Abstract
In larval lamprey, with increasing recovery times after a transection of the rostral spinal cord, there is a gradual recovery of locomotor behavior, and descending brain neurons regenerate their axons for progressively greater distances below the transection site. In the present study, spinal cord “conditioning lesions” (i.e., transections) were performed in the spinal cord at 30% body length (BL; normalized distance from the head) or 50% BL. After various “lesion delay times” (D), a more proximal spinal cord “test lesion” (i.e., transection) was performed at 10% BL, and then, after various recovery times (R), horseradish peroxidase was applied to the spinal cord at 20% BL to determine the extent of axonal regeneration of descending brain neurons. Conditioning lesions at 30% BL, lesion delay times of 2 weeks, and recovery times of 4 weeks (D-R = 2–4 group) resulted in a significant enhancement of axonal regeneration for the total numbers of descending brain neurons as well as neurons in certain brain cell groups compared to control animals without conditioning lesions. Experiments with hemiconditioning lesions, which reduce interanimal variability, confirmed that conditioning lesions do significantly enhance axonal regeneration and indicate that axotomy rather than diffusible factors released at the injury site is primarily involved in this enhancement. Results from the present study suggest that conditioning lesions “prime” descending brain neurons via cell body responses and enhance subsequent axonal regeneration, probably by reducing the initial delay and/or increasing the initial rate of axonal outgrowth.
Indexing terms: axotomy, reticulospinal neurons, spinal cord injury, conditioning lesion, test lesion
When neurons are axotomized, they often are “primed” for possible subsequent axonal regeneration. If the axons of these neurons then receive a second “test lesion,” often proximal to the first “conditioning lesion” (CL), regeneration can be enhanced compared with that of neurons without previous CLs. This phenomenon of enhanced axonal regeneration is called the “CL effect” (Forman et al., 1980). Because most studies of CL effects have been performed on nerves (e.g., sciatic nerve or optic nerve), crush injuries usually are employed for both the conditioning and the test lesions.
After an initial CL, there are a series of morphological, physiological, and biochemical changes in axotomized neurons (McQuarrie and Grafstein, 1982; Redshaw and Bisby, 1987; Perry et al., 1987; Jacob and McQuarrie, 1991; Tetzlaff et al., 1996). Investigating the effects of CLs potentially might clarify the mechanisms that regulate neural regeneration, and it might then be possible to manipulate these mechanisms to enhance axonal regeneration. For example, a CL of the peripheral branch of neurons in dorsal root ganglia (DRG) raises cAMP levels and promotes axonal regeneration of the central branches of these neurons, and this effect can be mimicked by injection of cAMP in DRG (Qiu et al., 2002).
The effects of CLs have been investigated in a number of different animal species. In rats with CLs of the sciatic nerve, after a subsequent more proximal nerve lesion, axonal regeneration of spinal motoneurons is enhanced compared with controls, including a reduction in the initial delay of regeneration, an increase in the rate of axonal outgrowth, and a shortening of recovery time of motor function (McQuarrie, 1978; Forman et al., 1980; McQuarrie and Grafstein, 1981; Navarro and Kennedy, 1990; Sjöberg and Kanje, 1990; Jacob and McQuarrie, 1991, 1993). Similar enhancement of axonal regeneration occurs in frog sciatic nerve following CLs (Carlsen, 1983; Perry et al., 1987; Edbladh and Edstrom, 1989). CL effects have also been found to enhance axonal regeneration of motoneurons in the facial nerves of rats (Tetzlaff et al., 1996). In goldfish with a CL of the optic nerve, after a subsequent more proximal nerve lesion, the initial delay of axonal regeneration of retinal ganglion cells is reduced, and the rate of axonal outgrowth is increased (Lanners and Grafstein, 1980; McQuarrie and Grafstein, 1981, 1982; Reich et al., 1990). Finally, in “old” rats, CLs induce axons of motoneurons to regenerate at growth rates typical of those in “young” animals (Jacob and Croes, 1998).
In general, there are at least two possible mechanisms for CL effects. The first is cell body responses. After a CL, morphological and metabolic changes in the cell bodies of axotomized neurons “prime” or switch the neurons to a “regenerative mode.” When a test lesion is then performed, either at or proximal to the first lesion, the initial delay of axonal regeneration is decreased, and the rate of axonal outgrowth is increased, compared to neurons without CLs (Carlsen, 1983; Oblinger and Lasek, 1984; McQuarrie, 1985; Jacob and McQuarrie, 1993). The second is environmental factors. Degenerative conditions in the vicinity of a CL potentially could make it easier for growth cones to physically elongate in this region, and neurotrophic factors released by glia and other cells at the lesion site might enhance axonal regeneration (Bisby and Keen, 1985; Navarro and Kennedy, 1990; Sjöberg and Kanje, 1990).
In larval lamprey, with increasing recovery times following a transection of the rostral spinal cord, there is a gradual recovery of locomotor behavior (Davis et al., 1993), and descending brain neurons regenerate their axons for progressively greater distances below the transection site (Davis and McClellan, 1994a,b). Double-labeling experiments indicate that axonal regeneration contributes significantly to restoration of descending brain-spinal cord projections following spinal cord injury (Zhang and Mc-Clellan, 1999). Although a few new descending brain-spinal cord projections are added, albeit very slowly, during larval life, they probably do not contribute substantially to restoration of these projections following spinal cord transection (Zhang et al., 2002).
At present, it is not known whether CLs enhance the restoration of descending brain-spinal projections in spinal-cord-transected lamprey or what mechanisms might be involved. Therefore, in the present study, CLs (i.e., spinal cord transections) were performed at one of two levels of the spinal cord in larval lamprey. The “lesion delay time,” which is the interval between a CL and a more proximal test lesion (i.e., spinal cord transection), and “recovery time,” which is the interval between spinal cord transection and retrograde labeling of descending brain neurons, were varied to determine the maximal effects on axonal regeneration. It was found that, with relatively rostral CLs and relatively short lesion delay times and recovery times, axonal regeneration of descending brain neurons was significantly enhanced relative to that in control animals without CLs. To our knowledge, this is the first demonstration of CL effects that involve spinal cord injury, and this provides an experimental system for studying some of the mechanisms that might regulate, and potentially be used to enhance, axonal regeneration. This work has been presented previously in abstract form (McClellan and Zhang, 2001).
MATERIALS AND METHODS
Animal care
Larval sea lamprey (Petromyzon marinus, 70–117 mm) were maintained in 2.5–10.0-gallon aquaria at 23–25°C and typically were fed weekly (Hanson et al., 1974). The procedures in this study have been approved by the Animal Use and Care Committee at the University of Missouri (protocol reference No. 1506-1).
CLs and spinal cord transections
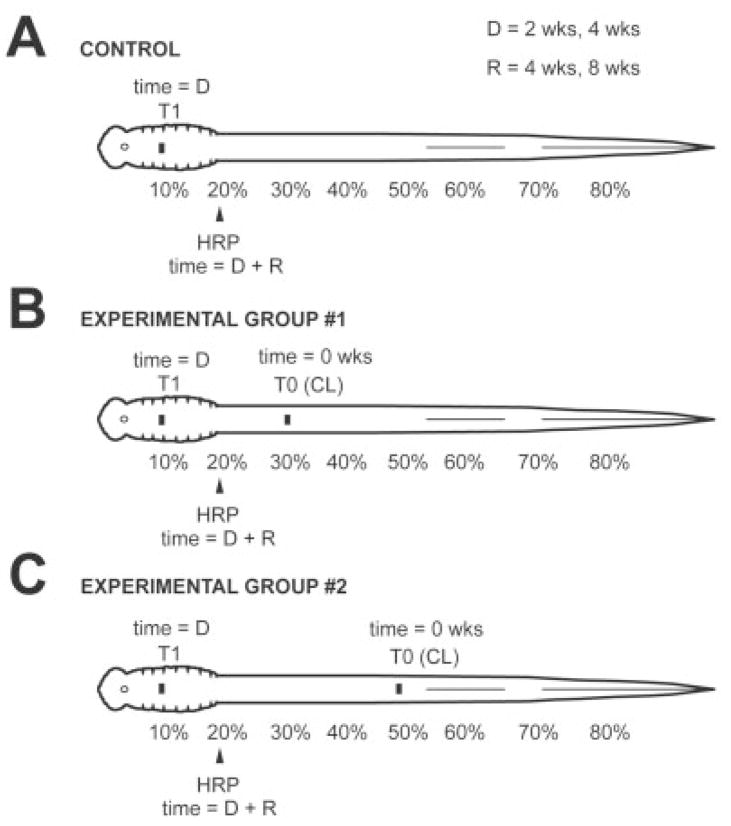
Prior to CLs or test lesions, lamprey were anesthetized in tricaine methanesulphonate (~200 mg/liter; Sigma, St. Louis, MO). For a CL, a 5–10-mm dorsal incision was made at 30% body length (BL; relative distance measured from the head) or 50% BL, and the spinal cord and overlying meninges were exposed and completely transected with iridectomy scissors, as previously described (Davis and McClellan, 1994a,b; see Fig. 1). After various lesion delay times (“D”; see below), the spinal cord was exposed at 10% BL and transected (test lesion). Animals were returned to their tanks for various recovery times (“R”; see below), after which time horseradish peroxidase (HRP) was applied to the spinal cord at 20% BL (see below) to retrogradely label descending brain neurons that regenerated their axons below the transection site at 10% BL. It should be noted that, after CLs at 30% or 50% BL, it is very unlikely that substantial numbers of axons from descending brain neurons retracted relatively long distances and were not severed by the transection at 10% BL (see below).
Fig. 1.
Diagrams showing experimental paradigm. A: Control group: The spinal cord was transected at 10% BL (T1), and, after recovery times of 4 or 8 weeks (R), HRP was applied to the spinal cord at 20% BL to label retrogradely descending brain neurons that regenerated their axons below the transection site. B: Experimental group 1: Animals were divided into different D-R groups, where D (2 or 4 weeks) is the lesion delay time between a conditioning lesion (T0) at 30% BL at time zero and spinal cord transection (T1) at 10% BL, and R (4 or 8 weeks) is the recovery time between spinal cord transection and application of HRP to the spinal cord at 20% BL. C: Experimental group 2: Similar to group 1, except conditioning lesions (T0) were performed at 50% BL.
Tracer application
Animals were anesthetized, and the spinal cord was exposed at 20% BL and completely transected. A Gelfoam pledget (Upjohn, Kalamazoo, MI; ~1 mm3) soaked in a 40% HRP (Sigma type VI) and 1% dimethyl sulfoxide (DMSO) solution was inserted between the transected ends of the spinal cord, and the incision was closed and sealed with cyanoacrylate (Super Glue Gel; Loctite Co., Rocky Hill, CT). The animals were returned to their tanks for 14 days to allow for retrograde transport of the tracer and labeling of descending brain neurons.
Experimental paradigm
Animals were divided into one control and two experimental groups (Fig. 1). Because in larval lamprey the numbers of descending projections are correlated with age and body length (Zhang et al., 2002), animals in the different groups were selected with similar mean body lengths (see Tables 1–3). In control animals (Fig. 1A), the spinal cord was transected at 10% BL (T1), and, after recovery times of 4 or 8 weeks (R), HRP was applied to the spinal cord at 20% BL to retrogradely label descending brain neurons that regenerated their axons through and beyond the transection site (n = 47). Experimental animals (Fig. 1B,C) were divided into several “D-R” groups, where D was the lesion delay time, and R was the recovery time. In experimental group 1 (Fig. 1B), a CL (see above) was made at 30% BL (T0), and, after a 2- or 4-week lesion delay time (D), the spinal cord was transected at 10% BL (T1). After a 4- or 8-week recovery time (R), HRP was applied to the spinal cord at 20% BL to determine the numbers of descending brain neurons that regenerated their axons below the transection site at 10% BL (n = 45). For experimental group 2 (Fig. 1C), conditions were similar to those described above, except that CLs (T0) were made in the spinal cord at 50% BL (n = 35).
TABLE 1.
Numbers of Descending Brain Neurons That Project to 20% BL in the Spinal Cord
| Cell group | 4-Week recovery1 | 8-Week recovery1 | Normal2 |
|---|---|---|---|
| N | 29 | 18 | 11 |
| Body length (mm) | 93.7 ± 9.5 | 84.9 ± 7.1 | 90.9 ± 6.1 |
| Di | 0.2 ± 0.53 | 6.9 ± 4.5 | 20.4 ± 9.2 |
| M cells (M1–M3) | 0.1 ± 0.4 | 2.6 ± 1.7 | 6.0 ± 0.0 |
| MRN | 1.1 ± 2.0 | 32.4 ± 9.5 | 55.3 ± 16.9 |
| I cells (I1–I4) | 0.5 ± 1.4 | 3.1 ± 1.9 | 8.0 ± 0.0 |
| aARRN | 3.1 ± 4.3 | 35.4 ± 9.8 | 61.0 ± 13.5 |
| IARRN | 2.2 ± 4.1 | 40.3 ± 10.7 | 69.0 ± 10.7 |
| mARRN | 2.2 ± 3.9 | 26.7 ± 5.1 | 38.0 ± 8.7 |
| B cells (B1–B5) | 0.1 ± 0.3 | 3.6 ± 1.9 | 9.6 ± 0.6 |
| Mau and AM cells | 0.1 ± 0.3 | 1.5 ± 1.1 | 4.0 ± 0.0 |
| aMRRN | 2.8 ± 4.5 | 33.0 ± 8.0 | 54.1 ± 11.9 |
| pMRRN | 7.6 ± 9.4 | 52.1 ± 11.7 | 72.1 ± 8.6 |
| PRRN | 25.7 ± 30.5 | 259.6 ± 49.5 | 539.5 ± 85.9 |
| ALV | 0.8 ± 1.5 | 10.9 ± 4.0 | 20.3 ± 2.7 |
| DLV | 0.5 ± 0.9 | 12.1 ± 8.1 | 57.7 ± 14.9 |
| PLV | 6.7 ± 9.2 | 58.4 ± 25.5 | 116.8 ± 28.0 |
| PON | 0.0 ± 0.0 | 0.8 ± 0.9 | 13.0 ± 3.0 |
Control animal groups (spinal cord transection at 10% BL, and following 4-week or 8-week recovery times, application of HRP to the spinal cord at 20% BL; see Fig. 1A).
For comparison purposes, the numbers of descending brain neurons in normal animals that are retrogradely labeled by application of HRP to the spinal cord at 20% BL; total numbers of labeled neurons = 1,141 ± 140 (data from Zhang et al., 2002).
Mean ± SD for numbers of labeled descending brain neurons on both sides of brain that projected to the HRP application site in the spinal cord at 20% BL.
TABLE 3.
Restoration of Descending Projections for 8-Week Recovery Times Following Conditioning Lesions
| CL at 30% BL |
CL at 50% BL |
|||
|---|---|---|---|---|
| 2–8 Group1 | 4–8 Group | 2–8 Group | 4–8 Group | |
| N | 8 | 8 | 7 | 8 |
| Body length (mm) | 88.5 ± 7.6 | 87.8 ± 5.4 | 89.4 ± 13.9 | 88.8 ± 8.1 |
| Di | 9.6 ± 4.92 | 7.6 ± 4.1 | 7.1 ± 3.4 | 5.5 ± 4.6 |
| M cells (M1–M3) | 2.4 ± 1.4 | 3.9 ± 1.4 | 3.3 ± 1.4 | 3.4 ± 1.4 |
| MRN | 25.6 ± 4.2 | 28.8 ± 8.5 | 30.9 ± 4.8 | 34.3 ± 8.3 |
| I cells (I1–I4) | 2.9 ± 0.8 | 4.3 ± 2.0 | 2.7 ± 1.1 | 4.4 ± 2.2 |
| aARRN | 38.4 ± 8.1 | 34.5 ± 5.2 | 31.9 ± 4.7 | 30.0 ± 8.5 |
| IARRN | 50.3 ± 13.9 | 48.9 ± 6.6 | 43.3 ± 6.1 | 39.5 ± 13.9 |
| mARRN | 23.0 ± 8.0 | 21.8 ± 2.1 | 20.0 ± 5.7 | 25.0 ± 5.2 |
| B cells (B1–B5) | 4.3 ± 1.3 | 4.0 ± 1.7 | 2.3 ± 1.0 | 4.9 ± 2.3 |
| Mau and AM cells | 1.9 ± 1.0 | 1.6 ± 0.5 | 1.1 ± 0.7 | 1.9 ± 0.8 |
| aMRRN | 32.3 ± 6.3 | 27.1 ± 6.3 | 31.3 ± 7.0 | 27.9 ± 7.1 |
| pMRRN | 53.6 ± 11.4 | 47.3 ± 7.9 | 44.1 ± 10.5 | 52.4 ± 7.1 |
| PRRN | 293.6 ± 64.4 | 244.1 ± 31.3 | 249.4 ± 43.9 | 237.1 ± 48.7 |
| ALV | 11.4 ± 4.3 | 12.3 ± 5.9 | 11.7 ± 2.4 | 11.9 ± 4.4 |
| DLV | 12.8 ± 8.9 | 9.4 ± 5.7 | 7.1 ± 3.7 | 11.3 ± 4.4 |
| PLV | 73.5 ± 34.4 | 58.0 ± 10.6 | 57.7 ± 31.8 | 65.6 ± 24.0 |
| PON | 1.8 ± 2.0 | 1.8 ± 2.4 | 0.4 ± 1.1 | 1.6 ± 1.5 |
Animal groups (“D-R” groups; see explanation in Materials and Methods, Table 2, and Fig. 1B, C).
Mean ± SD for numbers of labeled descending brain neurons on both sides of the brain (these values were not significantly different from controls at 8-week recovery times; see middle column in Table 1; one-way ANOVA).
First, based on previous studies (Swain et al., 1993; Davis and McClellan, 1994a), in larval sea lamprey the average numbers of descending brain neurons that project to 30% in the spinal cord are only about 6% greater than projections to 50% BL. Thus, in the present study, CLs at 30% BL and 50% BL transected the axons of approximately similar numbers of descending brain neurons. Second, in larval sea lamprey (80 –100 mm), injection of dye in Müller axons following spinal cord transections at ~30% BL indicates that these injured axons retract or “die back” an average of ~1.75 mm (Roederer et al., 1983). Similar results have been obtained with serial sections of the spinal cord rostral to a transection (Rovainen, 1976). Therefore, in the present study, CLs at 30% BL or 50% BL probably did not result in significant long-distance axonal die-back, and these axons very likely were transected by subsequent lesions made at 10% BL (Fig. 1B,C).
Processing of tissue
Lamprey were anesthetized, and the brains and a few segments of the rostral spinal cord were removed in oxygenated lamprey Ringer’s solution, as described previously (Davis and McClellan, 1994a). The tissue was transferred dorsal side up to a small rectangular strip of Sylgard (Corning, Midland, MI), the choroid plexus and meninges were removed, the cerebellar commissure was transected, and the obex was extended caudally. The tissue was pinned relatively flat and reacted en bloc at 0°C for 8 –25 minutes in a modified Hanker-Yates solution (Davis and McClellan, 1994a) consisting of 0.10% catechol, 0.05% phenylendiamine, and 0.075% H2O2 in 0.1 M Tris buffer (pH 7.4). After the reaction, the tissue was dehydrated in a series of ethanols, cleared in methyl salicylate, transferred to slides, and coverslipped with Permount (Fisher, Fair Lawn, NJ). Photomicrographs (see Fig. 5) were collected with a digital camera (Retiga 1300C; Q Imaging, Burnaby, British Columbia, Canada), and the contrast and brightness of digital images were adjusted slightly in PhotoShop (Adobe Systems, San Jose, CA) to improve the contrast between HRP-labeled descending brain neurons and background.
Fig. 5.
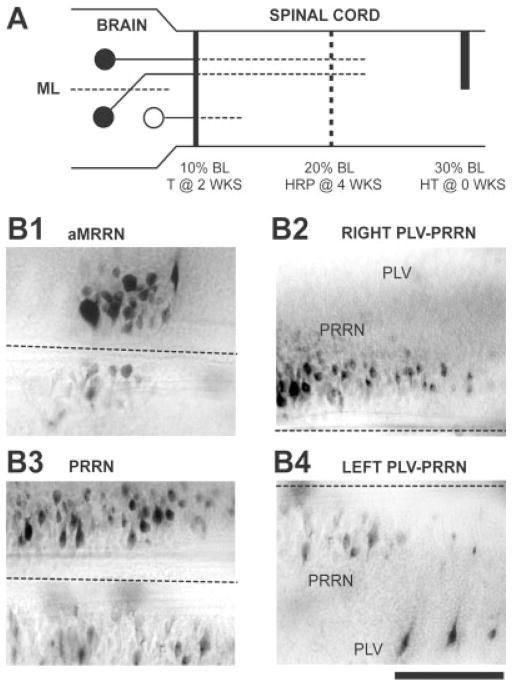
Contributions of axotomy to the conditioning lesion effect (see Table 4). A: A right hemitransection (HT) was made in the spinal cord at 30% BL, and, 2 weeks later, the spinal cord was transected (T) at 10% BL. The tracer HRP was applied to the spinal cord 4 weeks later at 20% BL. If axotomy is required for conditioning lesion effects, neurons that receive conditioning lesions (solid circles) should display enhanced axonal regeneration, whereas neurons not directly injured by the conditioning lesion (open circle) should not exhibit enhanced axonal growth. B: Photomicrographs from a brain showing asymmetrical labeling of descending brain neurons (rostral is to the left, right is upward; dashed lines indicate the midline). B1,3: There were higher numbers of labeled neurons in the right MRRN and PRRN, ipsilateral to the hemi-CL. B2,4: Larger numbers of labeled neurons were found in the right PRRN (B2) and left PLV (B4), ipsilateral and contralateral, respectively, to the hemi-CL (see solid circles in A). Scale bar = 200 μm.
Data collection
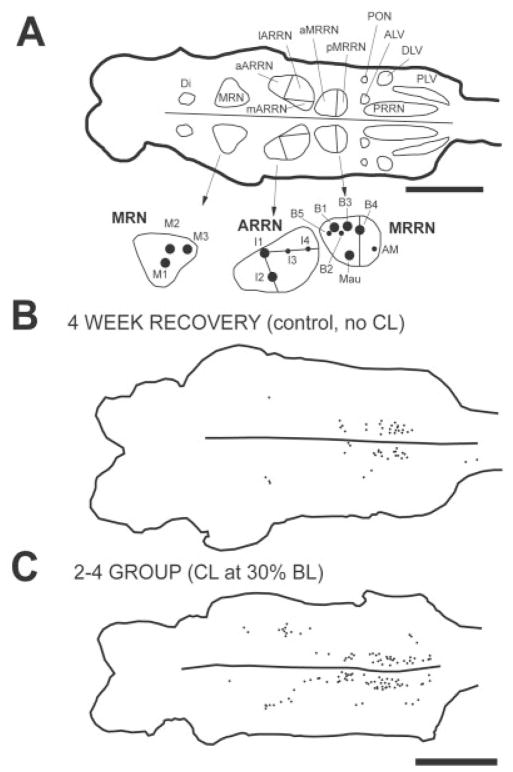
With a computer charting system (CCS; Warren Instruments, St. Louis, MO; see Tourtellotte et al., 1989), the outlines of the whole-mount brains were traced, and HRP-labeled descending brain neurons were marked and counted in 12 cell groups (Fig. 2A), as previously described (Davis and McClellan, 1994a,b). The minimum criteria for defining a labeled neuron included the presence of at least three individual HRP granules within the visible outline of the soma, as previously described (Davis and McClellan, 1994a,b). For neuronal cell counts, two types of mean values were calculated: 1) the total numbers of descending brain neurons for lamprey in a given animal group were averaged (Fig. 3), and 2) for lamprey in a given animal group, the numbers of neurons in each brain cell group were averaged (see Tables 1–3). For 4-week recovery times, the total number of labeled descending brain neurons in each of the four experimental animal groups (hatched and open bars in Fig. 3A) was compared to that in control animals (solid bar in Fig. 3A) with an unpaired t-test with Welch correction (i.e., parametric test for unequal SDs) or with a Mann Whitney U-test (nonparametric test), depending on whether the data were Gaussian, and P values were adjusted for α/4 (i.e., four comparisons; InStat 3.0; GraphPad Software, San Diego, CA). At 4-week recovery times and for a given cell group, the number of labeled neurons in each of the four animal groups (Table 2) was compared to that in control animals (Table 1) with a Mann Whitney U-test, and P values were adjusted for α/4 (i.e., four comparisons). For 8-week recovery times, the standard deviations were not significantly different in the various animal groups, so cell counts in experimental animals (Fig. 3B, Table 3) were compared to the count in controls (solid bar in Fig. 3B, Table 1) by one-way analysis of variance (ANOVA). Statistical significance was considered to be P ≤ 0.05.
Fig. 2.
A (top): Diagram of dorsal view of a brain from a normal larval lamprey (left is rostral, and right is caudal) that was generated by a computer charting system (CCS; Tourtellotte et al., 1989). Outlines of different cell groups were drawn around descending brain neurons that were retrogradely labeled by application of HRP to the spinal cord at 20% BL in a normal animal (see Materials and Methods). A (bottom): Enlargement of MRN, ARRN, and MRRN on the left side of the brain, showing large, identified Müller and Mauthner cells: M1–M3, mesencephalic cells; I1–I4, isthmus cells; B1–B5, bulbar cells; Mau, Mauthner cell; and AM, auxiliary Mauthner cell. B: Computer-generated diagram of the brain from a control animal showing descending brain neurons (dots) that were labeled by application of HRP to the spinal cord at 20% BL at 4 weeks after a spinal cord transection at 10% BL (see Fig. 1A). C: Labeled descending brain neurons (dots) in an animal in the D-R = 2–4 experimental group with a CL at 30% BL (see Fig. 1B). Scale bars = 1 mm.
Fig. 3.
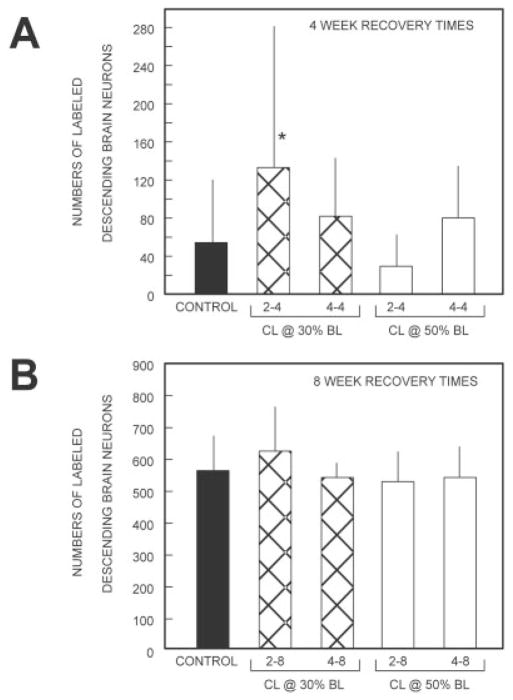
Histograms showing the average total numbers of HRP-labeled descending brain neurons in animals that had recovered for 4 weeks (A) or 8 weeks (B) following spinal cord transection (see Fig. 1; see also Tables 1–3). Means (bars) and standard deviations (SDs, vertical lines) were calculated by averaging the total numbers of labeled descending brain neurons for all animals within each given animal group. For 4-week recovery times (A), the total number of labeled descending brain neurons in each of the four experimental animal groups (hatched and open bars) was compared with that in control animals (solid bar) with an unpaired t-test with Welch correction (parametric test for unequal SDs) or with a Mann Whitney U-test (nonparametric test), depending on whether the data were Gaussian, and P values were adjusted for α/4 (i.e., four comparisons). For 8-week recovery times (B), the total cell counts for each experimental animal group were compared with the count in control animals by one-way analysis of variance (ANOVA). *P < 0.05.
TABLE 2.
Restoration of Descending Projections for 4-Week Recovery Times Following Conditioning Lesions
| CL at 30% BL |
CL at 50% BL |
|||
|---|---|---|---|---|
| 2–4 Group1 | 4–4 Group | 2–4 Group | 4–4 Group | |
| N | 22 | 7 | 12 | 8 |
| Body length (mm) | 92.3 ± 6.8 | 92.4 ± 5.9 | 102.4 ± 10.4 | 89.9 ± 4.8 |
| Di | 0.9 ± 1.52 | 0.9 ± 1.2 | 0.2 ± 0.4 | 0.0 ± 0.0 |
| M cells (M1–M3) | 0.5 ± 0.8 | 0.7 ± 1.1 | 0.2 ± 0.4 | 0.4 ± 0.5 |
| MRN | 3.5 ± 4.1 | 2.9 ± 4.1 | 0.4 ± 0.8 | 2.1 ± 1.4 |
| I cells (I1–I4) | 0.2 ± 0.5 | 1.1 ± 1.5 | 0.2 ± 0.4 | 1.1 ± 1.0 |
| aARRN | 7.5 ± 9.1 | 4.0 ± 2.9 | 0.6 ± 1.2 | 2.1 ± 2.4 |
| IARRN | 8.5 ± 10.0*3 | 5.0 ± 6.1 | 1.3 ± 3.2 | 4.0 ± 5.7 |
| mARRN | 6.5 ± 7.8 | 4.9 ± 3.5 | 1.3 ± 1.9 | 5.9 ± 4.6* |
| B cells (B1–B5) | 0.8 ± 1.0* | 0.7 ± 0.8 | 0.3 ± 0.5 | 0.4 ± 0.7 |
| Mau and AM cells | 0.1 ± 0.3 | 0.6 ± 0.5 | 0.0 ± 0.0 | 0.3 ± 0.7 |
| aMRRN | 11.4 ± 12.9* | 7.9 ± 5.0 | 2.4 ± 6.3 | 5.9 ± 5.1 |
| pMRRN | 16.0 ± 16.7 | 12.0 ± 8.5 | 5.8 ± 6.5 | 13.4 ± 9.3 |
| PRRN | 60.4 ± 71.1 | 33.3 ± 26.7 | 14.3 ± 14.8 | 35.1 ± 24.6 |
| ALV | 3.3 ± 4.0 | 1.0 ± 1.3 | 0.5 ± 1.4 | 0.5 ± 1.1 |
| DLV | 0.2 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.4 |
| PLV | 13.8 ± 14.5 | 7.7 ± 5.3 | 2.5 ± 3.5 | 9.4 ± 9.5 |
| PON | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Animal groups (“D-R” groups; “D” is the “lesion delay time” between a conditioning lesion and spinal cord transection at 10% BL, “R” is the “recovery time” (4 weeks) between spinal cord transection and application of HRP at 20% BL; see Fig. 1B, C).
Mean ± SD for numbers of labeled descending brain neurons on both sides of the brain.
Comparison to the numbers of descending brain neurons in control animals at the same recovery times (see left column in Table 1).
P ≤ 0.05 (for a given cell group, the cell counts for each of the four experimental groups were compared pairwise with the count in control animals at 4-week recovery times (left column in Table 1) with a Mann Whitney U-test, and P values were adjusted for α/4 (i.e., four comparisons).
Control experiment: role of axotomy for CL effects
In the present study, in the “2–4” group with CLs at 30% BL, the number of descending brain neurons that regenerated their axons was significantly greater than that in control animals (Fig. 3A, Tables 1, 2). To confirm these results and to reduce interanimal variability, hemi-CLs were performed in the spinal cord at 30% BL, so that only half of the descending brain neurons received CLs. In addition, these experiments were used to determine whether descending axons whose regeneration was enhanced by CLs might require direct axotomy or whether they could respond indirectly to more general changes in the spinal cord, such as release of diffusible factors from the injury site or other injured neurons. Three groups of animals were established: 1) control animals—identical to “control animals” described above with spinal cord transections at 10% BL and recovery times of 4 weeks (n = 40; Fig. 1A); 2) full CL at 30% BL—identical to the 2–4 group described above (n = 28; Fig. 1B); and 3) hemi-CL at 30% BL—similar to the 2–4 group described above, but a hemi-CL was performed on the right (n = 11) or left (n = 7) side of the spinal cord at 30% BL, so that only half of the descending brain neurons received CLs (n = 18; the left-right cell counts for left hemi-CLs were reversed so that all data were normalized for hemi-CLs on the right side; Fig. 5A). It should be noted that the midline of the spinal cord is clearly visible in the dissecting microscope and that hemi-CLs are relatively easy to perform. In addition, in a separate study, the extent of similar hemitransections was confirmed by intracellular recordings from reticulospinal neurons and recordings from the spinal cord above and below the lesion (McClellan, 2003).
For animals in each of the above three groups, the cell counts on left and right sides of the brain were determined for descending brain neurons in cell groups with largely ipsilateral projections (MRN, aMRRN, pMRRN, PRRN) or contralateral projections (PLV; Fig. 2; Shaw et al., 2002). The Sign test (Blaisdel, 1993) was applied to the numbers of animals in which the cell counts for a given cell group on the contralateral side or ipsilateral side of the brain were greater. Thus, if axotomy and not diffusible factors is primarily responsible for CL effects, the hemi-CL animals should display enhancement of axonal regeneration in ipsilateral cell groups of descending brain neurons with uncrossed projections and contralateral cell groups with crossed projections (solid circles in Fig. 5A). For example, if the cell counts in the right PRRN were greater in 10 brains and the counts in the left PRRN were greater in 2 brains, the Sign test would indicate that this asymmetry is not due to chance and that the cell counts for the right PRRN are significantly greater than those for the left nucleus (P = 0.039).
RESULTS
Labeled descending brain neurons at 4-week recovery times (R = 4)
Control animals
At 4-week recovery times after spinal cord transections at 10% BL (Fig. 1A), application of HRP to the spinal cord at 20% BL retrogradely labeled 54 ± 66 descending brain neurons (Fig. 2B, solid bar in Fig. 3A, Table 1; n = 29), and these cell counts were similar to those in a previous study (Davis and McClellan, 1994a). It should be noted that, in normal animals with a size range and age similar to those those used in the present study, on average ~1,140 descending brain neurons project to 20% BL (data in right column in Table 1 from Zhang et al., 2002). Thus, at recovery times of 4 weeks, about 5% of the normal numbers of descending brain neurons that project to 20% BL in the spinal cord regenerated their axons to this same level of the cord (Table 1). At 4-week recovery times following transections at 10% BL, the standard deviations for restored projections to 20% BL usually are relatively large (Fig. 3A, Tables 1, 2; see also Fig. 4), because the axons of descending brain neurons are just starting to arrive at this level of the spinal cord (Davis and McClellan, 1994a). Presumably, slight variations in the growth rates of these axons result in wide variations in the numbers of restored descending brain-spinal cord projections at these early recovery times.
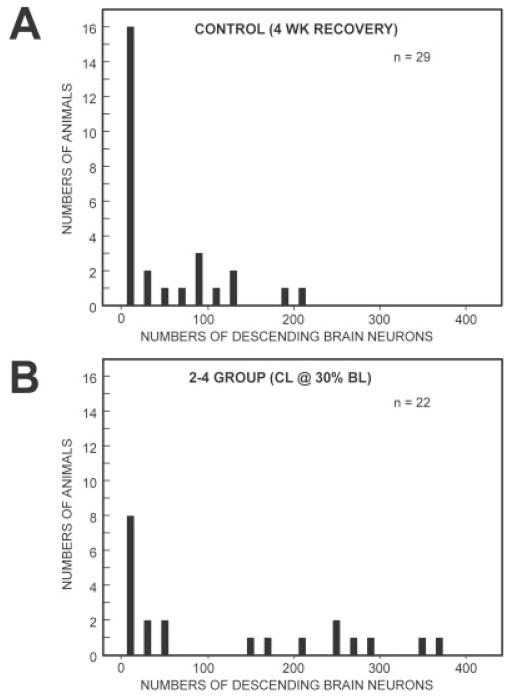
Fig. 4.
Distributions of the total numbers of labeled descending brain neurons that projected to 20% BL at recovery times of 4 weeks in control animals (A; Fig. 1A; individual animals in A were used to generated the data for the leftmost bar in Fig. 3A) and experimental animals (B) in the D-R = 2–4 group with conditioning lesions at 30% BL (Fig. 1B; individual animals in B were used to generated the data for second bar from the left in Fig. 3A).
Experimental group 1 (CLs at 30% BL)
In animals in which the spinal cord was transected 2 weeks after CLs (D = 2) and HRP was applied to the spinal cord after a 4-week recovery time (R = 4; 2–4 group), 133 ± 148 descending brain neurons were retrogradely labeled from 20% BL (Fig. 2C, second bar from left in Fig. 3A; n = 22). When 4 weeks had elapsed between CLs and spinal cord transections (4–4 group), 83 ± 60 descending brain neurons projected to 20% BL in the spinal cord (n = 7; Fig. 3A).
Experimental group 2 (CLs at 50% BL)
With lesion delay times of 2 weeks (D = 2) and recovery times of 4 weeks (R = 4; 2–4 group), 30 ± 33 descending brain neurons were retrogradely labeled from 20% BL (Fig. 3A, Table 2; n = 12). For 4-week lesion delay times (4–4 group), 80 ± 54 descending brain neurons projected to at least 20% BL in the spinal cord (n = 8; Fig. 3A).
Labeled descending brain neurons at 8-week recovery times (R = 8)
Control animals
For recovery times of 8 weeks following spinal cord transections at 10% BL (Fig. 1A), 579 ± 106 descending brain neurons were retrogradely labeled by application of HRP to the spinal cord at 20% BL (solid bar in Fig. 3B; n = 18), and these counts were similar to those in a previous study (Davis and McClellan, 1994a). Thus, in comparison to normal animals of the same size and age, at 8-week recovery times about 51% of the normal numbers of descending brain neurons that project to 20% BL had regenerated their axons to this level of the cord (Table 1; data in right column from Zhang et al., 2002).
Experimental group 1 (CLs at 30% BL)
When the spinal cord was transected 2 weeks after CLs were made (D = 2), and HRP was applied to the spinal cord after an 8-week recovery time (R = 8; 2–8 group), 637 ± 139 descending brain neurons were retrogradely labeled from 20% BL (Fig. 3B, Table 3; n = 8). When instead the CLs were performed 4 weeks before spinal cord transection (4–8 group), 555 ± 45 descending brain neurons were labeled (n = 8; Fig. 3B).
Experimental group 2 (CLs at 50% BL)
For animals with lesion delay times of 2 weeks (D = 2) and recovery times of 8 weeks (R = 8; 2–8 group), 544 ± 91 descending brain neurons were retrogradely labeled from 20% BL (Fig. 3B, Table 3; n = 7). When instead lesion delay times were 4 weeks (4–8 group), 557 ± 94 descending brain neurons were labeled (n = 8; Fig. 3B).
Comparison of the numbers of labeled descending brain neurons
Recovery times of 4 weeks
In the 2–4 experimental group with CLs at 30% BL, the total numbers of descending brain neurons that regenerated their axons to at least 20% BL (133 ± 148; second bar from left in Fig. 3A) were significantly greater than those in control animals (54 ± 66; solid bar in Fig. 3A; P ≤ 0.05, unpaired t-test with Welch correction; see also Fig. 2B vs. C). In the above-described control group, the numbers of labeled descending brain neurons varied from ~0 to ~200, and about half of the animals had very low cell counts (Fig. 4A). In the above-described experimental group, the numbers of labeled neurons varied from ~ to ~400, and there were proportionately fewer animals with very low cell counts (Fig. 4B). The broad distributions in these histograms lead to relatively large SDs (Fig. 3A) for the reasons noted above. Finally, in the above-described experimental group, descending brain neurons in the lARRN, B-cell group, and aMRRN regenerated their axons significantly better than in controls (Table 2 vs. Table 1; Mann Whitney U-test).
For the other three experimental groups at 4-week recovery times, CLs did not significantly enhance the total numbers of descending brain neurons that regenerated their axons relative to controls (Fig. 3A). Furthermore, for virtually all cell groups in these three other experimental groups, CLs did not significantly enhance axonal regeneration compared with controls (Tables 1, 2).
Recovery times of 8 weeks
The numbers of labeled descending brain neurons in the experimental groups with CLs (2–8 or 4–8 groups in Fig. 3B and Tables 3) were not significantly different from those for controls animals (solid bar in Fig. 3B, Table 1; overall P = 0.39, ANOVA). In summary, with CLs in the rostral spinal cord (30% BL) and relatively short lesion delay times (D = 2) and recovery times (R = 4), there was significant enhancement of axonal regeneration of descending brain neurons compared with that in control animals without CLs.
Control experiment: role of axotomy for CL effects
In the present study, in the 2–4 group with CLs at 30% BL, the total numbers of descending brain neurons that regenerated their axons were significantly greater than in controls (Fig. 3A). To confirm these results and reduce interanimal variability, hemi-CLs were performed in the spinal cord at 30% BL, so that only half of the descending brain neurons received Cls (Fig. 5A). In addition, hemi-CLs tested whether axotomy or diffusible factors contribute to the CL effect.
The left-right cell counts for retrograde labeling in cell groups with largely ipsilateral (MRN, aMRRN, pM-RRN, PRRN) or contralateral (PLV) projections (Shaw et al., 2001) were compared in control animals, animals with full CLs at 30% BL, and animals with hemi-CLs at 30% BL (Fig. 5A; see Materials and Methods). In the control and full-CL groups, the left-right cell counts of descending brain neurons in the above-described cell groups did not display a significant asymmetrical pattern (Table 4, sign test). In contrast, in the hemi-CL group, four of the five cell groups described above exhibited a significant asymmetrical pattern that was not due to random chance (Table 4, Sign test). In particular, the numbers of ipsilateral (contralateral) labeled neurons in cell groups with primarily uncrossed (crossed) projections (Shaw et al., 2002) were significantly greater (Fig. 5B,C). These experiments with hemi-CLs reduce interanimal variations and suggest that CLs significantly enhance axonal regeneration. In addition, the results suggest that axotomy rather than diffusible factors is primarily responsible for CL effects in the lamprey. It is likely that, under these conditions, hemi-CLs enhanced axonal regeneration of other descending brain neurons but that these other cell groups contain a greater mixture of crossed and uncrossed descending neurons (Shaw et al., 2002) that are not well suited to the present experimental paradigm.
TABLE 4.
Control Experiment to Test Whether Axotomy Is Critical for “Conditioning Lesion Effects”
| Control1 | Full CL2 | Hemi-CL3 (right side) | |
|---|---|---|---|
| N | 40 | 28 | 18 |
| Body length (mm) | 96 ± 9 | 97 ± 9 | 105 ± 8 |
| MRN | 24/65 | 5/12 | 0/7* |
| aMRRN | 4/10 | 10/14 | 1/8* |
| pMRRN | 9/14 | 12/13 | 8/10 |
| PRRN | 11/29 | 13/15 | 4/14* |
| PLV | 15/9 | 12/10 | 14/2** |
Transection at 10% BL (2 weeks) → HRP at 20% BL (6 weeks; see Fig. 1A).
Full CL at 30% BL (0 weeks) → transection at 10% BL (2 weeks) → HRP at 20% BL (6 weeks; see Fig. 1B).
Hemi-CL on right side at 30% BL (0 weeks) → transection at 10% BL (2 weeks) → HRP at 20% BL (6 weeks, (11 right and 7 left hemi-CLs; left-right cell counts for left hemi-CL’s were reversed so that the data were normalized for hemi-CLs on the right side, see Fig. 5A).
Numbers of animals in which the cell counts on the (4) contralateral (left) side or (5) ipsilateral (right) side of the brain were greater (see Fig. 5A).
P ≤ 0.05 (Sign test).
P ≤ 0.01 (Sign test).
DISCUSSION
Enhancement of axonal regeneration by CLs
In larval lamprey with a spinal CL at 30% BL, a 2-week lesion delay time, and a 4-week recovery time following a test lesion (D-R = 2–4 group), the total numbers of descending brain neurons that regenerated their axons to at least 20% BL were significantly greater compared to control animals without CLs (Figs. 2B,C, 3A). In addition, axonal regeneration of neurons in some cell groups was significantly enhanced compared to that in animals without CLs (Table 2 vs. Table 1). In the 2–4 experimental group, there were many more animals with substantial numbers of labeled descending brain neurons and relatively few animals with no retrograde labeling of neurons compared to controls (Fig. 4). Furthermore, experiments with hemi-CLs, which reduce interanimal variability, confirmed that CLs significantly enhance axonal regeneration (Fig. 5, Table 4). For virtually all other combinations of experimental conditions and CLs, axonal regeneration was not significantly enhanced compared to controls (Fig. 3, Tables 1–3).
Mechanisms for CLs from studies in other animals
Most CL studies involve peripheral nerves, so crush injuries usually are used for both Cls and test lesions. From these studies, at least two possible mechanisms have been proposed for the CL effect: cell body responses; and local environmental conditions.
Cell body responses
After a CL, axotomized neurons switch to a regenerating mode in which several biochemical changes in the cell favor axonal regeneration in response to a subsequent test lesion, including increases in RNA and protein synthesis (McQuarrie, 1985; Redshaw and Bisby, 1987), increase in cAMP levels (Qiu et al., 2002), decrease in neurofilament synthesis (Tetzlaff et al., 1996), up-regulation and acceleration of transport of slow component b (SCb) of axonal transport (Jacob and Mc-Quarrie, 1991), and increases in cytomatrix and structural proteins for some of the different components of axonal transport (McQuarrie and Grafstein, 1982; McQuarrie, 1986; Perry et al., 1987). After a subsequent test lesion, the initial delay of axonal regeneration is decreased, and the rate of axonal outgrowth is increased because axotomized neurons have already switched to a regenerating mode. Some experiments suggest that the cell body response plays a major role in the CL effect (McQuarrie and Grafstein, 1982; McQuarrie, 1985; Jacob and McQuarrie, 1991, 1993; Tetzlaff et al., 1996). For example, when frogs are maintained at 15°C, which is thought to inhibit the cell body response, CLs of the sciatic nerve do not produce enhanced axonal regeneration compared to the significant CL effect that occurs at 25°C (Carlsen, 1983).
Local environment
Other studies suggest that, after a CL, degenerative tissue in the vicinity of the lesion makes it physically easier for growth cones to elongate in this region, and the release of growth factors by glia and other cells might enhance outgrowth of axons (Bisby and Pollock, 1983; Bisby and Keen, 1985; Cho and So, 1989; Edbladh and Edstrom, 1989; Reich et al., 1990; Sjöberg and Kanje, 1990; Lu and Richardson, 1991). For example, when glia and other nonneuronal cells are temporarily frozen around an injury site in the rat sciatic nerve, CLs do not enhance axonal regeneration (Sjöberg and Kanje, 1990). In addition, CLs followed by test lesions at the same site usually induce a greater degree of axonal regeneration than when CLs are made distal to test lesions (Bisby and Pollock, 1983; Bisby, 1985; Bisby and Keen, 1985; Edbladh and Edstrom, 1989; Cho and So, 1989; Sjöberg and Kanje, 1990; Navarro and Kennedy, 1990). However, more distal CLs may also be less effective in eliciting cell body responses. Finally, [3H]thymidine studies demonstrate that axonal outgrowth begins several hours after a test lesion and before the cell body response to a CL is thought to have fully developed (Sjöberg and Kanje, 1990).
Possible mechanisms for CL effects in spinal-cord-transected lamprey
In the present study, CLs at 30% BL and relatively short lesion delay times and recovery times resulted in axonal regeneration from 10% to 20% BL that was enhanced compared to controls (2–4 group; Fig. 3A, Tables 1, 2; see also Table 4). First, in contrast to results in peripheral nerves (Sjoberg and Kanje, 1990), in the present study rostral CLs at 30% BL enhanced axonal regeneration to a greater degree than CLs at 50% BL. Notably, spinal cord transections in larval lamprey must be less than ~30 mm from neuronal cell bodies to produce significant morphological and neurophysiological changes in axotomized neurons (Yin et al., 1981). Although CLs at 30% BL are closer to the cell bodies of descending brain neurons than lesions at 50% BL, the CLs at 30% BL also eliminate more of the synapses that these neurons make with spinal targets and is a stronger stimulus for axonal regeneration (McClellan et al., in preparation; see also Yin and Selzer, 1983). Second, the number of brain neurons receiving CLs does not appear to be a significant factor in the present study, in that only about 6% more descending brain neurons project to 30% BL than to 50% BL (Swain et al., 1993; Davis and McClellan, 1994a). Third, CLs appear to exert their effects over relatively short recovery times (R), probably by increasing the initial rate of axonal outgrowth and/or decreasing the initial delay of regeneration. Also, with increasing recovery times (R), the CL effect subsides, possibly because many regenerating axons have made synapses below the transection site. Fourth, control experiments with hemi-CLs, which reduce interanimal variations, indicate that CLs significantly enhance axonal regeneration (Table 4, Fig. 5). Furthermore, these data suggest that axotomy rather than diffusible factors is primarily involved in promoting CL effects in the lamprey. However, the contribution of environmental factors to enhancement of axonal regeneration cannot be ruled out.
Preliminary results suggest that CLs do not appear to act primarily by causing a substantial reduction in axonal die-back. However, it is possible that CLs cause a slight reduction in die-back in the vicinity of the transection site (see Roederer et al. 1983; McHale et al., 1995), such that subsequent axonal regeneration starts from a less retracted position and is enhanced. Experiments are in progress to test this notion.
CONCLUSIONS
In the present study, the role of CLs in restoration of descending brain-spinal cord projections was investigated in larval lamprey. With CLs at 30% BL, lesion delay times of 2 weeks, and recovery times of 4 weeks (D-R = 2–4 group), regeneration of axons from descending brain neurons was significantly enhanced compared with that in control animals. Experiments with hemi-CLs at 30% BL (2–4 paradigm), which reduce interanimal variations, confirmed that CLs significantly enhance axonal regeneration and indicate that axotomy rather than diffusible factors is primarily responsible for the CL effect. To our knowledge, this is the first demonstration of a CL effect involving spinal cord injury. Cell body responses probably play a role in enhancing restoration of descending brain-spinal cord projections in larval lamprey with CLs. Recent studies strongly suggest that axonal regeneration is the major mechanism for restoration of descending brain-spinal cord projections and recovery of locomotor function in spinal cord-transected larval lamprey (Zhang and McClellan, 1999; Zhang et al., 2002). Thus, a better understanding of the mechanisms underlying the enhancement of axonal regeneration by CLs might suggest possible strategies for regulating regeneration under conditions in which axonal outgrowth is limited.
Acknowledgments
We are grateful to the Lamprey Control Units of the U.S. Fish and Wildlife Service at Millersburg, Michigan, and Ludington, Michigan, for help with lamprey collection. We thank Drs. Lori Thombs and Richard Madsen for help with the statistical analysis.
Grant sponsor: National Institutes of Health; Grant number: NS29043; Grant sponsor: American Paralysis Association; Grant number: MA1-9605; Grant sponsor: National Science Foundation; Grant number: IBN9817605 (all to A.D.M.).
Abbreviations
- ALV
anterolateral vagal group
- ARRN
anterior rhombencephalic reticular nucleus
- aARRN
anterior division of ARRN
- aMRRN
anterior division of MRRN
- BL
body length
- CL
conditioning lesion
- D
lesion delay times
- Di
diencephalic group
- DLV
dorsolateral vagal group
- HRP
horseradish peroxidase
- lARRN
lateral division of ARRN
- mARRN
medial division of ARRN
- MRN
mesencephalic reticular nucleus
- MRRN
middle rhombencephalic reticular nucleus
- PLV
posterolateral vagal group
- pMRRN
posterior division of MRRN
- PON
posterior octavomotor nucleus
- PRRN
posterior rhombencephalic reticular nucleus
- PT
posttransection
- R
recovery times
- RS
reticulospinal
LITERATURE CITED
- Bisby MA. Enhancement of the conditioning lesion effect in rat sciatic motor axons after superimposition of conditioning and test lesions. Exp Neurol. 1985;90:385–394. doi: 10.1016/0014-4886(85)90027-5. [DOI] [PubMed] [Google Scholar]
- Bisby MA, Keen P. The effect of a conditioning lesion on the regeneration rate of peripheral nerve axons containing substance P. Brain Res. 1985;336:201–206. doi: 10.1016/0006-8993(85)90646-8. [DOI] [PubMed] [Google Scholar]
- Bisby MA, Pollock B. Increased regeneration rate in peripheral nerve axons following double lesions: enhancement of the conditioning lesion phenomenon. J Neurobiol. 1983;14:467–472. doi: 10.1002/neu.480140607. [DOI] [PubMed] [Google Scholar]
- Blaisdel EA. Statistics in practice. Fort Worth, TX: Saunders College Publishing; 1993. [Google Scholar]
- Carlsen RC. Delayed induction of the cell body response and enhancement of regeneration following a condition/test lesion of frog peripheral nerve at 15°C. Brain Res. 1983;279:9–18. doi: 10.1016/0006-8993(83)90158-0. [DOI] [PubMed] [Google Scholar]
- Cho EYP, So K-F. Regrowth of retinal ganglion cell axons into a peripheral nerve graft in the adult hamster is enhanced by a concurrent optic nerve crush. Exp Brain Res. 1989;78:567–574. doi: 10.1007/BF00230244. [DOI] [PubMed] [Google Scholar]
- Davis GR, McClellan AD. Extent and time course of restoration of descending brainstem projections in spinal cord-transected lamprey. J Comp Neurol. 1994a;344:65–82. doi: 10.1002/cne.903440106. [DOI] [PubMed] [Google Scholar]
- Davis GR, McClellan AD. Long distance axonal regeneration of identified lamprey reticulospinal neurons. Exp Neurol. 1994b;127:94–105. doi: 10.1006/exnr.1994.1083. [DOI] [PubMed] [Google Scholar]
- Davis GR, Troxel MT, Kohler VJ, Grossmann EM, McClellan AD. Time course of locomotor recovery and functional regeneration in spinal-transected lamprey: kinematics and electromyography. Exp Brain Res. 1993;97:83–95. doi: 10.1007/BF00228819. [DOI] [PubMed] [Google Scholar]
- Edbladh M, Edstrom A. Environmental factors contribute to the enhanced regeneration of frog sciatic sensory axons by a conditioning lesion. Acta Physiol Scand. 1989;135:169–172. doi: 10.1111/j.1748-1716.1989.tb08564.x. [DOI] [PubMed] [Google Scholar]
- Forman DS, McQuarrie IG, Labore FW, Wood DK, Stone LS, Braddock CH, Fuchs DA. Time course of the conditioning lesion effect on axonal regeneration. Brain Res. 1980;182:180–185. doi: 10.1016/0006-8993(80)90842-2. [DOI] [PubMed] [Google Scholar]
- Hanson LH, King EL, Howell JH, Smith AJ. A culture method for sea lamprey larvae. Progressive Fish Culturist. 1974;36:122–128. [Google Scholar]
- Jacob JM, Croes SA. Acceleration of axonal outgrowth of motor axons from mature and old F344 rats after a conditioning lesion. Exp Neurol. 1998;152:231–237. doi: 10.1006/exnr.1998.6850. [DOI] [PubMed] [Google Scholar]
- Jacob JM, McQuarrie IG. Axotomy accelerates slow component b of axonal transport. J Neurobiol. 1991;22:570–582. doi: 10.1002/neu.480220603. [DOI] [PubMed] [Google Scholar]
- Jacob JM, McQuarrie IG. Acceleration of axonal outgrowth in rat sciatic nerve at one week after axotomy. J Neurobiol. 1993;24:356–367. doi: 10.1002/neu.480240308. [DOI] [PubMed] [Google Scholar]
- Lanners HN, Grafstein B. Effect of a conditioning lesion on regeneration of goldfish optic axons: ultrastructural evidence of enhanced outgrowth and pinocytosis. Brain Res. 1980;196:547–553. doi: 10.1016/0006-8993(80)90423-0. [DOI] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Inflammation near the nerve cell body enhances axonal regeneration. J Neurosci. 1991;11:972–978. doi: 10.1523/JNEUROSCI.11-04-00972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan AD. Axotomy alters spike frequency adaptation of reticulospinal neurons in larval lamprey. Soc Neurosci Abstr. 2003;29:42.21. [Google Scholar]
- McClellan AD, Zhang L. The effects of conditioning spinal cord lesions on subsequent axonal regeneration in spinal cord-transected larval lamprey. Soc Neurosci Abstr. 2001;27:960. [Google Scholar]
- McHale MK, Hall GF, Cohen MJ. Early cytoskeletal changes following injury of giant spinal axons in the lamprey. J Comp Neurol. 1995;353:25–37. doi: 10.1002/cne.903530105. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG. The effect of a conditioning lesion on the regeneration of motor axons. Brain Res. 1978;152:597–602. doi: 10.1016/0006-8993(78)91116-2. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG. Effect of conditioning lesion on axonal sprout formation at nodes of Ranvier. J Comp Neurol. 1985;231:239–249. doi: 10.1002/cne.902310211. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG. Structural protein transport in elongating motor axons after sciatic nerve crush. Effect of a conditioning lesion. Neurochem Path. 1986;5:153–164. doi: 10.1007/BF02842933. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B. Effect of a conditioning lesion on optic nerve regeneration in goldfish. Brain Res. 1981;216:253–264. doi: 10.1016/0006-8993(81)90128-1. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B. Protein synthesis and axonal transport in goldfish retinal ganglion cells during regeneration accelerated by a conditioning lesion. Brain Res. 1982;251:25–37. doi: 10.1016/0006-8993(82)91270-7. [DOI] [PubMed] [Google Scholar]
- Navarro X, Kennedy WR. The effect of a conditioning lesion on sudomotor axon regeneration. Brain Res. 1990;509:232–236. doi: 10.1016/0006-8993(90)90547-o. [DOI] [PubMed] [Google Scholar]
- Oblinger MM, Lasek RJ. A conditioning lesion of the peripheral axons of dorsal root ganglion cells accelerates regeneration of only their peripheral axons. J Neurosci. 1984;4:1736–1744. doi: 10.1523/JNEUROSCI.04-07-01736.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry GW, Krayanek SR, Wilson DL. Effects of a conditioning lesion on bullfrog sciatic nerve regeneration: analysis of fast axonally transported proteins. Brain Res. 1987;423:1–12. doi: 10.1016/0006-8993(87)90818-3. [DOI] [PubMed] [Google Scholar]
- Qui J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Redshaw JD, Bisby MA. Proteins of fast axonal transport in regenerating rat sciatic sensory axons: a conditioning lesion does not amplify the characteristic response to axotomy. Exp Neurol. 1987;98:212–221. doi: 10.1016/0014-4886(87)90237-8. [DOI] [PubMed] [Google Scholar]
- Reich JB, Burmeister DW, Schmidt JT, Grafstein B. Effect of conditioning lesions on regeneration of goldfish optic axons: time course of the cell body reaction to axotomy. Brain Res. 1990;515:256–260. doi: 10.1016/0006-8993(90)90604-a. [DOI] [PubMed] [Google Scholar]
- Roederer E, Goldberg NH, Cohen MJ. Modification of retrograde degeneration in transected spinal axons of the lamprey by applied dc current. J Neurosci. 1983;3:153–160. doi: 10.1523/JNEUROSCI.03-01-00153.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovainen CM. Regeneration of Müller and Mauthner axons after spinal transection in larval lampreys. J Comp Neurol. 1976;168:545–554. doi: 10.1002/cne.901680407. [DOI] [PubMed] [Google Scholar]
- Shaw AC, Holmes T, Johns JL, Thurman S, Jackson AW, Davis GR, McClellan AD. Pathfinding of regenerating descending axons in spinal cord-transected larval lamprey: functional anatomy. Soc Neurosci Abstr. 2001;27:960. [Google Scholar]
- Sjöberg J, Kanje M. The initial period of peripheral nerve regeneration and the importance of the local environment for the conditioning lesion effect. Brain Res. 1990;529:79–84. doi: 10.1016/0006-8993(90)90812-p. [DOI] [PubMed] [Google Scholar]
- Swain GP, Snedeker JA, Ayers J, Selzer ME. Cytoarchitecture of spinal-projecting neurons in the brain of the larval sea lamprey. J Comp Neurol. 1993;336:194–210. doi: 10.1002/cne.903360204. [DOI] [PubMed] [Google Scholar]
- Tetzlaff W, Leonard C, Krekoski CA, Parhad IM, Bisby MA. Reductions in motoneuronal neurofilament synthesis by successive axotomies: a possible explanation for the conditioning lesion effect on axon regeneration. Exp Neurol. 1996;139:95–106. doi: 10.1006/exnr.1996.0084. [DOI] [PubMed] [Google Scholar]
- Tourtellote WG, Lawrence DT, Getting PA, Van Hoesen GW. A graphics-oriented personal computer-based microscope charting system for neuroanatomical and neurochemical studies. J Neurosci Methods. 1989;29:43–57. doi: 10.1016/0165-0270(89)90107-6. [DOI] [PubMed] [Google Scholar]
- Yin HS, Selzer ME. Axonal regeneration in lamprey spinal neurons. J Neurosci. 1983;3:1135–1144. doi: 10.1523/JNEUROSCI.03-06-01135.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HS, Wellerstein KK, Selzer ME. Effects of axotomy on lamprey spinal neurons. Exp Neurol. 1981;73:750–761. doi: 10.1016/0014-4886(81)90210-7. [DOI] [PubMed] [Google Scholar]
- Zhang L, McClellan AD. Axonal regeneration of descending brain neurons in larval lamprey demonstrated by retrograde double labeling. J Comp Neurol. 1999;410:612–626. doi: 10.1002/(sici)1096-9861(19990809)410:4<612::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Zhang L, Palmer R, McClellan AD. Increase in descending brain-spinal cord projections with age in larval lamprey: Implications for spinal cord injury. J Comp Neurol. 2002;447:128–137. doi: 10.1002/cne.10208. [DOI] [PubMed] [Google Scholar]