Abstract
Cardiovascular disease (CVD) is more common in men and postmenopausal women than premenopausal women, suggesting vascular benefits of female sex hormones. Studies on the vasculature have identified estrogen receptors ERα, ERβ and a novel estrogen binding membrane protein GPR30, that mediate genomic and/or non-genomic effects. Estrogen promotes endothelium-dependent relaxation by inducing the production/activity of nitric oxide, prostacyclin, and hyperpolarizing factor, and inhibits the mechanisms of vascular smooth muscle contraction including [Ca2+]i, protein kinase C, Rho kinase and mitogen-activated protein kinase. Additional effects of estrogen on the cytoskeleton, matrix metalloproteinases and inflammatory factors contribute to vascular remodeling. However, the experimental evidence did not translate into vascular benefits of menopausal hormone therapy (MHT), and the HERS, HERS-II and WHI clinical trials demonstrated adverse cardiovascular events. The discrepancy has been partly related to delayed MHT and potential changes in the vascular ER amount, integrity, affinity, and downstream signaling pathways due to the subjects' age and preexisting CVD. The adverse vascular effects of MHT also highlighted the need of specific modulators of vascular sex hormone receptors. The effectiveness of MHT can be improved by delineating the differences in phramcokinetics and pharmacodynamics of natural, synthetic, and conjugated equine estrogens. Estriol, “hormone bioidenticals” and phytoestrogens are potential estradiol substitutes. The benefits of low dose MHT, and transdermal or vaginal estrogens over oral preparations are being evaluated. Specific ER modulators (SERMs) and ER agonists are being developed to maximize the effects on vascular ERs. Also, the effects of estrogen are being examined in the context of the whole body hormonal environment and the levels of progesterone and androgens. Thus, the experimental vascular benefits of estrogen can be translated to the outcome of MHT in postmenopausal CVD, as more specific modulators of sex hormone receptors become available and are used at the right dose, route of administration and timing, depending on the subject's age and preexisting cardiovascular condition.
Keywords: Estrogen, Progesterone, Testosterone, Endothelium, Vascular Smooth Muscle, Hypertension
INTRODUCTION
Sex differences in the incidence of cardiovascular disease (CVD) such as hypertension (HTN) and coronary heart disease (CHD) have been observed. CVD is more common in men than in women at 30 to 50yr of age. Also, the risk of CVD is greater in postmenopausal than premenopausal women, suggesting vascular benefits of estrogen and that the decline in its plasma level during menopause may contribute to the risk of CVD (Orshal et al. 2004). The cardiovascular benefits of estrogen have been supported by the identification of functional estrogen receptors (ERs) in the vasculature. ERs mediate long-term genomic effects that lead to gene transcription and expression of various proteins involved in vascular growth and remodeling. Estrogen also induces rapid non-genomic effects on endothelium-dependent vasodilation and on the mechanisms of vascular smooth muscle (VSM) contraction. Thus, the experimental evidence predicted vascular protective effects of female sex hormones in postmenopausal CVD (Dubey et al., 2004).
Menopausal hormone therapy (MHT) reduces hot flashes and mood swings and improves the social quality of life in menopausal women (Table 1). However, the usefulness of MHT in postmenopausal CVD is in dispute. Randomized clinical trials have evaluated the benefits of MHT in postmenopausal women (Vickers et al., 2007). The Heart and Estrogen/Progestin Replacement Study (HERS) and the Women's Health Initiative (WHI) trials highlighted the lack of benefit of combined estrogen plus progestin therapy for CVD (Table 2). Also, the WHI estrogen alone intervention phase was terminated early in 2004 due to increased risk of stroke and lack of effect of estrogen on the incidence of CVD in postmenopausal women with prior hysterectomy over 6.8 years (Table 3). The disappointing results of MHT in postmenopausal CVD have prompted investigations into the potential causes. Factors related to the subjects' age and preexisting cardiovascular condition have been implicated. Also, menopausal changes in vascular ERs have been suggested. That prompted the search for alternatives to conventional MHT and more specific modulators of vascular ER.
Table 1.
Representative Clinical Trials using MHT for Postmenopausal Hot Flashes
| Study | Objective | N | Age (yrs) |
Duration | Design | MHT Used | Outcome |
|---|---|---|---|---|---|---|---|
| Gordon et al., 1995 | To evaluate the efficacy and safety of a new, 7-day, transdermal E2 system in healthy postmenopausal women with hot flushes |
603 | 26 -73 | 11 weeks | Multicenter, randomized, double-blind, placebo- controlled |
Transdermal estradiol: 0.05, 0.1 mg/d CEE: 0.625 mg/d |
The seven-day, transdermal system effectively and safely treats post- menopausal vasomotor symptoms. |
| Speroff et al., 1996 | To determine the efficacy and local tolerance of a new matrix transdermal drug-delivery system that delivers 0.02 mg of E2 daily for 7 days for the relief of vasomotor symptoms |
324 | ≥ 50 naturally menopausal ≥ 35 surgically menopausal |
12 weeks | Randomized, double-blind, placebo- controlled |
Transdermal estradiol 0.02 mg /d |
This E2 transdermal system reduced vasomotor symptoms as early as the second week of therapy and was very well tolerated. The decrease in hot flush frequency was similar to that reported for oral and other transdermal estrogens, but at lower serum E2 concentrations. |
| Greendale et al., 1998 | To assess pair- wise differences between placebo, estrogen, and each of three estrogen-progestin regimens on selected symptoms. |
875 | 45-64 | 3 year | Randomized, placebo- controlled |
(CEE) + MPA 10 mg for 12 - 28 days OR MPA 2.5 mg daily, OR micronized progesterone 200 mg for 12 of 28 days |
Results confirm the usefulness of MHT for hot flashes, show that estrogen plus progestin causes breast discomfort, and demonstrate little influence of MHT on anxiety, cognition, or affective symptoms. |
| Notelovitz et al., 2000 | To compare the efficacy of different doses of E2 for relief of vasomotor symptoms in menopausal women. |
333 | 40-60 | 12 weeks | Randomized, double- masked, placebo- controlled |
0.25 mg, 0.5 mg, 1 mg, or 2 mg oral micronized 17b-E2, or placebo. |
Oral micronized E2 showed a dose response effect for reducing moderate and severe hot flushes in menopausal women. E2 1 mg appeared to be the most useful initial dose. |
Table 2.
The Heart and Estrogen/Progestin Replacement Studies (HERS) in Postmenopausal Women
| Study | Objective | N | Age (years) |
Duration | Design | MRT Used | Outcome |
|---|---|---|---|---|---|---|---|
| HERS Grady et al., 1998 | Whether HRT reduces CHD events in women with preexisting coronary disease |
2,763 | 44- 79, mean 66.7 |
4.1 years (1993 - 1998) |
Randomized, double- blinded |
Oral 0.625 mg CEE + 2.5 mg MPA/day or Placebo |
172 MI's and coronary deaths in HRT group, 176 in placebo; increase in events in first year; beneficial impact after 2 years |
| HERS-II Grady et al., 1992 | To determine if the risk reduction observed in the later years of HERS persisted and resulted in an overall reduced risk of CHD events with additional years of follow- up. |
2321 consented to follow -up |
mean 67 |
6.8 (2.7 years for HERS II) |
Randomized, blinded, placebo- controlled |
0.625 mg/d CEE and 2.5 mg of MPA or placebo during HERS; open- label hormone therapy was prescribed at personal physicians discretion during HERS II. |
Lower rates of CHD events among women in the hormone group in the final years of HERS did not persist during additional years of follow-up. After 6.8 years, hormone therapy did not reduce risk of cardiovascular events in women with CHD. |
| HERS - UA Simon et al., 2006 |
To examine the relation of MHT to serum uric acid (UA) levels and, in turn, the risk of CHD |
2763 | 44 – 79 |
4.1 years | Randomized, double- blind placebo- controlled |
CEE (0.625 mg/day) and MPA (2.5 mg/day) or placebo |
Treatment with Estrogen plus progestin lowered serum UA levels slightly, but neither baseline UA nor change in UA affected CHD risk. |
Table 3.
The Women's Health Initiative (WHI) Clinical Trial in Postmenopausal Women
| Study | Objective | N | Age (yrs) |
Duration | Design | MHT Used | Outcome |
|---|---|---|---|---|---|---|---|
| WHI Hsia, et al., 2006 | To examine the hypothesis that postmenopausal hormone therapy prevents CHD and assess the overall balance of risks and benefits |
10,739 | 50 - 79 (63.6) |
6.8 years |
Randomized placebo controlled |
CEE (0.625 mg/day) |
CEE provided no overall protection against myocardial infarction or coronary death in generally healthy postmenopausal women during a 7-year period of use. There was a suggestion of lower CHD risk with CEE among women 50 to 59 years of age at baseline. |
| WHI estrogen alone study Hendrix et al., 2006 |
To analyze subgroups, further elucidate the primary findings, and explore the relation between the results of this trial and the previously reported companion trial of CEE+MPA. |
10,739 | 50 to 79, mean 63.6 |
7.1 years |
Multicenter, Randomized, double- blind placebo- controlled |
CEE (0.625 mg/day) or placebo |
CEE provided no overall protection against myocardial infarction or coronary death in generally healthy postmenopausal women. |
| WHI Rossouw, 2002 | To assess the major health benefits and risks of the most commonly used combined hormone preparation in the United States |
16,608 | 50-79 | 5 years |
Multicenter, randomized, placebo- controlled |
CEE 0.625 mg/d, plus medroxyprogesterone acetate, 2.5 mg/d or placebo |
Overall health risks exceeded benefits from use of combined estrogen plus progestin for an average 5.2-year follow-up among healthy postmenopausal US women |
| WHI and WHI- CACS Manson et al., 2007 |
To examine the relationship between estrogen therapy and coronary-artery calcium |
1064 | 50- 59 |
7.4 years |
Randomized, double blind, placebo- controlled |
CEE (0.625 mg/day) or placebo |
Among women 50 to 59 years old at enrollment, the calcified-plaque burden in the coronary arteries after trial completion was lower in women assigned to estrogen than in those assigned to placebo. |
The purpose of this review is to provide an insight into the reasons why the beneficial vascular effects of estrogen observed in experimental studies did not translate into vascular benefits of MHT in clinical trials. We will first describe the current understanding of the interaction between estrogen, the vascular ER, and the post-receptor signaling mechanisms in the endothelium, VSM, and extracellular matrix (ECM). We will then discuss the discrepant effects of estrogen in clinical trials and the potential causes of MHT failure in CVD. The review will highlight how research into specific modulators of the vascular sex hormone receptors could help to maximize the effects of MHT in postmenopausal CVD. The effects of estrogen as a part of the whole body hormonal environment including progesterone and androgens will be considered. Throughout the review, we will discuss studies in human followed by those in experimental animals, and in large vessels followed by small vessels and vascular cells.
Estrogen Secretion and Metabolism
Estrogen is a major steroid sex hormone that primarily regulates the growth, development, and function of the female reproductive system. There is also substantial evidence for an effect of estrogen on the cardiovascular system. Understanding the pathways involved in the secretion and metabolism of estrogens would help determine the changes in estrogenic activity in adult and menopausal women.
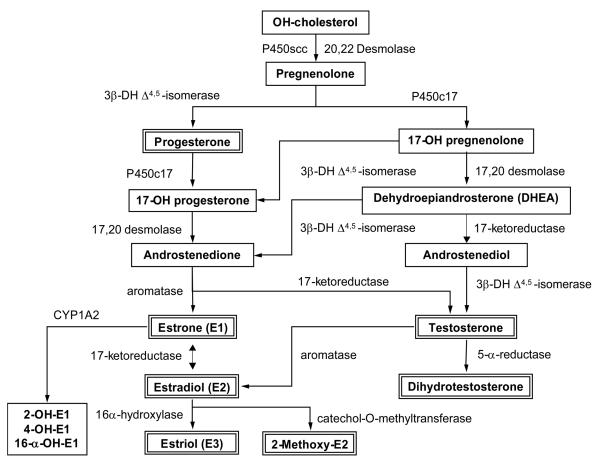
The major natural estrogens produced in women are estradiol (E2), estrone (E1) and estriol (E3). E2 is synthesized in the ovary and mainly circulates bound to sex hormone binding globulin, with less affinity to albumin. In normally cycling women, the ovarian follicle secretes 70 to 500 μg E2 per day, producing plasma estrogen levels of 210 pmol/L (58 pg/mL) in early follicular, 720 pmol/L (196 pg/mL) in late follicular and 490 pmol/L (129 pg/mL) in late luteal phase. Estrogens exist in a dynamic equilibrium of metabolic inter-conversions (Fig. 1). E2 half-life is ~3 hr and much of it is converted into E1 and E3. E2 removal is also accomplished by irreversible oxidation to hydroxy metabolites. Cytochrome p450 oxidative enzymes insert hydroxyl groups at the 2-, 4- or 16- position of the E1 and E2 molecule. The poly-OH derivatives are then conjugated with glucuronate or sulfate, or undergo methylation prior to excretion in urine. Estrogen also undergoes enterohepatic recirculation involving sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates in the intestine, followed by reabsorption. The OH metabolites of E1 and E2 have distinct ring position of the OH group and possess different estrogenic activity. The 2-OH-E1 metabolite has weak estrogenic activity, whereas the 4-OH-E1 and 16α-OH-E1 metabolites have strong estrogenic activity. The 2-OH-E2 inhibits VSM cell proliferation in vitro, while 16α-OH-E2 does not have similar inhibitory effects. In menopausal women, ovarian production of E2 is markedly diminished and the plasma estrogen level is reduced to <100 pmol/L (0 to 60 pg/mL), mostly in the form of E1. These estrogen levels are almost as low as those observed in males (15 to 25 pg/mL). The concentrations of 2-OH-E1 and 16α-OH-E1 in urine reflect the relative activity of the 2- and 16α-hydroxylation pathways. The ratio of urinary 2-OH-E2 to 16α-OH-E1 is a significant predictor of systolic blood pressure (BP) in menopausal women and may reflect the effects of 2-OH-E2, a potent inhibitor of VSM cell proliferation (Masi et al., 2006).
Fig.1.
Sex Steroid Biosynthesis. Biosynthesis of steroid hormones starts with conversion of cholesterol to pregnenolone through the cleavage of the cholesterol side chain by P450scc (Cytochrome P450, family 11, subfamily A, polypeptide 1). Pregnenolone is converted to progesterone though the 3β-DH delta4,5 isomerase pathway, or testosterone through the P450c17 pathway. Progesterone is converted to androstenedione through the 17,20 desmolase (P450c17) pathway. Androstenedione is then converted to E1 by aromatase in the extragonadal tissue, a pathway that increases with aging. Primary synthesis of E2 comes from a reversible reaction through the 17-ketoreductase pathway from E1. A secondary source of E2 comes from aromatization of testosterone in peripheral adipose tissue. E2 can be converted to E3 through hydroxylation by 16-α hydroxylase or to the highly active 2-methoxy-E2 through catechol-O-methlytransferase. Testosterone can also be converted to the more active dihydrotestosterone through the 5-α-reductase pathway.
E1 and E3 are formed in the liver from E2 or in peripheral tissues (muscle, adipose tissue, breast, bone) from androstenedione and androgens (Fig. 1). E3 is also produced by the placenta during pregnancy. The androstenedione secreted by the adrenal cortex is converted in peripheral tissues by aromatase into E1. Both E1 and the sulfate conjugated E1 are the most abundant circulating estrogens in menopausal women (Notelovitz, 2006). E1 is metabolized and cleared from the body by hydroxylation to OH-E1 and subsequent conversion to methoxy-E1, which is excreted in urine (Mitrunen & Hirrvonen, 2003).
Vascular Estrogen Receptors
Estrogen-induced cellular signaling is mediated through two ERs, ERα (NR3A1) and ERβ (NR3A2), both belonging to the nuclear receptor family of transcription factors (Heldring et al., 2007). ERs have been identified in endothelium, VSM and adventitial cells of human and experimental animals (Mendelsohn, 2002; Zhu et al., 2002; Mendelsohn et al., 2005). ERs have distinct tissue distribution, with ERβ being found in a wider range of tissues than ERα, and being the predominant ER in VSM (Orshal et al., 2004).
ERα and ERβ share a common structure of six distinct functional domains designated A-F (Fig. 2). The highly conserved domain C represents the DNA-binding domain (DBD) and has 97% similarity of amino acid sequence between ERα and ERβ. The DBD is necessary for specific interaction of ER with its DNA recognition sequence, the estrogen response element (ERE). Domain E, the ligand-binding domain (LBD), directs the specific interaction of ER with estrogen. LBD consists of a three-layered, antiparallel α-helical sandwich in which a central core layer of three helices packed between two additional layers of helices forms the ligand-binding cavity. The LBD domain is conserved, with 55% similarity in amino acid sequence between ERα and ERβ (Pettersson & Gustafsson, 2001). Beyond the conserved C and E domains, regions with considerable variability in amino acid sequence include the amino terminal A/B domain, the carboxy terminal F domain, and the centrally located hinge region, domain D. The amino terminal A/B domain contains a constitutively active transactivation region, activation function 1 (AF-1), while the hormone inducible AF-2, is present in the LBD domain E. The AF-1 and AF-2 regions are variable between ERα and ERβ, but both are important in enhancing estrogen-responsive gene expression (Kumar et al., 1987; Tora et al., 1989; Danielian et al., 1992; Metzger et al., 1995; Brzozowski et al., 1997; Pettersson & Gustafsson, 2001; Koide et al., 2007). Several splicing variants of ERα and ERβ have also been described (Orshal et al. 2004). An additional G protein-coupled 7-transmembrane receptor termed “GPR30” is structurally unrelated to ERα or ERβ and binds E2 with high affinity (Fig. 2) (Revankar et al., 2005; Thomas et al., 2005; Haas et al., 2007). Estrogen may also have other vascular effects independent of ER activation.
Fig 2.
Estrogen Receptor Structure. Three estrogen receptors that have been identified: ER-α and ER-β, and GPR30. Classical ERα and ERβ are nuclear receptors that share a common structure with 6 functional domains. The A/B domain is the transactivation domain, containing the activation function −1 (AF-1) and is least conserved domain with 24% homology and accounts for the main difference in size between ERα and ERβ. The C domain is the DNA binding domain (DBD) containing a dimerization interface. The D domain is the hinge region containing a nuclear localization signal and connects the DBD to the ligand binding domain (LBD). The E domain is the LBD containing the AF-2 region, and a dimerization interface that functions with the one present in the C domain. The F domain is the C terminal of ER. GPR30 is a G protein coupled receptor (GPCR) that also binds estrogen. It has an extracellular N terminal, seven transmembrane ( TM ) alpha helices, 3 exo-loops involved in ligand binding, 3 or 4 cyto-loops involved in G protein subunit binding, and a C terminal linked to the membrane through lipid addition, and also involved in binding G protein subunits.
Post-Receptor Signaling Mechanisms and Vascular Effects
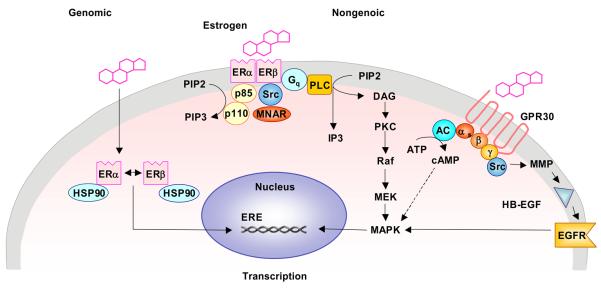
Estrogen induces both genomic and non-genomic effects in the vasculature (Gerhard et al., 1995; Farhat et al., 1996). Genomic effects of estrogen include increased gene transcription and cell growth and proliferation. Estrogen is a small lipophilic molecule that passively diffuses through the plasma membrane and form complexes with cytosolic ERs, which then localize in the nucleus (Fig. 3). In the absence of estrogen, the ERs exist as inactivated monomers bound with heat shock protein 90 (HSP90). Once estrogen binds to ER, the receptor undergoes conformational changes that result in dissociation of HSP90 and formation of a homo- or heterodimer with high affinity for estrogen and DNA (Pavao et al., 2001, Jacob et al., 2006) (Fig. 3). ER then binds to estrogen-responsive elements (EREs) residing in estrogen responsive genes, and recruits co-regulatory proteins to initiate changes in gene expression involved in cell growth, proliferation and differentiation (Brou et al., 1993; Robyr et al., 2000; Gougelet et al., 2007). ERs can also effect the transcription of genes by interacting with promoter bound proteins or through preventing the recruitment of other transcription factors (Jacob et al., 2006). Many of the estrogen-regulated genes also encode transcription factors (O'Lone et al., 2007).
Fig. 3.
ER-activated postreceptor mechanisms. In the genomic pathway, estrogen binds to cytoplasmic ER in the LBD, leading to ER dimerization and localization to the nucleus where the complex interacts with EREs to increase gene transcription. E2 via ER interacts with the SH2 domain of Src and activate Modulator of Nongenomic Action of Estrogen Receptor (MNAR). They interact with p85, a regulatory subunit of the PI3 kinase (PI3K) and lead to activation of the PI3K/Akt pathway). ERs may also activate phospholipase C (PLC) and increase diacylglycerol (DAG) production which leads to activation of MAPK. MAPK translocates to the nucleus where it increases gene transcription. GPR30 also binds E2 leading to activation of matrix metalloproteinase (MMP) with subsequent release of pro-heparan-bound epidermal growth factor (ProHB-EGF) and activation of epidermal growth factor receptor (EGFR). GPR30 also activates adenylyl cyclase (AC) and increases the generation of intracellular cAMP, which inhibits MAPK, thus, acting by different pathways to balance Erk-1/-2 (MAPK) activity.
ER studies on the aorta of wild-type and knockout mice have suggested that ERα primarily mediates estrogen-induced increase in gene expression, while ERβ may be responsible for estrogen-mediated decrease in gene expression. Also, estrogen induced activation of ERβ may mediate down-regulation of mRNAs for nuclear-encoded subunits in each of the major complexes of the mitochondrial respiratory chain (O'Lone et al., 2007).
Kim and Levin (2006) have shown that plasma membrane ERs may form dimers that bind to and activate G-protein subunits. While most plasma membrane ERα and ERβ form homodimers in the presence of E2, a small portion of the ER pool forms ERα/ERβ heterodimers (Razandi et al., 2004). Plasma membrane ER dimers can mediate genomic and non-genomic effects of estrogen. Non-genomic effects are rapid responses that occur too quickly to be mediated by gene transcription, are independent of protein synthesis, and typically involve modulation of membrane bound and cytoplasmic regulatory proteins (Simoncini et al., 2004). Estrogen binds to signal-generating ER on the plasma membrane of vascular cells and induces rapid non-genomic endothelium-dependent and -independent signaling cascades and vascular effects (Khalil, 2005; Leung et al., 2007). Non-genomic effects of estrogen are also mediated by non-ER mechanisms.
In addition to the effects of estrogen via ERα and ERβ there is evidence of a G protein-coupled receptor (GPCR) termed GPR30 that could be involved in non-genomic signaling by E2. GPR30 is a seven transmembrane-spanning receptor (7TMR) that specifically binds E2 with high affinity and acts independently of ERα and ERβ to promote rapid estrogen actions (Revankar et al., 2005; Thomas, 2005). GPR30 transduces intracellular signals through interactions with heterotrimeric G proteins (Fig. 3). Estrogen binding to GPR30 results in the dissociation of Gα-GTPase from the heterotrimeric Gαβγ complex. Dissociated Gβγ-subunit activates membrane associated matrix metalloproteinases (MMPs), with subsequent transactivation of epidermal growth factor receptor (EGFR) through the cleavage and release of pro-heparan-bound epidermal growth factor (ProHB-EGF) from the cell surface and transient activation of mitogen-activated protein kinase (MAPK) (Prenzel et al., 1999; Filardo et al., 2002; Filardo et al., 2005). This is supported by reports that E2 induces the phosphorylation of p38 and p42/44 MAPK (ERK-1/2) as well as proliferation and migration of porcine aortic endothelial cells (Geraldes et al., 2002). Gα-GTPase acts on membrane-associated adenylyl cyclase, generating the second messenger cAMP which in turn inhibits ERK-1/2 activity via a cAMP-dependent protein kinase mechanism (Soeder et al., 1999). These findings demonstrate how estrogen may act to balance ERK-1/2 activity through a single GPCR via two distinct G protein-dependent signaling pathways that have opposing effects on the EGF receptor and MAPK pathway (Filardo et al., 2002).
Estrogen and the Endothelium
The vascular endothelium is involved in the regulation of many processes including vascular tone and angiogenesis. Endothelium dysfunction can lead to various forms of CVD including HTN, CHD, stroke, and atherosclerosis (Kublickiene, 2008). Endothelial cells such as human umbilical vein endothelial cells (HUVECs) express both ERα and ERβ, and estrogen modulates endothelial cell function in human, experimental animal models and cultured cells (Mendelsohn, 2000).
E2 potentiates endothelium-dependent flow-mediated vasodilatation in the brachial artery of postmenopausal women (Chen et al., 1999; Pare et al., 2002; Kublickiene, 2005). Also, there is an association between endothelial dysfunction and reduced endogenous production of estrogens after natural or surgical menopause or premature ovarian failure in women with or without CHD (Taddei et al., 1996; Mercuro et al., 1999; Sanada et al., 2003; Kalantaridou et al., 2004).
Gender related differences in endothelial function have also been observed in experimental animal models. In spontaneously hypertensive rats (SHR) endothelium-dependent vascular relaxation is greater in females than males (Kauser & Rubanyi, 1995). Endothelium-induced vasodilation is also influenced by ER subtype. Selective ERα agonists improve endothelial dysfunction in blood vessels of OVX female SHR (Widder et al., 2003). Also, E2-induced vascular relaxation and NO production are greater in mice expressing only functional ERα (Nilsson BO et al., 2000; Darblade et al., 2002). E2 administration in OVX female mice in vivo causes rapid non-genomic arterial dilation and increased outer wall diameter of elastic and muscular arteries, as a result of ER-mediated stimulation of NO production. Rapid activation of both MAPK/ERK and phosphatidylinositol 3-kinase (PI3K) activity was found in the E2-exposed arteries, and inhibiting either kinase prevented E2-induced vasodilatation. Kinase activation and vasodilator responses to E2 were absent in either ERα or ERβ knockout mice, implicating both ER subtypes in mediating this E2 action. These results indicate that E2 modulation of arterial tone through plasma membrane ER and rapid signaling could underlie many of the observed actions of estrogen in vivo (Guo et al., 2005).
In vitro studies have also shown protective effects of estrogen on the endothelium. In small arteries isolated from healthy postmenopausal women not receiving MHT, the morphology and function of the endothelium are impaired, and these impairments are improved upon treating the isolated vessels with E2 (Kublickiene et al., 2005). Also, E2 induces the phosphorylation and activation of MAPK, and proliferation of endothelial cells via cytosolic and nuclear ERs (Barchiesi et al., 2002,).
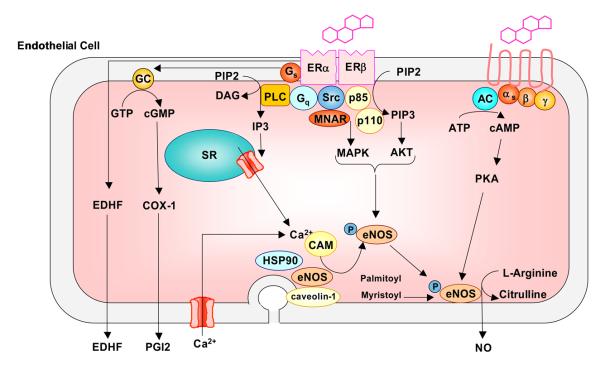
Some of the mechanisms of estrogen-induced endothelium-dependent vascular effects include modifications of the synthesis, release, and bioactivity of relaxing factors such as NO, PGI2 and endothelium-derived hyperpolarizing factor (EDHF) (Fig. 4), as well as contracting factors such as endothelin-1 (ET-1) and thromboxane A2 (TXA2) (Koledova & Khalil, 2007).
Fig. 4.
Effect of estrogen on endothelium-dependent pathways of vascular relaxation. E2 causes upregulation of COX-1 expression and PGI2 synthesis in endothelial cells and also increases endothelium derived hyperpolarizing factor (EDHF) production. Estrogen binds to endothelial surface membrane ER and activates phospholipase C (PLC), leading to the generation of inositol 1,4,5- triphosphate (IP3) and DAG. IP3 stimulates the release of stored Ca2+ from the endoplasmic reticulum, followed by influx of extracellular Ca2+. Ca2+ forms a complex with calmodulin (CAM), which causes initial activation of eNOS, its dissociation from caveolin-1, and its translocation to intracellular sites. Estrogen also activates phosphatidylinositol 3-kinase (PI3K), leading to transformation of phosphatidylinositol-4,5-bisphosphate (PIP2) into phosphatidylinositol 3,4,5-trisphosphate (PIP3), which activates Akt. ER-mediated activation of Akt or MAPK pathway causes phosphorylation of cytosolic eNOS and its second translocation back to the cell membrane where it undergoes myristoylation and palmitoylation to become fully activated. Fully activated eNOS promotes the transformation of L-arginine to L-citrulline and the production of NO, which diffuses through the endothelial cell and causes VSM relaxation.
Estrogen and NO
NO is a powerful vasodilator and relaxant of VSM. NO is produced from the transformation of L-arginine to L-citrulline by NO synthase (NOS). Three NOS isoforms have been described: NOS1 (neuronal nNOS) in neurons and innervated tissues such as smooth muscle, NOS2 (inducible iNOS) expressed ubiquitously, and NOS3 (endothelial eNOS) mainly in endothelium. iNOS is Ca2+ independent and may be involved in long-term regulation of vascular tone, whereas eNOS is Ca2+ dependent and plays a role in the short-term regulation of vascular tone (Nelson et al., 2000).
Estrogen mediated vasodilation has been attributed to increased eNOS transcription via genomic pathways as well as increased eNOS activity and NO production via non-genomic endothelial cell activation (Rahimian et. al., 2004). Studies in humans have shown a correlation between NO production and estrogen levels (Darkow et al., 1997; Meyer et al., 1997; Knot et al., 1999; Geary et al, 2000). Increased eNOS expression has been demonstrated in the uterine vasculature during estrogen treatment, in the follicular phase, and during pregnancy, suggesting that endothelial-derived NO is involved in the vasorelaxant actions of E2 (Ni et al., 1997, Magness et al, 1997; Nelson et al., 2000). Also, endothelial NO release is greater in arteries of females compared with males, and estrogen may mediate the gender differences in NO production (Kauser & Rubanyi, 1994; Wellman et al., 1996; Knot et al., 1999).
Gender differences in NO production have also been demonstrated in experimental animals. The inhibitory effect of the NOS inhibitor Nω−-Nitro-L-arginine methyl Ester (L-NAME) on acetylcholine (Ach)-induced relaxation is more pronounced in the mesenteric artery of female than male rats (Kähönen et al.,1998). Also, in isolated basilar arteries from OVX female rabbits, E2 treatment increases the response to NO in VSM cells, suggesting that estrogen plays a role in regulating vascular response to NO (Egami et al., 2005). Further evidence for estrogen involvement in NO production comes from a study using aortic rings isolated from wild type mice with trauma-induced hemorrhage. Aortic rings were either treated with E2, propylpyrazole triol (PPT, ER-α agonist), or diarylpropionitrile (DPN, ER-β agonist) or left untreated. Trauma hemorrhage increased ET-1 induced vasoconstriction, and treatment with E2 and DPN counteracted the vasoconstriction, while PPT had no effect (Ba et al., 2006). These data suggest that ERβ contributes to attenuation of E-1 mediated vasoconstriction, particularly in trauma hemorrhages, and that specific ERs may be involved in NO production.
Estrogen may also mediate NO effects that do not involve ER activation. In a study where NOS expression and vasorelaxant effects of E2 were measured in endothelium-denuded uterine arteries from nonpregnant and late pregnant rats, estrogen exerted direct acute vasorelaxant effects in smooth muscle that are mediated by mechanisms independent of ER activation (Scott et al., 2007). The effects of estrogen on NO production and vasodialation have also been observed in cultured endothelial cells, where estrogen has been shown to increase mRNA expression of eNOS (Rahimian et al., 2004).
Estrogen and Prostacyclin (PGI2)
The endothelial actions of estrogen may also involve the release of PGI2, a potent endogenous inhibitor of platelet aggregation and a strong vasodilator. PGI2 is a prostaglandin produced from free arachidonic acid through the catalytic activity of two cyclooxygenases, COX-1 and COX-2. Estrogen may modulate cross-talk between the NOS and COX pathways of vasodilation, and estrogen-induced increase in NO-mediated vasodilation may be associated with a decrease in the COX component (Case & Davison, 1999).
In postmenopausal women, the COX-2 pathway plays a specific role in the rapid E2-induced potentiation of cholinergic vasodilation (Calkin et al., 2002). Also, in OVX female monkeys with induced atherosclerosis, arteries treated with E2 show an increase in PGI2 production and the amount of PGI2 present is inversely correlated with plaque size, demonstrating that estrogens have a protective effect on the vasculature via inducing PGI2 synthesis (O'Sullivan et al., 2001). Estrogen also stimulates urinary excretion of COX-2-derived PGI2 metabolites in ERβ but not ERα deficient mice (Egan et. al., 2004), indicating that ERα may mediate the effects of E2 in PGI2 production.
Other studies have shown a different relation between COX and E2. COX inhibitors such as indomethacin inhibit a significant portion of endothelium-dependent vascular relaxation, and gender differences in indomethacin-sensitive vascular relaxation have been attributed to differences in COX products (Barber & Miller, 1997). Other studies have reported that indomethacin does not affect E2-induced relaxation in endothelium-intact coronary artery, suggesting that the release of vasodilator prostanoids may not be involved in E2-induced coronary relaxation (Jiang et. al., 1991). Still, other reports have added to the complexity of the estrogen-COX relationship. In a study on OVX female rats, administration of E2 was associated with upregulation of COX-2 in the uterus, whereas in the vena cava, COX-2 was down-regulated (Hertrampf et. al. 2005). Another study has shown that in the presence of E2 COX-2 was upregulated in human uterine microvascular endothelial cells, but not in human dermal microvascular endothelial cells (Tamura et. al., 2004). These findings indicate that COX modulation by E2 may be tissue specific.
Studies in cultured endothelial cells demonstrated a more positive relation between estrogen, COX and PGI2. E2 causes upregulation of COX-1 expression and PGI2 synthesis in endothelial cells (Orshal & Khalil, 2004). Also, E2 caused rapid ER mediated stimulation of PGI2 synthesis in ovine fetal pulmonary artery endothelial cells via a Ca2+-dependent, but MAPK-independent pathway (Sherman et al., 2002). Interestingly, PGI2 production by HUVECs is stimulated after exposure to serum from postmenopausal women treated with a mixture of phytoestrogens (Garcia-Martinez et al., 2003). Also, in cultured HUVECs treated with selective estrogen receptor modulators (SERMs), PGI2 synthesis is increased possibly by modification of activity or expression of COX-1 and COX-2 (Oviedo et al., 2005).
Estrogen and Endothelium-Derived Hyperpolarizing Factor (EDHF)
In some blood vessels treated with inhibitors of the NO and PGI2 pathways, the endothelium still releases EDHFs that cause hyperpolarization and relaxation of VSM (Vanhoutte, 2004). Estrogen-induced vascular relaxation could involve the release of EDHF. ER stimulation increases the production of EDHF, which activates K+ channels, causes hyperpolarization, and in turn inhibits Ca2+ influx via Ca2+ channels leading to VSM relaxation (Fig. 3) (Orshal & Khalil, 2004).
Ach-induced hyperpolarization and relaxation of mesenteric arteries are reduced in intact male and OVX female compared with intact female rats, and the differences in Ach responses are eliminated by K+ channel blockers. Also, the vascular hyperpolarizing and relaxation response to Ach is improved in E2-replaced OVX female rats, confirming that estrogen-deficient states attenuate vascular relaxation by EDHF (Orshal & Khalil, 2004; Nawate et al., 2005). In mesenteric arteries isolated from OVX female rats, E2 treatment increases the levels of EDHF (Burger et al., 2006). In addition, phytoestrogens induce vascular relaxation, particularly through production of EDHF (Woodman & Boujaoude, 2004). Thus, E2 and other estrogen alternatives may have a protective effect on the vasculature through EDHF-mediated vasodilation.
Estrogen and Endothelin (ET-1)
The endothelium releases other vascular modulators such as ET-1. ET-1 activates endothelial ETB1 receptors, which mediate the release of relaxing factors and cause vasodilation. ET-1 also stimulates ETA and ETB2 receptors in VSM and causes vasoconstriction. ET-1 induces greater contraction in mesenteric arteries of male deoxycorticosterone acetate (DOCA)-salt hypertensive rats than those of females. Ovariectomy in females is associated with increased ET-1 and possibly the ETB2 receptor mRNA in mesenteric arteries, and E2 replacement reverses these changes. Also, the ETB, agonist IRL-1620 induces smaller vasoconstriction in mesenteric arteries of intact compared with OVX females, and E2 replacement decreases IRL-1620-induced vasoconstriction in OVX females. These data suggest that estrogen attenuates ET-1/ETB receptor expression and their vascular responses in DOCA-salt hypertensive rats (David et al., 2001). E2 also modulates the expression and release of ET-1 in human endothelial cells (Bilsel et al., 2000). Prolonged E2 treatment of endothelial cells inhibits ET-1 production in response to serum, tumor necrosis factor-α, transforming growth factor β1, and AngII (Dubey et al., 2001). Also, ET-1 release is smaller in endothelial cells of female than those of male SHR.
Other endothelium-derived vasoactive factors include AngII and thromboxane. The Angiotensin Converting Enzyme (ACE) Insertion/Deletion(I/D) polymorphism appears to be involved in endothelial dysfunction in postmenopausal women, suggesting potential relation between estrogen users and the ACE-I/D polymorphism on endothelial function (Methot et al., 2006). Studies suggest that basal release of thromboxane from platelets is greater in raloxifene- compared to E2-treated OVX pigs. Raloxifene treatment, compared to E2, increases the production of contractile and proaggregatory prostanoids from venous endothelium and platelets. These differences, if found in humans, may contribute to the thrombotic risk with SERMs compared to natural estrogen (Lewis et al., 2006).
Estrogen and Mechanisms of VSM Contraction
Estrogen causes relaxation of endothelium-denuded vascular strips, suggesting direct effects of estrogen on VSM contraction mechanisms (Orshal & Khalil, 2004). VSM contraction is triggered by increases in [Ca2+]i due to Ca2+ release from the sarcoplasmic reticulum and Ca2+ entry from the extracellular space. Activation of protein kinases such as myosin light chain (MLC) kinase, Rho kinase, and MAPK as well as inhibition of MLC phosphatase have been suggested to contribute to VSM contraction (Horowitz et. al., 1996; Somlyo & Somlyo, 2000). PKC activation may increase the myofilament force sensitivity to [Ca2+]i and MLC phosphorylation, and thereby maintain vascular contraction (Fig. 5).
Fig. 5.
Effect of estrogen on the mechanisms of VSM contraction. An agonist (A) activates a specific receptor (R), stimulates membrane phospholipase (PLC), and increases the production of IP3 and diacylglycerol (DAG). IP3 stimulates Ca2+ release from the sarcoplasmic reticulum (SR). Also, the agonist stimulates Ca2+ entry through Ca+2 channels. Ca2+ binds CAM, activates myosin light chain (MLC) kinase, causes MLC phosphorylation, and initiates VSM contraction. DAG causes activation of PKC. PKC phosphorylates calponin (CaP) and/or activate a protein kinase cascade involving Raf, MAPK kinase (MEK), and MAPK, leading to phosphorylation of caldesmon (CaD) and an increase in the myofilament force sensitivity to Ca2+. Estrogen binds to plasma membrane ER, leading to inhibition of agonist-activated mechanisms of VSM contraction. Possible nongenomic effects of estrogen include activation of K+ channels, leading to membrane hyperpolarization, inhibition of Ca2+ entry through Ca2+ channels, and thereby inhibition of Ca2+-dependent MLC phosphorylation and VSM contraction. Estrogen may also inhibit PKC and/or the MAPK pathway through activation of plasma membrane ERs and thereby further inhibit VSM contraction. Estrogen-induced NO release from the endothelium activates guanylate cyclase in VSM leading to increased cGMP and stimulation of cGMP-dependent protein kinase (PKG). PKG decreases [Ca2+]i by stimulating Ca2+ extrusion pumps in the plasma membrane and Ca+2 uptake pumps in the sarcoplasmic reticulum and/or decrease the sensitivity of the contractile myofilaments to [Ca2+]i and thereby promote VSM relaxation. Estrogen also induces the release of PGI2 from the endothelium to activate the PGI2-cAMP pathway, or EDHF to activate Ca2+-activated K+ channels and to cause hyperpolarization and relaxation of VSM.
Estrogen and VSM [Ca2+]i
We have tested whether the sex differences in vascular tone are related to sex hormone-induced inhibition of the Ca2+ mobilization mechanisms of VSM contraction. In aortic segments isolated from intact and gonadectomized male and female Sprague-Dawley rats the contraction to the α-adrenergic agonist phenylephrine (Phe) is greater in vascular strips from intact male compared to female rats. There is no difference in Phe-induced contraction between intact and castrated males, suggesting that the enhanced vascular contraction may not be related to endogenous androgens. In contrast, Phe-induced contraction is greater in OVX compared with intact female rats, suggesting a role of estrogen in the reduced vascular response in females (Crews & Khalil, 1999).
In vascular strips incubated in Ca2+-free solution, Phe or caffeine produces a transient contraction due to Ca2+ release from the intracellular stores. The Phe- or caffeine-induced contraction in Ca2+-free solution is not different between intact and gonadectomized male and female rats, suggesting no sex differences in the intracellular Ca2+ release mechanism. Membrane depolarization by high KCl solution (96 mM) stimulates Ca2+ entry from the extracellular space through voltage-gated Ca2+ channels. KCl-induced contraction is reduced in vascular strips from intact female compared to male rats or OVX female rats. Also, both Phe and high KCl cause stimulation of 45Ca2+ influx that is reduced in intact female compared to male rats, suggesting that the reduced vasoconstriction in intact females is likely due to long-term effects of estrogen on the expression/permeability of voltage-gated Ca2+ channels (Crews & Khalil, 1999).
Although a significant component of estrogen-induced vascular relaxation involves the release of endothelium-derived relaxing factors, a considerable component is endothelium-independent. In endothelium-denuded porcine coronary artery strips, E2 (10−10 to 10−5 mol/L) causes concentration-dependent relaxation of prostaglandin F2α (PGF2α)-induced contraction. E2 does not affect caffeine-induced coronary contraction in Ca2+-free solution, suggesting that it does not affect Ca2+ release from the intracellular stores. On the other hand, E2 causes relaxation of high KCl-induced coronary artery contraction and inhibits PGF2α- and high KCl-induced Ca2+ influx, suggesting inhibitory effects of E2 on the Ca2+ entry mechanism of coronary VSM contraction (Crews & Khalil, 1999).
Because of the multiple effects of sex hormones on various vascular cells, measurement of contraction and [Ca2+]i in freshly isolated VSM cells avoids the contribution of other cell types to the overall vascular response. We have recently shown that PGF2α and high KCl cause 30 to 40% contraction of isolated coronary VSM cells. Sex hormones cause significant inhibition of VSM contraction in response to PGF2α and high KCl, with E2 being more potent than progesterone or testosterone. In fura-2 loaded coronary VSM cells incubated in a Ca2+-containing solution, PGF2α causes an initial, transient increase in [Ca2+]i (400 to 500 nM), mainly due to Ca2+ release from the intracellular stores, followed by maintained increase in [Ca2+]i (300 nM), mainly due to Ca2+ entry from the extracellular space. Topical application of sex hormones causes significant reduction in the maintained [Ca2+]i, with E2 being more effective than progesterone or testosterone (Murphy & Khalil, 1999).
In VSM cells incubated in Ca2+-free solution, both carbachol and caffeine cause a transient increase in [Ca2+]i to ~350 nM, and sex hormones do not affect this response, confirming that they do not affect the intracellular Ca2+ release mechanism. In contrast, high KCl causes maintained increase in [Ca2+]i (~400 nM), and E2 inhibits this response, confirming an effect on voltage-gated Ca2+ channels (Murphy & Khalil, 1999). These findings are supported by reports that E2 attenuates voltage-dependent Ca2+ current in A7r5 VSM cell line (Zhang et al., 1994).
If estrogen has protective vascular effects, one would predict that its inhibitory effects on the mechanisms of VSM contraction would be enhanced in CVD such as hypertension. We have examined the sex differences in VSM contraction and [Ca2+]i in aortic VSM cells isolated from intact and gonadectomized male and female WKY and SHR (Murphy & Khalil, 2000). In VSM cells of intact male WKY Phe (10−5 M) causes a transient increase in [Ca2+]i (~400 nM) followed by a maintained increase to ~200 nM. In intact male SHR, the Phe-induced maintained [Ca2+]i (~325 nM) is greater than that in WKY. The maintained [Ca2+]i is reduced in intact female compared to male WKY and SHR. Also, the reduction in female compared to male SHR (~38%) is greater compared to the respective reduction in female compared to male WKY (~25%). Similarly, the reduction in the high KCl-induced [Ca2+]i in female compared to male SHR (~31%) is greater than that observed in female compared with male WKY (~20%). These sex differences appear to be related to endogenous estrogen because they are eliminated in OVX female rats, and restored in E2-replaced OVX females. E2 also caused greater reduction of Phe- and high KCl-induced [Ca2+]i in VSM cells of OVX female SHR compared to WKY, suggesting enhanced vascular protective effects of E2 in animal models of genetic HTN (Murphy & Khalil, 2000).
Effect of Estrogen on Protein Kinase C (PKC)
PKC is a ubiquitous protein kinase that comprises a family of Ca2+-dependent and Ca2+-independent isoforms. PKC isoforms are expressed in different proportions in VSM of various vascular beds. During cell activation, PKC translocation to the cell surface may trigger a cascade of protein kinases that ultimately interact with the contractile myofilaments and cause VSM contraction (Salamanca et al., 2005).
The gender-related decrease in VSM contraction in female WKY rats compared with males is associated with reduction in the expression and activity of vascular α-, δ-, and ζ-PKC isoforms. Treatment of OVX females with E2 subcutaneous implants causes reduction in Phe and phorbol 12,13-dibutyrate (PDBu) contraction and PKC activity that is greater in SHR than in WKY. These findings suggest sex differences in VSM contraction and PKC activity that are possibly mediated by estrogen and are enhanced in the SHR model of genetic HTN (Kanashiro & Khalil, 2000).
Effect of Estrogen on Rho-Kinase
Rho Kinase is known to inhibit MLC phosphatase and to enhance the VSM myofilament sensitivity to [Ca2+]i. Rho kinase is upregulated in CVD and may play a role in the pathogenesis of coronary arteriosclerosis and vasospasm (Hiroki et al., 2004). Estrogen may inhibit Rho Kinase expression and activity. For instance, the expression of Rho-kinase may involve a PKC/NF-κB pathway that is inhibited by estrogen (Shimokawa & Takeshita, 2005). Also, the vasodilator response to the Rho kinase inhibitor Y-27632 is similar in OVX female and male rats, and E2 treatment of OVX rats normalizes the vasodilator effects of Y-27632 to those observed in intact females (Chrissobolis et al., 2004). In vivo studies have also shown that long-term inhibition of Rho Kinase causes regression of coronary arteriosclerosis (Hiroki et al., 2004). In cultured human coronary VSM cells, treatment with E2 causes a decrease in Rho Kinase mRNA expression, supporting that vascular Rho kinase's function is suppressed by estrogen (Hiroki et al., 2004).
Estrogen and the Extracellular Matrix (ECM)
The actin cytoskeleton forms the backbone of the cell, and its spatial organization is crucial for cell movement and migration. Modification of the form and positioning of actin fibers in relation to membrane anchoring structures such as integrins and focal adhesion complexes allow cell movement in the extracellular environment (Sheetz, 2001; Pollard et al., 2003). ERα interacts with the G protein Gα13 to induce activation of the RhoA/Rho-kinase pathway and phosphorylation of the actin-regulatory protein moesin, leading to remodeling of the actin cytoskeleton and endothelial cell migration (Simoncini et al., 2006).
Vascular remodeling occurs during all stages of atherosclerotic progression, and matrix metalloproteinases (MMPs), a family of zinc-binding proteolytic enzymes, are involved in these processes (Wingrove et al.,1998; Raffetto & Khalil, 2007). Increased MMP activity has been reported in numerous disease processes including tumor growth, arthritis and CVD. Also, MMP-induced ECM degradation within the atherosclerotic plaque may be involved in plaque instability and cardiovascular events. Studies suggest that changes in the levels of MMP-2, -9 and -10 in women receiving MHT may contribute to the potential risk of cardiovascular events and cancer (Lewandowski et al., 2006, Rodrigues et al., 2007).
Estrogen and Vascular Inflammation
Estrogen may influence the course of vascular disease via an effect on vascular inflammation. While vascular inflammation may partly account for the CVD risk in postmenopausal women, clinical trials demonstrate that MHT may impact the levels of soluble inflammatory markers (Miller et al., 2003).
Studies in experimental animals and vascular cells support an effect of estrogen on inflammatory factors. Tumor necrosis factor (TNF-α) is a proinflammatory cytokine involved in the pathology of vascular disorders (Bautista et al., 2005). Estrogen deficiency in OVX female rats is associated with increased serum levels of TNF-α and enhanced mesenteric artery sensitivity to Phe vasoconstriction (Arenas et al., 2005). Administration of TNF-α inhibitors or E2 replacement is associated with a decrease in Phe constriction of mesenteric arteries, and in the modulation of this vasoconstriction by the AT1R antagonist candesartan, as well as a reduction in the vascular expression of AngII, ACE, and AT1R. These findings suggest that up-regulation of TNF-α during estrogen deficiency may contribute to enhanced vascular constriction by altering the vascular AngII system (Arenas et al, 2006).
Estrogen may decrease the levels of inflammatory markers and thereby confer vasoprotective effects. In aortic segments from OVX mice, estrogen reduces expression of acute-phase protein genes and inhibits monocyte adhesion to endothelial cells (Gao et al., 2006). Also, estrogen inhibits secretion of IL-6 from Kupffer cells exposed to necrotic hepatocytes and reduces circulating levels of IL-6 in diethylnitrosamine-treated male mice (Prieto, 2008). In addition, in porcine aortic endothelial cells, E2 binding to ERα blocks the induction of CD40 and CD40L protein expression and prevents neutrophil adhesion to endothelial cells (Geraldes et al., 2006).
MHT in Clinical Trials
Although the experimental data provides evidence for beneficial vascular effects of estrogen, prospective MHT clinical trials do not support this concept (Table 4) (Hulley et al., 1998; Anderson et al., 2004). Also, while earlier observational studies have suggested vascular protective effects of MHT, these studies may have overestimated the vascular benefits of MHT due to selection biases and other limitations (Grodstein et al., 2003; Prentice et al., 2006). The causes of failure of MHT in postmenopausal CVD have been related to many factors including timing of MHT, preexisting CVD in the clinical trial participants, and the type, dose, and route of administration of MHT. Delineation of potential causes of MHT failure in CVD have suggested the adoption of different MHT strategies and prompted further research into specific modulators of vascular sex hormone receptors.
Table 4.
Other MHT clinical trials, and CVD in postmenopausal women
| Study | Objective | N | Age (yrs) |
Duration | Design | MRT Used | Outcome |
|---|---|---|---|---|---|---|---|
| ERA Herrington et al., 2000 |
To examine the effects of MHT on coronary atherosclerosis in women |
309 | mean 65.8 |
3.25 yeas | Three-arm, Randomized, double- blind, placebo- controlled |
0.625 mg CEE with or without 2.5 mg MPA/d |
Angiography detected no difference in disease progression despite a favorable effect on HDL (increase) and LDL(decrease). |
| RUTH Barrett-Connor et al., 2006 |
To determine the effect of raloxifene as compared with placebo on the incidence of coronary events and invasive breast cancer |
10,101 | mean age, 67.5 |
5.6 years. |
Multicenter, randomized, double- blind, placebo- controlled |
60 mg of raloxifene daily or placebo |
Raloxifene did not affect the risk of CVD. The benefits of raloxifene in reducing the risks of invasive breast cancer and vertebral fracture should be weighed against the risks of venous thromboembolism and fatal stroke. |
| ESTHER Canonico et al.,2007 |
To study the safety between the use of oral estrogen therapy and transdermal estrogen |
881 | 45 - 70 |
6 years | Multicenter, case-control study |
Current users of hormone therapy were classified according to both the route of estrogen administration and the type of progestogens. |
Oral but not transdermal estrogen is associated with an increased venous thromboembolism (VTE) risk. Norpregnane derivatives may be thrombogenic, whereas micronized progesterone and pregnane derivatives appear safe with respect to thrombotic risk. |
| WISDOM Vickers et al., 2007 |
To assess the long term risks and benefits of combined MHT vs. placebo, and oestrogen alone vs. combined MHT |
5692 | 50-69 | 10 years of treatment planned |
Multicenter, randomized, double blind, placebo- controlled |
CEE 0.625 mg/day or CEE plus MPA 2.5/5.0 mg/day |
MHT increases cardiovascular and thromboembolic risk when started many years after menopause. Results are consistent with the findings of WHI study and secondary prevention studies. |
Timing of MHT
An important cause of the discrepancy between the experimental evidence and the outcome of clinical trials may be the timing of MHT initiation in relation to the underlying state of the vasculature. It is possible that estrogen may be protective and not curative, and that degenerative changes in the vascular system associated with aging may not be reversible with MHT (Hurn et al., 2003; Ouyang et al., 2006). Consistent with this theory, clinical trials have shown no benefit of MHT in older postmenopausal women and some trials have suggested an increased risk of CHD during the first year of MHT (Hulley et al., 1998; Manson et al., 2003). The causes of failures may be related to age-related changes in the vascular ER number, affinity to estrogen, and postreceptor signaling mechanisms. Because the vascular expression or responsiveness of ERs may decrease with advanced age, MHT is expected to be more beneficial if started early in menopause rather than in older postmenopausal women.
MHT and Preexisting Cardiovascular Condition
The effects of MHT may be modulated by the subject's preexisting cardiovascular condition. Two major prospective clinical trials, HERS (Hulley et al., 1998) and WHI (Rossouw et al., 2002), have shown that MHT increased the risk of CVD in postmenopausal women (Table 2 & 3). The efficacy of MHT to protect against atherosclerosis seems dependent on the atherosclerotic state (Seli et al., 2007). A recent study on experimental animals has demonstrated that estrogen treatment increases apoptosis in VSM and is associated with decreased plaque formation in atherosclerosis. However, after the plaque is fully formed, the apoptotic effects of estrogen in VSM cells may contribute to plaque instability and lead to adverse cardiovascular events (Seli et al., 2007). Thus MHT administered early during perimenopause may decrease the development/progression of fibrous cap and atherogenic plaque. In contrast, in already established atherogenic plaques, estrogen may increase apoptosis, inflammation, MMP expression and neovascularization leading to lesion progression, plaque instability and rupture/hemorrhage. Also, studies in SHR have shown that E2 may protect against HTN-induced myocardial arterial remodeling in the early stages of HTN, but not when chronic alterations are established after prolonged HTN (Garcia et al., 2005). These observations may explain the reduced vascular benefits of MHT in postmenopausal women with preexisting CVD and further underscore the importance of timing of MHT (Koledova & Khalil, 2007).
ERs in CVD
Because ER subtypes have distinct vascular distribution and different binding affinity for estrogens, their vascular expression and activity may be modified in CVD. Studies in humans have demonstrated that ERβ expression is enhanced in the vascular wall of women with CVD, while ERα predominates in the control subjects, suggesting that selective targeting of ER subtypes may have an effect on the women's cardiovascular health (Cruz et al., 2008). Also, a recent study has examined the amount of ERα and ERβ in coronary arteries of premenopausal, postmenopausal with MHT, and non-MHT postmenopausal women. It was found that increased expression of ERβ is associated with advanced atherosclerosis and calcification regardless of age or hormone status (Christian et al., 2006).
Some animal studies suggest that ER subtypes work cooperatively to improve vascular health. In vivo kinase activation and arterial vasodilator responses to E2 are absent in either ERα or ERβ KO mice, indicating that both ER subtypes cooperate to impact vascular functions (Guo et al., 2005). Also, in aldosterone high-salt treated rats, both ERα and ERβ have protective effects against multiple types of CVD (Arias-Loza et al., 2007). However, other studies have suggested a role for specific ER subtypes in modulating the course of CVD. Apolipoprotein E KO mice (ApoE−/−) typically develop lipid-induced atherosclerosis. Studies in double KO mice deficient in ERα and apolipoprotein E (ApoE) indicate that ERα plays a major role in atherosclerosis reduction (Hodgin et al., 2001). When ApoE−/− mice are treated with E2, they exhibit 80% less lesions, and such benefit is abrogated in mice lacking both ERα and ApoE. Additional studies have shown that ERα is the main mediator of atherosclerosis mitigation, and the underlying mechanism includes ERα-mediated up-regulation of the athero-protective molecule PGI2 (Egan et al., 2004). Furthermore, in mice with genetic ERα deletion, the cardioprotective role of estrogen in ischemia/reperfusion injury is lost (Zhai et al., 2000; Wang et al., 2006). Thus, the vascular protective actions of estrogen including nongenomic vasodilation may be to a large extent mediated by ERα (Chen et al., 1999; Pare et al., 2002; Kublickiene et al., 2005). Contrasting studies show that E2 treatment reduces atherosclerotic progression equally in ApoE−/− mice and in ERα-deficient ApoE−/− mice, indicating that other ERs maybe involved in mediating the protective effects of estrogen. ERβ KO mice develop multiple functional abnormalities in VSM and blood vessels. Also, OVX ERβ KO mice demonstrate increased mortality, and increased clinical and biochemical markers of heart failure after experimental myocardial infarction (Pelzer et al., 2005). Also, in ERβ KO mice administration of estrogen produces vasoconstriction, which is opposite from the typical vasodilation seen in control mice when administered estrogen. Also, the ERβ KO mice develop extensive HTN as they age compared to normal mice (Zhu et al., 2002). In OVX SHR treated with ERβ specific agonists, the BP, vascular resistance and cardiac hypertrophy are reduced without promoting uterine growth. However, in OVX SHR treated with ERα selective agonists, endothelial dysfunction and cardiac hypertrophy are relieved, without lowering BP (Jazbutyte et al., 2008).
Thus, based on studies in human and animal models, ER subtypes seem to modulate vascular function in CVD in ways different from those observed in normal conditions. Further research into the role of ER subtypes in CVD could lead to improved MHT in postmenopausal women.
Type of MHT
The outcome of MHT in menopausal women could be affected by the type of estrogen used (Table 5). Natural estrogens are structurally similar to E2 produced in the ovaries, and are readily metabolized and excreted. Some of the commonly prescribed E2 are Estrace® and Gynodiol®. Synthetic estrogens are similar in structure to natural estrogens and could have measurable vascular effects, but may also have unwanted, adverse effects. Diethylstillbestrol, ethinyl estradiol (Levlen ®), estradiol benzoate (Celerin TM), cypionate and valerate are examples of synthetic estrogens. Mestranol is a produg that is demethylated in the body to ethinyl estradiol (Koledova et al., 2007).
Table 5.
Estrogen and estrogen-progestin products approved by US Food and Drug Administration (FDA) for postmenopausal women
| Product | Chemistry | Dosage |
|---|---|---|
|
ORAL Angeliq® Tablets |
estradiol - drospirenone | 1 mg estradiol - 0.5mg drospirenone |
| Enjuvia™ | Synthetic conjugated estrogens |
0.625 mg and 1.25 mg |
| Femhrt® | Norethindrone acetate/ ethinyl estradiol |
0.5 mg/2.5 microg 0.5 mg/5 microg |
| Prefest™ | Estradiol/norgestimate | 1.0 mg/d and 0.09mg/d |
| Premarin® | CEE | 0.3, 0.4, 0.625, 0.9 and 1.25 mg |
| Prempro™ | CEE/MPA | 0.3 mg/1.5 mg, 0.45mg/1.5 mg 0.625 mg/2.5 mg, 0.625 mg/5 mg |
|
TRANSDERMAL Alora® |
Estradiol | 0.025, 0.05, 0.075, 0.1 mg per day |
| Climara Pro™ | Estradiol/Levonorgestrel | 0.045 mg/d and 0.015 mg/d |
| CombiPatch® | Estradiol/norethindrone acetate (NETA) |
0.05 mg/d estradiol and 0.14 mg/d NETA 0.05 mg/d estradiol and 0.25 mg/d NETA |
| Estraderm® | Estradiol | 0.05 or 0.1 mg per day |
| Menostar® | Estradiol | 14 microg per 7days |
| Vivelle®, Vivelle-Dot® | Estradiol | 0.025, 0.0375, 0.05, 0.075, or 0.1mg |
|
TOPICAL EMULSION Estrasorb™ |
Estradiol | 0.05 mg/d |
|
INJECTION Delestrogen® |
Estradiol valerate | 10 mg every other week |
| Premarin® | CEE | 25 mg |
|
VAGINAL Femring® |
Estradiol acetate | 0.05 mg/d and 0.10 mg/d |
| Premarin® | CEE | 0.625 mg |
Conjugated equine estrogen (CEE) is a common form of MHT derived from urine of pregnant mares. CEE is a complex formulation containing multiple estrogens, including several estrogen forms not secreted by human ovary, as well as other biologically active steroids (Araújo et al., 2006). CEEs are metabolized in the body. Unconjugated estrogens (e.g. equilin) are absorbed more rapidly than conjugated estrogens (e.g. equilin sulfate), but they are soon conjugated in the liver (first-pass effect) and circulate together with E1-sulfate as a hormonally inert estrogen reservoir. Tissue enzymes such as sulfatases and 17β-hydroxysteroid dehydrogenases released from the bowel bacterial flora and the intestinal mucosa activate these estrogens to E1 and E2 (Notelovitz, 2006). These estrogens are excreted in the urine, along with glucronide and sulfate conjugates.
Studies have shown that CEE provided no overall coronary protection in women who had undergone prior hysterectomy, although there was a suggestion of lower CVD risk with CEE in women 50 to 59 years of age. Coronary risk with CEE appeared greater for women with more years since hysterectomy (Hsia et al., 2006; Hendrix et al., 2006). CEE and E2 have comparable effects on treatment of menopausal hot flashes and may have similar short term adverse effects (Nelson, 2004). However, CEE increases the risk of ischemic stroke in generally healthy postmenopausal women. The increased risk appeared to be present in all subgroups of women examined, including younger and more recently menopausal women.
Hormone “Bioidenticals” are being promoted for management of menopausal symptoms such as hot flashes and mood swings, but their vascular effects have not been thoroughly examined. Sterol analogs found in plants such as wild yam, are subjected to DNA engineering to form hormone that is bioidentical in chemical structure to the natural estriol (Koledova et al., 2007).
Phytoestrogens are steroidal molecules found in plants that have structural similarity with E2 and have estrogenic and/or antiestrogenic effects (Usui, 2006; Koledova et al., 2007). Phytoestrogens include isoflavones, coumestans, lignans, resveratrol, zearalenone, α-zearalanol, biochanin A and formononetin, which occur in either plants or their seeds (Usui, 2006; Koledova et al., 2007) (Table 6). Isoflavones have beneficial effects on C-reactive protein (CRP) levels, but not on other inflammatory biomarkers of CVD risk in postmenopausal women, and may improve vascular cell adhesion (VCAM-1) in ERβ gene polymorphic subgroup (Hall et al., 2006).
Table 6.
Modulators of Estrogen Receptors
| Agonists | Antagonists | SERMS | Phytoestrogens |
|---|---|---|---|
| Estradiol (natural) Estriol (natural) Estrone (natural) Chlorotrianisene Dienestrol Diethylstilbestrol Ethinylestradiol (ERα) Fosfestrol Mestranol Polyestradiol phosphate Quinestrol Propylpyrazole trispherol (PPT) 400x ERα Diarylpropionitrile (DPN) 30-70x ERβ |
MPP (ERα) RR-tetrahydrochrysene (ERβ) |
Tamoxifen 4-Hidroxytamoxifene Raloxifene Idoxifene Bazedoxifene Clomifene Toremifene Fulvestrant |
Genistein (ERβ) Biochanin A (ERβ) Coumestrol Daidzein Naringenin Taxifolin Zearalenone α-zearalanol |
In humans, the consumption of red wine reduces the incidence of mortality from CHD, the cardioprotective effect has been attributed to the polyphenol fraction of red wine (Das et al., 1999; Ruf, 1999). A key polyphenol in red wine is resveratrol, trans-3,5,4_-trihydroxystilbene, found in grape skin, which activates MAPK signaling through ERα and ERβ in endothelial cells (Klinge et al., 2005).
Genistein, a natural bioactive compound derived from legumes, has drawn wide attention because of its potential benefits in some human degenerative diseases. It has a weak estrogenic effect and is a non-specific tyrosine kinase inhibitor at pharmacological doses. Epidemiological studies show that genistein intake is inversely associated with the risk of CVD. Genistein can elicit vasodilator responses in human forearm in vivo as well as endothelium-dependent and -independent relaxation in arterial rings in vitro (Mahn et al., 2005). Genistein reduced the elevated BP and endothelial dysfunction in SHR, possibly due to increased eNOS activity as a result of increased calmodulin-1 expression and decreased superoxide generation (Crisafulli et al., 2005; Vera et al., 2007).
In a study on hypertensive animal models treated with quercetin (a flavonoid from many medicinal plants) higher eNOS activity, and decreased NADPH oxidase-mediated superoxide anion generation and reduced p47 protein expression were observed. These appear to be essential mechanisms for the improvement of endothelial function and the antihypertensive effects of chronic quercetin (Sánchez et al., 2006). Collectively, studies in humans, animals, and cellular experiments have demonstrated potential protective effects of phytoestrogens in the vasculature.
Dose of Estrogen in MHT
Because the plasma levels of estrogen decline rapidly during menopause, it was thought that MHT should be administered to restore the plasma estrogen to the levels observed in premenopausal women. However, this approach may not be the most effective. Estrogens are highly lipophilic compounds, and their circulating levels may not reflect their vascular tissue level. Also, hormones are extensively bound to plasma proteins, particularly globulin. Additionally, the hormone pharmacokinetics and volume of distribution may change during menopause particularly in women with liver or kidney disease. Thus, an estrogen dose that may seem normal in premenopausal women could produce superphysiological plasma levels in postmenopausal women. These observations have suggested that smaller doses of estrogen might be a safer strategy (Koledova et al., 2007). When comparing oral MHT, a low dose combination (0.3125 mg CEE) may be better than the conventional dose (0.625 mg CEE). Both combinations have the same effect on lipoproteins, flow-mediated vasodilation and plasma PAI-1 antigen levels, but the lower dose did not increase CRP or prothrombin fragment 1+2, and caused less reduction in anti-thrombin III than the conventional dose (Koh et al., 2004). Another clinical study in postmenopausal women showed an increase in the vascular inflammatory markers CRP and IL-6 with the conventional CEE dose, but not with the lower dose (Wakatsuki et. al., 2004). Thus, lower doses of MHT may still provide the beneficial results of the conventional dose, but with less associated risk of CVD. The Kronos Early Estrogen Prevention Study (KEEPS) began in mid-2005 as a randomized controlled multicenter 5-year clinical trial to evaluate the effectiveness of low dose CEE and weekly transdermal estradiol (both in combination with cyclic oral, micronized progesterone) in preventing progression of carotid intimal medial thickness and the accrual of coronary calcium in early menopausal women (aged 42-58 years) who are within 36 months of their final menstrual period, and would examine surrogate end points and risk factors for atherosclerosis (Harman et al., 2005).
Route of Administration of MHT
The outcome of MHT in postmenopausal women could also be affected by the route of administration. The combinations of estrogen used in clinical trials are usually in the oral pill form. Since estrogens are highly lipophilic, they are readily absorbed when taken orally. However, first-pass metabolism of estrogen in the liver could affect its cardiovascular effects. Synthetic estrogens such as ethinyl estradiol are not metabolized as rapidly and may be more effective than E2 when taken orally. Also, slow-release depot-forms of E2 that are available for parenteral administration, bypass the first-pass metabolism and are expected to be more effective.
Estrogen is absorbed through the skin because of its high lipophilicity. Topical estriol is used to manage the effects of aging on the skin and to decrease facial wrinkles. E2 patches such as Estraderm, Vivelle-Dot, Alora, and Climara are also used to minimize menopausal symptoms. The vascular effects of transdermal estrogens such as Estrasor benzoate, an E2 topical emulsion, have been tested. In recent studies markers of atherosclerosis and vascular endothelial function were compared in healthy postmenopausal women receiving oral CEE 0.625 mg/day or transdermal E2 gel 0.6 mg/day for 6 months. It was found that flow-mediated brachial artery vasodilation, a marker of endothelial function, was increased in both the oral and transdermal MHT groups. CRP levels increased in the oral but not the transdermal group, suggesting that transdermal estrogen might be better than the oral form in having less risk of vascular inflammation and atherosclerosis while offering similar vasodilation benefits (Ho et al. 2006; Shifren et. al., 2008).
Vaginal estrogen is available in the form of cream, tablets or rings for treatment of genitourinary symptoms, and maintenance of healthy mucosa in the vagina and urethra in postmenopausal women. Because of their low systemic absorption, vaginal estrogen preparations can be administered long-term. However, the potential long-term vascular effects of this treatment need to be carefully analyzed.
Selective ER modulators (SERMS)
In the search for alternatives to conventional MHT, recent studies have focused on the potential benefits of selective ER modulators (SERMs). A SERM is a non-steroidal molecule that binds with high affinity to ERs, but has tissue-specific effects distinct from estrogen (Table 6) (Park et al., 2002). Tamoxifen, a SERM with prominent estrogenic effects, has been studied extensively in breast cancer, and some studies have examined its effects on cardiovascular risk factors, the vascular system and the cerebral vasculature (Tsang et al., 2004). Tamoxifen exerts an estrogenic action, and causes rapid relaxation in blood vessels (Figtree et al., 2000; Hutchison et al., 2001). Also, in a study on OVX rats, tamoxifen demonstrated protective cardiovascular effects in myocardial ischemia-reperfusion injury due to its antioxidant properties (Ek et al., 2008). More studies are needed to examine the potential use of tamoxifen in MHT and its effects in postmenopausal CVD.
Raloxifene is another SERM that binds to ER and induces estrogenic effects in some tissues but estrogen antagonist effects in others (Fuchs-Young et al., 1995). The Raloxifene Use for The Heart (RUTH) clinical trail examined the risk-to-benefit ratio of raloxifene in preventing acute coronary events and invasive breast cancer. In postmenopausal women with CVD or at increased risk for CVD, treatment with raloxifene for a median of 5.6 years reduced the risk of invasive breast cancer but did not change the incidence of coronary events (Mosca et al., 2001). In another clinical study, raloxifene had a prothrombotic profile and increased coagulation profiles at 6 months into treatment, and decreased anti-coagulation profiles 12 months into treatment (Sgarabotto et al., 2007).
Ex vivo studies on rat renal and pulmonary artery rings and porcine arterial rings have shown that raloxifene inhibits VSM contraction and induces vascular relaxation in an endothelium-independent fashion through inhibition of Ca2+ influx via voltage-gated Ca2+ channels (Chan et al., 2005; Leung et al., 2005; Leung et al., 2007). Raloxifene and E2 have shown varying effects in human aortic smooth muscle cells (HASMCs) and HUVECs. Treatment with E2 or raloxifene increased both IGF-I and COX-2 mRNA expression in HUVECs, but reduced the serum-induced expression of COX-2 in HASMCs. Treatment by E2 and raloxifene induced recruitment of co-activator complexes and histone acetylation at both the IGF-I and COX-2 gene promoter in HUVECs, but decreased these parameters in HASMCs. These data indicate that SERMs may have different effects on multiple processes in different tissues. Although currently available SERMs show limited cardiovascular benefits, as new SERMs are developed and more studies are done on existing SERMs, they may be used in MHT to decrease the risk of postmenopausal CVD.
Estrogen metabolites and inter-conversions
Recent studies have compared the effects of naturally occurring estrogens and estrogen metabolites. Methoxy-E2 is a powerful estrogen agonist. Because of its non-feminizing properties methoxy-E2 may have cardiovascular benefits not only in postmenopausal women, but also in men with CHD. It should also be noted that estrogen is one of several sex hormones and many steroids in the body. Therefore the effects of E2 in vivo may be modified in the overall hormonal milieu. Sex hormones have a common origin from pregnanolone, and there is a continuous inter-conversions between E2 and other estrogens, as well as androgens (Fig 1). Administration of E2 may affect the hormonal balance and directly or indirectly affect the plasma levels of other sex hormones such as progesterone and testosterone.
Role of Progesterone
Plasma progesterone ranges from 1.5 nmol/L in the follicular phase to 35 nmol/L in the mid-luteal phase, and decreases to <1 nmol/L during menopause. Like estrogen, progesterone receptors have been identified in the endothelium and VSM of vascular beds of human as well as the mouse, rat, rabbit and primates. In addition to natural progesterone, synthetic progestins have been developed, such as medroxyprogesterone acetate (MPA), norethisterone, norgestimate, 3-keto-desogestrel, levonorgestrel, and gestodene. Also, a progesterone antagonist, RU486 (Mifepristone) is available (Koledova et al. 2007).
Similar to E2, progesterone has anti-atherosclerotic effects, decreases LDL, and increases HDL. Progesterone causes pulmonary vasodilation by activating both endothelium-dependant and -independent pathways. Also, progesterone stimulates eNOS expression, NO production and NO-mediated relaxation in rat aorta and ovine uterine artery. Progesterone may also cause direct nongenomic COX activation and increase vascular PGI2 production. Progesterone inhibits VSM proliferation/migration and facilitates the inhibitory effects of estrogen. Progesterone may alter the vascular effects of estrogen in MHT, and modify the effects of E2 on vascular contraction. Progesterone causes rapid relaxation of agonist- or KCl-induced contraction in endothelium-denuded porcine coronary artery, although with less potency than E2 (Crews & Khalil, 1999; Miller, 2003). The effects of progesterone on VSM [Ca2+]i are less clear, but acute application of progesterone decreases PGF2α- and high KCl-induced Ca2+ influx and [Ca2+]i in rabbit and porcine coronary VSM (Murphy & Khalil, 1999). Also, progesterone inhibits phorbol ester-induced contraction, PKC activation and translocation in VSM, an effect possibly mediated by increasing cAMP levels in VSM (Minshal et al., 2002). Also, like estrogen, progesterone may regulate the cytoskelelton (Fu et al., 2008).
However, the combined endothelium-dependent and endothelium-independent vasorelaxant effects of progesterone are less than those of estrogen. In effect, some studies have shown antagonistic effects of progesterone on the vasoprotective effects of estrogen. For example, progesterone counteracts the stimulatory effects of estrogen on NO production and vascular relaxation in canine coronary artery. In porcine coronary artery rings estrogen blocks the progesterone-induced endothelial dysfunction and reduction in NO production (Cox 2005). Also, progesterone antagonizes the vasoprotective effect of estrogen on antioxidant enzyme expression and function and enhances NADPH oxidase activity and the production of reactive oxygen species in OVX mice (Wassmann et al., 2005). Progesterone causes upregulation of vascular AT1R receptor mRNA and protein, an effect that promotes vasoconstriction (Nickenig et al., 2000; Dean et al., 2005). Although the anti-inflammatory effects of estrogen would attenuate ischemic brain injury, this vasoprotective benefit may be diminished in the presence of progestins (Sunday et al., 2006). Treatment of OVX females with either MPA or progesterone exacerbates the cerebrovascular inflammatory response to lipopolysacharides (LPS), whereas treatment with estrogen relieves the inflammatory responses. Also, CEE alone, but not in combination with MPA, inhibits aortic connective tissue remodeling after plasma lipid lowering in female monkeys (Register et al., 1998). Furthermore, MPA antagonizes the inhibitory effects of CEE on coronary artery atherosclerosis (Adam et al., 1997). These findings may have important implications in the use of multiple hormones in MHT. Because of the potentially complex interactions of estrogen and progesterone on the vasculature, more research is needed to determine the benefits of combined estrogen and progesterone in clinical trials.
Role of Androgens
Beyond estrogen and progesterone, androgens may play a role in determining the cardiovascular risk in postmenopausal women. Androgens are sex steroid hormones that control the development and maintenance of masculine secondary sexual characteristics. In women, androgens are important for maintaining bone mass, secondary sex characteristics and libido. Androgens are produced in the testis, adrenal glands, and ovaries.
The adrenal steroid dehydroepiandrosterone (DHEA) is a crucial precursor of human sex steroid biosynthesis. DHEA and its sulfate ester (DHEAS) are the most abundant steroids in the human circulation (Allolio & Arlt, 2002). DHEA and androstenedione do not have significant biological activity, but they are converted to testosterone which binds to and activates the androgen receptor. Testosterone is converted to dihydrotestosterone (DHT), which has five times higher binding affinity to the androgen receptor. Testosterone is aromatized peripherally, mainly in the adipose tissue, to estradiol (Fig. 1).
In adult females, the production rate of total testosterone is 250 μg/24 h, with 25% coming directly from the ovaries and 75% from non-ovarian sources such as the adrenals and extraglandular conversion (Adashi et al., 1981; Arlt et al., 1998). Therefore, in premenopausal women very low total plasma testosterone can be measured (1.0 to 1.5 nmol/L). However, with advanced age, circulating androgen levels decline because of a combination of decreasing adrenal production and ovarian failure (Davis et. al., 1996). While plasma levels of testosterone are reduced during menopause to 0.3-0.5 nmol/L, the androgen/estrogen ratio is increased in postmenopausal women (Wynne & Khalil, 2003).
In premenopausal women with polycystic ovary syndrome androgen excess has been associated with increased triglycerides and LDL, and reduced HDL-cholesterol (Rajkhowa et al., 1997; Pirwany et al., 2001). Also, hyperandrogenemic women demonstrate increased insulin resistance and incidence of CVD (Reinecke et al., 2002; Rexrode et al., 2003). Additionally, healthy postmenopausal women with a higher index of androgenicity, indicated by higher total testosterone, higher free androgen index, and lower sex hormone binding globulin, exhibit a pro-atherogenic risk profile, characterized by lower HDL-cholesterol and higher triglycerides, insulin resistance, and diastolic BP (Lambrinoudaki et al., 2006). Furthermore, observational studies in postmenopausal women that used self-reported high-dose intramuscular testosterone therapy for 1 year or longer have shown that in almost half of the hormone-users severe atherosclerosis of the aorta was present. These data suggest that high-dose testosterone therapy may adversely affect atherosclerosis in postmenopausal women and indicate that androgen replacement in these women may not be harmless (Hak et al., 2007). Androgens may also have adverse effects on the kidneys, particularly the rennin-angiotensin system, and may play a role in the development of hypertension (Reckelhoff, 2005). Selective androgen receptor antagonists such as flutamide, hydroxyflutamide and bicalutamide may minimize the harmful effect of androgens on atherosclerosis and the kidneys.
Interestingly, testosterone causes measurable relaxation of coronary artery. These findings argue against harmful effects of androgens on vascular function, and suggest that testosterone may confer protective effects on the coronary vessels in males (Crews & Khalil, 1999). A portion of testosterone-induced vasorelaxation is endothelium-independent and involves inhibition of Ca2+ influx and decreased [Ca2+]i in VSM (Murphy & Khalil, 1999). The vasorelaxant effect of testosterone is attenuated by K+ channel blockers, suggesting that activation of K+ channels such as the ATP-sensitive K+ channels (KATP) may be involved in the inhibitory effects of testosterone on VSM [Ca2+]i (Honda et al., 1999). In clinical trials testosterone confers symptomatic benefits in patients with coronary heart disease and heart failure, by acting as a vasodilator. Also, testosterone could be a potential treatment for patients with pulmonary artery hypertension, as vasodilator therapy remains the mainstay of treatment (Smith et al., 2006).
Conclusions and Perspective
Epidemiological studies suggest sex difference in the incidence of CVD, with an increase in postmenopausal compared with premenopausal women. ERα, ERβ, and GPR30 in the vasculature mediate estrogen-induced genomic and/or non-genomic effects. Estrogen promotes endothelium-dependent vascular relaxation, inhibits the cellular mechanism of VSM contraction, modulates the cytoskeleton, and the vascular inflammatory response. However, this experimental evidence has recently been challenged by the data from clinical trials. The discrepancy is likely related to changes in the vascular ERs amount, integrity, affinity to estrogen, and downstream signaling mechanisms, due to the subject age and preexisting cardiovascular condition. The beneficial vascular effects of estrogen could be translated to the outcome of MHT in postmenopausal CVD, as specific modulators of vascular sex hormone receptors become available and are used at the right dose, route of administration and timing depending on the subjects' age and preexisting cardiovascular condition. Also, the potential benefits/risks of combined therapy of multiple sex hormones and modulators of progesterone and androgen receptors should be examined.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Heart, Lung and Blood Institute (HL-65998, HL-70659). G.R.A. do Nascimento and Y.V.R. Barros were visiting trainees from Dr. Celia A. Kanashiro's group, at Universidade Estadual de Ciências da Saúde de Alagoas - UNCISAL, Maceio, Brazil.
Abbreviation List
- AC
adenylyl cyclase
- ACE
angiotensin-converting enzyme
- Ach
acetylcholine
- ApoE−/−
Apolipoprotein E KO mouse
- AR
androgen receptor
- AT1R
angiotensin II type 1 receptor
- BP
blood pressure
- CEE
conjugated equine estrogens
- CHD
coronary heart disease
- COX
cyclooxygenase
- CRP
C-reactive protein
- CVD
cardiovascular disease
- DHEA
dehydroepiandrosterone
- DHT
dihydrotestosterone
- E2
17β-Estradiol
- ECM
extracellular matrix
- EDHF
endothelium-derived hyperpolarizing factor
- αENaC
epithelial sodium channel
- ET-1
endothelin-1
- ER
estrogen receptor
- HASMCs
human aortic smooth muscle cells
- HERS
Heart and Estrogen/Progestin Replacement Study
- HSP
heat shock protein
- HTN
hypertension
- HUVECs
human umbilical vein endothelial cell
- KO
knockout
- L-NAME
Nω−-nitro-L-arginine methyl ester
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MCP
monocyte chemoattractant protein
- MISS
membrane-initiated steroid signaling
- MLCK
myosin light chain kinase
- MHT
menopausal hormone therapy
- MMP
matrix metalloproteinase
- MPA
medroxyprogesterone acetate
- NO
nitric oxide
- NOS
nitric oxide synthase
- OVX
ovariectomized
- PCOS
polycystic ovary syndrome
- PDBu
phorbol 12,13-dibutyrate
- PGI2
prostacyclin
- Phe
phenylephrine
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- RAS
renin-angiotensin system
- RUTH
Raloxifene Use for The Heart
- SERMs
selective estrogen receptor modulators
- SHR
spontaneously hypertensive rats
- TNF-α
tumor necrosis factor
- TXA2
thromboxane A2
- VCAM
vascular cell adhesion molecule
- VSM
vascular smooth muscle
- VTE
venous thromboembolism
- WHI
Women's Health Initiative
- WT
wild-type
- WKY
Wistar-Kyoto rats
REFERENCES
- Adams M, Thomas RC, Golden DL, Wagner JD, Williams JK. Mehtoxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery artherosclerosis. Arteriosclerosis, Thrombosis and Vascular Biology. 1997;17:217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- Adashi EY, Jones PB, Hsueh AJ. Synergistic effect of glucocorticoids on the stimulation of progesterone production by follicle-stimulating hormone in cultured rat granulosa cells. Endocrinology. 1981;109(6):1888–94. doi: 10.1210/endo-109-6-1888. [DOI] [PubMed] [Google Scholar]
- Adashi EY. The climacteric ovary as a functional gonadotropin-driven androgen-producing gland. Fertil Steril. 1994;62(1):20–7. doi: 10.1016/s0015-0282(16)56810-1. [DOI] [PubMed] [Google Scholar]
- Allolio B, Arlt W. DHEA treatment: myth or reality? Trends Endocrinol Metab. 2002;13(7):288–94. doi: 10.1016/s1043-2760(02)00617-3. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- de Araujo LF, Soares JM, Jr, Simões RS, Calió PL, Oliveira-Filho RM, Simões Mde J, Haidar MA, Baracat EC. Effect of conjugated equine estrogens and tamoxifen administration on thyroid gland histomorphology of the rat. Clinics. 2006;61(4):321–6. doi: 10.1590/s1807-59322006000400008. [DOI] [PubMed] [Google Scholar]
- Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Chronic tumor necrosis factor-alpha inhibition enhances NO modulation of vascular function in estrogen-deficient rats. Hypertension. 2005;46(1):76–81. doi: 10.1161/01.HYP.0000168925.98963.ef. [DOI] [PubMed] [Google Scholar]
- Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Tumor necrosis factor-alpha and vascular angiotensin II in estrogen-deficient rats. Hypertension. 2006;48(3):497–503. doi: 10.1161/01.HYP.0000235865.03528.f1. [DOI] [PubMed] [Google Scholar]
- Arias-Loza PA, Hu K, Dienesch C, Mehlich AM, König S, Jazbutyte V, Neyses L, Hegele-Hartung C, Heinrich Fritzemeier K, Pelzer T. Both estrogen receptor subtypes, α and β, attenuate cardiovascular remodeling in aldosterone salt-treated rats. Hypertension. 2007;50(2):432–8. doi: 10.1161/HYPERTENSIONAHA.106.084798. [DOI] [PubMed] [Google Scholar]
- Arlt W, Justl HG, Callies F, Reincke M, Hübler D, Oettel M, Ernst M, Schulte HM, Allolio B. Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J Clin Endocrinol Metab. 1998;83(6):1928–34. doi: 10.1210/jcem.83.6.4850. [DOI] [PubMed] [Google Scholar]
- Ba ZF, Lu A, Shimizu T, Szalay L, Schwacha MG, Rue LW, 3rd, Bland KI, Chaudry IH. 17β-Estradiol modulates vasoconstriction induced by endothelin-1 following trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2007;292(1):H245–50. doi: 10.1152/ajpheart.00809.2006. [DOI] [PubMed] [Google Scholar]
- Barber DA, Miller VM. Gender differences in endothelium-dependent relaxations do not involve NO in porcine coronary arteries. Am J Physiol. 1997;273(5 Pt 2):H2325–32. doi: 10.1152/ajpheart.1997.273.5.H2325. [DOI] [PubMed] [Google Scholar]
- Barchiesi F, Jackson EK, Gillespie DG, Zacharia LC, Fingerle J, Dubey RK. Mehtoxyestradiols mediate estradiol-induced antimitogenesis in human aortic SMCs. Hypertension. 2002;39(4):874–9. doi: 10.1161/01.hyp.0000013863.25970.ba. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK, Raloxifene Use for The Heart (RUTH) Trial Investigators Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355(2):125–37. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19(2):149–54. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- Bilsel AS, Moini H, Tetik E, Aksungar F, Kaynak B, Ozer A. 17Beta-estradiol modulates endothelin-1 expression and release in human endothelial cells. Cardiovasc Res. 2000;46(3):579–84. doi: 10.1016/s0008-6363(00)00046-8. [DOI] [PubMed] [Google Scholar]
- Brou C, Wu J, Ali S, Scheer E, Lang C, Davidson I, Chambon P, Tora L. Different TBP-associated factors are required for mediating the stimulation of transcription in vitro by the acidic transactivator GAL-VP16 and the two nonacidic activation functions of the estrogen receptor. Nucleic Acids Res. 1993;21(1):5–12. doi: 10.1093/nar/21.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engström O, Phman L, Green GL, Gustafson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389(6652):753–8. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- Burger NZ, Osol G, Gokina N. O-212: The effect of estrogen on EDHF function in the mesenteric vascular system. Fertility and Sterility. 2006;86(3):S91. [Google Scholar]
- Calkin AC, Sudhir K, Honisett S, Williams MR, Dawood T, Komesaroff PA. Rapid potentiation of endothelium-dependent vasodilation by estradiol in postmenopausal women is mediated via cyclooxygenase 2. J Clin Endocrinol Metab. 2002;87(11):5072–5. doi: 10.1210/jc.2002-020057. [DOI] [PubMed] [Google Scholar]
- Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Levesque H, Trillot N, Barrellier MT, Wahl D, Emmerich J, Scarabin PY, Estrogen and Thromboembolism Risk (ESTHER) Study Group Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and pregestogens: the ESTHER study. Circulation. 2007;155(7):840–5. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- Case J, Davison CA. Estrogen alters relative contributions of nitric oxide and cyclooxygenase products to endothelium-dependent vasodilation. J Pharmacol Exp Ther. 1999;291(2):524–30. [PubMed] [Google Scholar]
- Cassidy A, Albertazzi P, Lise Nielsen I, Hall W, Williamson G, Tetens I, Atkins S, Cross H, Manios Y, Wolk A, Steiner C, Branca F. Critical review of health effects of soyabean phyto-oestrogens in post-menopausal women. Proc Nutr Soc. 2006;65(1):76–92. doi: 10.1079/pns2005476. [DOI] [PubMed] [Google Scholar]
- Chan YC, Leung FP, Yao X, Lau CW, Vanhoutte PM, Huang Y. Raloxifene relaxes rat pulmonary arteries and veins: roles of gender, endothelium, and antagonism of Ca2+ influx. J Pharmacol Exp Ther. 2005;312(3):1266–71. doi: 10.1124/jpet.104.077990. [DOI] [PubMed] [Google Scholar]
- Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103(3):401–6. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheskis BJ, Greger JG, Naqpal S, Freedman LP. Signaling by estrogens. J Cell Physiol. 2007;212(3):610–7. doi: 10.1002/jcp.21253. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Budzyn K, Marley PD, Sobey CG. Evidence that estrogen suppresses rho-kinase function in the cerebral circulation in vivo. Stroke. 2004;35(9):2200–5. doi: 10.1161/01.STR.0000136951.85586.c8. [DOI] [PubMed] [Google Scholar]
- Christian RC, Liu PY, Harrington S, Ruan M, Miller VM, Fitzpatrick LA. Intimal estrogen receptor (ER)β, but not ERα expression, is correlated with coronary calcification and atherosclerosis in pre-and postmenopausal women. J. Clin Endocrinol Metab. 2006;91(7):2713–20. doi: 10.1210/jc.2005-2672. [DOI] [PubMed] [Google Scholar]
- Cox MW, Fu W, Chai H, Paladugu R, Lin PH, Lumsden AB, Yao Q, Chen C. Effects of progesterone and estrogen on endothelial dysfunction in porcine coronary arteries. J Surg Res. 2005;124(1):104–11. doi: 10.1016/j.jss.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Crews JK, Khalil RA. Antagonistic effects of 17β-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler Thromb Vasc Biol. 1999;19(4):1034–40. doi: 10.1161/01.atv.19.4.1034. [DOI] [PubMed] [Google Scholar]
- Crews JK, Khalil RA. Gender-specific inhibition of Ca2+ entry mechanisms of arterial vasoconstriction by sex hormones. Clin Exp Pharmacol Physiol. 1999;26(9):707–15. doi: 10.1046/j.1440-1681.1999.03110.x. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Altavilla D, Marini H, Bitto A, Cucinotta D, Frisina N, Corrado F, D'Anna R, Squadrito G, Adamo EB, Marini R, Romeo A, Cancellieri F, Buemi M, Squadrito F. Effects of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women. Menopause. 2005;12(2):186–92. doi: 10.1097/00042192-200512020-00013. [DOI] [PubMed] [Google Scholar]
- Cruz MN, Agewall S, Schenck-Gustafsson K, Kublickiene K. Acute dilatation to phytoestrogens and estrogen receptor subtypes expression in small arteries from women with coronary heart disease. Atherosclerosis. 2008;196(1):49–58. doi: 10.1016/j.atherosclerosis.2007.01.038. [DOI] [PubMed] [Google Scholar]
- Danielian PS, White R, Leese JA, Parker MG. Identification of a conserved region required for hormone dependant transcriptional activation by steroid hormone receptors. EMBO J. 1992;11(6):1025–33. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darblade B, Pendaries C, Krust A, Dupont S, Fouque MJ, Rami J, Chambon P, Bayard F, Arnal JF. Estradiol alters nitric oxide production in the mouse aorta through the alpha-, but not beta-, estrogen receptor. Circ Res. 2002;90(4):413–9. doi: 10.1161/hh0402.105096. [DOI] [PubMed] [Google Scholar]
- Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am J Physiol. 1997;272(6 Pt 2):H2765–73. doi: 10.1152/ajpheart.1997.272.6.H2765. [DOI] [PubMed] [Google Scholar]
- Das DK, Sato M, Ray PS, Maulik G, Engelman RM, Bertelli AA, Bertelli A. Cardioprotection of red wine: role of polyphenolic antioxidants. Drugs Exp Clin Res. 1999;25(2-3):115–20. [PubMed] [Google Scholar]
- David FL, Carvalho MH, Cobra AL, Nigro D, Fortes ZB, Rebouças NA, Tostes RC. Ovarian hormones modulate endothelin-1 vascular reactivity and mRNA expression in DOCA-salt hypertensive rats. Hypertension. 2001;38(3 Pt 2):692–6. doi: 10.1161/01.hyp.38.3.692. [DOI] [PubMed] [Google Scholar]
- Davis SR, Burger HG. Clinical review 82: androgens and the postmenopausal woman. J Clin Endocrinol Metab. 1996;81(8):2759–63. doi: 10.1210/jcem.81.8.8768824. [DOI] [PubMed] [Google Scholar]
- Dean SA, Tan J, O'brien ER, Leenen FH. 17beta-Estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;288(3):R759–66. doi: 10.1152/ajpregu.00595.2004. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK, Keller PJ, Imthurn B, Rosselli M. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. Hypertension. 2001;37(2 Part 2):640–4. doi: 10.1161/01.hyp.37.2.640. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Imthurn B, Zacharia LC, Jackson EK. Hormone replacement therapy and cardiovascular disease: what went wrong and where do we go from here? Hypertension. 2004;44(6):789–95. doi: 10.1161/01.HYP.0000145988.95551.28. [DOI] [PubMed] [Google Scholar]
- Egami R, Tanaka Y, Nozaki M, Koera K, Okuma A, Nakano H. Chronic treatment with 17beta-estradiol increases susceptibility of smooth muscle cells to nitric oxide. Eur J Pharmacol. 2005;520(1-3):142–9. doi: 10.1016/j.ejphar.2005.07.028. [DOI] [PubMed] [Google Scholar]
- Egan KM, Lawson JA, Fries S, Koller B, Rader DJ, Smyth EM, Fitzgerald GA. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306(5703):1954–7. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- Ek RO, Yildiz Y, Cecen S, Yenisey C, Kavak T. Effects of tamoxifen on myocardial ischemia-reperfusion injury model in ovariectomized rats. Mol Cell Biochem. 2008;308(1-2):227–35. doi: 10.1007/s11010-007-9633-0. [DOI] [PubMed] [Google Scholar]
- Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J. 1996;10(5):615–24. [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelium-dependent hyperpolarizations: past beliefs and present facts. Ann Med. 2007;39(7):495–516. doi: 10.1080/07853890701491000. [DOI] [PubMed] [Google Scholar]
- Figtree GA, Webb CM, Collins P. Tamoxifen acutely relaxes coronary arteries by an endothelium-, nitric oxide-, and estrogen receptor dependent mechanism. J Pharmacol Exp Ther. 2000;295(2):519–23. [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16(1):70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16(8):362–7. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Fu XD, Flamini M, Sanchez AM, Goglia L, Giretti MS, Genazzani AR, Simoncini T. Progestogens Regulate Endothelial Actin Cytoskeleton and Cell Movement via the Actin-Binding Protein Moesin. Mol Hum Reprod. 2008 doi: 10.1093/molehr/gan010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Fuchs-Young R, Glasebrook AL, Short LL, Draper MW, Rippy MK, Cole HW, Magee DE, Termine JD, Bryant HU. Raloxifene is a tissue-selective agonist/antagonist that functions through the estrogen receptor. Ann N Y Acad Sci. 1995;761:355–60. doi: 10.1111/j.1749-6632.1995.tb31392.x. [DOI] [PubMed] [Google Scholar]
- Gao H, Liang M, Bergdahl A, Hamrén A, Lindholm MW, Dahlman-Wright K, Nilsson BO. Estrogen attenuates vascular expression of inflammation associated genes and adhesion of monocytes to endothelial cells. Inflamm Res. 2006;55(8):349–53. doi: 10.1007/s00011-006-5194-z. [DOI] [PubMed] [Google Scholar]
- Garcia PM, Giménez J, Bonacasa B, Carbonell LF, Miguel SG, Quesada T, Hernández I. 17beta-estradiol exerts a beneficial effect on coronary vascular remodeling in the early stages of hypertension in spontaneously hypertensive rats. Menopause. 2005;12(4):453–9. doi: 10.1097/01.GME.0000151654.10243.01. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez MC, Hermenegildo C, Tarin JJ, Cano A. Phytoestrogens increase the capacity of serum to stimulate prostacyclin release in human endothelial cells. Acta Obstet Gynecol Scand. 2003;82(8):705–10. doi: 10.1080/j.1600-0412.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Estrogen reduces mouse cerebral artery tone through endothelial NOS- and cyclooxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol. 2000;279(2):H511–9. doi: 10.1152/ajpheart.2000.279.2.H511. [DOI] [PubMed] [Google Scholar]
- Geraldes P, Gagnon S, Hadjadj S, et al. Estradiol blocks the induction of CD40 and CD40L expression on endothelial cells and prevents neutrophil adhesion: an ERalpha-mediated pathway. Cardiovasc Res. 2006;71(3):566–73. doi: 10.1016/j.cardiores.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Geraldes P, Sirois MG, Bernatchez PN, Tanguay JF. Estrogen regulation of endothelial and smooth muscle cell migration and proliferation: role of p38 and p42/44 mitogen-activated protein kinase. Arterioscler Thromb Vasc Biol. 2002;22(10):1585–90. doi: 10.1161/01.atv.0000035393.11854.6a. [DOI] [PubMed] [Google Scholar]
- Gerhard M, Ganz P. How do we explain the clinical benefits of estrogen from bedside to bench. Circulation. 1995;92(1):5–8. doi: 10.1161/01.cir.92.1.5. [DOI] [PubMed] [Google Scholar]
- Gordon SF. Clinical experience with a seven-day estradiol transdermal system for estrogen replacement therapy. Am J Gynecol. 1995;173(3 Pt 2):998–1004. doi: 10.1016/0002-9378(95)90250-3. [DOI] [PubMed] [Google Scholar]
- Gougelet A, Mueller SO, Korach KS, Renoir JM. Oestrogen receptors pathways to oestrogen responsive elements: the transactivation function-1 acts as the keystone of oestrogen receptor (ER)beta-mediated transcriptional repression of ERalpha. J Steroid Biochem Mol Biol. 2007;104(3-5):110–22. doi: 10.1016/j.jsbmb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N, HERS Research Group Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS-II) JAMA. 2002;288(1):49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- Grady D, Applegate W, Bush T, Fuberg C, Riggs B, Hulley SB. Heart and Estrogen/progestin Replacement Study (HERS): design, methods, and baseline characteristics. Control Clin Trials. 1998;19(4):314–35. doi: 10.1016/s0197-2456(98)00010-5. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Reboussin BA, Hogan P, Barnabei VM, Shumaker S, Johnson S, Barrett-Connor E. Symptom relief and side effects of postmenopausal hormones: results from the Postmenopausal Estrogen/Progestin Interventions Trial. Obstet Gynecol. 1998;92(6):982–8. doi: 10.1016/s0029-7844(98)00305-6. [DOI] [PubMed] [Google Scholar]
- Greger JG, Fursov N, Cooch N, McLarney S, Freedman LP, Edwards DP, Cheskis BJ. Phosphorylation of MNAR promots estrogen activation of phophatidylinositol 3-kinase. Mol Cell Biol. 2007;27(5):1904–13. doi: 10.1128/MCB.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348(7):645–50. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133(12):933–41. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors alpha and beta. J Biol Chem. 2005;280(20):19704–10. doi: 10.1074/jbc.M501244200. [DOI] [PubMed] [Google Scholar]
- Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007;49(6):1358–63. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- Hak AE, Westendorp IC, Pols HA, Hofman A, Witteman JC. High-dose testosterone is associated with atherosclerosis in postmenopausal women. Maturitas. 2007;56(2):153–60. doi: 10.1016/j.maturitas.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Hall WL, Vafeiadou K, Hallund J, Bugel S, Reimann M, Koebnick C, Zunft HJ, Ferrari M, Branca F, Dadd T, Talbot D, Powell J, Minihane AM, Cassidy A, Nilsson M, Dahlman-Wright K, Gustafsson JA, Williams CM. Soy-isoflavone-enriched foods and markers of lipid and glucose metabolism in postmenopausal women: interactions with genotype and equol production. Am J Clin Nutr. 2006;83(3):592–600. doi: 10.1093/ajcn.83.3.592. [DOI] [PubMed] [Google Scholar]
- Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric. 2005;8(1):3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- Heldring N, Pike A, Anderson S, Matthews J, Cheng G, Hartman J, Tujaque M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol REv. 2007;87(3):905–31. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J, WHI Investigators Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation. 2006;113(20):2425–34. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- Herrington DM, Reboussin DM, Klein KP, Sharp PC, Shumaker SA, Synder TE, Geisinger KR. The estrogen replacement and artherosclerosis (ERA) study : study design and baseline characteristics of the cohort. Control Clin Trials. 21(3):257–85. doi: 10.1016/s0197-2456(00)00054-4. 200. [DOI] [PubMed] [Google Scholar]
- Hertrampf T, Schmidt S, Laudenbach-Leschowsky U, Seibel J, Diel P. Tissue-specific modulation of cyclooxygenase-2 (Cox-2) expression in the uterus and the v. cava by estrogens and phytoestrogens. Mol Cell Endocrinol. 2005;243(1-2):51–7. doi: 10.1016/j.mce.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hiroki J, Shimokawa H, Mukai Y, Ichiki T, Takeshita A. Divergent effects of estrogen and nicotine on Rho-kinase expression in human coronary vascular smooth muscle cells. Biochem Biophys Res Commun. 2005;326(1):154–9. doi: 10.1016/j.bbrc.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Ho JY, Chen MJ, Cheu WH, Yi YC, Tsai AC, Guu HF, Ho ES. Differential effects of oral conjugated equine estrogen and transdermal estrogen on atherosclerotic vascular disease risk markers and endothelial function in healthy postmenopausal women. Hum Reprod. 2006;21(10):2715–20. doi: 10.1093/humrep/del245. [DOI] [PubMed] [Google Scholar]
- Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor alpha is a major mediator of 17beta-estradiol's atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest. 2001;107(3):333–40. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H, Unemoto T, Kogo H. Different mechanisms for testosterone-induced relaxation of aorta between normotensive and spontaneously hypertensive rats. Hypertension. 1999;34:1232–1236. doi: 10.1161/01.hyp.34.6.1232. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev. 1996;76(4):967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, Pettinger M, Heckbert SR, Greep N, Crawford S, Eaton CB, Kostis JB, Caralis P, Prentice R, Women's Health Initiative Investigators Conjugated equine estrogens and coronary heart disease: the Women's Health Initiative. Arch Intern Med. 2006;166(3):357–65. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Brass LM. Estrogen and stroke: a balanced analysis. Stroke. 2003;34(2):338–41. doi: 10.1161/01.str.0000054051.88378.25. [DOI] [PubMed] [Google Scholar]
- Hutchison SJ, Chou TM, Chatterjee K, Sudhir K. Tamoxifen is an acute, estrogen-like, coronary vasodilator of porcine coronary arteries in vitro. J Cardiovasc Pharmacol. 2001;38(5):657–65. doi: 10.1097/00005344-200111000-00002. [DOI] [PubMed] [Google Scholar]
- Jacob J, Sebastian KS, Devassy S, Priyadarsini L, Farook MF, Shameem A, Mathew D, Sreeja S, Thampan RV. Membrane estrogen receptors: genomic actions and post transcriptional regulation. Mol Cell Endocrinol. 2006;246(1-2):34–41. doi: 10.1016/j.mce.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Jazbutyte V, Arias-Loza PA, Hu K, Widder J, Govindaraj V, von Poser-Klein C, Bauersachs J, Fritzemeier KH, Hegele-Hartung C, Neyses L, Ertl G, Pelzer T. Ligand-dependent activation of ER{beta} lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized spontaneously hypertensive rats. Cardiovasc Res. 2008;77(4):774–81. doi: 10.1093/cvr/cvm081. [DOI] [PubMed] [Google Scholar]
- Ji TH, Grossmann M, Ji I. G protein-coupled receptors. I. Diversity of receptor-ligand interactions. J Biol Chem. 1998;273(28):17299–302. doi: 10.1074/jbc.273.28.17299. [DOI] [PubMed] [Google Scholar]
- Jiang C, Sarrel PM, Lindsay DC, Poole-Wilson PA, Collins P. Endothelium-independent relaxation of rabbit coronary artery by 17beta- estradiol in vitro. Br J Pharmacol. 1991;104(4):1033–37. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähönen M, Tolvanen JP, Sallinen K, Wu X, Pörsti I. Influence of gender on control of arterial tone in experimental hypertension. Am J Physiol. 1998;275(1 Pt 2):H15–22. doi: 10.1152/ajpheart.1998.275.1.H15. [DOI] [PubMed] [Google Scholar]
- Kalantaridou SN, Naka KK, Papanikolaou E, Kazakos N, Kravariti M, Calis KA, Paraskevaidis EA, Sideris DA, Tsatsoulis A, Chrousos GP, Michalis LK. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab. 2004;89(8):3907–13. doi: 10.1210/jc.2004-0015. [DOI] [PubMed] [Google Scholar]
- Kanashiro CA, Khalil RA. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am J Physiol Cell Physiol. 2001;280(1):C34–45. doi: 10.1152/ajpcell.2001.280.1.C34. [DOI] [PubMed] [Google Scholar]
- Kauser K, Rubanyi GM. Gender difference in endothelial dysfunction in the aorta of spontaneously hypertensive rats. Hypertension. 1995;25(4 pt 1):517–23. doi: 10.1161/01.hyp.25.4.517. [DOI] [PubMed] [Google Scholar]
- Khalil RA. Sex hormones as potential modulators of vascular function in hypertension. Hypertension. 2005;46(2):249–54. doi: 10.1161/01.HYP.0000172945.06681.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Levin ER. Estrogen signaling in the cardiovascular system. Nucl Recept Signal. 2006;4:e013. doi: 10.1621/nrs.04013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM, Keynton RS. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem. 2005;280(9):7460–8. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Lounsbury KM, Brayden JE, Nelson MT. Gender differences in coronary artery diameter reflect changes in both endothelial Ca2+ and ecNOS activity. Am J Physiol Heart Circ Physiol. 1999;276(3 Pt 2):H961–9. doi: 10.1152/ajpheart.1999.276.3.H961. [DOI] [PubMed] [Google Scholar]
- Koh KK, Shin MS, Sakuma I, Ahn JY, Jin DK, Kim HS, Kim DS, Han SH, Chung WJ, Shin EK. Effects of conventional or lower doses of hormone replacement therapy in postmenopausal women. Arterioscler Thromn Vasc Biol. 2004;24(8):1516–21. doi: 10.1161/01.ATV.0000133683.65877.bc. [DOI] [PubMed] [Google Scholar]
- Koide A, Zhao C, Naganuma M, Abrams J, Deighton-Collins S, Skafar DF, Koide S. Identification of regions within the F domain of the human estrogen receptor alpha that are important for modulating transactivation and protein-protein interactions. Mol Endocrinol. 2007;21(4):829–42. doi: 10.1210/me.2006-0203. [DOI] [PubMed] [Google Scholar]
- Koledova VV, Khalil RA. Sex hormone replacement therapy and modulation of vascular function in cardiovascular disease. Expert Rev Cardiovasc Ther. 2007;5(4):777–89. doi: 10.1586/14779072.5.4.777. [DOI] [PubMed] [Google Scholar]
- Kublickiene K, Svedas E, Landgren BM, Crisby M, Nahar N, Nisell H, Poston L. Small artery endothelial dysfunction in postmenopausal women: in vitro function, morphology, and modification by estrogen and selective estrogen receptor modulators. J Clin Endocrinol Metab. 2005;90(11):6113–22. doi: 10.1210/jc.2005-0419. [DOI] [PubMed] [Google Scholar]
- Kumar V, Green S, Stack G, Berry M, Jin JR, Chambon P. Functional domains of the human estrogen receptor. Cell. 1987;51(6):941–51. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Lambrinoudaki I, Christodoulakos G, Rizos D, Economou E, Argeitis J, Vlachou S, Creatsa M, Kouskouni E, Botsis D. Endogenous sex hormones and risk factors for atherosclerosis in healthy Greek postmenopausal women. Eur J Endocrinol. 2006;154(6):907–16. doi: 10.1530/eje.1.02167. [DOI] [PubMed] [Google Scholar]
- Leung SW, Teoh H, Keung W, Man RY. Non-genomic vascular actions of female sex hormones: Physiological implications and signalling pathways. Clin Exp Pharmacol Physiol. 2007;34(8):822–6. doi: 10.1111/j.1440-1681.2007.04686.x. [DOI] [PubMed] [Google Scholar]
- Leung FP, Yao X, Lau CW, Ko WH, Lu L, Huang Y. Raloxifene relaxes rat intrarenal arteries by inhibiting Ca2+ influx. Am J Physiol Renal Physiol. 2005;289(1):F137–44. doi: 10.1152/ajprenal.00353.2004. [DOI] [PubMed] [Google Scholar]
- Leung HS, Seto SW, Kwan YW, Leung FP, Au AL, Yung LM, Yao X, Huang Y. Endothelium-independent relaxation to raloxifene in porcine coronary artery. Eur J Pharmacol. 2007;555(2-3):178–84. doi: 10.1016/j.ejphar.2006.10.035. [DOI] [PubMed] [Google Scholar]
- Lewandowski KC, Komorowski J, Mikhalidis DP, Bienkiewicz M, Tan BK, O'Callaghan CJ, Lewinski A, Prelevic G, Randeva HS. Effects of hormone replacement therapy type and route of administration on plasma matrix metalloproteinases and their tissue inhibitors in postmenopausal women. J Clinc Endocrinol Metab. 2006;91(8):3123–30. doi: 10.1210/jc.2005-2789. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Avsar M, Labreche P, Bracamonte M, Jayachandran M, Miller VM. Treatment with raloxifene and 17beta-estradiol differentially modulates nitric oxide and prostanoids in venous endothelium and platelets of ovariectomized pigs. J Cardiovasc Pharmacol. 2006;48(5):231–8. doi: 10.1097/01.fjc.0000247800.34991.a1. [DOI] [PubMed] [Google Scholar]
- Mahn K, Borrás C, Knock GA, Taylor P, Khan IY, Sugden D, Poston L, Ward JP, Sharpe RM, Viña J, Aaronson PI, Mann GE. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005;19(12):1755–7. doi: 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M, Women's Health Initiative Investigators Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349(6):523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Laudlam SE, Pettinger MB, Gass M, Margoslis KL, Nathan L, Ockene JK, Prentice RL, Robbins J, Stefanick ML, WHI and WHI-CACS Investigators Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356(25):2591–602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- Masi CM, Hawkley LC, Berry JD, Cacioppo JT. Estrogen metabolites and systolic blood pressure in a population-based sample of postmenopausal women. J Clin Endocrinol Metab. 2006;91:1015–1020. doi: 10.1210/jc.2005-2339. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N England J Med. 1999;340(23):1801–11. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME. Mechanisms of estrogen action in the cardiovascular system. J Steroid Biochem Mol Biol. 2000;74(5):337–43. doi: 10.1016/s0960-0760(00)00110-2. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol. 2002;90(1A):3F–6F. doi: 10.1016/s0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–7. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- Mercuro G, Longu G, Zoncu S, Cherchi A. Impaired forearm blood flow and vasodilator reserve in healthy postmenopausal women. Am Heart J. 1999;137(4 Pt 1):692–7. doi: 10.1016/s0002-8703(99)70225-5. [DOI] [PubMed] [Google Scholar]
- Méthot J, Hamelin BA, Arsenault M, Bogaty P, Plante S, Poirier P. The ACE-DD genotype is associated with endothelial dysfunction in postmenopausal women. Menopause. 2006;13(6):959–66. doi: 10.1097/01.gme.0000243576.09065.93. [DOI] [PubMed] [Google Scholar]
- Metzger D, Ali S, Bornert BM, Chambon P. Characterization of the amino-terminal transcription activation function of the human estrogen receptor in animal and yeast cells. J Biol Chem. 1995;270(16):9535–42. doi: 10.1074/jbc.270.16.9535. [DOI] [PubMed] [Google Scholar]
- Meyer MC, Cummings K, Osol G. Estrogen replacement attenuates resistance artery adrenergic sensitivity via endothelial vasodilators. Am J Physiol. 1997;272(5 Pt 2):H2264–70. doi: 10.1152/ajpheart.1997.272.5.H2264. [DOI] [PubMed] [Google Scholar]
- Miller AP, Chen YF, Xing D, Feng W, Oparil S. Hormone Replacement Therapy and Inflammation Interactions in Cardiovascular Disease. Hypertension. 2003;42(4):657–63. doi: 10.1161/01.HYP.0000085560.02979.0C. [DOI] [PubMed] [Google Scholar]
- Minshall RD, Pavcnik D, Browne DL, Hermsmeyer K. Nongenomic vasodilator action of progesterone on primate coronary arteries. J Appl Physiol. 2002;92(2):701–8. doi: 10.1152/japplphysiol.00689.2001. [DOI] [PubMed] [Google Scholar]
- Mitrunen K, Hirrvonen A. Molecular epidemiology of sporadic breast cancer: The role of polymorphic genes involved in oestrogen biosynthesis and metabolism. Molecular Research/Reviews in Mutation Research. 2003;544(1):9–41. doi: 10.1016/s1383-5742(03)00016-4. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Khalil RA. Decreased [Ca2+]i during inhibition of coronary smooth muscle contraction by 17β-estradiol, progesterone, and testosterone. J Pharmacol Exp Ther. 1999;291(1):44–52. [PubMed] [Google Scholar]
- Murphy JG, Khalil RA. Gender-specific reduction in contractility and [Ca2+]i in vascular smooth muscle cells of female rat. Am J Physiol Cell Physiol. 2000;278:C834–C844. doi: 10.1152/ajpcell.2000.278.4.C834. [DOI] [PubMed] [Google Scholar]
- Nawate S, Fukao M, Sakuma I, Soma T, Nagai K, Takikawa O, Miwa S, Kitabatake A. Reciprocal changes in endothelium-derived hyperpolarizing factor- and nitric oxide-system in the mesenteric artery of adult female rats following ovariectomy. Br J Pharmacol. 2005;144(2):178–89. doi: 10.1038/sj.bjp.0706091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SH, Steinsland OS, Wang Y, Yallampalli C, Dong YL, Sanchez JM. Increased Nitric Oxide Synthase Activity and Expression in the Huhman Uterine Artery During Pregnancy. Circulation Research. 2000;87(5):406–411. doi: 10.1161/01.res.87.5.406. [DOI] [PubMed] [Google Scholar]
- Nelson DH. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291(13):1610–20. doi: 10.1001/jama.291.13.1610. [DOI] [PubMed] [Google Scholar]
- Ni Y, Meyer M, Osol G. Gestation increases nitric oxide-mediated vasodilation in rat uterine arteries. American J of Obstetrics and Gynecology. 1997;176(4):856–64. doi: 10.1016/s0002-9378(97)70611-2. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Strehlow K, Wassmann S, Bäumer AT, Albory K, Sauer H, Böhm M. Differential effects of estrogen and progesterone on AT(1) receptor gene expression in vascular smooth muscle cells. Circulation. 2000;102(15):1828–33. doi: 10.1161/01.cir.102.15.1828. [DOI] [PubMed] [Google Scholar]
- Nilsson BO, Ekblad E, Heine T, Gustafsson JA. Increased magnitude of relaxation to oestrogen in aorta from oestrogen receptor beta knock-out mice. J Endocrinol. 2000;166(2):R5–9. doi: 10.1677/joe.0.166r005. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Gustafsson JA. Estrogen receptor transcription and transactivation. Basic aspects of estrogen action. Breast Cancer Res. 2000;2(5):360–6. doi: 10.1186/bcr81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notelovitz M, Lenihan J, McDermott M, Kreber I, Nanavati N, Arce JC. Initial 17beta-estradiol dose for treating vasomotor symptoms. Obstetrics & Gynecology. 2000;95:726–31. doi: 10.1016/s0029-7844(99)00643-2. [DOI] [PubMed] [Google Scholar]
- Notelovitz M. The Biologic and Pharmacologic Principles of Estrogen Therapy for Symptomatic Menopause. Med Gen Med. 2006;8(1):85. [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan Gerard M, Goodrish James A, Michael R. Adams. Increased prostacyclin synthesis by atherosclerotic arteries from estrogen treated monkeys. Life Sci. 2001;69:4395–401. doi: 10.1016/s0024-3205(01)01131-6. [DOI] [PubMed] [Google Scholar]
- O'Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, Bienkowska J, Mendelsohn ME, Hansen U. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;21(6):1281–96. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286(2):R233–49. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- Ouyang P, Michos ED, Karas RH. Hormone replacement therapy and the cardiovascular system lessons learned and unanswered questions. J Am Coll Cardiol. 2006;47(9):1741–53. doi: 10.1016/j.jacc.2005.10.076. [DOI] [PubMed] [Google Scholar]
- Oviedo PJ, Hermenegildo C, Tarín JJ, Cano A. Raloxifene promotes prostacyclin release in human endothelial cells through a mechanism that involves cyclooxygenase-1 and -2. Fertil Steril. 2005;83(6):1822–9. doi: 10.1016/j.fertnstert.2004.11.075. [DOI] [PubMed] [Google Scholar]
- Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res. 2002;90(10):1087–92. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- Park WC, Jordan VC. Selective estrogen receptor modulators (SERMS) and their roles in breast cancer prevention. Trends Mol Med. 2002;8(2):82–8. doi: 10.1016/s1471-4914(02)02282-7. [DOI] [PubMed] [Google Scholar]
- Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Clin Obstet Gynecol. 2007;50(2):354–61. doi: 10.1097/GRF.0b013e31804a838d. [DOI] [PubMed] [Google Scholar]
- Pavao M, Traish AM. Estrogen receptor antibodies: specificity and utility in detection, localization and analyses of estrogen receptor alpha and beta. Steroids. 2001;66(1):1–16. doi: 10.1016/s0039-128x(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Pelzer T, Loza PA, Hu K, Bayer B, Dienesch C, Calvillo L, Couse JF, Korach KS, Neyses L, Ertl G. Increased mortality and aggravation of heart failure in estrogen receptor-beta knockout mice after myocardial infarction. Circulation. 2005;111(12):1492–8. doi: 10.1161/01.CIR.0000159262.18512.46. [DOI] [PubMed] [Google Scholar]
- Pettersson K, Gustafsson JA. Role of estrogen receptor beta in estrogen action. Annu Rev Physiol. 2001;63:165–92. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- Pirwany IR, Fleming R, Greer IA, Packard CJ, Sattar N. Lipids and lipoprotein subfractions in women with PCOS: relationship to metabolic and endocrine parameters. Clin. Endocrinol (Oxf) 2001;54(4):447–53. doi: 10.1046/j.1365-2265.2001.01228.x. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112(4):453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Langer RD, Stefanick ML, Howard BV, Pettinger M, Anderson GL, Barad D, Curb JD, Kotchen J, Kuller L, Limacher M, Wactawski-Wende J, Women's Health Initiative Investigators Combined analysis of Women's Health Initiative observational and clinical trial data on postmenopausal hormone treatment and cardiovascular disease. Am J Epidemiol. 2006;163(7):589–99. doi: 10.1093/aje/kwj079. [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884–8. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Prieto J. Inflammation, HCC and sex: IL-6 in the centre of the triangle. J Hepatol. 2008;48(2):380–1. doi: 10.1016/j.jhep.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–59. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimian R, Chan L, Goel A, Poburko D, van Breemen C. Estrogen modulation of endothelium-derived relaxing factors by human endothelial cells. Biochem Biophys Res Commun. 2004;322(2):373–9. doi: 10.1016/j.bbrc.2004.07.137. [DOI] [PubMed] [Google Scholar]
- Rajkhowa M, Neary RH, Kumpatla P, Game FL, Jones PW, Obhrai MS, Clayton RN. Altered composition of high density lipoproteins in women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82(10):3389–94. doi: 10.1210/jcem.82.10.4318. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18(12):2854–65. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF. Sex steroids, cardiovascular disease, and hypertension: unanswered questions and some speculations. Hypertension. 2005;45(2):170–4. doi: 10.1161/01.HYP.0000151825.36598.36. [DOI] [PubMed] [Google Scholar]
- Register TC, Adams MR, Golden DL, Clarkson TB. Conjugated equine estrogens alone, but not in combination with medroxyprogesterone acetate, inhibit aortic connective tissue remodeling after plasma lipid lowering in female monkeys. Arterioscler Thromb Vasc Biol. 1998;18(7):1164–71. doi: 10.1161/01.atv.18.7.1164. [DOI] [PubMed] [Google Scholar]
- Reinecke H, Bogdanski J, Woltering A, Breithardt G, Assmann G, Kerber S, von Eckardstein A. Relation of serum levels of sex hormone binding globulin to coronary heart disease in postmenopausal women. Am J Cardiol. 2002;90(4):364–8. doi: 10.1016/s0002-9149(02)02490-6. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–30. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Rexrode KM, Manson JE, Lee IM, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108(14):1688–93. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- Robyr D, Gegonne A, Wolfee AP, Wahli W. Determinants of vitellogenin B1 promotor architecture. HFN3 and estrogen responsive transcription within chromatin. J Biol Chem. 2000;275(36):28291–300. doi: 10.1074/jbc.M002726200. [DOI] [PubMed] [Google Scholar]
- Rodríguez JA, Orbe J, Páramo JA. Metalloproteases, vascular remodeling and atherothrombotic syndromes. Rev Esp Cardio. 2007;60(9):959–67. doi: 10.1157/13109649. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Ruf JC. Wine and polyphenols related to platelet aggregation and atherothrombosis. Drugs Exp Clin Res. 1999;25(2-3):125–31. [PubMed] [Google Scholar]
- Salamanca DA, Khalil RA. Protein Kinase C Isoforms as Specific Targets for Modulation of Vascular Smooth Muscle Function in Hypertension. Biochem Pharmacol. 2005;70(11):1537–47. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada M, Higashi Y, Nakagawa K, Tsuda M, Kodama I, Kimura M, Chayama K, Ohama K. A comparison of low-dose and standard-dose oral estrogen on forearm endothelial function in early postmenopausal women. J Clin Endocrinol Metab. 2003;88(3):1303–9. doi: 10.1210/jc.2002-021147. [DOI] [PubMed] [Google Scholar]
- Sánchez M, Galisteo M, Vera R, Willar IC, Zarzuelo A, Tamargo J, Pérez-Vizcaíno F, Duarte J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endotelial dysfunction in spontaneous hypertensive rats. J Hypertension. 2006;24(1):75–84. doi: 10.1097/01.hjh.0000198029.22472.d9. [DOI] [PubMed] [Google Scholar]
- Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17 – estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol. 2007;293(6):H3713–9. doi: 10.1152/ajpheart.00736.2007. [DOI] [PubMed] [Google Scholar]
- Seli E, Guzeloglu-Kayisli O, Kayisli UA, Kizilay G, Arici A. Estrogen increases apoptosis in the arterial wall in a murine atherosclerosis model. Fertil Steril. 2007;88(4 Suppl):1190–6. doi: 10.1016/j.fertnstert.2007.01.132. [DOI] [PubMed] [Google Scholar]
- Sgarabotto M, Baldini M, Dei Cas A, Manotti C, Luciana Barilli A, Rinaldi M, Benassi L, Bacchi Modena A. Effects of raloxifene and continuous combined hormone therapy on haemostasis variables: a multicenter, randomized, double-blind study. Thromb Res. 2007;119(1):85–91. doi: 10.1016/j.thromres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat Rev Mol Cell Biol. 2001;2(5):392–6. doi: 10.1038/35073095. [DOI] [PubMed] [Google Scholar]
- Sherman TS, Chambliss KL, Gibson LL, Pace MC, Mendelsohn ME, Pfister SL, Shaul PW. Estrogen acutely activates prostacyclin synthesis in ovine fetal pulmonary artery endothelium. Am J Respir Cell Mol Biol. 2002;26(5):610–6. doi: 10.1165/ajrcmb.26.5.4528. [DOI] [PubMed] [Google Scholar]
- Shifren JL, Rifai N, Desindes S, McIlwain M, Doros G, Mazer NA. A Comparison of the Short-Term Effects of Oral Conjugated Equine Estrogens vs. Transdermal Estradiol on C-Reactive Protein, Other Serum Markers of Inflammation and Other Hepatic Proteins in Naturally Menopausal Women. J Clin Endocrinol Metab. 2008 Feb 26; doi: 10.1210/jc.2007-2193. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular disease. Arterioscler Thromb Vasc Biology. 2005;25(9):1767–75. doi: 10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- Simone JA, Lin F, Wittinghoff E, Bittner V, for the Heart and Estrogen-progestin Replacement Study (HERS) Research Group The Relation of Postmenopausal Hormone Therapy to Serum Eric Acid and the Risk of Coronary Heart Disease Events: The Heart and Estrogen-Progestin Replacement Study (HERS) Annals of Epidemiology. 2006;16(2):138–145. doi: 10.1016/j.annepidem.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Scorticati C, Mannella P, Fadiel A, Giretti MS, Fu XD, Baldacci C, Garibaldi S, Caruso A, Fornari L, Naftolin F, Genazzani AR. Estrogen receptor alpha interacts with Galpha13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol Endocrinol. 2006;20(8):1756–71. doi: 10.1210/me.2005-0259. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Mannella P, Fornari L, Caruso A, Varone G, Genazzani AR. Genomic and non-genomic effects of estrogens on endothelial cells. Steroids. 2004;69(8-9):537–42. doi: 10.1016/j.steroids.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Siow RC, Li FY, Rowlands DJ, de Winter P, Mann GE. Cardiovascular targets for estrogens and phtyoestrogens: transcriptional regulation of nitric oxide synthase and antioxidant defense genes. Free Radical Biology and Medicine. 2007;42(7):909–925. doi: 10.1016/j.freeradbiomed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Smith AM, Jones RD, Channer KS. The influence of sex hormones on pulmonary vascular reactivity: Possible vasodilator therapies for the treatment of pulmonary hypertension. Curr Vasc Pharmacol. 2006;4:9–15. doi: 10.2174/157016106775203090. [DOI] [PubMed] [Google Scholar]
- Soeder KJ, Snedden SK, Cao W, Della Rocca GJ, Daniel KW, Luttrell LM, Collins S. The beta3-adrenergic receptor activates mitrogen-activated protein kinase in adipocytes through a Gi-dependant mechanism. J Biol Chem. 1999;274(17):12017–22. doi: 10.1074/jbc.274.17.12017. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–85. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speroff L, Whitcomb RW, Kempfert NJ, Boyd RA, Paulissen JB, Rowan JP. Efficacy and local tolerance of a low-dose, 7-day matrix estradiol transdermal system in the treatment of menopausal vasomotor symptoms. Obstetrics & Gynecology. 1996;88(4 Pt 1):587–592. doi: 10.1016/0029-7844(96)00272-4. [DOI] [PubMed] [Google Scholar]
- Sunday L, Tran MM, Krause DN, Duckles SP. Estrogen and progestagens differentially modulate vascular proinflammatory factors. Am J Physiol Endocrinol Metab. 2006;291(2):E261–7. doi: 10.1152/ajpendo.00550.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28(4):576–82. doi: 10.1161/01.hyp.28.4.576. [DOI] [PubMed] [Google Scholar]
- Tamura M, Deb S, Sebastian S, Okamura K, Bulun SE. Estrogen up-regulates cyclooxygenase-2 via estrogen receptor in human uterine microvascular endothelial cells. Fertil Steril. 2004;81(5):1351–6. doi: 10.1016/j.fertnstert.2003.09.076. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989;59(3):477–87. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Tsang SY, Yao X, Chan FL, Wong CM, Chen ZY, Laher I, Huang Y. Estrogen and tamoxifen modulate cerebrovascular tone in ovariectomized female rats. Hypertension. 2004;44(1):78–82. doi: 10.1161/01.HYP.0000131659.27081.19. [DOI] [PubMed] [Google Scholar]
- Usui T. Pharmaceutical prospects of phytoestrogens. Endocr J. 2006;53(1):7–20. doi: 10.1507/endocrj.53.7. [DOI] [PubMed] [Google Scholar]
- Vera R, Sánchez M, Galisteo M, Villar IC, Jimenez R, Zarzuelo A, Pérez-Vizcaíno F, Duarte J. Chronic administration of genistein improves endothelial dysfunction in spontaneously hypertensive rats: involvement of eNOS, caveolin and calmodulin expression and NADPH oxidase activity. Clin Sci (Lond) 2007;112(3):183–91. doi: 10.1042/CS20060185. [DOI] [PubMed] [Google Scholar]
- Vickers MR, MacLennan AH, Lawton B, Ford D, Martin J, Meredith SK, DeStavola BL, Rose S, Dowell A, Wilkes HC, Darbyshire JH, Meade TW, WISDOM group Main morbidities recorded in the women's international study of long duration oestrogen after menopause (WISDOM): a randomised controlled trial of hormone replacement therapy in postmenopausal women. BMJ. 2007;335(7613):239. doi: 10.1136/bmj.39266.425069.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki A, Ikenoue N, Shinohara K, Watanabe K, Fukaya T. Effect of lower dosage of oral conjugated equine estrogen on inflammatory markers and endothelial function in healthy postmenopausal women. Arterioscler Thromb Vasc Biol. 2004;24(3):571–6. doi: 10.1161/01.ATV.0000115383.49802.0c. [DOI] [PubMed] [Google Scholar]
- Wang M, Crisostomo P, Wairiuko GM, Meldrum DR. Estrogen receptor-alpha mediates acute myocardial protection in females. Am J Physiol Heart Cir Physiol. 2006;290(6):H2204–9. doi: 10.1152/ajpheart.01219.2005. [DOI] [PubMed] [Google Scholar]
- Wassmann K, Wassmann S, Nickenig G. Progesterone antagonizes the vasoprotective effect of estrogen on antioxidant enzyme expression and function. Circ Res. 2005;97(10):1046–54. doi: 10.1161/01.RES.0000188212.57180.55. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Bonev AD, Nelson MT, Brayden JE. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca2+-dependant K+ channels. Circ Res. 1996;79(5):1024–30. doi: 10.1161/01.res.79.5.1024. [DOI] [PubMed] [Google Scholar]
- Widder J, Pelzer T, von Poser-Klein C, Hu K, Jazbutyte V, Fritzemeier KH, Hegele-Hartung C, Neyses L, Bauersachs J. Improvement of endothelial dysfunction by selective estrogen receptor-alpha stimulation in ovariectomized SHR. Hypertension. 2003;42(5):991–6. doi: 10.1161/01.HYP.0000098661.37637.89. [DOI] [PubMed] [Google Scholar]
- Wingrove CS, Garr E, Godslad If, Stevenson JC. 17beta-oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim Biophy Acta. 1998;1406(2):169–74. doi: 10.1016/s0925-4439(97)00097-5. [DOI] [PubMed] [Google Scholar]
- Woodman OL, Boujaoude M. Chronic treatment of male rats with daidzein and 17beta-oestradiol induces the contribution of EDHF to endothelium-dependent relaxation. Br J Pharmacol. 2004;141(2):322–8. doi: 10.1038/sj.bjp.0705603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne FL, Khalil RA. Testosterone and coronary vascular tone: implications in coronary artery disease. J. Endocrinol Invest. 2003;26(2):181–6. doi: 10.1007/BF03345150. [DOI] [PubMed] [Google Scholar]
- Zhang F, Ram JL, Standley PR, Sowers JR. 17 beta-Estradiol attenuates voltage dependant Ca2+ currents in A7r5 vascular smooth muscle cell line. Am J Physiol. 1994;266(4 Pt 1):C975–80. doi: 10.1152/ajpcell.1994.266.4.C975. [DOI] [PubMed] [Google Scholar]
- Zhai P, Eurell TE, Cooke PS, Lubahn DB, Gross DR. Myocardial ischemia-reperfusion injury in estrogen receptor-alpha knockout and wild-type mice. Am J Physiol Heart Circ Physiol. 2000;278(5):H1640–7. doi: 10.1152/ajpheart.2000.278.5.H1640. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295(5554):505–8. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]