Abstract
Background
Mammalian forkhead members of the class O (FOXO) transcription factors, including FOXO1, FOXO3a, and FOXO4, are implicated in the regulation of several biological processes, including the stress resistance, metabolism, cell cycle, apoptosis and DNA repair. The objectives of this study were to examine the molecular mechanisms by which FOXO transcription factors induced cell cycle arrest and apoptosis and enhanced anti-proliferative effects of sulforaphane (SFN, an active compound in cruciferous vegetables) in pancreatic cancer cells.
Results
Our data demonstrated that SFN inhibited cell proliferation and colony formation, and induced apoptosis through caspase-3 activation in pancreatic cancer cells. The inhibition of PI3K/AKT and MEK/ERK pathways activated FOXO transcription factors. SFN inhibited phosphorylation of AKT and ERK, and activated FOXO transcription factors, leading to cell cycle arrest and apoptosis. Phosphorylation deficient mutants of FOXO proteins enhanced FOXO transcriptional activity, and further enhanced SFN-induced FOXO activity and apoptosis. SFN induced the expression of p21/CIP1 and p27/KIP1, and inhibited the expression of cyclin D1.
Conclusion
These data suggest that inhibition of PI3K/AKT and ERK pathways acts together to activate FOXO transcription factor and enhances SFN-induced FOXO transcriptional activity, leading to cell cycle arrest and apoptosis.
Background
Cancer of the pancreas is the fourth leading cause of cancer death in the United States. This year approximately 32,000 Americans will die from cancer of the pancreas. With an overall 5-year survival rate of 3% [1], pancreatic cancer has one of the poorest prognoses among all cancers [2]. Only 20% of pancreatic cancer patients are eligible for surgical resection, which currently remains the only potentially curative therapy [3]. Unfortunately, many cancers of the pancreas are not resectable at the time of diagnosis. There are limited treatment options available for this disease because chemo- and radio-therapies are largely ineffective, and metastatic disease frequently redevelops even after surgery [1,2]. Therefore, developing effective strategies to prevent pancreatic neoplasms are of paramount importance.
Sulforaphane (SFN), a constituent of cruciferous vegetables, is a naturally occurring isothiocyanate with promising chemopreventive activity [4]. Epidemiological studies have shown that people who eat cruciferous vegetables have reduced incidence of breast and prostate cancer. SFN possesses anti-oxidant, anti-proliferative and anti-carcinogenic properties [5-7]. SFN is effective in preventing chemically induced breast [8,9], stomach [5] and colon [10] cancers in rats. We and others have shown that SFN inhibited the growth of prostate, breast, oral and squamous carcinoma xenografts [11-15]. SFN enhanced radiosensitivity of tumor cells in vitro and in vivo [16]. Furthermore, a pharmacokinetic study has demonstrated that it is rapidly absorbed and 82% bioavailable [17]. SFN induces a phase 2 enzyme, thereby neutralizing carcinogens before they can damage DNA [18,19]. SFN inhibits benzo[a]pyrene-DNA and 1,6-dinitropyrene-DNA adducts formation [20-23], and downregulates PI3K/AKT [24,25] and NFκB [12,26,27] pathways. We have recently demonstrated that SFN induces death receptors (DR4 and DR5) and proapoptotic members of Bcl-2 family, inhibits antiapoptotic Bcl-2 proteins, activates caspase(s), and enhances apoptosis-inducing potential of TRAIL in vitro [12]. In vivo, SFN inhibits growth of PC-3 cells orthotopically implanted in nude mice by inducing apoptosis and inhibiting tumor cell proliferation, metastasis and angiogenesis [12]. These studies strongly suggest that SFN can be developed as a cancer preventive agent.
PTEN (phosphatase and tensin homolog deleted on chromosome 10, also called MMAC1 or TEP1) is a tumor suppressor gene [28-30], which is frequently deleted or mutated in a wide range of human cancers, including glioblastoma [31], melanoma [32], and prostate [33], breast [34], and endometrial cancers [35]. While point mutations in PTEN rarely occur in pancreatic cancer [36,37], functional inactivation of PTEN through promoter methylation [38], loss of protein expression [39], reduction of mRNA levels [40], or loss of heterozygocity (LOH) of linked markers [37,41] occur with high frequency. Phosphatidylinositol 3,4,5-trisphosphate (PIP3) is a substrate of PTEN [42-44]. AKT is a serine-threonine protein kinase regulated by PIP3 that is implicated in survival signaling in a wide a variety of cells, including fibroblastic, epithelial, and neuronal cells [45]. PTEN increases sensitivity to cell death in response to several apoptotic stimuli by negatively regulating the PI3K/AKT pathway [43]. In addition to its role in regulating the PI3K/AKT cell survival pathway, PTEN also inhibits growth factor-induced Shc phosphorylation and suppresses the MAP kinase signaling pathway [46], suggesting that PTEN has roles in independent of PI3K/AKT signaling pathway. Hyperactivation of AKT is associated with resistance to apoptosis, increased cell growth, cell proliferation, metastasis, angiogenesis, and cellular energy metabolism [45,47-54]. Overexpression of AKT has been reported in a variety of human cancers, including pancreatic cancer, and cells expressing elevated levels of AKT are less sensitive to apoptosis stimuli [38,55-57]. Antagonizing PI3K activity negatively regulates AKT activity. Once activated, however, AKT exerts antiapoptotic effects through phosphorylation of substrates such as Bad [58,59] and caspase-9 [60] that directly regulate the apoptotic machinery, or human telomerase reverse transcriptase subunit [61], forkhead transcription family members [62,63] and IB kinases [64] that indirectly inhibit apoptosis [65]. Studies in pancreatic cancer cell lines have demonstrated that PI3K is required for growth and survival of tumor cells [66-68]. Furthermore, amplification or activation of AKT2 occurs in up to 60% of pancreatic cancer [39,69-71], supporting the participation of an activated PI3K-AKT axis in this disease.
FOXO subfamily of forkhead transcription factors include FOXO1a/FKHR, FOXO3a/FKHRL1, and FOXO4/AFX [72-75]. The PI3K pathway, via activation of its downstream kinase AKT, phosphorylates each of the FOXO proteins [62,76,77]. These phosphorylations result in impairment of DNA binding ability and increased binding affinity for the 14-3-3 protein [62,77]. Newly formed 14-3-3-FOXO complexes are then exported from the nucleus [78], thereby inhibiting FOXO-dependent transcription. Inhibition of the PI3K pathway leads to dephosphorylation and nuclear translocation of active FKHRL1, FKHR, and AFX; which induce cells cycle arrest and apoptosis [79]. Conversely, loss of PTEN activity results in increased AKT activity leading to inhibition of FOXO protein activity through phosphorylation and cytoplasmic sequestration. In addition, the data demonstrate that FOXO transcriptional activity controls cellular proliferation and apoptosis downstream of PTEN [80,81]. FOXO regulates cell cycle and apoptotic genes such as cyclin-dependent kinase inhibitor (CKI) p27KIP1 [78,80,82,83], Bim [84,85], Fas ligand [62], and Bcl-6 [86]. Consequently, activation of the PI3K pathway serves to repress FOXO-mediated growth arrest and apoptosis. However, regulation of FOXO target genes is multifactorial, and therefore other transcription factors and post-translation regulatory events will influence the final level of protein expression. Interestingly, overexpression of AKT, and inactivation and loss of PTEN are frequently observed in pancreatic cancer [39,66-71], indicating a potential role for FOXOs in modulating both cell cycle and apoptosis during tumorigenesis and treatment. Together, these results indicate that FOXO proteins are important downstream effectors of PTEN tumor suppressive activity; however, their molecular targets and mechanisms of action in pancreatic cancer are not well understood.
The Ras proteins are small (21 kDa) GTP-binding, membrane-associated proteins [87]. The Ras proteins transduce signals from ligand-activated tyrosine kinase receptors to downstream effectors [88]. Activating mutations can impair GTP hydrolysis and lead to constitutively activated Ras that impacts the cellular phenotype [89]. Oncogenic Ras can lead to cellular transformation [90], presumably by perturbing its signal transduction pathways. Ras regulates multiple signaling pathways [91]. Three major groups of MAP kinases are found in mammalian cells: extracellular signal-regulated protein kinase (ERK) [92], p38 MAP kinase [93], and c-Jun N-terminal kinase (JNK) [94-96]. MAP kinases regulate many cellular activities, which range from gene expression to mitosis, movement, metabolism, and apoptosis. These MAP kinases are activated by the dual phosphorylations of neighboring threonine and tyrosine residues in response to various extracellular stimuli [97,98]. Specifically, p38 and JNK have been implicated in stress-responsive signaling leading to the initiation of adaptive events such as gene expression, differentiation, metabolism, and apoptosis [94,95,99]. ERKs are often activated by growth signals, such as epidermal growth factor (EGF) or platelet-derived growth factor [100]. We have recently demonstrated that inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to regulate antiangiogenic effects of EGCG and SFN through activation of FOXO transcription factors [24,101].
Furthermore, FOXO transcription factors play a crucial role in the regulation of tissue homeostasis in organs such as the pancreas, and complex diseases such as diabetes and cancer. Unfortunately, the intracellular mechanisms by which SFN inhibits growth and induces apoptosis in pancreatic cancer cells through regulation of FOXO transcription factors have never been examined. The objectives of our study were to examine the molecular mechanisms by which FOXO transcription factors induce cell cycle arrest and apoptosis and enhances the anti-proliferative effects of SFN in pancreatic cancer cells. Our results demonstrate that inhibition of PI3K/AKT and ERK pathways activates FOXO transcription factors. SFN inhibited phosphorylation of AKT and ERK, and dephosphorylated FOXO transcription factors, leading to cell cycle arrest and apoptosis. Phosphorylation deficient mutants of FOXO proteins enhanced FOXO transcriptional activity, and further enhanced SFN-induced FOXO activity.
Results
Sulforaphane (SFN) inhibits cell growth in human pancreatic cancer cells
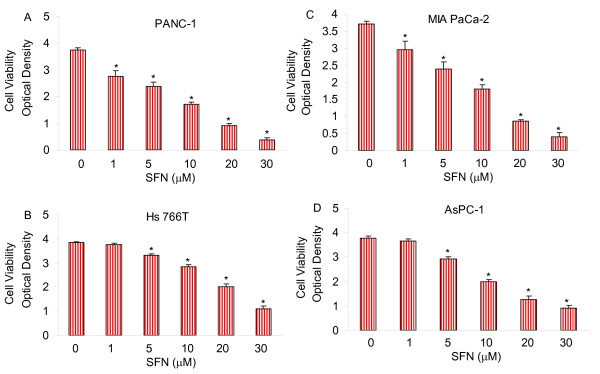
We first examined the effects of SFN on cell proliferation in four pancreatic cancer cell lines by XTT assay. We have selected four pancreatic cancer cell lines (MIA PaCa-2, AsPC-1, PANC-1 and Hs766T) because they have been derived from different pathological stages and may thus respond differently to SFN [102,103]. MIA PaCa-2 harbors a point mutation on Kras gene resulting in amino acid sunbstitution from the wild-type glycine to a valine at codon 12. AsPC-1 and PANC-1 harbor a point mutation on Kras gene resulting in amino acid substitution from glycine to aspartate. Hs766T cell line does not possess a point mutation in codon 12 of the Kras gene. SFN inhibited cell viability in a dose dependent manner (Fig. 1). PANC-1 and MIA PaCa-2 cell lines were most sensitive, AsPC-1 cell line was moderately sensitive, and Hs 766T cell line was least sensitive. These data suggest that SFN can be a viable agent for inhibiting pancreatic cancer cell proliferation.
Figure 1.
Effect of sulforaphane (SFN) on viability of pancreatic cancer cells. Pancreatic cancer (PANC-1, MIA PaCa-2, Hs766T and AsPC-1) cells were treated with SFN (0-30 μM) for 48 h. Cell viability was measured by XTT assay. Data represent the mean ± S.D. * = significantly different from respective controls, P < 0.05.
Sulforaphane inhibits colony formation in human pancreatic cancer cells
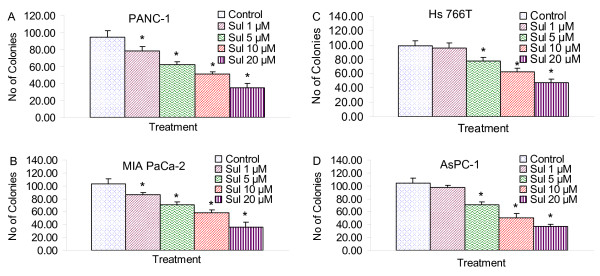
We next examined the effects of SFN on colony formation (a characteristic of cancer) on four pancreatic cancer cell lines by soft agar assay. SFN inhibited colony formation in a dose dependent manner (Fig. 2). Colonies formed by PANC-1 and MIA PaCa-2 cells were most sensitive, AsPC-1 cell line was moderately sensitive, and Hs 766T cell line was least sensitive. These data suggest that SFN can be used as a potent chemopreventive agent for pancreatic cancer.
Figure 2.
Effect of sulforaphane (SFN) on colony formation. Pancreatic cancer (PANC-1, MIA PaCa-2, Hs766T and AsPC-1) cells were treated with SFN (0-20 μM), and number of colonies were counted. Data represent the mean ± S.D. * = significantly different from respective controls, P < 0.05.
Sulforaphane induces caspase-3 activation in human pancreatic cancer cell
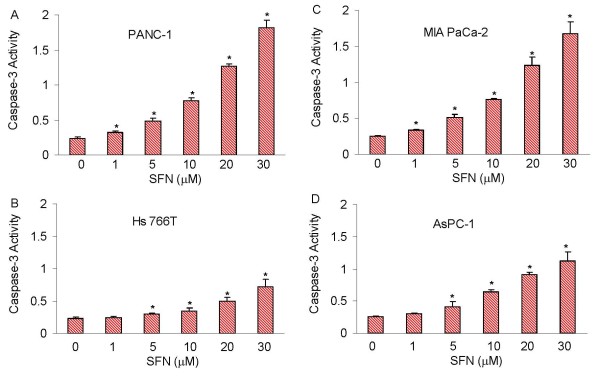
Most chemopreventive agents induce apoptosis through mitochondrial pathway, which activates caspase-3 [104]. We therefore examined whether SFN-induced apoptosis through caspase-3 activation in pancreatic cancer cell lines (Fig. 3). SFN induced caspase-3 activity in PANC-1, MIA PaCa-2, Hs 766T and AsPC-1 cells. However, a relatively high dose of SFN was required to activate caspase-3 in Hs 766T cells compared to other pancreatic cancer cell lines. These data suggest that SFN induced apoptosis through caspase-3 activation and may engage the mitochondria.
Figure 3.
Effect of sulforaphane (SFN) on caspase-3 activity. Pancreatic cancer PANC-1, MIA PaCa-2, Hs 766T and AsPC-1 cells were treated with SFN (0-30 μM) for 12 h and caspase-3 activity was measured as per manufacturer's instructions (EMD Biosciences). Data represent the mean ± S.D. * = significantly different from respective controls, P < 0.05.
Regulation and function of PI3K/AKT and MAP kinase pathways by sulforaphane
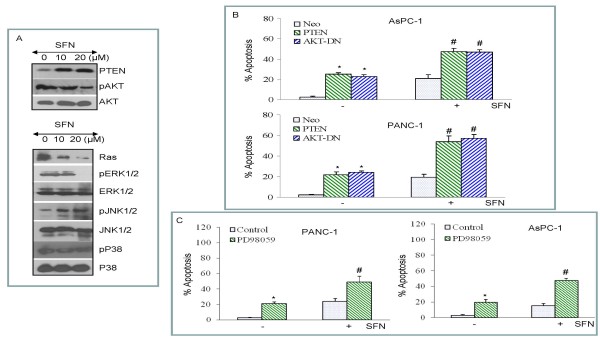
In most cancer cells, AKT is constitutively active and enhances cell proliferation [105]. In order to understand a relationship between PTEN and AKT in SFN-induced apoptosis, we measured the expression of PTEN and phosphorylation status of AKT in cells treated with SFN (Fig. 4A). SFN induces PTEN expression and inhibits AKT phosphorylation in pancreatic cancer PANC-1 cells. By comparison, SFN has no effect on total AKT expression. These data suggest that SFN inhibits cell proliferation by regulating PI3K/AKT pathway.
Figure 4.
Effects of sulforaphane (SFN) on the expression of PTEN, AKT, and MAP kinases; and the effects of PI3K/AKT and MAPK pathways on SFN-induced apoptosis. (A), PANC-1 cells were treated with or without SFN (0-20 μM) for 24 h. The cells were harvested and the expression of PTEN, phospho-AKT, AKT, Ras, phospho-ERK, ERK, phospho-JNK, JNK, phospho-p38 and p38 was measured by Western blotting. (B), PTEN and dominant negative AKT enhance SFN-induced apoptosis. AsPC-1 and PANC-1 cells were transiently transfected with empty vector (pcDNA3.1), PTEN wild type (PTEN-WT) or dominant negative AKT (AKT-DN) along with pCMV-LacZ vector (as transfection control) for 24 h. After medium replacement, cells were treated with SFN (10 μM) for 48 h and, apoptosis was measured by Live Dead Assay. Data represent the mean ± S.D. *, # = significantly different from respective controls, P < 0.05. (C), MEK inhibitor PD98059 enhances SFN-induced apoptosis. AsPC-1 and PANC-1 cells were pretreated with PD98059 (1 μM) followed by treatment with SFN (10 μM) for 48 h and, apoptosis was measured by Live Dead Assay. Data represent the mean ± S.D. *, # = significantly different from respective controls, P < 0.05.
Ras/Raf/MAP kinase pathway regulates many cellular activities, which range from gene expression to mitosis, movement, metabolism, and apoptosis [94,106-109]. We therefore examined the effects of SFN on the expression of Ras, and activation of ERK, JNK and p38 MAP kinases. SFN inhibited Ras expression in PANC-1 cells (Fig. 4A). Treatment of PANC-1 cells with SFN caused a decrease in ERK phosphorylation, and an increase in JNK phosphorylation. SFN has no significant effect on p38 MAP kinase activity in PANC-1 cells. These data suggest that SFN inhibits growth and induces apoptosis through regulation of Ras/Raf/MAP kinase pathway.
We next examined whether SFN induces apoptosis through PI3K/AKT pathway (Fig. 4B). Pancreatic cancer cells were transfected with empty vector, wild type PTEN, dominant negative AKT (DN-AKT), and apoptosis was measured. Overexpression of wild type PTEN or DN-AKT induced apoptosis in AsPC-1 and PANC-1 cells. Treatment of transfected cells with SFN further enhanced apoptosis. These data suggest that inhibition of PI3K/AKT pathway enhances SFN-induced apoptosis in pancreatic cancer cells.
We next examined whether inhibition of MEK/ERK pathway enhances SFN-induced apoptosis in pancreatic cancer cells. MEK1/2 inhibitor (PD98059) induced apoptosis in PANC-1 and AsPC-1 cells (Fig. 4C). PD98059 enhanced SFN-induced apoptosis. Overall, these data suggest that inhibition of PI3K/AKT and MEK/ERK pathways enhanced SFN-induced apoptosis.
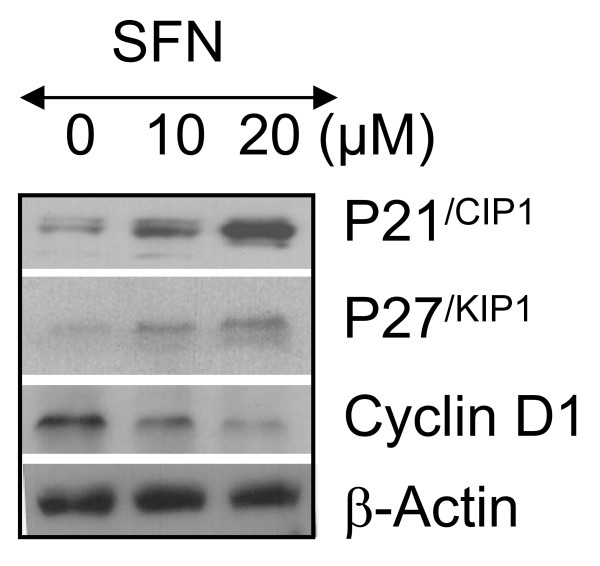
Sulforaphane induces p21/WAF1/CIP1, and p27/KIP1 and inhibits cyclin D1
PI3K/AKT signaling pathway may be involved in the control of the cell cycle progression most likely through mechanisms involving the activation of FOXO transcription factors [82]. We next examined the effects of SFN on cell cycle regulatory genes. SFN induced the expression cell cycle inhibitors p21/WAF1/CIP1 and p27/KIP1, and inhibited the expression of cyclin D1 in PANC-1 cells (Fig. 5). These data suggest that SFN causes growth arrest by regulating expression of cell cycle genes.
Figure 5.
Effects of sulforaphane (SFN) on cell cycle regulatory genes. PANC-1 cells were treated with SFN (0-20 μM) for 24 h. The expression of p21/CIP1, p27/KIP1 and cyclin D1 was measured by Western blotting. Anti β-actin antibody was used as a loading control.
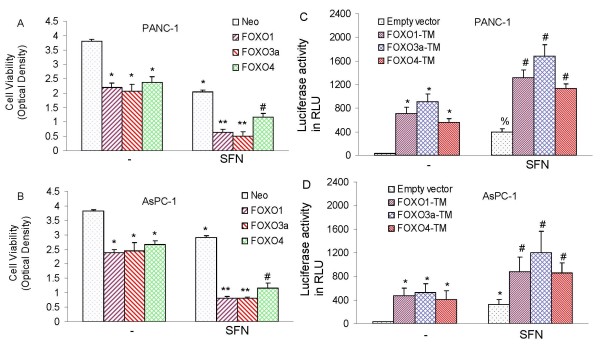
Overexpression of FOXO transcription factors inhibits cell viability and enhances FOXO transcriptional activity in pancreatic cancer cells
In order to examine whether FOXO transcription factors affect the ability of SFN to inhibit cell viability, pancreatic cancer cells were transfected with FOXO1, FOXO3a or FOXO4 (Fig. 6A and 6B). FOXO expression plasmids and FOXO-luciferase construct (pGL3-6X DBE) have previously been described [101]. Overexpression of FOXO1, FOXO3a, and FOXO4 inhibited cell viability in PANC-1 and AsPC-1 cells. The inhibitory effects of SFN on cell viability were further enhanced when pancreatic cancer cells were transfected with FOXO1, FOXO3a, and FOXO4. These data suggest that FOXO transcription factors can enhance the antiproliferative effects of SFN.
Figure 6.
Effects of FOXO transcription factors on cell viability and FOXO transcriptional activity. (A and B), PANC-1 and AsPC-1 cells were transiently transfected with plasmids expressing neo (pcDNA3.1), FOXO1, FOXO3a, or FOXO4 along with pCMV-LacZ vector (as transfection control). After transfection, cells were treated with or without SFN (10 μM) for 48 h, and cell viability was measured by XTT assay. Data represent the mean ± S.D. * = significantly different from respective controls, P < 0.05. (C and D), Phosphorylation deficient mutants of FOXO enhance sulforaphane-induced FOXO transcriptional activity in pancreatic cancer. PANC-1 and AsPC-1 cells were transiently transfected with empty vector or constructs encoding FOXO1-TM, FOXO3a-TM, or FOXO4-TM together with 6X DBE-luciferase for 24 h. After transfection, cells were washed with RPMI, treated with SFN (10 μM) for 24 h, and harvested for firefly/Renilla luciferase assays using the Dual-Luciferase Reporter Assay System (Promega). Luciferase counts were normalized using Renilla luciferase transfection control (pRL-TK; Promega). Data represent the mean ± S.D. * = significantly different from respective controls, P < 0.05.
We next examined whether SFN induces transcriptional activation of FOXO in the presence or absence phosphorylation deficient triple mutants of FOXO proteins (FOXO1-TM, FOXO3a-TM, or FOXO4-TM). PANC-1 and AsPC-1 cells were transfected with wild type FOXO promoter linked to a luciferase reporter gene in the presence or absence of plasmids expressing FOXO1-TM, FOXO3a-TM, or FOXO4-TM (Fig. 6C and 6D). After transfection, cells were treated with SFN for 24 h, and luciferase activity was measured. Transfection of cells with plasmids expressing FOXO1-TM, FOXO3a-TM, or FOXO4-TM induced FOXO transcriptional activity compared with the empty vector (control). SFN-induced FOXO transcriptional activity was further enhanced in the presence of FOXO1-TM, FOXO3a-TM, and FOXO4-TM. These data indicate that FOXO transcription factor may play a major role in mediating biological effects of SFN in pancreatic cancer cells.
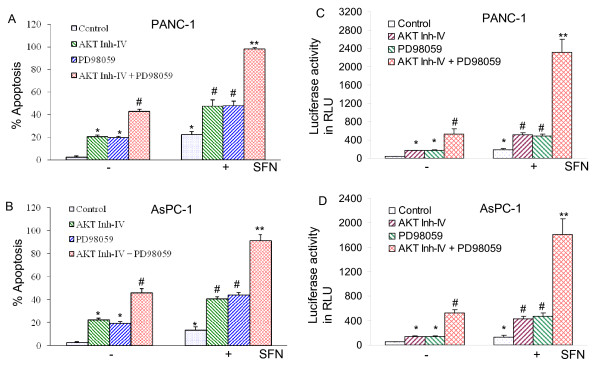
Inhibition of PI3K/AKT and MEK/ERK pathways synergistically/additively induces FOXO transcriptional activity and apoptosis in the presence or absence of sulforaphane
Since inhibition of PI3K/AKT and MEK/ERK pathways induce apoptosis in pancreatic cancer cells, we sought to examine whether these pathways act together to regulate SFN-induced apoptosis. AKT inhibitor (AKT Inh-IV) and MEK1/2 inhibitor (PD98059) synergistically/additively induced apoptosis in PANC-1 and AsPC-1 cells (Fig. 7A and 7B). AKT inhibitor and PD98059 alone enhanced SFN-induced apoptosis. Interestingly, the combination of AKT inhibitor and PD98059 with SFN induced more apoptosis than AKT inhibitor plus SFN or PD98059 plus SFN. These data suggest that inhibition of PI3K/AKT and MEK/ERK pathways act synergistically/additively to regulate apoptosis in the absence or presence of SFN.
Figure 7.
Inhibition of PI3K/AKT and MEK/ERK pathways synergistically/additively enhanced sulforaphane (SFN)-induced apoptosis and FOXO transcriptional activity in pancreatic cancer cells. (A and B), PANC-1 and AsPC-1 cells were pretreated with AKT inhibitor IV (1 μM) and/or MEK1/2 inhibitor PD98059 (10 μM) for 2 h, followed by treatment with SFN (10 μM) or DMSO (control) for 48 h. At the end of incubation period, cells were harvested and apoptosis was measured by TUNEL assay. Data represent mean ± SD. * = significantly different from respective controls, P < 0.05. (C and D), PANC-1 and AsPC-1 cells were transiently transfected with 6X DBE-luciferase construct for 24 h. After transfection, cells were pretreated with AKT inhibitor IV (1 μM) and/or MEK1/2 inhibitor PD98059 (10 μM) for 2 h, followed by treatment with SFN (10 μM) or DMSO (control) for 24 h. Cells were harvested for firefly/Renilla luciferase assays using the Dual-Luciferase Reporter Assay System (Promega). Luciferase counts were normalized using Renilla luciferase transfection control (pRL-TK; Promega). Data represent the mean ± S.D. *, #, ** = significantly different from respective controls, P < 0.05.
Since inhibition of PI3K/AKT and MEK/ERK pathways synergistically/additively induces apoptosis in pancreatic cancer cells, we sought to examine whether inhibition of these two pathways act together to regulate FOXO activity. AKT inhibitor (AKT Inh-IV) and MEK1/2 inhibitor (PD98059) synergistically induced FOXO transcriptional activity in AsPC-1 and PANC-1 cells (Fig. 7C and 7D). AKT inhibitor or PD98059 enhanced SFN-induced FOXO transcriptional activity. Interestingly, the combination of AKT Inh-IV and PD98059 with SFN induced greater FOXO transcriptional activity than AKT Inh-IV plus SFN or PD98059 plus SFN. These data suggest that inhibition of PI3K/AKT and MEK/ERK pathways acts synergistically/additively to regulate FOXO transcriptional activity in the absence or presence of SFN.
Discussion
Our study demonstrates, for the first time, that cancer preventive effects of SFN are regulated through activation of FOXO transcription factors. Specifically, we have demonstrated that (i) SFN induces apoptosis through caspase-3 activation, and causes growth arrest through induction of p21 and p27 and inhibition of cyclin D1; (ii) SFN induces apoptosis through inhibition of both PI3K/AKT and MEK/ERK pathways, and activation of FOXO transcription factors; (iii) inhibition of PI3K/AKT and MEK/ERK pathways acts together to enhance the activation of FOXO transcription factors; and (iv) phosphorylation deficient mutants of FOXO proteins further enhance SFN-induced FOXO activity and apoptosis. Our data are in agreement with others who demonstrated the anticancer activity of SFN in pancreatic cancer [110-112].
FOXO transcription factors play a crucial role in the regulation of tissue homeostasis in organs such as the pancreas and the ovaries and complex diseases such as diabetes and cancer [113-117]. FOXO transcription factors are emerging as critical transcriptional integrators among pathways regulating differentiation, proliferation, survival, and angiogenesis [118-121]. FOXO transcription factors regulate angiogenesis and postnatal neovascularization by regulation angiopoietin 2 (Ang2) and eNOS [121]. Gene expression profiling showed that FOXO1 and FOXO3a specifically regulate a nonredundant but overlapping set of angiogenesis- and vascular remodeling-related genes [121]. The FOXO1-deficient mice died around embryonic day 11 because of defects in the branchial arches and remarkably impaired vascular development of embryos and yolk sacs [118]. We have recently demonstrated that inhibition of the MEK/ERK and PI3K/AKT pathways synergistically induced FOXO transcriptional activity and inhibited angiogenesis (cell migration and capillary tube formation); these events were further enhanced in the presence of SFN [24]. Phosphorylation deficient mutants of FOXO enhanced antiangiogenic effects of SFN by activating the FOXO transcription factor. These studies suggest that activation of FOXO transcription factor by SFN could be an important physiological process to inhibit angiogenesis which may ultimately control tumor growth.
Activation of Kras has been shown to activate both PI3K/AKT and MAPK pathways [24,101,122-124]. Oxidative stress and activation of the JNK pathway induce the nucleocytoplasmic translocation of the pancreatic transcription factor Pdx-1, which leads to pancreatic β-cell dysfunction [125,126]. Furthermore, FOXO1/FKHR plays a role as a mediator between the JNK pathway and Pdx-1 [127]. Under oxidative stress conditions, FOXO1 changed its intracellular localization from the cytoplasm to the nucleus in the pancreatic β-cell line HIT-T15. The overexpression of JNK also induced the nuclear localization of FOXO1, but in contrast, suppression of JNK reduced the oxidative stress-induced nuclear localization of FOXO1, suggesting the involvement of the JNK pathway in FOXO1 translocation. In addition, oxidative stress or activation of the JNK pathway decreased the activity of AKT in HIT cells, leading to the decreased phosphorylation of FOXO1 following nuclear localization. Furthermore, adenovirus-mediated FOXO1 overexpression reduced the nuclear expression of Pdx-1, whereas repression of FOXO1 by FOXO1-specific small interfering RNA retained the nuclear expression of Pdx-1 under oxidative stress conditions. Activation of ERK has been shown to phosphorylate FOXO proteins, resulting in nuclear exclusion and transcriptional repression. In addition to ERK, direct phosphorylation of FOXO by AKT results in cytoplasmic retention and inactivation, inhibiting the expression of FOXO-regulated genes, which control the cell cycle, cell death, cell metabolism and oxidative stress [82,128,129]. Taken together, these studies demonstrate that dephosphorylation and activation of FOXO by inhibition of PI3K/AKT and MEK/ERK pathways has significant implication for pancreatic cancer treatment and prevention, where Kras is activated in about 90% patients.
In addition to phosphorylation, the acetylation/deacetylation of FOXO can be regulated by p300, Cbp (CREB-binding protein) and Pcaf (p300/CBP-associated factors) in response to oxidative stress or DNA binding, followed by deacetylation by class I and II histone deacetylases [130-132], including Sirt1, the NAD+-dependent deacetylase encoded by the ortholog of yeast longevity gene Sir2 [133]. Therefore, further studies are needed to examine the consequences of acetylation/deacetylation of FOXO transcription factors on anti-proliferative and anti-angiogenic effects of SFN.
In conclusion, we have demonstrated that SFN induces cell cycle arrest and apoptosis through regulation of FOXO transcription factors. Pharmacological and genetic inhibitions of PI3K/AKT and MEK/ERK pathways can have synergistic effects on the activation of FOXO transcription factors through dephosphorylation and nuclear retention. Thus, SFN appears to be as an attractive agent for pancreatic cancer prevention and treatment.
Methods
Reagents
Antibodies against PTEN, phospho-AKT, AKT, phospho-ERK, ERK, phospho-p38, p38, p21/CIP1, p27/KIP1, cyclin D1, and β-actin were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Enhanced chemiluminescence (ECL) Western blot detection reagents were from Amersham Life Sciences Inc. (Arlington Heights, IL). Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) assay kit was purchased from EMD Biosciences/Calbiochem (San Diego, CA). Sulforaphane was purchased from LKT Laboratories, Inc. (St. Paul, MN). Kits for Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) and caspase-3 assays were purchased from EMD Biosciences/Calbiochem (San Diego, CA).
Cell Culture
PANC-1, MIA PaCa-2, AsPC-1 and Hs 766T cells were obtained from the American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic (Invitrogen) at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Western Blot Analysis
Western blots were performed as we described earlier [134,135]. In brief, cells were lysed in RIPA buffer containing 1 × protease inhibitor cocktail, and protein concentrations were determined using the Bradford assay (Bio-Rad, Philadelphia, PA). Proteins were separated by 12.5% SDS/PAGE and transferred to membranes (Millipore, Bedford, MA) in a Tris (20 mM), glycine (150 mM) and methanol (20%) buffer at 55 V for 4 h at 4°C. After blocking in 5% nonfat dry milk in TBS, the membranes were incubated with primary antibodies at 1:1,000 dilution in TBS overnight at 4°C, washed three times with TBS-Tween 20, and then incubated with secondary antibodies conjugated with horseradish peroxidase at 1:5,000 dilution in TBS for 1 hour at room temperature. Membranes were washed again in TBS-Tween 20 for three times at room temperature. Protein bands were visualized on X-ray film using an enhanced chemiluminescence detection system.
Caspase-3 Assay
Cells (3 × 104 per well) were seeded in a 96-well plate with 200 μl culture medium. Approximately 16 h later, cells were treated with various doses of SFN to induce apoptosis. Casapse-3 activity was measured by a fluorometer as per manufacturer's instructions (EMD Biosciences).
Statistical Analysis
The mean and SD were calculated for each experimental group. Differences between groups were analyzed by one or two way ANOVA, followed by Bonferoni's multiple comparison tests using PRISM statistical analysis software (GrafPad Software, Inc., San Diego, CA). Significant differences among groups were calculated at P < 0.05.
List of abbreviations used
ANOVA: Analysis of Variance; PTEN: Phosphatase and Tensin Homolog Deleted on Chromosome 10; RIPA: Radio-Immunoprecipitation Assay; SDS-PAGE: Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis; SFN: Sulforaphane; TBS: Tris Buffer Saline.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SKR and SS performed the experiments. SS and RKS designed and wrote the manuscript. All the authors have read and approved the final manuscript.
Contributor Information
Sanjit K Roy, Email: skroy@gmail.com.
Rakesh K Srivastava, Email: rsrivastava@kumc.edu.
Sharmila Shankar, Email: sshankar@kumc.edu.
Acknowledgements
We thank our lab members for critical reading of the manuscript. We also thank Dr. Noboru Motoyama (National Institute for Longevity Sciences, Obu, Aichi, Japan) and Dr. Tatsuo Furuyama (Sonoda Women's University, Amagasaki, Hyogo, Japan) for providing FOXO expression plasmids and FOXO-luciferase construct (pGL3-6X DBE), respectively. This work was supported in part by the grants from the National Institutes of Health (R01CA125262 and RO1CA114469).
References
- Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- Magee CJ, Ghaneh P, Neoptolemos JP. Surgical and medical therapy for pancreatic carcinoma. Best Pract Res Clin Gastroenterol. 2002;16:435–455. doi: 10.1053/bega.2002.0317. [DOI] [PubMed] [Google Scholar]
- Yeo TP, Hruban RH, Leach SD, Wilentz RE, Sohn TA, Kern SE, Iacobuzio-Donahue CA, Maitra A, Goggins M, Canto MI. Pancreatic cancer. Curr Probl Cancer. 2002;26:176–275. doi: 10.1067/mcn.2002.129579. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Talalay P. Antioxidant functions of sulforaphane: a potent inducer of Phase II detoxication enzymes. Food Chem Toxicol. 1999;37:973–979. doi: 10.1016/S0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci USA. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Pezzuto JM. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:231–255. doi: 10.1023/A:1021254725842. [DOI] [PubMed] [Google Scholar]
- Weisburger JH. Antimutagens, anticarcinogens, and effective worldwide cancer prevention. J Environ Pathol Toxicol Oncol. 1999;18:85–93. [PubMed] [Google Scholar]
- Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- Shankar S, Ganapathy S, Srivastava RK. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis and angiogenesis. Clinical Cancer Res. 2008;14:1–16. doi: 10.1158/1078-0432.CCR-08-0903. [DOI] [PubMed] [Google Scholar]
- Jackson SJ, Singletary KW. Sulforaphane inhibits human MCF-7 mammary cancer cell mitotic progression and tubulin polymerization. J Nutr. 2004;134:2229–2236. doi: 10.1093/jn/134.9.2229. [DOI] [PubMed] [Google Scholar]
- Kim JH, Han Kwon K, Jung JY, Han HS, Hyun Shim J, Oh S, Choi KH, Choi ES, Shin JA, Leem DH. Sulforaphane Increases Cyclin-Dependent Kinase Inhibitor, p21 Protein in Human Oral Carcinoma Cells and Nude Mouse Animal Model to Induce G(2)/M Cell Cycle Arrest. J Clin Biochem Nutr. 2010;46:60–67. doi: 10.3164/jcbn.09-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho NP, Han HS, Leem DH, Choi IS, Jung JY, Kim HJ, Moon KS, Choi KH, Soh Y, Kong G. Sulforaphane enhances caspase-dependent apoptosis through inhibition of cyclooxygenase-2 expression in human oral squamous carcinoma cells and nude mouse xenograft model. Oral Oncol. 2009;45:654–660. doi: 10.1016/j.oraloncology.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Yu D, Sekine-Suzuki E, Xue L, Fujimori A, Kubota N, Okayasu R. Chemopreventive agent sulforaphane enhances radiosensitivity in human tumor cells. Int J Cancer. 2009;125:1205–1211. doi: 10.1002/ijc.24480. [DOI] [PubMed] [Google Scholar]
- Hanlon N, Coldham N, Gielbert A, Kuhnert N, Sauer MJ, King LJ, Ioannides C. Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br J Nutr. 2008;99:559–564. doi: 10.1017/S0007114507824093. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Kong AN. Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr Drug Metab. 2002;3:481–490. doi: 10.2174/1389200023337171. [DOI] [PubMed] [Google Scholar]
- Misiewicz I, Skupinska K, Kowalska E, Lubinski J, Kasprzycka-Guttman T. Sulforaphane-mediated induction of a phase 2 detoxifying enzyme NAD(P)H:quinone reductase and apoptosis in human lymphoblastoid cells. Acta Biochim Pol. 2004;51:711–721. [PubMed] [Google Scholar]
- Bacon JR, Williamson G, Garner RC, Lappin G, Langouet S, Bao Y. Sulforaphane and quercetin modulate PhIP-DNA adduct formation in human HepG2 cells and hepatocytes. Carcinogenesis. 2003;24:1903–1911. doi: 10.1093/carcin/bgg157. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye L, Coady JL, Wang JB, Wu Y. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- Conaway CC, Wang CX, Pittman B, Yang YM, Schwartz JE, Tian D, McIntee EJ, Hecht SS, Chung FL. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- Singletary K, MacDonald C. Inhibition of benzo[a]pyrene- and 1,6-dinitropyrene-DNA adduct formation in human mammary epithelial cells bydibenzoylmethane and sulforaphane. Cancer Lett. 2000;155:47–54. doi: 10.1016/S0304-3835(00)00412-2. [DOI] [PubMed] [Google Scholar]
- Davis R, Singh KP, Kurzrock R, Shankar S. Sulforaphane inhibits angiogenesis through activation of FOXO transcription factors. Oncol Rep. 2009;22:1473–1478. doi: 10.3892/or_00000589. [DOI] [PubMed] [Google Scholar]
- Jakubikova J, Sedlak J, Mithen R, Bao Y. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. Biochem Pharmacol. 2005;69:1543–1552. doi: 10.1016/j.bcp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lew KL, Xiao H, Herman-Antosiewicz A, Xiao D, Brown CK, Singh SV. D,L-Sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and Apaf-1. Carcinogenesis. 2007;28:151–162. doi: 10.1093/carcin/bgl144. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997;57:2124–2129. [PubMed] [Google Scholar]
- Wang SI, Puc J, Li J, Bruce JN, Cairns P, Sidransky D, Parsons R. Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res. 1997;57:4183–4186. [PubMed] [Google Scholar]
- Guldberg P, thor Straten P, Birck A, Ahrenkiel V, Kirkin AF, Zeuthen J. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res. 1997;57:3660–3663. [PubMed] [Google Scholar]
- Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- Rhei E, Kang L, Bogomolniy F, Federici MG, Borgen PI, Boyd J. Mutation analysis of the putative tumor suppressor gene PTEN/MMAC1 in primary breast carcinomas. Cancer Res. 1997;57:3657–3659. [PubMed] [Google Scholar]
- Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI, Li J, Parsons R, Ellenson LH. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57:3935–3940. [PubMed] [Google Scholar]
- Sakurada A, Suzuki A, Sato M, Yamakawa H, Orikasa K, Uyeno S, Ono T, Ohuchi N, Fujimura S, Horii A. Infrequent genetic alterations of the PTEN/MMAC1 gene in Japanese patients with primary cancers of the breast, lung, pancreas, kidney, and ovary. Jpn J Cancer Res. 1997;88:1025–1028. doi: 10.1111/j.1349-7006.1997.tb00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okami K, Wu L, Riggins G, Cairns P, Goggins M, Evron E, Halachmi N, Ahrendt SA, Reed AL, Hilgers W. Analysis of PTEN/MMAC1 alterations in aerodigestive tract tumors. Cancer Res. 1998;58:509–511. [PubMed] [Google Scholar]
- Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23:8571–8580. doi: 10.1038/sj.onc.1207902. [DOI] [PubMed] [Google Scholar]
- Altomare DA, Tanno S, De Rienzo A, Klein-Szanto AJ, Tanno S, Skele KL, Hoffman JP, Testa JR. Frequent activation of AKT2 kinase in human pancreatic carcinomas. J Cell Biochem. 2003;88:470–476. doi: 10.1002/jcb.10287. [DOI] [PubMed] [Google Scholar]
- Ebert MP, Fei G, Schandl L, Mawrin C, Dietzmann K, Herrera P, Friess H, Gress TM, Malfertheiner P. Reduced PTEN expression in the pancreas overexpressing transforming growth factor-beta 1. Br J Cancer. 2002;86:257–262. doi: 10.1038/sj.bjc.6600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn SA, Seymour AB, Hoque AT, Schutte M, da Costa LT, Redston MS, Caldas C, Weinstein CL, Fischer A, Yeo CJ. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res. 1995;55:4670–4675. [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/S0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- Kulik G, Weber MJ. Akt-dependent and -independent survival signaling pathways utilized by insulin-like growth factor I. Mol Cell Biol. 1998;18:6711–6718. doi: 10.1128/mcb.18.11.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Tamura M, Yamada KM. Tumor suppressor PTEN inhibits integrin- and growth factor-mediated mitogen-activated protein (MAP) kinase signaling pathways. J Cell Biol. 1998;143:1375–1383. doi: 10.1083/jcb.143.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Hengartner MO. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 2001;11:526–534. doi: 10.1016/S0962-8924(01)02173-0. [DOI] [PubMed] [Google Scholar]
- Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- Khwaja A. Akt is more than just a Bad kinase. Nature. 1999;401:33–34. doi: 10.1038/43354. [DOI] [PubMed] [Google Scholar]
- Chen X, Thakkar H, Tyan F, Gim S, Robinson H, Lee C, Pandey SK, Nwokorie C, Onwudiwe N, Srivastava RK. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene. 2001;20:6073–6083. doi: 10.1038/sj.onc.1204736. [DOI] [PubMed] [Google Scholar]
- Kandasamy K, Srivastava RK. Role of the phosphatidylinositol 3'-kinase/PTEN/Akt kinase pathway in tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in non-small cell lung cancer cells. Cancer Res. 2002;62:4929–4937. [PubMed] [Google Scholar]
- Cicenas J, Urban P, Vuaroqueaux V, Labuhn M, Kung W, Wight E, Mayhew M, Eppenberger U, Eppenberger-Castori S. Increased level of phosphorylated akt measured by chemiluminescence-linked immunosorbent assay is a predictor of poor prognosis in primary breast cancer overexpressing ErbB-2. Breast Cancer Res. 2005;7:R394–401. doi: 10.1186/bcr1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba S, Moriya T, Kimura W, Yamakawa M. Phosphorylated Akt/PKB controls cell growth and apoptosis in intraductal papillary-mucinous tumor and invasive ductal adenocarcinoma of the pancreas. Pancreas. 2003;26:250–257. doi: 10.1097/00006676-200304000-00008. [DOI] [PubMed] [Google Scholar]
- Yao Z, Okabayashi Y, Yutsudo Y, Kitamura T, Ogawa W, Kasuga M. Role of Akt in growth and survival of PANC-1 pancreatic cancer cells. Pancreas. 2002;24:42–46. doi: 10.1097/00006676-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Kang SS, Kwon T, Kwon DY, Do SI. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem. 1999;274:13085–13090. doi: 10.1074/jbc.274.19.13085. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Perugini RA, McDade TP, Vittimberga FJ Jr, Callery MP. Pancreatic cancer cell proliferation is phosphatidylinositol 3-kinase dependent. J Surg Res. 2000;90:39–44. doi: 10.1006/jsre.2000.5833. [DOI] [PubMed] [Google Scholar]
- Shah SA, Potter MW, Hedeshian MH, Kim RD, Chari RS, Callery MP. PI-3' kinase and NF-kappaB cross-signaling in human pancreatic cancer cells. J Gastrointest Surg. 2001;5:603–612. doi: 10.1016/S1091-255X(01)80102-5. discussion 612-603. [DOI] [PubMed] [Google Scholar]
- Bondar VM, Sweeney-Gotsch B, Andreeff M, Mills GB, McConkey DJ. Inhibition of the phosphatidylinositol 3'-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2002;1:989–997. [PubMed] [Google Scholar]
- Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci USA. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri BA, Huang L, Wood M, Cheng JQ, Testa JR. Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol Carcinog. 1998;21:81–86. doi: 10.1002/(SICI)1098-2744(199802)21:2<81::AID-MC1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer. 2003;89:2110–2115. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJ, Emanuel BS, Rovera G, Barr FG. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;5:230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics. 1998;47:187–199. doi: 10.1006/geno.1997.5122. [DOI] [PubMed] [Google Scholar]
- Hillion J, Le Coniat M, Jonveaux P, Berger R, Bernard OA. AF6q21, a novel partner of the MLL gene in t(6;11)(q21;q23), defines a forkhead transcriptional factor subfamily. Blood. 1997;90:3714–3719. [PubMed] [Google Scholar]
- Borkhardt A, Repp R, Haas OA, Leis T, Harbott J, Kreuder J, Hammermann J, Henn T, Lampert F. Cloning and characterization of AFX, the gene that fuses to MLL in acute leukemias with a t(X;11)(q13;q23) Oncogene. 1997;14:195–202. doi: 10.1038/sj.onc.1200814. [DOI] [PubMed] [Google Scholar]
- Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–8982. doi: 10.1128/MCB.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, Burgering BM, Raaijmakers JA, Lammers JW, Koenderman L, Coffer PJ. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1) Mol Cell Biol. 2000;20:9138–9148. doi: 10.1128/MCB.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers PF, Birkenkamp KU, Lam EW, Thomas NS, Lammers JW, Koenderman L, Coffer PJ. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J Cell Biol. 2002;156:531–542. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellini A, Tabellini G, Zweyer M, Bortul R, Tazzari PL, Billi AM, Fala F, Cocco L, Martelli AM. The phosphoinositide 3-kinase/Akt pathway regulates cell cycle progression of HL60 human leukemia cells through cytoplasmic relocalization of the cyclin-dependent kinase inhibitor p27(Kip1) and control of cyclin D1 expression. Leukemia. 2003;17:2157–2167. doi: 10.1038/sj.leu.2403111. [DOI] [PubMed] [Google Scholar]
- Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27:352–360. doi: 10.1016/S0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/S0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TT, Dowbenko D, Jackson A, Toney L, Lewin DA, Dent AL, Lasky LA. The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J Biol Chem. 2002;277:14255–14265. doi: 10.1074/jbc.M110901200. [DOI] [PubMed] [Google Scholar]
- Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Der CJ. Emerging concepts in the Ras superfamily of GTP-binding proteins. Faseb J. 1993;7:750–759. doi: 10.1096/fasebj.7.9.8330683. [DOI] [PubMed] [Google Scholar]
- Gibbs JB, Sigal IS, Poe M, Scolnick EM. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci USA. 1984;81:5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C, Weinberg RA. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982;29:161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Ulevitch RJ. Emerging targets for anti-inflammatory therapy. Nat Cell Biol. 1999;1:E39–40. doi: 10.1038/10032. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/S0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/S0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Woessmann W, Meng YH, Mivechi NF. An essential role for mitogen-activated protein kinases, ERKs, in preventing heat-induced cell death. J Cell Biochem. 1999;74:648–662. doi: 10.1002/(SICI)1097-4644(19990915)74:4<648::AID-JCB14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/S0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Lubinus M, Meier KE, Smith EA, Gause KC, LeRoy EC, Trojanowska M. Independent effects of platelet-derived growth factor isoforms on mitogen-activated protein kinase activation and mitogenesis in human dermal fibroblasts. J Biol Chem. 1994;269:9822–9825. [PubMed] [Google Scholar]
- Shankar S, Chen Q, Srivastava RK. Inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to enhance antiangiogenic effects of EGCG through activation of FOXO transcription factor. J Mol Signal. 2008;3:7. doi: 10.1186/1750-2187-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- Srivastava RK. Intracellular mechanisms of TRAIL and its role in cancer therapy. Mol Cell Biol Res Commun. 2000;4:67–75. doi: 10.1006/mcbr.2001.0265. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Mielke K, Herdegen T. JNK and p38 stresskinases--degenerative effectors of signal-transduction-cascades in the nervous system. Prog Neurobiol. 2000;61:45–60. doi: 10.1016/S0301-0082(99)00042-8. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, May WS, Duronio V, Mufson A. Serine/threonine phosphorylation in cytokine signal transduction. Leukemia. 2000;14:9–21. doi: 10.1038/sj.leu.2401657. [DOI] [PubMed] [Google Scholar]
- Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. Faseb J. 2000;14:6–16. [PubMed] [Google Scholar]
- Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Hutzen B, Willis W, Jones S, Cen L, Deangelis S, Fuh B, Lin J. Dietary agent, benzyl isothiocyanate inhibits signal transducer and activator of transcription 3 phosphorylation and collaborates with sulforaphane in the growth suppression of PANC-1 cancer cells. Cancer Cell Int. 2009;9:24. doi: 10.1186/1475-2867-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallifatidis G, Rausch V, Baumann B, Apel A, Beckermann BM, Groth A, Mattern J, Li Z, Kolb A, Moldenhauer G. Sulforaphane targets pancreatic tumor-initiating cells by NF-{kappa}B-induced anti-apoptotic signaling. Gut. 2009;58:949–63. doi: 10.1136/gut.2008.149039. [DOI] [PubMed] [Google Scholar]
- Pham NA, Jacobberger JW, Schimmer AD, Cao P, Gronda M, Hedley DW. The dietary isothiocyanate sulforaphane targets pathways of apoptosis, cell cycle arrest, and oxidative stress in human pancreatic cancer cells and inhibits tumor growth in severe combined immunodeficient mice. Mol Cancer Ther. 2004;3:1239–1248. [PubMed] [Google Scholar]
- Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH, Wright CV, White MF, Arden KC, Accili D. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Nakae J, Biggs WH, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- Xia SJ, Pressey JG, Barr FG. Molecular pathogenesis of rhabdomyosarcoma. Cancer Biol Ther. 2002;1:97–104. doi: 10.4161/cbt.51. [DOI] [PubMed] [Google Scholar]
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/S0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Nakayama K. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- Dejana E, Taddei A, Randi AM. Foxs and Ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim Biophys Acta. 2007;1775:298–312. doi: 10.1016/j.bbcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Chlench S, Mecha Disassa N, Hohberg M, Hoffmann C, Pohlkamp T, Beyer G, Bongrazio M, Da Silva-Azevedo L, Baum O, Pries AR, Zakrzewicz A. Regulation of Foxo-1 and the angiopoietin-2/Tie2 system by shear stress. FEBS Lett. 2007;581:673–680. doi: 10.1016/j.febslet.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Shen JN, Wang J, Huang G, Zhou JG. Oridonin induced apoptosis through Akt and MAPKs signaling pathways in human osteosarcoma cells. Cancer Biol Ther. 2007;6:261–268. doi: 10.4161/cbt.6.2.3621. [DOI] [PubMed] [Google Scholar]
- Schwab TS, Madison BB, Grauman AR, Feldman EL. Insulin-like growth factor-I induces the phosphorylation and nuclear exclusion of forkhead transcription factors in human neuroblastoma cells. Apoptosis. 2005;10:831–840. doi: 10.1007/s10495-005-0429-y. [DOI] [PubMed] [Google Scholar]
- Srivastava RK, Unterman TG, Shankar S. FOXO transcription factors and VEGF neutralizing antibody enhance antiangiogenic effects of resveratrol. Mol Cell Biochem. 2010;337:201–212. doi: 10.1007/s11010-009-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H, Kawamori D, Matsuoka TA, Kajimoto Y, Yamasaki Y. Oxidative stress and pancreatic beta-cell dysfunction. Am J Ther. 2005;12:529–533. doi: 10.1097/01.mjt.0000178773.31525.c2. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Kawamori D, Nakatani Y, Gorogawa S, Matsuoka TA. Oxidative stress and the JNK pathway as a potential therapeutic target for diabetes. Drug News Perspect. 2004;17:447–453. doi: 10.1358/dnp.2004.17.7.863704. [DOI] [PubMed] [Google Scholar]
- Kawamori D, Kaneto H, Nakatani Y, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem. 2006;281:1091–1098. doi: 10.1074/jbc.M508510200. [DOI] [PubMed] [Google Scholar]
- Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- Uddin S, Hussain AR, Siraj AK, Manogaran PS, Al-Jomah NA, Moorji A, Atizado V, Al-Dayel F, Belgaumi A, El-Solh H. Role of phosphatidylinositol 3'-kinase/AKT pathway in diffuse large B-cell lymphoma survival. Blood. 2006;108:4178–4186. doi: 10.1182/blood-2006-04-016907. [DOI] [PubMed] [Google Scholar]
- Daitoku H, Fukamizu A. FOXO transcription factors in the regulatory networks of longevity. J Biochem. 2007;141:769–774. doi: 10.1093/jb/mvm104. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Smidt MP. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends Biochem Sci. 2005;30:81–86. doi: 10.1016/j.tibs.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 2008;13:440–452. doi: 10.2741/2691. [DOI] [PubMed] [Google Scholar]
- Shankar S, Siddiqui I, Srivastava RK. Molecular mechanisms of resveratrol (3,4,5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol Cell Biochem. 2007;304:273–285. doi: 10.1007/s11010-007-9510-x. [DOI] [PubMed] [Google Scholar]