SUMMARY
Transforming growth factor-β1 (TGF-β1) has central functions in development, tissue maintenance, and repair and has been implicated in major diseases. We discovered that TGF-β1 contains several amphipathic helices and hydrophobic domains similar to apolipoprotein E (apoE), a protein involved in lipoprotein metabolism. Indeed, TGF-β1 associates with lipoproteins isolated from human plasma, cultured liver cells, or astrocytes, and its bioactivity was highest in high-density lipoprotein (HDL) preparations. Importantly, lipoproteins containing the apoE3 isoform had higher TGF-β levels and bioactivity than those containing apoE4, a major genetic risk factor for atherosclerosis and Alzheimer’s disease. Because TGF-β1 can be protective in these diseases an association with apoE3 may be beneficial. Association of TGF-β with different types of lipoproteins may facilitate its diffusion, regulate signaling, and offer additional specificity for this important growth factor.
INTRODUCTION
Transforming growth factor-β (TGF-β) is a cytokine with key roles in cell proliferation, differentiation, apoptosis, immune responses, tissue repair and extracellular matrix formation, and the prototype of a larger superfamily of growth factors that include activins and bone morphogenic proteins (Derynck and Zhang 2003). TGF-β acts by binding cell surface type I and type II receptor heterotetramers, to induce signal transduction via Smad-dependent or – independent pathways (Derynck and Zhang 2003). In the CNS, TGF-β protects neurons against age-related and excitotoxin-induced degeneration, decreases parenchymal amyloid deposition (Wyss-Coray et al. 2001; Brionne et al. 2003), promotes neurite outgrowth and is a potent anti-inflammatory agent (Ulich et al. 1991; Gillespie et al. 2001). In the vasculature, TGF-β regulates the properties and functions of all cell types present in the vascular wall and modulates atherosclerosis and restenosis (Singh and Ramji 2006).
TGF-β is synthesized as a precursor protein that is cleaved by furin-type proteases into a proregion, termed latency-associated peptide (LAP), and a bioactive peptide (TGF-β) (Dubois et al. 2001). Non-covalently linked heterodimers of the two proteins are secreted as a small latent complex (SLC). Alternatively, the SLC is secreted covalently linked with latent TGF-β binding proteins (LTBPs) in large latent complexes (LLC), which sequester TGF-β to the extracellular matrix (Saharinen and Keski-Oja 2000). Secreted TGF-β is present in tissues and plasma, but how this hydrophobic protein is transported through the body remains unclear.
To characterize the nature of secreted TGF-β, we size-fractionated human plasma and conditioned medium of cultured liver cells or primary astrocytes, and show that bioactive TGF-β co-elutes in fractions containing lipoproteins. Lipoproteins are spherical or discoidal particles composed of lipids and proteins that contain characteristic amphipathic lipid-binding domains such as apolipoprotein E (apoE). We found that TGF-β also contains such putative amphipathic lipid-binding domains. Prompted by the above findings and the fact that lipoproteins play an important role in the transport of hydrophobic molecules through an aqueous environment, we hypothesized that secreted TGF-β might associate with lipoproteins, which would facilitate its transport through the organism. Using different types of immunoprecipitation and immuno-electronmicroscopy (EM) we show that TGF-β1 indeed associates with lipoproteins. Moreover, we show that lipoproteins carrying apoE3 contain significantly more TGF-β protein and bioactivity than lipoproteins carrying apoE4, which could have significant implications for Alzheimer’s disease and cardiovascular disease.
RESULTS
Secreted TGF-β co-elutes with lipoproteins isolated from human plasma, cultured liver cells and primary astrocytes
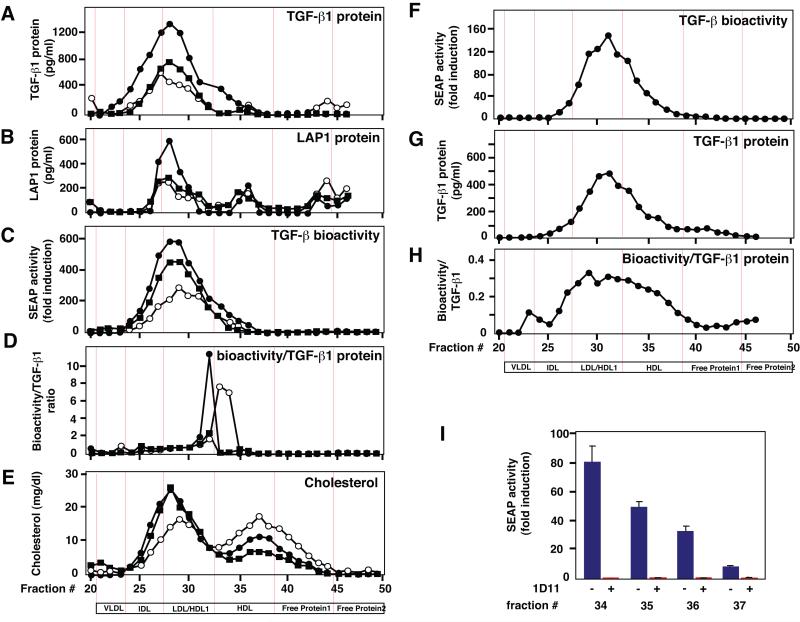
To determine whether secreted TGF-β bioactivity in plasma is transported as a single protein or in association with other molecules, we fractionated human plasma from different donors via Fast Protein Liquid Chromatography (FPLC) on a Superose-6 column. We found that TGF-β1 and LAP1 protein measured via ELISA eluted over a broad range of fractions (Fig. 1A and 1B). This is consistent with previous studies showing TGF-β1 protein elutes in different lipoprotein-containing plasma fractions (Grainger et al. 1997). We found that most of these fractions contained bioactive TGF-β based on measurements with the MFB-F11 bioassay (Tesseur et al. 2006) (Fig 1C). Interestingly, the ratio between the levels of bioactive TGF-β and TGF-β1 protein was highest in fractions 32 – 34 (Fig. 1D), demonstrating the presence of a highly bioactive TGF-β form. This is consistent with a recent report showing that HDL can increase TGF-β signaling (Chen et al. 2008).
Figure 1.
TGF-β co-elutes with lipoproteins. Human plasma from three different healthy donors was fractionated on Superose-6 by FPLC. A – B, TGF-β1 and LAP1 protein measured by specific ELISAs. C, TGF-β bioactivity measured with the MFB-F11 bioassay. D, The ratio between bioactive TGF-β and TGF-β1 protein levels was obtained by dividing the bioactive levels of each donor with its respective TGF-β1 protein levels. E, Cholesterol levels in individual fractions. F – G, TGF-β bioactivity and TGF-β1 protein in conditioned medium of McA/RH-7777 rat liver cells stably expressing apoE3, fractionated on Superose-6. H, Ratio between TGF-β bioactivity and TGF-β1 protein levels obtained as in D. I, TGF-β bioactivity is neutralized after incubation with a TGF-β antibody (1D11) in 4 separate HDL fractions. Values represent means or means ± SEM of triplicate measurements of individual fractions of one representative experiment.
Based on their size, TGF-β, LAP or SLC would have to elute in the free protein fractions (Table 1 and Fig. 1). Instead, most secreted TGF-β elutes in cholesterol-rich fractions containing Intermediate Density Lipoproteins (IDL), Low Density Lipoproteins (LDL) and HDL, with the highly bioactive TGF-β form eluting in the LDL and HDL fractions (Fig. 1D). Only small amounts of TGF-β1 or LAP1 protein were measured in the free protein fractions and they showed no bioactivity (Fig. 1A-C). In human serum TGF-β1 has also been shown to bind to α2-Macroglobulin (α2M) forming 720 kD tetrameric and 180 kD monomeric complexes (Webb et al. 1996). Based on that size these complexes are predicted to elute in the VLDL/IDL and HDL fractions respectively (Table 1). In our samples α2M eluted together with the IDL, LDL, HDL and free protein fractions and thus large molecular weight complexes consisting of TGF-β and α2M could account for some of the observed TGF-β bioactivity (Supplementary Fig. 1). Together, these fractionation studies indicate that TGF-β either associates with lipids or lipoproteins or with large molecular weight protein complexes, and that TGF-β1 bioactivity varies in different lipoprotein fractions.
Table 1.
protein sizes and their estimated elution pattern on Superose-6
| Protein | Size (kD) | Estimated fraction # | Estimated lipoprotein |
|---|---|---|---|
| TGF-β1 dimer | 25 | >45 | Free protein II |
| LAP1 monomer | 65 | >45 | Free protein II |
| SLC | 100 | 38-43 | Free protein I |
| LLC | 200-250 | 32-33 | HDL1/HDL |
| α-2M monomer | 165-180 | 33-37 | HDL |
| α-2M tetramer | 720 | 23-25 | VLDL/IDL |
TGF-β1 = Transforming Growth Factor-beta1, LAP1 = latency associated protein-1, SLC = small latent complex, LLC = large latent complex, α-2M = alpha-2-macroglobulin
The liver is the major source of lipoproteins in the periphery and secretes HDL into the bloodstream (Mahley 1988). To study the potential association of TGF-β with lipoproteins in a more defined system, we used McA/RH-7777 liver cells stably transfected with human apolipoprotein E3 (apoE3) producing mainly HDL-type lipoproteins (Huang et al. 1998). In humans, apoE exists in three isoforms apoE2, apoE3, and apoE4 of which apoE3 is the most common (Mahley 1988). Consistent with the results in human plasma, bioactive TGF-β and TGF-β1 protein produced by McA/RH-7777 liver cells elute in lipoprotein-rich fractions (Fig. 1F, G), and the ratio between bioactive TGF-β and TGF-β1 protein-levels is highest in the LDL/HDL fractions (Fig. 1H). Similar results were obtained with primary astrocytes, the main producers of lipoproteins in the brain (data not shown). Incubation of HDL-containing fractions with antibodies that neutralize all three isoforms of TGF-β showed a near 100% inhibition of bioactivity (Fig. 1I), suggesting that the measured bioactivity is indeed TGF-β and not a TGF-β related factor, such as activin. Comparison of the elution profile of recombinant SLC with culture supernatant from McA/RH-7777 cells, showed that LAP1 protein and TGF-β bioactivity in recombinant SLC overlap largely with the elution profile of TGF-β bioactivity in the supernatant (Supplementary Fig. 2), except for the larger size, LDL/HDL fractions (Supplementary Fig. 2A; shaded area). These results indicate that the fractions with highest relative TGF-β bioactivity contain mainly mature TGF-β and lend additional support for an association of TGF-β with HDL.
TGF-β1 contains putative lipid-binding helices
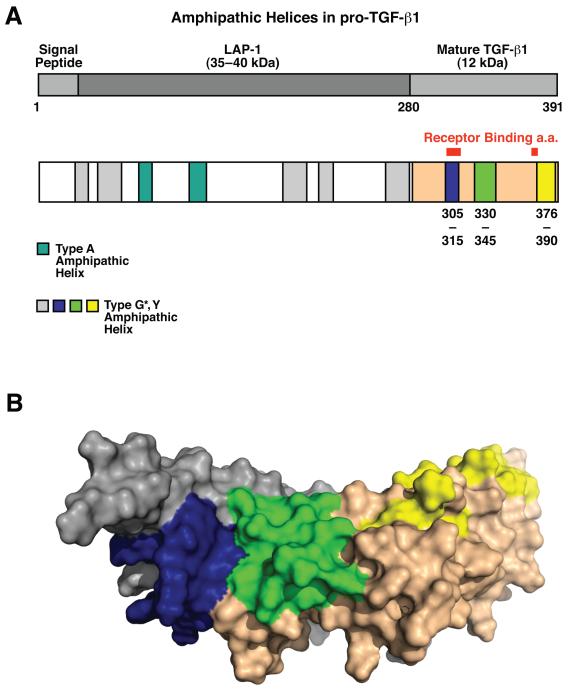
Proteins associated with lipoproteins, such as apolipoproteins contain characteristic amphipathic lipid-binding helices (Segrest et al. 1992). Computer modeling for amphipathic helices shows that the amino acid sequence of the human TGF-β1 precursor contains several domains predictive for lipid-binding motifs (Fig. 2A). LAP1 contains two class A helices and several G* helices that are high on the hydrophobicity scale, and mature TGF-β1 contains two G* helices that possess a high hydrophobicity moment of the non-polar face. Mapping the predicted G* lipid-binding regions onto the structure of TGF-β1 (PDB ID 1KLC, (Hinck et al. 1996)) reveals that dimerization of the growth factor juxtaposes G* domains from each monomer to form a continuous, potentially lipophilic, surface patch (Fig. 2B). These results support the possibility that TGF-β could bind to lipids or lipoproteins.
Figure 2.
TGF-β1 contains putative lipid binding domains. A, Schematic representation of lipid-binding amphipathic α-helices in pro-TGF-β1, identified by COMBO, COMMET, and CONSENSUS. Type G* or Y amphipathic helices (blue, and grey) are drawn to scale. Type G* amphipathic helices in mature TGF-β (blue, green and yellow) correspond to the same colored regions in B. B, Lipid-binding regions (blue, green and yellow) present in mature TGF-β1 form two putative lipid-binding domains. The blue region of the first chain forms a hydrophobic patch together with the green region of the second chain as shown. Grey and nude colors represent individual TGF-β1 peptides. Numbers shown correspond to amino acid numbering of pro-TGF-β.
TGF-β1 associates with lipoproteins
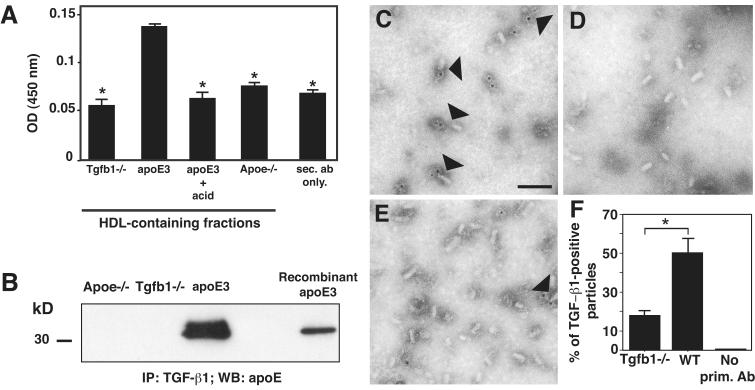
To determine whether TGF-β is directly associated with lipoproteins we studied supernatants from primary astrocyte cultures derived from transgenic mice expressing human apoE3 in astrocytes on a mouse apoE deficient background (GFAP-hapoE3; Raber et al. 2002), and from Tgfb1−/−, or ApoE−/− mice. ApoE producing astrocytes release lipoproteins which are similar in size to those found in human CSF or to plasma HDLs whereas ApoE−/− astrocytes produce no particles (Fagan et al. 1999). We developed a solid-phase immunoprecipitation assay, in which we captured apoE-containing lipoproteins on microtiter plates via an apoE-specific antibody. We used this method to avoid dissociation of the lipoprotein particles by the wash and elution buffers used in standard immunoprecipitation protocols. The specificity of the antibodies was confirmed by detecting recombinant apoE or TGF-β1 on Western blots (Supplementary Fig. 3). TGF-β1 protein was detected in HDL particles purified from primary astrocytes of GFAP-hapoE3 mice. In contrast, similar sized fractions from primary astrocytes of Tgfb1−/−, or ApoE−/− mice did not produce a TGF-β1 signal above that of secondary antibodies alone, which served as a control for the immunoprecipitation (Fig. 3A). In addition, no TGF-β1 protein was detected if apoE3-containing HDL was treated with acid to disintegrate lipoproteins – and presumably, release TGF-β1 from the particles (Fig. 3A).
Figure 3.
TGF-β1 associates with lipoproteins. A, HDL-containing fractions of conditioned media of apoE3-expressing, Apoe−/−, or Tgfb1−/− primary astrocytes were pooled and TGF-β1 protein was measured via solid-phase-immunoprecipitation. To disrupt lipoproteins, pooled HDL-fractions of conditioned medium of apoE3-expressing cells were transiently acidified before measurement (apoE3 + acid). Values represent means ± S.E.M. of triplicate measurements of one representative experiment. *, P < 0.01 by Tukey-Kramer when compared to apoE3. B, Total conditioned media of McA/RH-7777 cells stably expressing apoE3 or of Apoe−/−, or Tgfb1−/− primary astrocytes was purified over avidin columns and apoE was detected via western blotting. Recombinant apoE3 protein was used as a loading control. C, D and E, Immuno electron micrographs (EM) of HDL particles containing TGF-β1, obtained from conditioned media of wildtype (C, E) and Tgfb1−/− (D) primary astrocytes, purified via density-gradient ultracentrifugation, stained with an anti-TGF-β1 antibody. HDL particles associated with gold particles were visualized as positive staining. E, Control immuno-EM staining of HDL particles from wildtype astrocytes without primary antibody. Bar indicates 50 nm. F, Quantification of the ratio of immuno-positive HDL to total HDL in each field. Data are means ± S.E.M. of 17 and 11 fields for Tgfb1−/− and wildtype cells respectively. * P < 0.001 by ANOVA (Bonferoni). Two independent experiments showed similar results.
To immunoprecipitate TGF-β we developed a method in which cell culture supernatant is incubated with a biotinylated TGF-β1-specific antibody, loaded onto a sepharose-avidin column and gently eluted with excess biotin. Consistent with the previous experiment, immunoprecipitation with a TGF-β1 antibody resulted in pull-down of apoE in cell culture supernatants from McA/RH-7777 cells stably expressing human apoE3 but not in supernatants from primary astrocytes of Tgfb1−/−, or ApoE−/− mice (Fig. 3B). Interestingly, in vitro incubation of different concentrations of recombinant TGF-β1 or recombinant SLC with recombinant apoE, followed by immunoprecipitation with apoE antibodies did not result in pull down of TGF-β1 or SLC (data not shown). These results argue against a direct protein-protein interaction of TGF-β with apoE, and support the concept that this association requires lipids.
To gain additional evidence for an association of TGF-β1 with lipoproteins we used immuno-electron microscopy for TGF-β1. Immuno-gold labeling was detected in 50% of the HDL particles purified from wildtype primary astrocytes but only in 18% of those purified from Tgfb1−/− mice (Fig. 3C, 3E, and 3F). No staining was observed in wildtype HDL with the gold-tagged detection antibody alone (Fig. 3D). Part of the residual staining in Tgfb1−/− mice is likely due to the fixation procedures necessary for electronmicroscopy (Fig. 3E). Collectively, these results demonstrate that TGF-β1 associates with lipoproteins, including HDL-like particles secreted by primary astrocytes.
Lipoproteins containing apolipoprotein E3 carry significantly more bioactive TGF-β than those containing apolipoprotein E4
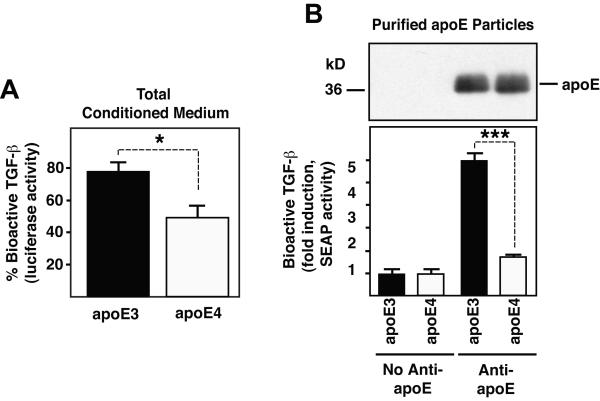
Apolipoprotein E4 (apoE4) is a major known genetic risk factor for atherosclerosis and sporadic late-onset Alzheimer’s disease (AD), and a susceptibility gene for other neurodegenerative diseases (Corder et al. 1993; Saunders et al. 1993; Strittmatter et al. 1993; Alberts et al. 1995; McCarron et al. 1998), but how apoE4 contributes to the pathogenesis of these diseases remains poorly understood. Because TGF-β1 has both neuroprotective and cardioprotective effects it is conceivable that its association with apoE-containing lipoproteins may mediate some of these effects. To determine whether apoE3- and apoE4-containing lipoproteins contain different amounts of TGF-β activity we analyzed HDL secreted by McA/RH-7777 cells stably expressing similar levels of human apoE3 or apoE4 (Huang et al. 1998). While the TGF-β elution profile was similar in supernatants separated on a Superose-6 column from either of these cell lines (data not shown), the amount of bioactive TGF-β relative to total levels was 30% higher in apoE3- compared to apoE4-containing medium (Fig. 4A). Purification of apoE3 and apoE4 containing lipoproteins from stably transfected McA/RH-7777 cells with the above described avidin-biotin immunoprecipitation method using a biotinylated monoclonal apoE antibody, showed that apoE4-containing lipoproteins carry only 29% of the amount of bioactive TGF-β of apoE3-containing lipoproteins (Fig. 4B). Immunoblotting for apoE, which can be taken as a relative measure for the amount of lipoproteins purified, shows that similar apoE levels were isolated from apoE3- and apoE4-containing conditioned medium (Fig. 4B). Together, these results show that both TGF-β levels and bioactivity are higher in lipoproteins containing apoE3 than in those containing apoE4.
Figure 4.
ApoE3-containing lipoproteins show higher TGF-β levels and activity than apoE4-containing lipoproteins. A, % of bioactive TGF-β present in conditioned medium of McA/RH-7777 cells stably expressing similar levels of human apoE3 and apoE4. B, Conditioned medium of McA/RH-7777 cells stably expressing human apoE3 or apoE4 was purified over avidin columns using biotinylated anti-apoE or no anti-apoE antibody. ApoE was detected via western blotting (top) and amount of bioactive TGF-β was measured with the MFB-F11 bioassay (below). Results shown are means ± SEM of triplicate measurements of one representative experiment. * P < 0.05, *** P < 0.001, Student’s t test.
DISCUSSION
Our findings provide novel evidence that TGF-β1 is associated with lipoproteins and that different apolipoprotein E isoforms modulate its level and bioactivity. The implications of such an association are potentially far reaching as our studies with lipoproteins containing either apoE3 or apoE4 indicate. Previous studies showed that lipoproteins are important carriers of lipid soluble vitamins, such as vitamin E and vitamin K (Traber et al. 1998; Schurgers and Vermeer 2002), and that lipophorins, the invertebrate homolog of the mammalian lipoproteins, carry Wnt in Drosophila (Panakova et al. 2005). In addition, a recent proteomic analysis of HDL suggests lipoproteins can carry proteins involved in lipid metabolism, proteinase inhibition, complement activation and the acute-phase response (Vaisar et al. 2007). These results support our finding that lipoproteins are associated with proteins other than the well-characterized apolipoproteins. More importantly, our study with human plasma, rat liver cells and mouse primary astrocytes provides biochemical and functional evidence that human and rodent lipoproteins can carry bioactive TGF-β1. Since lipoproteins are responsible for distributing and delivering lipids throughout the body, proteins such as TGF-β1 associated with lipoproteins could use this association to spread over long distances through the blood or interstitial fluids, in the periphery or the CNS. Cells taking up lipoproteins through specific receptors would also be able to internalize TGF-β1 or bring it in close proximity to the cell surface. Type II TGF-β receptors bind the “fingers” of the growth factor, partially overlapping with one of the hydrophobic surfaces (Hart et al. 2002), while leaving the other surface available for potential lipid binding. Subsequent binding of the Type I TGF-β receptor would occlude the remaining lipophilic portion (Kirsch et al. 2000), opening the possibility that lipoprotein association could regulate delivery of TGF-β to TGF-β receptors. Independent support for a possible role of lipids in TGF-β signaling comes also from recent studies describing lipoprotein-receptor related protein (LRP-1), a receptor for apoE, as a modulator of TGF-β signal transduction (Tseng et al. 2004; Cabello-Verrugio and Brandan 2007).
We observed that apoE3-containing lipoproteins carry more bioactive TGF-β than those containing apoE4. Since TGF-β1 has neuroprotective (Ren and Flanders 1996; Ren et al. 1997; Zhu et al. 2002; Brionne et al. 2003; Tesseur et al. 2006) and neurite outgrowth promoting (Gillespie et al. 2001) activities, its preferential association with apoE3-containing lipoproteins could help explain how these lipoproteins promote neurite outgrowth and protect neurons better than apoE4-containing lipoproteins. The differential association of TGF-β bioactivity with apoE3 versus apoE4 could have widespread implications for the differential functions of apoE isoforms in the CNS, and could at least in part explain why apoE4 carriers have an increased risk for developing AD and are more susceptible to neuronal injury. Likewise, both TGF-β1 and apoE3 exhibit anti-inflammatory action and both are protective in atherosclerosis and cardiovascular disease (Mahley 1988; Ulich et al. 1991; Singh and Ramji 2006). Therefore, it is possible that at least part of the beneficial effects of apoE3, are mediated by TGF-β associated with apoE3-containing lipoproteins. Based on these findings we propose that association of TGF-β with different types of lipoproteins will modulate its signaling properties, its diffusion and transport across tissues and provide additional specificity in regulating physiological and pathological processes.
EXPERIMENTAL PROCEDURES
Reagents, cells and mice
Human plasma was obtained from three healthy blood donors. After centrifugation for 20 minutes at 6000 g, plasma was transferred to a new tube and stored at 4°C until use. Procedures followed were in accordance with institutional guidelines.
McA/RH-7777 liver cells, stably transfected with human apolipoprotein (apo) E3 and apoE4 were described previously (Huang et al. 1998). C6 cells (SBE-SEAP/C6) and Tgfb1−/− fibroblasts (MFB-F11) stably transfected with a SBE-SEAP reporter gene were generated in our lab (Tesseur et al. 2006).
Mice expressing human apolipoprotein E3 in astrocytes of Apoe−/− mice (GFAP-apoE3/Apoe−/−; Raber et al. 2002) were obtained from Dr. L. Mucke (Gladstone Institutes San Francisco, University of California, San Francisco). Apoe−/− mice (C57BL/6J-Apoetm1Unc) were obtained from Jackson Laboratory (Bar Harbor, ME). Mice lacking one (Tgfb1−/+) or both (Tgfb1−/−) copies of the Tgfb1 gene ((Bonyadi et al. 1997)) were obtained from Dr. R. Akhurst (University of California, San Francisco).
Recombinant human TGF-β1 (rhTGF-β1) and recombinant latent human TGF-β1 (rSLC) were from R&D (Minneapolis, MN). Cholesterol was measured with Infinity cholesterol liquid reagent (ThermoTRACE, Melbourne, Australia).
To detect apoE, TGF-β and LAP, we tested a panel of antibodies and confirmed their specificity with ELISA and western blot (Supplementary Fig. 3). To detect ApoE we used polyclonal anti-apoE (KW, Gladstone Institutes, CA) and anti-human apoE (Calbiochem, La Jolla, CA), to detect TGF-β1 we used BAF240 and 1D11 (R&D, Minneapolis, MN), and to detect LAP1 we used MAB2461 and BAM2462 (R&D, Minneapolis, MN).
Cell culture
All tissue culture reagents were from Invitrogen (Carlsbad, CA). Cells were maintained in DMEM, 10% FBS, penicillin and streptomycin (D10). For conditioned media McA/RH-7777 cells expressing human apoE3 or apoE4 were seeded at 7.5 106 cells/10 cm2 plate in D10, allowed to attach, washed with PBS, and given 5 ml serum free DMEM containing penicillin and streptomycin. After 24 h conditioned medium was collected, centrifuged and transferred to a new tube.
Primary astrocyte culture
3-day old GFAP-apoE3/Apoe−/−, Apoe−/− and Tgfb1−/− pups were obtained from crosses between GFAP-apoE3/Apoe−/− mice with Apoe−/− mice, and from crosses between heterozygous Tgfb1−/+ mice. Pups were decapitated and cortex and hippocampus were dissected in HBSS medium. Tissue was cut into smaller pieces, trypsinized for 15 minutes at 37°C, washed with HBSS and further dissociated by pipetting up and down with pasteur pipettes. 10 ml DMEM supplemented with 10% FBS, Penicillin, Streptomycin, and Fungizone (D10F) was added, cell suspensions were transferred to T75 flasks and incubated at 37°C, 5% CO2. The next morning cells were washed with PBS and given fresh D10F. Individual cultures were genotyped by subjecting proteinase K-digested pup-tail tissue to touchdown PCR (Hecker and Roux 1996). When cells reached confluency they were split into T125 flasks.
T125 flasks with confluent astrocytes were incubated with 15 ml DMEM medium containing penicillin and streptomycin. After 24 h or 5 days, conditioned medium was collected, centrifuged, concentrated and separated by FPLC.
Gel filtration chromatography (FPLC)
Fast performance liquid gel-chromatography (FPLC) separates lipoproteins by size without disrupting their structure (Ordovas and Osgood 1998). Primary astrocyte or McA/RH-7777 cell conditioned media were concentrated 10-fold (Centricon-10, Amicon, Beverly, MA). 200 μl concentrated conditioned medium or undiluted human or mouse plasma was fractionated by gel filtration chromatography on a Superose-6 column (GE Healthcare, Piscataway, NJ) using PBS, 1 mM EDTA buffer at a flow rate of 4 ml/minute. 500 μl fractions were collected and analyzed for TGF-β bioactivity and for TGF-β1, LAP1, apoE and cholesterol contents.
Immunoelectron microscopy
Immunoelectron microscopy was performed with purified HDL particles obtained by density gradient ultracentrifugation. Purified HDL was placed on a carbon-coated electron microscopic grid. Nonspecific binding was blocked by incubation in PBS with 1% bovine plasma albumin (BSA) for 10 minutes. The grids were then placed on a droplet of PBS + 0.1% BSA, containing a TGF-β1-specific antibody (R&D, 500 ng/ml) or normal mouse IgG for 60 minutes, and washed with seven droplets of PBS, 1 minute each. The grids were placed on a droplet of avidin conjugated to 5-nm colloidal gold particles for 60 min (EY lab., San Mateo, CA; diluted 1: 20 in PBS, 0.1% BSA), passed over seven droplets of washing solution (PBS), and passed over another seven droplets of distilled water. Specimens were then negatively stained with 2% uranylacetate for 10 min, followed by incubation with a lead-staining solution for 5 min and observed using a transmission electron microscope (JEOL JEM-1200EX).
TGF-β bioactivity assay
This assay is a sensitive and specific assay to measure bioactive TGF-β via activation of latent TGF-β with acid and subsequent measurement of the activated TGF-β with MFB-F11 reporter cells (Tesseur et al. 2006). The amount of bioactive TGF-β is determined based on the induction level of the reporter above baseline (fold induction). Briefly, MFB-F11 cells were seeded in D10 at 4×104 cells/well in 96-well plates (Corning Inc., Corning, NY) and allowed to attach. The next day cells were washed with PBS and incubated in serum-free DMEM for 1-3 h. 2.5 μl 6M HCl was added to 150 μl sample. After 15 minutes samples were neutralized with 6M NaOH/1MHEPES. 50μl of the treated sample was added in triplicate to MFB-F11 cells. After 24 h, 10 μl supernatant was collected in 96 well plates and SEAP (Secreted Alkaline Phosphatase) activity was measured using Great EscApe SEAP Chemiluminescence kit 2.0 (BD biosciences, San Jose, CA) and a Lmax Microplate Luminometer (Molecular Devices, Sunnyvale, CA).
Immunoblotting
Equal amounts of fractionated sample were subjected to SDS-PAGE using 10% Tris-Glycine gels under reducing conditions, transferred to nitrocellulose membranes (Biorad, Hercules, CA) and probed with antibodies against human (Calbiochem, 1:8000), or mouse (mGoE, 1:2000) apolipoprotein E, followed by peroxidase-conjugated species-specific secondary antibodies. Binding of secondary antibodies was visualized by enhanced chemiluminescence (ECL, GE Healthcare, Piscataway, NJ).
TGF-β1 ELISA
96-well plates (Corning Inc., Corning, NY) were coated overnight at room temperature with 100 ng 1D11 (R&D, Minneapolis, MN) in PBS, and blocked with 5% Tween-20, 5% sucrose and 0.05% NaAz in PBS. Before loading, 500 μl sample was incubated for 10 minutes with 100 μl 1M HCl and neutralized with 100 μl 1.2N NaOH/0.5MHEPES. rhTGF-β1 (R&D, Minneapolis, MN) was used to prepare the standard curve. TGF-β1 was detected with BAF240 (400 ng/ml, R&D, Minneapolis, MN) followed by streptavidin-HRP (1:4000, Vector, Burlingame, CA) and 100 μl substrate solution (R&D, Minneapolis, MN). Reaction was stopped with 50 μl 1M H2SO4 and plates were read at 450 nm.
LAP1 ELISA
96-well plates were coated overnight at room temperature with 200 ng MAB2461 (R&D, Minneapolis, MN) in PBS, and blocked with 1% Block-Ace (Serotec, NC), 0.05% NaAz in PBS. Samples were loaded onto the plates and LAP1 was detected with BAM2462 (125 ng/ml, R&D, Minneapolis, MN), followed by Streptavidin-HRP (1:4000, Vector, Burlingame, CA) and 100 μl substrate solution. Reaction was stopped with 50 μl 1M H2SO4 and plates were read at 450 nm. RhLTGF-β1 (R&D, Minneapolis, MN) was used to prepare the standard curve.
Solid-phase IP
96-well plates were coated overnight at room temperature with anti-apolipoprotein E antibody (1:1000, Karl Weisgraber) in PBS. Plates were blocked with 5% Tween-20, 5% sucrose, 0.05% NaAz in PBS. Native or acid activated (see TGF-β1 ELISA) LDL/HDL1 fractions of astrocyte-conditioned medium were loaded on the plates. Antibody BAF240 (400 ng/ml, R&D, Minneapolis, MN) followed by streptavidin-HRP (1:4000, Vector, Burlingame, CA) and 100 μl substrate solution (R&D, Minneapolis, MN) was used to detect TGF-β1. Reaction was stopped with 50 μl 1M H2SO4 and plates were read at 450 nm.
Avidin-Biotin IP
Conditioned medium of apoE3 expressing McA/RH-7777 cells was incubated with biotinylated anti-apoE (Calbiochem, San Diego, CA), or biotinylated anti-TGF-β1 (R&D, Minneapolis, MN) antibody for 2 h at room temperature, and bound to monomeric Avidin agarose depending on the antibody used for IP.
ApoE3- and apoE4-containing Lipid Particle Production
Conditioned medium of apoE3 and apoE4 expressing McA/RH-7777 cells was incubated with 150 μg biotinylated monoclonal anti-apoE 2E8 (Karl Weisgraber, Gladstone Institute, UCSF, CA) for 5 h at room temperature and bound to monomeric Avidin agarose using the Immobilized Monomeric Avidin Kit (Pierce, Rockford, IL) according to manufacturers instructions. The gentle elution method at neutral pH with excess biotin allows for intact lipoprotein purification. The purified eluate was then concentrated using Centricon-10 (Millipore, Billerica, MA) and washed twice with equal amounts of DPBS (Invitrogen, Carlsbad, CA). The concentrated eluate was then used to detect TGF-β bioactivity via the MFB-F11 cell assay. ApoE protein levels were detected via immunoblotting in 6 μl purified eluate.
Computer Analysis
The amino acid sequence of human TGF-β1 was analyzed for helices using the program WHEEL and the amphipathic characteristic of identified helices were classified using COMBO, COMMET, and CONSENSUS (Segrest et al. 1992). TGF-β1 modeling was based on the previously published NMR structure (Hinck et al. 1996). Amphipathic helices identified in the primary sequence were mapped onto the NMR average structure of TGF-β1 (PDB code 1KLC). Similar results were obtained by mapping amphipathic helices onto the entire NMR ensemble (PDB codes 1KLA and 1LKD). Contiguous exposed surface patches were manually identified by visualization with PyMOL (http://pymol.org).
Statistical analysis
Differences between two means were assessed by Mann-Whitney U or Student’s t-test for nonparametric or parametric data, respectively. Differences among multiple means of data with parametric distribution were assessed by ANOVA followed by Tukey-Kramer post hoc test. Statistical analyses were performed with Statview 5.0 software (SAS Institute, Cary, NC) and GraphPad Prism 4.03 (GraphPad Software, Inc. La Jolla, CA).
Supplementary Material
Supplementary figure 1 Alpha-2-Macroglobulin co-elutes with lipoproteins in human plasma. Human plasma from three different healthy donors was fractionated on Superose-6 by FPLC. Alpha-2-Macroglobulin protein was detected via western blotting of aliquots of individual fractions collected by FPLC. All Western blots were performed at the same time.
Supplementary figure 2 Lipoprotein fractions with highest relative bioactivity levels contain mature TGF-β1. Recombinant SLC or Conditioned medium of McA/RH-7777 rat liver cells stably expressing human apoE3 was fractionated on superose-6. TGF-β bioactivity was measured via the TMLC bioassay (A, C), and LAP1 protein levels were measured via ELISA (B).
Supplementary figure 3 Example of western blots performed to test antibody specificity. Purified platelet derived human TGF-β1 (hTGF-β1), recombinant TGF-β1 (rTGF-β1), LAP1 (rLAP1) and apoE3 (rApoE3) and apoE4 (rApoE4) were separated on polyacrylamide gels, blotted onto nitrocellulose membranes and detected with the indicated antibodies.
ACKNOWLEDGEMENTS
We would like to thank Elisabeth Berber, Will Segal and Tiffany Seto for technical assistance, Dr. Lennart Mucke (University of California, San Francisco, San Francisco, CA) for providing us with the GFAP-apoE3 and GFAP-apoE4 mice, Dr. G.M. Anantharamiah (University of Alabama, Birmingham, AL) for identifying and classifying the amphipathic helices in TGF-β, and Dr. John Munger (New York University Medical Center, New York, NY) for TGF-β and LAP-specific antibodies. This research was funded by NIH grants NS40994 and AG23708 (TWC), the John Douglas French Alzheimer’s Foundation (IT) and the Alzheimer’s Association (IT).
Footnotes
COMPETING INTERESTS STATEMENT The authors declare no competing financial interests.
REFERENCES
- Alberts MJ, Graffagnino C, McClenny C, DeLong D, Strittmatter W, Saunders AM, Roses AD. ApoE genotype and survival from intracerebral haemorrhage. Lancet. 1995;346:575. doi: 10.1016/s0140-6736(95)91411-0. [DOI] [PubMed] [Google Scholar]
- Bonyadi M, Rusholme SAB, Cousins FM, Su HC, Biron CA, Farrall M, Akhurst RJ. Mapping of a major genetic modifier of embryonic lethality in TGFβ1 knockout mice. Nat. Genet. 1997;15:207–211. doi: 10.1038/ng0297-207. [DOI] [PubMed] [Google Scholar]
- Brionne TC, Tesseur I, Masliah E, Wyss-Coray T. Loss of TGF-beta1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40:1133–1145. doi: 10.1016/s0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- Cabello-Verrugio C, Brandan E. A novel modulatory mechanism of transforming growth factor-beta signaling through decorin and LRP-1. J Biol Chem. 2007;282:18842–18850. doi: 10.1074/jbc.M700243200. [DOI] [PubMed] [Google Scholar]
- Chen CL, Huang SS, Huang JS. Cholesterol modulates cellular TGF-beta responsiveness by altering TGF-beta binding to TGF-beta receptors. J Cell Physiol. 2008;215:223–233. doi: 10.1002/jcp.21303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dubois CM, Blanchette F, Laprise MH, Leduc R, Grondin F, Seidah NG. Evidence that furin is an authentic transforming growth factor-beta1-converting enzyme. Am J Pathol. 2001;158:305–316. doi: 10.1016/s0002-9440(10)63970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Holtzman DM, Munson G, Mathur T, Schneider D, Chang LK, Getz GS, Reardon CA, Lukens J, Shah JA, LaDu MJ. Unique lipoproteins secreted by primary astrocytes from wild type, apoE (-/-), and human apoE transgenic mice. J Biol Chem. 1999;274:30001–30007. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Bartlett PF, Marzella PL. LIF is more potent than BDNF in promoting neurite outgrowth of mammalian auditory neurons in vitro. Neuroreport. 2001;12:275–279. doi: 10.1097/00001756-200102120-00019. [DOI] [PubMed] [Google Scholar]
- Grainger DJ, Byrne CD, Witchell CM, Metcalfe JC. Transforming growth factor β is sequestered into an inactive pool by lipoproteins. J. Lipid Res. 1997;38:2344–2352. [PubMed] [Google Scholar]
- Hart PJ, Deep S, Taylor AB, Shu Z, Hinck CS, Hinck AP. Crystal structure of the human TβR2 ectodomain-TGF-β3 complex. Nat. Struct. Biol. 2002;9:203–208. doi: 10.1038/nsb766. [DOI] [PubMed] [Google Scholar]
- Hecker KH, Roux KH. High and low annealing temperatures increase both specificity and yield in touchdown and stepdown PCR. BioTechniques. 1996;20:478–485. doi: 10.2144/19962003478. [DOI] [PubMed] [Google Scholar]
- Hinck AP, Archer SJ, Qian SW, Roberts AB, Sporn MB, Weatherbee JA, Tsang ML-S, Lucas R, Zhang B-L, Wenker J, Torchia DA. Transforming growth factor β1: Three-dimensional structure in solution and comparison with the x-ray structure of transforming browth factor β2. Biochemistry. 1996;35:8517–8534. doi: 10.1021/bi9604946. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu XQ, Rall SC, Jr., Taylor JM, von Eckardstein A, Assmann G, Mahley RW. Overexpression and accumulation of apolipoprotein E as a cause of hypertriglyceridemia. J Biol Chem. 1998;273:26388–26393. doi: 10.1074/jbc.273.41.26388. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Sebald W, Dreyer MK. Crystal structure of the BMP-2–BRIA ectodomain complex. Nat. Struct. Biol. 2000;7:492–496. doi: 10.1038/75903. [DOI] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- McCarron MO, Muir KW, Weir CJ, Dyker AG, Bone I, Nicoll JAR, Lees KR. The apolipoprotein E ε4 allele and outcome in cerebrovascular disease. Stroke. 1998;29:1882–1887. doi: 10.1161/01.str.29.9.1882. [DOI] [PubMed] [Google Scholar]
- Ordovas JM, Osgood D. Preparative isolation of plasma lipoproteins using fast protein liquid chromatography (FPLC) Methods Mol Biol. 1998;110:105–111. doi: 10.1385/1-59259-582-0:105. [DOI] [PubMed] [Google Scholar]
- Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J Neurosci. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren RF, Flanders KC. Transforming growth factors-β protect primary rat hippocampal neuronal cultures from degeneration induced by β-amyloid peptide. Brain Res. 1996;732:16–24. doi: 10.1016/0006-8993(96)00458-1. [DOI] [PubMed] [Google Scholar]
- Ren RF, Hawver DB, Kim RS, Flanders KC. Transforming growth factor-β protects human hNT cells from degeneration induced by β-amyloid peptide: Involvement of the TGF-β type II receptor. Mol. Brain Res. 1997;48:315–322. doi: 10.1016/s0169-328x(97)00108-3. [DOI] [PubMed] [Google Scholar]
- Saharinen J, Keski-Oja J. Specific sequence motif of 8-Cys repeats of TGF-β binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-β. Mol. Biol. Cell. 2000;11:2691–2704. doi: 10.1091/mbc.11.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AM, Schmader K, Breitner JCS, Benson MD, Brown WT, Goldfarb L, Goldgaber D, Manwaring MG, Szymanski MH, McCown N, Dole KC, Schmechel DE, Strittmatter WJ, Pericak-Vance MA, Roses AD. Apolipoprotein E ε4 allele distributions in late-onset Alzheimer’s disease and in other amyloid-forming diseases. Lancet. 1993;342:710–711. doi: 10.1016/0140-6736(93)91709-u. [DOI] [PubMed] [Google Scholar]
- Schurgers LJ, Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta. 2002;1570:27–32. doi: 10.1016/s0304-4165(02)00147-2. [DOI] [PubMed] [Google Scholar]
- Segrest JP, Jones MK, De Loof H, Brouillette CG, Venkatachalapathi YV, Anantharamaiah GM. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J Lipid Res. 1992;33:141–166. [PubMed] [Google Scholar]
- Singh NN, Ramji DP. The role of transforming growth factor-beta in atherosclerosis. Cytokine Growth Factor Rev. 2006;17:487–499. doi: 10.1016/j.cytogfr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Engchild J, Salvesen GS, Roses AD. Apolipoprotein E: High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Zou K, Berber E, Zhang H, Wyss-Coray T. Highly sensitive and specific bioassay for measuring bioactive TGF-beta. BMC Cell Biol. 2006;7:15. doi: 10.1186/1471-2121-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber MG, Rallis M, Podda M, Weber C, Maibach HI, Packer L. Penetration and distribution of alpha-tocopherol, alpha- or gamma-tocotrienols applied individually onto murine skin. Lipids. 1998;33:87–91. doi: 10.1007/s11745-998-0183-0. [DOI] [PubMed] [Google Scholar]
- Tseng WF, Huang SS, Huang JS. LRP-1/TbetaR-V mediates TGF-beta1-induced growth inhibition in CHO cells. FEBS Lett. 2004;562:71–78. doi: 10.1016/S0014-5793(04)00185-1. [DOI] [PubMed] [Google Scholar]
- Ulich TR, Yin S, Guo K, Yi ES, Remick D, del Castillo J. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. Am J Pathol. 1991;138:1097–1101. [PMC free article] [PubMed] [Google Scholar]
- Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Yan F, Yu G, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-β1 promotes microglial amyloid-β clearance and reduces plaque burden in transgenic mice. Nat. Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yang G-Y, Ahlemeyer B, Pang L, Che X-M, Culmsee C, Klumpp S, Krieglstein J. Transforming growth factor-β1 increases bad phosphorylation and protects neurons against damage. J. Neurosci. 2002;22:3898–3909. doi: 10.1523/JNEUROSCI.22-10-03898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1 Alpha-2-Macroglobulin co-elutes with lipoproteins in human plasma. Human plasma from three different healthy donors was fractionated on Superose-6 by FPLC. Alpha-2-Macroglobulin protein was detected via western blotting of aliquots of individual fractions collected by FPLC. All Western blots were performed at the same time.
Supplementary figure 2 Lipoprotein fractions with highest relative bioactivity levels contain mature TGF-β1. Recombinant SLC or Conditioned medium of McA/RH-7777 rat liver cells stably expressing human apoE3 was fractionated on superose-6. TGF-β bioactivity was measured via the TMLC bioassay (A, C), and LAP1 protein levels were measured via ELISA (B).
Supplementary figure 3 Example of western blots performed to test antibody specificity. Purified platelet derived human TGF-β1 (hTGF-β1), recombinant TGF-β1 (rTGF-β1), LAP1 (rLAP1) and apoE3 (rApoE3) and apoE4 (rApoE4) were separated on polyacrylamide gels, blotted onto nitrocellulose membranes and detected with the indicated antibodies.