Abstract
The adult pancreas has considerable capacity to regenerate in response to injury. We hypothesized that after partial pancreatectomy (Px) in adult rats, pancreatic-duct cells serve as a source of regeneration by undergoing a reproducible dedifferentiation and redifferentiation. We support this hypothesis by the detection of an early loss of the ductal differentiation marker Hnf6 in the mature ducts, followed by the transient appearance of areas composed of proliferating ductules, called foci of regeneration, which subsequently form new pancreatic lobes. In young foci, ductules express markers of the embryonic pancreatic epithelium – Pdx1, Tcf2 and Sox9 – suggesting that these cells act as progenitors of the regenerating pancreas. The endocrine-lineage-specific transcription factor Neurogenin3, which is found in the developing embryonic pancreas, was transiently detected in the foci. Islets in foci initially resemble embryonic islets in their lack of MafA expression and lower percentage of β-cells, but with increasing maturation have increasing numbers of MafA+ insulin+ cells. Taken together, we provide a mechanism by which adult pancreatic duct cells recapitulate aspects of embryonic pancreas differentiation in response to injury, and contribute to regeneration of the pancreas. This mechanism of regeneration relies mainly on the plasticity of the differentiated cells within the pancreas.
Keywords: Regeneration, Pancreatic progenitors, Dedifferentiation
Introduction
Studies of the regeneration of organs have led to several different paradigms for stem and progenitor cells. Tissue-specific stem cells are well documented in tissues with a continuous and rapid turnover of cells, such as skin, intestine and testis, but are more elusive in tissues with a slower turnover. In the lung, several different types of stem cells have been characterized, but they appear to be dispensable for normal lung tissue homeostasis and are invoked with tissue injury (Giangreco et al., 2009). In the liver, even though both hepatic stem cells and oval cells are recognized as tissue-specific stem cells, regeneration occurs mainly by replication of existing differentiated hepatocytes (Fausto and Campbell, 2003). Pancreas tissues also turn over slowly but have substantial regenerative capacity in response to injury. Following partial pancreatectomy (Px), pancreatic-duct ligation and cerulein-induced pancreatitis, pancreatic endocrine, acinar and/or duct tissues regrow (Bonner-Weir et al., 1993; Gu and Sarvetnick, 1993; Jensen et al., 2005; Wang et al., 1995). The identification of pancreatic stem cells has been elusive but they might exist.
Following a 90% Px in the adult rat (Bonner-Weir et al., 1993; Bonner-Weir et al., 1983) regeneration is extensive. After 4 weeks, the endocrine and exocrine pancreas increased eightfold and sixfold, respectively. The regenerative mechanisms include enhanced replication of pre-existing differentiated cells, hypertrophy of β-cells and differentiation of whole new lobes of pancreas (Bonner-Weir et al., 1983; Brockenbrough et al., 1988; Xu et al., 1999). By 60 hours after Px surgery, duct-enriched areas (termed foci of regeneration or focal areas) appear; these specialized areas undergo proliferation and differentiation into all pancreatic cell types, forming new lobes of pancreas (Bonner-Weir et al., 1993). We have hypothesized that mature pancreatic ducts could act as facultative stem cells or a pool of potential progenitors both in vivo and in vitro (Bonner-Weir et al., 2004; Yatoh et al., 2007). This process could be accomplished through dedifferentiation to a progenitor-like phenotype and then redifferentiation through a pathway such as used during normal embryonic development. Indeed, in the cerulein-induced pancreatitis model, in which only acinar cells are lost, the ensuing acinar regeneration occurs predominantly through acinar cell dedifferentiation into cells that resemble embryonic pancreatic precursors that proliferate; their subsequent redifferentiation to acinar cells parallels that of embryonic pancreas development (Jensen et al., 2005).
Development of the mammalian pancreas occurs by the sequential differentiation of multipotent pancreatic progenitors located at foregut endoderm (Gittes, 2009; Slack, 1995). These pancreatic progenitors can be defined by the cadre of transcription factors, including several hepatocyte nuclear factors (Tcf1/2, FoxA2, Hnf4 and Hnf6/Onecut-1) as well as Pdx1, Hb9, Ptf1a-p48 and Sox9 (Collombat et al., 2006; Murtaugh, 2007). The endocrine progenitor cells then appear within this progenitor pool and are recognized by the expression of the pro-endocrine factor Neurogenin3 (Ngn3). Nkx2.2, Pax4, Pax6, MafA, MafB and Arx are expressed later and result in sequential differentiation and maturation of different lineages of hormone-producing islet cells (Collombat et al., 2006; Murtaugh, 2007; Nishimura et al., 2006). A more recent study showed that one of the acinar-associated enzymes carboxypeptidase a1 (Cpa1) is expressed in Pdx1+ Ptf1a+ cells located at distal ‘tip’ domains in developing pancreatic tubules, marking a distinct compartment of multipotent cells in the embryonic pancreas (Zhou et al., 2007). Although much progress has been made in understanding the expression and function of factors regulating organogenesis and differentiation in embryonic pancreas, less is known about their role in the expansion of pancreatic, and specifically β-cell, mass after birth or during regeneration.
In the Px model, in which all pancreatic cell types are regenerated, the molecular changes during regeneration are still unknown. In the current study, we define stages of the regeneration after Px and examine the expression of factors related to pancreas development. We show that: (1) rapid loss of differentiated phenotype in mature ducts is a very early event in pancreatic regeneration after Px; (2) the regenerating foci originate from and are continuous with the common pancreatic duct (CPD); (3) the proliferating ducts of these foci possess a molecular profile that is similar to pancreatic progenitors; and (4) the differentiating endocrine β-cells derived from these cells follow a maturation pathway similar to that in embryonic development. In summary, we provide strong evidence that mature pancreatic duct cells can regress and recapitulate the embryonic differentiation program during regeneration. Here, we map the molecular events mediating this process of dedifferentiation and redifferentiation to the different pancreatic cell types. Clues from regeneration models might provide new approaches to induce the formation of new β-cells in vivo.
Results
Early loss of Hnf6 and downstream duct-related transcription factors occurs before proliferation during regeneration
The expression of the transcription factor Hnf6 is an important marker of the duct epithelial phenotype. During development, Hnf6 is expressed throughout the undifferentiated ductal epithelium and becomes restricted to the ducts in the adult pancreas; the absence of Hnf6 expression results in perturbed ductal morphogenesis (Pierreux et al., 2006). A recent study using conditional gene inactivation showed that Hnf6 has an important role both at early and late pancreatic development stages and is essential for maintenance of adult pancreatic duct morphology (Zhang et al., 2009). Therefore, we examined the expression of Hnf6 as a marker of differentiated ductal epithelial cells in the pancreas following Px.
We found a rapid loss of Hnf6, which precedes decreased expression of other ductal markers and proliferation of the duct cells. By Px+4 hour Hnf6 mRNA was significantly (P<0.01) decreased whereas mRNA for Sox9 and Tcf2 (Hnf1β) were not significantly decreased until 16 hours (Fig. 1A). In our previous study, increase in proliferating (BrdU+) cells in the CPD was detected only from Px +20 hours onwards; after proliferation, duct cells transiently (48-72 hours) expressed Pdx1 protein without a change in levels of Pdx1 mRNA (Sharma et al., 1999). Consistent with those findings, mRNA expression of another replication marker Pcna was increased approximately 2.5-fold (P<0.01) in isolated Px +16 hour CPD compared with CPD from time-matched sham-operated rats (Fig. 1A) by real-time RT-PCR. Previously, we suggested that the ductal phenotype was lost with replication but had not examined the timing (Sharma et al., 1999). Here, the expression of Hnf6 was already decreasing (~50%) by Px +4 hours, reaching 20% of the level observed in the sham-operated control by 16 hours (Fig. 1A). This decrease in Hnf6 mRNA precedes that of two other transcription factors Sox9 and Tcf2 (Fig. 1A).
Fig. 1.
Duct dedifferentiation is an early molecular change in proliferating CPDs after 90% Px in the rat. (A) Hnf6, Sox9, Tcf2 and Pcna mRNA was measured by real-time RT-PCR in CPD after Px surgery. By 4 hours after Px, Hnf6 mRNA had significantly decreased (~50%), with further decrease to 20% of sham-operated control at 16 hours and then partial recovery. The loss of Hnf6 mRNA precedes decreases in Sox9 and Tcf2 mRNAs and increase in Pcna mRNA. n=3-5 pooled samples each from three to four individual CPD; data are mean ± s.e.m. *P<0.01 for comparison with sham control (ANOVA with Dunnett's post-hoc test). (B,C) Hnf6 (B) and Sox9 (C) proteins are expressed in many scattered cells of the CPD in sham-operated pancreas. At Px +1-2 days, staining for both proteins is dramatically reduced in intensity and in number of positive CPD cells. By Px +3-4 days, their expression is strong in most duct cells, but levels return to that of sham-operated control by Px +5-6 days. Scale bars: 50 μm. (D) Epithelial marker Cdh1 (E-cadherin) and duct functional marker Cftr mRNA were similarly examined by real-time RT-PCR. Cftr mRNA had the tendency to decrease by Px+16 hour and remained down at Px+1day, with subsequent recovery. Cdh1 mRNA was initially increased but then decreased, reaching significantly lower levels than sham control only by Px +1 day with recovery at Px +2 days. n=2-4 pooled samples from three to four individual CPDs; data are mean ± s.e.m. *P<0.01 for comparison with sham [ANOVA (P<0.0001) with Dunnett's post hoc test]. (E,F) Quantification of the percentage of proliferating cells in total CPD ducts and differential Hnf6 protein expression in these Ki67+ proliferating cells in sham-operated, Px +10 hours and Px +1 day rats. The percentage of Ki67+ duct cells is not changed at Px +10 days, but is significantly increased at Px +1 day. In sham-operated pancreas, most proliferating CPD cells express Hnf6high as shown in F. By Px +10 hours, most cells are still not proliferating but are Hnf6low or Hnf6undetectable. At Px +1day, Hnf6 protein expression remains low but most of the cells are now in cell cycle and proliferating. Changes in populations of Hnf6 expression were statistically significant compared to sham-operated control; data are mean ± s.e.m. *P<0.01 for comparison with sham (ANOVA with Dunnett's post-hoc test).
Decreased Hnf6 protein also preceded proliferation. In adult rat pancreas, Hnf6 protein was expressed in ductal cells of the CPD (Fig. 1B), many small pancreatic ducts and some centroacinar cells (data not shown). At Px +1 day and +2 days, both the immunostaining intensity and number of positive cells for Hnf6 decreased in the CPD (Fig. 1B), indicating that duct cells change their molecular profile within 48 hours of Px surgery. At Px +3-4 days, both the number of Hnf6-expressing cells and their intensity greatly increased (Fig. 1B), but returned to near-sham level by Px +5-7 days (Fig. 1B). A similar expression pattern was observed for Sox9 (Fig. 1C). It is noted that, for unknown reasons, Hnf6 and Sox9 protein expression increased, whereas levels of their RNA did not (Fig. 1A). This pattern was similar to what we previously reported for Pdx1 expression in Px rats (Sharma et al., 1999). In addition to duct-associated transcription factors, mRNA expression of an epithelial marker, e-cadherin (Cdh1), and a functional duct marker, cystic fibrosis transmembrane conductance regulator (Cftr), decreased by Px +1 day, the period when ducts in the CPD begin to proliferate (Fig. 1D). Thus, the reduction of Hnf6 might initiate the loss of the differentiated phenotype and the dedifferentiation of ducts to a progenitor-cell-like phenotype.
In our previous studies using either 12 hour or 6 hour incorporation of BrdU before sacrifice, labeled cells were increased compared with the control at Px +24-36 hours (Bonner-Weir et al., 1993; Sharma et al., 1999); importantly there was no increase in BrdU-labeled cells at either 12 or 18 hours after Px. To further examine the relationship between loss of Hnf6 and proliferation, we co-stained for Hnf6 and the proliferation marker Ki67 in the CPD from sham-operated, Px +10 hours and Px +1 day rats (Fig. 1E,F). As with PCNA, Ki67 expression was induced during early G1 and was maintained until mitosis, during which it is degraded (Gerdes et al., 1984). In sham-operated rats (n=5) most epithelial cells in the CPD were Hnf6+ and 15.3±1.3% were Ki67+. At Px +10 hours (n=3), a greater proportion of the cells had decreased intensity of Hnf6 staining (more Hnf6low) than in the sham-operated control, and yet the percentage of Ki67+ cells was unchanged (12.4±6.6%). At Px +1 day (n=4), there was no further change in Hnf6 staining, but now 60.3±13.8% of the ductal epithelial cells were positive for Ki67. These data indicate that duct proliferation only occurs after the loss of Hnf6 protein. The dynamic expression pattern of Hnf6 protein in the CPD after Px might reflect its requirement in both maintaining duct differentiation (Px +1-2 days) and regulating growth of pancreatic progenitors (Px +3-4 days) during regeneration.
Extensive branching morphogenesis of the CPD forms foci of regeneration
Following rapid expansion of the ductal cells of the CPD at Px +1-2 days, areas of regenerating ductules are transiently seen starting at approximately 60 hours after Px (Bonner-Weir et al., 1993; Sharma et al., 1999). These areas are well-defined structures consisting of abundant stromal cells, increasingly branched ductules, and differentiating acinar and islet cells. Their appearance follows that of the proliferation of the CPD and their disappearance coincides with the growth of the pancreatic remnant (Bonner-Weir et al., 1993). The lack of a cell-lineage-tracing system in rats precludes the use of genetic-lineage tracing to show that the regenerating foci are derived from the CPD. Instead we examined the continuity of the ducts with the CPD in three ways.
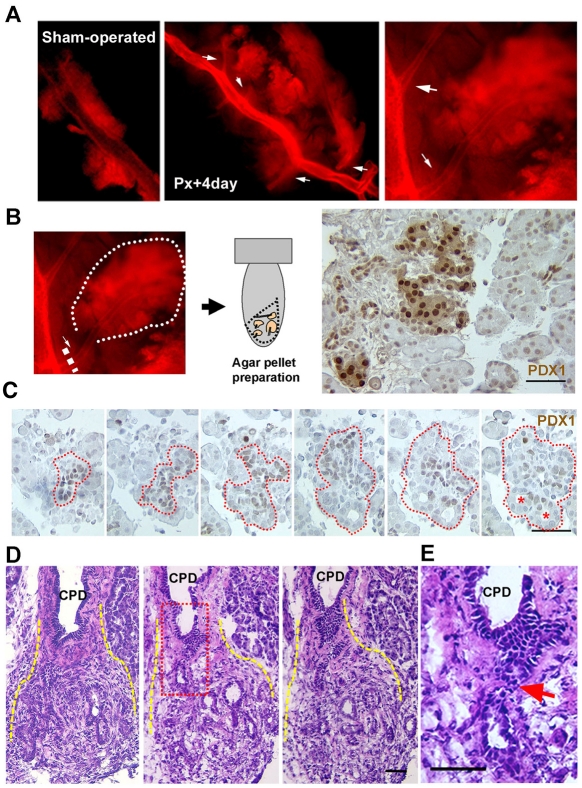
In the first, injection of eosin-gelatin solution into the pancreatic ductal tree allowed the visualization of its three-dimensional structure (for experimental details, see the Materials and Methods). There were consistently more branches along the CPD from Px +4 day rats (n=4) than from sham-operated rats (n=3) (Fig. 2A), suggesting that the CPD is probably the origin of the regenerating foci. To further characterize these branching tissues, we dissected them from the CPD and performed Pdx1 immunostaining to determine whether their histology resembled regenerating foci (Sharma et al., 1999). These CPD-derived tissues were comprised of stroma and Pdx1+ ducts (Fig. 2B,C). Then, on serial sections (Fig. 2C), we observed the smaller-branching Pdx1+ ducts and the open lumen structures seen in the foci (Sharma et al., 1999). Additionally, sequential transverse sections of isolated CPDs from Px rats (illustrated in supplementary material Fig. S1A) showed the continuity of the duct epithelium of foci and the CPD (Fig. 2D,E and supplementary material Fig. S1B). These data show that the foci tissues, with enriched duct and stroma profiles, are indeed continuous with, and probably originated from, the epithelium of the CPD.
Fig. 2.
The regenerating foci develop from the CPD. (A) In ductal casts made by injection of eosin and gelatin through the CPD, more numerous branches (arrows) from the CPD are seen at Px +4 days compared with sham-operated animals. The image on the far right is at higher magnification. (B,C) Pdx1 immunostaining of the branched tissues cut from Px +4 day CPD (n=7). Areas enriched in Pdx1+ duct profiles and stroma tissues resemble the young regenerating foci (detailed below) observed in pancreatic sections. Images from serial sections (C) show the branching of Pdx1+ ducts into open-lumen ductal structures as in foci. Red-dotted lines highlight gradual expansion from a small duct profile into more branching Pdx1+ ducts (red asterisks). The sections were collected every 10 μm. (D) In these consecutive sections transversely cut along an isolated CPD, the continuity of the ductal epithelium of the CPD and the branching ductules of an early-forming focal area (yellow lines) is shown. (E) High-magnification image of the red square in D; the arrow highlights the direct connection between CPD and a duct in foci. Scale bars: 50 μm.
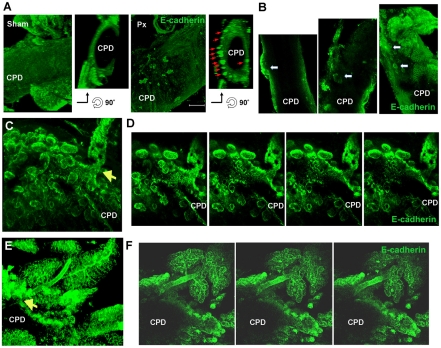
Finally, images of whole-mount immunostaining for the epithelial marker E-cadherin and progenitor marker Pdx1 in isolated CPDs from Px rats were taken as Z-stacks of optical sections and viewed either as reconstructed three-dimensional structures or as single frames. At Px +2 days, a number of E-cadherin+ structures began to protrude from the CPD (Fig. 3A,B); these structures extended to form elongated structures by Px +3-4 days (Fig. 3C-F). The structures expressed Pdx1 protein in branching networks with higher levels of Pdx1 expression at the tips of these structures (supplementary material Fig. S2). The Pdx1 expression was similar to that of the young regenerating foci in sectioned tissues from Px +3-4 day rats (see below, Fig. 6A). Together, these results show the continuity of the CPD and the emerging branching regenerating foci, thus suggesting that the regenerating foci originate from mature ducts, i.e. the CPD.
Fig. 3.
Expansion of epithelium from the CPD is detected in Px rats using whole-mount immunostaining for E-cadherin. (A) In three-dimensional reconstructions from Z-stacks of optical sections, small branches of E-cadherin+ cells were detected on the surface of Px +2 day CPD but not in sham-operated CPD. Orientation of the three-dimensional images to give transverse section of CPD clearly shows clusters of E-cadherin+ cells ‘budding’ from CPD (red arrows). Scale bar: 200 μm. (B-F) Although much of the surface of CPD is smooth, budding structures (arrows) can be seen along the CPD of Px +3 day rats (B). At higher magnification, some areas had extensive budding E-cadherin+ structures shown three-dimensionally (C) and in consecutive single frames (D). In Px+4 day CPD, elongated structure of new lobe seen in rotated three-dimensional view (E) and then in single frames (F) with clear connection to CPD (arrow).
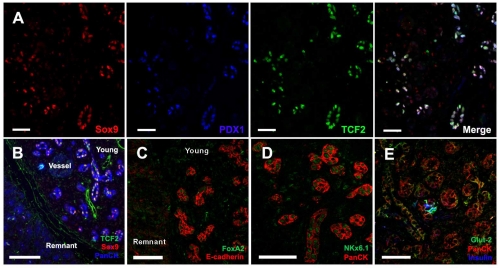
Fig. 6.
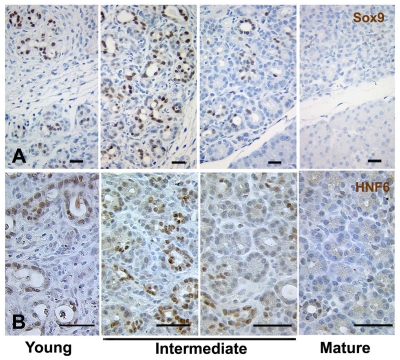
A set of shared progenitor markers is detected in ducts of young regenerating foci at Px +3-4 days. Immunostaining showed that ducts in young regenerating foci express proteins, mainly transcription factors, normally expressed in embryonic ductal progenitors. (A) Multiple progenitor proteins Sox9 (red), Tcf2 (green) and Pdx1 (blue) are expressed in the same ductules, as seen in embryonic duct epithelium. (B) Sox9 and Tcf2 co-localize in PanCk+ (blue) ductal epithelium in young foci, but not in acini of remnant tissues. (C) FoxA2 protein (green) is induced in E-cadherin+ (red) ductal epithelium. (D) Nkx6.1 (green) is expressed in ductal epithelium of foci (PanCk in red). (E) Glut-2 (green) is also detected in insulin (blue)-negative ductules (PanCk, red). Scale bars: 50 μm.
Regeneration following 90% Px is an asynchronous process and several stages of regenerating foci are observed within a single pancreas
Large numbers of foci can be detected within a single pancreas after Px (Fig. 4A); the number peaks at Px +3-4 days (Fig. 4B). The foci have well-defined boundaries and increasingly complex cellular organization; we suggest they are new lobes in formation. Based on their similarity with branching and differentiation seen in the developing rat pancreas (supplementary material Fig. S3A), we classified them by cellular composition and morphology: a young stage, which is predominantly stromal with a few duct tubules and an occasional hormone-positive cell; an intermediate stage, with more ductal profiles that result from further branching, but cells are mostly cuboidal without much specialization; and a complex, mature stage, which has densely packed ductal profiles and differentiating acinar cells and islets (Fig. 4C). Indeed, immunostaining of these foci showed enrichment of E-cadherin+ amylase− duct epithelium in young foci and amylase+ cells in more mature foci (Fig. 4D), implying duct-to-acinar differentiation, as seen in development of the rat embryonic pancreas (supplementary material Fig. S3B). These observations suggest that the foci reflect different stages of the regeneration process within the same pancreas, prompting us to examine the dynamics of gene expression within the stages.
Fig. 4.
Progressive stages of regenerating foci expanding from CPD are observed within a single pancreas at Px +3-4 day rats. (A) Low-magnification images of H&E-stained Px +3-4 day pancreatic sections show stages of regenerating foci labeled: (1) young foci, (2) intermediate foci and (3) mature foci. (B) Clearly identifiable regenerating foci were counted on one random section of each post-Px pancreas. Each symbol represents an individual rat; numbers of animals given in parentheses. Black bar indicates mean. (C) Higher magnification of the areas in A. (C,D) At Px +3-4 days, the ‘age’ of foci could be classified by tissue composition using (C) H&E staining or (D) immunostaining for E-cadherin (green) and amylase (red) (DAPI in blue). Ductules in young foci show regular open lumens without amylase staining; as the cells differentiate into acinar cells, the lumens become smaller and amylase is expressed. Scale bars: 50 μm.
Embryonic progenitor markers are expressed in ducts of young foci and are decreased in differentiating foci
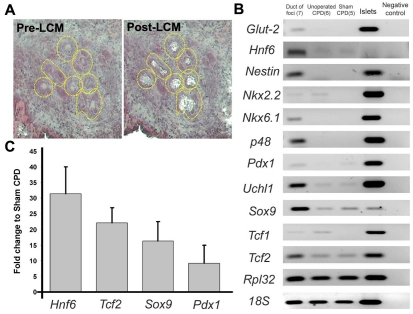
Since we hypothesized that differentiation of regenerating foci recapitulates pancreatic development, we examined the expression of markers of pancreatic or endocrine progenitors during development (Collombat et al., 2006; Murtaugh, 2007) in newly formed foci. The use of laser-capture microdissection (LCM) allowed the selective isolation of the newly formed ductal epithelium of young regenerating foci for examination of the gene expression profile (Fig. 5A). The enrichment of duct epithelium in LCM samples was confirmed by increased expression of the epithelial markers Cdh1 (3.6-fold, n=8 isolated ducts from young foci from Px +3-4 day rats) and Ck20 (cytokeratin 20; 7.2-fold) and decreased the mesenchymal gene Vim (vimentin; 50% lower) in LCM-captured ducts compared with whole foci. By semi-quantitative RT-PCR, expression of numerous pancreatic progenitor markers, including Hnf6, Nkx6.1, Ptf1a and Pdx1, was enhanced in LCM-captured ducts from foci compared with mature CPD ducts of control (both unoperated and sham-operated) rats (Fig. 5B). The ducts of foci had 10- to 30-fold more Pdx1, Hnf6, Sox9 and Tcf2 mRNA than CPDs from sham-operated rats by real-time PCR analysis (Fig. 5C).
Fig. 5.
Progenitor marker mRNAs are enriched in LCM-excised duct epithelium of young regenerating foci compared with mature ducts (CPDs). (A) LCM enables specific excision of duct epithelium from young regenerating foci (yellow-dotted lines); from more mature foci, the RNA quality is compromised by the exocrine enzymes. (B) By semi-quantitative RT-PCR, pancreatic progenitor markers show greater expression in LCM-captured ducts from regenerating foci than in mature control ducts (CPD) from unoperated and sham-operated rats. Sample numbers given in parentheses. Ribosomal proteins L32 (Rpl32) and 18S were used as internal control. (C) Fold change of Pdx1, Sox9, Tcf2 and Hnf6 mRNA in LCM-captured ducts was compared with mature (sham-operated CPD) ducts using real time RT-PCR analysis, with Rpl32 as reference gene. n=3 samples of pooled LCM-captured ducts (each from three to four Px +3-4 day rats). Data presented as mean ± s.e.m.
Furthermore, the progenitor marker proteins were strongly expressed in focal regions, as seen with immunostaining (Fig. 6). In young foci, the Pdx1+ ductal epithelium co-expressed Sox9 and Tcf2 (Fig. 6A,B), which are only observed in early pancreatic progenitor tubules (Maestro et al., 2003; Seymour et al., 2007). Similarly, other proteins expressed in insulin-negative pancreatic progenitors, including FoxA2 (Fig. 6C), Nkx6.1 (Fig. 6D) and Glut2 (Fig. 6E) (Lee et al., 2005; Oster et al., 1998; Pang et al., 1994), were found in young regenerating foci. The initial strong expression of Hnf6 and Sox9 protein in ducts of young foci waned as the foci matured (Fig. 7A,B), with a similar pattern for Tcf2 protein (data now shown). This decrease in progenitor marker expression as the foci mature suggests a dynamic differentiation process in regenerating foci after Px.
Fig. 7.
Progressive loss of progenitor proteins Sox9 and Hnf6 in ducts in differentiating foci. Sox9 (A) and Hnf6 (B) proteins are strongly expressed in ducts of young foci but decrease in maturing foci. Scale bars: 50 μm.
The endocrine progenitor marker Neurogenin 3 is activated during regeneration
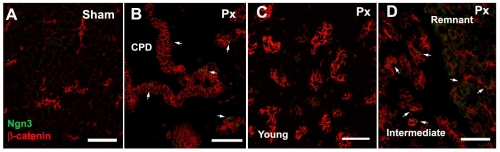
For pancreatic epithelial cells to enter the endocrine lineage, they must first activate expression of the pro-endocrine transcription factor Ngn3 (Apelqvist et al., 1999; Gradwohl et al., 2000). In the developing mouse pancreas, Ngn3 expression peaks at E14.5 and then slowly decreases to undetectable levels after birth (Wang et al., 2009; White et al., 2008); its expression in individual cells is brief. By immunostaining, Ngn3 protein was detected only in the ducts of intermediate foci (Fig. 8D), scattered cells in the CPD (Fig. 8B) and some centroacinar cells in the remnant (Fig. 8D); Ngn3 expression was not detected in sham-operated pancreas (Fig. 8A), in very young foci (Fig. 8C) or in mature foci (data not shown). This finding suggested that Ngn3 is activated transiently after Px, mimicking its developmental expression in a narrow window of time.
Fig. 8.
Ngn3 is activated in intermediate foci in Px +3-4 day rats. (A-D) Ngn3 (green) is expressed in scattered cells (arrows) in the CPD (B) and in scattered centroacinar cells (D) in remnants, as well as clustered in ductules (β-catenin, red) of intermediate stage regenerating foci (D). However, no Ngn3-positive cells were found in sham-operated pancreas (A) or young regenerating foci (C). Scale bars: 50 μm.
Similar differentiation programs are seen in both developing and regenerating endocrine pancreas
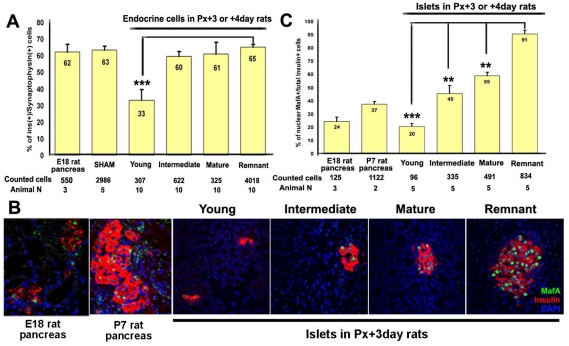
Both insulin+ and glucagon+ cells form within the regenerating foci (Bonner-Weir et al., 1993) and the total number of endocrine cells increase as the foci mature (Hayashi et al., 2003). To assess the percentage of endocrine cells that were β-cells in the foci, sections double-stained for insulin (β-cell) and synaptophysin (pan-endocrine) cells were analyzed. In young foci, the endocrine mass consisted of small clusters or single cells with low proportion of insulin+ synaptophysin+ cells; the proportion of β-cells significantly increased as the foci matured (Fig. 9A).
Fig. 9.
Composition of islet (percentage of β-cells) and expression of MafA in foci resemble that in developing pancreas. (A) Insulin-expressing cells were often observed as scattered insulin+ cells in young foci and as islets in remnant or more mature foci. The proportion of insulin+ cells was measured as the number of insulin+ cells as percent of total synaptophysin+ cells in foci. Insulin+ cells comprised a lower percentage of endocrine cells in young regenerating foci than in islets of remnant. Cells counted are from five sham, five Px +3 days, five Px +4 days and three E18.5 embryonic pancreas. Data presented as mean ± s.e.m. (B,C)In young regenerating foci, MafA is expressed in only a portion of insulin+ cells (~20%) as in embryonic pancreas. This percentage of insulin+ MafA+ cells progressively increased to ~55% in mature foci, which is less than that (~85-90%) in remnant pancreases of same animals. Cells counted are from five Px +3-4 days, three E18 embryonic and two P7 postnatal rat pancreases. Data presented as mean ± s.e.m. **P<0.01, ***P<0.001.
We further examined the development of β-cells in regenerating foci by immunostaining for known pancreatic progenitor and β-cell markers. As insulin+ cells ‘bud’ from ductules, expression of Sox9, a crucial transcription factor expressed in pancreatic progenitors (Lynn et al., 2007), diminished (supplementary material Fig. S4A). Similarly, in patterns reminiscent of embryonic pancreas development, Nkx2.2 (supplementary material Fig. S4B), Nkx6.1 (Fig. 6D) and Glut2 (Fig. 6E) were expressed in insulin-negative ducts in younger foci, but were restricted to insulin+ cells as the foci matured. Maf factors are considered to be essential for endocrine differentiation (Artner et al., 2007; Nishimura et al., 2008), with the transition from MafB+ insulin+ to MafA+ insulin+ cells, which is a late step in development, even occurring after birth (Nishimura et al., 2006). Although available MafB antibodies do not react with rat tissue, the percentage of total MafA+ total insulin+ cells was lower in young regenerating foci than in mature foci (Fig. 9B,C), suggesting that these β-cells are immature. Interestingly, even in mature foci, the proportion of MafA+ insulin+ cells still had not reached that of islets of the remnant, suggesting that the transition from an immature to a mature β-cell was incomplete at 1 week after Px.
Discussion
Here, we examined the molecular mechanisms underlying the regeneration seen in 90% Px rats. The main mechanisms for restoring tissue mass during pancreas regeneration are replication of pre-existing differentiated cells, hypertrophy of individual β-cells and differentiation from stem or progenitor cells (Bonner-Weir et al., 1993; Bonner-Weir et al., 2004; Bouwens and Rooman, 2005; Xu et al., 1999). Using a cellular-lineage-tracing system, it was suggested that self-replication of pre-existing β-cells is the main mechanism that contributes to increased β-cell mass, even in 50% Px mice (Dor et al., 2004); however, the contributions from stem or progenitor cells cannot be ruled out owing to the low labeling efficiency. More recently, several studies provided further evidence that the stem or progenitor cells located in different pancreatic pools can be activated for the repair process under various physiological challenges and injuries. The in vivo cerulein-induced pancreatitis model supported the concept that pancreatic acini dedifferentiated and subsequently redifferentiated following a genetic program resembling that of embryonic pancreatic precursors, but the authors clearly stated there were no duct-like intermediates (Jensen et al., 2005). Xu and co-workers showed activation of Ngn3+ cells either located within or next to ducts during regeneration after duct ligation in mice (Xu et al., 2008). Lineage-tracing studies from our laboratory showed that ducts expressing carbonic anhydrase II give rise to both endocrine and exocrine cells after birth and after pancreatic ductal ligation (Inada et al., 2008). Our current data in support of the notion that ducts contribute to the pancreatic regeneration show that the regeneration follows a dedifferentiation-redifferentiation paradigm, in which mature ducts dedifferentiate or regress to a progenitor-like state and then recapitulate the normal development program to form all differentiated pancreatic cell types. It is still not clear whether all the cells can equally form all different pancreatic cell types. Our interpretation (Kushner et al., 2010) of a recent paper is that duct cells are heterogeneous in their potential to act as pancreatic progenitors. This analysis is consistent with the results from Solar et al. (Solar et al., 2009) showing that cells expressing enough Hnf1β to have effective Cre-recombination-mediated excision do not give rise to endocrine cells. Further work is needed to define the population(s) of duct cells that act as endocrine progenitors.
During development, Hnf6 is initially expressed throughout the pancreatic epithelium, but becomes restricted to the ducts, where it has been described as the master regulator for maintenance of the differentiated ductal phenotype (Clotman et al., 2002; Pierreux et al., 2006; Zhang et al., 2009). Thus, changes in Hnf6 might be necessary to trigger the dedifferentiation (CPDs in Px +1-2 day rats) to potential progenitors required for regeneration. The rebound expression of Hnf6 protein in the CPD and mRNA and protein in foci could be interpreted either as ducts simply redifferentiating back to their mature phenotype or their gaining the phenotype of embryonic pancreatic progenitors (Maestro et al., 2003). The transcriptional profile of LCM-isolated ducts from young-intermediate foci of Px +3-4 day rats (Fig. 5B) supports the latter interpretation because Ptf1a and Nkx6.1 are expressed in embryonic pancreatic progenitors and not in mature ducts (Fig. 5B) (Burlison et al., 2008; Jensen et al., 1996). Similar rapid falls of Hnf6 mRNA and protein were seen during bile-duct obstruction (Holterman et al., 2002; Tan et al., 2006). If these drops after bile-duct ligation are blunted by adenoviral overexpression of Hnf6, the wave of proliferation necessary for repair does not occur (Holterman et al., 2002), suggesting a complex role for Hnf6 during regeneration in ducts, both in liver and pancreas.
A striking finding in our study was the detection of a continuum of regenerating foci within an individual pancreas. This continuum makes it possible to examine dynamic molecular changes at different stages of regeneration without the confounding influence of intra-animal bias. Indeed, the decreasing expression of progenitor proteins (Fig. 7) and increasing maturity of islets (Fig. 9) in more mature foci provide direct evidence of progressive differentiation. The appearance of a wide range of foci facilitated the detection of Ngn3+ endocrine progenitors during regeneration. In a previous study using Ngn3-GFP mice, no Ngn3+ cells were found during regeneration after 50% Px (Lee et al., 2006). However, in the duct-ligation model, Ngn3+ cells were seen within or next to ducts, although their origin was unknown (Xu et al., 2008). This discrepancy might be due to: (1) differences in the severity of injury as stimulus for regeneration and/or (2) weak GFP signal in 50% Px mice. Ngn3 immunostaining was detected in the remnants of Px +3-4 day rats (Fig. 8) in a few cells of the CPD and an occasional centroacinar cell; this latter finding supports the suggestion that centroacinar cells could also be precursors during regeneration (Stanger et al., 2005). Indeed, previous studies showed progenitor markers ubiquitin carboxyl-terminal esterase L1 (Uchl1, or pgp9.5), Pdx1 and Epiplakin1 expressed in centroacinar cells (Hosotani et al., 2004; Suzuki et al., 2003; Yokoyama-Hayashi et al., 2002; Yoshida et al., 2008).
The regenerating foci we have described are seen not only after partial pancreatectomy in rodents, but also in other physiological or experimental conditions under which pancreatic remodeling occurs, such as pancreatitis (Bockman et al., 1982), chemical-induced carcinogenesis (Jimenez et al., 1999; Rao and Reddy, 1980) and some diabetic animals (Lipsett and Finegood, 2002; Wang et al., 2005). The term ‘tubular complexes’ has been used to describe both degenerative structures from injured acini or proliferative structures as we described; both structures are sometimes seen in the same pancreas. The degenerative structures partially resemble our regenerating foci, but they have residual amylase expression (which we do not see in the early foci, see Fig. 4D), an irregular luminal surface (also not seen in our ductal profiles) and less epithelial proliferation than in our foci. More recently, similar structures containing proliferating ductules were observed in sitaglipin-treated human islet amyloid polypeptide transgenic (HIP) rats (Matveyenko et al., 2009). The regenerating foci in some of these other experimental systems also express pancreatic progenitor markers, such as Pdx1, Ngn3, Nestin and Uchl1 proteins (Wang et al., 2005), suggesting that the induction of tubular complexes or regenerating foci could be a common initiating step in pancreatic regeneration. These observations underscore the importance of duct cells functioning as progenitors in regulating pancreatic mass (Inada et al., 2008; Trivedi et al., 2001).
Clues for the regulatory factors that promote differentiation of these progenitors into mature pancreatic cells might come from the knowledge of pancreatic organogenesis. During early development, distinct signals released from adjacent structures including notochord (Tgfβ), aorta (Vegf) and cardiac mesoderm (Fgfs and Bmps) are thought to be essential for the patterning of pancreatic endoderm (Deutsch et al., 2001; Kim et al., 1997; Lammert et al., 2001). In regenerating foci of Px rats, these local signaling molecules might arise from the mesenchymal (stromal) tissue surrounding the proliferating ductules. Examination of molecular ‘crosstalk’ between ductules and stroma in foci to explore triggers for differentiation of duct progenitors in regenerating foci will be important.
The dedifferentiation-redifferentiation paradigm seen in regenerating pancreas after Px can be observed in other tissues of different organisms. The clearest model might be the regenerating limbs and tails of amphibians (Slack, 1983; Slack et al., 2008). It has been shown by tracking dextran-injected myotubes, that muscle cells could acquire plasticity during limb regeneration to form cartilage in the salamander Ambystoma mexicanum (axolotl) (Echeverri and Tanaka, 2002). Similar mechanisms might apply to the regeneration process in higher vertebrates (Odelberg, 2005). For example, after the resection of ventricular apex in adult zebrafish, cardiac cells close to the surgery site undergo epithelial-to-mesenchymal transition (EMT), a process that occurs in developing heart and is considered to be ‘dedifferentiation’, to regenerate the endothelial cells and smooth muscle that form new vessel tissues (Lepilina et al., 2006). However, in the mammalian heart, regeneration is mainly through the resident stem cells rather than through heart-derived dedifferentiated cells (Ausoni and Sartore, 2009). Using a genetic lineage tracing system, it was shown that, through a proliferation-dedifferentiation process, differentiated cells [lung Clara cells (Rawlins et al., 2009) and renal tubular epithelium (Humphreys et al., 2008)] were the main sources of multiple cell types in normal growth and repair after tissue injury. These data suggest that cell plasticity is a more general mechanism for repair and regeneration and that the early step of dedifferentiation often accompanies an increasing proliferation index and activation of factors essential for wound healing and tissue remodeling (Carlson, 2005).
In conclusion, our data demonstrate the important role pancreatic ducts have in regeneration after injury. Although we have previously shown that self-duplication of both acinar and endocrine cells contribute to regeneration (Brockenbrough et al., 1988), the present data support the additional contribution of the dedifferentiation or regression of differentiated duct epithelial cells to less-differentiated progenitors, which expand and then redifferentiate into differentiated endocrine and exocrine cells following the embryonic developmental program. This mechanism of regeneration does not rely on self-duplication or on stem cells, but rather relies on the plasticity of the differentiation of cells within an organ.
Materials and Methods
Partial pancreatectomy (Px) in adult rats
90% Px was performed as previously described (Bonner-Weir et al., 1983) in young adult male Sprague-Dawley (SD) rats, weighing 95-110 g (TACONIC Laboratories). Morning-fed blood glucose values and body weights were measured over the first week after surgery. Animals were sacrificed by overdose of anesthesia at Px +4 hours, +10 hours, +16 hours and +1-7 days. Consistent with our previous observations (Bonner-Weir et al., 1983), body weight gain was blunted for the first several days but by Px +7 days, the weight gain was similar for Px and sham control (data not shown); blood glucose levels only slightly differed starting at day 4 (104.4±19.2% of day 0 value; n=52) and were stably maintained until day 7. All animal procedures were approved by the Joslin Institutional Animal Care and Use Committee.
Isolation of rat islets and CPDs
Rat islets were isolated using a collagenase digestion protocol described before (Gotoh et al., 1985). Using a modification of this procedure, CPDs were isolated (Sharma et al., 1999). Briefly, 1 ml M199 medium containing 1.5 mg/ml collagenase (Boehringer Mannheim, Indianapolis, IN) was injected into the parenchyma of the pancreatic remnant of Px rats, or remnant equivalent tissue from sham-operated rats. After incubation at 37°C for 40 minutes with intermittent shaking, the tissue was washed three times with M199 medium containing serum and CPDs (one per animal) were dissected. The CPDs were then redigested in collagenase solution for another 5-10 minutes and further dissection removed contaminating tissue (stroma, islet, exocrine and blood vessels). Most of the stromal tissue is removed using this isolation protocol. For gene-expression analysis, at various times after surgery two to four isolated CPDs were pooled as an individual sample for RNA extraction; the pooled CPDs from time-matched sham-operated rats was used as a control to normalize the results. All data were obtained from two to five pooled samples.
Three-dimensional ductal casts of the regenerating pancreas
To investigate the direct continuity of the mature ducts and the regenerating foci, we made three-dimensional casts of the ductal tree at Px +4 days. In anesthetized animals, the duodenal end of the portions of the common bile duct that passes through the pancreas, CPD, was ligated and then 2-3 ml mixture of 5% eosin (v/v) and 5% gelatin (w/v; dissolved in warm PBS) was slowly injected into the hepatic end of the common bile duct. The injected gelatin was solidified by placing ice on the tissues. The CPDs were dissected, and connective tissue or surrounding acini was mechanically teased away, keeping the branching ducts intact. Tissues were then fixed in 4% paraformaldehyde (PFA, Fisher Scientific), dehydrated in ethanol and cleared with methyl salicylate (Sigma). Tissues were stored in methyl salicylate until imaging. The images were taken using a Nikon SMZ800 fluorescent dissecting microscope.
In some (n=7) Px +3 or 4 day rats, the tissues were isolated without fixation. The branches extending from CPDs from these samples were dissected, fixed in 4% PFA and pooled (n=3-4) and enrobed in GenePure LE agarose (w/v 2%, ISC BioExpress) to make paraffin-embedded blocks for histological examination of regenerating foci.
Transverse sectioning of isolated CPDs
To determine the continuity of CPDs and regenerating foci, cryoblocks containing isolated CPDs from Px +2, 3 or 4 day rats were sectioned transversely. To obtain transverse sections of the long tubular structure, an isolated CPD was frozen in OCT in horizontal orientation; then this ‘first block’ was turned 90 degrees and re-embedded. An average of 1000 consecutive sections were collected and stained with hematoxylin (Meyer's hematoxylin solution, Sigma) and Eosin (Shandon eosin Y, Thermo Scientific) (H&E).
Whole-mount immunofluorescent staining and construction of three-dimensional images
Whole-mount immunofluorescent staining for dissected CPDs was carried out using a protocol modified from embryonic pancreas (Ahnfelt-Ronne et al., 2007; Jorgensen et al., 2007). In brief, the isolated CPDs (three sham-operated, three Px +2 days, two Px +3 days and two Px +4 days) were hand dissected to remove excess acinar tissue, fixed in 4% PFA, equilibrated in cold absolute methanol, and then incubated in Dent's bleach (methanol:DMSO:H2O2, 4:1:1) at room temperature for 2 hours. The bleaching step is crucial because it facilitates antibody penetration and reduces autofluorescent background (Alanentalo et al., 2007). Tissues were then rehydrated in PBS, blocked in 0.5% TNB buffer [supplied with Tyramide Signal Amplification (TSA) fluorescein or Cy5 system, Perkin Elmer] and incubated in primary antibody. TSA amplification followed the manufacturer's instructions. Stained tissues were equilibrated in absolute methanol and immersed in BABB (a 1:2 mixture of benzyl alcohol to benzyl benzoate; both from Sigma). Finally, the tissues were mounted in BABB in concavity slides (Fisher Scientific) with coverslips. The slides can be stored at 4°C without loss of fluorescence intensity for months. All images of whole-mount CPD immunostaining were taken using the Zeiss LSM 710 Live Confocal microscope in the Joslin DERC Advanced Microscopy Core as optical Z-sections using Zeiss LSM software. Raw files were analyzed and representative images were exported using Zen 2009 Light Edition.
Laser-capture microdissection (LCM) on ducts in regenerating foci
LCM was performed on an Arcturus Pixcell lle using manufacturer's protocols as previously modified for pancreas (Ahn et al., 2007; Laybutt et al., 2003; Laybutt et al., 2002). Pancreas was embedded in Tissue Tek OCT medium and immediately frozen by immersion in chilled 2-methybutane. Frozen blocks were stored at −80°C until sectioning (8 μm). Sections adjacent to those used for LCM were stained with H&E to determine the landmarks of ‘young’ foci of regeneration. For LCM, frozen sections were rapidly incubated sequentially in 70% ethanol (fixation), in DEPC-treated water (rehydration), H&E, and dehydrated in 70, 95 and 100% ethanol with a final step in xylene. Once air-dried, selective cells [duct or combined (duct+stroma) of whole foci] of early regenerating foci were captured under appropriate amplitude and pulse duration adjusted to allow complete tissue capture by 7.5 μm laser beam. To obtain good quality RNA, the sampling was done within 15 minutes of bringing the sections to room temperature. As a result of the digestive enzymes in the acini, only young foci yielded good quality RNA.
Qualitative and real-time RT-PCR analysis
Total RNA for CPDs isolated at Px +4 hours, +16 hours and +1-7 days was extracted using TRIzol (Invitrogen). RNA from LCM-captured tissues was extracted using Arcturus PicoPure RNA Isolation Kit following the manufacturer's instructions. Purified RNA concentration was measured by NanoDrop TM 1000 Spectrophotometer (Thermo Scientific); reverse transcription was carried out using SuperScript III First-Strand Synthesis System (Invitrogen). Qualitative PCR reactions containing the mixture of cDNA, ReddyMixTM PCR Master Mix (ABgene) and sense and antisense primers (supplementary material Table S1) were processed in a thermal cycler (Bio-Rad) for indicated cycles. Samples were separated in 1.2% agarose gel. Real-Time PCR was performed using ABI Prism® SDS 7900 PCR machine And SYBR-based detection (Applied Biosystems). Relative quantification analysis was used to determine the relative expression of target genes between different samples. Ribosomal protein L32 and S25 genes were used as the reference genes, and quantification was performed using the ΔΔCT method (Livak and Schmittgen, 2001).
Immunohistochemistry and image processing
For immunostaining, pancreas was excised, and either fixed in 4% PFA, embedded in paraffin and sectioned (5 μm) or embedded in Tissue Tek OCT medium and immediately frozen by immersion in chilled 2-methybutane. Frozen blocks were stored at −80°C until sectioning (8 μm). Primary antibodies are listed in supplementary material Table S2. All biotinylated and fluorescent-conjugated (Texas Red, Cy2, Cy3, Cy5 or AMCA) antibodies were from Jackson ImmunoResearch; streptavidin-conjugated Alexa Fluor 488 antibody was obtained from Invitrogen. Antigen retrieval was performed by either microwave treatment in 10 mM citrate acid buffer or in a PickCell 2100 antigen retriever in Retrievagen A solution (BD Biosciences). Peroxidase-based immunohistochemistry (ABC kit, Vector Labs) used chromagen 3,3′-diaminobenzidine (DAB, Sigma) and counterstaining of Harris's Hematoxylin (Sigma). For immunofluorescence, either TSA system (Perkin Elmer) or biotin-streptavidin method was used. DAPI was used for nuclear staining. Stained sections were examined on Olympus BH-2 microscope or in confocal mode on a Zeiss LSM 410 microscope. Final images were compiled using Adobe Photoshop. All immunostaining results were reproducibly examined in at least five individual animals unless otherwise stated. For the percentage of MafA+ insulin+ in each stage of regenerating foci, more than 20 random microscopic fields of pancreatic sections from five individual Px rats were quantified using NIH ImageJ software. Data are displayed as mean ± s.e.m.
Statistical analysis
Using GraphPad Prism 4, one-way ANOVA analyses followed by Dunnett's comparison test were done to compare mRNA changes (Fig. 1A,D), percentage of Ki67+ cells in total CPD ducts and Hnf6 expression in total Ki67+ CPD ducts (Fig. 1E). The percentages of insulin+ synaptophysin+ and MafA+ insulin+ cells among individual cell populations (Fig. 9) were compared using two-tailed unpaired Student's t-test.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institutes of Health R01 DK 66056 (S.B.-W.), P30 DK36836 [Joslin Diabetes and Endocrinology Research Center (DERC) Advanced Microscopy Core] as well as the Diabetes Research and Wellness Foundation and an important group of private donors. W.-C.L. was supported by a JDRF postdoctoral fellowship (3-2008-72); J.M.R. by funds from the NIH/NIDDK (K01 DK076791); and W.N. by the Mary K. Iacocca Fellowship. Mouse monoclonal anti-ngn3 and anti-nkx6.1 antibodies (both generated by Ole D. Madsen) and mouse anti-nkx2.2 (generated by Thomas M. Jessell) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa, IA 52242. We thank Christopher J. Cahill for superb technical support and Dr Loeken (Joslin Diabetes Center) for the use of fluorescent dissecting microscope. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/16/2792/DC1
References
- Ahn Y. B., Xu G., Marselli L., Toschi E., Sharma A., Bonner-Weir S., Sgroi D. C., Weir G. C. (2007). Changes in gene expression in beta cells after islet isolation and transplantation using laser-capture microdissection. Diabetologia 50, 334-342 [DOI] [PubMed] [Google Scholar]
- Ahnfelt-Ronne J., Jorgensen M. C., Hald J., Madsen O. D., Serup P., Hecksher-Sorensen J. (2007). An improved method for three-dimensional reconstruction of protein expression patterns in intact mouse and chicken embryos and organs. J. Histochem. Cytochem. 55, 925-930 [DOI] [PubMed] [Google Scholar]
- Alanentalo T., Asayesh A., Morrison H., Loren C. E., Holmberg D., Sharpe J., Ahlgren U. (2007). Tomographic molecular imaging and 3D quantification within adult mouse organs. Nat. Methods 4, 31-33 [DOI] [PubMed] [Google Scholar]
- Apelqvist A., Li H., Sommer L., Beatus P., Anderson D. J., Honjo T., Hrabe de Angelis M., Lendahl U., Edlund H. (1999). Notch signalling controls pancreatic cell differentiation. Nature 400, 877-881 [DOI] [PubMed] [Google Scholar]
- Artner I., Blanchi B., Raum J. C., Guo M., Kaneko T., Cordes S., Sieweke M., Stein R. (2007). MafB is required for islet beta cell maturation. Proc. Natl. Acad. Sci. USA 104, 3853-3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausoni S., Sartore S. (2009). From fish to amphibians to mammals: in search of novel strategies to optimize cardiac regeneration. J. Cell Biol. 184, 357-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockman D. E., Boydston W. R., Anderson M. C. (1982). Origin of tubular complexes in human chronic pancreatitis. Am. J. Surg. 144, 243-249 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Trent D. F., Weir G. C. (1983). Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J. Clin. Invest. 71, 1544-1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S., Baxter L. A., Schuppin G. T., Smith F. E. (1993). A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes 42, 1715-1720 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Toschi E., Inada A., Reitz P., Fonseca S. Y., Aye T., Sharma A. (2004). The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr. Diabetes 5, 16-22 [DOI] [PubMed] [Google Scholar]
- Bouwens L., Rooman I. (2005). Regulation of pancreatic beta-cell mass. Physiol. Rev. 85, 1255-1270 [DOI] [PubMed] [Google Scholar]
- Brockenbrough J. S., Weir G. C., Bonner-Weir S. (1988). Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes 37, 232-236 [DOI] [PubMed] [Google Scholar]
- Burlison J. S., Long Q., Fujitani Y., Wright C. V., Magnuson M. A. (2008). Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev. Biol. 316, 74-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson B. M. (2005). Some principles of regeneration in mammalian systems. Anat. Rec. B New Anat. 287, 4-13 [DOI] [PubMed] [Google Scholar]
- Clotman F., Lannoy V. J., Reber M., Cereghini S., Cassiman D., Jacquemin P., Roskams T., Rousseau G. G., Lemaigre F. P. (2002). The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development 129, 1819-1828 [DOI] [PubMed] [Google Scholar]
- Collombat P., Hecksher-Sorensen J., Serup P., Mansouri A. (2006). Specifying pancreatic endocrine cell fates. Mech. Dev. 123, 501-512 [DOI] [PubMed] [Google Scholar]
- Deutsch G., Jung J., Zheng M., Lora J., Zaret K. S. (2001). A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 128, 871-881 [DOI] [PubMed] [Google Scholar]
- Dor Y., Brown J., Martinez O. I., Melton D. A. (2004). Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41-46 [DOI] [PubMed] [Google Scholar]
- Echeverri K., Tanaka E. M. (2002). Mechanisms of muscle dedifferentiation during regeneration. Semin. Cell Dev. Biol. 13, 353-360 [DOI] [PubMed] [Google Scholar]
- Fausto N., Campbell J. S. (2003). The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech. Dev. 120, 117-130 [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. (1984). Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 133, 1710-1715 [PubMed] [Google Scholar]
- Giangreco A., Arwert E. N., Rosewell I. R., Snyder J., Watt F. M., Stripp B. R. (2009). Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc. Natl. Acad. Sci. USA 106, 9286-9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes G. K. (2009). Developmental biology of the pancreas: a comprehensive review. Dev. Biol. 326, 4-35 [DOI] [PubMed] [Google Scholar]
- Gotoh M., Maki T., Kiyoizumi T., Satomi S., Monaco A. P. (1985). An improved method for isolation of mouse pancreatic islets. Transplantation 40, 437-438 [DOI] [PubMed] [Google Scholar]
- Gradwohl G., Dierich A., LeMeur M., Guillemot F. (2000). neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 97, 1607-1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu D., Sarvetnick N. (1993). Epithelial cell proliferation and islet neogenesis in IFN-g transgenic mice. Development 118, 33-46 [DOI] [PubMed] [Google Scholar]
- Hayashi K. Y., Tamaki H., Handa K., Takahashi T., Kakita A., Yamashina S. (2003). Differentiation and proliferation of endocrine cells in the regenerating rat pancreas after 90% pancreatectomy. Arch. Histol. Cytol. 66, 163-174 [DOI] [PubMed] [Google Scholar]
- Holterman A. X., Tan Y., Kim W., Yoo K. W., Costa R. H. (2002). Diminished hepatic expression of the HNF-6 transcription factor during bile duct obstruction. Hepatology 35, 1392-1399 [DOI] [PubMed] [Google Scholar]
- Hosotani R., Ida J., Kogire M., Fujimoto K., Doi R., Imamura M. (2004). Expression of pancreatic duodenal hoemobox-1 in pancreatic islet neogenesis after surgical wrapping in rats. Surgery 135, 297-306 [DOI] [PubMed] [Google Scholar]
- Humphreys B. D., Valerius M. T., Kobayashi A., Mugford J. W., Soeung S., Duffield J. S., McMahon A. P., Bonventre J. V. (2008). Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2, 284-291 [DOI] [PubMed] [Google Scholar]
- Inada A., Nienaber C., Katsuta H., Fujitani Y., Levine J., Morita R., Sharma A., Bonner-Weir S. (2008). Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci. USA 105, 19915-19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J., Serup P., Karlsen C., Nielsen T. F., Madsen O. D. (1996). mRNA profiling of rat islet tumors reveals nkx 6.1 as a beta-cell-specific homeodomain transcription factor. J. Biol. Chem. 271, 18749-18758 [DOI] [PubMed] [Google Scholar]
- Jensen J. N., Cameron E., Garay M. V., Starkey T. W., Gianani R., Jensen J. (2005). Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology 128, 728-741 [DOI] [PubMed] [Google Scholar]
- Jimenez R. E., Z'Graggen K., Hartwig W., Graeme-Cook F., Warshaw A. L., Fernandez-del Castillo C. (1999). Immunohistochemical characterization of pancreatic tumors induced by dimethylbenzanthracene in rats. Am. J. Pathol. 154, 1223-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen M. C., Ahnfelt-Ronne J., Hald J., Madsen O. D., Serup P., Hecksher-Sorensen J. (2007). An illustrated review of early pancreas development in the mouse. Endocr. Rev. 28, 685-705 [DOI] [PubMed] [Google Scholar]
- Kim S. K., Hebrok M., Melton D. A. (1997). Notochord to endoderm signaling is required for pancreas development. Development 124, 4243-4252 [DOI] [PubMed] [Google Scholar]
- Kushner J. A., Weir G. C., Bonner-Weir S. (2010). Ductal origin hypothesis of pancreatic regeneration under attack. Cell Metab. 11, 2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert E., Cleaver O., Melton D. (2001). Induction of pancreatic differentiation by signals from blood vessels. Science 294, 564-567 [DOI] [PubMed] [Google Scholar]
- Laybutt D. R., Sharma A., Sgroi D. C., Gaudet J., Bonner-Weir S., Weir G. C. (2002). Genetic regulation of metabolic pathways in beta-cells disrupted by hyperglycemia. J. Biol. Chem. 277, 10912-10921 [DOI] [PubMed] [Google Scholar]
- Laybutt D. R., Glandt M., Xu G., Ahn Y. B., Trivedi N., Bonner-Weir S., Weir G. C. (2003). Critical reduction in beta-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J. Biol. Chem. 278, 2997-3005 [DOI] [PubMed] [Google Scholar]
- Lee C. S., Sund N. J., Behr R., Herrera P. L., Kaestner K. H. (2005). Foxa2 is required for the differentiation of pancreatic alpha-cells. Dev. Biol. 278, 484-495 [DOI] [PubMed] [Google Scholar]
- Lee C. S., De Leon D. D., Kaestner K. H., Stoffers D. A. (2006). Regeneration of pancreatic islets after partial pancreatectomy in mice does not involve the reactivation of neurogenin-3. Diabetes 55, 269-272 [PubMed] [Google Scholar]
- Lepilina A., Coon A. N., Kikuchi K., Holdway J. E., Roberts R. W., Burns C. G., Poss K. D. (2006). A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 127, 607-619 [DOI] [PubMed] [Google Scholar]
- Lipsett M., Finegood D. T. (2002). beta-cell neogenesis during prolonged hyperglycemia in rats. Diabetes 51, 1834-1841 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402-408 [DOI] [PubMed] [Google Scholar]
- Lynn F. C., Smith S. B., Wilson M. E., Yang K. Y., Nekrep N., German M. S. (2007). Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc. Natl. Acad. Sci. USA 104, 10500-10505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestro M. A., Boj S. F., Luco R. F., Pierreux C. E., Cabedo J., Servitja J. M., German M. S., Rousseau G. G., Lemaigre F. P., Ferrer J. (2003). Hnf6 and Tcf2 (MODY5) are linked in a gene network operating in a precursor cell domain of the embryonic pancreas. Hum. Mol. Genet. 12, 3307-3314 [DOI] [PubMed] [Google Scholar]
- Matveyenko A. V., Dry S., Cox H. I., Moshtaghian A., Gurlo T., Galasso R., Butler A. E., Butler P. C. (2009). Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes 58, 1604-1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh L. C. (2007). Pancreas and beta-cell development: from the actual to the possible. Development 134, 427-438 [DOI] [PubMed] [Google Scholar]
- Nishimura W., Kondo T., Salameh T., El Khattabi I., Dodge R., Bonner-Weir S., Sharma A. (2006). A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev. Biol. 293, 526-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura W., Rowan S., Salameh T., Maas R. L., Bonner-Weir S., Sell S. M., Sharma A. (2008). Preferential reduction of beta cells derived from Pax6-MafB pathway in MafB deficient mice. Dev. Biol. 314, 443-456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odelberg S. J. (2005). Cellular plasticity in vertebrate regeneration. Anat. Rec. B New. Anat. 287, 25-35 [DOI] [PubMed] [Google Scholar]
- Oster A., Jensen J., Edlund H., Larsson L. I. (1998). Homeobox gene product Nkx 6.1 immunoreactivity in nuclei of endocrine cells of rat and mouse stomach. J. Histochem. Cytochem. 46, 717-721 [DOI] [PubMed] [Google Scholar]
- Pang K., Mukonoweshuro C., Wong G. G. (1994). Beta cells arise from glucose transporter type 2 (Glut2)-expressing epithelial cells of the developing rat pancreas. Proc. Natl. Acad. Sci. USA 91, 9559-9563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierreux C. E., Poll A. V., Kemp C. R., Clotman F., Maestro M. A., Cordi S., Ferrer J., Leyns L., Rousseau G. G., Lemaigre F. P. (2006). The transcription factor hepatocyte nuclear factor-6 controls the development of pancreatic ducts in the mouse. Gastroenterology 130, 532-541 [DOI] [PubMed] [Google Scholar]
- Rao M. S., Reddy J. K. (1980). Histogenesis of pseudo-ductular changes induced in the pancreas of guinea pigs treated with N-methyl-N-nitrosourea. Carcinogenesis 1, 1027-1037 [DOI] [PubMed] [Google Scholar]
- Rawlins E. L., Okubo T., Xue Y., Brass D. M., Auten R. L., Hasegawa H., Wang F., Hogan B. L. (2009). The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4, 525-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour P. A., Freude K. K., Tran M. N., Mayes E. E., Jensen J., Kist R., Scherer G., Sander M. (2007). SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc. Natl. Acad. Sci. USA 104, 1865-1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Zangen D. H., Reitz P., Taneja M., Lissauer M. E., Miller C. P., Weir G. C., Habener J. F., Bonner-Weir S. (1999). The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes 48, 507-513 [DOI] [PubMed] [Google Scholar]
- Slack J. M. (1983). Positional information in the forelimb of the axolotl: properties of the posterior skin. J. Embryol. Exp. Morphol. 73, 233-247 [PubMed] [Google Scholar]
- Slack J. M. (1995). Developmental biology of the pancreas. Development 121, 1569-1580 [DOI] [PubMed] [Google Scholar]
- Slack J. M., Lin G., Chen Y. (2008). The Xenopus tadpole: a new model for regeneration research. Cell Mol. Life Sci. 65, 54-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solar M., Cardalda C., Houbracken I., Martin M., Maestro M. A., De Medts N., Xu X., Grau V., Heimberg H., Bouwens L., et al. (2009). Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev. Cell 17, 849-860 [DOI] [PubMed] [Google Scholar]
- Stanger B. Z., Stiles B., Lauwers G. Y., Bardeesy N., Mendoza M., Wang Y., Greenwood A., Cheng K. H., McLaughlin M., Brown D., et al. (2005). Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell 8, 185-195 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Kadoya Y., Sato Y., Handa K., Takahashi T., Kakita A., Yamashina S. (2003). The expression of pancreatic endocrine markers in centroacinar cells of the normal and regenerating rat pancreas: their possible transformation to endocrine cells. Arch. Histol. Cytol. 66, 347-358 [DOI] [PubMed] [Google Scholar]
- Tan Y., Yoshida Y., Hughes D. E., Costa R. H. (2006). Increased expression of hepatocyte nuclear factor 6 stimulates hepatocyte proliferation during mouse liver regeneration. Gastroenterology 130, 1283-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi N., Hollister-Lock J., Lopez-Avalos M. D., O'Neil J. J., Keegan M., Bonner-Weir S., Weir G. C. (2001). Increase in beta-cell mass in transplanted porcine neonatal pancreatic cell clusters is due to proliferation of beta-cells and differentiation of duct cells. Endocrinology 142, 2115-2122 [DOI] [PubMed] [Google Scholar]
- Wang G. S., Rosenberg L., Scott F. W. (2005). Tubular complexes as a source for islet neogenesis in the pancreas of diabetes-prone BB rats. Lab. Invest. 85, 675-688 [DOI] [PubMed] [Google Scholar]
- Wang R. N., Kloppel G., Bouwens L. (1995). Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 38, 1405-1411 [DOI] [PubMed] [Google Scholar]
- Wang S., Jensen J. N., Seymour P. A., Hsu W., Dor Y., Sander M., Magnuson M. A., Serup P., Gu G. (2009). Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc. Natl. Acad. Sci. USA 106, 9715-9720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P., May C. L., Lamounier R. N., Brestelli J. E., Kaestner K. H. (2008). Defining pancreatic endocrine precursors and their descendants. Diabetes 57, 654-668 [DOI] [PubMed] [Google Scholar]
- Xu G., Stoffers D. A., Habener J. F., Bonner-Weir S. (1999). Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48, 2270-2276 [DOI] [PubMed] [Google Scholar]
- Xu X., D'Hoker J., Stange G., Bonne S., De Leu N., Xiao X., Van de Casteele M., Mellitzer G., Ling Z., Pipeleers D., et al. (2008). Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197-207 [DOI] [PubMed] [Google Scholar]
- Yatoh S., Dodge R., Akashi T., Omer A., Sharma A., Weir G. C., Bonner-Weir S. (2007). Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes 56, 1802-1809 [DOI] [PubMed] [Google Scholar]
- Yokoyama-Hayashi K., Takahashi T., Kakita A., Yamashina S. (2002). Expression of PGP9.5 in ductal cells of the rat pancreas during development and regeneration: can it be a marker for pancreatic progenitor cells? Endocr. J. 49, 61-74 [DOI] [PubMed] [Google Scholar]
- Yoshida T., Shiraki N., Baba H., Goto M., Fujiwara S., Kume K., Kume S. (2008). Expression patterns of epiplakin1 in pancreas, pancreatic cancer and regenerating pancreas. Genes Cells 13, 667-678 [DOI] [PubMed] [Google Scholar]
- Zhang H., Ables E. T., Pope C. F., Washington M. K., Hipkens S., Means A. L., Path G., Seufert J., Costa R. H., Leiter A. B., et al. (2009). Multiple, temporal-specific roles for HNF6 in pancreatic endocrine and ductal differentiation. Mech. Dev. 126, 958-973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Law A. C., Rajagopal J., Anderson W. J., Gray P. A., Melton D. A. (2007). A multipotent progenitor domain guides pancreatic organogenesis. Dev. Cell 13, 103-114 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.