Abstract
Objective:
To identify and characterize treatment compliance profiles of glaucoma patients and evaluate the association with intraocular pressure (IOP).
Methods:
A computerized device (Travalert®) that recorded daily instillation times and eye-drop counts was given for 3 months. Patients were declared compliant when at least 2 drops were instilled per day. Compliance rates were calculated for weekdays and weekends, separately, over 8 consecutive weeks. A principal components analysis (PCA) was followed by an ascendant hierarchical classification (AHC) to identify compliance groups.
Results:
140 patients were recruited (mean age 65.5 years; 51.8% female) of whom 83.6% had primary open-angle glaucoma with mean IOP 23.9 mmHg before Travalert® use. 60.7% were treated with DuoTrav® (travoprost timolol fixed combination) and 39.3% with travoprost. The PCA identified two axes (compliance and treatment weeks). The AHC identified 3 compliance groups: ‘high’ (56.6%, approx. 80% compliance), ‘medium’ (21.2%, approx. 50% compliance), and ‘low’ (22.1%, approx. 20% compliance). Demographics and glaucoma parameters did not predict low compliance. Final mean IOP was 16.1 mmHg, but higher in the low compliance group (17.7 mmHg, P = 0.02).
Conclusions:
Compliance measurement by a medical device showed compliance rates <80% by 50% (approx.) of patients, significantly impacting IOP control. No demographic or glaucoma variable was associated with low compliance.
Keywords: glaucoma, compliance, efficacy, intraocular pressure control
Introduction
Glaucoma occurs in about 2% of the population aged more than 40 years.1–7 It is estimated that glaucoma affects more than 500,000 people in England and Wales alone, and more than 70 million worldwide.8
Topical treatments have been reported to delay onset and the worsening of glaucoma,9 but they must be instilled for life or until a curative treatment becomes available. When the intraocular pressure (IOP) is poorly controlled, sufferers may eventually notice a severe restriction of visual fields or even a loss of central vision.10 Though blindness from glaucoma is uncommon, it is responsible wholly or in part for 13% of patients on the blind register in England and Wales.11
It is also reported that patients with chronic medical conditions self-administer only 30%–70% of their prescribed medication, and about 50% discontinue treatment in the initial months of therapy.12 Glaucoma is no exception, as reported by similar (50%) rates of treatment compliance published in the ophthalmic literature.13–21
Friedman et al13 analyzed pharmacy claims data and patients’ clinical files and calculated a ‘medication possession ratio’ (MPR) to estimate adherence with topical glaucoma therapy. The ratio expressed the number of days when a patient possessed eye-drops as a proportion of the observation period. They reported mean MPR values from 0.63 to 0.68 that were independent of the visual field mean deviation. Within 6 months, half of all new patients had discontinued therapy. A French study analyzed self-declared treatment compliance and found that 68% of patients stated they were fully compliant, 16% did not follow the administration schedule, and only 6% admitted they had forgotten instillations on certain days. The principal reasons given for compliance failure were forgetfulness, time constraints, or a poor doctor relationship.17,22
Glaucoma surgery is, at least for some patients, a way to avoid compliance issues. Breusegem et al23 found that patients having had trabeculectomy added with topical ketorolac or fluorometholone had a significantly reduced need for additional postoperative IOP-lowering medication. Tube implant during glaucoma surgery24,25 has shown promising results requiring confirmatory results.
The effect of deficient compliance on IOP control and visual impairment has been reported by 2 authors. Konstas et al26 found that noncompliant patients, determined by 2 independent observers using a formal questionnaire, had higher IOP values (22.9 versus 18.5 mmHg) and more severe visual field loss (10.8 versus 7.0 dB, mean defect). Forsman et al,27 applying a retrospective analysis, described associations between the incidence of blindness and poor treatment compliance.
Various methods are used to measure treatment compliance, eg, patient questionnaires, physicians’ reports, and pharmacy claims data, with high possible bias on the relevance of the findings. However, more objective methods exist, such as Travalert® (Alcon Inc, Fort Worth, Texas, USA), an electronic device that accomplishes several objectives aimed at improving treatment adherence.28 The device reminds patients when to instill their glaucoma medication and gives physicians objective compliance data for treatment decisions. The dosing aid monitors compliance electronically and makes it possible to track a patient’s dosing history. Other devices exist to help patients by reminding them.29
Travalert® (Alcon, Inc., Fort Worth, TX, USA) is a computerized bottle holder30 that reminds patients to instill their drops, assists with administering drops, and records dosing times. It is used with travoprost (Travatan®, Alcon Inc, Fort Worth, Texas, USA) and the travoprost/timolol fixed combination (Duotrav®, Alcon Inc, Fort Worth, Texas, USA). The ophthalmologist programs the number and timing of instillations before giving Travalert® to the patient and recovers accurate dosing data at the patient’s next visit. During each control visit, Travalert® is connected to the ophthalmologist’s computer, and all instillation dates and times are listed. The number of instillations properly performed (correct time and quantity) are expressed as a compliance rate.
The aims of our study were (1) to assess the convenience of using Travalert®, (2) to measure patient compliance with Travalert®, (3) to evaluate associations between compliance and IOP control, and (4) to identify risk factors of poor compliance.
Materials and methods
The present survey was conducted according to French law. The protocol was reviewed and approved by the Comité consultatif sur le traitement de l’information en matière de recherche dans le domaine de la santé and the Commission nationale informatique et liberté.31–33
Selected ophthalmologists were those specialized in glaucoma treatment. Centers were required to keep accurate and accessible patient records and be able to devote the necessary resources prior to participating in this survey. The study was an ‘open label’ design in patients with glaucoma or ocular hypertension (OHT) who, after consenting, were required to meet the inclusion/exclusion criteria. Patients visited their ophthalmologist 2 times: when prescribing Travalert® and at the next control visit where compliance data were collected (ie, the interval between visits was not fixed by the protocol).
As this was an observational survey, the protocol did not specify how ophthalmologists should inform their patients, apart from telling them how to use Travalert®. Lastly, how doctors discussed the results with patients was not standardized. At the time of this study Travalert® was EC labeled and available to ophthalmologists and patients. Prescription drugs were reimbursed by the French sick funds, and physicians’ fees for time spent completing the study case report forms was approved by the Conseil National de l’Ordre des Médecins.
Patients were required to meet the following inclusion criteria: (1) a diagnosis of primary open-angle glaucoma (POAG) or treated OHT, (2) age 18 years or above, (3) Travalert® used for at least 4 weeks, (4) ability to read and understand French, (5) received information on the study’s purpose and gave consent prior to the study, and (6) medical records accessible and properly documented.
Conversely, patients with the following characteristics were excluded from the survey: (1) age below 18 years, (2) secondary glaucoma (congenital, inflammatory, neovascular, or following cataract surgery), (3) closed-angle glaucoma, (4) participation in a concomitant clinical trial or observational survey, (5) severe co-morbidities directly affecting compliance (eg, severe depression), (6) severe dry-eye (>5 instillations per day), (7) numerous non-glaucoma treatments judged by the ophthalmologist as likely to impact indirectly on glaucoma drug compliance, (8) medical records lacking relevant retrospective data, or (9) refusal to participate. To be close to usual practice, there was no exclusion criteria related to lack of efficacy and or local tolerance (eg, hyperemia).
The following information was collected: inclusion and exclusion findings, socio-demography, POAG/OHT history, current treatment, IOP and visual acuity (measured at diagnosis, and both before and after Travalert®). Data were transcribed from the patients’ records to the case report forms by the ophthalmologists.
The utility of Travalert® in glaucoma management was assessed by an 8 item, 6-point rating scale scored from ‘1’ (no help at all) to ‘6’ (very positive help) that assessed the following dimensions: (1) instillation help, (2) time reminder, (3) use of the instillation listing, (4) compliance index, (5) clinical interpretation, (6) discussion with the patient, (7) treatment decision, and (8) global evaluation (average of previous dimensions). These questions were formulated with glaucoma experts during a focus group meeting. The objective was to capture the information they would need before incorporating compliance measurements into their treatment decisions. The questions were answered by investigators. Case report forms were not anonymized so to enable patient-based quality control.
The statistical analysis was conducted with SAS, release 9.2 (SAS Institute, NC, USA). The average delay between the two visits yielded daily compliance data over an 8-week period. A patient was declared compliant on any given day when at least 2 drops (one in each eye) were instilled. The diurnal instillation pattern (morning or evening) was not taken into account. The compliance rate of each patient was calculated for the entire 8 weeks and for weekdays and weekends separately. We used an automatic classification algorithm to identify compliance groups that did not specify the number of groups, in preference to pre-defined rules based on prior knowledge. The reasons for the decision were as follows: (1) complexity of the daily compliance information structure, (2) scarcity of available prior information on compliant patients, and (3) need for homogenous patient groups (in terms of compliance profiles) to enhance the likelihood of detecting IOP differences. A principal components analysis (PCA)34 was followed by an ascendant hierarchical classification (AHC)35 to identify compliance groups. With this type of analysis, the number of groups is not identified a priori. A distance is computed between each patient, and the number of the group is identified according to a dendrogram. The algorithm minimizes within group variance and maximizes between group variance. Characteristics of compliance groups were compared using Chi-squared tests or ANOVA. All statistical tests were 2-sided with alpha fixed at 5%.
Results
The study population was comprised of 140 patients recruited by 17 physicians. Travalert® documentation was completed for 113 patients (80.7%), the main reason of not participating to the analysis being less than 8 weeks of compliance measurements. No major selection bias was observed for this subgroup, which was similar to the original population in terms of socio-demographics and clinical factors (POAG versus OHT, IOP, and co-morbidities). This manuscript reports findings in the population with documented compliance (n =113).
Sexes of eligible patients were balanced (females 51.8%), and the mean age was 65.5 ± 12.1 years. Most patients (64.0%) were retired and did not live alone (77.0%). The frequency of POAG (83.6%) was higher than ocular hypertension. Most patients were prescribed the travoprost/timolol combination Duotrav® (60.7%). The remainder received travoprost (39.3%), and treatments were generally used alone (77.1%). More females (70.8%) were treated with Duotrav® than males (50.7%, P = 0.015) and patients with POAG were more frequently prescribed the combination (P < 0.05). Mean time since glaucoma onset was 4.7 ± 5.5 years, but it was longer for patients treated with Duotrav® (5.6 ± 5.9 years) than for travoprost (3.6 ± 4.7 years, P = 0.06) although statistically not significant. At inclusion, 56.4% of patients exhibited at least one non-ocular comorbidity and 31.4% had at least one ocular comorbidity, the most frequent being unoperated cataract (14.3%) or retinal detachment (4.3%). Glaucoma treatment was mainly pharmacological prior to the use of Travalert®, with only 17.9% of patients receiving surgery and 10.0% having laser therapy. Lastly, no differences were found between the 3 groups of compliance on general co-morbidities (cardiovascular diseases, dementia, hepatic disease, renal disease, cancer, chronic pulmonary disease, autoimmune disease, neurologic disease, gastrointestinal disease, metabolic diseases, other diseases) (Table 1).
Table 1.
Socio-demographics and medical parameters according to compliance group
| Low n = 25 | Medium n = 24 | High n = 64 | P-valuea | |
|---|---|---|---|---|
| Gender: | ||||
| Male | 16 (64.0%) | 13 (54.2%) | 29 (46.0%) | 0.30 |
| Female | 9 (36.0%) | 11 (15.8%) | 35 (54.0%) | |
| Age | 67.4 (14.8) | 68.8 (8.8) | 65.3 (11.9) | 0.44 |
| POAG | 19 (76.0%) | 22 (91.7%) | 57 (89.1%) | 0.22 |
| Normal pressure glaucoma | 3 (12.0%) | 1 (4.2%) | 1 (1.6%) | 0.07 |
| Retinal detachment | 2 (8.0%) | 1 (4.2%) | 3 (4.7%) | 0.85 |
| Not operated cataract | 3 (12.0%) | 2 (8.3%) | 12 (18.8%) | 0.49 |
| Diabetic retinopathy | 1 (4.0%) | 1 (4.2%) | 0 (0%) | 0.19 |
| Uveitis | 0 (0%) | 1 (4.2%) | 0 (0%) | 0.21 |
| IOP at diagnosis (mmHg) | 25.0 (6.1) | 23.6 (4.2) | 24.0 (6.3) | 0.73 |
| Time since glaucoma diagnosis (years) | 4.0 (7.4) | 3.6 (3.4) | 5.4 (5.8) | 0.47 |
| Previous surgery | 6 (24.0%) | 3 (12.5%) | 12 (18.8%) | 0.61 |
| Previous laser | 3 (12.0%) | 3 (12.5%) | 6 (9.4%) | 0.77 |
| Monotherapy treatment | 18 (72.0%) | 21 (87.5%) | 46 (71.9%) | 0.32 |
Note:
Comparison of the 3 compliance groups.
Abbreviations: POAG, primary open-angle glaucoma; IOP, intraocular pressure.
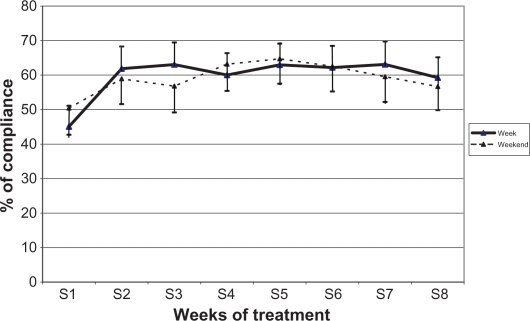
Compliance data were recovered from the Travalert® device, which recorded each instillation including times and number of drops. When entire weeks were combined, mean compliance was 60.0% (±25.9%) with little difference between weekdays (58.9%) and weekends (60.4%). Also, compliance was virtually stable over time, apart from the first week where it fluctuated from 60.3% to 63.6% during weekdays, and from 56.7% to 63.3% during the weekend (Figure 1).
Figure 1.
Average compliance rates over the study period (IC 95%). N = 113 at all time points.
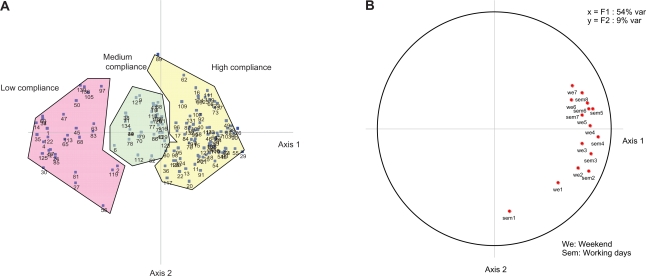
Compliance information was explored by the PCA and yielded 2 main axes. The first axis explained 53% of the variance and the second 9%. Eigen values of other axes (measuring axis variance contributing to total variance) reached less than unity and were ignored (ie, their inclusion did not add information). Figure 2b depicts the resulting factorial structure. Axis 1 depicts compliance findings and shows better compliance in later treatment weeks. Axis 2 depicts temporal findings and shows lower compliance in earlier weeks. Figure 2a plots individual patients within the factorial structure plan, according to the 3 compliance groups identified by the subsequent AHC analysis, and shows that the groups were ordered consistently along Axis 1. When all 8 weeks were merged, treatment compliance days amounted to 22.1% for the low compliance group, 51.1% for the medium compliance group, and 78.1% for the high compliance group.
Figure 2.
Principal component analysis results. A) Principal component analysis patients plotted after HCA. B) Principal component analysis factorial structure.
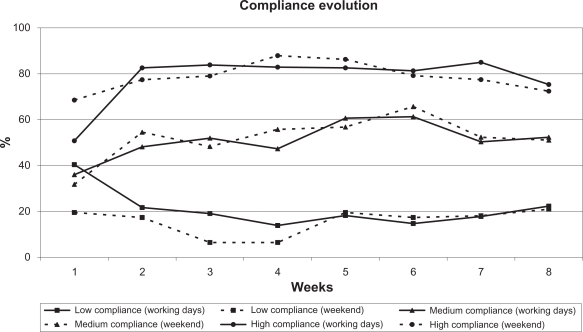
Percent compliance identified by Travalert® during 8 weeks of treatment is shown in Figure 3 according to compliance group. The low compliance group featured 2 characteristics: (1) decreasing compliance during the first two weeks, and (2) very low compliance on weekends.
Figure 3.
Percent compliance rates over 8 weeks of treatment, during weekdays and weekends, according to compliance group. N = 113 at all time points.
Table 2 shows mean maximum IOP values measured at the final visit for the compliance groups in Figure 2b, and shows no statistically significant IOP difference between the 3 groups. However, on comparing the low compliance group with the combined medium and high compliance groups, maximum IOP values were higher in low compliance patients (P = 0.02). This difference persisted after adjusting for IOP at diagnosis.
Table 2.
Maximum IOP of each compliance group at the last visit
| Cluster 1: Low compliance n = 25 | Cluster 2: Medium compliance n = 24 | Cluster 3: High compliance n = 64 | P-value | |
|---|---|---|---|---|
| Max IOP (mmHg) | ||||
| Mean (sd) | 17.7 (5.3) | 15.8 (3.3) | 15.6 (3.3) | 0.069 |
| Median (Min–Max) | 17.0 (12–40) | 15.0 (10–24) | 16.0 (9–25) | |
| Cluster 1: Low compliance n = 25 | Clusters 2 and 3: Medium and high compliance n = 88 | |||
| Max IOP (mmHg) | ||||
| Mean (sd) | 17.7 (5.3) | 15.7 (3.3) | 0.021 | |
| Median (Min–Max) | 17.0 (12–40) | 15.0 (9–25) | ||
Note: Max IOP: highest IOP eye measurements.
Abbreviation: IOP, intraocular pressure.
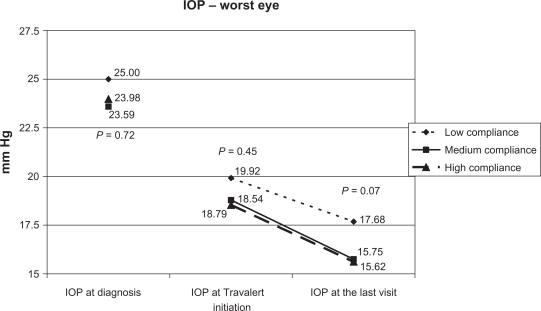
Figure 4 depicts mean IOP values in the worst affected eye at diagnosis, at Travelert® initiation, and on the last study visit for the 3 compliance groups. After 8 weeks of treatment, worst-eye IOP values were higher (P = 0.04) in the low compliance group.
Figure 4.
Mean IOP values in the worst affected eye at salient visits.
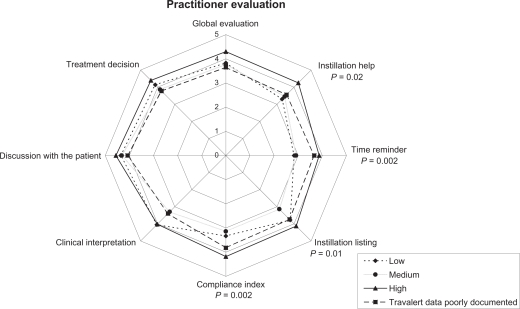
The utility of Travalert® for various aspects of glaucoma management was evaluated by the physicians (rating: 1 ‘none’ to 6 ‘high’) and analyzed for the 3 compliance groups (Figure 5). Physicians’ ratings of Travalert® differed between compliance groups. For example, the high compliance group was given a higher ‘global evaluation’ score than the other groups, accompanied by significantly higher scores for the ‘instillation help’, ‘time reminder’, and ‘compliance index’ dimensions. In other respects, ‘instillation listing’ and ‘compliance interpretation’ scores were similar for low and high compliance groups and significantly higher than that for the medium compliance group.
Figure 5.
Practitioner evaluations relative to patient compliance groups. P-value: comparison between compliance groups.
Patients (n = 27) providing insufficient data for clustering into any of the 3 compliance groups received scores close to the low compliance group, or even lower.
Discussion
The objectives of this study were to identify patient compliance profiles, risk factors for poor compliance, and consequences of poor compliance on IOP control. To do so, we used a computerized bottle holder (Travalert®) that reminded patients when to instill their eye-drops, assisted with the administration, and recorded dosing times of travoprost (Travatan®) and the travoprost/timolol fixed combination Duotrav®.
A total of 140 patients with POAG or OHT were included of which 113 (80.7%) were able to use the device and thereby report daily compliance figures over 8 successive weeks. This represented a high acceptance rate and a high proportion of patients able to monitor treatment daily.
Global treatment compliance during the 8 weeks was 60.0%, denoting conversely that 40% of the instillations were missed. These proportions agree with published reports of compliance from various countries.13,14–18,36,37 However, our number (n = 27) of low compliance patients is not big enough to give precise estimates on non compliance at a population level.
The fact that compliance improved during the first week indicates a possible learning effect with Travalert® use. No major differences were found between weekdays and weekends, except with low compliance patients during the first 4 weeks. The latter would suggest that compliance questions might well focus on weekends when poor IOP control is observed in early weeks of treatment.
As Travalert® provided an objective measurement of compliance, we did not need to rely on patients’ or ophthalmologists’ declarations. It is important to note here that although patients agreed to participate in the study and were aware that Travalert® recorded their instillations, some still did not take their medication. This could be because they disliked the device and so stopped using it. Unfortunately, patients’ satisfaction with Travalert® was not recorded. The reliability of the Travalert® has also been questioned:20,21 eye drops were reliably recorded by the device only after each full lever depression. Also, in case of more than 1 iterative depression, we counted only on instillation. Lastly, it is possible that the patient is squeezing the container and placing a drop into the sink or manipulating the monitoring device by pressing the lever even though they are not trying to place a drop in their eye. These are some limitations in terms of compliance measurements, since Travalert® cannot actually record the eye drop going onto the surface of the eye.
Clusters of patients’ compliance rates were identified by a PCA and further analyzed by an AHC. This objective approach did not depend on any a priori knowledge of what a compliance rate should be to control glaucoma progression. Hence, we created homogenous groups of compliance profiles and were able to extract relevant information (PCA) and minimize intra-group variance (AHC). The reliability of our classification was tested against measured IOP values. With this approach we identified 56.6% of patients showing high compliance and 22.1% low compliance.
This approach is different from other analyses where attempts were made to link last intake time with IOP control, the day of the visit. We were not able to identify a cluster of patients who took their instillation the days before the visit in the purpose to please their doctor, maybe because the size of this cluster was too small to be identified. Our poor compliant cluster patients were constantly missing their instillations; their lack of IOP control occurred every day and is therefore likely to be associated with disease progression.
Our 3 compliance groups were comparable on socio-demographic, eye and general co-morbidities, and glaucoma parameters. This suggests that compliance prediction might involve factors not considered as strictly medical. For example, Pappa et al38 recently reported that an immature, defensive personality style increased the risk for non-compliance with glaucoma treatments. We shall deal further with this matter in a later paper reporting our Eye-Drop Satisfaction Questionnaire results. Other factors such as disease knowledge, patient-to-clinician relationship, and treatment characteristics39,40 would be worth being explored when IOP is not controlled.
After 8 weeks of using Travalert®, IOP values decreased in all 3 compliance groups (low compliance: −2.28 mmHg; medium: −3.04 mmHg; high: −2.92 mmHg). The better IOP control after 8 weeks of Travalert® might be explained by either an observational bias or the instillation reminder of the medical device. It is also important to note that the gain in IOP after 8 weeks of Travalert® was lower in the low compliance group. However, the mean IOP of medium and high compliance groups was lower (−2.0 mmHg, P = 0.02) at the last visit than for low compliance patients. The difference was clinically relevant and supported the findings of Konstas et al.26 Nonetheless, it should be noted that all patients who used Travalert®, even those with low compliance, achieved an average IOP of 17 mmHg.
Lastly, though the difference did not reach statistical significance, IOP values of the low compliance group were higher at diagnosis than the values of other patients. This would support a behavioral explanation for poor compliance, eg, low compliance patients might possibly be invested less in healthcare and delayed their POAG/OHT diagnosis. This hypothesis is supported by the absence of correlation between IOP at diagnosis and IOP at the last visit, suggesting that our results are not confounded by glaucoma severity.
Practitioners rated global Travalert® satisfaction high. All individual dimensions rated above the midscale point (3.5). When collecting data, practitioners did not know the patients’ compliance groups, hence it is interesting that they rated satisfaction on 2 dimensions (‘instillation listing’ and ‘clinical interpretation’) similarly for the low and high compliance groups. This suggests that information collected by Travalert® was relevant to its IOP lowering effect.
Our study has several limitations. First, our sample was not recruited randomly and we applied inclusion and exclusion criteria, so extrapolation of compliance prevalence rates to the wider glaucoma population in France would be questionable. Second, consenting patients knew that their compliance could be evaluated, even though Travalert® was presented as an instrument to remind and help them instill eye-drops. Compliance records might have changed patient behavior resulting into overestimated compliance estimates. Third, our follow-up period was limited to 8 weeks, which was short for a life-long treatment. Fourth, we measured IOP only, omitting MD (mean deviation) and PSD (pattern standard deviation). Fifth, our patient sample was not large enough to identify more than 3 groups of compliance reliably, thus necessitating an average IOP difference >2 mmHg for statistical significance. Hence, our results need confirmation in a larger sample. Also, we were unable to address the effects of compliance on visual impairment. Sixth, about one quarter of the patients had an additional glaucoma treatment beside travaprost or DuoTrav and the compliance of the former was not followed up by Travalert. Seventh, our experimental design does not allow us to state that Travalert® is a device that has a utility at lowering IOP. A randomized clinical trial against a control group with several IOP measurements should be conducted to confirm this hypothesis.
Conclusions
Most patients were able to record their compliance accurately with Travalert®. The present survey illustrates that adherence to IOP-lowering treatment remains a problem. Poor compliance was associated with decreased IOP control. Lastly, factors predicting compliance were unrelated to socio-demographic and glaucoma parameters.
Acknowledgments
Acknowledgments and Disclosure
This study was supported by a grant from Alcon France SA, Rueil-Malmaison, France. The analysis was performed by Cemka Eval, Bourg-la-Reine, France. Dr Gilles Berdeaux is employed by Alcon France. Prof Nordmann, Prof Baudouin, Prof Renard, and Prof Denis participated in clinical trials sponsored by Alcon and are appointed as clinical experts. The results of this manuscript were presented at the International Society for Pharmaco-Economics and Outcome Research congress, Athens, November 2008.
References
- 1.Weih LM, Nanjan M, McCarty CA, Taylor HR. Prevalence and predictors of open-angle glaucoma: results from the visual impairment project. Ophthalmology. 2001;108:1966–1972. doi: 10.1016/s0161-6420(01)00799-0. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Smith W, Attebo K, et al. Prevalence of open-angle glaucoma in Australia. The Blue Mountains eye study. Ophthalmology. 1996;103:1661–1669. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 3.Klein BEK, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992;99:1499–1504. doi: 10.1016/s0161-6420(92)31774-9. [DOI] [PubMed] [Google Scholar]
- 4.Rudnicka AR, Mt-Isa S, Owen CG, et al. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci. 2006;47:4254–4261. doi: 10.1167/iovs.06-0299. [DOI] [PubMed] [Google Scholar]
- 5.Bron A, Baudouin C, Nordmann JP, et al. [Prevalence of intraocular hypertension and glaucoma in a nonselected French population] J Fr Ophtalmol. 2006;29:635–641. doi: 10.1016/s0181-5512(06)73824-4. French. [DOI] [PubMed] [Google Scholar]
- 6.Nizankowska MH, Kaczmarek R. Prevalence of glaucoma in the Wroclaw population. The Wroclaw Epidemiological Study. Ophthalmic Epidemiol. 2005;12:363–371. doi: 10.1080/09286580500212904. [DOI] [PubMed] [Google Scholar]
- 7.Antón A, Andrada MT, Mujica V, et al. Prevalence of primary open-angle glaucoma in a Spanish population: the Segovia Study. J Glaucoma. 2004;13:371–376. doi: 10.1097/01.ijg.0000133385.74502.29. [DOI] [PubMed] [Google Scholar]
- 8.Broman AT, Quigley HA. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:253–254. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 10.McKinnon SJ, Goldberg LD, Peeples P, et al. Current management of glaucoma and the need for complete therapy. Am J Manag Care. 2008;14(Suppl 1):S20–S27. [PubMed] [Google Scholar]
- 11.Bunce C, Wormald R. Leading causes of certification for blindness and partial sight in England and Wales. BMC Public Health. 2006;6:58. doi: 10.1186/1471-2458-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA. 2002;288:2880–2883. doi: 10.1001/jama.288.22.2880. [DOI] [PubMed] [Google Scholar]
- 13.Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS) Invest Ophthalmol Vis Sci. 2007;48:5052–5057. doi: 10.1167/iovs.07-0290. [DOI] [PubMed] [Google Scholar]
- 14.Quigley HA, Friedman DS, Hahn SR. Evaluation of practice patterns for the care of open-angle glaucoma compared with claims data: the Glaucoma Adherence and Persistency Study. Ophthalmology. 2007;114:1599–1606. doi: 10.1016/j.ophtha.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 15.Nordstrom BL, Friedman DS, Mozaffari E, et al. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140:598–606. doi: 10.1016/j.ajo.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 16.Bour T, Blanchard F, Segal A. [Therapeutic observance and life of patients with primary open-angle glaucoma. Apropos of 341 cases in the department of Marne] J Fr Ophtalmol. 1993;16:380–391. French. [PubMed] [Google Scholar]
- 17.Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg. 1995;26:233–236. [PubMed] [Google Scholar]
- 18.Wilensky J, Fiscella RG, Carlson AM, et al. Measurement of persistence and adherence to regimens of IOP-lowering glaucoma medications using pharmacy claims data. Am J Ophthalmol. 2006;141(Suppl 1):S28–S33. doi: 10.1016/j.ajo.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid study. Ophthalmology. 2009;116(2):191–199. doi: 10.1016/j.ophtha.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Cronin TH, Kahook MY, Lathrop KL, Noecker RJ. Accuracy and performance of a commercially available dosing aid. Br J Ophthalmol. 2007;91(4):497–499. doi: 10.1136/bjo.2006.103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman DS, Jampel HD, Congdon NG, Miller R, Quigley HA. The tarvatan dosing aid accurately records when drops are taken. Am J Ophthalmol. 2007;143(4):699–701. doi: 10.1016/j.ajo.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 22.Winfield AJ, Jessiman D, Williams A, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol. 1990;74:477–480. doi: 10.1136/bjo.74.8.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breusegem C, Spielberg L, Van Ginderdeuren R, et al. Preoperative nonsteroidal anti-inflammatory drug or steroid and outcomes after trabeculectomy a randomized controlled trial. Ophthalmology. Epub 2010 Apr 10. [DOI] [PubMed]
- 24.Freedman J. What is new after 40 years of glaucoma implants. J Glaucoma. Epub 2010 Feb 22. [DOI] [PubMed]
- 25.Papaconstantinou D, Georgalas I, Karmiris E, et al. Trabeculectomy with OloGen versus trabeculectomy for the treatment of glaucoma: a pilot study. Acta Ophthalmol. 2010;88(1):80–85. doi: 10.1111/j.1755-3768.2009.01753.x. [DOI] [PubMed] [Google Scholar]
- 26.Konstas AG, Maskaleris G, Gratsonidis S, Sardelli C. Compliance and viewpoint of glaucoma patients in Greece. Eye. 2000;14:752–756. doi: 10.1038/eye.2000.197. [DOI] [PubMed] [Google Scholar]
- 27.Forsman E, Kivelä T, Vesti E. Lifetime visual disability in open-angle glaucoma and ocular hypertension. J Glaucoma. 2007;16:313–319. doi: 10.1097/IJG.0b013e318033500f. [DOI] [PubMed] [Google Scholar]
- 28.Flowers B, Wand M, Piltz-Seymour J, et al. the Travatan Dosing Aid Study Group Patients’ and physicians’ perceptions of the travoprost dosing aid: an open-label, multicenter study of adherence with prostaglandin analogue therapy for open-angle glaucoma or ocular hypertension. Clin Ther. 2006;28:1803–1811. doi: 10.1016/j.clinthera.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Kass MA, Meltzer DW, Gordon M. A miniature compliance monitor for eyedrop medication. Arch Ophthalmol. 1984;102:1550–1554. doi: 10.1001/archopht.1984.01040031266033. [DOI] [PubMed] [Google Scholar]
- 30.TRAVALERT® Dosing Aid. http://www.alcon.com/en/professionals/product-advances.asp
- 31.Directive 2001/20/EC of the European parliament and of the council of 2001 Apr 4. http://eur-lex.europa.eu/smartapi/cgi/sga_doc?smartapi!celexapi!prod!CELEXnumdoc&lg=en&numdoc=32001L0020&model=guicheti
- 32.Comité consultatif sur le traitement de l’information en matière de recherche dans le domaine de la santé. http://www.enseignementsuprecherche.gouv.fr/cid20537/cctirs.html
- 33.La commission nationale de l’informatique et des libertés http://www.cnil.fr/
- 34.Lebart L, Morineau A, Warwick KM. Multivariate descriptive statistical analysis: correspondence analysis and related techniques for large matrices. New York: John Willey & Sons; 1984. [Google Scholar]
- 35.Anderberg MR. Cluster analysis for applications. New York: Academic Press; 1973. [Google Scholar]
- 36.Kholdebarin R, Campbell RJ, Jin YP, Buys YM. Multicenter study of compliance and drop administration in glaucoma. Can J Ophthalmol. 2008;43:454–461. doi: 10.1139/i08-076. [DOI] [PubMed] [Google Scholar]
- 37.Traverso CE, Walt JG, Stern LS, Dolgitser M. Pharmacotherapy compliance in patients with ocular hypertension or primary open-angle glaucoma. J Ocul Pharmacol Ther. 2009;25:77–82. doi: 10.1089/jop.2008.0079. [DOI] [PubMed] [Google Scholar]
- 38.Pappa C, Hyphantis T, Pappa S, et al. Psychiatric manifestations and personality traits associated with compliance with glaucoma treatment. J Psychosom Res. 2006;61:609–617. doi: 10.1016/j.jpsychores.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 39.Regnault A, Viala-Danten M, Gilet H, Berdeaux G. Scoring and psychometric properties of the Eye-Drop Satisfaction Questionnaire (EDSQ), an instrument to assess satisfaction and compliance with glaucoma treatment. BMC Ophthalmol. 2010;10:1. doi: 10.1186/1471-2415-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordmann JP, Denis P, Vigneux M, Trudeau E, Guillemin I, Berdeaux G. Development of the conceptual framework for the Eye-Drop Satisfaction Questionnaire (EDSQ) in glaucoma using a qualitative study. BMC Health Serv Res. 2007;7:124. doi: 10.1186/1472-6963-7-124. [DOI] [PMC free article] [PubMed] [Google Scholar]