Summary
Lupus-prone NZB/W F1 mice develop glomerulonephritis after T helper cell dependent isotype switching of autoantibody secretion from IgM to IgG at about six months of age. We compared innate immune natural killer (NK) T cells and conventional T cells for their capacity to help spontaneous in vitro immunoglobulin and autoantibody secretion of innate immune (B-1 and marginal zone) and conventional (follicular) B cell subsets from NZB/W F1 mice. We found that purified NKT cells not only increased spontaneous secretion of IgM and IgM anti-dsDNA antibodies by B-1 and marginal zone B cells, but also facilitated secretion of IgG anti-dsDNA antibodies predominantly by B-1 B cells. Little IgM or IgG anti-dsDNA antibodies was secreted by follicular B cells, and conventional T cells failed to provide potent helper activity to any B cell subset. All combinations of T and B cell subsets from normal C57BL/6 mice failed to generate vigorous IgM and IgG secretion. NZB/W NKT cell helper activity was blocked by anti-CD1 and anti-CD40L mAbs. In conclusion, direct interactions between innate immune T and B cells form a pathway for the development of IgM and IgG lupus autoantibody secretion in NZB/W mice.
Keywords: B cells, T cells, Systemic lupus erythematosus, Autoantibodies
Introduction
Systemic lupus erythematosus is an autoantibody mediated disease that causes injury to multiple organ systems [1, 2]. In the NZB/W F1 hybrid mouse model of lupus, spontaneous secretion of IgM and IgM anti-double stranded (ds) DNA antibodies by B cells is observed in young mice, and isotype switching to IgG, especially pathogenic IgG2a autoantibodies, is associated with the onset of glomerulonephritis and proteinuria [3-5].
In NZB/W mice, the innate immune B-1 and MZ B cells have been reported to spontaneously secrete IgM and IgM autoantibodies in vitro, and conventional follicular (FO) B cells fail to spontaneously secrete these antibodies [6, 7]. In addition, the B-1 and MZ B cells are expanded in the spleen of NZB/W mice as they age, and their percentages are increased among all B cells as compared to non-autoimmune mice at the expense of the FO B cells [8, 9]. Absolute numbers of NKT cells in multiple organs and B-1 B cells in the peritoneum are also increased in NZB/W mice as compared to non-autoimmune strains [8, 10, 11]. In contrast, B-1 B cells are not expanded in lupus-prone MRL/lpr mice that fail to express the Fas receptor[8]. Initial reports that NKT cells were reduced in lupus prone mouse strains including NZB/W mice as judged by PCR analysis of Vα14 mRNA [12] were contradicted by Lsequent reports that enumerated NKT cells by staining with NK1.1 markers and CD1d tetramers [10, 11, 13]
Since NKT cells can interact with B cells via the recognition of B cell surface CD1d by the invariant Vα14 TCR, this interaction may contribute to helper activity for NZB/W B cells to secrete immunoglobulins and autoantibodies [7, 10, 13-17]. Transfer of transgenic T cells that recognize CD1d receptors can induce lupus in BALB/c nu/nu mice, and treatment of lupus-prone NZB/W mice with anti-CD1d mAb ameliorated lupus disease activity [7, 13, 18]. The ability of freshly isolated CD4+ T cells from NZB/W mice to help spontaneous IgM and IgM anti-dsDNA antibody secretion of NZB/W B cells in vitro was lost when NKT cells were depleted, even though the latter cells accounted for about 5% of the CD4+ T cells [13]. On the other hand, NKT cells purified with a CD1d-tetramer reagent provided potent T cell helper activity for the IgM and IgM anti-dsDNA secretion [13]. However, no helper activity was detected for isotype switching to total IgG1, total IgG2a or IgG anti-dsDNA antibody secretion in these 5 day cultures [13].
Switching to IgG isotypes is critical for development of glomerulonephritis [4, 5], and IgG2a anti-dsDNA antibodies have been reported to bind to the glomerular basement membrane and cause glomerular injury [2, 3, 5]. In view of the importance of the IgG antibodies, we modified the culture system in the current study and extended the duration of the culture period from 5 to 10 days to determine whether NZB/W NKT cells and/or conventional T cells can provide help for IgG switching of NZB/W B cells. In addition, our previous studies [13] did not determine which Lset of NZB/W B cells, innate immune B-1 and MZ B cells, and/or adaptive immune FOB cells can interact with NKT cells to secrete immunoglobulins and autoantibodies. In the current study, we found that by using the modified culture system, the NKT cells provided potent help for secretion of IgG1, IgG2a and IgG anti-dsDNA antibodies. Among the B-1, MZ, and FOB cell subsets, the B-1 B cells were induced by the NKT cells to secrete the highest levels of IgG anti-dsDNA antibodies. Surprisingly, conventional T cells failed to help IgM or IgG secretion of the B-1, MZ, and FO B cells. NKT cell help was blocked by anti-CD1d and anti-CD40L mAbs.
Results
α-galactosylceramide (α-GalCer) activation of NKT cells requires antigen presenting cells (APC's) in C57BL/6 but not NZB/W mice
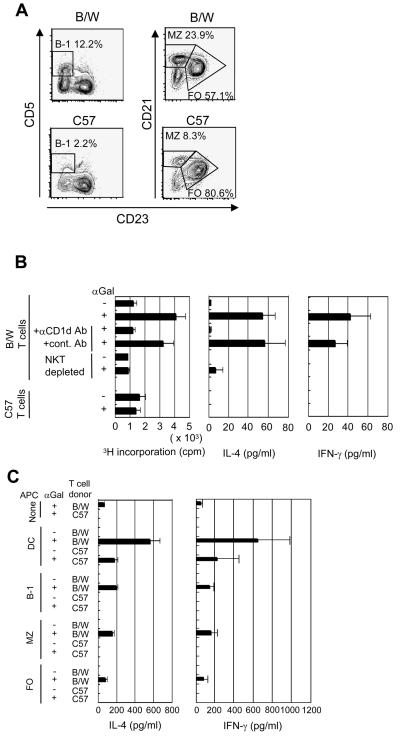
In non-autoimmune strains of mice, APC's such as dendritic cells or B cells are required to induce NKT cell activation in vitro with the stimulatory ligand, α-GalCer, associated with CD1d [19]. In the current study, we compared the APC requirements for NKT cell activation in non-autoimmune C57BL/6 mice, and in lupus-prone NZB/W mice. Purified C57BL/6 or NZB/W T cells were stimulated with 100 ng/ml α-GalCer, a concentration previously determined to provide optimum activation of NKT cells in the presence of APC's. Gated CD11c+TCRαβ−B220−NK1.1− cells were used as the source of dendritic cells, and gated TCRαβ+CD11c−B220− cells were used as the source of T cells. B cell subsets were separated by gating on B220+ cells, and identifying B-1 B cells as CD5+CD23lo, MZ B cells as CD23 loCD21hi, and FO B cells as CD23hiCD21lo cells (Fig. 1A).
Figure 1. Different requirements of APC's for in vitro activation of NKT cells from NZB/W and C57BL/6 mice.
(A) CD5 or CD21 versus CD23 expression on gated B220+ spleen cells from NZB/W and C57BL/6 mice. Boxes in left panels enclose CD5+CD23 lo (B-1) B cells, and boxes in right panels enclose CD21hiCD23lo (MZ) B cells or CD21loCD23hi (FO) B cells. Percentages of cells in each box are shown. B cell subsets were sorted based on thresholds for each box. (B) 3H-thymidine incorporation in supernatants of 72 hour cultures, and concentration of IL-4 and IFN-γ in supernatants of 48 hour cultures containing 2×105 purified T cells with and without stimulation by 100 ng/ml α-GalCer. Bars show mean and brackets show standard errors of pooled data from two to three replicate experiments with triplicate wells in each experiment. In some cultures, anti-CD1d or control mAbs were added for blocking, and in others T cells were depleted of CD1d-tetramer+ cells before culture. (C) IL-4 and IFN-γ concentrations in supernatants from 48 hour cultures of purified T cells alone, purified T cells and B cell subsets or dendritic cells with or without α-GalCer stimulation. Means and standard errors are from pooled data as described above.
NKT cell dependent activation was measured by 3H-thymidine incorporation at 72 hours, and by secretion of IL-4 and IFN-γ in culture supernatants at 48 hours. C57BL/6 T cells showed no significant difference in 3H-thymidine incorporation with or without α-GalCer in the medium (Fig. 1B). Unexpectedly, NZB/W purified T cells increased 3H-thymidine incorporation four fold (p=0.004) above background after stimulation with α-GalCer. The proliferative response was reduced to background level when the purified T cells were depleted of NKT cells using CD1d-tetramer staining and sorting, or when anti-CD1d mAb was added to the purified T cell cultures (Fig. 1B). The results show that the proliferative response was dependent on NKT cell activation, but we did not determine the phenotype of the proliferating cells.
The ability of α-GalCer to activate NKT cells from NZB/W but not C57BL/6 mice in the absence of APC's was reflected also in the secretion of IL-4 and IFN-γ. Purified C57BL/6 T cells secreted no detectable cytokines in the culture supernatant with or without α-GalCer stimulation, and NZB/W T cells secreted about 50 pg/ml of IL-4 (p<0.0001) and 40 pg/ml IFN-γ (p=0.04) in cultures with α-GalCer as compared to background (Fig. 1B). NZB/W T cell dependent_cytokine secretion was reduced to background when anti-CD1d mAb was added to the cultures or when NKT cells were depleted.
Addition of purified dendritic cells to cultures with T cells and α-GalCer markedly increased cytokine secretion such that supernatants from C57BL/6 cells contained about 200 pg/ml IL-4 and IFN-γ, and supernatants from NZB/W cells contained about 600 pg/ml of both cytokines (Fig. 1C). Addition of purified B-1, MZ, or FO B cells from C57BL/6 mice could not Lstitute for the dendritic cells in facilitating C57BL/6 NKT cell dependent secretion of cytokines in response to α-GalCer stimulation, but addition of B-1 and MZ B cells from NZB/W mice to the NZB/W T cell cultures significantly increased IL-4 cytokine secretion (IL-4: B-1, p<0.0001, MZ B, p=0.008, IFN-γ: B-1, p=0.04, MZ B, p=0.07) (Fig. 1C). Thus the requirements of NZB/W NKT cell dependent activation differed from that of C57BL/6 NKT cells in that only the former was activated by α-GalCer alone with augmentation in the presence of B cells.
Purified NZB/W NKT cells help NZB/W B cells to spontaneously secrete IgM, IgM anti-dsDNA antibodies, IgG, and IgG anti-dsDNA antibodies
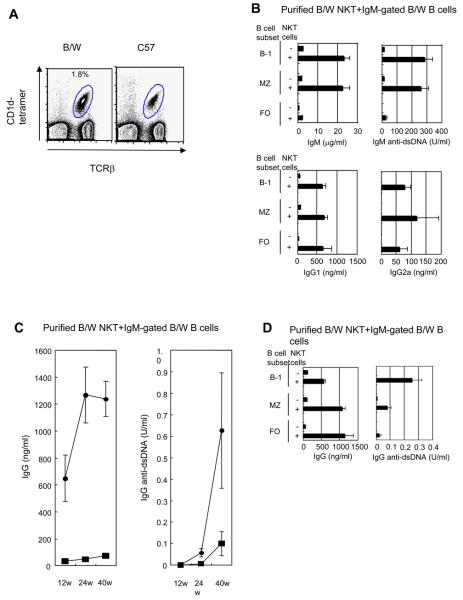
Since purified NZB/W NKT cells have been reported to augment the IgM but not IgG secretion of NZB/W total B cells in vitro in 5 day cultures [10, 13], we measured immunoglobulin secretion from purified NZB/W B cell subsets for both IgM and IgG after co-culture with or without purified NKT cells for 10 days. We reasoned that switching from IgM to IgG secretion in vitro may require an increase in the duration of the cultures. Splenic NKT cells were identified as TCRαβ+CD1d-tetramer+ cells shown in Figure 2A, and there was an increased percentage (~2%) in the spleen of NZB/W as compared to C57BL/6 mice (~1%). The TCRαβ+CD1d-tetramer+ cells were invariant NKT cells, since the CD1d-tetramer reagent identifies the invariant Vα14 TCR [20]. About 95% of the sorted NKT cells from NZB/W mice were CD4+, and about 88% from C57BL/6 mice were CD4+ (data not shown). Purified B cell subsets for these experiments were first enriched using anti-CD19 mAb beads, and then gated on IgM+ cells instead of B220+ cells in order to assess immunoglobulin isotype switching to IgG.
Figure 2. NKT cells provide helper activity for spontaneous antibody secretion and isotype switching of NZB/W B cell subsets.
(A) CD1d-tetramer versus TCRαβ staining of spleen cells. NKT cells (CD1d-tetramer+TCRαβ+) are enclosed in ellipses, and percentages of enclosed cells are shown. Sorting of NKT cells was based on thresholds for enclosed cells. (B) Mean concentrations of IgM, IgM anti-dsDNA, IgG1, and IgG2a antibodies in supernatants from 10 day cultures of 1×105 sorted NZB/W B cell subsets with and without sorted 5×104 NKT cells in 6-10 experiments. (C) Mean concentrations of total IgG and IgG anti-dsDNA antibodies in cultures of sorted total IgM+ B cells from 12, 24, and 40 week old NZB/W mice with or without the addition of sorted NKT cells. (■)-B cells alone, (●)-B and NKT cells. Data are from 2 to 8 cultures at each time point. (D) Total IgG and IgG anti-dsDNA antibodies in cultures of sorted B cell subsets from 24 week old NZB/W mice with or without the addition of NKT cells.
Co-culture of NZB/W NKT cells with B-1 B cells and MZ B cells increased IgM and IgM anti-dsDNA antibody secretion about 11-14 fold (p= <0.0001 to 0.003) and about 23 fold (p=0.0004 to 0.0005) respectively (Fig. 2B). FO B cells secreted background levels of IgM and IgM anti-dsDNA antibodies after culture with NKT cells. Furthermore, all B cell subsets that were co-cultured with NKT cells secreted over 500 ng/ml of IgG1 and about 60-120 ng/ml IgG2a. The IgG levels were significantly increased (p= <0.0001 to 0.03), as compared to cultures with B cell subsets alone. These results indicated that NKT cells provide help for the B cell subsets to switch to IgG secretion (Fig. 2B). The lack of augmentation of IgM secretion of FO B cells by NKT cells may be due to either an inability to secrete IgM in the culture system, or a more rapid switching to IgG secretion induced by NKT cells.
Since the concentrations of IgM in cultures of NKT cells and B-1 or MZ B cells from 12 week old NZB/W mice were considerably higher than that of IgG, and since IgG anti-dsDNA antibodies were not detected (less than 0.01 U/ml), we determined whether secretion of IgG antibodies would increase using cells from older NZB/W mice. Accordingly, 2×105 sorted IgM+ total B cells were cultured with 5×104 NKT cells from 12, 24, or 40 week old mice. Figure 2C shows the total IgG and IgG anti-dsDNA antibody concentrations of cultures of the sorted B cells with or without the sorted NKT cells. Supernatants from cultures of the B cells alone contained less than 80 ng/ml of total IgG using mice up to 40 week old. When NKT cells were cultured with B cells, then there was an increase in IgG concentrations at 24 and 40 weeks to about 1200 ng/ml. The increases at all time points were significant (p= <0.0001 to 0.001). Although secretion of IgG anti-dsDNA antibodies were less than 0.01 U/ml in NKT and B cell cultures at 12 weeks of age, these antibodies were detected at 24 weeks of age (0.06 U/ml) and increased about 10 fold to about 0.6 U/ml at 40 weeks of age (Fig. 2C). Cultures of B cells alone from mice at 24 and 40 weeks contained 0.01 and 0.1 U/ml of IgG anti-dsDNA antibodies, respectively. The increased antibody level after addition of NKT cells at 24 weeks was significant (p=0.03).
In further studies, we examined the ability of NKT cells to augment IgG anti-dsDNA antibody secretion from purified B cell subsets using 24 week old NZB/W mice. As shown in Figure 2D, NKT cells significantly increased total IgG secretion from all B cell subsets (p= <0.0001 to 0.001). Interestingly, the IgG anti-dsDNA antibody secretion by the combination of NKT cells and B-1 B cells was more than ten fold higher than that of NKT cells and FO B cells (p=0.006), and significantly higher than that of NKT cells and MZ B cells (p=0.04).
Purified conventional T cells fail to help NZB/W B cells to secrete IgM, IgM anti-dsDNA antibodies, and IgG
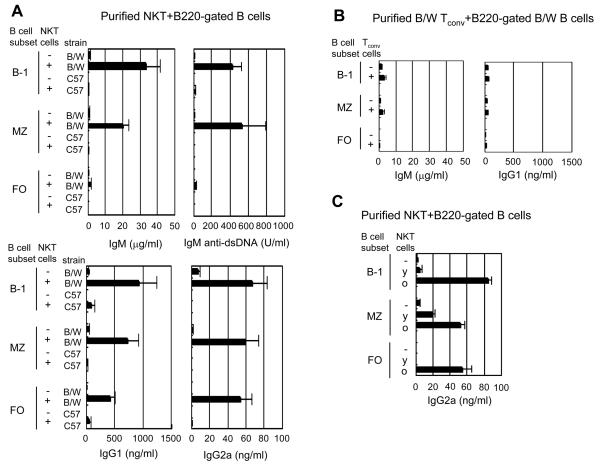
In further studies, we compared the ability of conventional (non-NK) T cells and NKT cells from NZB/W and C57BL/6 mice to help B cell secretion of immunoglobulin and autoantibodies. In these experiments, B cell subsets were sorted using B220 gating instead of IgM gating in order to remove the possible contribution of crosslinking of IgM receptors to the spontaneous B cell activation. Purified NZB/W NKT cells co-cultured with NZB/W B-1 B cells or MZ B cells secreted markedly increased amounts of IgM (p= <0.0001 to 0.001) and IgM anti-dsDNA antibodies (p=0.0007 to 0.03) (Fig. 3A) as compared to cultures of B cell subsets alone. Although FO B cells plus NKT cells secreted low levels of IgM and IgM anti-dsDNA antibodies as compared to the other subsets, all the NZB/W B cell subsets cultured with NKT cells secreted significantly increased amounts of IgG1 (p=0.0003 to 0.008) and IgG2a (p=0.0003 to 0.002) that were above 400 ng/ml and 50 ng/ml respectively (Fig. 3A). In contrast, cultures of purified NKT cells and B cells subsets from C57BL/6 mice contained less than 0.6 μg/ml IgM, less than 15 U/ml IgM anti-dsDNA antibodies, less than 80 ng/ml IgG1, and undetectable levels of IgG2a secretion (Fig. 3A). We did not assay for IgG2c, the predominant IgG2 isotype in C57BL/6 mice. In order to determine whether sorting C57BL/6 B cells with anti-IgM instead of anti-B220 mAb could enhance the ability of B cells to secrete IgM and IgG after interaction with C57BL/6 NKT cells, we repeated the above experiments using IgM+ gated B cells. The results of the experiments were similar to those with B220+ gated B cells, and secretion of IgM and IgG was minimal (≤1μg/ml IgM, <10 ng/ml IgG1, <3 ng/ml IgG2a). The results indicate that autoimmune abnormalities in both NZB/W NKT cells and B cells are likely to contribute to the high levels of spontaneous immunoglobulin secretion.
Figure 3. NKT cells but not conventional T cells provide help for spontaneous antibody secretion of B cells from NZB/W mice.
(A) Concentrations of IgM, IgM anti-dsDNA, IgG1, and IgG2a antibodies in supernatants from 10 day cultures of 1×105 C57BL/6 or NZB/W B cell subsets with or without 5×104 NKT cells. Results are pooled data from three to four independent experiments. (B) Concentrations of antibodies from cultures of NZB/W B cell subsets with or without sorted conventional T cells. Results are pooled data from three independent experiments. (C) Concentrations of IgG2a from cultures of 4 week old NZB/W B cell subsets with or without 4 week old (y) or 24 week old (O) NZB/W NK T cells. Results are from three experiments.
We also determined whether NZB/W conventional T cells (non-NKT cells; TCRαβ+CD1d-tetramer−) help NZB/W B cells to secrete immunoglobulins. Cultures of conventional T cells and B cell subsets did not show significantly increased secretion of IgM (p>0.2), or IgG isotypes (IgG1, p= >0.05 to 0.5) as compared to cultures of B cell subsets alone (Fig. 3B). IgG2a was not detected in supernatants from cultures of conventional T cells and B cell subsets, and IgM anti-dsDNA levels were less than 20 U/ml (data not shown). In further experiments, we compared the NKT cell helper activity in young (4 week old) and old (24 week old) NZB/W mice using 4 week old B cells to determine whether the activity is related to age. Figure 3C shows that the helper activity of the old NKT cells was markedly increased (p=0.0002 to 0.009) as compared to young NKT cells for augmenting the secretion of IgG2a from all 4 week old B cell subsets. Interestingly, helper activity for IgM secretion was not significantly different between young and old NKT cells (data not shown).
NKT cell augmentation of antibody secretion by B cells is dependent on interactions with CD1d and CD40
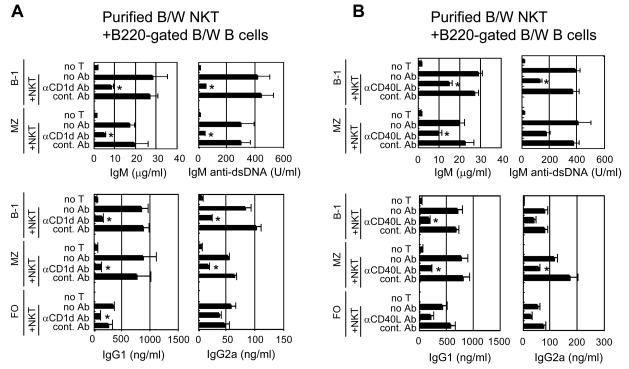
Since the purified NZB/W NKT cells augmented antibody secretion, the dependence of the augmentation on cell-cell interactions via key surface receptors remained to be determined. Accordingly, we added anti-CD1d mAb to the cultures of purified NKT cells and B cell subsets obtained from 12 week old NZB/W mice to inhibit the interaction between the NKT cell TCR and CD1d. As shown in Figure 4A, the addition of the anti-CD1d mAb significantly decreased the concentration of IgM, and IgM anti-dsDNA antibodies secreted by the B-1 B cells (p=0.004 to 0.03), and by the MZ B cells (p=0.003 to 0.02). Similarly, the addition of anti-CD1d mAb significantly reduced IgG1 secretion of all three subsets (p=0.001 to 0.01), and IgG2a secretion of the B-1 and MZ B cells (p=0.0001 to 0.001). No significant reduction (p>0.07) of antibody secretion was observed when isotype matched irrelevant mAb was used instead of anti-CD1d mAb (Fig. 4A).
Figure 4. Anti-CD1d and anti-CD40L mAbs inhibit spontaneous antibody secretion in cultures of NZB/W B cell subsets and NKT cells.
(A) Concentrations of antibodies in culture supernatants from 1×105 sorted NZB/W B cell subsets alone or in combination with 5×104 NKT cells with or without the addition of anti-CD1d or control mAbs. Results are pooled data from three independent experiments. (B) Concentrations of antibodies in 10 day culture supernatants as above with or without the addition of anti-CD40L or control mAbs. Results are pooled data from four independent experiments. Asterisks show cultures in which anti-CD1d or anti-CD40L mAb significantly reduced antibody concentrations compared to cultures with control mAbs.
The interactions between T cells and B cells or APC's that result in disease activity in lupus-prone mice has been shown to be dependent upon the binding of the co-stimulatory ligand CD40L to CD40 [21, 22]. In order to determine the dependence of the NKT cell augmentation of antibody secretion in vitro on this interaction, anti-CD40L mAb was added to the NKT cell/B cell cultures from NZB/W mice. As shown in Figure 4B, the addition of the anti-CD40L mAb significantly reduced IgM secretion of the B-1 and MZ B cells (p=0.0009 to 0.01), as well as the IgM anti-dsDNA antibody secretion of the B-1 B cells (p=0.0004). Although, the MZ B cell secretion of IgM anti-dsDNA was reduced, statistical significance was not achieved (p=0.07). A similar pattern of reduction of IgG1 and IgG2a secretion was found for all B cell subsets, and statistically significant reductions were achieved for IgG1 secretion by B-1 and MZ B cells (p=0.001 to 0.003), and for IgG2a secretion for MZ B cells (p=0.01) (Fig. 4B). Addition of control mAb failed to spontaneously reduce antibody secretion in any of the cultures (p>0.2).
In similar experiments, we attempted to inhibit the secretion of IgM and IgG by adding anti-IL-4, anti-IFN-γ, or anti-IL-10 mAbs to cultures of NZB/W NKT cells and B cell subsets. In contrast to the blocking activity observed with anti-CD1d and anti-CD40L mAbs, we found no significant blocking activity with the anti-cytokine mAbs in any of the culture combinations (data not shown).
Discussion
The results of the present study show that there are abnormalities in the function of both innate immune NKT cells and innate immune B-1 and MZ B cells from lupus-prone NZB/W mice. Purified dendritic cells from C57BL/6 mice were required to activate the C57BL/6 NKT cells in the presence of the NKT cell TCR specific ligand α-GalCer, and all purified C57BL/6 B cell subsets failed to function as APC's to induce activation with α-GalCer. In contrast, NZB/W B-1 and MZ B cells significantly augmented NKT cell activation, and α-GalCer still activated NZB/W NKT cells without B cells or dendritic cells. The results suggest that NZB/W NKT cells are abnormal, and can be activated via ordinarily weak antigen presentation by CD1d on T and B cells.
NZB/W NKT cells markedly augmented the secretion of IgM, IgG, and anti-dsDNA antibodies by NZB/W B cells in vitro in the current study using 10 day cultures. The use of 10 day cultures to study in vitro IgG secretion has been reported before [10]. Our previous study using 5 day cultures showed augmentation of IgM secretion but not IgG [13] because switching to IgG secretion required a longer duration of culture. The ability of young (4 week old) NZB/W NKT cells to help young NZB/W B cells secrete IgG2a in vitro in the current study was minimal, and helper activity became considerably more potent for young B cells when NKT cells were obtained from 6 month old NZB/W mice. This age related change in the function of NZB/W NKT cells may help explain the reported inability of an NZB/W NKT cell line derived from 5 week old mice to help NZB/W B cells to secrete IgM but neither IgG2a nor IgG anti-dsDNA antibodies_in a 10 day culture system [10]. In addition, the NKT cell line was irradiated (3,000 rad) before addition to the culture, whereas the NKT cells used in the current study were fresh unirradiated cells. Secretion of IgG2a anti-dsDNA antibodies in cultures of NZB/W NKT cells and B-1 B cells was also markedly increased when cells from 6 month old mice were compared to one month old mice. We have not observed these age related changes in NKT and B cells from non-autoimmune C57BL/6 mice. It is of interest that NKT cell lines from humans with systemic lupus show a pattern of helper activity for IgG and IgG anti-dsDNA secretion by autologous B cells that is similar to that reported here for old NZB/W NKT cells (Engleman E., Stanford University, personal communication).
Although the in vitro interaction between the NZB/W NKT cells and NZB/W B-1 B cells gave rise to the most vigorous secretion of IgG2a anti-dsDNA antibodies as compared to interactions of conventional T cells or NKT cells with other B cell subsets, further studies are required to show that the NKT cell/B-1 B cell interaction in vivo is a requirement for the in vivo generation of IgG2a anti-dsDNA antibodies and/or a requirement for the development of lupus kidney disease. Evidence that supports an important contribution of T cells that recognize CD1d to the development of lupus disease activity include the induction of lupus in nu/nu BALB/c mice injected with transgenic anti-CD1d T cells [18], the amelioration of lupus in NZB/W mice after treatment with anti-CD1d mAb [7, 13], the worsening of lupus in NZB/W mice after treatment with α-GalCer [13], and the amelioration of lupus in NZB/W mice after treatment with glycolipid that binds to CD1d and antagonizes NKT cell cytokine secretion (S. Morshed et al, submitted for publication).
Additional evidence that B-1B cells contribute to lupus in NZB/W mice include the amelioration of disease in NZB/W xid mice [23], and reduction of disease severity by relative depletion of peritoneal B-1 B cells [24]. The role of NKT cells in the development of lupus in mouse strains other than the NZB/W mice is variable. Although in vivo activation of NKT cells with α-GalCer improved lupus induced by the injection of pristane in BALB/c mice, administration of α-GalCer worsened lupus in B10.PLJ mice injected with pristane [25]. In addition, deficiency of NKT cells in CD1d−/− BALB/c mice worsened lupus nephritis compared to wild-type mice after injection of pristane [26], but did not affect lupus kidney or skin disease in MRL/lpr mice [27]. Reports of decreased NKT cells in humans with lupus and other autoimmune diseases have been limited to examination of the blood, and may not reflect the changes in the other lymphoid tissues [28-30]. Some of these studies have erroneously identified NKT cells as CD56+ T cells [30], and our recent study showed that neither CD56+ T cells nor CD161+ T cells accurately reflect the number of invariant NKT cells in human blood samples [31]. In addition, decreased in vitro responses of NKT cells to α-Galcer in lupus patients[28] may reflect the impact of immunosuppressive drugs, and decreased numbers of NKT cells may reflect in vivo activation and TCR down regulation as has been shown in mice [32-34].
We are currently studying whether the adoptive transfer of purified NZB/W NKT cells and B-1 B cells to irradiated NZB/W mice will result in the rapid generation of IgG2a anti-dsDNA antibodies and the development of lupus kidney disease. A previous study showed that the transfer of an NZB/W T cell line that had been immunized to idiotypic segments of the anti-dsDNA antibody molecule into young NZB/W hosts was able to generate anti-dsDNA antibodies in vivo [9]. Thus, T cells other than the NKT cells can contribute to the production of pathogenic autoantibodies in NZB/W mice. The helper activity of the NZB/W NKT cells in the current study was dependent on cellular interactions via CD1d and CD40/CD40L receptors as judged by blocking experiments with receptor specific and control mAbs. The in vitro blocking with the anti-CD1d and anti-CD40L mAbs can explain the ability of these mAbs to ameliorate lupus disease activity in vivo [13, 21, 22]. Two recent papers indicate that NKT cells may have a protective role in the early development of lupus in NZB/W mice or in the late development of lupus in non-autoimmune prone mice that are more than 24 months of age [35, 36]. CD1d−/− NZB/W mice lacking CD1d dependent invariant and non-invariant NKT cells were reported to develop nephritis earlier than wild-type NZB/W mice [35]. In view of the age dependent abnormalities of NZB/W NKT cells observed in the current study, it is possible that the NKT cells in young mice are protective, and change to pathogenic cells as the disease process accelerates at about six months of age. In non-autoimmune mice, the NKT cells may be protective even at very late time points because they do not develop the age related abnormalities observed in the lupus-prone NZB/W mice [36].
The failure of freshly isolated NZB/W conventional T cells to provide helper activity in our in vitro system could have been explained by their lack of activation before culturing with B cells. Our previous studies, showed that NKT cells are partially activated by staining with the CD1d tetramers [13]. However, NZB/W conventional T cells also failed to provide helper activity after polyclonal activation in vitro with anti-CD3 and anti-CD28 mAbs (data not shown). Cultures of NZB/W total T cells augmented IgG secretion of B cells more than ten fold only after α-GalCer was added to the cultures, and α-GalCer augmentation was reversed by NKT cell depletion ( data not shown). In conclusion, the spontaneous in vitro interaction between purified NKT cells and purified B-1 B cells results in the vigorous secretion of IgM, IgG, IgM and IgG anti-dsDNA antibodies in NZB/W mice. This interaction may play an important role in the development of lupus disease activity observed in older mice.
Materials and Methods
Mice
NZB/W and C57BL/6 female mice were purchased from the Jackson Laboratories (Bar Harbor, ME), and were maintained in the Stanford University Department of Comparative Animal Medicine until they were used for experiments at 12 weeks of age, unless otherwise stated in the text. All animal protocols were reviewed and approved by the Stanford Administrative Panels on Laboratory Animal Care.
Reagents
Anti-mouse CD19, IgG1 magnetic beads were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). FITC-conjugated mAbs recognizing mouse CD5 (53-7.3), CD11c (HL3), CD21 (7G6), B220 (RA3-6B2), I-Ab (25-9-17), I-Ad (39-10-8), PE-conjugated mAbs recognizing CD1d (1B1), CD23 (B3B4), IgM (R6-60.2), APC-conjugated mAbs recognizing B220 (RA3-6B2), TCRβ (H57-597), NK1.1 (PK136), streptavidin, and biotin conjugated CD23 (B3B4) were purchased from BD Biosciences (San Diego, CA). PE-conjugated PBS-57 glycolipid-loaded CD1d tetramers were obtained from the NIH Tetramer Facility, Rockville MD. CD1d-dimers were purchased from BD Biosciences. α–GalCer was kindly provided from Dr. Paul Savage (Brigham Young University, UT). Purified anti-mouse CD3ε (145-2C11) and CD28 (37.51) for T cell stimulation and purified anti-mouse CD1d (1B1) and CD40L (MR1) for blocking experiments were purchased from BD Biosciences. Anti-IL-4 (11B11), anti-IL-10 (JES5-16E3), and anti-IFN-γ (R4-6A2) mAbs used for blocking experiments were purchased from BD Biosciences.
Cell preparation
For B cell subset sorting, spleen cells were incubated with anti-mouse CD19 beads and CD19+ cells were enriched on MACS columns (Miltenyi Biotec) according to the manufacture's protocol. Then cells were incubated with various combinations of mAbs and sorted into subsets using a highly modified FACStar instrument (Becton Dickinson, Milpitas, CA). For dendritic cells, spleen cells were incubated with anti-mouse CD11c beads and CD11c+ cells were enriched on MACS columns. Then the cells were incubated with mAbs and sorted. For NKT cells, spleen cells were first incubated with α–GalCer-loaded CD1d dimers and PE-CD1d-tetramers, washed, and then incubated with anti-mouse IgG1 beads. After enrichment on MACS columns, cells were incubated with PE-CD1d tetramers again and anti-TCRβ mAb, and sorted. Sorted conventional T cells were obtained by staining spleen cells for TCRβ versus CD1d-tetramers, and sorting TCRβ+tetramer− cells. Flow cytometric data were analyzed with FlowJo software (Treestar, San Carlos, CA). All cell suspensions were stained with propidium iodide to exclude dead cells. The purity of sorted cell populations was always more than 95% except B-1 B cells in C57BL/6 mice, which was 90-95%.
Cell culture and ELISA
Combinations of 1×105 sorted B cell subsets, 5×104 dendritic cells, 5×104 NKT or 5×104 conventional T cells were cultured in 96-well round-bottomed plates in RPMI medium supplemented with 10% FCS, 1×10−5 M β-mercaptoethanol, 2 mM glutamine, and 100 μg/ml penicillin and streptomycin (hereafter referred as cRPMI). Cells were incubated at 37°C with 5% CO2. Measurements of IL-4 or IFN-γ in culture supernatants were performed using an ELISA kit according to the manufacture's protocol (IL-4: BioSource, Camarillo, CA, IFN-γ: BD Biosciences). Measurements of IgM, IgG, IgG1, and IgG2a in culture supernatants were performed using an ELISA as described previously [18]. IgM or IgG anti-dsDNA Abs were captured using deproteinized calf thymus DNA. Anti-dsDNA titers are expressed as units per milliliter using a reference-positive standard of pooled sera from 9-mo-old NZB/W mice. Details of the anti-dsDNA assay have been described previously [18].
In vitro proliferative response
Sorted splenic T cells (TCRβ+CD11c−B220−I-A−) were incubated (2×105 cells) with or without 100 ng/ml α–GalCer in cRPMI with 10% FCS in 96-well round-bottomed plates for 72 hrs at 37°C in 5% CO2. 3H-thymidine (1 μCi/well) was added 20 hrs before cells were harvested. 3H-thymidine (New England Nuclear, Boston, MA) incorporation was measured in a liquid scintillation counter (Betaplate: Wallac, Turku, Finland). All assays were performed in triplicate wells.
Pre-activation of T cell subset
To pre-activate NKT cells, spleen cells were cultured in the presence of α–GalCer (100 ng/ml) for 2hrs before purification. To pre-activate conventional T cells, sorted TCRαβ+CD1d-tetramer− spleen cells were cultured in 24 well plates coated anti-CD3 mAb (10 μg/ml) with medium containing anti-CD28 mAb (5 μg/ml) for 18 hrs in cRPMI with 10% FCS.
Blockade experiments
In some cell cultures, mAbs were added to the medium to inhibit immunoglobulin secretion. For CD1d blockade, 20 μg/ml of rat anti-mouse CD1d mAb or irrelevant rat IgG2b mAb were used. For CD40L blockade, 20 μg/ml of rat anti-mouse CD40L mAb or control rat IgG3 mAb were used.
Statistical analyses
Differences in mean immunoglobulin secretion, cytokine secretion and proliferation were analyzed using the two-tailed Student's t test. In experiments in which the majority of determinations in a given group were undetectable, then the Fisher's exact test was used. A value of p < 0.05 was considered statistically significant.
Acknowledgements
We thank Mary Hansen for assistance in the submission of the manuscript. We thank the NIH tetramer facility for providing CD1d-tetramer. This study was supported in part by grants from the NIH, NIAID RO1 AI-40093 and NIAMSD 1 RO1 AR-51748 to Dr. Strober.
Abbreviations
- ds
double stranded
- dsDNA
double stranded DNA
- α-GalCer
α-galactosylceramide
- β-GalCer
β-galactosylceramide
Footnotes
Conflict of Interest: There is no financial conflict of interest.
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–1368. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 3.Datta SK, Patel H, Berry D. Induction of a cationic shift in IgG anti-DNA autoantibodies. Role of T helper cells with classical and novel phenotypes in three murine models of lupus nephritis. J Exp Med. 1987;165:1252–1268. doi: 10.1084/jem.165.5.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert PH, Dixon FJ. Pathogenesis of the glomerulonephritis of NZB/W mice. J Exp Med. 1968;127:507–522. doi: 10.1084/jem.127.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebling F, Hahn BH. Restricted subpopulations of DNA antibodies in kidneys of mice with systemic lupus. Comparison of antibodies in serum and renal eluates. Arthritis Rheum. 1980;23:392–403. doi: 10.1002/art.1780230402. [DOI] [PubMed] [Google Scholar]
- 6.Okada T, Abe M, Takiura F, Hirose S, Shirai T. Distinct surface phenotypes of B cells responsible for spontaneous production of IgM and IgG anti-DNA antibodies in autoimmune-prone NZB x NZW F1 mice. Autoimmunity. 1990;7:109–120. doi: 10.3109/08916939008993383. [DOI] [PubMed] [Google Scholar]
- 7.Zeng D, Lee MK, Tung J, Brendolan A, Strober S. Cutting edge: a role for CD1 in the pathogenesis of lupus in NZB/NZW mice. J Immunol. 2000;164:5000–5004. doi: 10.4049/jimmunol.164.10.5000. [DOI] [PubMed] [Google Scholar]
- 8.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuster H, Martin T, Marcellin L, Garaud JC, Pasquali JL, Korganow AS. Expansion of marginal zone B cells is not sufficient for the development of renal disease in NZBxNZW F1 mice. Lupus. 2002;11:277–286. doi: 10.1191/0961203302lu191oa. [DOI] [PubMed] [Google Scholar]
- 10.Forestier C, Molano A, Im JS, Dutronc Y, Diamond B, Davidson A, Illarionov PA, Besra GS, Porcelli SA. Expansion and hyperactivity of CD1d-restricted NKT cells during the progression of systemic lupus erythematosus in (New Zealand Black x New Zealand White)F1 mice. J Immunol. 2005;175:763–770. doi: 10.4049/jimmunol.175.2.763. [DOI] [PubMed] [Google Scholar]
- 11.Morshed SR, Mannoor K, Halder RC, Kawamura H, Bannai M, Sekikawa H, Watanabe H, Abo T. Tissue-specific expansion of NKT and CD5+B cells at the onset of autoimmune disease in (NZBxNZW)F1 mice. Eur J Immunol. 2002;32:2551–2561. doi: 10.1002/1521-4141(200209)32:9<2551::AID-IMMU2551>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Mieza MA, Itoh T, Cui JQ, Makino Y, Kawano T, Tsuchida K, Koike T, Shirai T, Yagita H, Matsuzawa A, Koseki H, Taniguchi M. Selective reduction of V alpha 14+ NK T cells associated with disease development in autoimmune-prone mice. J Immunol. 1996;156:4035–4040. [PubMed] [Google Scholar]
- 13.Zeng D, Liu Y, Sidobre S, Kronenberg M, Strober S. Activation of natural killer T cells in NZB/W mice induces Th1-type immune responses exacerbating lupus. J Clin Invest. 2003;112:1211–1222. doi: 10.1172/JCI17165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schofield L, McConville MJ, Hansen D, Campbell AS, Fraser-Reid B, Grusby MJ, Tachado SD. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 15.Sonoda KH, Stein-Streilein J. CD1d on antigen-transporting APC and splenic marginal zone B cells promotes NKT cell-dependent tolerance. Eur J Immunol. 2002;32:848–857. doi: 10.1002/1521-4141(200203)32:3<848::AID-IMMU848>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, Dellabona P, Abrignani S. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med. 2003;197:1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bezbradica JS, Stanic AK, Matsuki N, Bour-Jordan H, Bluestone JA, Thomas JW, Unutmaz D, Van Kaer L, Joyce S. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J Immunol. 2005;174:4696–4705. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- 18.Zeng D, Dick M, Cheng L, Amano M, Dejbakhsh-Jones S, Huie P, Sibley R, Strober S. Subsets of transgenic T cells that recognize CD1 induce or prevent murine lupus: role of cytokines. J Exp Med. 1998;187:525–536. doi: 10.1084/jem.187.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Huang W, Schiffer LE, Mihara M, Akkerman A, Hiromatsu K, Davidson A. Effects of anti-CD154 treatment on B cells in murine systemic lupus erythematosus. Arthritis Rheum. 2003;48:495–506. doi: 10.1002/art.10929. [DOI] [PubMed] [Google Scholar]
- 22.Early GS, Zhao W, Burns CM. Anti-CD40 ligand antibody treatment prevents the development of lupus-like nephritis in a subset of New Zealand black x New Zealand white mice. Response correlates with the absence of an anti-antibody response. J Immunol. 1996;157:3159–3164. [PubMed] [Google Scholar]
- 23.Steinberg BJ, Smathers PA, Frederiksen K, Steinberg AD. Ability of the xid gene to prevent autoimmunity in (NZB X NZW)F1 mice during the course of their natural history, after polyclonal stimulation, or following immunization with DNA. J Clin Invest. 1982;70:587–597. doi: 10.1172/JCI110651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami M, Yoshioka H, Shirai T, Tsubata T, Honjo T. Prevention of autoimmune symptoms in autoimmune-prone mice by elimination of B-1 cells. Int Immunol. 1995;7:877–882. doi: 10.1093/intimm/7.5.877. [DOI] [PubMed] [Google Scholar]
- 25.Singh AK, Yang JQ, Parekh VV, Wei J, Wang CR, Joyce S, Singh RR, Van Kaer L. The natural killer T cell ligand alpha-galactosylceramide prevents or promotes pristane-induced lupus in mice. Eur J Immunol. 2005;35:1143–1154. doi: 10.1002/eji.200425861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang JQ, Singh AK, Wilson MT, Satoh M, Stanic AK, Park JJ, Hong S, Gadola SD, Mizutani A, Kakumanu SR, Reeves WH, Cerundolo V, Joyce S, Van Kaer L, Singh RR. Immunoregulatory role of CD1d in the hydrocarbon oil-induced model of lupus nephritis. J Immunol. 2003;171:2142–2153. doi: 10.4049/jimmunol.171.4.2142. [DOI] [PubMed] [Google Scholar]
- 27.Yang JQ, Saxena V, Xu H, Van Kaer L, Wang CR, Singh RR. Repeated alpha-galactosylceramide administration results in expansion of NK T cells and alleviates inflammatory dermatitis in MRL-lpr/lpr mice. J Immunol. 2003;171:4439–4446. doi: 10.4049/jimmunol.171.8.4439. [DOI] [PubMed] [Google Scholar]
- 28.Kojo S, Adachi Y, Keino H, Taniguchi M, Sumida T. Dysfunction of T cell receptor AV24AJ18+, BV11+ double-negative regulatory natural killer T cells in autoimmune diseases. Arthritis Rheum. 2001;44:1127–1138. doi: 10.1002/1529-0131(200105)44:5<1127::AID-ANR194>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 29.van der Vliet HJ, von Blomberg BM, Nishi N, Reijm M, Voskuyl AE, van Bodegraven AA, Polman CH, Rustemeyer T, Lips P, van den Eertwegh AJ, Giaccone G, Scheper RJ, Pinedo HM. Circulating V(alpha24+) Vbeta11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin Immunol. 2001;100:144–148. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- 30.Green MR, Kennell AS, Larche MJ, Seifert MH, Isenberg DA, Salaman MR. Natural killer T cells in families of patients with systemic lupus erythematosus: their possible role in regulation of IgG production. Arthritis Rheum. 2007;56:303–310. doi: 10.1002/art.22326. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T, Dejbakhsh-Jones S, Strober S. Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities. J Immunol. 2006;176:211–216. doi: 10.4049/jimmunol.176.1.211. [DOI] [PubMed] [Google Scholar]
- 32.Harada M, Seino K, Wakao H, Sakata S, Ishizuka Y, Ito T, Kojo S, Nakayama T, Taniguchi M. Down-regulation of the invariant Valpha14 antigen receptor in NKT cells upon activation. Int Immunol. 2004;16:241–247. doi: 10.1093/intimm/dxh023. [DOI] [PubMed] [Google Scholar]
- 33.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, Bouillet P, Strasser A, Smyth MJ, Godfrey DI. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang JQ, Wen X, Liu H, Folayan G, Dong X, Zhou M, Van Kaer L, Singh RR. Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model. Arthritis Rheum. 2007;56:1219–1233. doi: 10.1002/art.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sireci G, Russo D, Dieli F, Porcelli SA, Taniguchi M, La Manna MP, Di Liberto D, Scarpa F, Salerno A. Immunoregulatory role of Jalpha281 T cells in aged mice developing lupus-like nephritis. Eur J Immunol. 2007;37:425–433. doi: 10.1002/eji.200636695. [DOI] [PubMed] [Google Scholar]