Abstract
A variety of pathogenic insults cause synthesis of tumor necrosis factor (TNF)α in the brain, resulting in sickness behavior. Here we used TNF-receptor (TNF-R)2-deficient and wild-type mice to demonstrate that the reduction in social exploration of a novel juvenile, the increase in immobility and the loss of body weight caused by central TNFα (i.c.v., 50 ng/mouse) are blocked by central pre-treatment with the multifunctional peptide, insulin-like growth factor (IGF-I; i.c.v., 300 ng/mouse). These results establish that sickness behavior induced by central TNFα via the TNF-R1 (p55) is directly opposed by IGF-I in the brain.
Keywords: Sickness behavior, TNF receptor, IGF-I, gene deficiency, central nervous system
1. Introduction
During the course of numerous pathogenic insults, the immune and central nervous systems interact to induce adaptive responses known as sickness behavior (Bluthé et al., 2000; Dantzer, 2004a; Dantzer, 2004b). This behavioral syndrome consists of a variety of physiological responses, which include symptoms of reduced appetite, weight loss, immobility and reduced interest in the physical and social environment (Dantzer, 2001). These symptoms of sickness can be mimicked in mice by systemic or central administration of the two major pro-inflammatory cytokines, interleukin (IL)-1β and TNFα (Bluthé et al., 1991; Bluthé et al., 2000; Bluthé et al., 1994). In order to develop therapeutic approaches to improve quality of life during infectious, autoimmune and neoplastic diseases, it is important to develop approaches that can oppose the behavioral responses induced by pro-inflammatory cytokines [reviewed in (Kelley et al., 2003)].
IGF-I is a multifunctional peptide that is essential for normal growth and development and provides neuroprotective and repair properties within the brain (Carro et al., 2003). IGF-I can oppose the pro-inflammatory activity of TNFα. When given in vivo, IGF-I decreases systemic induction of TNFα during both septic shock (Inoue et al., 1995) and burn wounds (Spies et al., 2001). Reciprocally, over-expression of TNFα causes retardation of brain growth and alters central nervous system architecture in parallel with depressed IGF-I expression in the cerebellum (Ye et al., 2003). We have extended these in vivo experiments by showing that IGF-I opposes the sickness-inducing properties of the cytokine inducer lipopolysaccharide (LPS) when both compounds are administered into either the lateral ventricles (i.c.v.) of the brain (Dantzer et al., 1999) or peripherally in the form of an intraperitoneal (i.p.) injection (Johnson et al., 2005). Sickness behavior induced by LPS requires pro-inflammatory cytokine secretion (Johnson et al., 1997; Segreti et al., 1997). We recently discovered that centrally-administered IGF-I inhibits sickness behavior induced by centrally injected TNFα in CD-1 mice (Bluthé et al., 2006). Collectively, these results suggest a multifaceted interaction between IGF-I and pro-inflammatory cytokines and that some of the major beneficial effects of IGF-I are related to its ability to antagonize the pro-inflammatory cytokine TNFα.
The biologic activities of TNFα are mediated by two structurally related but functionally distinct receptors belonging to the TNF-R gene family, TNF-R1 (p55) and TNF-R2 (p75) (Vandenabeele et al., 1995). It is unknown whether IGF-I acts by impairing the activity of TNF-R1 or TNF-R2 in the brain. Here we demonstrate that IGF-I blocks the sickness-inducing properties of central TNFα in both TNF-R2-deficient and wild-type mice, both of which have fully functional TNF-R1 receptors. These results firmly establish that IGF-I opposes the activity of TNF-R1 that are expressed in the brain. This finding indicates that the balance between TNFα and IGF-I in the brain is critical for regulating the expression of sickness behaviors.
2. Materials and methods
2.1 Genetic Strains of Mice and Animal Housing
Adult mice carrying the TNFrsf1btm1Mwm (TNF-R2−/−) mutation (Erickson et al., 1994) were purchased at 7 to 10 weeks of age from the Jackson Laboratory (Bar Harbor, Maine, USA). These inbred mice were been back-crossed to C57BL/6j mice for at least six generations by null × null sibling breedings. Heterozygotes were produced by crossbreeding with wild-type (WT) C57BL/6j mice, and these animals were then interbred to produce TNF-R2−/− mice. Adult C57BL/6j mice at 7 to 10 weeks of age from Jackson Laboratory were used as control WT mice. All mice were maintained under standard rodent colony conditions in polycarbonate transparent plastic cages with corn cob litter in a temperature- (23 ± 1 °C) and humidity- (40 %) controlled room. This room was maintained on a 12–12 h light/dark cycle (lights off at 09:00 h). Animals were provided free access to food and water. Juvenile male Crl:CD1(ICR)BR mice (3–4 weeks of age) were used to provide stimuli for the social investigation behavioral paradigm. These mice were group-housed in cages (40 × 25 × 15 cm) in a different room from the WT and TNF-R2−/− mice. Prior to initiation of all experiments, WT and TNF-R2−/− mice were handled for 5 min each day for one week. Behavioral tests were initiated during the dark phase. All Animal protocols were approved by the Institutional Animal Care and Use Committee. All behavioral data were obtained by trained observers blinded to the genotype and treatment of experimental mice.
2.2 Surgical Procedures
Mice were anesthetized with an i.p. mixture of ketamine and xylazine (13 mg/kg and 0.9 mg/kg at 0.1 ml/10 g body weight, respectively). Animals were placed in a Kopf stereotaxic instrument (Tujunga, CA, USA) and surgically implanted unilaterally with a stainless-steel guide cannula (23-gauge, 7 mm length) 0.6 mm posterior to bregma, 1.5 mm lateral and 2 mm below the skull surface at the point of entry, which was 1 mm above the lateral ventricle (Paxinos and Franklin, 2001). Mice were allowed to recover for two weeks prior to initiation of all behavioral tests. In order to confirm cannula placement in the lateral ventricles, a solution of India ink was injected into the guide cannula at the end of each experiment. The brain of every mouse was then removed, sliced and the site of injection was verified. All treatments were administered to non-anesthetized animals i.c.v. over 90 sec in a volume of one microliter by gravity through a 30-gauge needle placed through the guide cannulae and into the lateral ventricle.
2.3 Treatments
Recombinant murine TNFα (R & D Systems Inc., Minneapolis, MN, USA) was dissolved in artificial cerebrospinal fluid (aCSF; NaHCO3 26.2 mM; glucose 10 mM; NaCl 120 mM; Na2HPO4 1 mM; KCl 2.5 mM; MgCl2 1 mM; CaCl2 2.5 mM). Based upon previous experiments with CD1 mice (Bluthé et al., 2006), the optimal amount of TNFα to induce sickness behavior was determined in a dose-response experiment performed in C57BL/6j mice (i.c.v. 25, 50 or 100 ng/mouse). This experiment established that the optimal amount of TNFα required to induce sickness behavior in C57BL/6j mice is 50 ng given i.c.v. (data not shown). Recombinant human IGF-I (Intergen, Purchase, NY, USA) was dissolved in aCSF. Based on previous experiments showing the ability of 100 and 1,000 ng IGF-I injected i.c.v. to inhibit behavioral changes induced by i.c.v. LPS or TNFα in outbred mice (Bluthé et al., 2006; Dantzer et al., 1999), the present experiments were performed with one dose of IGF-I (300 ng/mouse). Each mouse received only one combination of treatments, and aCSF (1 μl) was used as the control for TNFα or IGF-I injections.
2.4 Behavioral Measurements
Beginning 24 h prior to initiation of all experiments, mice were individually housed in polycarbonate transparent plastic cages (26 × 20 × 14 cm). Three standard, accepted criteria were used to determine the degree of TNFα-induced sickness behavior: (a) Duration of social exploration directed toward the novel juvenile during the first 4 min following introduction into the home cage of the adult mouse to be tested. (b) Amount of time the test animal remained immobile during this 4-min observation period. and (3) Change in body weight over a 24-h period following treatment. Behavioral observations were recorded using a video camera under red light illumination between 09:00 h and 17:00 h (dark phase) and scored by an observer blind to the experimental treatments.
2.5 Experimental Design and Statistical Analyses
Both WT and TNF-R2−/− mice were treated i.c.v. with IGF-I or aCSF immediately before being treated with either TNFα or aCSF in a completely randomized design (n=6). Duration of social exploration and immobility were analyzed by a two-way ANOVA with strain (2 levels: WT and TNF-R2−/−) and treatment (4 levels: aCSF + aCSF, IGF-I + aCSF, aCSF + TNFα or IGF-I + TNFα) as the between-subject factors and time (four levels: 0, 2, 6 and 24 h) as the within-subject factor. The change in body weight was analyzed by a one-way ANOVA. Post-hoc comparisons were conducted with the protected LSD. All results were summarized and presented as the mean ± SEM.
3. Results
3.1. Central IGF-I abrogates the reduction in body weight caused by central TNFα in both WT and TNF-R2−/− mice
Body weight was significantly reduced 24 h after i.c.v. treatment with TNFα [F(3, 40) = 12.9, p<0.001] (Table 1). Post-hoc comparisons revealed that aCSF + TNFα decreased body weight in both WT (p<0.001) and TNF-R2−/− (p<0.001) mice. This reduction in body weight was completely abolished by central IGF-I treatment in both strains of mice (p<0.001).
Table 1.
IGF-I reverses the loss of body weight induced by injections of central TNFα.
| Strain | Treatment | Change in Body Weight (g) |
|---|---|---|
| WT | aCSF + aCSF | 1.35 ± 0.23 |
| aCSF + TNFα | −1.49 ± 0.41* | |
| IGF-I + aCSF | 0.96 ± 0.35 | |
| IGF-I + TNFα | 2.27 ± 0.51# | |
|
| ||
| TNF-R2−/− | aCSF + aCSF | 0.75 ± 0.32 |
| aCSF + TNFα | −1.53 ± 0.21* | |
| IGF-I + aCSF | 0.36 ± 0.10 | |
| IGF-I + TNFα | 0.33 ± 0.07# | |
Body weight changes were determined for WT (top) and TNF-R2-deficient (bottom) mice from weights obtained before and 24 h after i.c.v. treatment. Each value represents the mean ± SEM
n=6/group
p<0.001 compared to the aCSF + aCSF treatment within strain
p<0.001 compared to the aCSF + TNFα treatment within strain.
3.2. The decline in social exploration induced by central TNFα in both WT and TNF-R2−/− mice is blocked by central IGF-I
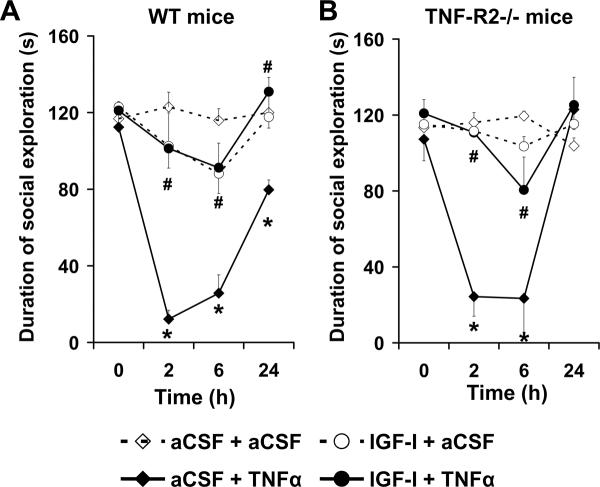
Average baseline duration of social exploration of a novel juvenile ranged from 112±7 s to 123±10 s for WT mice (time 0; Fig. 1A) and from 107±11 s to 121±7 s for the TNF-R2−/− mice (time 0; Fig. 1B) over the four treatments. The experimental treatments induced significant changes in duration of social exploration [F(3, 40) = 34.8, p<0.0001] in a time- [F(3, 120) = 35.2, p<0.0001] and time × treatment- [F(9, 120) = 13.8, p<0.0001] dependent manner but independently of the strain (Fig. 1 A–B). As expected, duration of social exploration remained stable following administration of both i.c.v. aCSF + aCSF and IGF-I + aCSF for both the WT (Fig. 1A) and TNF-R2−/− strains of mice (Fig. 1B). The aCSF + TNFα treatment significantly reduced duration of social exploration at 2, 6 and 24 h for the WT (Fig. 1A) and at 2 and 6 h for the TNF-R2−/− (Fig. 1B) mice. These effects were completely abolished by central IGF-I treatment at all time points for the WT (Fig. 1A) and TNF-R2−/− (Fig. 1B) strains of mice.
Fig. 1.
Central administration of IGF-I abrogates the reduction in social exploration caused by central TNFα in both WT and TNF-R2−/− mice. Mice were pretreated i.c.v. with either aCSF or IGF-I (300 ng/1 μl/mouse) and then injected centrally with either aCSF or TNFα (50 ng/1 μl/mouse). Duration of social exploration was measured at 0, 2, 6 and 24 h post-treatment in WT (A) and TNF-R2−/− (B) mice. Each value represents the mean ± SEM (n=6/group; * p<0.01 compared to aCSF + aCSF and# comparing IGF-I + TNFα to aCSF + TNFα).
3.3. Central IGF-I impairs the increase in immobility caused by central TNFα in TNF-R2−/− mice
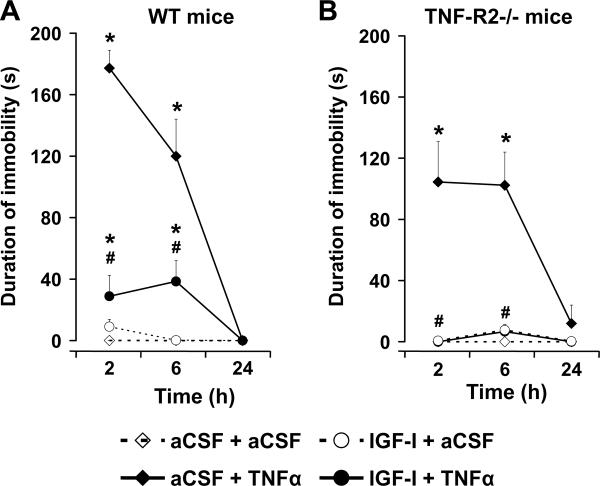
All mice were fully active at initiation of experiments (immobility equal to zero), which is why duration of immobility is not presented in Fig. 2. The experimental treatments induced significant changes of the duration of immobility [F(3, 40) = 64.6, p<0.0001] in a time- [F(2, 80) = 45.5, p<0.0001], time × strain- [F(2, 80) = 6.2, p<0.01], time × treatment- [F(6, 80) = 30.0, p<0.0001] and time × strain × treatment- [F(6, 80) = 2.8, p<0.05] dependent manner (Fig. 2 A–B). Mice remained fully active following i.c.v. aCSF + aCSF and IGF-I + aCSF treatments for both the WT (Fig. 2A) and TNF-R2−/− (Fig. 2B) mouse strains. Conversely, aCSF + TNFα increased duration of immobility at 2 and 6 h for both the WT and TNF-R2−/− mice. At the earliest time point (2 h), the increase in duration of immobility caused by aCSF + TNFα was significantly (p<0.05) lower in TNF-R2−/− than in WT mice. However, this strain difference was no longer apparent at either 6 or 24 h. More importantly, central IGF-I significantly reversed TNFα-induced immobility at both 2 and 6 h for WT mice (Fig. 2A) and TNF-R2−/− mice (Fig. 2B)
Fig. 2.
Immobility caused by central administration of TNFα is inhibited by IGF-I in both WT and TNF-R2−/− mice. Mice were treated centrally with either aCSF or IGF-I (300 ng/1 μl/mouse) and then injected i.c.v. with either aCSF or TNFα (50 ng/1 μl/mouse). Duration of immobility was measured at 2, 6 and 24 h post-treatment in WT (A) and TNF-R2−/− (B) mice. Each value represents the mean ± SEM (n=6/group; * p<0.01 compared to aCSF + aCSF and# comparing IGF-I + TNFα to aCSF + TNFα).
4. Discussion
These results establish that mice lacking functional TNF-R2s display similar symptoms of sickness behavior as WT mice following administration of TNFα centrally into the lateral ventricles into the brain. These data also show that sickness behavior induced by central TNFα is antagonized by pretreatment with centrally administered IGF-I in both WT and TNF-R2−/− mice. In order to conduct these experiments, i.c.v. surgeries were performed on both WT and TNF-R2−/− mice, both of which express functional TNF-R1 receptors and display sickness behavior following TNFα injection. Both strains of mice were pretreated i.c.v. with IGF-I prior to i.c.v. administration of an optimal amount of TNFα, as determined in preliminary experiments. Since TNF-R2−/− mice lack TNF-R2 receptors in both the periphery and brain, we chose to administer both TNFα and IGF-I into the lateral ventricles of the brain. The behavioral effects of central TNFα in both WT and TNR-R2−/− mice were similar to those reported in other experiments that administered human TNFα to inbred DBA/2 and outbred CD-1 mice that express normal levels of both TNF-R1 and TNR-R2 (Bluthé et al., 1991; Bluthé et al., 1994). Consistent with previous findings that human TNFα acts as a relatively pure agonist of murine TNF-R1s (Lewis et al., 1991), we predicted that TNF-R2−/− mice would behaviorally respond to central injections of recombinant murine TNFα. Here we confirm this idea and significantly extend it by using murine inbred genetic models that express functional central WT receptors as well as mice that express TNF-R1 but do not express TNF-R2. These new results clearly establish that even in the absence of TNF-R2, central TNFα is sufficient to induce robust sickness behaviors and that these sickness behaviors are blocked by central administration of IGF-I. These new experimental data strongly reinforce the emerging concept that the balance of TNFα in the brain in relation to anti-inflammatory cytokines, such as the IL-1 receptor antagonist, IL-10 or, as shown here, IGF-I, is critical for determining the extent of sickness behavior.
The current study shows that central injection of IGF-I (300 ng) alone does not affect body weight or sickness behavior, as assessed by investigation of a juvenile mouse or duration of immobility of both WT and TNF-R2−/− mice. Conversely, when injected immediately before central TNFα treatment, central IGF-I abolishes the reduction in social exploration and body weight and significantly inhibits immobility in both strains of mice. Although IGF-I is well-known to be active in the central nervous system [reviewed in (McCusker et al., 2006)], the mechanism responsible for the ability of IGF-I to antagonize the behavioral effects of TNFα remains elusive. One of the first questions that must be answered is whether IGF-I antagonizes the central effects of TNF-R1 or TNF-R2. Here we directly addressed this important issue by directly administering IGF-I, TNFα or both into the lateral ventricles of the brain. The behavioral responses of WT and TNF-R2−/− mice were nearly the same, with only a minor difference in full recovery of social investigation of TNF-R2−/− at 24 h (Fig. 1B) and a reduction in immobility at 2 h in TNF-R2−/− mice in response to central TNFα (Fig. 2B). Collectively, these results offer little support for the idea that central TNF-R2 receptors protect against TNF-induced sickness. More importantly, the results establish that IGF-I blocks TNFα-induced sickness behavior by a central mechanism in mice that display functional TNF-R1 receptors but lack TNF-R2 receptors. It is possible that IGF-I may have similar protective properties when administered either earlier or later in relation to TNFα, but chronic injections of IGF-I may be needed for this protection. For example, repeated rather than acute i.c.v. injections of IGF-I (1,000) are needed to protect mice against kainate-induced loss of cognitive impairment (Bluthé et al, 2005). Similarly, the anti-depressant properties of IGF-I are apparent as long a six days following a single i.c.v. injection (Hoshaw, Malberg and Lucki, 2005).
The beneficial effects of IGF-I on TNFα-induced sickness behavior could be explained by either direct or indirect processes. First, some studies have established that IGF has the potential to depress TNFα signaling. For example, microglial-derived IGF-II inhibits TNF-activation of JNK in oligodendrocytes cultured in vitro (Nicholas et al., 2002). IGF-I has also been shown to stimulate dephosphorylation of Iκß by astrocytes, thus preventing its degradation and diminishing NFκβ activation (Pons and Torres-Aleman, 2000). Similarly, TNFα-induced NFκβ activation is downregulated by IGF-I treatment of human colonic adenocarcinoma cells (Vallee et al., 2003). Second, IGF-I may reduce the production of cytokines in the brain that are synthesized in response to TNFα. We have previously established that IL-1β activity is necessary for TNFα-induction of most symptoms of sickness behavior (Bluthé et al., 1991; Bluthé et al., 1994). Therefore, the beneficial effect of IGF-I may be secondary to impairment in the ability of TNFα to induce central synthesis of IL-1β. Finally, IGF-I and TNFα may inversely regulate the availability of the major excitatory neurotransmitter of the mammalian central nervous system, glutamate. For example, recent studies have assessed the fundamental role of TNFα and IGF-I in the availability of brain glutamate. IGF-I enhances glutamate transport activity and stimulates expression of the glial glutamate transporter, GLAST, in rat cortical astrocytes cultures (Suzuki et al., 2001). If similar effects occur in vivo, IGF-I could temper extracellular glutamate activity. Conversely, TNFα treatment promotes glutamate release through the TNF-R1 pathway in primary cultures of mouse microglia (Takeuchi et al., 2006). Metabotropic glutamate receptor activity is required for the febrile and behavioral changes in response to i.p. LPS, a pro-inflammatory cytokine-dependent effect (Weiland et al., 2006). This finding that metabotropic glutamate receptors are involved in sickness behavior supports the possibility that the opposing actions of IGF-I and TNFα on sickness behavior might somehow involve the neurotransmitter glutamate.
Major depressive disorders can develop on a background of cytokine-induced sickness behavior (Dantzer et al., 2006), and TNFα appears to play an important role in these processes. Indeed, in patients with major depressive disorder, a reduction in serum TNFα is directly correlated with psychopathological improvement (Lanquillon et al., 2000). Recently, experiments using mice deficient in either TNF-R1 or TNF-R2 suggest that both receptors can exert similar effects on depressive-like behaviors but with different magnitudes (Simen et al., 2006). This finding suggests that selective inhibition of either receptor may be effective in treating at least some components of depressive-like behavior. Like sickness behavior, IGF-I and another growth factor, brain-derived neurotrophic factor, have recently been shown to exhibit antidepressant activity (Hoshaw et al., 2005). These results are not inconsistent with the idea that IGF-I may effectively modify behaviors by counteracting the actions of pro-inflammatory cytokines. Collectively, new results reported here support and extend the idea that the balance between pro-inflammatory cytokines and growth factors in the brain are critical for determining the degree of sickness behavior following exposure to pathogenic insults.
Acknowledgments
This research was supported NIH grants to KWK (MH 51569) and RD (MH71349).
References
- Bluthé RM, Dantzer R, Kelley KW. Interleukin-1 mediates behavioural but not metabolic effects of tumor necrosis factor alpha in mice. Eur J Pharmacol. 1991;209:281–283. doi: 10.1016/0014-2999(91)90184-r. [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Frenois F, Kelley KW, Dantzer R. Pentoxifylline and insulin-like growth factor-I (IGF-I) abrogate kainic acid-induced cognitive impairment in mice. J. Neuroimmunol. 2005;169:50–58. doi: 10.1016/j.jneuroim.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Kelley KW, Dantzer R. Effects of insulin-like growth factor-I on cytokine-induced sickness behavior in mice. Brain Behav Immun. 2006;20:57–63. doi: 10.1016/j.bbi.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthé RM, Layé S, Michaud B, Combe C, Dantzer R, Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447–4456. [PubMed] [Google Scholar]
- Bluthé RM, Pawlowski M, Suarez S, Parnet P, Pittman Q, Kelley KW, Dantzer R. Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology. 1994;19:197–207. doi: 10.1016/0306-4530(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Nunez A, Torres-Aleman I. Brain repair and neuroprotection by serum insulin-like growth factor I. Mol Neurobiol. 2003;27:153–162. doi: 10.1385/MN:27:2:153. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004a;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Innate immunity at the forefront of psychoneuroimmunology. Brain Behav Immun. 2004b;18:1–6. doi: 10.1016/j.bbi.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthé RM, Castanon N, Kelley KW, Konsman JP, Layé S, Lestage J, Parnet P. Cytokines, sickness behavior and depression. In: Adler R, Dantzer R, Glaser R, Heijnen C, Irwin M, Padgett D, Sheridan J, editors. Psychoneuroimmunology. Fourth Edition Elsevier; San Diego: 2006. pp. 281–318. [Google Scholar]
- Dantzer R, Gheusi G, Johnson RW, Kelley KW. Central administration of insulin-like growth factor-1 inhibits lipopolysaccharide-induced sickness behavior in mice. Neuroreport. 1999;10:289–292. doi: 10.1097/00001756-199902050-00015. [DOI] [PubMed] [Google Scholar]
- Erickson SL, de Sauvage FJ, Kikly K, Carver-Moore K, Pitts-Meek S, Gillett N, Sheehan KC, Schreiber RD, Goeddel DV, Moore MW. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 1994;372:560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Inoue T, Saito H, Fukushima R, Inaba T, Lin MT, Fukatsu K, Muto T. Growth hormone and insulinlike growth factor I enhance host defense in a murine sepsis model. Arch Surg. 1995;130:1115–1122. doi: 10.1001/archsurg.1995.01430100093018. [DOI] [PubMed] [Google Scholar]
- Johnson DR, O'Connor JC, Dantzer R, Freund GG. Inhibition of vagally mediated immune-to-brain signaling by vanadyl sulfate speeds recovery from sickness. Proc Natl Acad Sci USA. 2005;102:15184–15189. doi: 10.1073/pnas.0507191102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RW, Gheusi G, Segreti S, Dantzer R, Kelley KW. C3H/HeJ mice are refractory to lipopolysaccharide in the brain. Brain Res. 1997;752:219–226. doi: 10.1016/s0006-8993(96)01454-0. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(Suppl 1):S112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Lewis M, Tartaglia LA, Lee A, Bennett GL, Rice GC, Chen EY, Goeddel DV. Cloning and expression of cDNAs for two distinct murine tumor necrosis factors demonstrate one receptor is species specific. Proc Natl Acad Sci USA. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker RH, Strle K, Broussard SR, Dantzer R, Bluthé RM, Kelley KW. Crosstalk between insulin-like growth factors and pro-inflammatory cytokines. In: Adler R, Dantzer R, Glaser R, Heijnen C, Irwin M, Padgett D, Sheridan J, editors. Psychoneuroimmunology. Fourth Edition Elsevier; San Diego: 2006. pp. 171–191. [Google Scholar]
- Nicholas RS, Stevens S, Wing MG, Compston DA. Microglia-derived IGF-2 prevents TNFalpha induced death of mature oligodendrocytes in vitro. J Neuroimmunol. 2002;124:36–44. doi: 10.1016/s0165-5728(02)00011-5. [DOI] [PubMed] [Google Scholar]
- Johnson DR, O'Connor JC, Dantzer R, Freund GG. Inhibition of vagally mediated immune-to-brain signaling by vanadyl sulfate speeds recovery from sickness. Proc Natl Acad Sci U S A. 2005;102:15184–15189. doi: 10.1073/pnas.0507191102. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press; New York: 2001. [Google Scholar]
- Pons S, Torres-Aleman I. Insulin-like growth factor-I stimulates dephosphorylation of ikappa B through the serine phosphatase calcineurin (protein phosphatase 2B) J Biol Chem. 2000;275:38620–38625. doi: 10.1074/jbc.M004531200. [DOI] [PubMed] [Google Scholar]
- Segreti J, Gheusi G, Dantzer R, Kelley KW, Johnson RW. Defect in interleukin-1beta secretion prevents sickness behavior in C3H/HeJ mice. Physiol Behav. 1997;61:873–878. doi: 10.1016/s0031-9384(96)00611-7. [DOI] [PubMed] [Google Scholar]
- Simen BB, Duman CH, Simen AA, Duman RS. TNFα signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Spies M, Nesic O, Barrow RE, Perez-Polo JR, Herndon DN. Liposomal IGF-1 gene transfer modulates pro- and anti-inflammatory cytokine mRNA expression in the burn wound. Gene Ther. 2001;8:1409–1415. doi: 10.1038/sj.gt.3301543. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Ikegaya Y, Matsuura S, Kanai Y, Endou H, Matsuki N. Transient upregulation of the glial glutamate transporter GLAST in response to fibroblast growth factor, insulin-like growth factor and epidermal growth factor in cultured astrocytes. J Cell Sci. 2001;114:3717–3725. doi: 10.1242/jcs.114.20.3717. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- Vallee S, Fouchier F, Bremond P, Briand C, Marvaldi J, Champion S. Insulin-like growth factor-1 downregulates nuclear factor kappa B activation and upregulates interleukin-8 gene expression induced by tumor necrosis factor alpha. Biochem Biophys Res Commun. 2003;305:831–839. doi: 10.1016/s0006-291x(03)00866-0. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Declercq W, Beyaert R, Fiers W. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- Weiland TJ, Anthony-Harvey-Beavis D, Voudouris NJ, Kent S. Metabotropic glutamate receptors mediate lipopolysaccharide-induced fever and sickness behavior. Brain Behav Immun. 2006;20:233–245. doi: 10.1016/j.bbi.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Ye P, Price W, Kassiotis G, Kollias G, D'Ercole AJ. Tumor necrosis factor-alpha regulation of insulin-like growth factor-I, type 1 IGF receptor, and IGF binding protein expression in cerebellum of transgenic mice. J Neurosci Res. 2003;71:721–731. doi: 10.1002/jnr.10512. [DOI] [PubMed] [Google Scholar]