Abstract
In humans, we tested the hypothesis that a flat interface nerve electrode (FINE) placed around the femoral nerve trunk can selectively stimulate each muscle the nerve innervates. In a series of intraoperative trials during routine vascular surgeries, an eight-contact FINE was placed around the femoral nerve between the inguinal ligament and the first nerve branching point. The capability of the FINE to selectively recruit muscles innervated by the femoral nerve was assessed with electromyograms (EMGs) of the twitch responses to electrical stimulation. At least four of the six muscles innervated by the femoral nerve were independently and selectively recruited in all subjects. Of these, at least one muscle was a hip flexor and at least two were knee extensors. Results from the intraoperative experiments were used to estimate the potential for the electrode to restore knee extension and hip flexion through functional electrical stimulation. Normalized EMGs and biomechanical simulations were used to estimate joint moments and functional efficacy. Estimated knee extension moments exceed the threshold required for the sit-to-stand transition.
1. Introduction
Each year 12 000 Americans sustain a spinal cord injury (SCI) that can result in partial to complete loss of sensation and function below the injury (Nobunaga et al 1999, National Spinal Cord Injury Statistical Center 2008). By activating peripheral motor nerves, functional electrical stimulation (FES) produces coordinated contractions of paralyzed muscles, partially restoring lost function. Epimysial and intramuscular electrodes have restored short-duration standing and stepping function to select individuals with paraplegia (Scheiner et al 1994, Triolo et al 1995, 1996, 2001, Daly et al 2001, Guiraud et al 2006). These systems require multiple surgical sites to deploy electrodes in multiple muscles. Full recruitment of large muscles with distributed motor points such as those of the anterior thigh is challenging with a single muscle-based electrode (Uhlir et al 2004).
An alternative to placing an electrode in or on a muscle is placing an electrode near, on, around or in a nerve. An extraneural cuff electrode has the potential to fully recruit the entire population of axons innervating a target muscle because of its close proximity to the axons. It also requires less current than muscle-based electrodes. This reduces the energy required for stimulation and may extended stimulator battery life. Additionally, a single multi-contact nerve electrode can reduce surgery time because it requires only a single site for implantation.
Selective and graded muscle contractions have been reported with spiral nerve cuff electrodes placed around the cat sciatic nerve (Sweeney et al 1990, Veraart et al 1993, Grill and Mortimer 1996, Tarler and Mortimer 2003, 2004, 2007), with a penetrating electrode array placed on the cat sciatic nerve (Branner and Normann 2000, Branner et al 2001, 2004, McDonnall et al 2004a, 2004b), and with intrafascicular electrodes placed within the tibial nerve of cats (Nannini and Horch 1991, Yoshida and Horch 1993).
The femoral nerve is a target for lower extremity standing and walking neuroprostheses. It innervates six muscles. The vastus lateralis, vastus medialis and vastus intermedius contribute to knee extension. The rectus femoris extends the knee and flexes the hip. The sartorius contributes to hip and knee flexion. The pectineus is a weak hip flexor.
In humans, the spiral nerve cuff electrode has been used to activate branches of the human femoral nerve (Fisher et al 2008, Polasek et al 2009). Fisher et al (2008) placed a multi-contact spiral nerve cuff electrode on the distal branch of the femoral nerve innervating the vasti in chronic human trials. Compared to muscle-based electrodes, the legs supported 50% more body weight during standing and the maximal standing time increased fivefold using the nerve cuff. In acute experiments, Polasek et al (2009) showed that stimulation with a four-contact spiral electrode placed around the femoral nerve selectively recruited hip flexors but did not reliably recruit knee extensors.
Schiefer et al (2008) used computer models to design a flat interface nerve electrode (FINE) that independently recruited muscles innervated by the femoral nerve. The flat configuration of the FINE (figure 1) is a closer match to the broad, flat geometry of the femoral nerve trunk found in vivo (figure 2). Prior acute and chronic animals studies have demonstrated selective stimulation with the FINE (Tyler and Durand 2002, Leventhal and Durand 2003).
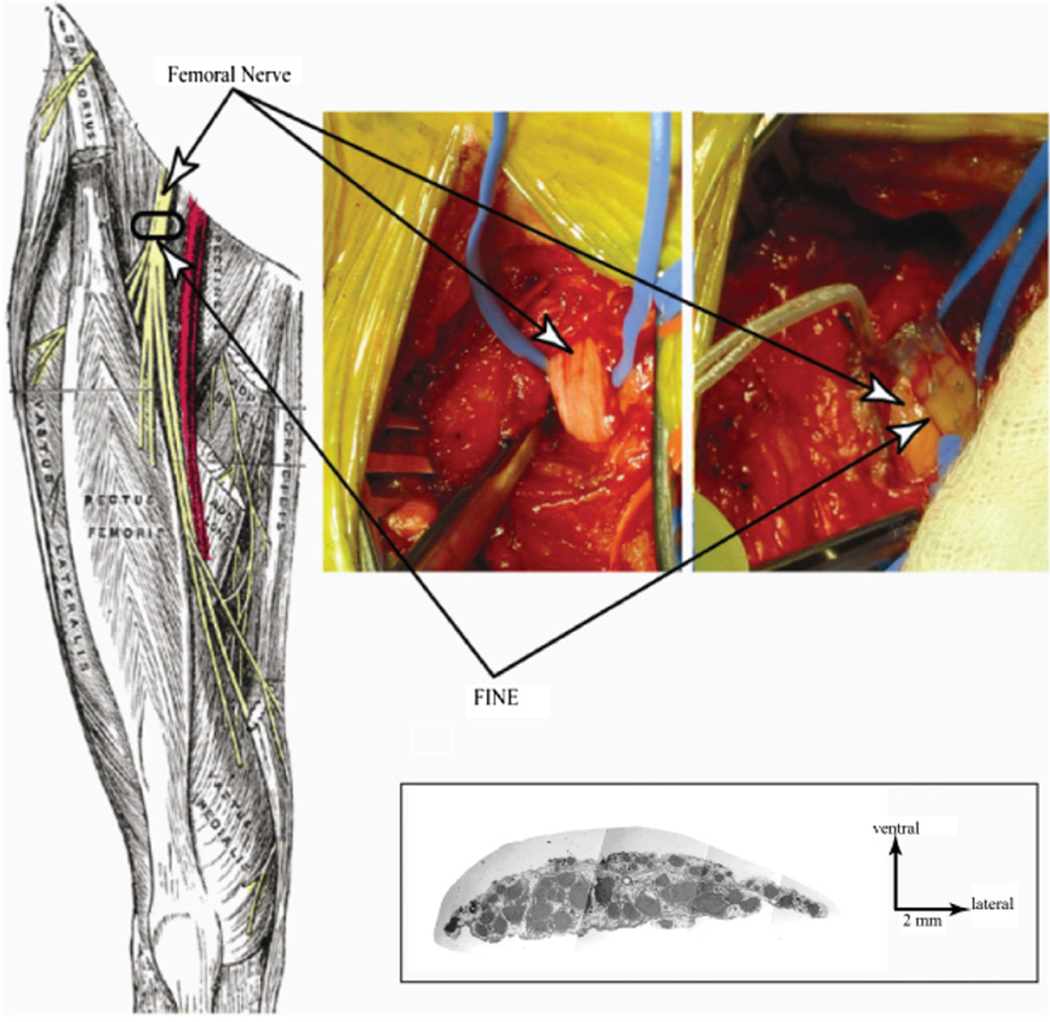
Figure 1.
A FINE similar to the one pictured here was used in intraoperative experiments. Contacts were offset to maximize the spatial volume that was stimulated. Top view (top) shows the offset contacts. Side view (middle) shows the lumen through which the femoral nerve passed. Side view (bottom) shows the open FINE and the button designed to keep the FINE closed. Scale on right is in mm.
Figure 2.
The target location for FINE placement is along the femoral nerve distal to the inguinal ligament and proximal to nerve branching (left) (Gray 1918). The exposed femoral nerve prior to implanting the FINE remained oblong in cross section (upper middle). The FINE placed around the exposed femoral nerve (upper right). Histological examination of a cadaver’s femoral nerve shows that its width is greater than its height (lower right) (Schiefer et al 2008).
To validate the computer models and determine if a FINE is appropriate for a chronic clinical standing and stepping neural prosthesis, we tested the hypothesis that a FINE placed proximally on the femoral nerve trunk will selectively activate at least four of the six muscles innervated by the femoral nerve and can separately recruit either knee extensors or hip flexors.
2. Methods
2.1. Subject recruitment
Seven subjects were recruited from a pool of patients scheduled for lower extremity vascular surgery that routinely exposes the femoral artery and nerve. Prior to the surgery, a therapist performed a manual muscle test (MMT) to evaluate baseline strength of the subject’s anterior thigh muscles. This test was performed again at a follow-up appointment after the surgery to confirm equivalent functional performance following the experiment. All surgeries were conducted at the Louis Stokes Cleveland Department of Veterans Affairs Medical Center (LSCDVAMC), Cleveland, OH. The Institutional Review Board of the LSCDVAMC approved the study and the subjects provided consent prior to participation.
2.2. FINE design
The FINE had eight platinum–iridium contacts: four on the upper inner surface (visible in figure 1) and four on the lower inner surface (not visible in figure 1). Upper contacts were offset from lower contacts. Each contact was laser cut (Norman Noble, Cleveland, OH) and spot welded to seven-strand, stainless steel, perfluoroalkoxy (PFA)-coated leads (Technical Development Laboratory, Case Western Reserve University). The silicone housing was fabricated and assembled with the leads at a commercial fabricator of medical-grade silicone devices (Point Medical Corporation, Crown Point, IN). The aperture exposing each embedded contact was 0.5 mm in diameter. FINE luminal dimensions were 10 mm wide × 1.5 mm tall × 7 mm along the nerve. Assembled FINEs were double-peel packed in Tyvek pouches (Dupont, Midland, MI), sealed and gas sterilized in ethylene oxide by a contract sterilization facility (Ethox International, Buffalo, NY) prior to clinical use.
2.3. FINE implant and stimulation procedure
To implant the FINE, a 2 cm section of the femoral nerve was exposed distal to the inguinal ligament, but proximal to nerve branching (figure 2). The width and height of the nerve were measured by the surgeon using a paper ruler. The exposed nerve was verified to be the femoral nerve by its response to stimulation delivered with a hand-held probe.
The surgeon put the FINE on the femoral nerve and inserted a 13 mm needle electrode subcutaneously near the incision as the stimulation current return electrode (figure 3). The FINE leads were connected to a custom-designed stimulator (Crishtronics, Cleveland, OH) that delivered monopolar, charge-balanced, biphasic, cathodic-first, square pulses. Pulse amplitude ranged from 0.04 to 5.0 mA with a resolution of 5 µA at or below 1.25 mA or 20 µA above 1.25 mA. Pulse width ranged from 10 to 500 µs with a resolution of 2 µs.
Figure 3.
Experimental setup for testing the FINE on the femoral nerve. A custom current-controlled stimulator delivered stimulus pulses to the FINE. Differential EMG was collected from each of the six muscles innervated by the femoral nerve. EMG was referenced to a ground patch placed on the contralateral leg, amplified, filtered and collected.
2.4. EMG recording procedures
During pre-operative preparation in the operating room, a surface reference electrode (2 in × 4 in, Nicolet-VIASYS, Madison, WI) was placed over a bony protuberance, usually the patella, contralateral to the leg undergoing surgery. During surgery, the surgeon placed pairs of electromyogram (EMG) needle electrodes (27 gauge, Axon Systems, Hauppauge, NY) 1 cm apart into the six muscles innervated by the femoral nerve (Lee and DeLisa 2000, Perotto et al 2005) (table 1). The response to stimulation with a hand-held stimulator attached to each pair of electrodes verified electrode placement.
Table 1.
Anatomical landmarks for EMG (Lee and DeLisa 2000, Perotto et al 2005).
| Muscle | EMG needle placement |
|---|---|
| Sartorius | 5–10 cm distal to the anterior superior illiac spine (ASIS) on a line directed from the ASIS to the medial epicondyle |
| Rectus femoris | Middle third of the femur halfway between the lateral and medial borders of the thigh |
| Vastus lateralis | At the top of the lower third of the of the thigh above the lateral border of the patella |
| Vastus intermedius | Halfway between the patella and the ASIS |
| Vastus medialis | 5–10 cm above the medial border of the patella |
| Pectineus | 1 cm lateral to the pubic tubercle |
Each pair of EMG electrodes was connected to a differential pre-amplifier (B&L Engineering, Tustin, CA) with a gain of 325 and a bandwidth of 12–2975 Hz (figure 3). Input impedance of the pre-amplifiers was 1 GΩ. Programmable amplifiers (1902, Cambridge Electronic Design (CED), Cambridge, UK) further amplified and filtered the responses. The CED amplifiers had a variable programmable gain allowing for overall signal gain of 1155–1155 000. Gain was selected such that a twitch response to a 2 mA, 250 µs biphasic cathodic-first square pulse was full-scale without saturating the amplifiers. The CED amplifiers clamped the EMG signal for 3 ms starting at the onset of stimulation to prevent amplifier saturation and remove stimulus artifact. AC coupling removed any dc drift from the electrode– tissue interface. Signals were low-pass-filtered at 1 kHz. A laptop running a custom MATLAB software suite was used to interface with the amplifiers and stimulator. Data were sampled at 2.4 kHz using a National Instruments A/D data acquisition (DAQ) connector box (BNC-2110, National Instruments, Webster, TX) and a PCMCIA card (6036E).
2.5. Recruitment curve generation
Pulse-width-modulated (PWM) and/or pulse-amplitude-modulated (PAM) EMG response recruitment curves were acquired for each contact of the FINE. For PWM, the pulse width was varied between 10 and 500 µs with a fixed amplitude of 0.3, 0.5, 0.8 or 1.2 mA. For PAM, the amplitude was varied between 0.4 and 2 mA with a fixed pulse width of 10 or 50 µs. Modulation proceeded according to an adaptive binary search in which an algorithm compared the EMG responses at consecutive stimuli. If the difference in sequential responses was greater than a set maximum value, a new stimulus was added to the sequence at the midpoint between the two stimulus levels. Each EMG response was calculated using the average of the response to three identical stimuli.
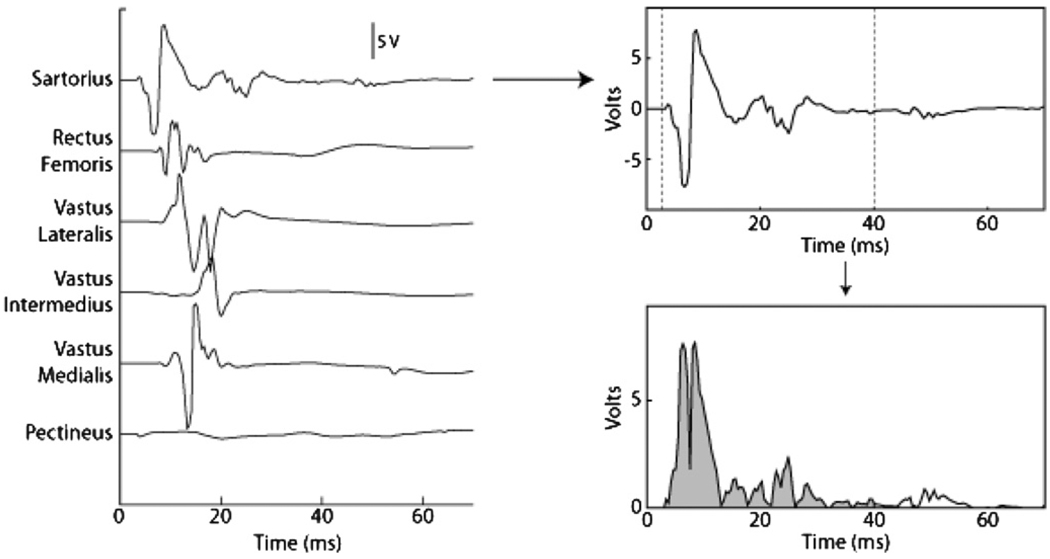
The muscle response to each stimulus was quantified as the rectified and integrated EMG. The integration window was specific for each muscle and set to capture the m-wave (figure 4). During an experiment, the largest quantified response of each muscle across all trials was defined as the maximum activation of that muscle. A muscle’s response to a given stimulus was quantified as the percentage activation that that muscle exhibited relative to the maximum response observed for that muscle over all trials.
Figure 4.
Protocol used to quantify the EMG response to nerve stimulation. The twitch response of the six muscles innervated by the femoral nerve (left). The twitch response of the sartorius (upper right). The EMG was rectified and integrated from 3 to 40 ms (dashed vertical lines) to quantify the twitch (lower right).
2.6. Selectivity
Selectivity, Si, for a muscle of interest, i, was defined as
| (1) |
The recruitment benefit, RBi, was defined as the normalized twitch response of target muscle i; the recruitment cost, RCi,j, was the normalized twitch response of muscle j when targeting muscle i; and N was the total number of muscles from which EMGs were recorded. Si ranged from −1.0 to 1.0. Si of −1.0 indicated that the stimulus parameters did not activate the target muscle, but instead fully activated all other muscles. Si of 1.0 indicated that the stimulus parameters resulted in full activation of only the target muscle.
The mean of the individual costs was used to calculate selectivity. Individual costs were therefore equally weighted. The weighting of an individual cost did not increase as the recruitment of a non-target muscle increased because a cost threshold was applied. Based on prior work, the cost threshold was defined as 10% of the maximum activation observed for any non-target muscle (Tyler and Durand 2002, Tarler and Mortimer 2003, Polasek et al 2007), which has been shown to correspond to the first visible or palpable muscle twitch (Polasek et al 2007). The maximum selectivity for each muscle was found when all costs remained below this threshold. That is, the cost of activating any of the five non-target muscles (RCi,j) could not exceed 0.10 of the maximum activation observed for those muscles over the duration of the experiment. Therefore, costs were limited such that non-target muscle recruitment was negligible. The overall selectivity of the FINE was quantified as
| (2) |
Selectivity was also calculated with respect to motion at the knee or hip. For this, RCi,j was a function of the twitch response of only the non-agonist muscles. For knee extension, agonists were considered to be the three vasti. For hip flexion, agonists were considered to be the rectus femoris, sartorius and pectineus. When RCi,j was a function of non-agonists only, N−1 was the number of non-agonist muscles.
2.7. Estimated joint moments
Measurement of muscle moment was not possible during intraoperative trials. Therefore, moments and the resulting function were estimated using a SIMM (software for interactive musculoskeletal modeling, Musculographics Inc., SantaRosa, CA) biomechanical model (Wickiewicz et al 1983, Brand et al 1986, Delp 1990, Delp et al 1990, Friederich and Brand 1990, Schiefer et al 2008). To estimate the joint moment generated as a result of stimulation, the maximum static moment produced by the muscle in the SIMM model was scaled by its activation level or the percentage of the maximum EMG elicited. This is an indirect estimate of the expected functional response to an implanted FINE.
3. Results
3.1. Participation, duration and thresholds
Seven subjects consented to the study. Valid data were collected from the last four subjects. Software and hardware problems prevented collection of a full data set during the first experiment. The second subject had a high stimulus threshold prior to placing the FINE, confirmed with a hand-held stimulator. Muscle twitches normally occur when stimulating the nerve at 2 mA with a pulse width below 50 µs, but in subject 2, the twitch threshold was 8 mA at pulse widths exceeding 100 µs. High thresholds prevented adequate stimulation within the charge density safety limits of the FINE. In the third subject, the nerve was embedded in scar tissue from previous surgeries and had an abnormal branching pattern. The surgeon removed the subject from the study because an adequate implant location could not be safely found.
On average, the threshold to elicit muscle contraction was 21 ± 18 nC. In subjects 4 through 7, the width across the nerve was between 8.0 and 10.0 mm. The height of the nerve was between 1.0 and 1.5 mm.
Each experiment required approximately 1 h. Thirty minutes were required to select, place and verify placement of the EMG needle electrodes. During this time the FINE was tested for continuity. FINE implantation and removal was accomplished using standard surgical tools and in less than 1 min. Data collection required 30 ± 7 min.
3.2. MMT scores
For subjects 1 and 4 through 7, post-operative MMT scores matched or exceeded pre-operative MMT scores, indicating that the FINE had no adverse effects on the nerve. Post-operative scores were not collected for subject 2 due to scheduling conflicts nor for subject 3 because this subject was removed from the study without having a FINE placed on the nerve. On average, follow-up MMTs were performed 32 days after surgery (20–54 days).
3.3. Recruitment curves and selectivity
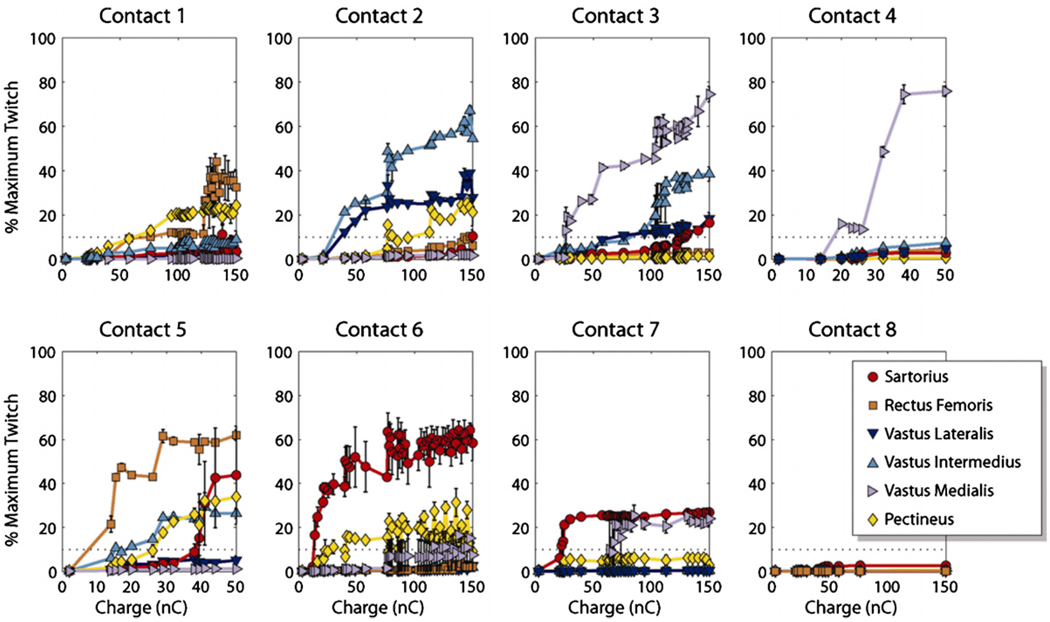
Representative recruitment curves are shown in figure 5. Each curve was obtained independently with a single active contact. Curves for contacts 1–3 and 6–8 were PWM. Curves for contacts 4 and 5 were PAM. Contacts 2 through 4 selectively recruited knee extensors. Contacts 1 and 5 through 7 selectively recruited hip flexors. Contact 8 did not recruit any muscle above threshold.
Figure 5.
Recruitment curves generated for subject 4 at each contact. Each curve was obtained independently with only a single contact active. Contacts 1–3 and 6–8 were fixed at 0.3 mA. Contacts 4 and 5 were fixed at 10 µs. The dashed line at 10% represents threshold.
3.3.1. RCi,j ⩽ 0.10 for all non-target muscles
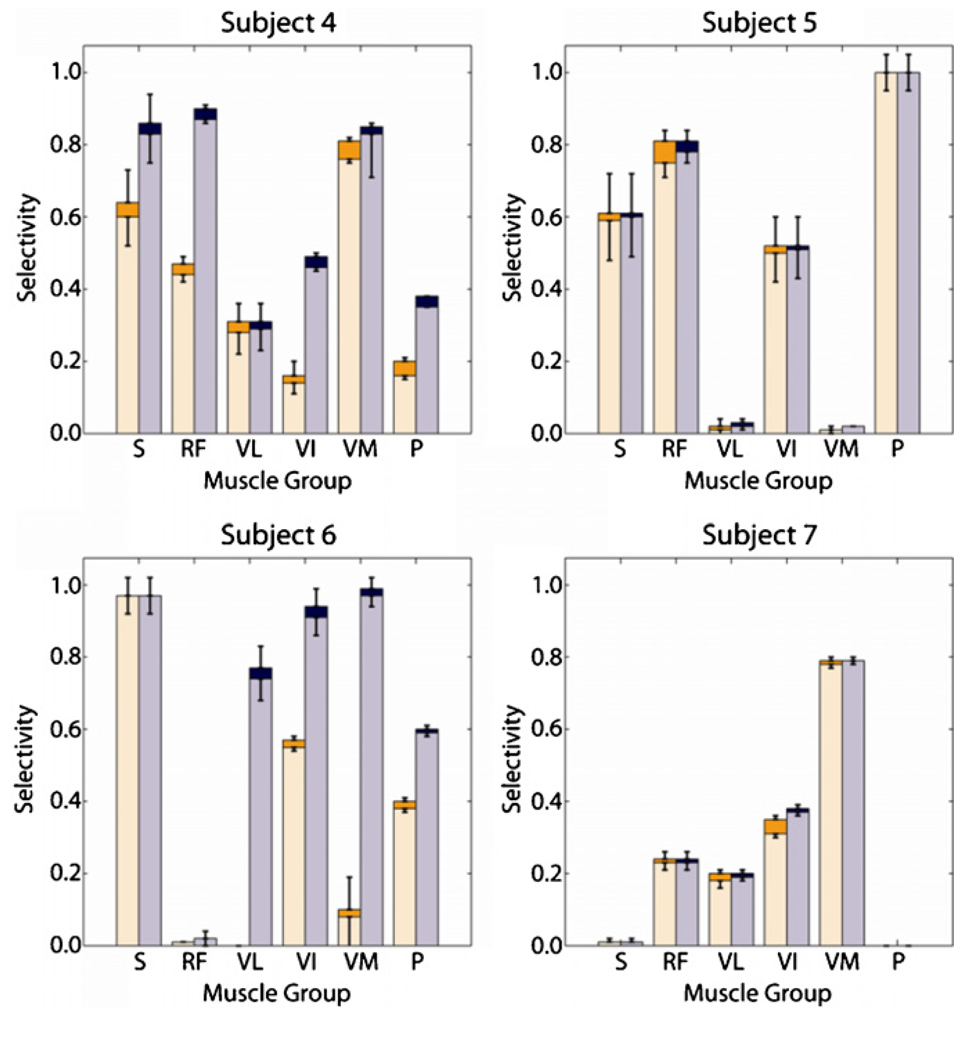
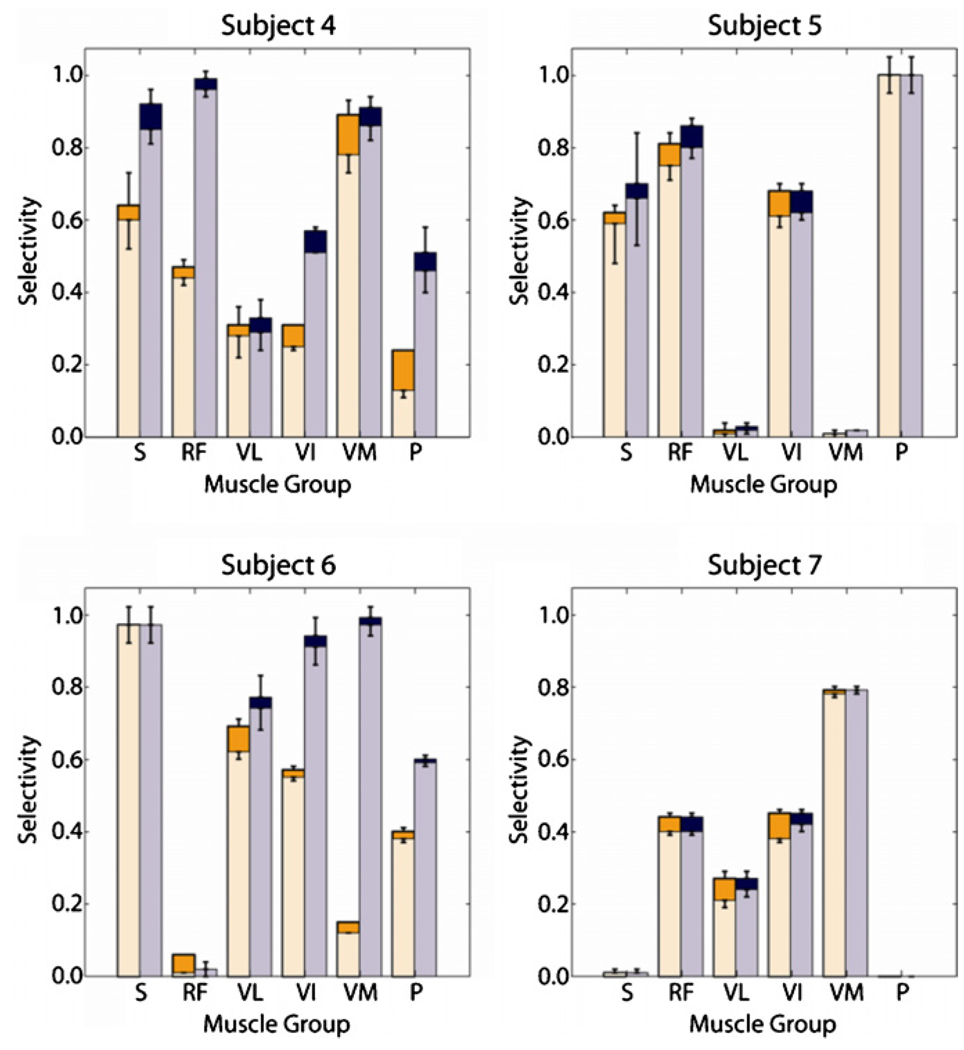
The maximum selectivity (shown by the lower bar in each set of stacked bars) and corresponding recruitment (shown by the higher bar in each set of stacked bars) obtained for each muscle in each subject under the strictest cost criterion was plotted (figure 6, the left bars in each set of grouped bars). Eighteen of the twenty-three recorded muscles and at least four muscles in each subject were independently and selectively recruited above threshold. When selectivity was maximized for each muscle, the average costs associated with spillover to non-target muscles were 0.02 ± 0.02 across all muscles and subjects.
Figure 6.
The maximized selectivity (lower of stacked bars) and corresponding recruitment (higher of stacked bars) for each muscle within each subject is shown under two regimes: (1) costs were a function of all non-target muscles and did not exceed 10% for any non-target muscle (left of grouped bars) and (2) costs were a function of all non-agonist muscles and did not exceed 10% for any non-agonist muscle (right of grouped bars). Subject 7 did not exhibit pectineus twitches.
Relaxation of the cost constraint resulted in an increase in the number of muscles that were activated and the level to which they were activated. When the maximum recruitment costs (max(RCi,j)), originally capped at 0.10, were capped at 0.20, the recruitment benefit associated with the maximized selectivity of at least half of the target muscles exceeded 0.40 in all subjects (figure 7).
Figure 7.
Same as figure 6, except that costs were limited to 20% instead of 10%, illustrating an increase in recruitment for many muscles. The largest increase occurred for vastus lateralis recruitment in subject 6 when costs were a function of all non-target muscles.
3.3.2. RCi,j ⩽ 0.10 for non-agonist muscles
In all subjects, the maximum selectivity for activation of the target muscles increased when costs were computed as a function of only the non-agonist muscles, and thus, when the contribution of the agonist muscles was ignored (figure 6, the right bars in each set of grouped bars). The number of subjects in which the muscle was selectively recruited, the degree to which the muscle was selectively recruited, or both increased for five of the six target muscles in all subjects. Nineteen of the twenty three recorded muscles and at least four muscles in each subject were selectively recruited above threshold. Although in subject 6, the vastus lateralis was not selectively stimulated under the stricter cost criterion, the recruitment benefit associated with the maximized selectivity increased to 0.77 ± 0.06 when vastus intermedius and vastus medialis activation could exceed threshold, indicating that the knee extensors were recruited simultaneously. Average costs associated with spillover to non-target muscles were 0.02 ± 0.01.
3.4. Estimated joint moment
The normalized activation levels of each muscle in response to a stimulus applied to a contact were used to estimate the cumulative moments at the knee and hip. The maximum estimated knee extension moment for each subject was found while varying the acceptable hip flexion moment across all stimuli applied to each contact (figure 8, left panel). When the estimated knee extension moment was maximized while restricting hip flexion moment to no more than 5 N m—approximately 10% of the maximum moment produced by simultaneous contraction of the hip flexors—knee extension moment averaged 82 ± 48 N m. In all subjects, estimated knee extension moment exceeded the 35 N m sit-to-stand transition threshold (Rodosky et al 1989, Kotake et al 1993, Uhlir et al 2000). 35 N m is approximately 16% of the maximum moment produced by simultaneous contraction of knee extensors.
Figure 8.
The maximum estimated knee extension moment increased as the acceptable estimated hip moment increased (left). The dashed line marks the sit-to-stand transition threshold, which all subjects exceeded with minimal hip flexion moment. The maximum estimated hip flexion moment increased as the acceptable estimated knee moment increased (right). Hip flexion was estimated to occur with a negligible knee extension in three subjects. Hip flexion sufficient for gait was marked by the dashed line. Curves were constructed with single contact stimulation.
The same technique was applied for hip flexion (figure 8, right panel). When costs were limited to 10%, an estimated 5 N m of hip flexion, or approximately 17% of that required for gait, could be obtained in all subjects. The maximum estimated hip flexion moment exceeded the estimated 30 N m required for gait (Winter 1991, Piazza and Delp 1996) in one subject but resulted in excess of 30 N m of estimated knee extension.
4. Discussion
Data from this study, which is the first time a FINE has been tested in humans, support the hypothesis that the electrode can selectively activate components of the femoral nerve. Under the strictest cost criterion, i.e. non-target muscles could not be activated above threshold, at least four of the six muscles were independently and selectively activated in all subjects and all six muscles were independently and selectively activated in one subject. At least one hip flexor and two knee extensors were selectively activated in each subject. In all subjects, at least half of the muscles were selectively activated to or above 24% of maximal activation. In two subjects, each head of the quadriceps could be selectively activated. In similar experiments conducted with a spiral nerve cuff electrode placed around the femoral nerve trunk, stimulation resulted in selective recruitment of no more than three muscles and only the sartorius could be consistently recruited in all subjects [24].
The threshold to elicit muscle contraction was 21 ± 18 nC. This was not significantly different from the 25 ± 17 nC found during chronic studies with a spiral nerve cuff implanted on upper extremity nerves in humans (p = 0.25, one-sided t-test) (Polasek et al 2007), the 18 ± 12 nC obtained during intraoperative evaluation of the spiral nerve cuff on the human femoral nerve (p = 0.38, one-sided t-test) (Polasek et al 2009) or the 23 ± 8 nC and 29 ± 17 nC obtained in chronic studies with a spiral nerve cuff implanted on the distal femoral nerve in humans (p = 0.38, p = 0.14, respectively; one-sided t-test) (Fisher et al 2008). The thresholds obtained for the FINE were significantly less than the 311 ± 100 nC (Kilgore et al 2003) reported for intramuscular electrodes in the upper extremity (p ≪ 0.01; one-sided t-test).
The criteria used to define selectivity differ depending on the desired outcome. Activation of a non-target muscle may be acceptable if it performs a function that aids or is synergistic with the target muscle, e.g. two knee extensors. When activation of agonists was not included in the recruitment costs, RC (equation (1)), the selectivity is an indication of functional muscle selectivity. Functional muscle selectivity was high for all subjects and the maximum functional selective activation of a muscle was also high. At least four muscles could be functional selectively recruited to a level of 20% in all subjects, and in three of four subjects, these muscles could be functional selectively activated to at least 49%.
When calculating selectivity, the costs of all muscles were weighted equally and activation of sensory fibers did not contribute to costs. A neuroprosthesis user with a complete SCI is unlikely to experience discomfort from activated sensory fibers. Therefore, this measure is a sufficient indication of selectivity if the goal is to maximize the percentage of a muscle that is selectively activated. However, each muscle’s maximum moment is not equal. If the goal is to maximize the functional response, then the costs associated with recruiting a muscle should be weighted by its contribution to the functional output. If the user maintains sensation, then activation of sensory fibers should also be incorporated into the cost calculation.
Results were obtained over a short period of time during which only a small portion of the stimulus space was explored. An anatomically based finite element method modeling study suggested that selectivity increased when using multiple contacts to recruit non-overlapping populations of axons innervating the same muscle (Schiefer et al 2008). A thorough exploration of stimulus space and the addition of field shaping techniques (Grill et al 1991) should result in even higher selectivity, but will require long-term experiments not yet conducted.
In all subjects, two to five contacts were selective for knee extensors and one to five contacts were selective for hip flexors. Of eight contacts, one or two could not produce selective recruitment. In two subjects, the non-selective contact simultaneously recruited both hip flexors and knee extensors. In three cases, contacts at the outer edges of the cuff did not recruit any muscle above threshold. In these cases, the nerve was smaller than the FINE and it was likely that the current shunted through extraneural fluid to the return electrode. Continuities of all leads were measured post-operatively and all were intact. Therefore, lack of muscle stimulation was not a result of broken leads.
The data indicate that a knee extension moment sufficient for the sit-to-stand transition can be obtained with minimal hip flexion moment using a single contact. Since multiple contacts on each FINE could selectively recruit multiple knee extension muscles, the FINE stimulation has functional redundancy. Using multiple contacts, carousel stimulation (sequential stimulation with different contacts in the electrode) is possible (Koller et al 1996). This would extend the duration an activity can be performed by delaying the onset of fatigue.
Moment estimates were made under the assumption that the selectivity obtained in a chronic trial will not be significantly less than that obtained in these experiments. Others have shown that selectivity obtained with a nerve cuff electrode during acute trials is comparable to that obtained during chronic trials (Grill and Mortimer 1998, Leventhal and Durand 2004, Polasek et al 2007).
It was assumed that EMG can be used to estimate moment. Normalized EMG reflects the maximum isometric force if the maximum voluntary contraction for the muscle is known (Bogey et al 2005). Here, the maximum voluntary contraction for a muscle was based on SIMM simulations that did not account for muscle weakness due to atrophy. Others have reduced muscle strength by 50% to account for atrophy after SCI (Acosta 2002). Even with a reduction in muscle strength by 50%, the estimated knee extension moment elicited by the FINE exceeded 35 N m.
Another assumption was that EMG from a single pair of recording electrodes in a muscle represents the electrical activity of the entire muscle. Others have shown that normalized EMG obtained with microwires represents whole-muscle activity in the human soleus (Bogey et al 2000). While data do not exist to support this for muscles of the anterior thigh, inaccuracies due to this assumption will be small and not affect the results.
5. Conclusion
This study indicates that a single eight-contact FINE placed on the femoral nerve trunk selectively recruits muscles innervated by the femoral nerve. Models indicate that selective muscle recruitment from a FINE on the femoral nerve trunk generates sufficient knee extension moment without hip flexion for a sit-to-stand transition and for the knee extension phase of gait. Further, selective activation of hip flexion without knee extension supplements hip flexion for the swing phase of gait. Multiple contacts on a single FINE would achieve sufficient knee extension moment from different muscles, demonstrating functional redundancy. Therefore, a single FINE on the common femoral nerve is a valuable component of a lower extremity neuroprosthesis.
Acknowledgments
The authors thank Mr Matt Eiseman, who recruited subjects, and Ms Lisa Boggs, PT, who conducted MMTs.
Contributor Information
M A Schiefer, Email: matthew.schiefer@case.edu.
D J Tyler, Email: dustin.tyler@case.edu.
References
- Acosta AM. PhD thesis. Cleveland, OH: Department of Biomedical Engineering, Case Western Reserve University; 2002. Musculoskeletal modeling of the shoulder and elbow in cervical spinal cord injury. [Google Scholar]
- Bogey RA, et al. Comparison of across-subject EMG profiles using surface and multiple indwelling wire electrodes during gait. J. Electromyogr. Kinesiol. 2000;10:255–259. doi: 10.1016/s1050-6411(00)00015-8. [DOI] [PubMed] [Google Scholar]
- Bogey RA, et al. An EMG-to-force processing approach for determining ankle muscle forces during normal human gait. IEEE Trans. Neural Syst. Rehabil. Eng. 2005;13:302–310. doi: 10.1109/TNSRE.2005.851768. [DOI] [PubMed] [Google Scholar]
- Brand RA, et al. The sensitivity of muscle force predictions to changes in physiologic cross-sectional area. J. Biomech. 1986;19:589–596. doi: 10.1016/0021-9290(86)90164-8. [DOI] [PubMed] [Google Scholar]
- Branner A, Normann RA. A multielectrode array for intrafascicular recording and stimulation in sciatic nerve of cats. Brain Res. Bull. 2000;51:293–306. doi: 10.1016/s0361-9230(99)00231-2. [DOI] [PubMed] [Google Scholar]
- Branner A, et al. Selective stimulation of cat sciatic nerve using an array of varying-length microelectrodes. J. Neurophysiol. 2001;85:1585–1594. doi: 10.1152/jn.2001.85.4.1585. [DOI] [PubMed] [Google Scholar]
- Branner A, et al. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans. Biomed. Eng. 2004;51:146–157. doi: 10.1109/TBME.2003.820321. [DOI] [PubMed] [Google Scholar]
- Daly JJ, et al. Performance of an intramuscular electrode during functional neuromuscular stimulation for gait training post stroke. J. Rehabil. Res. Dev. 2001;38:513–526. [PubMed] [Google Scholar]
- Delp SL. PhD thesis. Stanford University; 1990. Surgery simulation: a computer graphics system to analyze and design musculoskeletal reconstructions of the lower extremity. [Google Scholar]
- Delp SL, et al. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans. Biomed. Eng. 1990;37:757–767. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- Fisher LE, et al. Standing after spinal cord injury with four-contact nerve-cuff electrodes for quadriceps stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2008;16:473–478. doi: 10.1109/TNSRE.2008.2003390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich JA, Brand RA. Muscle fiber architecture in the human lower limb. J. Biomech. 1990;23:91–95. doi: 10.1016/0021-9290(90)90373-b. [DOI] [PubMed] [Google Scholar]
- Gray H. Anatomy of the Human Body. Philadelphia: Lea & Febiger; 1918. [Google Scholar]
- Grill WM, Jr, Mortimer JT. Quantification of recruitment properties of multiple contact cuff electrodes. IEEE Trans. Rehabil. Eng. 1996;4:49–62. doi: 10.1109/86.506402. [DOI] [PubMed] [Google Scholar]
- Grill WM, Mortimer JT. Stability of the input–output properties of chronically implanted multiple contact nerve cuff stimulating electrodes. IEEE Trans. Rehabil. Eng. 1998;6:364–373. doi: 10.1109/86.736150. [DOI] [PubMed] [Google Scholar]
- Grill WM, et al. Selective activation of peripheral nerve fascicles: use of field steering currents; Proc. 13th Int. Conf. IEEE Eng. Med. Biol. Soc; 1991. [Google Scholar]
- Guiraud D, et al. An implantable neuroprosthesis for standing and walking in paraplegia: 5-year patient follow-up. J. Neural Eng. 2006;3:268–275. doi: 10.1088/1741-2560/3/4/003. [DOI] [PubMed] [Google Scholar]
- Kilgore KL, et al. Durability of implanted electrodes and leads in an upper-limb neuroprosthesis. J. Rehabil. Res. Dev. 2003;40:457–468. doi: 10.1682/jrrd.2003.11.0457. [DOI] [PubMed] [Google Scholar]
- Koller R, et al. Influence of different conditioning methods on force and fatigue resistance in chronically stimulated skeletal muscles. Pacing Clin. Electrophysiol. 1996;19:222–230. doi: 10.1111/j.1540-8159.1996.tb03314.x. [DOI] [PubMed] [Google Scholar]
- Kotake T, et al. An analysis of sit-to-stand movements. Arch. Phys. Med. Rehabil. 1993;74:1095–1099. doi: 10.1016/0003-9993(93)90068-l. [DOI] [PubMed] [Google Scholar]
- Lee HJ, DeLisa JA. Surface Anatomy for Clinical Needle Electromyography. New York: Demos Medical; 2000. [Google Scholar]
- Leventhal DK, Durand DM. Subfascicle stimulation selectivity with the flat interface nerve electrode. Ann. Biomed. Eng. 2003;31:643–652. doi: 10.1114/1.1569266. [DOI] [PubMed] [Google Scholar]
- Leventhal DK, Durand DM. Chronic measurement of the stimulation selectivity of the flat interface nerve electrode. IEEE Trans. Biomed. Eng. 2004;51:1649–1658. doi: 10.1109/TBME.2004.827535. [DOI] [PubMed] [Google Scholar]
- McDonnall D, et al. Interleaved, multisite electrical stimulation of cat sciatic nerve produces fatigue-resistant, ripple-free motor responses. IEEE Trans. Neural Syst. Rehabil. Eng. 2004a;12:208–215. doi: 10.1109/TNSRE.2004.828425. [DOI] [PubMed] [Google Scholar]
- McDonnall D, et al. Selective motor unit recruitment via intrafascicular multielectrode stimulation. Can. J. Physiol. Pharmacol. 2004b;82:599–609. doi: 10.1139/y04-047. [DOI] [PubMed] [Google Scholar]
- Nannini N, Horch K. Muscle recruitment with intrafascicular electrodes. IEEE Trans. Biomed. Eng. 1991;38:769–776. doi: 10.1109/10.83589. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center. Spinal Cord Injury: Facts and Figures at a Glance. Department of Physical Medicine and Rehabilitation, University of Alabama at Birmingham; 2008. [Google Scholar]
- Nobunaga AI, et al. Recent demographic and injury trends in people served by the Model Spinal Cord Injury Care Systems. Arch. Phys. Med. Rehabil. 1999;80:1372–1382. doi: 10.1016/s0003-9993(99)90247-2. [DOI] [PubMed] [Google Scholar]
- Perotto AO, et al. Anatomical Guide for the Electromyographer: The Limbs and Trunk. Springfield, MA: Charles C Thomas; 2005. [Google Scholar]
- Piazza SJ, Delp SL. The influence of muscles on knee flexion during the swing phase of gait. J. Biomech. 1996;29:723–733. doi: 10.1016/0021-9290(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Polasek KH, et al. Human nerve stimulation thresholds and selectivity using a multi-contact nerve cuff electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 2007;15:76–82. doi: 10.1109/TNSRE.2007.891383. [DOI] [PubMed] [Google Scholar]
- Polasek KH, et al. Intraoperative evaluation of the spiral nerve cuff electrode on the femoral nerve trunk. J. Neural Eng. 2009;6:066005. doi: 10.1088/1741-2560/6/6/066005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodosky MW, et al. The influence of chair height on lower limb mechanics during rising. J. Orthop. Res. 1989;7:266–271. doi: 10.1002/jor.1100070215. [DOI] [PubMed] [Google Scholar]
- Scheiner A, et al. Design and clinical application of a double helix electrode for functional electrical stimulation. IEEE Trans. Biomed. Eng. 1994;41:425–431. doi: 10.1109/10.293216. [DOI] [PubMed] [Google Scholar]
- Schiefer MA, et al. A model of selective activation of the femoral nerve with a flat interface nerve electrode for a lower extremity neuroprosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 2008;16:195–204. doi: 10.1109/TNSRE.2008.918425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JD, et al. A nerve cuff technique for selective excitation of peripheral nerve trunk regions. IEEE Trans. Biomed. Eng. 1990;37:706–715. doi: 10.1109/10.55681. [DOI] [PubMed] [Google Scholar]
- Tarler MD, Mortimer JT. Comparison of joint torque evoked with monopolar and tripolar-cuff electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 2003;11:227–235. doi: 10.1109/TNSRE.2003.816867. [DOI] [PubMed] [Google Scholar]
- Tarler MD, Mortimer JT. Selective and independent activation of four motor fascicles using a four contact nerve-cuff electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 2004;12:251–257. doi: 10.1109/tnsre.2004.828415. [DOI] [PubMed] [Google Scholar]
- Tarler MD, Mortimer JT. Linear summation of torque produced by selective activation of two motor fascicles. IEEE Trans. Neural Syst. Rehabil. Eng. 2007;15:104–110. doi: 10.1109/TNSRE.2007.891377. [DOI] [PubMed] [Google Scholar]
- Triolo R, et al. Effects of stimulated hip extension moment and position on upper-limb support forces during FNS-induced standing—a technical note. J. Rehabil. Res. Dev. 2001;38:545–555. [PubMed] [Google Scholar]
- Triolo RJ, et al. Inter-rater reliability of a clinical test of standing function. J. Spinal. Cord Med. 1995;18:14–22. doi: 10.1080/10790268.1995.11719375. [DOI] [PubMed] [Google Scholar]
- Triolo RJ, et al. Standing and walking with FNS: technical and clinical challenges. In: Harris G, editor. Human Motion Analysis. New York: IEEE Press; 1996. pp. 318–350. [Google Scholar]
- Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 2002;10:294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- Uhlir JP, et al. The use of selective electrical stimulation of the quadriceps to improve standing function in paraplegia. IEEE Trans. Rehabil. Eng. 2000;8:514–522. doi: 10.1109/86.895955. [DOI] [PubMed] [Google Scholar]
- Uhlir JP, et al. Performance of epimysial stimulating electrodes in the lower extremities of individuals with spinal cord injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2004;12:279–287. doi: 10.1109/TNSRE.2004.827224. [DOI] [PubMed] [Google Scholar]
- Veraart C, et al. Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Trans. Biomed. Eng. 1993;40:640–653. doi: 10.1109/10.237694. [DOI] [PubMed] [Google Scholar]
- Wickiewicz TL, et al. Muscle architecture of the human lower limb. Clin. Orthop. 1983;179:275–283. [PubMed] [Google Scholar]
- Winter DA. Biomechanics of Motor Control and Human Gait. Waterloo, Ontario, Canada: University of Waterloo Press; 1991. [Google Scholar]
- Yoshida K, Horch K. Selective stimulation of peripheral nerve fibers using dual intrafascicular electrodes. IEEE Trans. Biomed. Eng. 1993;40:492–494. doi: 10.1109/10.243412. [DOI] [PubMed] [Google Scholar]