Abstract
In the lamprey, spinal locomotor activity can be initiated by pharmacological microstimulation in several brain areas: rostrolateral rhombencephalon (RLR); dorsolateral mesencephalon (DLM); ventromedial diencephalon (VMD); and reticular nuclei. During DLM- or VMD-initiated locomotor activity in in vitro brain/spinal cord preparations, application of a solution that focally depressed neuronal activity in reticular nuclei often attenuated or abolished the locomotor rhythm. Electrical microstimulation in the DLM or VMD elicited synaptic responses in reticulospinal (RS) neurons, and close temporal stimulation in both areas evoked responses that summated and could elicit action potentials when neither input alone was sufficient. During RLR-initiated locomotor activity, focal application of a solution that depressed neuronal activity in the DLM or VMD abolished or attenuated the rhythm. These new results suggest that neurons in the RLR project rostrally to locomotor areas in the DLM and VMD. These latter areas then appear to project caudally to RS neurons, which probably integrate the synaptic inputs from both areas and activate the spinal locomotor networks. These pathways are likely to be important components of the brain neural networks for the initiation of locomotion and have parallels to locomotor command systems in higher vertebrates.
Keywords: locomotion, command, descending, reticulospinal, central pattern generators
Locomotor “command” systems in the brain activate central pattern generators (CPGs) in the spinal cord to initiate locomotor behavior (reviewed in Grillner, 1981). In a wide variety of vertebrates, locomotor activity can be initiated by “pharmacological microstimulation” (e.g. focal ejection of neurotransmitters or their agonists) or electrical microstimulation in at least four brain “locomotor areas”: (a) specific reticular nuclei that contain reticulospinal (RS) neurons which activate spinal CPGs (Noga et al., 1988; Bernau et al., 1991); (b) “mesencephalic locomotor region” (MLR; Shik et al., 1966; Kashin et al., 1974; Parker and Sinnamon, 1983; Garcia-Rill and Skinner, 1987a,b; Bernau et al., 1991; Uematsu and Todo, 1997); (c) “subthalamic locomotor region” (SLR; Orlovsky, 1969; Parker and Sinnamon, 1983); and (d) “pontomedullary locomotor strip” (PLS; Kazennikov et al., 1981; Selionov and Shik, 1984; Beresovskii and Bayev, 1988; Noga et al., 1988). Lesions or blocking neuronal activity in medullary reticular nuclei abolishes MLR- or PLS-initiated locomotor activity (Shefchyk et al., 1984; Garcia-Rill and Skinner, 1987a; Bernau et al., 1991), and stimulation in the MLR or SLR elicits synaptic responses in RS neurons (Orlovsky, 1970; Garcia-Rill and Skinner, 1987b).
In the lamprey, a lower vertebrate, spinal locomotor activity also can be initiated by pharmacological or electrical microstimulation in specific brain areas (Fig. 1A, B): reticular nuclei, such as the anterior (ARRN), middle (MRRN), and posterior (PRRN) rhombencephalic reticular nuclei; ventral thalamus in the ventromedial diencephalon (VMD); dorsolateral mesencephalon (DLM); caudomedial mesencephalon, thought to be the lamprey MLR (not shown in Fig. 1B); and rostrolateral rhombencephalon (RLR; McClellan and Grillner, 1984; Hagevik et al., 1996; El Manira et al., 1997; Paggett et al., 2000; Sirota et al., 2000). Although electrical microstimulation can activate dendrites, cell bodies, and axons of passage, pharmacological microstimulation with neurotransmitters or their agonists is thought to activate, as a rule, cell bodies and dendrites, where postsynaptic receptors are primarily located (Goodchild et al., 1982).
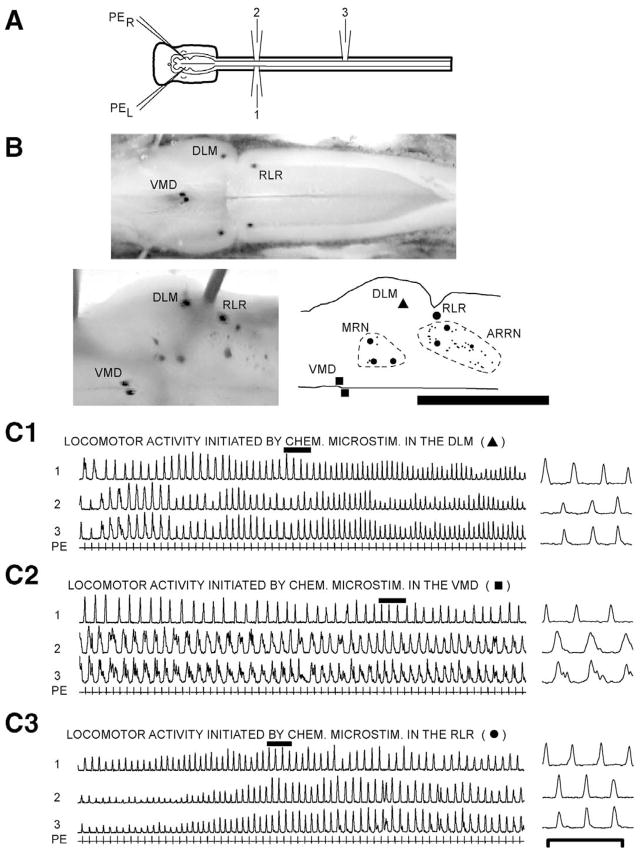
Fig. 1.
(A) In vitro brain/spinal cord preparation showing ventral root recording electrodes (1–3) and micropipettes (PEL and PER) for pharmacological microstimulation. (B) Dorsal view of the brain (left is rostral, right is caudal) at different steps in the experiment. (Upper) Live brain from an in vitro experiment showing Alcian-marked pharmacological microstimulation sites in the VMD, DLM, and RLR used to initiate locomotor activity in C. (Lower left) Enlargement of the right side of the brain immediately after reaction for HRP showing the above marked locomotor areas as well as HRP labeled RS neurons. (Lower right) Computer-generated diagram of the right side of the brain after histological processing showing the VMD, DLM, and RLR as well as labeled RS neurons (small dots are unidentified neurons; larger dots are Müller cells) in the mesencephalic reticular nucleus (dotted outline) and ARRN (dotted outline) (this type of diagram was used to generate summary diagrams, such as in Fig. 2B; see text for other abbreviations). Scale bar=1.6 mm (upper), 1.0 mm (lower). (C) Integrated spinal locomotor burst activity (1–3; τ=50 ms), consisting of left-right alternation (1↔2) and a rostrocaudal phase lag (2→3), initiated by pharmacological microstimulation using PE (see text) in (C1) DLM, (C2) VMD, and (C3) RLR. Pharmacological microstimulation began prior to the left side of the recordings, and these recordings began after a stable locomotor rhythm had developed (see text). Locomotor patterns on the right are expanded regions during the solid bars from the records on the left. Scale bar=10 s (left recordings), 2.5 s (right recordings).
In the lamprey, RS neurons are thought to activate the spinal locomotor CPGs (McClellan, 1988), but it is not known which reticular nuclei contribute to the initiation of locomotion, or how the different higher brain locomotor areas are interconnected. This information is critical for understanding how brain locomotor command systems function and activate the spinal CPGs to initiate locomotor behavior. In the present study, two aspects of locomotor command function were examined. First, does DLM- or VMD-initiated locomotor activity require all or only specific reticular nuclei. Second, are the VMD and/or DLM required for locomotor activity initiated from the RLR. Preliminary accounts of this study have appeared in abstract form (McClellan, 1989; Paggett et al., 2000).
EXPERIMENTAL PROCEDURES
Experimental preparation
In vitro brain/spinal cord preparations (n=41 animals; range: 73–125 mm) from larval sea lamprey (Petromyzon marinus) were dissected (McClellan and Hagevik, 1997; Fig. 1A) and transferred to a recording chamber containing lamprey Ringer’s solution (6–9°C; McClellan, 1990) and 15 mg/l D-tubocurarine (22 mM). Curare probably did not significantly altered the in vitro rhythms by blocking GABA receptors (Caputi et al., 2003), since in vitro locomotor activity initiated from the brain is virtually identical in the absence of curare and with as much as 150 mg/l (220 mM) curare applied to the spinal cord (i.e. 10× the concentration used in the present study; n=3; Hinton and McClellan, unpublished observations). In contrast, 40 μM bicuculline applied to the spinal cord severely disrupts brain-initiated locomotor activity (Hagevik and McClellan, 1994). In the present study, suction electrodes were placed on spinal ventral roots usually at approximately 20% body length (BL; relative distance from the head) and approximately 40% BL to record locomotor activity, which was integrated to better reveal the burst activity (τ=50 ms; 1–3, Fig. 1C). The procedures in this study have been approved by the Animal Use and Care Committee at the University of Missouri. All efforts were made to minimize the number of animals used and their suffering.
Initiation of locomotor activity
Two micropipettes were filled with both 5 mM D-glutamate and 5 mM D-aspartate in Ringer’s solution (pH=7.4; Fast Green added to visualize ejection bolus), and the micropipette tips were broken off and positioned approximately 25–50 μm below the dorsal surface of the brain (i.e. brain is approximately 300–400 μm thick) where many neuronal cell bodies are located (Niewenhuys, 1977). Subsequently, pharmacological agents were focally pressure ejected bilaterally in brain locomotor areas (DLM, VMD, or RLR) to initiate locomotor activity (approximately 5–15 ms pulses at 1 Hz; 20 psi; Fig. 1; McClellan and Hagevik, 1997). The above concentrations of glutamate/aspartate, as well as those for the blocking agents discussed below, are about five times those used for bath application in lamprey experiments. However, if the micropipette tips in the present study are assumed to be continuous point sources, it can be shown that the concentrations of the above agents will decrease very rapidly with distance from the micropipette tips (Curtis, 1964). Thus, brain neurons in the vicinity of the micropipette tips were exposed to average concentrations of the agents that were much lower than in the micropipettes and likely were within physiological ranges.
Blocking in reticular nuclei during DLM- or VMD-evoked locomotion
After VMD- or DLM-initiated locomotor activity reached a steady state and burst amplitudes were relatively constant, a solution that depresses neuronal activity (i.e. “blocking solution”; the terms “blocking” and “blockade” will be used, although activity may be partially blocked) was applied bilaterally in reticular nuclei to test if these nuclei are necessary for VMD- or DLM-initiated locomotor activity (n=18 animals). In most experiments, stimulation in brain locomotor areas initiated well-coordinated locomotor activity in the spinal cord. However, because relatively long duration episodes of locomotor activity that could be repeated many times were required for blocking experiments, in a given animal, not all combinations of stimulation and blocking were always performed (Table 1). The “blocking micropipettes” (see Fig. 2A) contained the following solution (pH=7.4): 1.0 mM GABA and 1.0 mM glycine; 2.5 mM kynurenic acid; zero-calcium Ringer’s solution; and Fast Green. The micropipettes tips were positioned in reticular nuclei approximately 25–50 μm below the dorsal surface of the brain (see Fig. 2A, B), and “blocking solution” was pressure ejected with approximately 5–15 ms pulses delivered simultaneously with pulses for pharmacological microstimulation. Several control experiments were performed to determine the distance over which the blocking solution depressed neuronal activity (see Results).
Table 1.
Summary of neural activity blocking experiments
| Stim. areaa | Block areab | Type of block of locomotor activity |
||
|---|---|---|---|---|
| Completec | Partiald | No blocke | ||
| VMDf,g | ARRN | 3 | 7 | 0 |
| MRRN | 0 | 2 | 4 | |
| PRRN | 2 | 6 | 3 | |
| DLMf,g | ARRN | 2 | 4 | 2 |
| MRRN | 2 | 5 | 2 | |
| PRRN | 4 | 6 | 3 | |
| RLRf,h | VMD | 3 | 2 | 0 |
| RLRf,h | DLM | 3 | 1 | 0 |
Brain areas in which pharmacological microstimulation was applied to initiate spinal locomotor activity (see Experimental Procedures).
Brain areas in which a solution that depresses neuronal activity (i.e., “blocking solution,”; see Experimental Procedures) was ejected.
Numbers of animals in which ejection of blocking solution completely abolished burst activity for at least 5 s (usually much longer; see Experimental Procedures for criteria for complete and partial blockade of locomotor activity).
Numbers of animals in which the amplitudes of locomotor burst activity were clearly decreased for at least 5 s (usually much longer) during ejection of blocking solution.
Numbers of animals in which there was no obvious decrease in burst amplitudes during the ejection of blocking solution.
Because repeatable, long duration episodes of locomotor activity were required for blocking experiments, in a given animal, not all combinations of stimulation and blocking were always performed.
Total number of animals=18.
Total number of animals=5.
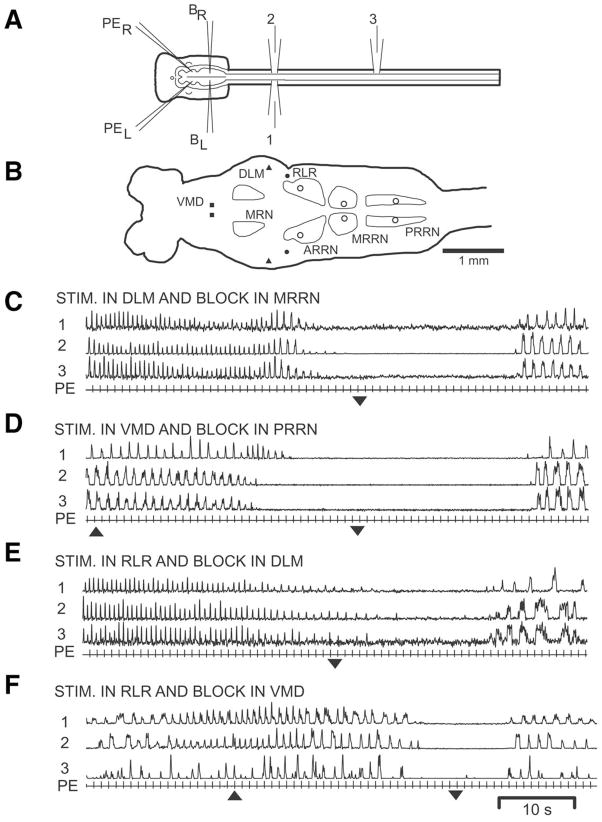
Fig. 2.
(A) In vitro preparation showing pharmacological microstimulation pipettes (PEL and PER), blocking micropipettes (BL and BR), and ventral root recording electrodes (1–3). (B) Diagram of brain (left) and rostral spinal cord showing locomotor areas in the VMD, DLM, and RLR as well as sites in the ARRN, MRRN, and middle PRRN where blocking solution was applied (open circles; see Experimental Procedures). (C, D) Spinal locomotor activity (1–3) initiated by pharmacological microstimulation using PE in the (C) DLM or (D) VMD prior to, during, and after bilateral focal PE of blocking solution (block ON=up arrowhead, block OFF=down arrowhead) in the (C) MRRN and (D) PRRN. (E, F) Spinal locomotor activity initiated by pharmacological microstimulation in the RLR prior to, during, and after bilateral focal PE of blocking solution in the (E) DLM and (F) VMD. Note in “C” and “E” that the onset of ejection of blocking solution began approximately 20 s before the beginning of the record, but after a steady state locomotor rhythm had been established (see text, Fig. 1C).
Several criteria were used to categorize complete or partial blockade of spinal locomotor activity: (a) clear attenuation of locomotor activity during blockade compared with control locomotor patterns in the same preparations (see Fig. 1C vs. Fig. 2C, D); (b) attenuation or abolishment of bursting during blockade for at least 5 s (usually much longer; see Fig. 2C, D); (c) blockade repeatable in at least two consecutive episodes; and (d) following blockade, burst activity recovered and increased in amplitude. Pharmacological microstimulation as well as blocking sites were marked for later analysis by focal pressure ejection (PE) of 1–2% Alcian Blue in Ringer’s solution (Harnischfeger, 1979; see Fig. 1B).
DLM- and VMD-evoked synaptic responses in RS neurons
Electrical microstimulation in the VMD and/or DLM (1 ms, cathodal constant current pulses, 5–100 μA, at 1–10 Hz) was used to evoke synaptic responses in RS neurons that were recorded with micropipettes filled with 5 M KAc (approximately 60–100 MΩ). In some experiments, two double-barrel micropipettes were employed, with one pair of barrels used for pharmacological microstimulation to locate the VMD or DLM (n=3 animals, six RS neurons). When the micropipettes were positioned in these locomotor areas, electrical microstimulation was applied to the DLM and/or VMD via the second pair of barrels, which contained Ringer’s solution. In other experiments, single-barrel pharmacological stimulation micropipettes were used to locate the VMD and DLM, and then the tips of separate single-barrel electrical microstimulation micropipettes that contained Ringer’s solution were positioned in these locomotor areas (n=7 animals, 44 RS neurons). For electrical stimulation in three of these experiments (21 neurons), Ringer’s solution in the bath contained high calcium and high magnesium (3× or 5× the normal concentrations) to attenuate polysynaptic pathways (Berry and Penthreath, 1976). Orthodromic responses recorded from the spinal cord were used to confirm the presence of descending axons (Fig. 3A, 3D3).
Fig. 3.
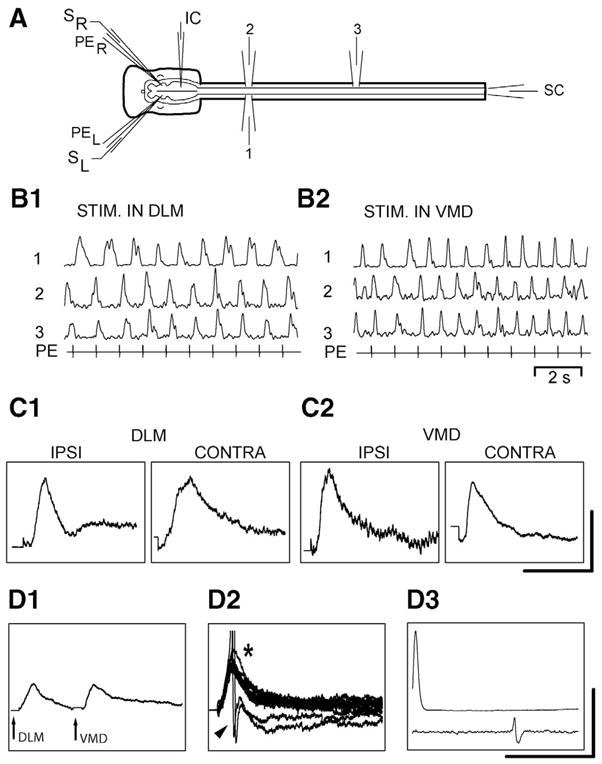
(A) In vitro preparation showing bilateral double-barrel micropipettes with pharmacological microstimulation barrels (PEL and PER) and electrical microstimulation barrels (SL and SR), intracellular microelectrode (IC), ventral root recording electrodes (1–3), and spinal cord recording electrode (SC). (B) Spinal locomotor activity (1–3) elicited by pharmacological microstimulation using PE in the (B1) DLM and (B2) VMD. (C) Averaged synaptic responses in RS neurons elicited by electrical microstimulation in the (C1) DLM (25 μA) and (C2) VMD (“ipsi”=40 μA, “contra”=10 μA; same preparation as in B). C1 (both panels) and C2 (stim “ipsi”) are from an unidentified left MRRN neuron and C2 (stim “contra”) is from an identified B1 neuron (Müller cell) in the left MRRN. (D1, D2) Different preparation, in which synaptic responses were recorded from an identified B4 neuron (Müller cell) in the right MRRN during electrical microstimulation in the right DLM (12.5 μA) and right VMD (12.5 μA) with (D1) a delay (averaged response) or (D2) simultaneous stimulation (superimposed responses; *=summated responses; arrowhead=action potentials). (D3) Intracellular action potentials (top) evoked orthodromic spikes in the SC (bottom). Scale bars=(C1) 1 mV/50 ms (left) and 1 mV/100 ms (right); (C2) 1 mV/100 (left) and 2 mV/50 ms (right), (D1, D2) 5 mV/50 ms; (D3) 80 mV/25 ms (amplitude scale applies to top intracellular trace only).
Blocking in the DLM or VMD during RLR-initiated locomotor activity
The RLR, DLM, and VMD were located with pharmacological microstimulation and marked with Alcian Blue. Subsequently, during pharmacological stimulation in the RLR, “blocking solution” (see above) was focally pressure ejected bilaterally in the DLM or VMD to test whether these areas are necessary for RLR-initiated spinal locomotor activity (n=5 animals). The criteria for complete or partial blockade of spinal locomotor activity were the same as those described above.
Histological processing
In nine of the 20 experiments in which blocking solution was ejected in reticular nuclei (see Figs. 1, 2C, 2D), approximately 14 days before setting up in vitro preparations, animals were anesthetized, and horseradish peroxidase (HRP) was applied to the transected spinal cord at 60% BL (relative distance from the head), as previously described (Davis and McClellan, 1994). This procedure pre-labeled RS neurons that were later used to confirm the locations of Alcian Blue marked blocking sites within reticular nuclei (see below). Locomotor activity in these HRP-labeled preparations was indistinguishable from that in preparations without the retrograde tracer.
Following in vitro experiments in which RS neurons were prelabeled with HRP and stimulation/blocking sites were marked with Alcian Blue (Fig. 1B, top), whole-mount brains were histologically processed for HRP (Fig. 1B, lower left), placed on slides, and coverslipped with Permount, as previously described (Davis and McClellan, 1994). For all other experiments, the brains were processed without the HRP reaction step. With a computer-microscope marking/tracing system, the outlines of the brains were traced, stimulation and blocking sites were marked, and, if applicable, the outlines of reticular nuclei were traced around groups of HRP-labeled RS neurons (e.g. diagrams such as Fig. 1B, lower right were used to generate summary diagrams, such as Fig. 2B).
RESULTS
Stimulation in higher order locomotor areas in the brain
In in vitro brain/spinal cord preparations (Fig. 1A), pharmacological microstimulation in the DLM, VMD, or RLR (Fig. 1B) initiated spinal locomotor activity (Fig. 1C; PE) that often could be sustained for 60–120 s and that consisted of left-right alternation (1↔2) and a rostrocaudal phase lag (2→3), similar to those in previous studies (e.g. see McClellan and Hagevik, 1997). In semi-intact preparations, in which the brain and rostral spinal cord are exposed and the caudal body is relatively intact, we have shown that stimulation in these brain areas initiates well-coordinated swimming movements (Jackson and McClellan, 2001). Pharmacological microstimulation in the caudomedial mesencephalon, which is thought to represent the lamprey MLR (e.g. Fig. 8A in Sirota et al., 2000), did not reliably elicit spinal locomotor activity, at least with the agents used in the present study (however, see Discussion).
Typically, within approximately 4–40 s after the onset of pharmacological microstimulation, locomotor activity reached a “steady state,” at which time the burst amplitudes remained relatively constant (Fig. 1C2). However, there often was a small, very gradual decline in burst amplitudes during episodes, and in a few cases, there was a relatively slow modulation of burst amplitudes superimposed on the overall locomotor rhythm (Fig. 1C1, 1C3). These patterns were easily distinguished from partial or complete blockade of locomotor activity during ejection of “blocking solution” (see below).
“Blocking” in reticular nuclei during DLM- or VMD-initiated locomotor activity
During pharmacological microstimulation in the VMD or DLM (Fig. 1A, B), ejection of “blocking solution” (see Experimental Procedures) in the ARRN (not shown), MRRN (Fig. 2C), or PRRN (Fig. 2D) often abolished or partially blocked (25%–60% decrease in burst amplitudes) spinal locomotor activity (Table 1). Approximately 4–60 s after the onset of blocking, spinal locomotor activity was maximally attenuated. During maximal blockade of the rhythm, Fast Green in the blocking solution stained a relatively small region of the brain that was less than or equal to the width of the reticular nucleus in which blockade was applied. Within approximately 2–20 s after termination of blocking, locomotor burst activity recovered, although burst activity did not always fully return to pre-blockade levels.
In some experiments, blocking in reticular nuclei did not noticeably affect spinal locomotor activity (Table 1). In these preparations, VMD- or DLM-initiated locomotor activity was very robust, and reduction of activity in only a fraction of the total RS neuron population probably was insufficient to attenuate spinal locomotor patterns.
Several control experiments also were performed. First, in experiments in which blocking in reticular nuclei abolished or attenuated DLM- (n=7) or VMD- (n=6) initiated spinal locomotor activity, ejection of blocking solution approximately 200 μm lateral to reticular nuclei did not noticeably affect the rhythm (not shown). In addition, trigeminal-evoked synaptic responses in RS neurons were not noticeably affected when blocking solution was pressure ejected at a distance greater than approximately 200 μm (eight neurons, n=3 preparations; not shown). Thus, long distance effects of the blocking solution appeared to be minimal, suggesting that application of blocking solution in reticular nuclei is largely confined to these neural structures. Second, in partitioned in vitro brain/spinal cord preparations, application of blocking solution to the rostral spinal cord did not abolish DLM- or VMD-initiated spinal locomotor activity in the caudal spinal cord (n=2; not shown). Thus, the blocking solution probably had little or no effect on axonal conduction.
DLM- and VMD-evoked synaptic responses in RS neurons
In in vitro preparations (Fig. 3A), pharmacological microstimulation in the DLM or VMD initiated locomotor activity (Fig. 3B), and brief electrical microstimulation in these same areas elicited depolarizing synaptic responses in RS neurons (50 neurons, n=10 animals; Fig. 3C). Furthermore, for 33 RS neurons in five of the above animals, close temporal stimulation in the DLM and VMD elicited summated (*) synaptic responses in RS neurons that could produce action potentials (arrowhead) when neither input alone was sufficient (Fig. 3D1, 3D2). Stimulation in the DLM and/or VMD elicited depolarizing synaptic responses in both unidentified RS neurons and Müller cells.
“Blocking” in the DLM or VMD during RLR-initiated locomotor activity
During RLR-initiated locomotor activity in in vitro preparations (Fig. 2A, B), ejection of blocking solution in the DLM (Fig. 2E) or VMD (Fig. 2F) abolished or attenuated (approximately 75% decrease in burst amplitudes) the spinal locomotor rhythm (Table 1). Approximately 20–65 s after the onset of blocking, spinal locomotor activity was maximally attenuated, and approximately 10–60 s after the termination of blocking, locomotor burst activity recovered.
In control experiments, (n=3 preparations; not shown), blocking in the DLM during stimulation in the VMD, or vice versa, did not appear to attenuate spinal locomotor activity. Furthermore, during stimulation in the VMD or DLM, blocking in the RLR did not produce obvious effects on in vitro locomotor activity.
DISCUSSION
Organization of locomotor areas in the lamprey brain
Projections from the RLR to the VMD and DLM
Results from blocking experiments in the present study suggest that neurons in the RLR project rostrally to the DLM and VMD (Fig. 2E, F). In support of this finding, complete transverse lesions at the mesencephalic–rhombencephalic border abolish RLR-initiated locomotor activity (Paggett et al., 2000 and unpublished observations). In addition, ejection of retrograde tracer in the DLM or VMD labels neurons in the vicinity of the RLR (Paggett et al., 2000 and unpublished observations). These are new and important results because, in general, synaptic inputs to locomotor areas in the diencephalon and mesencephalon from other brain locomotor areas have not been well studied, particularly rostrally projecting inputs. Furthermore, the DLM locomotor area has not been studied in other vertebrates.
In larval lamprey, locomotor activity can be initiated by pharmacological microstimulation in a “locomotor strip” along the lateral rhombencephalon (LR), close to the trigeminal sensory nuclei (Hagevik et al., 1996). Ejection of retrograde tracers in the RLR labels neurons in the LR (Paggett et al., unpublished observations). Thus, trigeminal sensory inputs, which can initiate escape locomotor behavior (McClellan, 1990), might result in activation of neurons in the RLR, which then project rostrally to diencephalic and mesencephalic locomotor areas.
Projections from the DLM and VMD to reticular nuclei
The blocking experiments (Fig. 2; Table 1) and intracellular recordings (Fig. 3C) suggest that neurons in the DLM and VMD project to RS neurons to initiate spinal locomotor activity. However, no one group of RS neurons appears to be primarily responsible for activation of spinal CPGs (Paggett et al., 2000). Results from dual stimulation in both the DLM and VMD suggest that RS neurons summate the inputs from these locomotor areas (Fig. 3D), similar to that proposed for MLR and hypothalamic inputs to RS neurons in the rat (Sinnamon et al., 1987). In general, synaptic integration of multiple locomotor inputs to RS neurons has not been well studied. In support of these data, blocking synaptic transmission in the entire brain of a partitioned in vitro preparation always abolishes VMD- and DLM-initiated spinal locomotor activity (Paggett et al., 2000). In addition, application of anatomical tracers to reticular nuclei retrogradely labels neurons in the vicinity of the VMD and DLM, but not the RLR (Paggett et al., 2000 and unpublished observations; also see El Manira et al., 1997).
Results from the present study support a working model for part of the locomotor command system in the lamprey brain:
Although this model undoubtedly contains additional components, it can serve as a framework to gain new and important insights into the operation of locomotor command systems in a vertebrate nervous system.
Results from other lamprey studies
In adult lamprey, electrical microstimulation in the ventral thalamus, in the vicinity of the VMD, elicits rhythmic ventral root activity (El Manira et al., 1997), but the functional identity of this activity has not been tested. Brief electrical stimulation in the ventral thalamus elicits synaptic responses in RS neurons, and manual application of a retrograde tracer to reticular nuclei with an “insect pin” labels neurons in the ventral thalamus (El Manira et al., 1997).
In the lamprey, electrical microstimulation in the caudomedial mesencephalon, which is thought to represent the lamprey MLR, initiates spinal locomotor activity (McClellan and Grillner, 1984) and elicits synaptic responses in RS neurons (McClellan, 1989; Sirota et al., 2000). In the present study, pharmacological microstimulation in the caudomedial mesencephalon did not reliably elicit spinal locomotor activity, at least with the agents used. This discrepancy may be due to the “very small size” of the MLR (Sirota et al., 2000) or the fact that different agents were used to stimulate in the brain than those used in the present study.
Brain locomotor areas in other vertebrates
Locomotor activity can be initiated by stimulation in specific brain areas: reticular nuclei; MLR; SLR; and PLS (see introduction). Lesions or blocking activity in medullary reticular nuclei abolishes MLR- or PLS-initiated locomotor activity (Shefchyk et al., 1984; Garcia-Rill and Skinner, 1987a; Bernau et al., 1991), stimulation in the MLR or SLR elicits synaptic responses in RS neurons (Orlovsky, 1970; Garcia-Rill and Skinner, 1987b), and anatomical experiments reveal projections from the MLR and SLR to reticular nuclei (Steeves and Jordan, 1984; Bernau et al., 1991). The PLS is in the vicinity of the trigeminal sensory nucleus (Bayev et al., 1988; Beresovskii and Bayev, 1988), and sensory neurons in the medulla, including those in trigeminal sensory nuclei, project to the MLR (Bayev et al., 1988). Thus, several features of locomotor command systems are conserved in widely divergent vertebrates, including the lamprey.
Summary
In larval lamprey, during locomotor activity initiated from the RLR, depression of neuronal activity in the DLM or VMD abolished or attenuated spinal locomotor activity. Depression of neuronal activity in reticular nuclei often attenuated or abolished DLM- or VMD-initiated locomotor activity, and electrical microstimulation in the DLM and VMD elicited synaptic responses in RS neurons that summated. These new results suggest that higher order locomotor areas in the DLM and VMD receive rostrally projecting inputs from the RLR. The DLM and VMD then project caudally to RS neurons, which appear to summate the inputs from these areas and activate the spinal CPGs for locomotion. These pathways are likely to be important components of the brain command systems for the initiation of locomotion.
Acknowledgments
Supported by NIH grant NS29043, NSF grant IBN-9817905, and American Paralysis Association grant MA1-9605 awarded to A.D.M. We thank Ryan Wolf and Emily Fotovich for help with some of the data analysis.
Abbreviations
- ARRN
anterior rhombencephalic reticular nucleus
- BL
body length
- CPG
central pattern generator
- DLM
dorsolateral mesencephalon
- HRP
horseradish peroxidase
- LR
lateral rhombencephalon
- MLR
mesencephalic locomotor region
- MRRN
middle rhombencephalic reticular nucleus
- PE
pressure ejection
- PLS
pontomedullary locomotor strip
- PRRN
posterior rhombencephalic reticular nucleus
- RLR
rostrolateral rhombencephalon
- RS
reticulospinal
- SLR
subthalamic locomotor region
- VMD
ventromedial diencephalon
References
- Bayev KV, Beresovskii VK, Kebkalo TG, Savoskina LA. Afferent and efferent connections of brainstem locomotor regions: study by means of horseradish peroxidase (HRP) transport technique. Neuroscience. 1988;26:871–891. doi: 10.1016/0306-4522(88)90106-6. [DOI] [PubMed] [Google Scholar]
- Beresovskii VK, Bayev KV. New locomotor regions of the brainstem revealed by means of electrical stimulation. Neuroscience. 1988;26:863–869. doi: 10.1016/0306-4522(88)90105-4. [DOI] [PubMed] [Google Scholar]
- Bernau NA, Puzdrowski RL, Leonard RB. Identification of the midbrain locomotor region and its relation to descending locomotor pathways in the Atlantic stingray, Dasyatis sabina. Brain Res. 1991;557:83–94. doi: 10.1016/0006-8993(91)90119-g. [DOI] [PubMed] [Google Scholar]
- Berry MS, Penthreath VW. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976;105:1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- Caputi L, Bengtson CP, Guatteo E, Bernardi G, Mercuri NB. D-Tubocurarine reduces GABA responses in rat substantia nigra dopamine neurons. Synapse. 2003;47:236–239. doi: 10.1002/syn.10164. [DOI] [PubMed] [Google Scholar]
- Curtis DR. Microelectrophoresis. In: Natsuk WL, editor. Physical techniques in biological research: electrophysiological methods. New York: Academic Press; 1964. pp. 144–190. [Google Scholar]
- Davis GR, McClellan AD. Extent and time course of restoration of descending brainstem projections in spinal-transected lamprey. J Comp Neurol. 1994;344:65–82. doi: 10.1002/cne.903440106. [DOI] [PubMed] [Google Scholar]
- El Manira A, Pombal MA, Grillner S. Diencephalic projection to reticulospinal neurons involved in the initiation of locomotion in adult lampreys Lampetra fluviatilis. J Comp Neurol. 1997;389:603–616. [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. The mesencephalic locomotor region: I. Activation of a medullary projection site. Brain Res. 1987a;411:1–12. doi: 10.1016/0006-8993(87)90675-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. The mesencephalic locomotor region: II. Projections to reticulospinal neurons. Brain Res. 1987b;411:13–20. doi: 10.1016/0006-8993(87)90676-7. [DOI] [PubMed] [Google Scholar]
- Goodchild AK, Dampney RAL, Bandler R. A method for evoking physiological responses by stimulation of cell bodies, but not axons of passage, within localized regions of the central nervous system. J Neurosci Methods. 1982;6:351–363. doi: 10.1016/0165-0270(82)90036-x. [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapods and fish. In: Brooks V, editor. Handbook of physiology, motor control. Baltimore, MD: American Physiological Society, Waverly Press; 1981. pp. 1179–1236. [Google Scholar]
- Hagevik A, McClellan AD. Coupling of spinal locomotor networks in larval lamprey revealed by receptor blockers for inhibitory amino acids: neurophysiology and computer modeling. J Neurophysiol. 1994;72:1810–1829. doi: 10.1152/jn.1994.72.4.1810. [DOI] [PubMed] [Google Scholar]
- Hagevik A, Oxner-McGaha A, McClellan AD. Pharmacological microstimulation in brain locomotor regions in larval lamprey. Soc Neurosci Abstr. 1996;22:1372. [Google Scholar]
- Harnischfeger G. An improved method for extracellular marking of electrode tip positions in nervous tissue. J Neurosci Meth. 1979;1:195–200. doi: 10.1016/0165-0270(79)90017-7. [DOI] [PubMed] [Google Scholar]
- Jackson AW, McClellan AD. Locomotor behavior initiated by chemical microstimulation in higher locomotor centers and reticular nuclei in semi-intact preparations of larval lamprey. Soc Neurosci Abstr. 2001;27:2481. [Google Scholar]
- Kashin SM, Feldman AG, Orlovsky GN. Locomotion of fish evoked by electrical stimulation of the brain. Brain Res. 1974;82:41–47. doi: 10.1016/0006-8993(74)90891-9. [DOI] [PubMed] [Google Scholar]
- Kazennikov OV, Selionov VA, Shik ML, Yakovloeva GV. The rhombencephalic “locomotor region” in turtles. Neurophysiology. 1981;12:251–257. [PubMed] [Google Scholar]
- McClellan AD. Brainstem command systems for locomotion in the lamprey: localization of descending pathways in the spinal cord. Brain Res. 1988;457:338–349. doi: 10.1016/0006-8993(88)90704-4. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Reticulospinal neurons in the lamprey: biophysical properties and inputs from the MLR. Soc Neurosci Abstr. 1989;15:505. [Google Scholar]
- McClellan AD. Locomotor recovery in spinal-transected lamprey: role of functional regeneration of descending axons from brainstem locomotor command neurons. Neuroscience. 1990;37:781–798. doi: 10.1016/0306-4522(90)90108-g. [DOI] [PubMed] [Google Scholar]
- McClellan AD, Grillner S. Activation of “fictive” swimming by electrical microstimulation of “locomotor command regions” in the brainstem of the lamprey. Brain Res. 1984;300:357–362. doi: 10.1016/0006-8993(84)90846-1. [DOI] [PubMed] [Google Scholar]
- McClellan AD, Hagevik A. Descending control of turning locomotor activity in larval lamprey: neurophysiology and computer modeling. J Neurophysiol. 1997;78:214–228. doi: 10.1152/jn.1997.78.1.214. [DOI] [PubMed] [Google Scholar]
- Niewenhuys R. The brain of the lamprey in a comparative perspective. Ann NY Acad Sci. 1977;299:97–145. doi: 10.1111/j.1749-6632.1977.tb41902.x. [DOI] [PubMed] [Google Scholar]
- Noga BR, Kettler J, Jordan LM. Locomotion produced in mesencephalic cats by injections of putative transmitter substances and antagonists into the medial reticular formation and the pontomedullary locomotor strip. J Neurosci. 1988;8:2074–2086. doi: 10.1523/JNEUROSCI.08-06-02074.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovsky GN. Spontaneous and induced locomotion of the thalamic cat. Biofizika. 1969;14:1095–1102. [Google Scholar]
- Orlovsky GN. Connections between reticulospinal neurons and locomotor regions of the brain stem. Biofizika. 1970;15:58–64. [Google Scholar]
- Paggett KC, Jackson AW, McClellan AD. Locomotor projections to reticular nuclei in larval lamprey. Soc Neurosci Abstr. 2000;26:158. [Google Scholar]
- Parker JM, Sinnamon HM. Forward locomotion elicited by electrical stimulation in the diencephalon and mesencephalon of the awake rat. Physiol Behav. 1983;31:581–587. [PubMed] [Google Scholar]
- Selionov VA, Shik ML. Medullary locomotor strip and column in the cat. Neuroscience. 1984;13:1267–1278. doi: 10.1016/0306-4522(84)90297-5. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Jell RM, Jordan LM. Reversible cooling of the brainstem reveals areas required for mesencephalic locomotor region evoked treadmill locomotion. Exp Brain Res. 1984;56:257–262. doi: 10.1007/BF00236281. [DOI] [PubMed] [Google Scholar]
- Shik ML, Orlovsky GN, Severin FV. Control of walking by means of electrical stimulation of the mid-brain. Biophysics. 1966;11:756–765. [Google Scholar]
- Sinnamon HM, Ginzburg RN, Kurose GA. Midbrain stimulation in the anesthetized rat: direct locomotor effects and modulation of locomotion produced by hypothalamic stimulation. Neuroscience. 1987;20:695–707. doi: 10.1016/0306-4522(87)90120-5. [DOI] [PubMed] [Google Scholar]
- Sirota M, Di Prisco GV, Dubuc R. Stimulation of the mesencephalic locomotor region elicits controlled swimming in semi-intact lampreys. Eur J Neurosci. 2000;12:4081–4092. doi: 10.1046/j.1460-9568.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- Steeves JD, Jordan LM. Autoradiographic demonstration of the projections from the mesencephalic locomotor region. Brain Res. 1984;307:263–276. doi: 10.1016/0006-8993(84)90480-3. [DOI] [PubMed] [Google Scholar]
- Uematsu K, Todo T. Identification of the midbrain locomotor nuclei and their descending pathways in the teleost carp, Cyprinus carpio. Brain Res. 1997;773:1–7. doi: 10.1016/s0006-8993(97)00619-7. [DOI] [PubMed] [Google Scholar]