Abstract
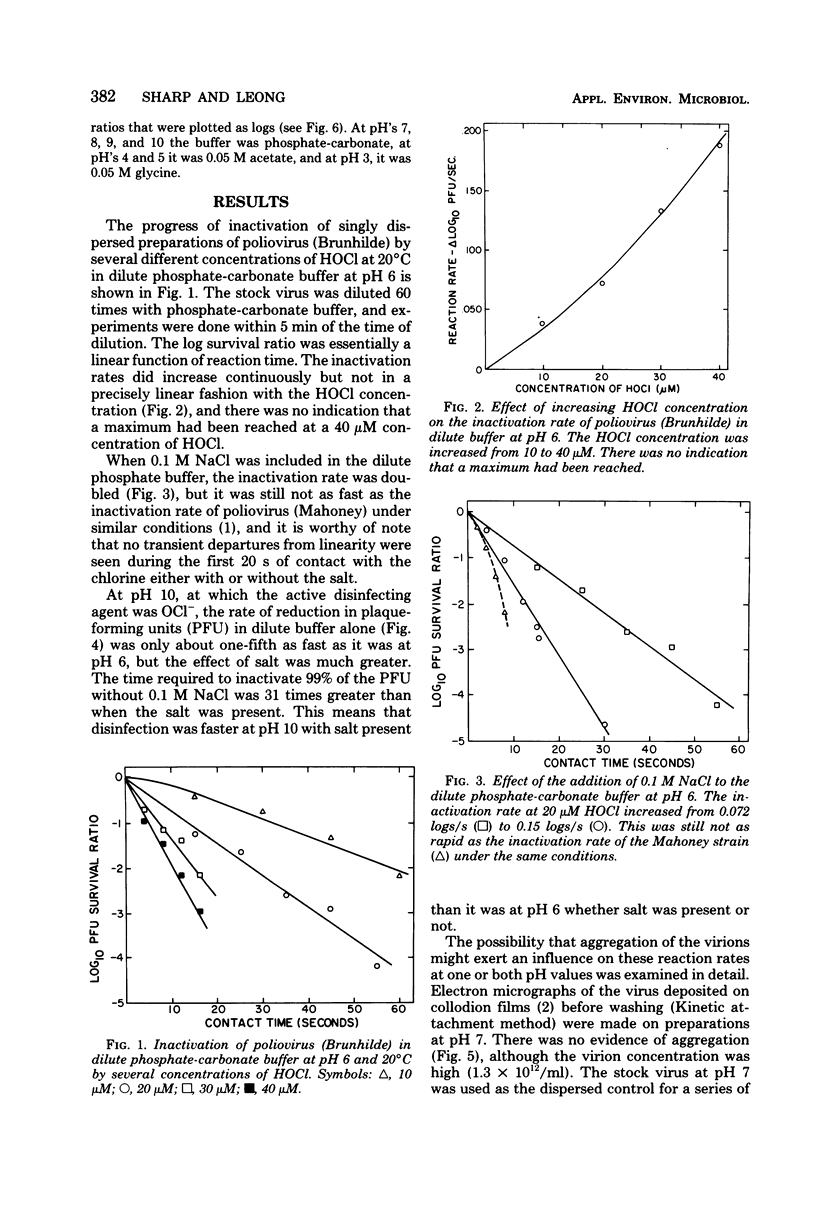
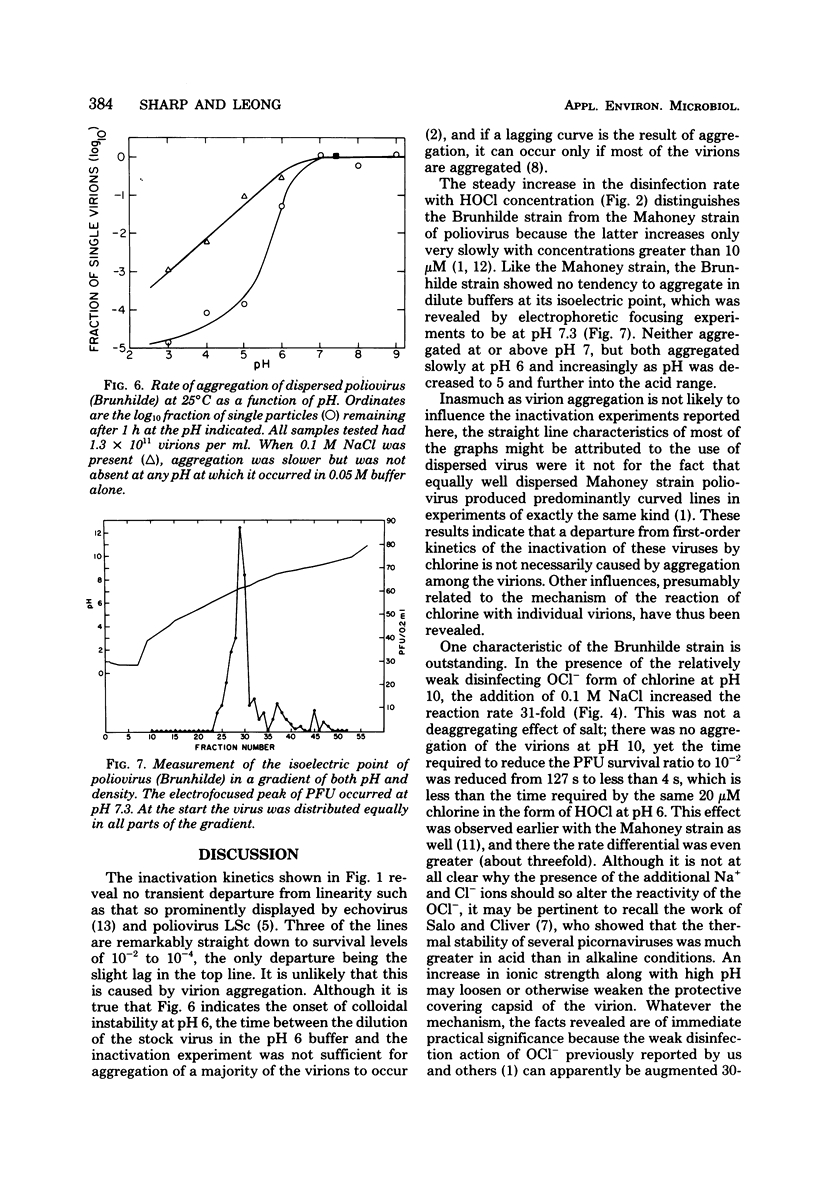
Like the Mahoney strain, the Brunhilde strain of poliovirus aggregated slowly in dilute phosphate-carbonate buffer at pH 6 but not at all at or above pH 7. Infectivity decreased at rates approximately proportional to the concentration of free chlorine present at pH 6 over the entire range of 5 to 40 micrometer. The addition of 0.1 M NaCl to the buffer increased the rate about twofold, but this strain was still twice as resistant as the Mahoney strain. At pH 10, inactivation was much slower than at pH 6, but when 0.1 M NaCl was added, the rate was increased 31-fold, making the OCl- at pH 10 over three times more effective than HOCl at pH 6.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Floyd R., Sharp D. G. Viral aggregation: effects of salts on the aggregation of poliovirus and reovirus at low pH. Appl Environ Microbiol. 1978 Jun;35(6):1084–1094. doi: 10.1128/aem.35.6.1084-1094.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Viral aggregation: quantitation and kinetics of the aggregation of poliovirus and reovirus. Appl Environ Microbiol. 1978 Jun;35(6):1079–1083. doi: 10.1128/aem.35.6.1079-1083.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka R. S., Ackermann W. W. Evidence for conformational states of poliovirions: effects of cations on reactivity of poliovirions to guanidine. Proc Soc Exp Biol Med. 1975 Apr;148(4):1070–1074. doi: 10.3181/00379727-148-38690. [DOI] [PubMed] [Google Scholar]
- Mandel B. Characterization of type 1 poliovirus by electrophoretic analysis. Virology. 1971 Jun;44(3):554–568. doi: 10.1016/0042-6822(71)90369-2. [DOI] [PubMed] [Google Scholar]
- Salo R. J., Cliver D. O. Effect of acid pH, salts, and temperature on the infectivity and physical integrity of enteroviruses. Arch Virol. 1976;52(4):269–282. doi: 10.1007/BF01315616. [DOI] [PubMed] [Google Scholar]
- Sharp D. G., Floyd R., Johnson J. D. Initial fast reaction of bromine on reovirus in turbulent flowing water. Appl Environ Microbiol. 1976 Feb;31(2):173–181. doi: 10.1128/aem.31.2.173-181.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. G., Floyd R., Johnson J. D. Nature of the surviving plaque-forming unit of reovirus in water containing bromine. Appl Microbiol. 1975 Jan;29(1):94–101. doi: 10.1128/am.29.1.94-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. G., Young D. C., Floyd R., Johnson J. D. Effect of ionic environment on the inactivation of poliovirus in water by chlorine. Appl Environ Microbiol. 1980 Mar;39(3):530–534. doi: 10.1128/aem.39.3.530-534.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDENKOPF S. J. Inactivation of type 1, poliomyelitis virus with chlorine. Virology. 1958 Feb;5(1):56–67. doi: 10.1016/0042-6822(58)90005-9. [DOI] [PubMed] [Google Scholar]
- Young D. C., Johnson J. D., Sharp D. G. The complex reaction kinetics of ECHO-1 virus with chlorine in water. Proc Soc Exp Biol Med. 1977 Dec;156(3):496–499. doi: 10.3181/00379727-156-39965. [DOI] [PubMed] [Google Scholar]
- Young D. C., Sharp D. G. Partial reactivation of chlorine-treated echovirus. Appl Environ Microbiol. 1979 Apr;37(4):766–773. doi: 10.1128/aem.37.4.766-773.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]




