Abstract
The function of occludin remains elusive. Proposed roles include maintenance of tight junction barriers, signaling and junction remodeling. To investigate a potential role in mediating cytokine-induced changes in barrier properties, we measured barrier responses to interferon-γ plus TNFα in control, occludin-overexpressing and occludin knockdown MDCK II monolayers. MDCK cells show a complex response to cytokines characterized by a simultaneous increase in the transepithelial electrical resistance and a decrease in the barrier for large solutes. We observed that overexpression of occludin increased and occludin knockdown decreased sensitivity to cytokines as assessed by both these parameters. It is known that caveolin-1 interacts with occludin and is implicated in several models of cytokine-dependent barrier disruption; we found that occludin knockdown altered the subcellular distribution of caveolin-1 and that partitioning of caveolin into detergent-insoluble lipid rafts was influenced by changing occludin levels. Knockdown of caveolin decreased the cytokine-induced flux increase, whereas the increase in the electrical barrier was unaltered; the effect of double knockdown of occludin and caveolin was similar to that of occludin single knockdown, consistent with the possibility that they function in the same pathway. These results demonstrate that occludin is required for cells to transduce cytokine-mediated signals that either increase the electrical barrier or decrease the large solute barrier, possibly by coordinating the functions of caveolin-1.
Keywords: Occludin, Tight junction, Caveolin-1, Lipid rafts, TNFα, IFNγ
Introduction
Tight junctions form the physiological and structural paracellular barrier and demarcate the apical and basolateral domains of polarized epithelial cells. Although a very large number of structural and signaling proteins have been identified at this specialized cell-cell contact (Gonzalez-Mariscal et al., 2003; Schneeberger and Lynch, 2004; Guillemot et al., 2008), the exact composition of individual tight junctions shows some tissue-specific variability. For example, among the integral membrane proteins associated with the tight junction different members of the claudin family of proteins are variably expressed in different tight junctions (Furuse and Tsukita, 2006; Van Itallie and Anderson, 2006). By contrast, the transmembrane protein occludin is an invariant component, found in all epithelial and endothelial tight junctions.
Occludin has been the subject of considerable interest since its discovery by Tsukita and co-workers in 1993 (Furuse et al., 1993). Overexpression studies demonstrated that it could influence the function of tight junction barriers (Balda et al., 2000), but the subsequent demonstration that the occludin knockout mouse had physiologically and structurally normal tight junctions (Saitou et al., 2000; Schulzke et al., 2005) argued against a critical role for occludin as a barrier-forming protein. However, despite having apparently normal barrier properties, the null phenotype includes numerous abnormalities in many organs.
Occludin is a member of a larger protein family of Marvel (MAL-related proteins for vesicle trafficking and membrane link)-domain-containing proteins (Sanchez-Pulido et al., 2002). Most of these proteins, for example synaptophysins, synaptogyrins and MAL (myelin and lymphocyte proteins) family members, are involved in vesicle trafficking, although their exact functions are unclear. Of note, many studies have demonstrated that occludin endocytosis is a common feature of tight junction injury and is closely associated with physiological barrier disruption (for a review, see Yu and Turner, 2008). Along with a putative role in trafficking, a second characteristic shared by many Marvel domain family members is an association with lipid rafts, cholesterol and/or caveolin. Occludin itself was shown to be a component of a detergent-resistant membrane microdomain (Nusrat et al., 2000; Lambert et al., 2005; Lynch et al., 2007) and several studies have implicated the importance of a cholesterol-rich membrane domain in tight junction function. For example, removal of cholesterol from wild-type MDCK cells results in decreased transepithelial electrical resistance (TER), but has no effect on TER in occludin-depleted MDCK cells (Yu et al., 2005). In addition, occludin has also been demonstrated in co-immunoprecipitation experiments to interact with the cholesterol-binding protein caveolin-1 (Nusrat et al., 2000; Lynch et al., 2007). There is significant evidence that occludin functions within a lipid microdomain, although it remains unclear whether occludin plays an active or passive role in organizing this microdomain.
Caveolin-1 is a small, ubiquitously expressed integral membrane protein that is involved in both clathrin-independent endocytosis (Pelkmans and Helenius, 2002; Nichols, 2003) and in formation of cholesterol-rich membrane microdomains. These microdomains can act as signaling platforms through their interactions with G-protein subunits, receptor and non-receptor protein kinases and small GTPases, among other proteins (Head and Insel, 2007). Several studies have implicated the importance of caveolin-1-dependent occludin endocytosis in tight junction disruption. For example, in vivo administration of TNFα (Marchiando et al., 2010) results in occludin endocytosis and increased epithelial permeability; neither occludin endocytosis nor increased permeability occur after cytokine administration to caveolin-1 knockout mice.
We hypothesized that although occludin might be dispensable for baseline barrier function, it might be required to mediate some signaling events that normally result in barrier remodeling and that caveolin-1 might be involved in this interaction. To test this hypothesis, we focused on stimuli that result in caveolin-dependent occludin endocytosis, in particular treatment of MDCK cells with TNFα plus IFNγ and with Latrunculin A (Shen and Turner, 2005). We found that barrier changes were increased in cells overexpressing occludin and decreased in occludin knockout cells. In addition, we found that depletion of occludin resulted in alterations in caveolin-1 distribution and partitioning in detergent-insoluble rafts. Together these results suggest an active requirement for occludin in mediating barrier responses to cytokines, perhaps through an effect on caveolin-1 or other lipid microdomain signaling processes at the junction.
Results
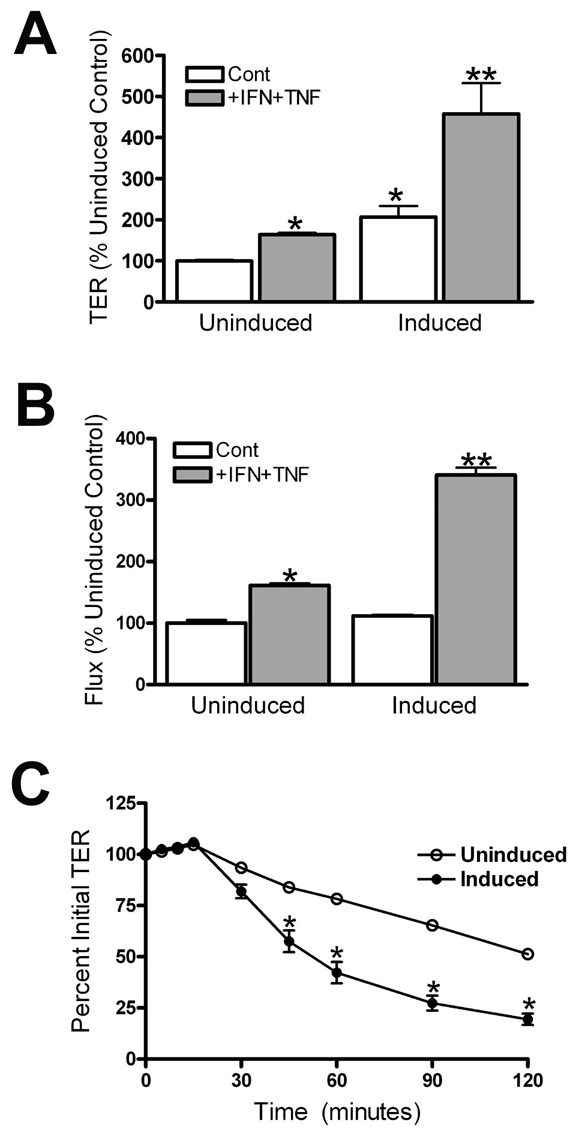
Overexpression of occludin increases baseline TER but not baseline flux
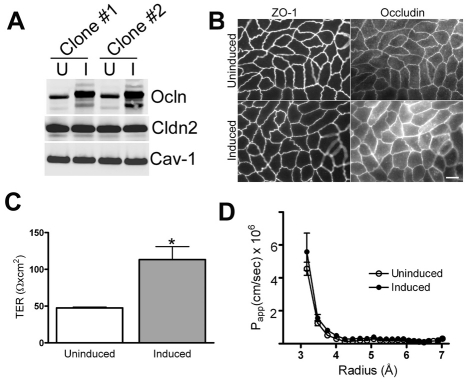
Occludin expression was increased about fourfold compared with control levels by removal of doxycycline in MDCK II Tet-Off cell lines (Fig. 1A), but there were no changes in the levels of claudin-2 or caveolin-1. Immunofluorescence analysis of uninduced and induced cell lines (Fig. 1B) showed that induction of occludin had no effect on ZO-1 staining, but it did result in increased immunofluorescence signal both at cell contacts and in intracellular compartments. Comparison of TER of uninduced and induced cell lines (Fig. 1C) demonstrates a greater than twofold increase in TER in induced cells; this increase is larger than has been reported elsewhere (McCarthy et al., 1996; Balda et al., 1996), but this difference is probably due to different cell handling procedures, as described in the Materials and Methods section. We further characterized the permeability of non-charged solutes using a graded series of PEG oligomers (Van Itallie et al., 2008) (Fig. 1D). This method has the advantage of separately revealing the permeability of the small claudin-based pores (less than 4 Å radius) from the leak pathway for larger solutes (larger than 4 Å radius). Although others have previously reported increased mannitol flux after occludin overexpression (McCarthy et al., 1996; Balda et al., 1996), we did not see flux differences between uninduced and induced cell lines. This is unlikely to represent a difference in sensitivity between the measurement techniques, since the PEG method can detect small changes in flux, but might be due to differences in occludin expression levels, clonal differences or culture conditions. Under our conditions we consistently found that increasing occludin levels increases TER but not the flux for larger solutes or the size discrimination of the junction.
Fig. 1.
Characterization of occludin-overexpressing MDCK cell lines. (A) Immunoblots of two cell lines that were uninduced (U) or induced (I) to express human occludin (Ocln) show an approximate fourfold increase in occludin levels, but no change in claudin-2 (Cln2) or caveolin-1 (Cav-1) expression. (B) Immunofluorescence imaging of ZO-1 and occludin in uninduced and induced cells reveals no change in the distribution or intensity of ZO-1 after occludin induction, but that the occludin signal in induced cells is brighter and more is present in intracellular compartments compared with uninduced cells. Scale bar: 8 μm. (C) Occludin induction (grey bar) increases TER by approximately twofold when compared with uninduced cells (white bar), from 47.6±1.3 Ω×cm2 to 113.3±17.6 Ω×cm2, *P<0.002. (D) Measurement of the size-dependence of paracellular permeability assessed with PEG oligomers reveals no change in flux for uncharged molecules in the size range of 3-7 Å after occludin induction (closed circles). All experiments were performed with two separate clonal cell lines at least twice.
Overexpression of occludin increases physiologic responses to treatment with proinflammatory cytokines or Latrunculin A
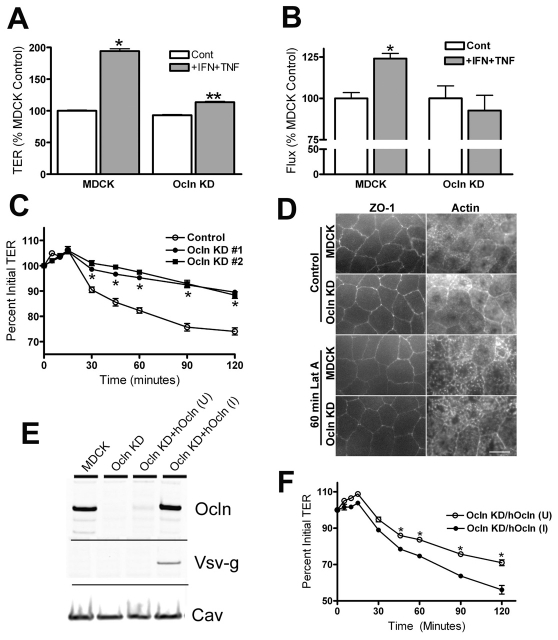
Co-treatment of monolayers of MDCK cells with IFNγ and TNFα is known to increase both TER and paracellular flux (Patrick et al., 2006). To test the role of occludin in the response to cytokines, we compared the TER of MDCK cells uninduced or induced to express human occludin after 24 hours of cytokine treatment. Exposure to 100 ng/ml IFNγ and 30 ng/ml TNFα resulted in a significant increase in TER (Fig. 2A), as previously described (Patrick et al., 2006). Occludin-overexpressing cells had higher basal TER, and the magnitude of their response to cytokine treatment was larger than in the uninduced cells (2.2-fold compared with 1.7-fold). Paracellular flux was similarly enhanced by occludin overexpression as measured by permeability of fluorescein-labeled 3-kDa dextran (Fig. 2B). This tracer size is larger than the claudin pores and reveals permeability of the leak pathway. Dextran flux was increased less than twofold after cytokine treatment of uninduced cells, but more than threefold in cells overexpressing occludin. Both occludin and ZO-1 localization was still continuous at the tight junction after cytokine treatment in both uninduced or induced cells. However, there appeared to be more intracellular occludin staining in the occludin-overexpressing cells after cytokine treatment compared with untreated induced cells (supplementary material Fig. S1).
Fig. 2.
Occludin overexpression increases cytokine responses. (A) Cytokine treatment results in a 1.7-fold increase in TER (from 49±1 to 83±3 Ω×cm2) in uninduced MDCK cells and in a greater than 2-fold increase in TER (from 100±13 to 222±36 Ωs×cm2) in cells overexpressing occludin. (B) Measurement of paracellular flux with fluorescein-labeled 3-kDa dextran after cytokine treatment of MDCK cells uninduced and induced to express human occludin shows a similar increase in response. Dextran flux is similar in both uninduced and induced untreated cell lines; flux increases 60% after cytokine treatment in uninduced cells and more than threefold in cells overexpressing occludin. *P<0.05 compared with uninduced controls; **P<0.02 when compared with cytokine-treated uninduced cells. All experiments were performed with two separate clonal cell lines, two or more times. (C) Time course of reduction of TER in cells uninduced and induced to overexpress occludin in response to Latrunculin A (added at 0 minutes). The electrical barrier is significantly more sensitive to disruption when occludin is overexpressed.
Shen and Turner (Shen and Turner, 2005) demonstrated that depolymerization of F-actin with Latrunculin A results in a concentration- and time-dependent drop in TER in MDCK II cells. This disruption is dependent on occludin internalization. To determine if overexpression of occludin might increase the sensitivity to Latrunculin A, we compared the time course of the drop in TER after administration of 0.5 μM Latrunculin A to uninduced and induced MDCK cells (Fig. 2C). Occludin overexpression resulted in a faster drop in TER after Latrunculin A administration compared with the response in uninduced MDCK cells. Thus, elevating occludin makes the barrier more responsive to both cytokines and an actin-depolymerizing drug.
Knockout of occludin in MDCK II cells does not alter the baseline barrier properties as measured by electrical resistance or size-dependence of permeability for non-charged solutes
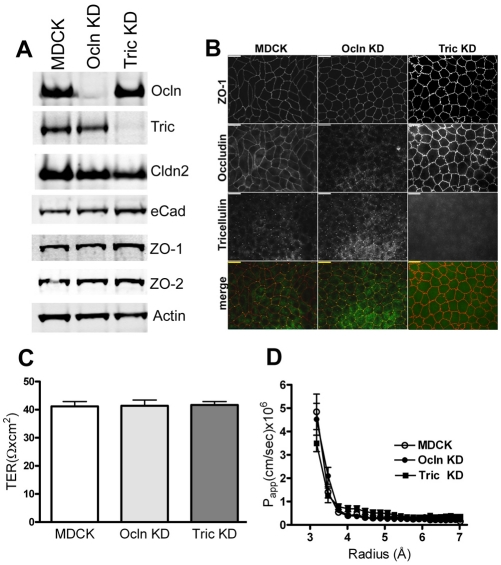
Since overexpression of occludin increased the magnitude of the barrier responses to cytokines, we next asked whether knockdown (KD) of occludin (Ocln) had the opposite effect. We first confirmed the previously published behavior of occludin KD cells and compared the results with tricellulin knockdown cells generated in our laboratory. Consistent with previously published results (Yu et al., 2005) we found that KD of Ocln in MDCK cell monolayers does not alter the levels (Fig. 3A) or localization (Fig. 1B) of several other tight junction and adherens junction proteins. We found a similar lack of effect of tricellulin KD on the levels of other tight junction proteins (Fig. 3A, right) and their distribution (Fig. 3B, right panel). All clonal knockdown lines expressed less than 5% of the control occludin or tricellulin levels and some faint staining in the Ocln KD cell lines is visible in Fig. 3 and in other figures. Unlike a previous report (Ikenouchi et al., 2008), we did not find that occludin knockdown altered tricellulin distribution. This might reflect variation in the degree of occludin knockdown or in antibody sensitivity. We normally see a very low level of bicellular staining with our tricellulin antibody and this is not significantly altered in the occludin-depleted cells. In addition, we did not observe a change in occludin distribution in tricellulin-depleted cells as was reported in occludin-depleted Eph4 cells (Ikenouchi et al., 2005).
Fig. 3.
Characterization of occludin and tricellulin knockdown (KD) MDCK cell lines reveals no changes in expression and localization of other proteins and barrier function. (A) Immunoblots of occludin (Ocln), tricellulin (Tric), claudin-2 (Cldn2), E-cadherin (eCad), ZO-1, ZO-2 and actin in MDCK II Tet-Off parental cells and representative Ocln KD and Tric KD cell lines. Ocln and Tric KDs expressed less than 5% of the respective endogenous protein levels; there were no consistent changes in the expression levels of the other tight and adherens junction proteins tested. Actin was used as a loading control. (B) Co-immunolocalization of ZO-1, Ocln and Tric in control and KD cell lines shows no consistent changes in cell shape or tight junction protein localization. Scale bars: 10 μm; the merged images show ZO-1 (red) and tricellulin (yellow). (C,D) Measurement of TER (C) and size-dependence of paracellular permeability assessed with PEG oligomers (D) revealed no significant change in barrier characteristics in control MDCK, Ocln KD and Tric KD monolayers; at least three separate clonal KD cell lines were compared for each measurement, each assayed in triplicate.
Also, consistent with results published by Yu and coworkers (Yu et al., 2005), we observed no significant change in TER after depletion of occludin (Fig. 3C), or in the permeability for uncharged solutes (Fig. 3D) as determined by the PEG profiling assay. This is consistent with published findings that occludin depletion does not alter barrier permeability for non-charged solutes, which are larger than the claudin pores as assessed with single tracers (Saitou et al., 1998; Yu et al., 2005; Schulzke et al., 2005). Furthermore, we saw no significant alteration in barrier properties in tricellulin-depleted MDCK II cells, as measured by either TER (Fig. 3C) or by permeability for PEG oligomers (Fig. 3D). This finding differs from a previous study performed in Eph4 cells (Ikenouchi et al., 2005) but might represent a cell-specific difference.
Knockdown of occludin attenuates barrier disruption induced by either pro-inflammatory cytokines or Latrunculin A
To determine if the effects of IFNγ and TNFα on paracellular permeability requires occludin, we treated parental and Ocln KD cells with these cytokines as described above and measured TER and flux of fluorescent 3-kDa dextran. After 24 hours of combined treatment with IFNγ and TNFα, MDCK II parental cells showed nearly a twofold increase in TER, whereas cytokine treatment of Ocln KD cells resulted in a TER increase of only 20% (Fig. 4A). Ocln KD cells similarly showed less responsiveness to cytokine treatment than did control cells when assessed by changes in dextran flux; in MDCK parental cells flux was significantly increased, whereas treatment of Ocln KD cells failed to increase dextran flux above that observed in untreated Ocln KD cells (Fig. 4B). Again, ZO-1 localization was unaltered after cytokine treatment in either control or Ocln KD cells (supplementary material Fig. S2). Thus, responses of the barrier to cytokines are directly related to occludin levels whether occludin is increased or decreased from control levels.
Fig. 4.
Ocln KD cell lines are less sensitive to proinflammatory cytokines. (A) Treatment of parental MDCK II Tet-Off cells resulted in a 1.9-fold increase in TER (from 56±2 to 107±6 Ω×cm2), while treatment of occludin KD cells increased TER only 1.2-fold above untreated levels (from 52±2 to 62±6 Ω×cm2). (B) Treatment of MDCK control and Ocln KD monolayers with TNFα and IFNγ resulted in a significant increase in flux of fluorescent 3-kDa dextran in control cells but not in Ocln KD cells. (C) Administration of Latrunculin A (0.5 μM) to monolayers of control MDCK and two separate Ocln KD cell lines resulted in time-dependent decreases in TER in all cell lines; however, the decrease in Ocln KD cells is significantly less than in MDCK controls at all time points longer than 30 minutes. (D) Rhodamine-phalloidin labeling of F-actin (right panels) at the cellular level of maximal ZO-1 staining intensity (left panels) in untreated MDCK (top row) and Ocln KD cells (second row) revealed similar actin localization; 60 minutes of 0.5 μM Latrunculin A (Lat A) treatment of MDCK (third row) and Ocln KD cells (bottom row) did not alter continuous ZO-1 staining in either cell line but resulted in similar changes in actin localization. (E) Immunoblot analysis of MDCK control cells, Ocln KD cells and Ocln KD cells transfected with a Tet-responsive vector containing Vsv-g-tagged human Ocln either uninduced (U) or induced (I) to express Ocln-Vsv-g; blots were probed for Ocln, using an antibody that recognizes both canine and human occludin, Vsv-g and caveolin-1; the results shown are representative of two similar clones; occludin induction levels are 1.5-fold control values. (F) Re-expression of an Ocln transgene restores Latrunculin A sensitivity. Treatment of Ocln KD cells (two independent clonal cell lines) uninduced (U) and induced (I) to express human Ocln with 0.5 μM latrunculin A demonstrates induction of Ocln is associated with increased sensitivity to latrunculin A and thus results in a larger decrease in TER (*P<0.05 or less, t-test).
To determine if the observed drop in electrical resistance after Latrunculin A treatment also required occludin, we compared the time course of TER after treatment with Latrunculin A in MDCK parental and Ocln KD cells. As previously reported, MDCK II control cells showed a concentration- and time-dependent decrease in TER (time course for 0.5 μM shown in Fig. 4C), although the drop in TER we observed was slower and less extensive than that reported previously (Shen and Turner, 2005). Depletion of occludin, but not tricellulin (not shown), resulted in a significant decrease in the rate of TER drop in response to Latrunculin A compared to its effect on the parental cell line. A similar reduced sensitivity to Latrunculin A in Ocln KD cells was evident at two other Latrunculin A doses tested (0.25 and 1 μM; not shown). In spite of the relative difference in sensitivity to Latrunculin A seen in parental MDCK and Ocln KD cell lines, disruption of the actin filament pattern was similar in both lines after treatment (Fig. 4D).
Inducible expression of occludin in KD cell lines restores sensitivity to Latrunculin A
To verify that the decreased sensitivity to Latrunculin A was due to depletion of occludin and not to variation among clonal cell lines, we tested the effect of Latrunculin A on Ocln KD cell lines that were engineered to inducibly express a human occludin transgene. Induction of occludin by removal of doxycycline resulted in occludin levels that were similar to those in control cells (Fig. 4E). When Ocln KD cells uninduced or induced to re-express occludin were treated with 0.5 μM Latrunculin A, there was a significant increase in the sensitivity of the induced cells compared with their uninduced controls (Fig. 4F). Latrunculin A sensitivity was restored to levels observed in the parental lines (compare with Fig. 4C).
Depletion of occludin alters localization of caveolin-1
A recent study by Turner and co-workers (Marchiando et al., 2010) using caveolin-1 knockout mice demonstrated that caveolin-1 is required for TNFα regulation of tight junctions in vivo. In addition, previous studies demonstrated that caveolin-1 is widely distributed throughout the cell on the apical and basolateral cell membranes and in intracellular vesicles but that a fraction colocalizes and is physically associated with occludin. For example, occludin co-immunoprecipitates with caveolin (Nusrat et al., 2000; Lynch et al., 2007), the two proteins colocalize at the tight junction at the ultrastructural level as revealed by immuno-gold techniques (Nusrat et al., 2000), and when endocytosis is stimulated they can be found in the same vesicles (Shen and Turner, 2005; Stamatovic et al., 2009). These observations led us to investigate whether depleting occludin might alter the distribution of caveolin-1. To compare the distribution of caveolin in control and Ocln KD cells, we co-plated both cell types on Transwell filters. Confocal maximum density projection images of caveolin-1, occludin and ZO-1 in confluent mixed cell populations (Fig. 5) revealed normal ZO-1 localization (Fig. 5, top left) in both parental and Ocln KD cells. Occludin immunofluorescent analysis of the mixed cell population (Fig. 5, top right) revealed parental (Ocln) and Ocln KD cell patches; the boundaries between these patches are outlined with a dotted line. The dotted line overlaid on the caveolin-1 signal (Fig. 5, Cav-1, lower right) revealed that caveolin in the Ocln KD cells appeared primarily associated with the lateral cell membranes, with reduced intracellular staining relative to the adjacent occludin-positive cells (Fig. 5, lower right). This immunofluorescence suggested that Ocln KD cells might have lower levels of caveolin; however, in spite of the apparent difference in caveolin distribution, caveolin protein levels are indistinguishable in control and knockdown cell lines as determined by immunoblotting (Fig. 4E).
Fig. 5.
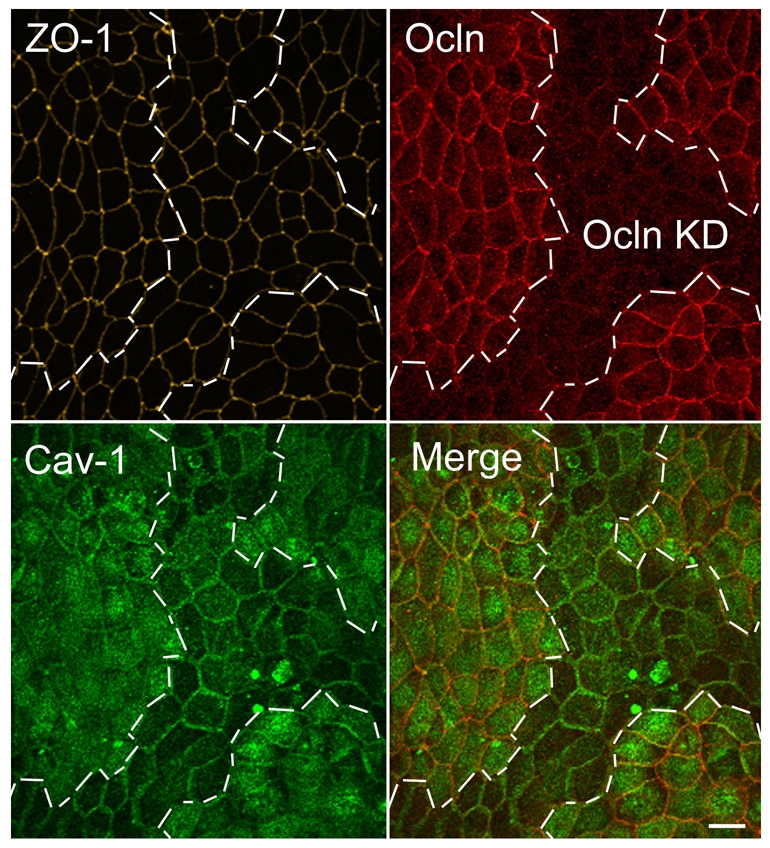
Maximum density confocal projection of occludin and caveolin-1 immunofluorescence in mixed populations of MDCK control and Ocln KD cells reveals global differences in caveolin distribution. MDCK parental and Ocln KD cells were co-cultured on Transwell filters, fixed and stained for Ocln, ZO-1 and caveolin and imaged using confocal microscopy. All sections through cells were collapsed to reveal the overall distribution of caveolin; boundaries of parental and KD are delineated with white dashed lines. ZO-1 immunofluorescence is identical in control and KD cells (yellow, top right). Ocln immunofluorescence (top right panel, red) identifies control and KD cells. Caveolin (bottom left panel, green) staining is brighter in control cells than in KD cells and more appears to be present in intracellular vesicles and/or on the apical surface. The merged image (right panel, red Ocln red; green caveolin) demonstrates brighter apical membrane and/or cytosolic staining of caveolin in Ocln-positive cells and more obvious lateral membrane staining in Ocln KD cells. Scale bar: 10 μm.
Depletion or overexpression of occludin alters the fraction of caveolin-1 associated with detergent-insoluble lipid rafts
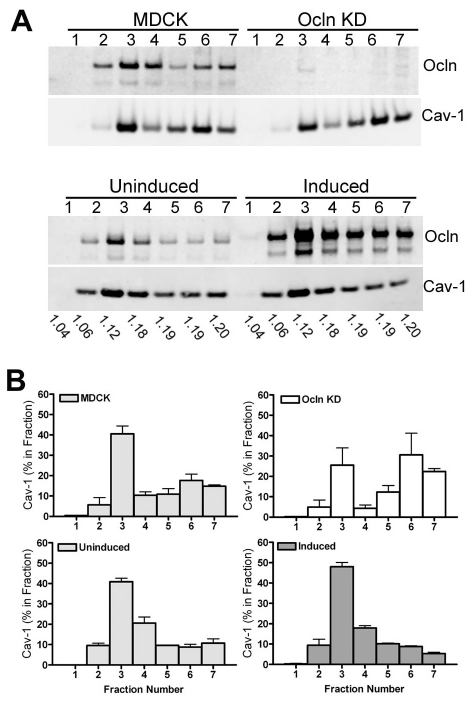
As noted above, some caveolin and occludin are normally associated with detergent-insoluble lipid rafts. Although only a fraction of the total cell caveolin would be expected to interact with occludin, we examined whether depletion or overexpression of occludin led to a detectable alteration of the fraction of caveolin that is associated with the Triton-X-100-insoluble membrane fraction. Sucrose gradient fractionation of Ocln KD cells compared with control MDCK cells (Fig. 6A, top panel) revealed a small but consistent shift of caveolin from low density (centered around 1.12 g/ml) raft-associated fractions to higher density (centered around 1.19 g/ml) non-raft fractions (quantified from several replicate gradients in Fig. 6B, top panels); this shift is reversed in the MDCK II cell induced (Fig. 6A,B, bottom panels) to express human occludin.
Fig. 6.
Partitioning of caveolin-1 with low-density lipid rafts is influenced by the level of Ocln. (A) Immunoblot of representative sucrose density gradient fractions from parental MDCK, Ocln KD (top panels) and MDCK cells uninduced (U) and induced (I) to express the human Ocln transgene (bottom panels). The density of each fraction is shown below the immunoblot. (B) Quantification of replicate sucrose gradients reveals a shift in caveolin-1 from less to more dense fractions in Ocln KD cells compared with the MDCK parental cell lines (compare top panels) and a shift in the opposite direction in Ocln-overexpressing cells (bottom two panels); each gradient was repeated with at least two different clonal cell lines.
Knockdown of caveolin-1 does not alter the cytokine-induced change in TER but does alter responsiveness of the pathway for large solutes
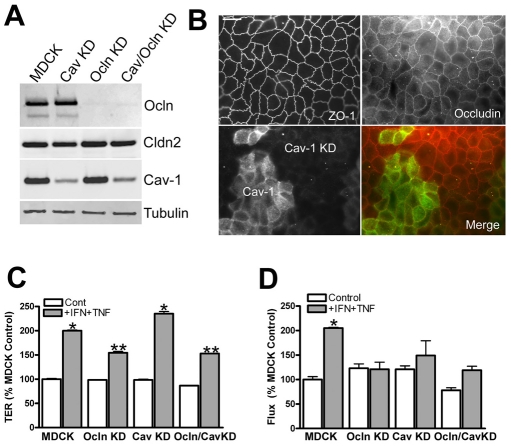
Because we showed that occludin can regulate the subcellular distribution of caveolin-1 and caveolin-1 controls several signaling pathways, we asked whether KD of cavolin-1 would also alter responsiveness of the barrier to cytokines. Stable caveolin-1 knockdown cell lines were generated using both the MDCK Tet-Off parental line to make a single KD and the Ocln KD cells to make a double KD. Immunoblot comparison of parental cells, caveolin-1 KD, Ocln KD and the double occludin-caveolin KDs (Fig. 7A) demonstrate our cavolin-1 KDs still express 15-20% of endogenous caveolin-1; this was in spite of testing many clonal cell lines and the use of the same sequence previously reported to result in expression levels of 5-10% of controls (Manninen et al., 2005). Caveolin knockdown does not alter occludin levels and there appeared to be small differences in claudin-2 levels, which was not seen in other KD cell lines; α-tubulin was used as a loading control. Knockdown of caveolin-1 does not alter occludin distribution (Fig. 7B); the localization of ZO-1 (7B, top left) and occludin (7B, top right panel) is identical in a mixed population of caveolin-1-expressing cells and caveolin-1 knockdown cells (Fig. 7B, lower right panel). Thus, caveolin-1 does not obviously regulate occludin location but occludin influences caveolin-1 location.
Fig. 7.
Caveolin KD does not affect occludin levels or distribution but does decrease the sensitivity of flux to cytokines. (A) Immunoblot analysis of KD cells lines reveals partial KD of caveolin (80-85%) in both parental and occludin KD lines. Claudin-2 levels appear slightly lower in the Cav-1 KD line, but this was not seen in other KD lines; α-tubulin was used as a loading control. (B) Cav-1 KD does not alter occludin or ZO-1 immunolocalization; this is best seen in a cell line with a mixed population of caveolin-expressing and KD cells: ZO-1 (top left) and occludin (top right) are similar in all cells; caveolin-1 (green, bottom left), and merged with occludin (red, bottom right). Scale bar: 5 μm. (C) Occludin but not caveolin KD decreases sensitivity to cytokines as measured by TER, but (D) both occludin and caveolin KD decrease sensitivity to cytokine treatment as measured by dextran flux; the effect of the double occludin-caveolin KD was not different from that of occludin KD alone. *P<0.02 compared with MDCK controls, **P<0.05 compared with cytokine-treated MDCK cells; each experiment was repeated with at least two separate clonal cell lines.
The measurement of TER in parental and knockdown cells (Fig. 7C) reveals little difference in baseline TER among all cell lines, but cytokine treatment of both parental MDCK cells and caveolin KD cells results in a twofold increase in electrical resistance, whereas the occludin and occludin–caveolin double KD cells show only a 50% increase in resistance. Flux measurement in the same cell lines shows little difference in baseline flux (Fig. 7D), but after cytokine treatment, flux is significantly increased only in the MDCK parental cells. Both occludin and caveolin KD decrease the cytokine effect on tight junction barrier properties, and the double knockdown is similar to the Ocln KD alone, suggesting caveolin and occludin might function in the same pathway.
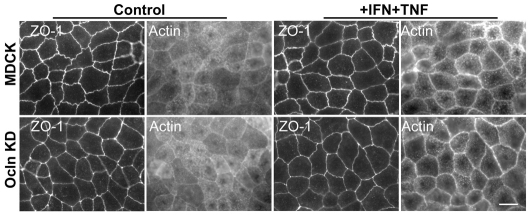
Occludin expression levels do not mediate alterations in cytokines responses via differential changes in actin localization
Co-treatment of MDCK II cells with IFNγ and TNFα is known to change F-actin localization (Patrick et al., 2006). To determine whether the differential responses of parental MDCK and Ocln KD cells to cytokines correlated with effects of Ocln KD on F-actin distribution, we compared actin localization in both cell lines before and after cytokine treatment. ZO-1 and F-actin localization in control cells (Fig. 8, left panels) demonstrated that at the cellular level of maximal ZO-1 staining intensity, F-actin appeared as a thin ring and in a speckled apical location, perhaps associated with microvilli in both parental and Ocln KD cells. After cytokine treatment (Fig. 8, right panels), actin appeared condensed into a brighter peri-junctional ring in both parental MDCK cells and in Ocln KD cells. Immunofluorescent localization of myosin 2B was also more distinct in the peri-junctional ring after cytokine treatment, but this change was also indistinguishable between parental and Ocln KD cells (supplementary material Fig. S2). Thus, cytokines induce a similar peri-junctional focusing of F-actin and myosin 2B independent of the occludin levels.
Fig. 8.
Occludin does not mediate changes in cytokine sensitivity via differential changes in actin localization after cytokine treatment. Fluorescent-actin localization in parental MDCK parental or occludin KD cells at the level of ZO-1 (left four panels) reveals diffuse apical actin and a thin peri-junctional actin ring. Treatment with IFNγ +TNFα (right four panels) caused no appreciable change in ZO-1 staining and a similar increased concentration of peri-junctional actin in both the parental and KD cells. Scale bar: 10 μm.
Discussion
There are two major new findings revealed by our studies. First, we demonstrate that occludin plays an active required role in cytokine-induced changes in tight junction barrier properties. Overexpression of occludin increases whereas knockdown decreases the response of MDCK cells to treatment with IFNγ plus TNFα. Second, knockdown of occludin plays an active role in the cellular location of caveolin-1. Increasing occludin levels increases association of caveolin-1 with detergent-resistant lipid rafts, whereas occludin KD results in increased caveolin in non-raft fractions. By contrast, KD of caveolin-1 does not alter occludin distribution. Finally, similar to the occludin KD, knockdown of caveolin is also associated with decreased responsiveness to cytokines, as measured by flux, but unlike occludin KD the increase in TER still occurs. Together, these results demonstrate that occludin is a required component in modulating the response of tight junction barriers to cytokines and suggests that in part, it might act through coordinating the functions of caveolin-1.
Cytokine treatment of intestinal cells results in increases in paracellular flux and decreases in TER, and many studies have described that disruption of tight junction barriers after exposure to IFNγ and TNFα results in displacement of tight junction proteins and in particular, occludin (Capaldo and Nusrat, 2009). More recently, it has been recognized that occludin endocytosis is a common feature of responses not only to cytokine treatment but also to exposure to bacteria, toxins (Yu and Turner, 2008) or some viruses (Coyne et al., 2007; Liu et al., 2009). This endocytosis coincides with and is required for barrier disruption, since blocking endocytosis prevents cytokine- or toxin-induced increases in tight junction permeability (Shen and Turner, 2005; Clayburgh et al., 2005; Schwarz et al., 2007). In addition, there is recent in vivo evidence for cytokine-induced occludin endocytosis, using genetically modified mice expressing GFP-occludin (Marchiando et al., 2010). However, there was no direct evidence that occludin is required for the barrier loss in any of the above experiments. Our results are the first to demonstrate that occludin is required for changes in tight junction barrier responses to cytokines and that the cytokine-induced changes in TER and flux are proportional to occludin levels.
In contrast to intestinal cells, the barrier changes observed in MDCK II cells differ in a noteworthy way. Although cytokines still induce an increase in paracellular flux there is no loss of continuity in tight junction staining for ZO-1 or occludin (this study) (Patrick et al., 2006) and TER actually increases, indicating that the electrical barrier is tightened after cytokine administration (Patrick et al., 2006). This reveals that occludin is not only involved in disrupting the barrier, presumably through endocytosis, but can also transduce signals that tighten the electrical barrier. TER reveals the instantaneous status of the paracellular space where small ions move during an imposed electrical field. By contrast, flux studies are typically performed over 1-3 hours and reveal dynamic instabilities or transient breaks in the barrier, which allow larger molecules to pass. Consequently, in MDCK cells occludin is required for cytokines to disrupt the macromolecular barrier either through endocytosis or altering the cytoskeleton as well as to enhance the electrical barrier.
Occludin endocytosis has been reported to occur through a variety of different mechanisms (Ivanov et al., 2005; Yu and Turner, 2008), although several recent studies have highlighted a role for caveolin in this process (Shen and Turner, 2005; Coyne et al., 2007; Marchiando et al., 2010). The most compelling of these is the recent demonstration that caveolin-1 knockout mice show decreased intestinal epithelial sensitivity to cytokines coincident with decreased occludin endocytosis, consistent with a requirement for caveolin-1 in this pathway (Marchiando et al., 2010). Consistent with this result, we find in MDCK cells that caveolin-1 knockdown also blunts the responses to cytokines. However, although endocytosis is demonstrably an important component of the response to cytokines (Foerg et al., 2007), another role for caveolin is the organization of lipid-dependent signaling domains (Wei et al., 1999).We speculate that extra occludin in MDCK cells could be acting to enhance association with, and therefore the response to, these signaling molecules; occludin KD could have the opposite effect. This hypothesis is supported not only by the occludin-dependent change in caveolin localization demonstrated in this study, but also by published studies demonstrating that occludin can immunoprecipitate caveolin (Nusrat et al., 2000; Lynch et al., 2007). Furthermore, caveolin but not occludin binds cholesterol, yet the disruption to the function of tight junction barriers caused by removal of cholesterol is dependent on the presence of occludin (Yu et al., 2005). Cholesterol removal decreases caveolin levels (Furuchi and Anderson, 1998) and thus lipid-raft-associated signaling molecules; it is possible that altered signaling through this complex can affect barrier function only when occludin is present to recruit caveolin and lipid-raft molecules to the junction. Candidate signaling molecules include src or G-protein subunits (Head and Insel, 2007).
Endocytosis after cytokine treatment of epithelial cells is induced in response to phosphorylation and activation of myosin II, either by myosin light chain kinase (Zolotarevsky et al., 2002; Clayburgh et al., 2005; Wang et al., 2005; Ma et al., 2005) or Rho-activated kinase (Utech et al., 2005). Activation is associated with a condensation of filamentous actin and myosin into the perijunctional ring (Clayburgh et al., 2005; Patrick et al., 2006). In our studies we observed a similar level of actomyosin condensation in response to cytokines in control and occludin KD cells. This suggests myosin activity is not dependent on occludin and supports the model in which myosin activation and cytoskeletal alterations are upstream of occludin-dependent steps. This finding is supported by published data (Shen and Turner, 2005) showing that inhibition of occludin endocytosis by Latrunculin A blocks junction disruption but not the actin reorganization induced by Latrunculin A. Additionally, cytokine treatment of caveolin-1 KD animals (Marchiando et al., 2010) fails to result in barrier disruption, in spite of an observed increase in myosin light chain phosphorylation.
In summary, these data are the first demonstration the occludin is required for cytokine-induced tight junction remodeling and increasing occludin levels actually increase the responses. We also show that occludin can affect the location and lipid raft partitioning of caveolin-1. Since caveolin-1 is required for cytokine-induced barrier changes in vivo (Marchiando et al., 2010), our results raise the possibility that occludin enables caveolin function at the tight junction where it participates in signals required for barrier remodeling. Whether occludin is mainly involved in endocytosis-dependent tight junction remodeling, as suggested by other studies, or is also an organizer of signaling proteins during remodeling remains unresolved, but will provide the focus for further investigation.
Materials and Methods
Antibodies
All antibodies were purchased from Zymed/Invitrogen (Carlsbad, CA) unless otherwise noted. Polyclonal guinea pig occludin antibodies were from HyCult Biotechnology (Uden, Netherlands); the anti-ZO-1 rat monoclonal was a gift from Bruce Stevenson (Stevenson et al., 1986). Polyclonal caveolin-1 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA), myosin 2B antibody was from Cell Signaling Technology (Beverly, MA), and actin, β-catenin and E-cadherin (DECMA) antibodies and Rhodamine-phalloidin were purchased from Sigma Chemical Co. (St Louis, MO).
Cell lines
Occludin-overexpressing MDCK II cell lines have been previously described (Medina et al., 2000). Stable MDCK II cell lines depleted of occludin (Ocln KD) were generously provided by Eveline Schneeberger, Massachusetts General Hospital, Charlestown, MA (Yu et al., 2005); additional cell lines were generated in our laboratory using the occludin knockdown vector in pSuper also provided by Eveline Schneeberger. Rescue constructs for the Ocln KD cells were constructed using a full-length human occludin cDNA with VSV-g tag on the C-terminus cloned into the tetracycline-inducible pTRE vector (Medina et al., 2000); to prevent inhibition of this construct by the pSuper occludin knockdown vector, the human occludin sequence was modified by the introduction of two silent mutations (Quikchange, Stratagene, LaJolla, CA) in the sequence complimentary to the knockdown vector. The oligonucleotide sequence used in this site-directed mutagenesis was: 5′-GACTATAGAGAAGAAAGTGAGGAATACATGGCTGCTGCTGATGA-3′. Stable cells were generated by co-transfection with pSVZeo (Invitrogen) using Lipofectamine (Invitrogen) and selected using 1 mg/ml Zeocin (Invitrogen). Tricellulin KD cells were similarly constructed in the MDCK II Tet-Off cell background (Clontech, Mountain View, CA) using either pTER (generously provided by M. van de Wetering and H. Clevers (Hubrecht Laboratory, Center for Biomedical Genetics, Utrecht, The Netherlands) or pSuper vectors (Oligoengine, Seattle, WA); the antisense oligonucleotides used were 5′-GGTACAACCTGGTGGGCTA-3′ and 5′-CCCAGACTTCTACTCAAGT-3′; vectors containing each sequence were combined for transfection with Lipofectamine. pSVzeo was co-transfected with pSuper knockdown vectors and stable cell lines were selected with 1 mg/ml Zeocin. Stable cell lines depleted of caveolin-1 were generated using RVH-1-puro caveolin knockdown vector generously provided by Kai Simons, Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany (Manninen et al., 2005) or pSuper containing the same knockdown sequence (5′-GATGTGATTGCAGAACCAG-3′) cotransfected with pSVZeo and selected as above with 1 mg/ml Zeocin.
Immunoblot and immunofluorescence analyses
Immunoblot and immunofluorescence analyses were in general performed as previously described (Van Itallie et al., 2009). Widefield images were acquired on a Nikon E800 microscope using a 60× Plan Apo lens and an Orca ER cooled CCD camera controlled with Metamorph imaging software package (version 6.0) Filter sets and dye combinations were as previously described (Fanning et al., 2002). Confocal images were acquired using a Zeiss LSM510 Meta using a 63 Pan Apo lens. Confocal stacks and image projections were made using LSM image browser 3.2. Adobe Photoshop version 7.0 was used for contrast adjustment and montage assembly.
Barrier assays: PEG and fluorescent-dextran flux and measurement of transepithelial electrical resistance
Polyethylene glycol profiling to measure the size dependence of permeability of non-charged solutes was described recently (Van Itallie et al., 2008); all measurements were performed on triplicate filters and at least three different clonal cell lines. Fluorescent dextran permeability was measured as previously described (Van Itallie et al., 2009); these measurements were also performed on triplicate filters and at least two different clonal cell lines. TER was determined using plate electrodes positioned on either side of the monolayers placed in a Plexiglas cup attached to an EVOM (Epithelial Volt Ohm Meter; WPI, Sarasota, FL); two Transwell filters were left blank to determine the contribution of filter and medium in the absence of cells to TER measurements. All measurements were performed on a minimum of triplicate wells and the TER value of the blank filter was subtracted from the reading of the filter with cells. Statistical analysis (ANOVA, Dunnett's and t-tests) were performed using GraphPad software (GraphPad Prism, San Diego, CA).
Cytokine treatment
Because maximal Ocln induction takes 4 days, Ocln-overexpressing cells were plated in T25 flasks without or with 50 ng/ml doxycycline for 2 days and then plated at confluent density without or with doxycycline (4×105 cells/1.1 cm2 12-well Transwell filters). MDCK, Ocln KD, Cav-1 KD and Ocln–Cav-1 KD cells were also plated at confluent density (4×105 cells/1.1 cm2 12-well Transwell filters) but in medium without doxycycline; medium was changed after 24 hours. At 48 hours, the medium was changed in half the wells and the in other half the fresh medium was supplemented with 30 ng/ml TNFα (Calbiochem, EMD Biosciences, Inc., La Jolla, CA) and 100 ng/ml IFNγ (Biosource, Camarillo, CA). After incubation for 24 hours with cytokines, cells were washed once and equilibrated with Hank's balanced salt solution for 20 minutes at 37°C. After 20 minutes, basolateral Hank's was replaced with 0.7 ml fresh Hank's and apical Hank's was replaced with 0.2 ml fresh Hank's containing 0.5-1.0 mg/ml 3-kDa fluorescent dextran (Molecular Probes, Invitrogen, Carlsbad, CA). Flux assay was performed as above.
Latrunculin A treatment
Monolayers were plated at confluent density (4×105 cells/1.1 cm2 12-well filter) and medium was changed after 24 hours; Ocln-overexpressing and rescue cells were plated as above. After 48 hours, the medium was changed to Hank's balanced salt solution supplemented with 15 mM HEPES, pH 7.4 (HBSS) as described by Shen and Turner (Shen and Turner, 2005) and allowed to equilibrate for 60 minutes at 37°C. Basolateral medium was then changed to fresh HBSS containing differing doses of latrunculin A (Invitrogen, Carlsbad, CA). TER was measured at the indicated intervals following latrunculin A administration.
Detergent-resistant membrane preparation
Detergent-resistant membranes were prepared as described earlier (Van Itallie et al., 2005) with some modifications. MDCK and Ocln KD cells were lysed in ice-cold 1% Triton X-100 in 25 mM Tris-HCl, pH 7.5, 5 mM EDTA (TNE). Cells were solubilized by passing them through a 26 gauge needle 40 times and the resulting homogenate was brought to 40% sucrose; 0.5 ml of this solution was placed in the bottom of a 2.2 ml Ultraclear centrifuge tube, overlaid with 1 ml 38% sucrose in TNE and finally with 0.7 ml 5% sucrose in TNE. Step gradients were centrifuged at 166,000 g for 3 hours in a Beckman TLS-55 rotor, 0.3 ml fractions were removed and added to an equal volume of 4× SDS sample buffer. The resulting fractions were analyzed by SDS-PAGE and immunoblot analysis as described above. The density of fractions post centrifugation was assessed in duplicate blank gradients using a Bausch and Lomb refractometer (Rochester, NY).
Supplementary Material
Acknowledgments
We thank Eveline Schneeberger for her generous gift of the occludin KD cell lines. Imaging was partially supported by an anonymous grant to the Michael Hooker Microscopy Facility at the University of North Carolina at Chapel Hill. This work was supported by grants from the National Institutes of Health, DK45134 (J.M.A., C.M.V.I.), P30 DK034987 and DK61397 (J.M.A., A.S.F.). Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/16/2844/DC1
References
- Balda M. S., Whitney J. A., Flores C., Gonzalez S., Cereijido M., Matter K. (1996). Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 134, 1031-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda M. S., Flores-Maldonado C., Cereijido M., Matter K. (2000). Multiple domains of occludin are involved in the regulation of paracellular permeability. J. Cell Biochem. 78, 85-96 [PubMed] [Google Scholar]
- Capaldo C. T., Nusrat A. (2009). Cytokine regulation of tight junctions. Biochim. Biophys. Acta 1788, 864-871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayburgh D. R., Barrett T. A., Tang Y., Meddings J. B., Van Eldik L. J., Watterson D. M., Clarke L. L., Mrsny R. J., Turner J. R. (2005). Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J. Clin. Invest 115, 2702-2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne C. B., Shen L., Turner J. R., Bergelson J. M. (2007). Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe 2, 181-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning A. S., Ma T. Y., Anderson J. M. (2002). Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 16, 1835-1837 [DOI] [PubMed] [Google Scholar]
- Foerg C., Ziegler U., Fernandez-Carneado J., Giralt E., Merkle H. P. (2007). Differentiation restricted endocytosis of cell penetrating peptides in MDCK cells corresponds with activities of Rho-GTPases. Pharm. Res. 24, 628-642 [DOI] [PubMed] [Google Scholar]
- Furuchi T., Anderson R. G. (1998). Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK). J. Biol. Chem. 273, 21099-21104 [DOI] [PubMed] [Google Scholar]
- Furuse M., Tsukita S. (2006). Claudins in occluding junctions of humans and flies. Trends Cell Biol. 16, 181-188 [DOI] [PubMed] [Google Scholar]
- Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. (1993). Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 123, 1777-1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Betanzos A., Nava P., Jaramillo B. E. (2003). Tight junction proteins. Prog. Biophys. Mol. Biol. 81, 1-44 [DOI] [PubMed] [Google Scholar]
- Guillemot L., Paschoud S., Pulimeno P., Foglia A., Citi S. (2008). The cytoplasmic plaque of tight junctions: a scaffolding and signalling center. Biochim. Biophys. Acta 1778, 601-613 [DOI] [PubMed] [Google Scholar]
- Head B. P., Insel P. A. (2007). Do caveolins regulate cells by actions outside of caveolae? Trends Cell Biol. 17, 51-57 [DOI] [PubMed] [Google Scholar]
- Ikenouchi J., Furuse M., Furuse K., Sasaki H., Tsukita S., Tsukita S. (2005). Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 171, 939-945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J., Sasaki H., Tsukita S., Furuse M., Tsukita S. (2008). Loss of occludin affects tricellular localization of tricellulin. Mol. Biol. Cell 19, 4687-4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. I., Nusrat A., Parkos C. A. (2005). Endocytosis of the apical junctional complex: mechanisms and possible roles in regulation of epithelial barriers. BioEssays 27, 356-365 [DOI] [PubMed] [Google Scholar]
- Lambert D., O'Neill C. A., Padfield P. J. (2005). Depletion of Caco-2 cell cholesterol disrupts barrier function by altering the detergent solubility and distribution of specific tight-junction proteins. Biochem. J. 387, 553-560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Yang W., Shen L., Turner J. R., Coyne C. B., Wang T. (2009). Tight junction proteins claudin-1 and occludin control hepatitis C virus entry and are downregulated during infection to prevent superinfection. J. Virol. 83, 2011-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch R. D., Francis S. A., McCarthy K. M., Casas E., Thiele C., Schneeberger E. E. (2007). Cholesterol depletion alters detergent-specific solubility profiles of selected tight junction proteins and the phosphorylation of occludin. Exp. Cell Res. 313, 2597-2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T. Y., Boivin M. A., Ye D., Pedram A., Said H. M. (2005). Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G422-G430 [DOI] [PubMed] [Google Scholar]
- Manninen A., Verkade P., Le Lay S., Torkko J., Kasper M., Fullekrug J., Simons K. (2005). Caveolin-1 is not essential for biosynthetic apical membrane transport. Mol. Cell. Biol. 25, 10087-10096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiando A. M., Shen L., Graham W. V., Weber C. R., Schwarz B. T., Austin J. R., Raleigh D. R., Guan Y., Watson A. J., Montrose M. H., et al. (2010). Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J. Cell Biol. 189, 111-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K. M., Skare I. B., Stankewich M. C., Furuse M., Tsukita S., Rogers R. A., Lynch R. D., Schneeberger E. E. (1996). Occludin is a functional component of the tight junction. J. Cell Sci. 109, 2287-2298 [DOI] [PubMed] [Google Scholar]
- Medina R., Rahner C., Mitic L. L., Anderson J. M., Van Itallie C. M. (2000). Occludin localization at the tight junction requires the second extracellular loop. J. Membr. Biol. 178, 235-247 [DOI] [PubMed] [Google Scholar]
- Nichols B. (2003). Caveosomes and endocytosis of lipid rafts. J. Cell Sci. 116, 4707-4714 [DOI] [PubMed] [Google Scholar]
- Nusrat A., Parkos C. A., Verkade P., Foley C. S., Liang T. W., Innis-Whitehouse W., Eastburn K. K., Madara J. L. (2000). Tight junctions are membrane microdomains. J. Cell Sci. 113, 1771-1781 [DOI] [PubMed] [Google Scholar]
- Patrick D. M., Leone A. K., Shellenberger J. J., Dudowicz K. A., King J. M. (2006). Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma modulate epithelial barrier function in Madin-Darby canine kidney cells through mitogen activated protein kinase signaling. BMC Physiol 6, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L., Helenius A. (2002). Endocytosis via caveolae. Traffic 3, 311-320 [DOI] [PubMed] [Google Scholar]
- Saitou M., Fujimoto K., Doi Y., Itoh M., Fujimoto T., Furuse M., Takano H., Noda T., Tsukita S. (1998). Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 141, 397-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M., Furuse M., Sasaki H., Schulzke J. D., Fromm M., Takano H., Noda T., Tsukita S. (2000). Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 11, 4131-4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pulido L., Martin-Belmonte F., Valencia A., Alonso M. A. (2002). MARVEL: a conserved domain involved in membrane apposition events. Trends Biochem. Sci. 27, 599-601 [DOI] [PubMed] [Google Scholar]
- Schneeberger E. E., Lynch R. D. (2004). The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 286, C1213-C1228 [DOI] [PubMed] [Google Scholar]
- Schulzke J. D., Gitter A. H., Mankertz J., Spiegel S., Seidler U., Amasheh S., Saitou M., Tsukita S., Fromm M. (2005). Epithelial transport and barrier function in occludin-deficient mice. Biochim. Biophys. Acta 1669, 34-42 [DOI] [PubMed] [Google Scholar]
- Schwarz B. T., Wang F., Shen L., Clayburgh D. R., Su L., Wang Y., Fu Y. X., Turner J. R. (2007). LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology 132, 2383-2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Turner J. R. (2005). Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol. Biol. Cell 16, 3919-3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic S. M., Keep R. F., Wang M. M., Jankovic I., Andjelkovic A. V. (2009). Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J. Biol. Chem. 284, 19053-19066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B. R., Siliciano J. D., Mooseker M. S., Goodenough D. A. (1986). Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 103, 755-766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utech M., Ivanov A. I., Samarin S. N., Bruewer M., Turner J. R., Mrsny R. J., Parkos C. A., Nusrat A. (2005). Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol. Biol. Cell 16, 5040-5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie C. M., Anderson J. M. (2006). Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68, 403-429 [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M., Gambling T. M., Carson J. L., Anderson J. M. (2005). Palmitoylation of claudins is required for efficient tight-junction localization. J. Cell Sci. 118, 1427-1436 [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M., Holmes J., Bridges A., Gookin J. L., Coccaro M. R., Proctor W., Colegio O. R., Anderson J. M. (2008). The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J. Cell Sci. 121, 298-305 [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M., Fanning A. S., Bridges A., Anderson J. M. (2009). ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 20, 3930-3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Anders R. A., Wang Y., Turner J. R., Abraham C., Pfeffer K., Fu Y. X. (2005). The critical role of LIGHT in promoting intestinal inflammation and Crohn's disease. J. Immunol. 174, 8173-8182 [DOI] [PubMed] [Google Scholar]
- Wei Y., Yang X., Liu Q., Wilkins J. A., Chapman H. A. (1999). A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 144, 1285-1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A. S., McCarthy K. M., Francis S. A., McCormack J. M., Lai J., Rogers R. A., Lynch R. D., Schneeberger E. E. (2005). Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am. J. Physiol. Cell Physiol. 288, C1231-C1241 [DOI] [PubMed] [Google Scholar]
- Yu D., Turner J. R. (2008). Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim. Biophys. Acta 1778, 709-716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotarevsky Y., Hecht G., Koutsouris A., Gonzalez D. E., Quan C., Tom J., Mrsny R. J., Turner J. R. (2002). A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 123, 163-172 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.