Abstract
Children with closed head injuries often experience significant and persistent disruptions in their social and behavioral functioning. Studies with adults sustaining a traumatic brain injury (TBI) indicate deficits in emotion recognition and suggest that these difficulties may underlie some of the social deficits. The goal of the current study was to examine if children sustaining a TBI exhibit difficulties with emotion recognition in terms of emotional prosody and face emotion recognition and to determine (1) how these abilities change over time and (2) what, if any, additional factors such as sex, age, and socioeconomic status (SES) affected the findings. Results provide general support for the idea that children sustaining a TBI exhibit deficits in emotional prosody and face emotion recognition performance. Further, although some gains were noted in the TBI group over the two-years following injury, factors such as SES and age at injury influenced the trajectory of recovery. The current findings indicate the relationship between TBI and emotion recognition is complex and may be influenced by a number of developmental and environmental factors. Results are discussed in terms of their similarity to previous investigations demonstrating the influence of environmental factors on behavioral recovery following pediatric TBI, and with regard to future investigations that can further explore the link between emotion recognition deficits and long-term behavioral and psychosocial recovery.
Keywords: Children, Head Injury, Emotion Processing, Face Perception
Introduction
Children sustaining a moderate to severe traumatic brain injury (TBI) associated with closed head trauma often experience significant and long-lasting behavioral and psychosocial difficulties (Andrews, Rose, & Johnson, 1998; Fay, Yeates, Wade, Drotar, & Stancin, 2009; Levin et al., 2004; Yeates et al., 2007). Research conducted with TBI survivors also suggests that TBI results in emotion recognition deficits (Bornhofen & McDonald, 2008a; McDonald, 2005) which may contribute to or exacerbate social difficulties (Bornhofen & McDonald, 2008a; Hornak, Rolls, & Wade, 1996; Watts & Douglas, 2006).
The first studies demonstrating emotion recognition deficits following TBI involved adult patients and demonstrated that brain injured individuals were impaired at recognizing facial emotions (Braun, Baribeau, Ethier, Daigneault, & Proulx, 1989; Jackson & Moffat, 1987; Prigatano & Pribram, 1982). Subsequent investigations have confirmed such findings (Allerdines & Alfano, 2006; Croker & McDonald, 2005; Green, Turner, & Thompson, 2004; Henry, Phillips, Crawford, Ietswaart, & Summers, 2006; Hopkins, Dywan, & Segalowitz, 2002; Ietswaart, Milders, Crawford, Currie, & Scott, 2008; Lew et al., 2005; McDonald, Flanagan, Rollins, & Kinch, 2003; Spell & Frank, 2000; Watts & Douglas, 2006). Other studies have extended this line of research to the auditory modality, showing that individuals sustaining a TBI demonstrate deficits on tasks of emotional prosody recognition (Hornak et al., 1996; Milders, Fuchs, & Crawford, 2003; Spell & Frank, 2000). Emotion recognition deficits also occur in patients with various neurologic conditions including stroke (Adolphs, Damasio, Tranel, & Damasio, 1996; Calder, Keane, Antoun, & Young, 2000; Charbonneau, Scherzer, Aspirot, & Cohen, 2003; Kucharska-Pietura, Phillips, Gernand, & David, 2003), epilepsy (Adolphs, Tranel, & Damasio, 2001; Meletti et al., 2009), and neurodegenerative disorders such as Parkinson’s and Huntington’s disease (Ariatti, Benuzzi, & Nichelli, 2008; Hayes, Stevenson, & Coltheart, 2007)
Although various studies have verified the existence of emotion recognition deficits following brain injury in adults, very few have examined this ability in children (Tonks, Williams, Frampton, Yates, & Slater, 2007a, 2007b, 2007c). In an initial study, Pettersen (1991) demonstrated that, compared to controls, children and adolescents sustaining a TBI exhibited deficits in interpreting emotions in both the visual and verbal domains and made more errors discriminating positive and negative emotional stimuli. Turkstra and colleagues (2001) indicated that adolescents with a history of TBI performed worse than age-matched peers on tasks of emotion recognition and social conversation skills. Snodgrass and Knott (2006) also found evidence of emotion recognition and advanced theory of mind deficits in a group of 6–12 year-old children sustaining a TBI.
Tonks et al. (2007b) evaluated a group of 18 9–17 year-old children following mild, moderate, or severe TBI on a variety of emotion recognition tasks as well as several measures of behavioral functioning. Children within the TBI group performed significantly worse on most tasks of emotion recognition than non-injured controls. Another recent study by Tonks and colleagues (2008) used a case series design to demonstrate emotion processing deficits can occur in combination with or apart from other cognitive deficits in children sustaining a pediatric head injury. The investigators concluded these initial data suggest that, similar to adults, children sustaining a TBI are at risk for deficits in emotion recognition; however, they emphasize that findings from both studies are limited by small, heterogeneous samples
The extent of emotion recognition deficits and trajectory of their recovery following pediatric TBI are important variables to examine because researchers postulate that recognition of others’ emotions may be a necessary component of accurate social cognition (Lemerise & Arsenio, 2000). Leppanen & Hietanen (2001) demonstrated that emotion recognition accuracy (especially the identification of the emotion of surprise) was significantly related to social adjustment in typically developing school-aged girls. Similarly, in a sample of economically disadvantaged children, Izard and colleagues (2001) found that children’s performance on a task of facial emotion recognition at age five was related to their social competence and academic performance at age nine.
The central goal of the current investigation was to examine the trajectory of emotional processing performance over time in children sustaining a TBI. We were also interested in characterizing which, if any, external variables (e.g., sex, age at injury, or socioeconomic status (SES) affected outcomes). These latter variables are especially important to consider as previous research demonstrates that variables such as age, sex, and SES may not only impact recovery from brain injury (Anderson, Barrash, Bechara, & Tranel, 2006; Ratcliff et al., 2007; Yeates et al., 2004; Yeates et al., 1997), but may also impact emotion recognition skills in children (Herba & Phillips, 2004).
We used two tasks of emotion perception – emotional prosody and face emotion recognition – representing the auditory and visual domains respectively, to examine emotional processing in children sustaining a TBI as compared with a group of children who sustained orthopedic injury (OI). Based upon previous findings with children and adults, we hypothesize that, relative to children with OI, children sustaining a TBI will exhibit difficulties on both types of emotion recognition tasks at baseline, but will show gradual improvement in performance over the two-year follow-up period. We also hypothesize that the pattern of performance will be affected by age and SES, with older age at injury and higher SES being associated with better recovery of emotion recognition skills.
Method
Participants
All procedures were approved by the institutional review boards at each participating organization, and complied with the National Institute of Health (NIH) policies on human subjects. Participants with a non-penetrating TBI or an OI were recruited from participating institutions after they had become medically stable. Inclusion of the OI group was intended to control for risk factors predisposing children to injury, and to equate for non-specific factors such as maturation or stress resulting from hospitalization.
The current investigation examined participants at five time points; baseline (within one month after injury), three months, 12 months, 18 months, and 24 months after injury. A total of 144 children (69 children with orthopedic injury (OI) and 75 children with TBI) participated in the current study. There were 50 (72.5%) males and 19 (27.5%) females in the OI group, and 49(65.3%) males and 26 (34.7%) females in the TBI group. However, not all participants had complete data for each time point. More specifically, 57 (28 OI, 29 TBI) participants had five complete visits, 33 (15 OI, 18 TBI) participants had four complete visits, 16 (5 OI, 11 TBI) participants had three complete visits, 18 (8 OI, 10 TBI) participants had two complete visits, and 20 (13 OI, 7 TBI) participants had only one complete visit.
Participant demographics, including ethnicity, sex, and socioeconomic composite index (SCI) (Yeates et al., 1997) are shown in Table 1. Participants ranged in age from seven to 17 years of age at the time of injury
Table 1.
Demographic characteristics of patient population
| TBI (n = 75) | OI (n = 69) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age of Injury (yrs) | 13.34 | 2.77 | 7.10 – 17.22 | 12.02 | 2.49 | 7.05 – 16.56 |
| SCI | −0.04 | 0.84 | −1.87 – 1.75 | 0.08 | 0.84 | −1.52 – 1.89 |
| Glasgow Coma Score | 7.97 | 4.43 | 3 – 15 | 14.99 | 0.12 | 14 – 15 |
| Sex Distribution | 49 male ; 26 female | 50 male ; 19 female | ||||
| Ethnicity Distribution | 11 African American ; 32 Caucasian; 30 Hispanic ; 1 Am Indian; 1 Biracial |
26 African American ; 20 Caucasian; 20 Hispanic; 1 Asian; 2 Biracial |
||||
| Mechanism of Injury | 25 inside auto/truck/bus ; 7 RV/offroad; 7 motorcycle/moped; 5 bicycle ; 12 fall; 4 sports/play; 12 hit by motor vehicle ; 2 other |
2 inside auto/truck/bus ; 1 RV/off road ; 6 motorcycle/moped; 5 bicycle ; 13 fall ; 31 sports/play; 1 hit by object ; 4 hit by motor vehicle ; 4 other |
||||
Note: Not all subjects had data for all time points
The TBI group was composed of children who experienced moderate to severe closed head injuries and who had a recorded Glasgow Coma Scale (GCS; Teasdale & Jennett, 1974) score reported in hospital records. Moderate TBI was defined by lowest post-resuscitation GCS scores of 9 to 12 or by GCS scores of 13 to 15 with brain lesions (contusions, hematomas) indicated by computed tomography (CT) scans. GCS scores of 3 to 8 defined severe TBI. See Table 1 for a description of the participants in terms of GCS score and mechanism of injury. The hospitalized OI patients had mild to moderate orthopedic injuries as defined by the Abbreviated Injury Scale (Copes, Lawnick, Champion, & Sacco, 1988). All participants were English speaking, had never previously been hospitalized for a head injury, were not injured as a result of abuse, and did not have a previous diagnosis of mental retardation or pervasive developmental disorder.
Procedures
Testing commenced following the procurement of parental consent and child assent. Children were assessed at five time points during the first two years post injury: baseline (within 1 month), 3 months (± 1 month), 12 months (± 2 months), 18 months (± 2 months), and 24 months (± 2 months) post injury. Possible moderating variables relating to age at injury, SES, sex, and ethnicity were also collected on each child/family. Participants were assessed on the capacity to recognize emotions from voice cues (emotional prosody task) and visual cues (face emotion recognition) in addition to other neurocognitive and behavioral measures not reported here. Each participant also completed two control procedures, including a separate test of phonological discrimination and a face identity recognition procedure built into the face emotion recognition task.
Measures
Socioeconomic composite index (SCI) (Yeates et al., 2004; Yeates et al., 1997).
As used in the original study (Yeates et al., 1997) and as operationalized in the present investigation, the SCI was based on three variables: maternal education, coded on a seven point scale with values representing < 7 years education to attainment of a graduate degree; annual family income, based off an eight point scale ranging from < $20,000 to >$60,000 as part of the Life Stressors and Resources Scale (LISRES) (Moos & Moos, 1994); and the Duncan occupational status index (Stevens & Featherman, 1981). These three variables were transformed into z-scores and then averaged together to yield a composite z-score which was standardized (mean = 0, SD = 1).
Emotional Prosody Task (Adolphs & Tranel, 1999; Adolphs et al., 2001; Levin, 2006)
During this task, children listened to a digital recording of the same female voice speaking four semantically neutral sentences using eight different emotional prosodic contours: happy, sad, angry, neutral, afraid, surprised, disgusted, and sleepy. Thus, each sentence was heard eight times and each prosodic contour was heard four times. The child indicated which emotion was expressed by pointing to the name of the emotion printed beneath a stylized picture of a face emotion. The 32 sentences of 3-second duration were played in random order. The child proceeded at his/her own pace with a minimum of 10 seconds between sentences. Performance was evaluated in terms of the number of sentences correctly identified. This task has been shown to be sensitive to changes in cortical volume following pediatric TBI (Levin, 2006).
Phonological Discrimination (Hanten & Martin, 2000)
In the phonological discrimination test (Hanten & Martin, 2000), subjects made a “matching” or “not-matching” judgment immediately after the oral presentation of two nonsense words, which were either identical or varied by a single phoneme (e.g., "bolap-bolab"). The purpose of this test was to identify low-level perceptual phonological deficits that might account for or contribute to poor performance in the prosody task.
Face Emotion Recognition (Adolphs et al., 2001)
This task was used to test the ability to recognize facial emotions as opposed to facial identity. Faces were drawn from the 1976 Eckman series of faces (Ekman, 1976). Children sorted an array of 24 photographs of faces of six different adults each displaying four emotions: angry, happy, surprised, or disgusted. In the experimental condition (face emotion recognition), children were instructed to sort the photographs into four groups comprising six people expressing the same emotion (e.g., all happy). In the control condition (face identity recognition), children were then instructed to sort the 24 photographs into 6 groups, each comprising photos of the same person regardless of the emotion expressed. Each task was scored separately for accuracy of recognizing the emotion displayed (by sorting into the correct emotion group) or the identity of a person based on their facial features; completion time to sort the photographs was recorded. Our pilot data with TBI participants indicated the reliability for this task for children with TBI was moderately high (internal consistency coefficient = 0.75).
Statistical Analysis
T-tests were used to compare the groups on continuous variables such as age at injury, SCI, and phonological discrimination whereas the Chi-square test was used for categorical variables (e.g., sex). The nature of the emotional processing data is longitudinal, with missing data points secondary to patient unavailability. Therefore, we used growth curve analysis and applied a generalized linear mixed model approach to model the changes in the total number of correct responses on both the emotional prosody and face identity and face emotion recognition tasks over time. The generalized linear mixed model is similar to the general linear mixed model and can handle missing data. A binomial distribution was assumed for the data, with a link function of logit for the dependent variable. The total number of items was 32 for the emotional prosody task and 24 for the face emotion recognition task. General linear mixed models were applied to the time variables.
Polynomial functions of time since injury (interval) with linear, quadratic, and cubic terms were tested in level 1 of the model with injury group. The interval was centered at 12 months – as we believed this to be an ideal time point to observe group differences and understand changes in performance. For the polynomial terms, the intercept represents the level of the dependent variable, that is, the level of performance at 12 months, the slope (linear term) represents the rate of change in the dependent variable at 12 months, the quadratic represents the change in slope between intervals, and the cubic term represents the change in the quadratic term, that is, the change in the shape of the trajectory over time.
Control tasks (e.g., phonological discrimination for emotional prosody and face identity recognition time for face emotion recognition time) were entered in the models for their respective procedures. Age at injury, phonological discrimination, and face identity recognition time were centered at their grand means to facilitate interpretation when significant interactions were observed. Three-way interactions of group by every time invariant variable and each term of the polynomials of the interval were tested and deleted from higher to lower order for non-significant interactions. The same procedure was followed for two-way interactions. The figures reflecting the changes over time were made from the fitted values at the corresponding level of each involved term. When there was a significant interaction with a continuous variable, we plotted the group means at three points of the continuous variable, mean, mean-1sd and mean+1sd. Only statistically significant findings are reported in the results presented below.
Results
Demographic Variables
Groups did not demonstrate differences in the demographic variables of SCI t(135) = 0.85, p = 0.396, and sex χ2(1, N = 144) = 0.851, p = 0.356. The groups differed on ethnicity and age at injury, there were more African Americans in the OI group and more Caucasians and Hispanics in the TBI group, Fisher’s Exact test, p = 0.0096. TBI participants were significantly older (M = 13.34, SD = 2.77) at the time of injury than OI participants were (M = 12.02, SD = 2.49), t(142) = −2.99, p = 0.003. Demographic variables (e.g., age at injury, SCI, and sex) that were significantly related to the dependent measures were entered into the models for each task with group as time-invariant variables. When variables such as age, sex, socioeconomic status were not significantly related to outcome, they were dropped from the model to preserve power.
Prosodic Processing Tasks
Emotional Prosody
As may be anticipated, GCS score was significantly related to emotion prosody performance. In fact, results from a Spearman correlation indicated that this relationship grew stronger with time (Table 2). There was a significant cubic recovery pattern for both groups F(1, 222) = 7.67, p = 0.006. This indicates that participants improved at a faster rate from baseline to 12 months, then slowed down until 18 months. After 18 months, the performance of the participants again improved but at a slower rate than before 12 months. The changing rate for the slope and the quadratic term did not significantly differ between TBI and OI participants – indicating that TBI and OI participants had similar patterns of change.
Table 2.
Spearman correlations of emotional recognition with GCS score at all time points.
| Emotional Prosody - Total Correct |
Face Emotion Sorting - Total Correct |
|||
|---|---|---|---|---|
| r | p | r | P | |
| Baseline | 0.297 | 0.02 | 0.158 | 0.236 |
| 3 months | 0.33 | 0.01 | 0.246 | 0.056 |
| 12 months | 0.394 | 0.007 | 0.335 | 0.022 |
| 18 months | 0.402 | 0.012 | 0.03 | 0.846 |
| 24 months | 0.487 | < 0.001 | 0.293 | 0.056 |
Note: Not all subjects had data for all time points
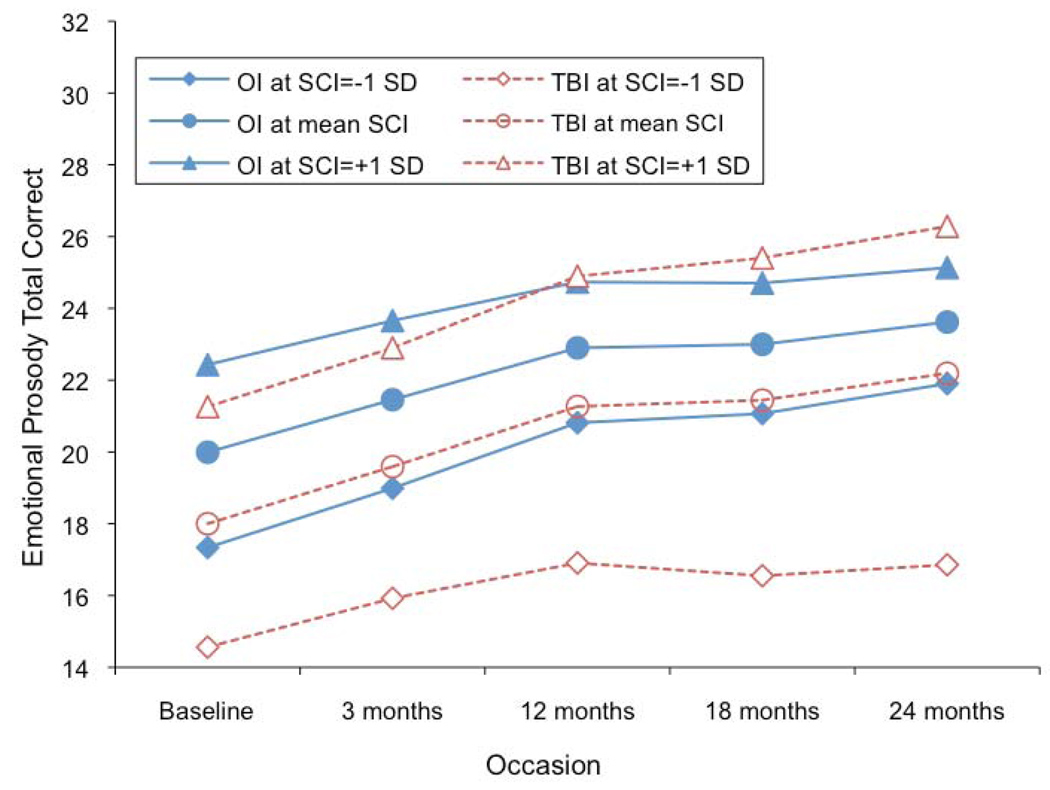
However, groups significantly differed on the slope and this difference depended on SCI, yielding a three way interaction of slope by group by SCI, F(1,222) = 7.73, p = 0.0059 (see Fig. 1). Group means were plotted at three points of SCI, mean (0), mean-1sd (−1) and mean+1sd (1). Thus, among participants with lower SCI scores, OI participants demonstrated a faster rate of recovery in scores when compared to TBI participants. When SCI = −1, OI participants recovered faster and performed better than TBI participants. This difference increased with time; that is, the disparity between low SCI OI and low SCI TBI participants increased as the study progressed. The slope difference between groups decreased when SCI increased, and the TBI participants eventually demonstrated a faster rate of recovery than OI participants when SCI was high. For example, when SCI = 1, OI participants recovered faster and outperformed TBI participants before 12 months, but after 12 months TBI participants began outperforming OI participants due to a faster increase in the rate of recovery. As noted in the paragraph above, the change rate of slope and curvature did not significantly differ by group. A variance covariance test showed that the intercept did not significantly relate to the slope (p = 0.4799), indicating that baseline performance did not account for the slower rate of recovery
Figure 1.
Emotional Prosody performance over time based on group and SCI
Age at injury also affected the slope of change, F(1,222) = 14.83, p = 0.0002. This effect did not differ by group, indicating that younger age at injury was associated with a faster rate of recovery in emotional prosody performance for both groups.
Phonological Discrimination
Because phonological processing may contribute to task performance on the emotional prosody task, at baseline we administered a phonological discrimination task to both groups. OI participants had significantly higher phonological discrimination performance (M = 27.194, SD = 2.797) than TBI participants (M = 26.098, SD = 3.526), t(136) = 2.01, p = 0.046. We re-analyzed emotional prosody performance taking into account phonological discrimination performance by adding the phonological processing variable and the interaction term with other terms in the previous model. Phonological discrimination performance was significantly related with emotional prosody performance in both groups, but age at injury affected the relation, F(1, 215) = 4.05, p = 0.0455, showing that the relationship was stronger in younger participants. Phonological discrimination performance did not impact the differences in recovery patterns for emotional prosody between the groups.
In summary (see Table 3), as a group, TBI participants exhibited a faster rate of recovery (i.e., faster rate of performance improvement) when compared to OI controls. However, there was a significant group by slope by SCI interaction. Thus, among participants with a lower SCI score, OI participants consistently demonstrated a faster rate of improvement in emotional prosody performance. Conversely, TBI participants with a higher SCI score exhibited a significantly faster rate of improvement in emotional prosody performance, especially after 12 months. These patterns were not changed when phonological discrimination performance was controlled for in the analysis – suggesting that differences in phonological processing did not account for the divergent patterns of emotional prosody performance between groups. Finally, younger age at injury was associated with a faster rate of recovery in emotional prosody performance for both groups
Table 3.
Recovery Pattern for Prosody and Face Emotion Sorting Total Correct
| Test | Fixed Effects | Estimate | t | p |
|---|---|---|---|---|
| Prosody Total Correct | Group (OI vs. TBI) | 0.2391 | 2.08 | .0390 |
| Age | .02157 | 1.02 | .3067 | |
| Age * Slope | −.00333 | −3.85 | .0002 | |
| SCI | .5704 | 6.30 | <.0001 | |
| SCI * Group | −.2684 | −2.02 | .0442 | |
| Slope * SCI | .01157 | 3.26 | .0013 | |
| Slope * Group | −.00162 | −.34 | .7310 | |
| Slope * SCI * Group | −.01492 | −2.78 | .0059 | |
| Changing rate of Slope (Quadratic) | −.00104 | −2.62 | .0094 | |
| Changing in Shape (Cubic) | .00012 | 2.77 | .0061 | |
| Face Emotion Sorting Total Correct |
Group | .38800 | 3.64 | .0003 |
| Age | .00812 | .33 | .7426 | |
| SCI | .20720 | 3.24 | .0014 | |
| Gender (Female vs. Male) | .36960 | 3.17 | .0017 | |
| Slope | −.00283 | −.31 | .7572 | |
| SCI * Slope | .01033 | 2.10 | .0368 | |
| Gender * Slope | .02088 | 2.28 | .0234 | |
| Age * Slope | .00479 | 1.59 | .1138 | |
| Quadratic | −.00131 | −2.51 | .0128 | |
| Age * Quadratic | .00045 | 2.37 | .0187 | |
| Cubic | .00012 | 1.85 | .0654 | |
| Age * Cubic | −.00005 | −2.45 | .0150 | |
| Face Emotion Sorting Time |
Group | −12.2538 | −1.89 | .0598 |
| SCI | −9.6523 | −2.53 | .0121 | |
| Slope | −.7683 | −2.76 | .0067 | |
| Face Sorting Time | Group | −24.5556 | −3.79 | .0002 |
| SCI | −11.9894 | −3.35 | .0010 | |
| Age | −.1140 | −.07 | .9432 | |
| Slope | −1.9893 | −5.83 | <.0001 | |
| Group * Slope | 1.1399 | 2.28 | .0236 | |
| Age * Slope | .2440 | 2.62 | .0095 | |
| Quadratic | .1091 | 3.07 | .0025 | |
| Age * Quadratic | −.02897 | −2.26 | .0250 | |
Face Processing Tasks
Face Identity Recognition
On this task, most participants for both groups had > 90% accuracy. Although the OI participants outperformed the TBI participants at the baseline (χ12 = 7.55, p = .006), 80% of the OI participants had > 90% accuracy, while 56.67% of the TBI participants had > 90% accuracy. At the three month occasion (χ12 =4.63, p = .032), 84.31% of the OI participants had > 90% accuracy, while 66.67% of the TBI participants had > 90% accuracy. There was no significant group difference in terms of total correct for the remaining occasions.
In terms of reaction time, OI participants performed faster than TBI participants F(1, 171) = 14.33, p = 0.0002. This difference became smaller with time secondary to the faster rate of recovery of TBI participants when compared to OI participants, F(1,171) = 5.22, p = 0.0236. There was also a significant quadratic recovery pattern, reflecting a faster recovery from baseline to 12 months, after which time the rate of recovery slowed down. The rate of change in slope did not significantly differ between groups.
Face Emotion Recognition
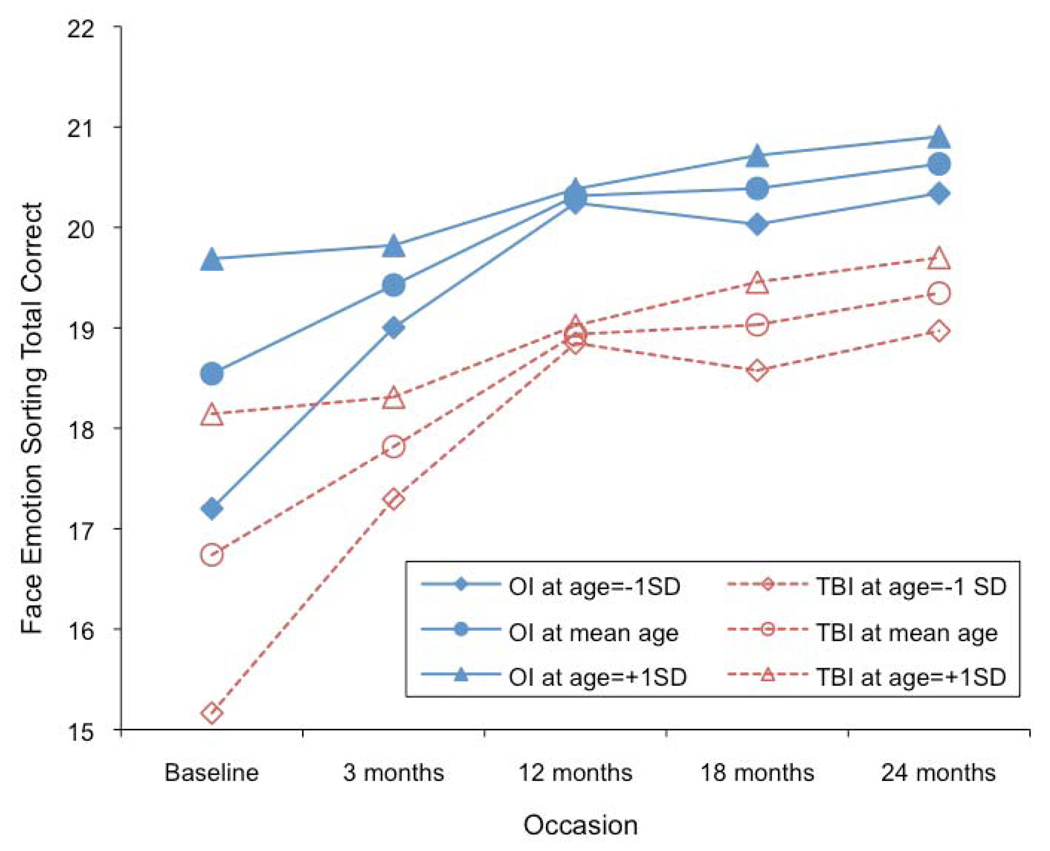
Although there were some relationships between GCS score and face emotion recognition performance, these findings were inconsistent throughout the two-year follow up period (See Table 2). There was a significant cubical change pattern, and age at injury affected the change rate, although the pattern did not differ by group. Older participants started with a higher intercept at baseline, but they exhibited a smaller increase in rate of recovery until 12 months. Between 12 and 18 months, younger participants exhibited a decreasing rate of improvement in performance, and after 18 months, all participants showed a similarly increasing rate of recovery. Although groups didn’t differ in these patterns, OI patients consistently outperformed TBI patients at all time points, F(1, 236) = 13.24, p = 0.0003. There was an effect for sex; female participants performed better and had a faster recovery rate than male participants. Performance was also influenced by SCI. Participants with higher SCI scores performed better and had a faster recovery rate than participants with lower SCI scores. There were no significant group by sex or group by SCI interactions (see Fig. 2).
Figure 2.
Face Emotion Sorting performance over time based on group and SCI
With regard to reaction time, both groups demonstrated a linear trend of improvement F(1, 120) = 7.60, p = 0.0067, but these changes were not significantly different by group. There was a marginally significant group difference on the intercept with OI participants tending to be faster than TBI participants F(1, 233) = 3.58, p = 0.0598. This group difference did not significantly change with time.
In summary (see Table 3), most participants performed well on face identity recognition, and any group differences that existed disappeared after three months post-injury. OI participants consistently outperformed TBI participants on face emotion recognition. In both groups, younger age was associated with a faster initial rate of recovery. Likewise, for both groups, females and individuals with a higher SCI score generally faired better than their male and lower SCI peers.
Relationship of Emotional Prosody to Face Emotion Recognition
In order to investigate if performance of individual participants was related on the two emotion recognition tasks, we performed basic Spearman correlations between total correct on the emotional prosody and face emotion recognition tasks for each occasion. Results for OI and TBI participants are presented in Table 4. Briefly, moderate correlations were observed in both groups at the baseline and 24-month occasions.
Table 4.
Spearman correlations of emotional prosody recognition with face emotion recognition at all time points.
| TBI (n = 75) | OI (n = 69) | |||
|---|---|---|---|---|
| r | p | r | p | |
| Baseline | 0.445 | 0.001 | 0.333 | 0.01 |
| 3 months | 0.211 | 0.112 | 0.197 | 0.161 |
| 12 months | 0.039 | 0.806 | 0.346 | 0.019 |
| 18 months | 0.066 | 0.688 | 0.106 | 0.55 |
| 24 months | 0.524 | < 0.001 | 0.354 | 0.034 |
Note: Not all subjects had data for all time points
Discussion
The current study explored emotion processing in both the auditory and visual domains following pediatric TBI. Although complex and influenced by environmental and developmental factors, results provide general support for our hypothesis that children sustaining a TBI exhibit difficulties on auditory and visual tasks of emotion recognition. However, results regarding environmental factors and age were more complex than we initially anticipated.
We found that, as a group, TBI participants demonstrate a faster rate of improvement in performance especially during the initial 12 months after injury. This is not surprising, as previous research demonstrates that the most significant recovery following pediatric brain injury occurs within the first year (Yeates et al., 2002). Therefore, TBI participants would be expected to show a greater rate of improvement as their performance is not only indicative of cognitive recovery but may also be influenced by maturation. Conversely, the baseline performance of OI participants is likely not significantly influenced by their injury; thus, their improvement over time will largely reflect maturational factors only.
The pattern becomes more complex when results are examined with regards to the influence of socioeconomic factors, as measured by the SCI. After 12 months, children sustaining a TBI with relatively high SCI (>+1 SD above the mean) experience a good recovery. The rapid improvement resulting from this accelerated upward trajectory causes the high SCI TBI group to outperform their high SCI OI peers as the study progressed. Conversely, children sustaining a TBI who have relatively lower SCI (< −1 SD below the mean) consistently exhibited a slower rate of recovery and were always outperformed by their low SCI OI peers. The differences in recovery patterns between the low SCI TBI and OI groups grew more pronounced as time progressed.
The current data cannot disambiguate whether these findings within the low SCI TBI group result from attenuated recovery or lack of age-expected gains. However, the percentage of severe patients in both low SCI and high SCI are very similar (72.7% vs. 77.8%). Thus, it is not likely that GCS score independently of SCI accounted for the differences between the low and high SCI groups.
Age at injury also influenced recovery, with younger children making faster gains in emotional prosody performance. Although somewhat attenuated by controlling for phonological discrimination performance, the patterns of recovery remained generally stable. Therefore, even though phonological processing difficulties may contribute to emotional prosody performance, they appear not to alter the trajectory of recovery within the TBI group.
Children sustaining a TBI exhibited consistently poorer performance on face emotion recognition when compared to the OI controls. Most participants in both groups scored above 90% accuracy on the face identity recognition task, and group differences in terms of total number of individuals not meeting the 90% cut off score disappeared after the three month assessment. These findings suggest the deficit displayed by the TBI group on face emotion recognition was specific to emotional stimuli and likely not the result of a more general deficit in face perception.
Although their baseline performance was lower, younger children demonstrated a faster recovery rate during the first year after injury when compared to older children on face emotion recognition. Females and individuals with a higher SCI score consistently outperformed and recovered faster than their male and low SCI peers on this task.
Participants injured at younger ages displayed a more rapid recovery in both tasks. Although the relationship between age at injury and recovery was somewhat different than we hypothesized, in some ways, this makes intuitive sense, as greater gains would be expected to occur in younger children as they are continuing to develop emotion recognition skills (Herba & Phillips, 2004). However, younger age at injury has also been associated with worse prognosis (Anderson et al., 2006; Hanten et al., 2009). Many of the studies to find this relationship have dealt with children injured before the age of five. Therefore, it is conceivable that within this age range (six-17 year-old) children who were relatively younger at the time of injury may still be expected to display a significantly faster rate of recovery as the recovery continues to be confounded with skills acquisition.
A faster recovery rate is not necessarily indicative of a better overall prognosis. Specifically, although younger children exhibit a more rapid recovery trajectory, their eventual performance may level off at a point far below what they may have obtained in the absence of an injury or below that of their peers who sustained an injury of similar severity at an older age. Similar to the current findings Hanten and colleagues (2009) used a prospective cohort to demonstrate that younger children displayed accelerated recovery of reading skills when compared to their older peers. The authors suggested that despite the increased rate of recovery noted in the prospective study, the retrospective results suggested that the longer-term prognosis for recovery was somewhat less optimistic.
It is also possible that age and mechanism of injury are confounded in the current sample. For example, it could be the case that there were more high-speed car crashes in the older group which lead to more brain lesions or a greater amount of white matter damage either of which could be influencing the current findings. A post-hoc analysis of the data revealed that although individuals sustaining a TBI secondary to a high-speed car crash tended to be slightly older, the difference in age was not statistically significant (p > .05).
The relationship of SCI to performance in both tasks is interesting when viewed in the context of other studies that have revealed group differences in recovery patterns based upon family influences (e.g., Catroppa & Anderson, 2003; Hanten et al., 2009; Hanten et al., 2008; Taylor et al., 2002; Taylor, Yeates, Wade, Drotar, & Klein, 1999; Yeates et al., 2004; Yeates et al., 1997). For example, Yeates and colleagues (1997) demonstrated that children in stable family environments had relatively better behavioral outcomes six and 12 months after severe head injury when compared to children residing in more stressed family systems. These investigators later showed that children with severe TBI exhibited poorer social outcomes compared to children with orthopedic injuries, especially when residing in families with limited resources and/or with significant family dysfunction (Yeates et al., 2004). Catroppa and Anderson (2003) found that children with low SES demonstrated poorer recovery of intellectual skills over a two year follow-up period.
It is interesting to speculate on the differences observed between the emotional prosody and the face emotion recognition tasks. First, it is unclear if the tasks differed in difficulty and if this may have differentially impacted the findings. The pattern of results do not overtly indicate this possibility, but the differences between groups on the two control tasks suggests that face perception may be a more robust ability than phonological processing. Second, sex effects were only observed within the face emotion sorting task. The precise reason for this difference is unknown; however, there is some research suggesting that there is a small (but statistically significant) female advantage for the processing of facial expression information (McLure, 2000). Thus, because there were no group by sex interactions, it is possible that the significantly faster rate of recovery observed in female participants is primarily secondary to development. Third, although SCI played a role in both tasks in terms of the pattern of recovery, the interaction between SCI, group, and rate of recovery was noted in the emotional prosody task only. Conflicting results involving emotional prosody performance in childhood TBI have been previously obtained, (see Snodgrass & Knott, 2006; Tonks et al., 2007b; Tonks et al., 2008; Turkstra et al., 2001); therefore, it is possible this type of procedure is sensitive to vagaries of the sample.
The present investigation has some significant advantages compared to previous studies – mainly, a substantially larger cohort, the analysis of data at five distinct time points over a two year follow-up period, and the ability to control for variables such as sex, age at injury, and SES due to the fairly large number of participants. Thus, we believe that the present results are fairly representative of the pattern of emotional prosody performance following childhood TBI. Findings from the phonological discrimination task suggest that lower-level language processing may play a role in the current results. Given that children with TBI frequently exhibit a number of language processing difficulties (Dennis, Purvis, Barnes, Wilkinson, & Winner, 2001; Ewing-Cobbs, Levin, Eisenberg, & Fletcher, 1987; Hanten et al., 2009), the interaction of language skills with SES and task performance may underlie the slightly different pattern observed between emotional prosody and face emotion recognition. However, it should be noted that the results of our correlational analysis suggested that many of the children who exhibited difficulties on one task of emotion recognition were the same children who demonstrated difficulties on the other task, at least at the baseline and 24-month occasions. This finding suggests the possibility that many children sustaining a TBI display a general deficit in emotion processing although it should be noted that OI participants exhibited a similar pattern of correlations. As previously discussed, this type of general emotion processing deficit may influence other aspects of social interaction (Lemerise & Arsenio, 2000) and may have long-term repercussions for future social skill acquisition and rehabilitation following pediatric TBI (Yeates et al., 2007).
Although it was not a goal of the present study to link emotion recognition deficits directly to brain imaging, it is interesting to briefly discuss those brain regions that are affected by TBI and that may be recruited during processing of emotional information. Previous research suggests that tasks involving emotional prosody recruit brain regions such as core language areas (Kotz et al., 2003) as well as fronto-opercular, fronto-temporal, and some subcortical areas bilaterally (Buchanan et al., 2000; Kotz et al., 2003). Other studies indicate that damage to either hemisphere may impair emotional prosody performance (Pell, 2006), although likely for different reasons. Research suggests a significant role for brain regions such as the fusiform gyrus, superior temporal sulcus, occipital face area, anterior part of the middle temporal gyrus, and orbitofrontal cortex in face emotion recognition (Dekowska, Kuniecki, & Jaskowski, 2008). Whereas a recent study found that emotional pictures (not necessarily pictures of faces) produced extensive activation within frontal brain regions (Brazdil et al., 2009).
The processing of emotional information regardless of modality recruits an extensive brain network including various frontal and temporal areas, regions which are sensitive to TBI (Wilde et al., 2005). Further, other research indicates that in addition to defuse damage, children sustaining moderate and severe TBIs often have focal frontal brain lesions that endure long past the acute stage (Levin et al., 1997) as well as diffuse white matter anomalies that may affect functional connectivity of cortical and subcortical circuits (Levin et al., 2008). Given the significant involvement of frontal structures likely in combination with a widely distributed network of other brain areas for the accurate processing and interpretation of emotional information, one can conjecture that various metrics of brain structure and function (cortical volumetrics, diffusion tensor imaging, functional activation, etc.) perturbed in TBI can be associated with the emotion processing deficits shown in the current investigation. Thus, it will be important for future studies to examine the relationship between various measures of brain structure and function and processing of visual and verbal emotional information.
Limitations and Future Directions
The current study has several important limitations that should be mentioned. First, as is the case with longitudinal data, not every subject can be followed-up at each time point. However, our retention rate does not appear significantly more problematic than other similar investigations. Second, the results are based on the findings from one task of emotional prosody and one task of face emotion recognition. Thus, more tasks may have yielded more robust and detailed results. Similarly, the tasks used have been used in previous research but not with children. It is possible that the use of other procedures with more child-specific stimuli may have slightly changed the nature of our findings. Finally, previous studies with adults have suggested that the perception of negative emotions is particularly perturbed after closed head injury (Bornhofen & McDonald, 2008b); however, due to the restricted number of trials of specific emotions in the tasks used, we were unable to investigate if this finding was born out in pediatric TBI. Therefore, it would be useful for future studies to investigate if recognition of specific emotions is altered following pediatric TBI or if deficits are more indicative of generalized deficits.
Regardless of these limitations, the current study is the first of which we are aware to systematically examine a large group of children with moderate to severe TBI on tasks of emotion recognition over a two-year follow-up period. The findings are in keeping with previous investigations that suggest the importance of environmental and developmental factors on recovery from childhood TBI. They open the way for future studies to examine in more detail family factors that influence emotion recognition performance, and studies examining the extent to which emotion recognition difficulties contribute to the psychosocial deficits that are so common and potentially debilitating following pediatric head injury.
Acknowledgments
This work was supported by NINDS grant # NS-21889 to HSL and by a T32 postdoctoral fellowship to ATS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Damasio H, Tranel D, Damasio AR. Cortical systems for the recognition of emotion in facial expressions. Journal of Neuroscience. 1996;16(23):7678–7687. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D. Intact recognition of emotional prosody following amygdala damage. Neuropsychologia. 1999;37:1285–1292. doi: 10.1016/s0028-3932(99)00023-8. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H. Emotion Recognition From Faces and Prosody Following Temporal lobectomy. Neuropsychology. 2001;15(3) doi: 10.1037//0894-4105.15.3.396. [DOI] [PubMed] [Google Scholar]
- Allerdines MD, Alfano DP. Neuropsychological correlates of impaired emotion recognition following traumatic brain injury. Brain and Cognition. 2006;60:193–217. doi: 10.1016/j.bandc.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. Journal of the International Neuropsychological Society. 2006;12(2):224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Andrews TK, Rose FD, Johnson DA. Social and behavioural effects of traumatic brain injury in children. Brain Injury. 1998;12(2):133–138. doi: 10.1080/026990598122755. [DOI] [PubMed] [Google Scholar]
- Ariatti A, Benuzzi F, Nichelli P. Recognition of emotions from visual and prosodic cues in Parkinson's disease. Neurological Sciences. 2008;29(4):219–227. doi: 10.1007/s10072-008-0971-9. [DOI] [PubMed] [Google Scholar]
- Bornhofen C, McDonald S. Comparing strategies for treating emotion perception deficits in traumatic brain injury. Journal of Head Trauma and Rehabilitation. 2008a;23(2):103–115. doi: 10.1097/01.HTR.0000314529.22777.43. [DOI] [PubMed] [Google Scholar]
- Bornhofen C, McDonald S. Emotion perception deficits following traumatic brain injury: A review of the evidence and rationale for intervention. Journal of the International Neuropsychological Society. 2008b;14:511–525. doi: 10.1017/S1355617708080703. [DOI] [PubMed] [Google Scholar]
- Braun CM, Baribeau JM, Ethier M, Daigneault S, Proulx R. Processing of pragmatic and facial affective information by patients with closed-head injuries. Brain Injury. 1989;3(1):5–17. doi: 10.3109/02699058909008068. [DOI] [PubMed] [Google Scholar]
- Brazdil M, Roman R, Urbanek T, Chladek J, Spok D, Marecek R, et al. Neural correlates of affective picture processing - A depth ERP study. NeuroImage. 2009;47(376–383) doi: 10.1016/j.neuroimage.2009.03.081. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lutz K, Mirzazade S, Specht K, Shah NJ, Zilles K, et al. Recognition of emotional prosody and verbal components of spoken language: an fMRI study. Brain Research Cognitive Brain Research. 2000;9(3):227–238. doi: 10.1016/s0926-6410(99)00060-9. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane T, Antoun FN, Young AW. Impaired recognition and experience of disgust following brain injury. Nature Neuroscience. 2000;3(11):1076–1078. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson V. Recovery and predictors of intellectual ability two years following paediatric traumatic brain injury. Rehabilitation. 2003;13(5):517–536. [Google Scholar]
- Charbonneau S, Scherzer BP, Aspirot D, Cohen H. Perception and production of facial and prosodic emotions by chronic CVA patients. Neuropsychologia. 2003;41:605–613. doi: 10.1016/s0028-3932(02)00202-6. [DOI] [PubMed] [Google Scholar]
- Copes WS, Lawnick M, Champion HR, Sacco WJ. A comparison of Abbreviated Injury Scale: 1980 and 1985. Journal of Trauma. 1988;28:78–86. doi: 10.1097/00005373-198801000-00011. [DOI] [PubMed] [Google Scholar]
- Croker V, McDonald S. Recognition of emotion from facial expression following traumatic brain injury. Brain Injury. 2005;19(10):787–799. doi: 10.1080/02699050500110033. [DOI] [PubMed] [Google Scholar]
- Dekowska M, Kuniecki M, Jaskowski P. Facing facts: Neuronal mechanisms of face perception. Acta neurobiologiae experimentalis. 2008;68:229–252. doi: 10.55782/ane-2008-1692. [DOI] [PubMed] [Google Scholar]
- Dennis M, Purvis K, Barnes MA, Wilkinson M, Winner E. Understanding of literal truth, ironic criticism, and deceptive praise following childhood head injury. Brain and Language. 2001;78(1):1–16. doi: 10.1006/brln.2000.2431. [DOI] [PubMed] [Google Scholar]
- Ekman P. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists; 1976. [Google Scholar]
- Ewing-Cobbs L, Levin HS, Eisenberg HM, Fletcher JM. Language functions following closed head injury in children and adolescents. Experimental Neuropsychology. 1987;9:575–592. doi: 10.1080/01688638708410770. [DOI] [PubMed] [Google Scholar]
- Fay TB, Yeates KO, Wade SL, Drotar DD, Stancin T. Predicting longitudinal patterns of functional deficits in children with Traumatic Brain Injury. Neuropsychology. 2009;23(3):271–282. doi: 10.1037/a0014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green REA, Turner GR, Thompson WF. Deficits in facial emotion perception in adults with recent traumatic brain injury. Neuropsychologia. 2004;42:133–141. doi: 10.1016/j.neuropsychologia.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Hanten G, Li X, Newsome MR, Swank P, Chapman SB, Dennis M, et al. Oral reading and expressive language after childhood traumatic brain injury: Trajectory and correlates of change over time. Topics in Language Disorders. 2009;29(3):236–248. [Google Scholar]
- Hanten G, Martin RC. Contributions of phonological and semantic short-term memory to sentence processing: Evidence from two cases of closed head injury in children. Journal of Memory & Language. 2000;43(2):335. [Google Scholar]
- Hanten G, Wilde EA, Menefee DS, Li X, Vasquez C, Swank P, et al. Correlates of Social Problem Solving During the First Year After Traumatic Brain Injury in Children. Neuropsychology. 2008;22(3):357–370. doi: 10.1037/0894-4105.22.3.357. [DOI] [PubMed] [Google Scholar]
- Hayes CJ, Stevenson RJ, Coltheart M. Disgust and Huntington's disease. Neuropsychologia. 2007;45(6):1135–1151. doi: 10.1016/j.neuropsychologia.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Henry JD, Phillips LH, Crawford JR, Ietswaart M, Summers F. Theory of mind following traumatic brain injury: the role of emotion recognition and executive dysfunction. Neuropsychologia. 2006;44(10):1623–1628. doi: 10.1016/j.neuropsychologia.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Herba C, Phillips M. Annotation: Development of facial expression recognition from childhood to adolescence: behavioral and neurological perspectives. Journal of Child Psychology and Psychiatry. 2004;45(0):1–14. doi: 10.1111/j.1469-7610.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- Hopkins MJ, Dywan J, Segalowitz SJ. Altered electrodermal response to facial expression after closed head injury. Brain Injury. 2002;16(3):245–257. doi: 10.1080/02699050110103346. [DOI] [PubMed] [Google Scholar]
- Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioral changes following ventral frontal lobe damage. Neuropsychologia. 1996;34:247–261. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Ietswaart M, Milders M, Crawford JR, Currie D, Scott CL. Logitudinal aspects of emotion recognition in patients with traumatic brain injury. Neuropsychologia. 2008;46:148–159. doi: 10.1016/j.neuropsychologia.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Izard C, Fine S, Schultz D, Mostow A, Ackerman B, Youngstrom E. Emotion knowledge as a predictor of social behavior and academic competence in children at risk. Psychological Science. 2001;12(1):18–23. doi: 10.1111/1467-9280.00304. [DOI] [PubMed] [Google Scholar]
- Jackson HF, Moffat NJ. Impaired emotional recognition following sever head injury. Cortex. 1987;23:293–300. doi: 10.1016/s0010-9452(87)80039-4. [DOI] [PubMed] [Google Scholar]
- Kotz S, Meyer M, Alter K, Besson M, von Cramon DY, Friederici AD. On the lateralization of emotional prosody: And event-related functional MR investigation. Brain and Language. 2003;86:366–376. doi: 10.1016/s0093-934x(02)00532-1. [DOI] [PubMed] [Google Scholar]
- Kucharska-Pietura K, Phillips ML, Gernand W, David AS. Perception of emotions from faces and voices following unilateral brain damage. Neuropsychologia. 2003;41:1082–1090. doi: 10.1016/s0028-3932(02)00294-4. [DOI] [PubMed] [Google Scholar]
- Lemerise EA, Arsenio WF. An integrated model of emotion processes and cognition in social information processing. Child Development. 2000;71(1):107–118. doi: 10.1111/1467-8624.00124. [DOI] [PubMed] [Google Scholar]
- Leppanen JM, Hietanen JK. Emotion recognition and social adjustment in school-aged girls and boys. Scand J Psychol. 2001;42(5):429–435. doi: 10.1111/1467-9450.00255. [DOI] [PubMed] [Google Scholar]
- Levin HS. Neuroplasticity and brain imaging research: implications for rehabilitation. Archives of Physical Medicine and Rehabilitation. 2006;87(12 Suppl):1. doi: 10.1016/j.apmr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Roberson G, Li X, Ewing-Cobbs L, Dennis M, et al. Prediction of cognitive sequelae based on abnormal computed tomography findings in children following mild traumatic brain injury. Journal of Neurosurgery Pediatrics. 2008;1:461–470. doi: 10.3171/PED/2008/1/6/461. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Zhang L, Swank P, Ewing-Cobbs L, Dennis M. Changes in working memory after traumatic brain injury in children. Neuropsychology. 2004;18:240–247. doi: 10.1037/0894-4105.18.2.240. [DOI] [PubMed] [Google Scholar]
- Levin HS, Mendelsohn D, Lilly MA, Yeakley J, Song J, Scheibel RS, et al. Magnetic resonance imaging in relation to functional outcome of pediatric closed head injury: a test of the Ommaya-Gennarelli model. Neurosurgery. 1997;40(3):432–441. doi: 10.1097/00006123-199703000-00002. [DOI] [PubMed] [Google Scholar]
- Lew HL, Poole JH, Chiang JYP, Lee EH, Date ES, Warden D. Event-related potential in facial affect recognition: Potential clinical utility in patients with traumatic brain injury. Journal of Rehabilitation Research and Development. 2005;42(1):29–34. doi: 10.1682/jrrd.2004.05.0056. [DOI] [PubMed] [Google Scholar]
- McDonald S. Are you crying or laughing? Emotion recognition deficits after severe traumatic brain injury. Brain Impairment. 2005;56(6):56–67. [Google Scholar]
- McDonald S, Flanagan S, Rollins J, Kinch J. TASIT: A new clinical tool for assessing social perception after traumatic brain injury. Journal of Head Trauma and Rehabilitation. 2003;18(3):219–238. doi: 10.1097/00001199-200305000-00001. [DOI] [PubMed] [Google Scholar]
- McLure EB. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychological Bulletin. 2000;126:424–453. doi: 10.1037/0033-2909.126.3.424. [DOI] [PubMed] [Google Scholar]
- Meletti S, Benuzzi F, Cantalupo G, Rubboli G, Tassinari CA, Nichelli P. Facial emotion recognition impairment in chronic temporal lobe epilepsy. Epilepsia. 2009;50(6):1547–1549. doi: 10.1111/j.1528-1167.2008.01978.x. [DOI] [PubMed] [Google Scholar]
- Milders M, Fuchs S, Crawford JR. Neuropsychological Impairments and Changes in Emotional and Social Behaviour Following Severe Traumatic Brain Injury. Journal of Clinical and Experimental Neuropsychology. 2003;25(2):157–172. doi: 10.1076/jcen.25.2.157.13642. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos BS. LISRES-A: Life Stressors and Social Resources Inventory - Adult Form Manual. Odessa: Psychological Assessment Resources, Inc; 1994. [Google Scholar]
- Pell MD. Cerebral mechanisms for understanding emotional prosody in speech. Brain and Language. 2006;96:221–234. doi: 10.1016/j.bandl.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Pettersen L. Sensitivity to emotional cues and social behavior in children and adolescents after head injury. Percept Mot Skill. 1991;73(3 Pt 2):1139–1150. doi: 10.2466/pms.1991.73.3f.1139. [DOI] [PubMed] [Google Scholar]
- Prigatano GP, Pribram KH. Perception and memory for facial affect following brain injury. Perceptual and Motor Skills. 1982;54:859–869. doi: 10.2466/pms.1982.54.3.859. [DOI] [PubMed] [Google Scholar]
- Ratcliff JJ, Greenspan AI, Goldstein FC, Stringer AY, Bushnik T, Hammond F, et al. Gender and traumatic brain injury: Do the sexes fare differently. Brain Injury. 2007;21(10):1023–1030. doi: 10.1080/02699050701633072. [DOI] [PubMed] [Google Scholar]
- Snodgrass C, Knott F. Theory of mind in children with traumatic brain injury. Brain Injury. 2006;20(8):825–833. doi: 10.1080/02699050600832585. [DOI] [PubMed] [Google Scholar]
- Spell LA, Frank E. Recognition of nonverbal communication of affect following traumatic brain injury. Journal of Nonverbal Behavior. 2000;24(4):285–300. [Google Scholar]
- Stevens G, Featherman DL. A revised socioeconomic index of occupational status. Social Science Research. 1981;10:364–395. [Google Scholar]
- Taylor HG, Wade SL, Stancin T, Yeates KO, Drotar DD, Minich N. A Prospective Study of Short-and Long-Term Outcomes After Traumatic Brain Injury in Children: Behavior and Achievement. Neuropsychology. 2002;16(1):15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Yeates KO, Wade SL, Drotar DD, Klein SK. Influences on First-Year Recovery From Traumatic Brain Injury in Children. Neuropsychology. 1999;13(1):76–89. doi: 10.1037//0894-4105.13.1.76. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tonks J, Williams WH, Frampton I, Yates P, Slater A. Assessing emotion recognition in 9–15-years olds: Preliminary analysis of abilities in reading emotions from faces, voices and eyes. Brain Injury. 2007a;21(6):623–629. doi: 10.1080/02699050701426865. [DOI] [PubMed] [Google Scholar]
- Tonks J, Williams WH, Frampton I, Yates P, Slater A. Reading emotions after child brain injury: A comparison between children with brain injury and non-injured controls. Brain Injury. 2007b;21(7):731–739. doi: 10.1080/02699050701426899. [DOI] [PubMed] [Google Scholar]
- Tonks J, Williams WH, Frampton I, Yates P, Slater A. The neurological bases of emotional dys-regulation arising from brain injury in childhood: A 'when and where' heuristic. Brain Impairment. 2007c;8(2):143–153. [Google Scholar]
- Tonks J, Williams WH, Frampton I, Yates P, Wall SE, Slater A. Reading emotions after childhood brain injury: Case series evidence of dissociation between cognitive abilities and emotional expression processing skills. Brain Injury. 2008;22(4):325–332. doi: 10.1080/02699050801968303. [DOI] [PubMed] [Google Scholar]
- Turkstra LS, McDonald S, DePompeii R. Social Information Processing in Adolescents: Data from Normally Developing Adolescents and Preliminary Data from Their Peers with Traumatic Brain Injury. Journal of Head Trauma Rehabilitation. 2001;16(5):469–483. doi: 10.1097/00001199-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Watts AJ, Douglas AM. Interpreting facial expression and communication competence following severe traumatic brain injury. Aphasiology. 2006;20(8):707–722. [Google Scholar]
- Wilde EA, Hunter JV, Newsome MR, Scheibel RS, Bigler ED, Johnson JL, et al. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J Neurotrauma. 2005;22(3):333–344. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, et al. Social outcomes in childhood brain disorder: a heuristic integration of social neuroscience and developmental psychology. Psychological Bulletin. 2007;133(3):535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Swift E, Taylor HG, Wade SL, Drotar DD, Stancin T, et al. Short-and long-term social outcomes following pediatric traumatic brain injury. Journal of the International Neuropsychological Society. 2004;10:412–426. doi: 10.1017/S1355617704103093. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Drotar DD, Wade SL, Klein S, Stancin T, et al. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. Journal of the International Neuropsychological Society. 1997;3:617–630. [PubMed] [Google Scholar]
- Yeates KO, Wade SL, Stancin T, Taylor HG, Drotar DD, Minich N. A Prospective Study of Short-and Long-Term Neuropsychological Outcomes After Traumatic Brain Injury in Children. Neuropsychology. 2002;16(4):514–523. doi: 10.1037//0894-4105.16.4.514. [DOI] [PubMed] [Google Scholar]