Abstract
Objective
Optical coherence tomography (OCT) is a diagnostic imaging modality that combines low coherence light with inter ferometry to produce high-resolution cross-sectional images of living tissues. Using this technology, we have imaged in vivo the human tympanic membrane (TM) in the office clinic setting and characterized TM microstructure in normal and pathologic conditions.
Study Design
Prospective clinical trial.
Materials and Methods
The normal and diseased TMs in 10 adult subjects were examined. Each subject underwent direct microscopic examination before OCT imaging to provide visual coregistration of associated subsites including the anulus fibrosus, pars tensa, pars flaccida, and umbo. The probe from the imaging system (1,310-nm central wavelength, 15-μm coherence length, Niris; Imalux, Cleveland, OH, USA) was introduced into the ear canal to obtain lateral cross-sectional images.
Results
Systematic imaging of the TM was performed with characterization of the epithelial and collagenous layers. The overall TM thickness was clearly demonstrated and quantified.
Conclusion
The ability to noninvasively study middle ear microstructures in vivo is essential in the treatment of diseases of the ear. OCT may provide the otologist/neurotologist with the ability to 1) image pathology such as cholesteatoma, dimeric TMs, and chronic otitis media; 2) gauge the response to pharmacological therapy; and 3) monitor postsurgical changes after tympanoplasty and other procedures. OCT may provide a means to optimize the diagnosis and management of patients with middle ear disease.
Keywords: In vivo, Optical coherence tomography, Tympanic membrane
Optical coherence tomography (OCT) is a novel, noninvasive imaging technique that uses low-coherence interferometry to produce cross-sectional images of tissue microstructures (1). It uses light to discern differences in tissue microstructure and uses coherence gating to localize the origin of the reflected optic signal. OCT is analogous to B-mode ultrasonography but uses broadband near-infrared light to produce images with a resolution approaching that of light microscopy (10–15 μm) (1,2). OCT relies on differences in tissue optical properties to generate contrast, with axial resolution on the order of 10 μm and of a depth of penetration of approximately 1 to 2 mm, depending on turbidity. Axial resolution is inversely proportional to the coherence length of the sources. Lateral resolution is diffraction limited and therefore dependent on the optical design of each device. Although OCT has been used as a measurement tool in ossiculoplasties and stapedectomies (3), in vivo use of OCT in the human tympanic membrane (TM) has not previously been reported.
In the head and neck, OCT applications have primarily focused on characterizing cancer and other disease processes in the larynx (4), because OCT is ideal for imaging the thin, layered structures of the vocal fold epithelium and lamina propria. Most OCT applications in the study of the middle and inner ear have focused on either animal investigations or human temporal bone studies (5–10). In this study, we use OCT to image and characterize the TM in both normal and pathologic conditions. Successful OCT imaging of the TM will allow the clinician to obtain structural information on the TM, supplementing planar two-dimensional images obtained during routine clinical examination. This is the first report on the in vivo high-resolution cross-sectional anatomy of human TM using OCT.
MATERIALS AND METHODS
Ten patients being evaluated for middle ear disease at the University of California-Irvine Medical Center were examined under protocol approved by the human subjects institutional review board at the University of California, Irvine. Images were obtained using an OCT workstation (Niris; Imalux, Cleveland, OH, USA) and associated probe. The device light source is a super luminescent diode, which emits light at 1,310 nm with a coherence length of 15 μm. Images are generated by raster scanning the single mode fiber at the tip of the probe across the region of interest. Images are acquired at a frame rate of 1 Hz. Image intensity is proportional to the amount of reflected light in a given region of interest. Image size was 1.87 mm laterally (~10-μm resolution) and 2.56 mm in depth (~7-μm resolution). To obtain images, the probe was inserted through a speculum into the external auditory canal using an operative otoscope. Imaging time was less than 3 minutes in each subject. The structural anatomy of normal TMs were obtained in all four quadrants. Disease processes imaged including dimeric membranes, regions of tympanosclerosis, cholesteatoma, and granulation tissue at the periphery of a perforation.
RESULTS
Images were obtained from normal TM (Fig. 1), dimeric TM (Fig. 2), tympanosclerosis (Fig. 3), cholesteatoma (Fig. 4), granulation tissue from a perforation (Fig. 5), and hyperkeratosis (Fig. 6). The normal TM that is imaged in Figure 1 in the anterosuperior quadrant had a thickness of 88 μm, whereas the dimeric TM (Fig. 2) had a thickness of 29 μm. The thickness of the TM in the patient with tympanosclerosis (Fig. 3) or cholesteatoma (Fig. 4) could not be measured because of the opacity of the structures. The thickness of the hyperkeratotic TM in Figure 6 was 169 μm.
FIG. 1.
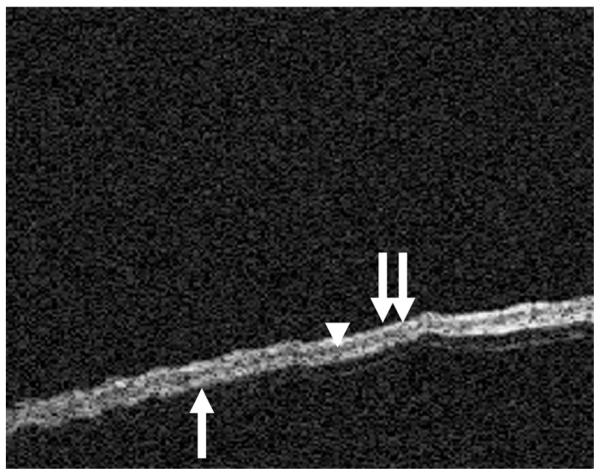
OCT image of the normal TM. The trilaminar structure of the TM can be seen with the outer squamous layer (double arrows), middle fibrous layer (arrowhead), and medial cuboidal epithelium (single arrow).
FIG. 2.
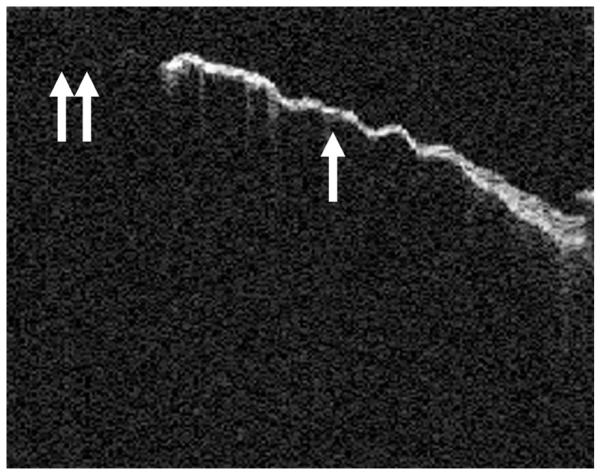
OCT of a dimeric TM. The fusion of the squamous epithelial layer and the mucosal layer without the presence of a middle fibrous layer can be seen. The thin nature of this TM (arrow) compared with the normal TM in Figure 1 can be seen. The dimeric TM in this figure is abutting a perforation (double arrows).
FIG. 3.
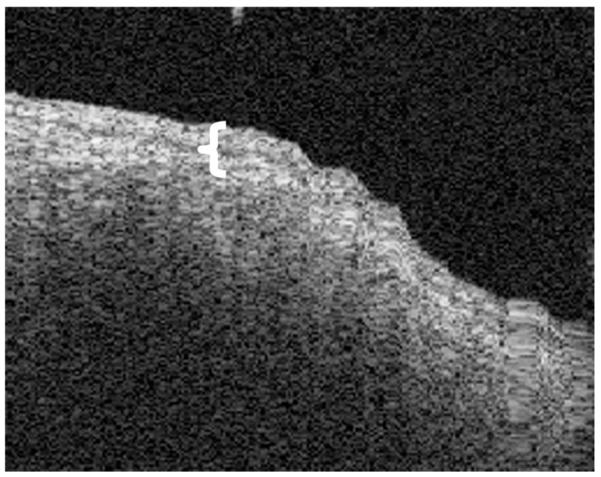
OCT image of a tympanosclerotic plaque. The image shows that the squamous epithelial layer is somewhat thicker (brackets). The calcified fibrosis prevents clear imaging of the medial layer of the TM.
FIG. 4.
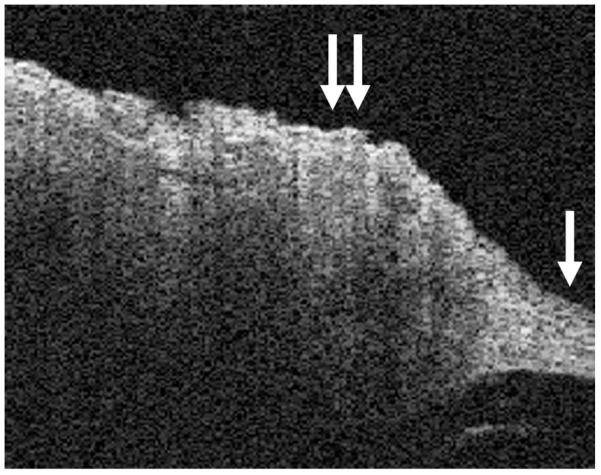
This figure shows the transition from the normal TM in the right lower corner (single arrow) to cholesteatoma (double arrows), with significant hyperkeratosis.
FIG. 5.
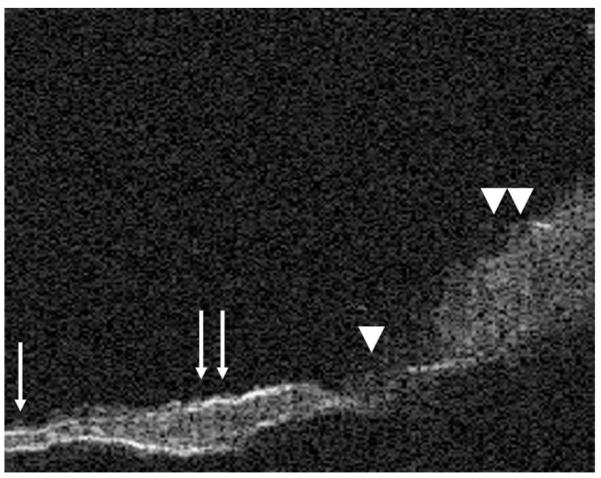
This figure shows a thinned TM on the left with a very thin fibrous layer (single arrow), which then transitions into a normal TM (double arrows) with a thicker fibrous middle layer. The small perforation can be seen in the middle of the image (arrowhead), and granulation tissue is seen on the right side of the image (double arrowhead).
FIG. 6.
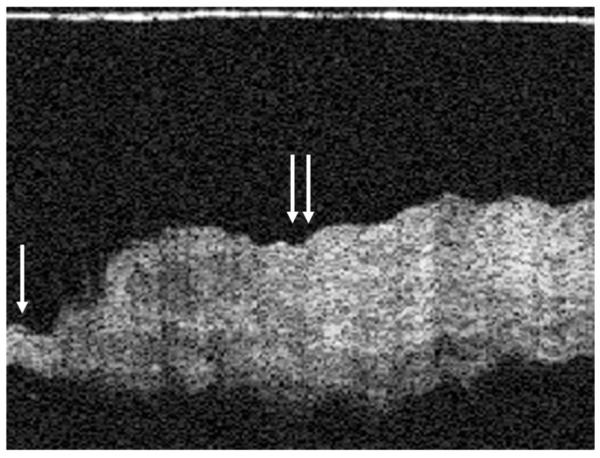
Hyperkeratosis of the TM seen using OCT. The normal TM can be seen on the left (single arrow) and the hyperkeratotic area on the right (double arrows).
DISCUSSION
This is the first report of in vivo structural imaging of the TM in cross-section using OCT. OCT provides a means to accurately gauge and measure TM thickness in the office. In addition, detailed information can be obtained on pathologic conditions such as granulation tissue, tympanosclerosis, and dimeric TMs.
The only in vivo clinical study of OCT in the ear is to use it as a measuring tool during ossiculoplasty (3). Using OCT to image the TM and the middle ear was performed by Pitris et al. (10) in temporal bones. The authors demonstrated that three-dimensional images of the middle ear can be obtained in an in vitro setting. The image acquisition times for the system that Pitris et al. (10) used was 10 to 30 seconds per image. This gave them the ability to obtain much higher resolution images than we were able to with our system. In a clinical setting, the patient’s movement would preclude obtaining images with a system of that speed. In our system, images were obtained at 1 frame per second, which gave us the ability to easily characterize the TM and associated abnormalities commonly observed in out patient otology.
The high resolution of OCT imaging (10 μm), lack of ionization radiation, speed, and capability for office based use are obvious advantages of this technology. The resolution significantly exceeds that of traditional computed tomography (0.4 mm) and magnetic resonance imaging (3.0 mm). In addition, OCT is an ideal complement to conventional microscopy where only a planar en face image of TM and middle ear surface topology is obtained. OCT permits the surgeon to visualize tissue structure in depth, which was not previously possible in the office. Unlike high-frequency ultrasound (~150-μm resolution), OCT imaging can be performed either in contact or near contact without the use of an impedance or refractive index matching gel and, again at higher resolution, albeit with a moderate sacrifice in imaging depth. These advantages will increase the possibility of its clinical use when the probe sizes are further reduced, and the image resolution is improved in commercial systems. Developing OCT technology with improved resolution, increased imaging speed, and true three-dimensional volumetric imaging is an active focus of our research group, and we think that OCT imaging systems might also be incorporated into otologic microscopy systems just as they are in ophthalmology.
The limitations of an OCT device for use in TM and middle ear imaging are related to the calcification and thickness of the TM as well as intervening bone of the ear canal or malleus. Because OCT relies on tissue optical properties to generate image contrast, optically dense structures (e.g., bone or tympanosclerosis, blood) will reflect or absorb incident light at the surface and limit signal penetration to deeper structures. In addition, as the optical beam penetrates deeper into turbid media such as living tissues, scattering prevents collection of adequate coherent photons to generate images.
CONCLUSION
This study demonstrates the feasibility of imaging the human TM in vivo. As this imaging modality is non contact and uses a safe near-infrared light source, OCT may provide a means to image microstructural changes in the TM and middle ear while preserving the TM’s integrity. Further research is needed to develop smaller probes that can obtain higher resolution images with greater depth.
Acknowledgments
This work was supported by the Flight Attendant Medical Research Institute (32456) and the Air Force Office of Scientific Research (F49620-00-1-0371).
Footnotes
Presented at the American Otologic Society Meeting, May 2–3, 2008, Orlando, FL, USA.
REFERENCES
- 1.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brezinski ME, Tearney GJ, Boppart SA, Swanson EA, Southern JF, Fujimoto JG. Optical biopsy with optical coherence tomography: feasibility for surgical diagnostics. J Surg Res. 1997;71:32–40. doi: 10.1006/jsre.1996.4993. [DOI] [PubMed] [Google Scholar]
- 3.Heermann R, Hauger C, Issing PR, Lenarz T. Application of optical coherence tomography (OCT) in middle ear surgery [in German] Laryngorhinootologie. 2002;81:400–5. doi: 10.1055/s-2002-32213. [DOI] [PubMed] [Google Scholar]
- 4.Wong BJ, Jackson RP, Guo S, et al. In vivo optical coherence tomography of the human larynx: normative and benign pathology in 82 patients. Laryngoscope. 2005;115:1904–11. doi: 10.1097/01.MLG.0000181465.17744.BE. [DOI] [PubMed] [Google Scholar]
- 5.Wong BJ, de Boer JF, Park BH, et al. Optical coherence tomography of the rat cochlea. J Biomed Opt. 2000;5:367–70. doi: 10.1117/1.1310165. [DOI] [PubMed] [Google Scholar]
- 6.Wong BJ, Zhao Y, Yamaguchi M, et al. Imaging the internal structure of the rat cochlea using optical coherence tomography at 0.827 micron and 1.3 micron. Otolaryngol Head Neck Surg. 2004;130:334–8. doi: 10.1016/j.otohns.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Choudhury N, Song G, Chen F, et al. Low coherence interferometry of the cochlear partition. Hear Res. 2006;220:1–9. doi: 10.1016/j.heares.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Pau HW, Lankenau E, Just T, Hüttmann G. Imaging of Cochlear Structures by Optical Coherence Tomography (OCT) [in German] Laryngorhinootologie. 2008 doi: 10.1055/s-2007-995725. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Pau HW, Lankenau E, Just T, Behrend D, Hüttmann G. Optical coherence tomography as an orientation guide in cochlear implant surgery. Acta Otolaryngol. 2007;127:907–13. doi: 10.1080/00016480601089408. [DOI] [PubMed] [Google Scholar]
- 10.Pitris C, Saunders KT, Fujimoto JG, Brezinski ME. High-resolution imaging of the middle ear with optical coherence tomography: a feasibility study. Arch Otolaryngol Head Neck Surg. 2001;127:637–42. doi: 10.1001/archotol.127.6.637. [DOI] [PubMed] [Google Scholar]


