbio237076a0. [DOI] [PubMed] [Google Scholar]


