Abstract
A series of substituted aryl amide derivatives of 6-naltrexamine, 3 designed to be metabolically stable were synthesized and used to characterize the structural requirements for their potency to binding and functional activity of human mu (μ), delta (δ) and kappa (κ) opioid and nociceptin (NOP) receptors. Binding assays showed that 4–10 had subnanomolar Ki values for μ and κ opioid receptors. Functional assays for stimulation of [35S] GTPγS binding showed that several compounds acted as partial or inverse agonists and antagonists of the μ and δ, κ opioid or NOP receptors. The compounds showed considerable stability in the presence of rat, mouse or human liver preparations and NADPH. The inhibitory activity on the functional activity of human cytochrome P450s was examined to determine any potential inhibition by 4–9. Only modest inhibition of CYP3A4, CYP2C9 and CYP2C19 was observed for a few of the analogs. As a representative example, radiolabeled 6 was examined in vivo and showed reasonable brain penetration. The inhibition of ethanol self-administration in rats trained to self-administer a 10% (w/v) ethanol solution, utilizing operant techniques showed 5–8 to have very potent efficacy (ED50 values 19–50 μg/kg).
Keywords: Beta naltrexamides, Alcohol cessation agents, Metabolism, In vitro–in vivo studies
1. Introduction
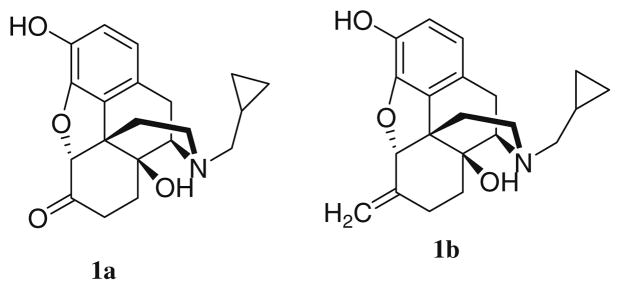
Worldwide, dependence on alcohol is a serious public health disorder with significant social and economic consequences. In 1994, naltrexone (compound 1a, Fig. 1) was approved by the United States FDA for treatment of alcoholism. Compound 1a, along with acamprosate (C10H20CaN2O8S2) and disulfiram (C10H20N2S4) are the only agents currently available to treat alcohol dependence. Compound 1a has shown benefit for treating alcoholism in heavy drinkers1 and in a number of clinical studies linked to moderate to severe alcoholism.1,2 However, 1a is not successful in treating all alcoholics and adverse effects including intolerable nausea3 and hepatotoxicity4 confound treatment of patients with liver disease. It may be that metabolic bioactivation of 1a to a reactive metabolic intermediate contributes to the hepatotoxicity observed. Diminished effect over time, relatively low bioavailability2 and possibly relatively low affinity for δ and κ opioid receptors5 or genetic variability of the opioid receptors6 may explain the less than consistent efficacy of 1a.7 Nalmefene (compound 1b, Fig. 1), possesses superior pharmaceutical properties compared with 1a4 but 1b also suffers from hepatotoxic side effects.
Figure 1.
Chemical structures of 1a and 1b.
Studies using rodent animal models have shown that 1a decreases alcohol self-administration,8,9 suggesting that these types of agents may prevent the reinforcing effects of alcohol consumption. 1 However, some opioid receptor antagonists decrease both ethanol and sucrose intake in rats.10 Certain opioid receptor agonists stimulate food consumption in preclinical animal models of obesity and opioid receptor antagonists inhibit energy-rich food consumption.11 It may be that opioid receptor antagonists prevent central reward mechanisms that share common neural substrates responsible for the development of alcohol dependence.12
Opioid receptors are well-characterized receptors and numerous studies suggest that alcohol interacts with endogenous opioid systems13,14 (e.g., 1a is a pure opioid μ receptor antagonist with no agonist activity and no abuse potential). Antagonizing opioid receptors decrease the effects of alcohol-mediated pleasure-inducing endogenous opioids. By attenuating the positive reinforcing effects of alcohol consumption, opioid receptor antagonists have direct effects on alcohol-seeking behavior.10 A decrease in alcohol consumption by antagonism of opioid receptors suggests direct effects on this reinforcement system and animal studies have shown that μ-, δ- and κ-opioid receptors contribute to alcohol-induced reinforcement.15,16
Previously, 6-alpha and 6-beta-N-heterocyclic17 naltrexamines were synthesized and displayed considerable antagonist potency and selectivity for the μ-opioid receptor. In a separate study, N-aryl amide naltrexamine18 derivatives were synthesized and showed considerable potency and selectivity for κ-opioid receptors. At the outset, we hypothesized that κ-opioid receptor antagonists would show alcohol consumption cessation in animal models. Most electron-rich N-aryl amide naltrexamines18 were not potent at alcohol consumption cessation in animal models. However, one electron-deficient aryl amide of naltrexamine (i.e., 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4′-chloro) benzamido] morphinan) showed considerable efficacy as an in vivo alcohol cessation agent.18 We thus investigated highly electron-deficient aryl amides of naltrexamine as alcohol cessation agents.
Herein, we report on the rationale for and the design of a class of metabolically stable compounds that have mixed potency and efficacy as μ-, δ- and κ-opioid and NOP-receptor partial agonists, inverse-agonists and/or antagonists as alcohol self-administration cessation agents. Partial agonist agents are anticipated to show a dual action by inhibiting alcohol reinforcement and stimulating dopamine release to decrease craving. The rationale for the work described herein was to develop long-lived, metabolically stable analogues of 1a or 1b by replacing the metabolically labile 6-keto or 6-methylene groups, respectively, with an amide moiety, thus leading to agents with more sustained pharmacological activity and potentially less hepatotoxicity.
2. Results
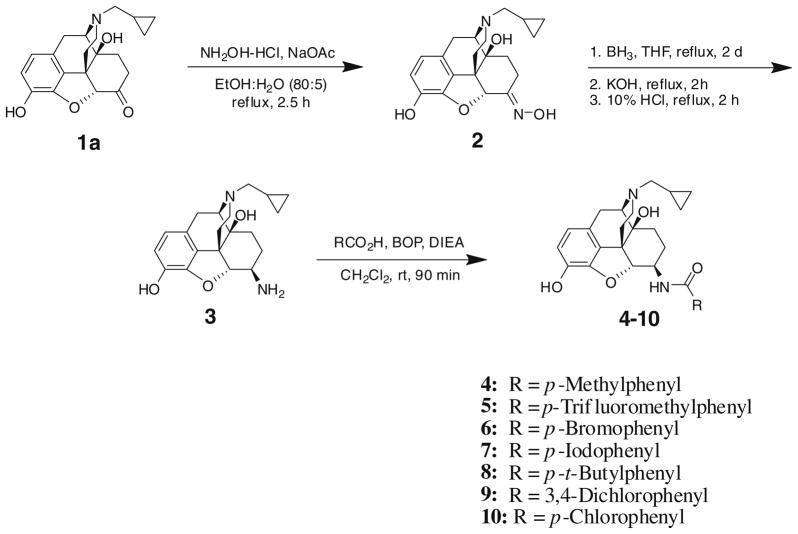
The chemical synthesis of a series of substituted aryl amide derivatives of 6-β-naltrexamine 4–10 was efficiently accomplished (Scheme 1) and used to characterize the structural requirements for binding to and functional activity of human μ-, δ-, κ-opioid and nociceptin receptors. Compound 1a was converted to its oxime 2 in a quantitative yield using hydroxylamine hydrochloride in the presence of sodium acetate in refluxing ethanol.19 Reduction of the oxime 2 to the corresponding amine 3 was accomplished by heating 2 with borane-tetrahydrofuran complex for 2 days. Following an aqueous workup, amine 3 was obtained as a 1:9 (α/β) mixture of diastereomers. The diastereomers were separated by chromatography on silica gel and the stereochemistry at the C-6 position was determined on the basis of the size of the NMR coupling constant, J5,6. The amine 3 (only the beta diastereomer was used in this work because previous work18 showed little stereoselectivity in opioid binding for β versus α diastereomers) was coupled either with a carboxylic acid in the presence of benzotriazol-1-yl-oxy-tris-(dimethylamino) phosphonium hexafluorophosphate (BOP) and diisopropylethylamine or alternatively, with an acid chloride in triethylamine. The product was treated with potassium carbonate in methanol to remove the byproduct resulting from esterification of the 3-position hydroxyl group, giving amides 4–10 in moderate to high yields (60–97%). While the BOP coupling procedure resulted in less esterification at the 3-position compared with the acid chloride method, some esterification at the 3-position could not be avoided. Thus, it was found to be more convenient to run the reaction with an excess of the acid chloride, form the intermediate amide ester and then purify the amide ester before base hydrolysis to afford the desired amide.
Scheme 1.
Chemical synthesis of compounds 2–10.
The binding of compounds 1a, 1b, 4–10 to the μ-, δ- and κ-opioid receptors was determined in a competitive binding assay with the following radioligands: [3H] [D-ala,2 N-MePhe,4 Gly-ol] enkephalin, 11 ([3H] DAMGO, μ-opioid receptor agonist), [3H] [D-ala,2 D-leu5] enkephalin, 12 ([3H] DADLE, δ-opioid receptor agonist) and [3H] (5a,7a,8b)-(+)-N-methyl-N-(7-[1-pyrrolidinyl]-1-oxaspiro[4,5] dec-8-yl)-benzene-acetamide, 13 ([3H] U69593, κ-opioid receptor agonist), 14 [D-Pen2,5]-enkephalin (DPDPE), 15, naltrindole, 16 (−)-trans-(1S,2S)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl) cyclohexyl] benzene acetamide hydrochloride ((−) U50,488), and 17, norbinaltorphimine. Results of binding to the individual receptors, and the ratios of δ- and κ-binding relative to μ- were summarized and listed in Table 1. The amides 4–10 were between 4 and 10-fold more potent at the δ-opioid receptors than 1a and 1b (Ki <4 nM for 4–10 compared to 16.3 and 13.3 nM for 1a and 1b, respectively). The 3,4-dichlorophenyl amide and the bulky t-butyl and iodo phenyl amide analogs were the most potent with Ki values around 1 nM. Compared to 1a and 1b, binding was also improved with regard to the κ-opioid receptor. The phenyl amide derivatives afforded a Ki of <0.4 nM compared to 0.8 nM for 1a and 1.03 nM for 1b. The p-methyl phenyl analog 4 bound to the κ receptor with the greatest affinity (Ki = 0.11 nM) suggesting that a smaller group at the para position was favored for κ receptor binding. Finally, adding an aryl amide at the 6-position on the naltrexamine core (i.e., 4–10) did not significantly change the affinity for binding to the μ-receptor compared to 1a and 1b, (i.e., Ki values between 0.3 and 1.09 nM were observed). All the compounds examined had at least 2–3-fold greater potency for the κ-receptor compared to the μ- or δ-receptor. Compound 5 was about sevenfold more selective for the κ-receptor compared with the δ-receptor.
Table 1.
Inhibition values and selectivity for μ, δ and κ opioid binding
| Compda |
Ki (nM) ± SEM |
Selectivity |
|||
|---|---|---|---|---|---|
| μ | δ | κ | δ/μ | κ/μ | |
| 1ab | 0.30 ± 0 | 16.31 ± 1.10 | 0.81 ± 0.02 | 49 | 2.5 |
| 1bb | 0.91 ± 0.10 | 13.26 ± 0.75 | 1.03 ± 0.19 | 15 | 1.1 |
| 4 | 0.34 ± 0.05 | 3.6 ± 0.3 | 0.11 ± 0.02 | 11 | 0.32 |
| 5 | 0.47 ± 0.05 | 2.5 ± 0.3 | 0.34 ± 0.05 | 5.3 | 0.72 |
| 6 | 0.88 ± 0.10 | 2.2 ± 0.3 | 0.29 ± 0.04 | 2.5 | 0.33 |
| 7 | 0.82 ± 0.10 | 1.4 ± 0.2 | 0.37 ± 0.05 | 1.7 | 0.45 |
| 8 | 1.09 ± 0.20 | 1.4 ± 0.1 | 0.37 ± 0.06 | 1.3 | 0.34 |
| 9 | 0.48 ± 0.07 | 1.0 ± 0.1 | 0.34 ± 0.04 | 2.1 | 0.71 |
| 10 | 0.61 ± 0.09 | 2.6 ± 0.3 | 0.23 ± 0.03 | 4.3 | 0.38 |
| 11 | 0.9 | ||||
| 17 | 0.8 | ||||
| Salvonorin A | 0.8 | ||||
Ki values were expressed as the mean ± SEM of two determinations.
Compounds 4–10 were salts as described in the Methods.
Data was taken from Ref. 18.
A functional assay was also run in order to evaluate the opioid receptor-mediated activation of its associated G protein. Compounds 4–10 were evaluated using the [35S] GTPγS assay.20 In this assay, a compound’s potency or affinity for the receptor was associated with its EC50 value for stimulating [35S] GTPγS binding. Agonist activity of each compound was determined at the μ-, δ-, κ-opioid and NOP-receptors, and compared to the standard selective full agonists, 11, [D-pen2, D-pen5]-enkephalin, 14 (DPDPE), 13 and nociceptin, 15, respectively. Table 2 summarizes the EC50 and Emax values for compounds 4–16 in the presence of cloned human cell membranes containing the μ, δ- or κ-opioid or NOP receptors.
Table 2.
Stimulation of [35S] GTPγS binding at opioid receptors by compounds 4–10 and the opioid agonists, 11, 14, 16 and 15
| Compda | μ |
δ |
κ |
NOP |
||||
|---|---|---|---|---|---|---|---|---|
| EC50 | Emax | EC50 | Emax | EC50 | Emax | EC50 | Emax | |
| 4 | >10 μM | 0 | 0.14 ± 0.1 nM | 10 ± 9.6 | >10 μM | 0 | >10 μM | 0 |
| 5 | 16 ± 2.1 nM | 63 ± 14 | 5.1 ± 0.2 nM | 28 ± 6.5 | 9.9 ± 1.7 nM | 36 ± 6.4 | >10 μM | 0 |
| 6 | 4.5 ± 0.5 nM | 38 ± 4.3 | 0.2 ± 0.1 nM | 46 ± 3.7 | 0.1 ± 0.1 nM | 42 ± 3.9 | >10 μM | 0 |
| 7 | 8.8 ± 1.7 nM | 53 ± 2.8 | 5.1 ± 2.9 nM | 45 ± 6.2 | 29 ± 3.4 nM | 28 ± 3.1 | >10 μM | 0 |
| 8 | >10 μM | 0 | >10 μM | 0 | >10 μM | 0 | >10 μM | 0 |
| 9 | 2.3 ± 1.4 nM | 85 ± 7.4 | 1.4 ± 1.4 nM | 85 ± 35 | 0.9 ± 0.1 nM | 80 ± 7.4 | >10 μM | 0 |
| 10 | 6.8 ± 1.7 nM | 46 ± 3.8 | 42 ± 3.1 nM | 22 ± 1.7 | 7.1 ± 2.7 nM | 31 ± 2.6 | >10 μM | 0 |
| 11 | 8.2 ± 1.4 nM | 124 ± 7.9 | — | — | — | — | — | 0 |
| 14 | — | — | 15 ± 2.6 nM | 76 ± 4.8 | — | — | — | 0 |
| 15 | — | — | — | — | — | — | 3.9 ± 0.5 nM | 109 ± 11 |
| 16 | — | — | — | — | 0.4 ± 0.2 nM | 54 ± 14 | — | 0 |
Compounds 4–10 were salts as described in the Methods. Emax values are expressed as mean ± SEM percentage of basal [35S] GTPγS binding stimulation.
Para-alkyl substituted 4 and 8 were either very weak agonists or completely not functional suggesting that electron donating groups might be detrimental to functional activity. The 3,4-dichlorophenyl derivative 9 was found to stimulate GTPγS binding as a full agonist at μ-, δ- and κ-opioid receptors (Emax ~80–85%), with an EC50 value in the low nanomolar range (EC50 = 2.3, 1.4, 0.9 for μ-, δ-, κ-receptors, respectively). Compounds 5–7 and 10 were partial agonists (Emax values between 28% and 63%) at μ-, δ-, and κ-opioid receptors (Table 2). Compounds 4–10 had very low affinity for the NOP receptor and did not stimulate agonist-induced GTPγS binding.
In a second functional assay, compounds 4–10 were evaluated as inverse-agonists. Compounds 4 and 8 were found to be partial inverse-agonists at the μ- and κ-receptors. Compounds 4–10 were found to potently decrease basal binding at NOP and compounds 7 and 9 were found to have high affinity as inverse-agonists. Compound 8 was also observed to be a potent inverse-agonist at δ-and κ-receptors, with less potent inverse-agonism at the μ-receptor (Table 3). Compound 4 was found to be a potent inverse-agonist at μ-and κ-receptors with decreased potency (albeit with high efficacy) at the NOP receptor. Compound 6 was also observed to display inverse-agonism at the δ-receptor albeit at higher concentrations (i.e., 10 nM–10 μM), in addition to potent agonism at lower concentrations (i.e., 10 pM–10 nM).
Table 3.
Inhibition of basal [35S] GTPγS binding at opioid receptors by compounds 4–10a
| Compd | μ |
δ |
κ |
NOP |
||||
|---|---|---|---|---|---|---|---|---|
| EC50 | Emax | EC50 | Emax | EC50 | Emax | EC50 | Emax | |
| 4 | 8.9 ± 0.2 nM | 33 ± 2.4 | >10 μM | 0 | 2.4 ± 0.4 nM | 20 ± 1.2 | 135 ± 31 nM | 138 ± 4.8 |
| 5 | >10 μM | 0 | >10 μM | 0 | >10 μM | 0 | 20 ± 11 nM | 104 ± 4.4 |
| 6 | >10 μM | 0 | 66 ± 1.8 nM | 35 ± 4.8 | >10 μM | 0 | 94 ± 33 nM | 92 ± 7.4 |
| 7 | >10 μM | 0 | >10 μM | 0 | >10 μM | 0 | 3.6 ± 1.5 nM | 173 ± 6.3 |
| 8 | 4.0 ± 0.1 μM | 46 ± 1.6 | 0.3 ± 0.1 nM | 28 ± 9.1 | 0.4 ± 1.2 nM | 60 ± 2.6 | 15 ± 2.6 nM | 92 ± 2.9 |
| 9 | >10 μM | 0 | >10 μM | 0 | >10 μM | 0 | 0.1 ± 0.4 nM | 87 ± 6.9 |
| 10 | >10 μM | 0 | >10 μM | 0 | >10 μM | 0 | 88 ± 19 nM | 320 ± 6.8 |
Compounds 4–10 were salts as described in the Methods. Emax values are expressed as mean ± SEM percentage of basal [35S] GTPγS binding stimulation.
High affinity compounds that showed low or partial agonist activity in the GTPγS binding experiments were tested for inhibition of agonist-induced GTPγS binding at each receptor. Compound 4 produced strong inhibition at δ- and κ-receptors and potent inhibition at μ-receptors, but not at the NOP-receptor (Table 4). Compound 5 produced potent inhibition at both κ- and NOP-receptors, but not at μ- or δ-receptors. Compound 6 produced very potent inhibition at the κ-receptor but no detectable inhibition at μ-, δ-or NOP receptors. Compounds 7 and 8 did not produce any detectable inhibition at any opioid receptor examined. Compounds 4, 5 and 6 appear to possess mixed activity as either agonists, inverse-agonists or antagonists for each of the μ-, δ- and κ-opioid and NOP-receptors. As described below, further kinetic analysis was done to characterize the pharmacological properties of these latter compounds.
Table 4.
Inhibition of agonist-stimulated [35S] GTPγS binding at opioid receptors by compounds 4–8a compared to 1a, 17 and 18
| Compd |
Ki |
|||
|---|---|---|---|---|
| μ | δ | κ | NOP | |
| 4 | 6.2 ± 1.9 nM | 0.1 ± 0.02 nM | 15 ± 1.4 pM | >10 μM |
| 5 | >10 μM | >10 μM | 637 ± 10 pM | 4.2 ± 0.3 nM |
| 6 | >10 μM | >10 μM | 0.3 ± 0.2 pM | >10 μM |
| 7 | >10 μM | >10 μM | >10 μM | >10 μM |
| 8 | >10 μM | >10 μM | >10 μM | >10 μM |
| 1a | 3.6 ± 0.2 nM | 66.8 ± 12.6 nM | 42 ± 4.0 pM | >10 μM |
| 17 | — | 0.3 ± 0.1 nM | — | — |
| 18 | — | — | 4.8 ± 2.3 pM | — |
Compounds 4–8 were salts as described in the Methods. Values are expressed as mean (±SEM) Ki for inhibition of 11 (1 μM), 14 (200 nM, 16 (2 μM) and 15 (NOP; 1 μM) basal [35S] GTPγS binding stimulation was performed at μ-, δ-,κ-opioid and nociceptin (NOP) receptors, respectively.
3. Structure–activity studies
The SAR of the aromatic amide portion of the opioid derivatives was examined. Despite the limited number of compounds studied, a few conclusions could be reached. In general, electron withdrawing para-monosubstituted or meta, para-disubstituted aromatic groups showed the greatest potency and efficacy for the μ-receptor (Table 2). Thus, compound 10 (the 4-chloro-substituted aromatic amide) showed significant affinity for the μ-receptor and had EC50 values in the low nM range (Tables 1 and 2). Electron-rich aryl-substituted compounds 4 and 8 showed no detectable stimulation of [35S] GTPγS binding. Compounds 4 and 6 possessed the greatest potency against the δ receptor but aside from compound 9, the compounds tested did not markedly stimulate [35S] GTPγS binding. With the exception of the electron-rich aryl-substituted compounds 4 and 8, all of the compounds examined had relatively good potency for the κ-receptor. The efficacy of 4–10 for the κ receptor largely paralleled that observed for the μ receptor. No detectable potency for the NOP receptor was observed for compounds 4–10 (Table 2). In summary, the opioid receptors appear to favor binding of compounds with highly electron-deficient and lipophilic substituents at the meta and para position of C-6 substituted aromatic amides of naltrexamine. The electronic effect of the aromatic substituent on the in vivo ED50 value was more pronounced (see in vivo analysis, below).
4. Metabolic stability
As a prelude to studying the test compounds in vivo, TLC- and HPLC-based analytical methods and biochemical assays were used to assess the metabolic stability of selected compounds in the presence of rat, mouse and human liver preparations and the appropriate NADPH generating system. These studies were done to ascertain the stability of the compounds toward oxidative metabolism in advance of more detailed studies with highly purified human CYPs and FMO3 as well as to determine if the compounds possessed sufficient metabolic stability for in vivo studies. Compared to 1b, the candidate compounds 4–9 were quite metabolically stable in the presence of liver preparations from all three species examined (i.e., rat, mouse, human) with the exception that 6 and 8 possessed similar metabolic stability as 1b in the presence of rat liver microsomes (Table 5). Compounds 4 and 5 remained unchanged in the mouse and human liver microsomes for the length of the experiment. Compounds 6–9 were also very stable in the mouse and human liver microsomes with a half life greater than 112 min. Similarly, 4–9 were stable in mouse liver microsomes and compounds that were metabolically stable in the presence of mouse or human liver microsomal preparations did not afford evidence of significant amounts of metabolite formation based on inspection of the HPLC profiles (data not shown). In the presence of rat liver microsomes, overall, the compounds were somewhat less metabolically stable, but the half life values observed did not preclude evaluation of the compounds in vivo. The lack of metabolic instability, however, may have been the result of inhibition of CYP-dependent metabolism. To examine this point more carefully, the effect of 4–9 on inhibition of selected CYPs was examined.
Table 5.
Metabolic stability of 4–9a in the presence of liver preparations
5. CYP Inhibition
It is known that cyclopropyl methyl-containing amines can inhibit CYP.21,22 To understand the metabolic stability data described above and to examine the possible extent and selectivity of CYP inhibition, selected compounds (i.e., 4–9) were examined along with 1b for their ability to inhibit selective functional activities of human CYP enzymes. The observed percent inhibition for selective functional inhibition of CYP-3A4, -2B6, -2C9, -2C19 and -2D6 were reported in Table 6. The enzyme assays were done using standard conditions as previously described.23 Compounds 4–9 were weaker inhibitors than 1b for the CYPs studied except in the case of CYP2C19 that appeared to be more sensitive than 1b to inhibition by 5, 8, and 9. In general, the enzymes mainly involved in inhibition by 4–9 were CYP3A4 and 2C19. In addition, compound 6 inhibited CYP2B6 with greater potency than 1b. Replacement of the C-6 exo methylene group of 1b with an aryl amide group in this series attenuated the inhibitory potency toward CYP. This suggests a significant contribution of the C-6 moiety in the interaction of 1b with CYP and for the C-6 substituted amides examined herein, it suggests a decreased interaction with CYP. Because CYP3A4 and CYP2D6 often make significant contributions to opioid metabolism, adverse drug–drug interactions, metabolic bioactivation and therefore possible side-effects, this new synthetic class of opioid analog is attractive. Decreased interaction with CYP in part may explain some of the metabolic stability observed for the compounds in this and related series.24 On the basis of the data from the in vitro metabolism studies, we judged the compounds to be sufficiently stable and of low CYP inhibitory potency to study them in vivo in an animal model of ethanol self-administration. Compound 6 was selected to examine the putative metabolism in greater detail.
Table 6.
Percent inhibition of CYP3A4, CYP2B6, CYP2C9, CYP2C19 and CYP2D6 by selected naltrexamides
| Compda | Percent inhibitionb |
||||
|---|---|---|---|---|---|
| CYP3A4 | CYP2B6 | CYP2C9 | CYP2C19 | CYP2D6 | |
| 1b | 60 | 5 | 35 | 17 | 31 |
| 4 | 36 | 6 | 12 | 7 | NDc |
| 5 | 29 | 9 | 9 | 52 | 12 |
| 6 | 36 | 18 | 8 | 19 | 12 |
| 7 | 41 | 6 | 7 | 17 | ND |
| 8 | 13 | 9 | 5 | 49 | ND |
| 9 | 11 | 5 | 7 | 39 | 10 |
Compounds 4–9 were salts as described in the Methods.
Percent inhibition in the presence of 10 μM test compound. The test compound was preincubated for 2–5 min with the enzyme and cofactor and then the appropriate substrate was added and the rate of product was monitored and compared with the complete system without the test compound present. Values are the average of 2–3 determinations. The range of the values never exceeded 10–15%.
ND, no detectable inhibition was observed at the concentration of the test compound examined.
6. In vitro and in vivo studies with compound 6
A radiometric assay and an HPLC assay were set up to examine the possible metabolism of radiolabeled 6. Compound 6 was chosen as a representative compound to study because its radiosynthesis was very efficient. To confirm the results from the radiometric studies, we developed an HPLC method to analyze N-oxygenated and amide hydrolysis of compound 6. In the presence of rat liver microsomes and after extractive work-up and HPLC analysis, compound 6 hydrolysis was linearly dependent on time (0–15 min) and protein concentration (i.e., 0–0.4 mg of protein). However, the rate of hydrolysis was quite low and ranged between 0.7 and 0.9 nmol/min/mg of protein. No significant amount of the N-oxide of 6 was detected. In the presence of human liver microsomes, compound 6 hydrolysis was linearly dependent on time (0–30 min) and protein concentration (i.e., 0–0.5 mg of protein). The rate of hydrolysis in human liver microsomes was lower than for rat liver microsomes and ranged between 0.2 and 0.5 nmol/min/mg of protein. In contrast to rat liver microsomes, in the presence of human liver microsomes a significant amount of 6 N-oxide was formed (i.e., 10–23 pmol/min/mg of protein). Formation of the N-oxide of 6 was dependent on pH; the rate doubled upon going from pH 7.4 to pH 10. Highly purified human FMO3 catalyzed the formation of 6 N-oxide (i.e., 0.9–1.1 nmol/min/mg of protein) but this rate was quite low. In summary, overall, the metabolism of 6 was quite low and the data agreed with the relative metabolic stability described above (Table 5).
The oxalate salt of radiolabeled 6 was administered to two groups of three male Wistar rats via oral gavage (400 μg/kg) and iv (100 μg/kg) route of administration. After oral administration, the Tmax was 57 min and the apparent T1/2 was 2.5 h. After iv administration, the Tmax was 22 min and the T1/2 was 45 min. A separate group of three male Wistar rats was administered the oxalate salt of radiolabeled 6 via the oral route of administration and killed after 1.5 h. Brain tissue and blood was immediately procured and chilled on ice and prepared for analysis as described in the Methods section. The amount of radiolabeled 6 oxalate present in each animal at 1.5 h was determined by examining an aliquot of brain homogenate and plasma by scintillation counting. The amount of radiolabeled 6 in brain tissue and plasma was 6.5 ± 0.8 ng/gm and 2.8 ± 0.3 ng/mL, respectively. The brain tissue/plasma ratio of 2.3 at the time of measurement suggested that adequate brain concentrations of 6 was present to proceed with in vivo alcohol self-administration cessation studies.
7. In vivo alcohol self-administration studies
In vivo studies were intended to test the effects of compounds 4–10 on baseline ethanol (EtOH) intake in rats trained to self-administer a 10% (w/v) ethanol solution, utilizing an operant technique model. This model is commonly used to examine the effects of novel compounds on reinforcing effects of ethanol.25–30 Control groups consisting of rats trained to orally self-administer a 0.025% saccharin (SACC) solution were used to examine non-specific effects of the experimental compounds. 1b hydrochloride was used as a positive control. Initially, dose range studies were conducted and if compounds appeared biologically active, more detailed studies were conducted. Preliminary determinations, showed that 4–8 and 10 possessed ED50 values of 0.25, 0.019, 0.042, 0.038, 0.05 and 0.5 mg/kg, respectively. Because compound 10 showed inhibition of alcohol self-administration with an ED50 of approximately 0.5 mg/kg and was considerably less potent than the other compounds examined, it was not studied further. Additionally, after sc administration of 0.025 mg/kg of 9, a potent decrease in alcohol consumption was observed (i.e., 77%), but 9 also caused profound analgesia and consequently further studies were not pursued with this compound. Compounds 5–8 were then administered sc in a separate drug-naive cohort of rats using a within-subjects Latin Square dose design. Results from testing compounds 5–8 at doses ranging from 0.00625 to 0.05 mg/kg showed significant effects in the alcohol self-administration model (Table 7). For 1b [F = 13.1, P <0.0001], 5 [F = 5.3, P <0.006], and 6 [F = 7.3, P <0.001], treatment with opioid 30 min prior to testing had an overall effect on operant self-administration of 10% ethanol. Compared with vehicle, post hoc analysis of 1b, 5 and 6 showed that doses of 0.0125, 0.025 and 0.05 mg/kg significantly inhibited operant self-administration of 10% ethanol. For compounds 7 [F = 5.7, P <0.004] and 8 [F = 4.9, P <0.008], treatment had an overall effect on operant self-administration of 10% ethanol. Compared with vehicle, post hoc analysis showed that only a dose of 0.05 mg/kg significantly inhibited operant self-administration of 10% ethanol. To test whether the effect of the compounds were selective for ethanol, the effect of 1b and 5–8 on self-administration of saccharin (0.025%) (Table 8) was examined. Treatment with 1b [F = 1.0, P = 0.4135] and 8 [F = 0.68.7, P = 0.578] did not have an overall effect on the operant self-administration of saccharin compared with vehicle. Compound 5 [F = 6.06, P = 0.0065], compound 6 [F = 4.52, P = 0.019], and compound 7 [F = 3.7, P = 0.037] did have an overall effect on saccharin self-administration. In light of these non-specific effects, post-hoc analysis of a dose of 0.025 mg/kg for 5 and a dose of 0.05 mg/kg for 6 showed that these doses were the only doses examined that significantly inhibited self-administration of saccharin, compared with vehicle. The ED50 value for ethanol self-administration observed for hydrochlorides of 1a and 1b in similar experiments31 was approximately 0.5 and 0.04 mg/kg, respectively. The efficacy for inhibition of ethanol self-administration by 5–8 compared very favorably to that of 1b, and in some cases, (i.e., compounds 5, 6 and 7) were apparently more efficacious.
Table 7.
Effect of 5–8a on the number of ethanol self-administrations in rats
| Compd | N | Vehicle | Dose (μg/kg) |
|||
|---|---|---|---|---|---|---|
| 6.25 | 12.5 | 25 | 50 | |||
| 1b | 10 | 39.6 ± 3.2 | NDc | 26.1 ± 3.8b | 22.2 ± 3.4b | 17.1. ± 1.5b |
| 5 | 10 | 30.6 ± 3.9 | 26.4 ± 3.6 | 20.5 ± 3.2b | 12.8 ± 1.6b | NDc |
| 6 | 10 | 41.1 ± 6.0 | NDc | 29.7 ± 3.9b | 25.6 ± 3.5b | 19.3 ± 2.6b |
| 7 | 10 | 33.3 ± 5.5 | NDc | 25.0 ± 2.7 | 24.7 ± 3.7 | 13.3 ± 1.5b |
| 8 | 10 | 39.2 ± 5.4 | NDc | 35.6 ± 5.7 | 28.0 ± 3.1 | 19.9 ± 4.1b |
Compounds 5–8 were salts as described in the Methods.
Statistically significant compared to vehicle-treated rats (P <0.05).
ND, no data collected at this dose based on preliminary screening in a separate cohort of rats showing no efficacy at this dose (for 6.25 μg/kg dose) or total suppression of saccharin controls (for 50 μg/kg dose).
Table 8.
Effect of 5–8a on the number of saccharin self-administrations in 1 h in rats
| Compd | N | Vehicle | Dose (μg/kg) |
|||
|---|---|---|---|---|---|---|
| 6.25 | 12.5 | 25 | 50 | |||
| 1b | 6 | 21.0 ± 10.6 | NDc | 17.5 ± 6.0 | 7.3 ± 1.9 | 13.7. ± 5.6 |
| 5 | 6 | 33.3 ± 7.2 | 23.8 ± 6.8 | 24.8 ± 8.0 | 10.0 ± 3.1b | NDc |
| 6 | 6 | 31.5 ± 9.1 | NDc | 11.3 ± 3.6 | 13.2 ± 2.3 | 10.5 ± 5.2b |
| 7 | 6 | 16.8 ± 7.0 | NDc | 6.0 ± 1.7 | 6.2 ± 3.0 | 4.8 ± 1.6 |
| 8 | 6 | 14.0 ± 7.1 | NDc | 16.2 ± 8.8 | 6.2 ± 2.3 | 7.2 ± 3.5 |
Compounds 5–8 were salts as described in the Methods.
Statistically significant compared to vehicle-treated rats (P <0.05).
ND, no data collected at this dose based on preliminary screening in separate cohort of rats showing no efficacy at this dose (for 6.25 μg/kg dose) or total suppression of saccharin controls (for 50 μg/kg dose).
8. In vivo SAR
The effect of the C-6 meta- or para-aryl amide substituent of the opioid on the relative efficacy of compounds 5–9 to inhibit ethanol self-administration in vivo was examined with regression correlation analysis using various physical organic parameters.32 A plot of the log ED50 value versus the electronic substituent sigma values provided a linear correlation with a slope of rho (ρ) value of 1.55 and an R2 value of 0.925. A plot of the log ED50 value versus the hydrophobicity substituent pi values provided a less linear correlation with a slope of 1.35 and an R2 value of 0.59. Likewise, an examination of steric effects with a plot of the log ED50 value versus the steric substituent values (Fs) provided a non-linear correlation with a slope of −0.793 and an R2 value of 0.563. On the basis of the R2 value and the goodness of fit the suggestion is that the in vivo ED50 values for alcohol cessation can be explained to a great extent by the C-6 meta-or para-aryl amide electronic substituent effects and to a much less extent on the basis of hydrophobicity or steric effects.
9. Conclusion
A series of C-6 aryl-substituted amide derivatives of 6-β-naltrexamine where the amide was appended with substituted aromatic groups was prepared by the reaction of 6-β-naltrexamine, compound 3b, with aryl acid chlorides or carboxylic acids. The compounds were evaluated as ligands for the human μ-, δ-, κ- and nociceptin opioid receptors with the goal of identifying a potent and selective alcohol self-administration cessation agent. Originally, metabolically stable saccharide derivatives of opioids were studied as pain medication candidates.24 It was noted that glucuronides were less potent than glucose derivatives but certain substituted aryl amides showed significant selectivity for the κ-receptor.33 Recently published17 related studies have shown that 6β-N-heterocyclic substituted naltrexamides were μ opioid receptor selective. In a previous study18 we initially examined glucose derivatives as alcohol cessation agents but the compounds did not possess significant potency in vitro against the δ- and κ-receptors and were not efficacious in vivo to warrant further investigation. Next, we synthesized analogs of saccharides where C-6 aryl amide substituents mimicked the polar functionalities of a saccharide and the compounds retained significant potency. In good agreement with that described previously, 34 carboxyl-containing C-6 aryl amides possessed poor affinity for the μ- and κ-opioid receptors.18 On that basis, we made a number of analogs to explore SAR and in brief, electron-deficient aryl amides appeared to show the greatest promise in terms of potency and opioid receptor antagonism. As shown in Table 2, not all of the compounds tested stimulated full [32S] GTPγS binding at the μ-, δ- and κ-receptors. Compounds 4 and 8 showed no functional agonism of the μ-receptor. Addition of a meta-chloro substituent to 10 provided a compound (i.e., 9) that elicited considerable potency to all three receptors. Thus, introduction of small substituent changes can result in dramatic biological effects in this series. Surprisingly, a Hammett relationship was not observed for 4–10 on the basis of in vitro data but a strong Hammett relationship was observed for C-6 aryl amide substituents and alcohol cessation ED50 values in vivo. The finding from in vivo studies that 5–8 were much more potent as ethanol cessation agents compared with 1a suggests that the partial agonist activity at all three opioid receptors might be necessary for full ethanol cessation activity. We surmise that the in vivo efficacy of 5–8 is dependent on interaction with all three μ-, δ-, and κ-opioid receptors because a compound such as 4 that is highly selective for the δ-opioid receptor is not optimal for alcohol cessation in vivo. The profile of opioid receptor binding coupled with the metabolic stability of 5–8 contributes to the optimal functional activity as alcohol self-administration cessation agents in vivo.
Hepatotoxicity of 1a and 1b is a concern because, generally, the liver of individuals that abuse ethanol is severely compromised. Because a relationship between metabolic bioactivation and hepatotoxicity has been established,35 the design of the compounds described herein may afford a decrease in the metabolic lability of 1b analogs by providing compounds with increased bioavailability as a result of modification of the C-6 position. As shown in Tables 4 and 5, we identified compounds that possessed greater metabolic stability and generally less propensity to interact with hepatic CYP than 1b. It may be that decreasing the affinity of opioid derivatives described herein for metabolic enzymes and increasing the metabolic stability results in a class of compounds with less potential for hepatotoxicity.
On the basis of the results of the binding and functional assay and metabolic stability studies shown herein, selected compounds were evaluated for their effects in an animal model of ethanol self-administration. One objective was to try to determine which opioid receptor contributed to ethanol self-administration cessation. Based on limited data presented above, it appears that individual antagonism of μ-, δ- or κ-receptors alone is not sufficient for ethanol self-administration cessation. It may be that potent κ-antagonism can compensate for weak δ-antagonism but what is more likely is that ethanol self-administration cessation efficacy requires partial agonism of all three opioid receptors but requires strong κ-opioid antagonism. Such agents (i.e., 5–7) showed efficacious in vivo activity in an animal model of ethanol self-administration in rats trained to self-administer a 10% (w/v) ethanol solution. This is in agreement with a recent study that showed that an opioid with strong κ-receptor antagonism (i.e., 1b) was more effective at ethanol self administration cessation than an opioid with broad receptor antagonism (i.e., 1a).31 Consequently, compounds such as 5–7 and related agents may represent an exciting lead for developing the next generation of opioid compounds useful in the treatment of alcohol abuse.
10. Methods
10.1. Chemicals
Hydrochloride salts of 1a and 1b were obtained from Tyco Mallincrodt (St. Louis, MO). Compounds 9 and 10 and their HCl salts were prepared as previously described.18 NADP+, glucose-6-phosphate, glucose-6-phosphate dehydrogenase, diethylenetriaminepentaacetic acid (DTPA), MgCl2, 11 [D-Ala,2 N-MePhe,4 Gly5-ol]-enkephalin (DAMGO), 14 [D-Pen2,5]-enkephalin (DPDPE), 15, naltrindole 16 (−)-trans-(1S,2S)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl) cyclohexyl] benzene acetamide hydrochloride ((−) U50,488), 17, norbinaltorphimine 18, guanosine 5′-[γ-thio] triphosphate tetralithium salt (GTPγS), guanosine 5′-diphosphate sodium salt (GDP), 2-hydroxy-ethylpiperazine-N-2-ethane sulfonic acid (HEPES), DL-dithiothreitol, tricine, ethylenediaminetetraacetic acid (EDTA), ethanol and saponin were all obtained from Sigma–Aldrich Chemical Company (St. Louis, MO) and were used as received. All of the solvents and buffers were obtained in the highest grade commercially available from VWR (San Diego, CA). [35S]-Guanosine 5′-(γ-thio) triphosphate ([35S]-GTPγS) (250 μCi; 9.25 MBq) was supplied from Perkin–Elmer (Boston, USA). [14C] Bromobenzoic acid was purchased from American Radiolabeled Chemicals, Inc., (St. Louis, MO). Sodium chloride was purchased from Fisher Scientific (Fairlawn, USA). Zeocin™ 100mg/mL and Geneticin 50mg/mLwere both purchased from Invitrogen™ (Carlsbad, CA). Wheatgerm Agglutinin SPA Beads were purchased from Amersham Biosciences (Little Chalfont, England), Protein Assay Reagent was purchased from BIORAD (Hercules, USA) and the mammalian protease inhibitor tablet was purchased from Roche Diagnostics, (Indianapolis, IN).
10.2. General procedures
Synthetic chemical reactions were run under a positive pressure of nitrogen with magnetic stirring at ambient temperature using oven-dried glassware unless otherwise indicated. Air- and moisture-sensitive liquids were transferred via syringe through rubber septa. Silica gel (230–400 mesh) was used for column chromatography. DMF was dried by filtration through a column of neutral alumina and stored over activated 4 Å molecular sieves under nitrogen prior to use. All other solvents and reagents were used as received. 1H NMR and 13C NMR were recorded at 300.0 and 75.4 MHz, respectively, on a Varian Mercury 300 instrument. Chemical shifts were reported in ppm (δ) relative to CDCl3 at 7.26 ppm and 77 ppm, respectively. NMR spectra were recorded in CDCl3 unless stated otherwise. Melting points were reported uncorrected. High resolution mass spectra were obtained with a VG7070 spectrometer with an Opus V3.1 and DEC 3000 Alpha Station data system at the University of California, Riverside or a Waters LCT Premier instrument operating in the ESI mode at the University of California, Irvine. Analytical purities were determined by straight and reversed-phase HPLC using a Hitachi D2500 Hitachi Chromato-integrator, an L-6000 Hitachi pump and an L-4200 UV–vis Hitachi detector (285 nM). When an AXXI-chrom normal phase column (5 μm i.d. × 4.6 mm o.d. × 250 mm) was used (condition A), the mobile phase was MeOH/2-propanol/HClO4 (55:45:0.01, v:v) at a flow rate of 1 mL/min. When a reversed-phase system was used (condition B) HPLC was done with an L-7100 Hitachi pump, an L-7400 UV–vis Hitachi detector (285 nM), an L-7200 Hitachi autosampler and a D-7000 Hitachi chromato-integrator employing a Supleco reversed-phase column (5 μm × 4.6 mm × 250 mm). The mobile phase was 20% 0.05 M tetrabutylammonium hydroxide and 80% methanol using isocratic elution at a flow rate of 1 mL/min. Elemental analysis was done by NuMega Resonance labs inc. San Diego, CA.
Microsomes from rat, mouse and human liver expressing functional human cytochrome P-450s were purchased from BD Gentest (Woburn, MA) or made in house and microsomes from baculovirus-infected cells co-expressing cytochrome P-450s (3A4, 2B6, 2C9, 2C19and2D6), NADPH-cytochromeP-450 reductase and cytochrome b5 (BACULOSOMES®) were purchased from PanVera LLC (Madison, WI). Human flavin-containing monooxygenase form 3 (FMO3) was prepared in house according to the method described before.36 The receptor binding studies were conducted by the National Institute of Mental Health Psychoactive Drug Screening Program (Chapel Hill, NC) following a previously described procedure.37
10.3. Naltrexone oxime (2)
Compound 1a (500 mg, 1.46 mmol, 1 equiv), NH2OH-HCl (147 mg, 2.12 mmol, 1.5 equiv) and NaOAc (294 mg, 3.58 mmol, 2.5 equiv) were dissolved in absolute ethanol (8 mL) and the mixture was heated at reflux for 2.5 h and then concentrated to dryness. Water (20 mL) was added and the mixture was made basic with K2CO3 and extracted with CHCl3. The CHCl3 extract was washed with brine, dried over Na2SO4, filtered and concentrated to give a white solid (463 mg, 89%): ESI-MS m/z 357 (MH+); 1H NMR (CDCl3) δ 6.75 (d, J = 8.2 Hz, 1H), 6.61 (d, J = 8.2 Hz, 1H), 5.0 (s, 1H), 3.15 (m, 2H), 2.65–1.30 (m, 10H), 0.86 (m, 1H), 0.56 (m, 2H), 0.20 (m, 2H).
10.4. α6–Naltrexamine (3a) and β6–naltrexamine (3b)
Compound 2 (5.83 g, 16.3 mmol, 1 equiv) was dissolved in THF (40 mL) and transferred by cannula over 10 min to a solution of BH3. THF (300 mL, 300 mmol, 1 M solution in THF, 18 equiv) held at 10 °C. A white precipitate formed and then slowly dissolved as the reaction was heated at reflux for 48 h. The solution was cooled to room temperature and water (10 mL) and 1 N KOH (200 mL) was added slowly. The solution was then reheated at reflux for 2 h. The pH was decreased to 2.5 with 10% HCl (225 mL) and the solution was heated at reflux for an additional 2 h. The THF was removed under vacuum and the aqueous solution was made basic (pH 8–9) with K2CO3. The mixture was extracted with CHCl3 (4 × 150 mL) and the extract was dried over Na2SO4, filtered and concentrated. The resulting oil was purified by chromatography on SiO2 (26 × 60 cm, elution with CH3CN/MeOH/NH4OH, 25/5/1, v/v/v) providing 3b (β-diastereomer) (2.14 g, 38%) as a white-yellow solid: Rf = 0.2; 1H NMR (CDCl3 with two drops of CD3OD) δ 6.61 (d, J = 8.1 Hz, 1H), 6.49 (d, J = 8.1 Hz, 1H), 4.17 (d, J = 7.5 Hz, 1H), 3.39–0.45 (20 H); MS m/z 343 (MH+). An additional 0.64 g (12%) of material consisting of a mixture of α- and β-diastereomers was also isolated. Repeated chromatography gave an analytically pure sample of the α-diastereomer, compound 3a: Rf = 0.16; 1H NMR δ 6.65 (d, J = 8.1 Hz, 1H), 6.46 (d, J = 8.1 Hz, 1H), 4.50 (d, J = 3.0 Hz, 1H), 3.34 (dt, J = 3.9, 12.6 Hz, 1H), 3.04 (t, J = 6.6 Hz, 1H), 2.95 (s, 1H), 2.63–0.29 (17H); ESI-MS m/z 343 (MH+).
10.5. General procedure for amidation of 6-β-naltrexamine with an acid chloride
6-β-Naltrexamine (104 mg, 0.3 mmol, 1 equiv) was dissolved in CH2Cl2 (4 mL) and NEt3 (0.13 mL, 0.93 mmol, 3.1 equiv) and a substituted benzoyl chloride (0.73 mmol, 2.4 equiv) were added. The solution was stirred for 2 h at room temperature and concentrated to dryness. The residue was filtered through a column of SiO2 (CH2Cl2/MeOH, 20/1, v/v). The resulting solid was dissolved in anhydrous methanol (3 mL) and K2CO3 (300 mg, 2.2 mmol, 7.3 equiv) was added. The mixture was stirred at room temperature for 12 h, concentrated and purified by SiO2 chromatography.
10.6. General procedure for the amidation of 6-β-naltrexamine with a carboxylic acid
6-β-Naltrexamine (100 mg, 0.29 mmol, 1 equiv), the substituted benzoic acid (0.58 mmol, 2 equiv) and BOP (258 mg, 0.58 mmol, 2 equiv) were dissolved in CH2Cl2 (3 mL). To this solution, Pr2EtN (0.15 mL, 0.88 mmol, 3 equiv) was added and the mixture was stirred at room temperature for 2 h. The solution was concentrated and filtered through a short column of SiO2 (eluted with EtOAc) providing a white material. This product was dissolved in MeOH (3 mL) and K2CO3 (300 mg, 2.2 mmol, 7.5 equiv) was then added. The mixture was stirred at room temperature for 3 h and concentrated to dryness. The residue was purified by SiO2 chromatography (CH2Cl2/MeOH, 20/1, v/v) to provide the target compound.
10.6.1. 17-Cyclopropylmethyl-3, 14β-dihydroxy-4, 5α-epoxy-6 β-[(4′-methyl) benzamido] morphinan (4)
Compound 4 was synthesized according to the general procedure described above; 6-β-naltrexamine (100 mg, 0.29 mmol, 1 equiv), p-toluoyl chloride (0.09 mL, 0.7 mmol, 2.4 equiv) and tri-ethylamine (0.13 mL, 0.91 mmol, 3.1 equiv) were combined in dichloromethane followed by basic hydrolysis with K2CO3 to give the title compound as a white solid (107 mg, 79%). Rf = 0.04 (CHCl3/MeOH, 20/1, v/v); mp = 207.6 °C; ESI/MS m/z = 461 (MH+); 1H NMR (CDCl3/CD3OD, 9/1, v/v) δ 7.68 (d, J = 8.1 Hz, 2H), 7.23 (d, J = 8.1 Hz, 2H), 6.67 (d, J = 8.1 Hz,, 2H), 6.51 (d, J = 8.1 Hz, 1H), 4.40 (d, J = 6.6 Hz, 1H), 4.15–4.05 (m, 1H), 3.09–2.96 (m, 2H), 2.60 (m, 2H), 2.34 (s, 3H), 2.12–1.40 (m, 6H) 0.50 (m, 2H), 0.09 (m, 2H); 13C NMR (CDCl3/CD3OD, 9/1, v/v) δ 168.1, 142.8, 142.1, 139.9, 131.0, 130.4, 128.9, 128.1, 127.3, 126.7, 123.7, 118.6, 93.0, 70.4, 62.3, 61.9, 59.0, 49.6, 48.7, 47.3, 22.6, 9.3, 3.9, 3.6; HRMS calcd for C28H33N2O4 461.2440, found 461.2440.
10.6.2. 17-Cyclopropylmethyl-3,14β-dihydroxy-4, 5α-epoxy-6 β-[(4′-methyl) benzamido] morphinan-oxalate (4-oxalate salt)
Compound 4 (50 mg, 0.11 mmol, 1 equiv) was converted to its oxalate salt using 1 equiv of oxalic acid in methanol (3 mL). Anal. Calcd for C30H34N2O8+3H2O: C, 59.59; H, 6.67; N, 4.63. Found: C, 60.35; H, 6.81; N, 4.59. HPLC (Condition A) tR = 5.14 min (99%).
10.6.3. 17-Cyclopropylmethyl-3,14β-dihydroxy-4, 5α-epoxy-6β-[(4′-trimethylfluoro) benzamido] morphinan (5)
Compound 5 was synthesized according to the general procedure described above; 6-β-naltrexamine (100 mg, 0.29 mmol, 1 equiv), 4-(trifluoromethyl) benzoyl chloride (0.12 mL, 0.73 mmol, 2.5 equiv) and triethylamine (0.12 mL, 0.88 mmol, 3 equiv) were combined in dichloromethane followed by basic hydrolysis with K2CO3 to give the title compound as a white solid (117 mg, 78% yield). Rf = 0.11 (CHCl3/MeOH, 20/1, v/v); mp = 157.5 °C; ESI/MS m/z = 515 (MH+); 1H NMR δ 7.92 (d, J = 8.4 Hz, 2H), 7.63 (d, J = 8.4 Hz, 2H), 6.67 (d, J = 7.8 Hz,, 2H), 6.53 (d, J = 7.8 Hz, 1H), 4.60 (d, J = 5.4 Hz, 1H), 4.16–4.13 (m, 1H), 3.15–1.44 (m, 11H), 0.54 (m, 2H), 0.13 (m, 2H); 13C NMR δ 166.2, 142.6, 139.4, 137.4, 130.5, 128.1, 127.3, 125.4, 125.1, 124.3 121.8, 119.4, 118.1, 92.6, 70.4, 62.3, 61.9, 59.2, 51.3, 50.8, 47.2, 22.6, 9.3, 3.9; HRMS calcd for C28H30F3N2O4 515.2158, found 515.2137.
10.6.4. 17-Cyclopropylmethyl-3,14β-dihydroxy-4, 5α-epoxy-6β-[(4′-trimethylfluoro) benzamido] morphinan-oxalate (5-oxalate salt)
The amide product was converted to its oxalate salt using 1 equiv of oxalic acid dihydrate in methanol. Anal. Calcd for C30H31F3N2O8+2H2O: C, 56.25; H, 5.51; N, 4.37. Found: C, 56.79; H, 5.93; N, 4.38. HPLC (condition A) tR = 4.80 min (98%).
10.6.5. 17-Cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4′-bromo) benzamido] morphinan (6)
Compund 6 was synthesized according to the general procedure described above; 6-β-naltrexamine (70 mg, 0.2 mmol, 1 equiv), p-bromobenzoic acid (62 mg, 0.31 mmol, 1.5 equiv), BOP (137 mg, 0.31 mmol, 1.5 equiv) and N,N-diisopropylethylamine (0.11 mL, 0.61 mmol, 3 equiv) were combined in dichloromethane (2 mL) followed by basic hydrolysis with K2CO3 to give the title compound as a white foam (101 mg, 94%). Rf = 0.02 (CH2Cl2/MeOH, 20/1, v/v); ESI/MS m/z = 525 (MH+); 1H NMR δ 7.71 (d, J = 8.1 Hz, 2H), 7.51 (d, J = 8.1 Hz, 2H), 6.73 (d, J = 7.8 Hz,, 2H), 6.52 (d, J = 7.8 Hz, 1H), 4.56 (d, J = 6.0 Hz, 1H), 4.16–4.13 (m, 1H), 3.12–1.46 (m, 11H), 0.52 (m, 2H), 0.12 (m, 2H); 13C NMR δ 166.1, 143.2, 139.9, 133.4, 132.5, 131.7, 130.5, 129.1, 128.2, 125.4, 124.1, 121.8, 119.4, 118.1, 92.6, 70.2, 62.4, 61.9, 59.3, 47.1, 37.6, 36.8, 36.7, 35.9, 9.3, 3.9; HRMS calcd for C27H30BrN2O4 525.1389, found 525.1382.
10.6.6. 17-Cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4′-bromo) benzamido] morphinan-oxalate (6-oxalate salt)
The amide product was converted to its oxalate salt using 1 equiv of oxalic acid dihydrate in methanol. Anal. Calcd for C27H29BrN2O4+2C2H2O4+H2O: C, 51.46; H, 4.88; N, 3.87. Found: C, 51.66; H, 5.16; N, 3.24. HPLC (condition A) tR = 5.15 min (98%).
10.6.7. 17-Cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4′-iodo) benzamido] morphinan (7)
Compound 7 was synthesized according to the general procedure described above; β-naltrexamine (50 mg, 0.20 mmol, 1 equiv), p-iodobenzoic acid (55 mg, 0.22 mmol, 1.1 equiv), BOP (97 mg, 0.22 mmol, 1.1 equiv) and N,N-diisopropylethylamine (0.08 mL, 0.44 mmol, 2.2 equiv) were combined in dichloromethane (2 mL) followed by basic hydrolysis with K2CO3 to give the title compound as a white foam (83 mg, 97%). Rf = 0.05 (CH2Cl2/MeOH, 20/1, v/v); ESI/MS m/z = 572.9 (MH+); 1H NMR δ 7.68 (d, J = 8.1 Hz, 2H), 7.55 (d, J = 8.1 Hz, 2H), 6.72 (d, J = 8.1 Hz, 2H), 6.51 (d, J = 8.1 Hz, 1H), 4.56 (d, J = 6 Hz, 1H), 4.11–4.08 (m, 1H), 3.1–1.44 (m, 11H), 0.51 (m, 2H), 0.11 (m, 2H); 13C NMR δ 166.4, 143.2, 140, 137.8, 137.1, 133.9, 130.5, 129.1, 128.2, 124, 119.4, 118.1, 92.3, 70.2, 62.4, 62.0, 59.2, 47.2, 37.6, 36.8, 36.7, 35.9, 9.3, 3.9; HRMS calcd for C27H30IN2O4 573.1250, found 573.1237.
10.6.8. 17-Cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4′-iodo) benzamido] morphinan-oxalate (7-oxalate salt)
The amide product was converted to its oxalate salt using 1 equiv of oxalic acid dihydrate in methanol. Anal. Calcd for C29H31IN2O8+2H2O: C, 49.87; H, 5.05; N, 4.01. Found: C, 49.44; H, 5.23; N, 5.74. HPLC (condition A) tR = 5.20 min (98%).
10.6.9. 17-Cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4′-t-butyl) benzamido] morphinan (8)
Compound 8 was synthesized according to the general procedure described above; β-naltrexamine (50 mg, 0.15 mmol, 1 equiv), 4-t-butylbenzoyl chloride (0.14 mL, 0.70 mmol, 4.7 equiv) and NEt3 (0.07 mL, 0.88 mmol, 5.9 equiv) were combined in dichloromethane (2 mL) followed by basic hydrolysis with K2CO3 to give the title compound as a white solid (47 mg, 64%). Rf = 0.09 (CH2Cl2/MeOH, 20/1, v/v); mp = 151.1 °C; ESI/MS m/z = 503 (MH+), 501 (MH−); 1H NMR δ 7.75 (d, J = 8.4 Hz, 2H), 7.40 (d, J = 8.4 Hz, 2H), 6.72 (d, J = 7.8 Hz, 2H), 6.55 (d, J = 7.8 Hz, 1H), 4.53 (d, J = 5.7 Hz, 1H), 4.21–4.18 (m, 1H), 3.14–1.44 (m, 11H), 0.54 (m, 2H), 0.13 (m, 2H); 13C NMR δ 167.2, 143.1, 139.5, 137.4, 131.4, 130.6, 127.3, 126.6, 125.5, 124.5, 119.4, 118.0, 93.2, 70.1, 62.4, 62.0, 59.3, 49.6, 47.2, 34.2, 31.3, 31.0, 9.3, 3.9; HRMS calcd for C31H39N2O4 503.2910, found 503.2893.
10.6.10. 17-Cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4′-t-butyl) benzamido] morphinan-oxalate (8-oxalate salt)
The amide product was converted to its oxalate salt using 1 equiv of oxalic acid dihydrate in methanol. Anal. Calcd for C33H40N2O8+2H2O: C, 63.04; H, 7.05; N, 4.46. Found: C, 62.58; H, 7.23; N, 4.49. HPLC (condition A) tR = 5.32 min (98%).
10.6.11. Radiosynthesis of [14C]-17-Cyclopropylmethyl-3,14βdihydroxy-4,5α-epoxy-6β-[(4′-bromo) benzamido] morphinan ([14C] (6a)
In a dry screw cap culture tube was placed 6-β-naltrexamine (10 mg, 29.9 μmol, 3 equiv), 4-bromobenzoic acid (2 mg, 9.9 μmol, 1 equiv), 100 μCi of carboxyl-labeled [14C]-bromobenzoic acid and BOP (4.4 mg, 9.95 μmol, 1 equiv). The mixture was dissolved in dichloromethane (0.2 mL). The mixture was placed under an atmosphere of argon and i-Pr2EtN (5.1 μL, 29.9 μmol, 3 equiv) was added and stirred at room temperature for 4 h. The mixture was evaporated to dryness and purified by PTLC (CH2Cl2/MeOH, 8/1, v/v) Rf = 0.54, to give 4.9 mg, 91% product yield and 70% radioactivity yield. The product gave an ESI/MS m/z = 527 (MH+) and 529 (MH++14C). Conversion of a portion of the product to an oxalate salt gave radiolabeled 6a oxalate salt.
10.6.12. 17-Cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4′-bromo) benzamido] morphinan N-oxide
A solution of compound 6 (10 mg, 29.9 μmol, 1 equiv) in dichloromethane (0.3 mL) was combined with meta-chloroperbenzoic acid (4 mg, 23 μmol, 0.8 equiv) and allowed to stir at room temperature for 5 h. The mixture was evaporated to dryness and purified with PTLC (EtOAc/MeOH, 20/1, v/v) to give 6.1 mg of the desired N-oxide (60% yield). Rf = 0.06; ESI/MS m/z = 541 (MH+).
10.7. General procedures for cell culture
HEK293 cells stably transfected with FLAG-tagged mouse μ-and κ-opioid and human nociceptin receptors or hemaglutinin-tagged mouse δ-opioid receptors were confirmed with Fluorescence Activated Cell Sorter (FACS) analysis and confocal microscopic visualization of cells on coverslips stained with fluorescent antibodies (SF: M1 & Alexa IgG2b; HA: HA11 & Alexa IgG1). Cells were cultured under 7% CO2 in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum in the presence of 0.4 mg/mL Zeocin (for μ-and δ-receptor cells), 0.5 mg/mL of Geneticin (for κ-receptor cells), or 0.2 mg/mL hygromycin (for NOP-receptor cells) to select for the presence of the transfected plasmid (pcDNA3.1Zeo and pcDNA3.1) that codes for both the opioid receptor and antibiotic resistance.
10.8. General procedure for membrane preparation
HEK293 cells expressing the μ-, δ-, κ- and nociceptin (NOP) receptors were grown in 10 cm dishes. When the cells were nearly 100% confluent, cells were washed twice with ice-cold phosphate buffered saline and scraped from the dishes with a HME lysis buffer (pH 7.5; 100 mM HEPES, 8 mM MgCl2, 4 mM EDTA, 10 mg/mL saponin and one mammalian protease inhibitor tablet). The material was pelleted (14,000 rpm, 15 min, 4 °C) and resuspended in HME buffer. Following a rapid freeze (N2)/thaw cycle, the material was sonicated at 4 °C, repelleted and resuspended in HME buffer and stored at −80 °C until used. Protein concentrations of membrane samples were determined by visible spectophotometry (595 nm) using the BIORAD protein assay reagent and found to be 5.8 μg/μL (μ), 7.3 μg/μL (δ), 8.6 μg/μL (κ) and 3.5 μg/μL (NOP).
10.9. General procedure for [35S] GTPγS binding assay
Triplicate assays were done in 96-well plates on ice with each reaction containing [35S] GTPγS (50 pM), cell membrane (10 μg protein), GDP (5 μM), and SPA beads (0.5 mg) with assay buffer (pH 7.5; 50 mM HEPES, 100 mM NaCl, 5 mM MgCl2, 10 mg/mL saponin) and the opioid ligands as before.18 Non-specific binding was determined in the presence of GTPγS (10 μM). Single drug dose–response curves (0.1 nM–10 μM) of [35S] GTPγS stimulated binding were done at each opioid receptor with each compound and compared to the standard opioid agonist compounds 11, 14, 16 and 15 for the μ-, δ-, κ- and nociceptin receptors, respectively. Inhibition of opioid agonist-stimulated [35S]-GTPγS binding of selective opioid agonists 11 (1 μM), 14 (200 nM), (−) 16 (2 μM) and 15 (1 μM) for the μ-, δ-, κ- and nociceptin receptors, respectively, were done in the presence of varying concentrations (10 pM–10 μM) of each compound. Membranes and GDP were incubated with the antagonists for 30 min, before the opioid agonists, [35S] GTPγS and SPA beads were added. Assay plates were shaken for 45 min at 25 °C, and then centrifuged (1500 rpm, 5 min, 25 °C) before [35S] GTPγS-stimulated binding was assessed using the NXT TOPCOUNTER.
10.10. Rat and mouse liver microsome and human liver S-9 stability assays
A typical assay mixture contained rat or mouse liver microsomes or human liver S-9 (0.4–0.5 mg of protein), 100 μM potassium phosphate buffer (pH 7.4), 40 μM test compound, an NADPH-generating system consisting of 0.5 mM NADP+, 0.5 mM glucose-6-phosphate, 5 IU/mL glucose-6-phosphate dehydrogenase, 1 mg/mL DTPA and 7 mM MgCl2 for a final incubation volume of 0.1 mL.23 Incubations were run for 0, 10, 25, 40 and 60 min in air with shaking at 37 °C in a water bath and were terminated by the addition of 1 mL CH2Cl2/2-propanol (3:1, v:v). After centrifugation at 13,000 rpm for 5 min, the organic fraction was collected and the solvent was removed with a stream of argon. The residue was reconstituted in methanol (200 μL), centrifuged at 13,000 rpm for 5 min and the supernatant was analyzed by high-performance liquid chromatography with an Axxi-chrom normal-phase column (4.6 mm × 250 mm, 5 μm) or with a Supelco reversed-phase HS F5 pentafluorophenyl column (4.6 mm × 250 mm, 5 μm) as described above. Standard conditions utilized an isocratic, ternary-solvent system consisting of solvents A (methanol), B (isopropanol) and C (aqueous 70% HClO4) set at a flow rate of 1.5 mL/min (straight-phase), or A, D (water) and E (HCO2H) set at a flow rate of 1.0 mL/min (reversed-phase), λ = 254 nm with retention times (tR) evaluated in min.
10.11. CYP inhibition assays
To measure CYP3A4 activity, testosterone 6-hydroxylation, was determined as previously described.23 To measure CYP2C9, diclofenac hydroxylase activity was measured.38 For determination of CYP2B6, CYP2C19 and CYP2D6 activity, isozyme specific Vivid Blue substrate O-dealkylation was determined via a modified Panvera Vivid Assay Protocol as previously described.23
10.12. Metabolism studies of compound 6
As a representative example, metabolic incubations were done with 6 in the presence of human or rat liver microsomes or highly purified human FMO3. The incubation mixture contained the NADPH-generating system as described above, 1 mg/mL DTPA and 7 mM MgCl2, 0.4 mg of microsomes or 10 μg of human FMO3 in a total volume of 0.25 mL combined and mixed at 4 °C. The incubation was initiated by the addition of 6 (30 μM) and placed in a 37 °C shaking incubator. At the appropriate time, the incubation was stopped by the addition of 2 volumes of ice cold acetonitrile (for the TLC assay) and an aliquot was directly placed on an LK5DF preabsorbent TLC plate (Whatman, Maidstone, UK) using an eluant of EtAOc/MeOH/NH4OH, 20/5/0.2, v/v) that separated compound 6, 6-N-oxide and bromobenzoic acid with Rf values of 0.58, 0.28 and 0.11, respectively. For analysis, 50 μg of 6, 6-N-oxide and bromobenzoic acid was used as TLC standards and the UV–vis bands corresponding to these regions were scraped and placed in scintillation vials for counting and quantification. For the HPLC assay, the incubation was stopped by the addition of isopropanol/CH2Cl2 (3/1, v/v), mixed thoroughly and the organic layer was separated by centrifugation. The organic extracts were evaporated to dryness, taken up in MeOH and the products were separated by HPLC (i.e., Supleco column (4.6 mm × 25 cm, 5 μm) with a mobile phase of CH3CN/potassium phosphate buffer, 1/1, v/v, pH 3) that separated 3b, 6-N-oxide and 6, with retention volumes of 4.1, 8.4 and 9.2 mL, respectively) at 235 nm. The analytes were quantified on the basis of HPLC peak height.
10.13. In vivo distribution studies with compound 6
Animal studies in male Wistar rats (275–310 g) with jugular vein and femoral artery catheters were administered radiolabeled 6 oxalate salt (100 μg/kg iv and 400 μg/kg, oral). For plasma analysis, blood was obtained from the catheters at various time points up to 8 h and centrifuged at 4 °C. An aliquot of plasma was counted by scintillation counting. Brain distribution of radiolabeled 6 oxalate salt was also investigated in male Wistar rats administered 400 μg/kg by the oral route of administration. After 90 min post dosing, animals were anesthetized by ip ketamine/xylazine and blood samples were obtained by cardiac puncture. Brain tissues were immediately removed, weighed, homogenized with a mortar and pestle in borate buffer (pH 8.5)/acetonitrile, 1/1, v/v), centrifuged and an aliquot was measured by scintillation counting.
10.14. General procedure for oral ethanol and saccharin operant self-administration training
Ethanol or saccharin (SACC) self-administration training was conducted in standard operant cages (Coulbourn Instruments, PA) located in sound-attenuated, ventilated cubicles. Two 35-ml syringes dispensed either ethanol/SACC or water through plastic tubing into two stainless steel drinking cups mounted 4 cm above the grid floor and centered on the front panel of each chamber. Each drinking cup held 2 reinforcer deliveries (0.1 ml fluid/reinforcer). Two retractable levers were located 4.5 cm to either side of the drinking cups. Fluid delivery and recording of operant responses were controlled by a microcomputer.
Briefly, animals were trained to voluntarily self-administer 10% (w/v) ethanol (n = 10) or saccharin (n = 6) by the oral route using the saccharin fadeout method39 and were tested for their response for ethanol or saccharin solution in a two-lever free choice situation. Once baseline ethanol and saccharin intake were achieved (i.e., when responding across three consecutive days varied less than 20% and response rates correspond to pharmacologically relevant blood alcohol levels (BALs)), dose response testing for each compound commenced. To allow for a complete dissipation of any carry-over effects, a one week washout period, where rats were re-baselined during daily 30 min operant sessions, occurred between testing of each compound.
10.15. Data analyses
Non-specific [35S] GTPγS-stimulated binding was determined in the presence of unlabeled GTPγS (10 μM) with ligand-induced [35S] GTPγS-stimulated binding expressed as a percentage of the basal stimulation:% basal stimulation = (ligand count − no treatment)/basal stimulation × 100%, where basal stimulation = no treatment − non-specific binding. Calculated EC50 values represent the concentration of the compound required to produce 50% maximal stimulation of [35S] GTPγS binding. The apparent functional Ki values were determined using the Cheng and Prusoff equation (Ki = IC50/(1 + [L]/ED50),40 where IC50 is the concentration that produces 50% inhibition of opioid selective agonist-stimulated [35S] GTPγS binding, [L] is the concentration of agonist and ED50 is the concentration of agonist that produces 50% maximal stimulation of [35S] GTPγS binding as determined from agonist-response curves. All statistics, regression analyses and determination of EC50, IC50 and Ki values were done using GraphPad Prism® (version 4.02, GraphPad, San Diego, CA). Data from in vitro binding or functional assays were analyzed by non-linear regression using a sigmoidal curve with variable slope.
10.16. Ethanol self-administration analysis
Data were collected on-line simultaneously from multiple operant chambers. Results of the operant procedure are reported as mean cumulative number of bar presses for ethanol or saccharin. In general, tests for homogeneity of variance were first performed on the data. If the scores did not violate the assumption of homogeneity of variance, appropriate analyses of variance (ANOVA) were done. Data were analyzed using the StatView statistical package on a PC-compatible computer. Mixed-design ANOVAs were used with drug treatments as a within-subjects factor (i.e., repeated measures design for drug treatment). A priori analysis examining individual drug doses to vehicle control dose was conducted using paired t-tests. Significant drug effects were defined as having p <0.05 compared to vehicle-treated rats.
Acknowledgments
The authors thank Dr. James MacDougall, Mr. Luke Guo and Mr. John Buza for their help with some of the synthetic and analytical work described herein (supported in part by a grant from the David Copley Foundation). The authors thank Ms. Meike Motika for help with some of the animal work. Receptor binding data was generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # NO1MH32004 (NIMH PDSP). This work was financially supported by a grant from the National Institutes of Health (Grant AA016029 to MA).
Abbreviations
- BOP
benzotriazol-1-yl-oxy-tris-(dimethylamino) phosphonium hexafluorophosphate
- ESI
electrospray ionization
- DTPA
diethylenetriaminepenta-acetic acid
- GTP
guanosine 5′-triphosphate
- TLC
thin layer chromatography
- HPLC
high-performance liquid chromatography
References and notes
- 1.Bouza C, Angeles M, Munoz A, Amate JM. Addiction. 2004;99:811. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 2.Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Am J Psychiatry. 1999;156:1758. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- 3.Croop RS, Faulkner EB, Labriola DF. Arch Gen Psychiatry. 1997;54:1130. doi: 10.1001/archpsyc.1997.01830240090013. [DOI] [PubMed] [Google Scholar]
- 4.Mason BJ, Salvato FR, Williams LD, Ritvo EC, Cutler RB. Arch Gen Psychiatry. 1999;56:719. doi: 10.1001/archpsyc.56.8.719. [DOI] [PubMed] [Google Scholar]
- 5.Tabakoff B, Hoffman PL. Life Sci. 1983;32:197. doi: 10.1016/0024-3205(83)90031-0. [DOI] [PubMed] [Google Scholar]
- 6.Oslin DW, Berrettini WH, O’Brien CP. Addict Biol. 2006;11:397. doi: 10.1111/j.1369-1600.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 7.Roozen HG, de Waart R, van der Windt DA, van den Brink W, de Jong CA, Kerkhof AJ. Eur Neuropsychopharmacol. 2006;16:311. doi: 10.1016/j.euroneuro.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin D, Grant ER, Pohorecky LA. Brain Res. 1993;621:137. doi: 10.1016/0006-8993(93)90309-b. [DOI] [PubMed] [Google Scholar]
- 9.Stromberg MF, Mackler SA, Volpicelli JR, O’Brien CP. Alcohol. 2001;23:109. doi: 10.1016/s0741-8329(00)00137-3. [DOI] [PubMed] [Google Scholar]
- 10.Pastor R, Aragon CM. Neuropsychopharmacology. 2006;31:1489. doi: 10.1038/sj.npp.1300928. [DOI] [PubMed] [Google Scholar]
- 11.Reid LD. Am J Clin Nutr. 1985;42:1099. doi: 10.1093/ajcn/42.5.1099. [DOI] [PubMed] [Google Scholar]
- 12.Yeomans MR, Gray RW. Neurosci Biobehav Rev. 2002;26:713. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 13.Grisel JE, Grahame NJ, Mogil JS, Belknap JK, Low MJ. Soc Neurosci Abstr. 1995;21:1699. [Google Scholar]
- 14.Gianoulakis C, Krishnan B, Thavundayil J. Arch Gen Psychiatry. 1996;53:250. doi: 10.1001/archpsyc.1996.01830030072011. [DOI] [PubMed] [Google Scholar]
- 15.Herz A. Psychopharmacology (Berl) 1997;129:99. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- 16.Ulm RR, Volpicelli JR, Volpicelli LA. J Clin Psychiatry. 1995;56:5. [PubMed] [Google Scholar]
- 17.Li G, Aschenbach LC, Chen J, Cassidy MP, Stevens DL, Gabra BH, Selley DE, Dewey WL, Westkaemper RB, Zhang Y. J Med Chem. 2009;52:1416. doi: 10.1021/jm801272c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghirmai S, Azar MR, Polgar WE, Berzetei-Gurske I, Cashman JR. J Med Chem. 2008;51:1913. doi: 10.1021/jm701060e. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed MS, Portoghese PS. J Org Chem. 1986;51:105. [Google Scholar]
- 20.Traynor JR, Nahorski SR. Mol Pharmacol. 1995;47:848. doi: 10.1016/S0026-895X(25)08634-1. [DOI] [PubMed] [Google Scholar]
- 21.Tullman RH, Hanzlik RP. Drug Metab Rev. 1984;15:1163. doi: 10.3109/03602538409033560. [DOI] [PubMed] [Google Scholar]
- 22.Bondon A, Macdonald TL, Harris TM, Guengerich FP. J Biol Chem. 1989;264:1988. [PubMed] [Google Scholar]
- 23.Denton TT, Zhang X, Cashman JR. Biochem Pharmacol. 2004;67:751. doi: 10.1016/j.bcp.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 24.MacDougall JM, Zhang XD, Polgar WE, Khroyan TV, Toll L, Cashman JR. J Med Chem. 2004;47:5809. doi: 10.1021/jm049554t. [DOI] [PubMed] [Google Scholar]
- 25.Heyser CJ, Schulteis G, Koob GF. Alcohol Clin Exp Res. 1997;21:784. [PubMed] [Google Scholar]
- 26.Heyser CJ, Roberts AJ, Schulteis G, Koob GF. Alcohol Clin Exp Res. 1999;23:1468. [PubMed] [Google Scholar]
- 27.Heyser CJ, Moc K, Koob GF. Neuropsychopharmacology. 2003;28:1463. doi: 10.1038/sj.npp.1300175. [DOI] [PubMed] [Google Scholar]
- 28.June HL, Grey C, Warren-Reese C, Durr LF, Ricks-Cord A, Johnson A, McCane S, Williams LS, Mason D, Cummings R, Lawrence A. Alcohol Clin Exp Res. 1998;22:2174. [PubMed] [Google Scholar]
- 29.Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH. J Pharmacol Exp Ther. 2000;293:1002. [PubMed] [Google Scholar]
- 30.Holter SM, Spanagel R. Psychopharmacology (Berl) 1999;145:360. doi: 10.1007/s002130051069. [DOI] [PubMed] [Google Scholar]
- 31.Walker BM, Koob GF. Neuropsychopharmacology. 2008;33:643. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowry TH, Richardson KS. Mechanism and Theory in Organic Chemistry. 3. Harper and Row; New York: 1987. [Google Scholar]
- 33.Cashman JR, MacDougall JM. Curr Top Med Chem. 2005;5:585. doi: 10.2174/1568026054367647. [DOI] [PubMed] [Google Scholar]
- 34.Stevens WC, Jr, Jones RM, Subramanian G, Metzger TG, Ferguson DM, Portoghese PS. J Med Chem. 2000;43:2759. doi: 10.1021/jm0000665. [DOI] [PubMed] [Google Scholar]
- 35.Doss GA, Baillie TA. Drug Metab Rev. 2006;38:641. doi: 10.1080/03602530600959466. [DOI] [PubMed] [Google Scholar]
- 36.Brunelle A, Bi YA, Lin J, Russell B, Luy L, Berkman C, Cashman J. Drug Metab Dispos. 1997;25:1001. [PubMed] [Google Scholar]
- 37.Roth BL, Laskowski MB, Coscia CJ. J Biol Chem. 1981;256:10017. [PubMed] [Google Scholar]
- 38.Yano JK, Denton TT, Cerny MA, Zhang X, Johnson EF, Cashman JR. J Med Chem. 2006;49:6987. doi: 10.1021/jm060519r. [DOI] [PubMed] [Google Scholar]
- 39.Rassnick S, Pulvirenti L, Koob GF. Alcohol. 1993;10:127. doi: 10.1016/0741-8329(93)90091-2. [DOI] [PubMed] [Google Scholar]
- 40.Cheng Y, Prusoff WH. Biochem Pharmacol. 1973;22:3099. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]