Abstract
Munc13 is a multidomain protein of presynaptic active zones that mediates the priming and plasticity of synaptic vesicle exocytosis, but the mechanisms involved remain unclear. Here, we use biophysical, biochemical, and electrophysiological approaches to demonstrate that the central C2B-domain of Munc13 functions as a Ca2+-regulator of short-term synaptic plasticity. The crystal structure of the C2B-domain revealed an unusual Ca2+-binding site with an amphipathic α-helix. This configuration confers onto the C2B-domain unique Ca2+-dependent phospholipid-binding properties favoring phosphatidylinositolphosphates. A mutation that inactivated Ca2+-dependent phospholipid binding to the C2B-domain did not alter neurotransmitter release evoked by isolated action potentials, but depressed release evoked by action potential trains. In contrast, a mutation that increased Ca2+-dependent phosphatidylinositolbisphosphate binding to the C2B-domain enhanced release evoked by isolated action potentials and by action potential trains. Our data suggest that during repeated action potentials, Ca2+- and phosphatidylinositolphosphate-binding to the Munc13 C2B-domain potentiate synaptic vesicle exocytosis, thereby offsetting synaptic depression induced by vesicle depletion.
INTRODUCTION
Synaptic transmission is initiated when Ca2+-influx during an action potential triggers neurotransmitter release1. Synaptic transmission is not a constant point-to-point transfer of information from one neuron to the next, but changes as a function of use, rendering synapses elementary computational units of the brain2. Many different types of use-dependent synaptic plasticity have been described, among which presynaptic short-term plasticity stands out because it is universally present at synapses, and can alter synaptic transmission more than 10-fold3. Short-term plasticity is of central importance for information processing by the brain; for example, it may underlie working memory formation in cortex4.
At first approximation, presynaptic short-term plasticity results from two opposing processes3. Repeated action potentials deplete the readily-releasable pool (RRP) of synaptic vesicles, thereby inducing synaptic depression. At the same time, Ca2+-influx during repeated action potentials causes accumulation of residual Ca2+, thereby inducing synaptic facilitation. As a consequence, a high release probability usually results in synaptic depression because the RRP becomes depleted, whereas a low release probability usually results in synaptic facilitation because vesicle depletion is delayed but accumulating residual Ca2+ increases Ca2+-triggering.
Considerable evidence, however, indicates that presynaptic plasticity is an active, regulated, and synapse-specific process that goes beyond a passive response dictated by the release probability and RRP size. For example, RIM1α and Munc13 are active zone proteins that interact with each other, and form a heterotrimeric complex with the synaptic vesicle protein Rab35–9. Mutations in each of these three proteins induce changes in short-term synaptic plasticity that cannot be accounted for by corresponding alterations in residual Ca2+, release probability, or RRP8,10–14. These and other observations indicate that Ca2+ not only triggers release, but that during stimulus trains, the residual Ca2+ accumulating between action potentials regulates release by independent mechanisms.
At present, the major Ca2+-regulator in short-term synaptic plasticity is thought to be calmodulin15. Calmodulin regulates neurotransmitter release by multiple mechanisms, including a direct modulation of Ca2+-channels16–18, activation of protein kinases19, regulation of synaptic vesicle priming via the cytoskeleton20, and binding to Munc13-1 and -2 (13). Moreover, calmodulin acts in presynaptic long-term plasticity by activating adenylate cyclase during mossy-fiber LTP21,22. However, the large variety of different types of presynaptic plasticity with distinct spatio-temporal profiles suggests that calmodulin is unlikely to account for all forms of short-term plasticity.
Munc13s and RIMs are essential for priming synaptic vesicles, and are additionally involved in short- and long-term synaptic plasticity8,10. Three Munc13 isoforms (Munc13-1, -2, and -3, of which Munc13-2 is expressed in two principal isoforms called bMunc13-2 and ubMunc13-2) function in synaptic vesicle exocytosis5,10,23,24. In addition, two ubiquitously expressed Munc13 isoforms (BAP-3 and Munc13-4 probably act in non-synaptic forms of exocytosis25–27. Munc13s have variable N-terminal sequences, but contain similar central and C-terminal domains: a C2B-domain, a large Munc13-homology region (the MUN domain14), and a Ca2+-independent C2C-domain (Fig. 1A). It seems likely that the canonical Munc13-domains, i.e. their C2B-, MUN- and C2C-domains, mediate their shared functions, whereas their variable N-terminal domains modulate these functions. Consistent with this notion, the calmodulin-binding motif and the C1-domain of Munc13-1 are involved in short-term synaptic plasticity12,13, whereas its MUN domain mediates its priming function14,28,29.
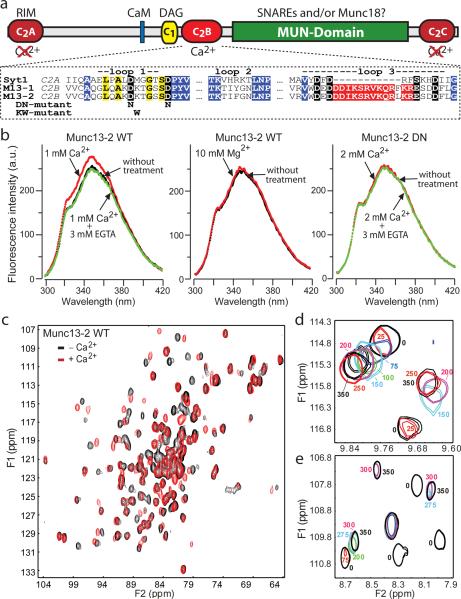
Figure 1. The Munc13-1 C2B-domain is a Ca2+-binding module.
a. Domain organization of Munc13-1 and bMunc13-2. The binding activities of various domains are indicated above (CaM = calmodulin; DAG = diacylglycerol), and their presumed Ca2+-binding ability below the domains. An alignment of the Ca2+-binding loops from the synaptotagmin-1 C2A-domain, the Munc13-1 C2B-domain, and the Munc13-2 C2B-domain is shown below the domain organization (residues 162–241 from rat synaptotagmin-1 [acc. # X52772]; 695–779 from rat Munc13-1 [acc. # U24070]; and 619–703 from rat bMunc13-2 [acc. # AF159706]). In the alignment, conserved sequences are highlighted (black = Ca2+-binding residues; yellow = top loop; blue = β-strands; red = conserved charged sequence in loop 3 specific for the Munc13 C2B-domains). The two C2B-domain mutations analyzed (“DN” and “KW”) are described at the bottom.
b. Fluorescent emission spectra of WT and DN-mutant Munc13-2 C2B-domains without and with 1 mM Ca2+ plus/minus EGTA, or with 10 mM Mg2+ (for data on Munc13-1 and for individual spectra, see Supplementary Fig. 1).
c. 1H-15N HSQC spectra of the Munc13-1 C2B-domain in the absence (black contours) and presence (red contours) of 0.5 mM Ca2+.
d, e. Ca2+-binding to the Munc13-1 C2B-domain monitored with 1H-15N HSQC spectra. The diagrams show expansions of superpositions of selected 1H-15N HSQC spectra acquired during a titration of Ca2+ from 0 to 0.7 mM. The contours are color coded according to the Ca2+-concentration (indicated in μM next to the contours).
The C2B-domains of all Munc13 isoforms, but not their C2A- and C2C-domains, contain the requisite Ca2+-binding residues of C2-domains30 (Fig. 1A), suggesting that Munc13's may universally bind Ca2+ via their C2B-domains. However, previous attempts to demonstrate Ca2+-binding to the Munc13 C2B-domain failed5. We now show that the rat Munc13 C2B-domain binds Ca2+, and contains an unusual α-helix in its top Ca2+-binding loops. The Munc13 C2B-domain exhibits Ca2+-dependent phospholipid binding with an unexpected PIP- and PIP2-specificity that differs from that of other C2-domains, and that mediates the Ca2+-dependent regulation of short-term synaptic plasticity by the Munc13 C2B-domain. Thus, our data reveal that the Munc13 C2B-domain functions as a Ca2+-regulator of short-term synaptic plasticity by interfacing with PIP and PIP2.
RESULTS
The Munc13 C2B-domain is a Ca2+-binding module
The C2B-domains of all Munc13 isoforms contain the canonical Ca2+-binding sites of C2-domains30 (Fig. 1a), but exhibit only limited sequence homology to other C2-domains, hindering prediction of domain boundaries. Thus, we first examined the minimum sequence necessary to obtain an autonomously folded Munc13 C2B-domain, and identified a C2B-domain fragment that was soluble and monomeric (residues 675–820 in Munc13-1).
To determine whether the C2B-domain binds Ca2+, we recorded fluorescence spectra of purified C2B-domains from Munc13-1 and Munc13-2 in the absence or presence of Ca2+ or Mg2+ (Fig. 1b and Supplementary Fig. 1). For Munc13-2, we also examined a mutant C2B-domain in which two canonical aspartates (D629 and D635) in the presumptive Ca2+-binding sites were replaced by asparagines (referred to as the DN-mutant, Fig. 1a). Application of Ca2+ but not Mg2+ enhanced the intrinsic fluorescence of the wild-type (WT) C2B-domains, but had no effect on DN-mutant C2B-domain, suggesting that the Munc13 C2B-domains specifically bind Ca2+ (Fig. 1b).
We next acquired 1H-15N heteronuclear single quantum correlation (HSQC) spectra of the Munc13-1 C2B-domain in the absence and presence of Ca2+ (Fig. 1c, black and red contours, respectively). Ca2+ induced extensive cross-peak changes as expected for a Ca2+-binding module. During Ca2+-titrations, some cross-peaks exhibit progressive Ca2+-induced shifts (e.g. Fig. 1d, upper left corner). Other cross-peaks disappeared during the titration, or shifted to different parts of the spectrum (Figs. 1d and 1e), suggesting that the exchange between Ca2+-free and Ca2+-bound states is slow on the NMR time scale. The curved, progressive Ca2+-induced shifts of some cross-peaks, and the differential shifts between cross-peaks (Figs. 1d and 1e), show that at least two Ca2+-ions bind to the C2B-domain. At a C2B-domain concentration of 120 μM, no major cross-peak shifts occurred beyond 250–300 μM Ca2+, demonstrating that Ca2+-binding was saturable. The cross-peak movements can be fitted to a binding model with two Ca2+-binding sites and a KD of <100 μM.
Crystal structure of Ca2+-free and Ca2+-bound C2B-domain
To study how Ca2+ binds to the Munc13 C2B-domain, we crystallized the Ca2+-free and Ca2+-bound Munc13-1 C2B-domain. The Ca2+-free and Ca2+-bound C2B-domain cystals exhibited distinct space groups (C2221 and P43212, respectively). Using diffraction data and the crystal structure of the synaptotagmin-1 C2A-domain31 as a search model for molecular replacement, we determined the structure of the Ca2+-free Munc13-1 C2B-domain to a resolution of 1.90 Å (Table 1). The resulting model was then employed for molecular replacement together with diffraction data to determine the structure of the Ca2+-bound C2B-domain to a resolution of 1.37 Å (Fig. 2).
Table 1.
Data collection and refinement statistics for the Munc13-1 C2B domain
| Munc13-1 C2B-domain Ca2+-free | Munc13-1 C2B-domain Ca2+-bound | |
|---|---|---|
| Data collection | ||
| Space group | C2221 | P43212 |
| Cell dimensions | ||
| a, b, c (Å) | 42.57, 101.14, 68.04 | 55.94, 55.94, 89.98 |
| α, β, γ (°) | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 |
| Resolution (Å)* | 26.43-1.90 (1.93-1.90) | 30.00-1.37 (1.39-1.37) |
| Rmerge (%) | 5.0 (37.9) | 5.5 (46.4) |
| I/σI | 33.1 (2.4) | 40.8 (3.1) |
| Completeness (%) | 86.5 (47.5) | 99.8 (96.4) |
| Redundancy | 7.5 (3.3) | 12.6 (6.1) |
| Refinement | ||
| Resolution (Å) | 26.00 – 1.90 (1.94-1.90) | 30.00 – 1.37 (1.40-1.37) |
| No. reflections (unique/Rfree) | 9,905/501 | 30,262/1,546 |
| Rwork / Rfree | 0.217 (0.263) / 0.282 (0.318) | 0.170 (0.208) / 0.193 (0.231) |
| No. atoms | 1,019 | 1,352 |
| Protein | 935 | 1,175 |
| Ligand/ion | 19/2 | 12/4 |
| Water | 63 | 161 |
| B-factors | ||
| Protein | 43.9 | 17.3 |
| Ligand/ion | 45.7/49.0 | 14.7/15.5 |
| Water | 45.9 | 18.4 |
| R.m.s. deviations | ||
| Bond lengths (Å) | 0.017 | 0.018 |
| Bond angles (°) | 1.579 | 1.813 |
For each structure, data was collected from one crystal.
Values in parentheses are for highest-resolution shell.
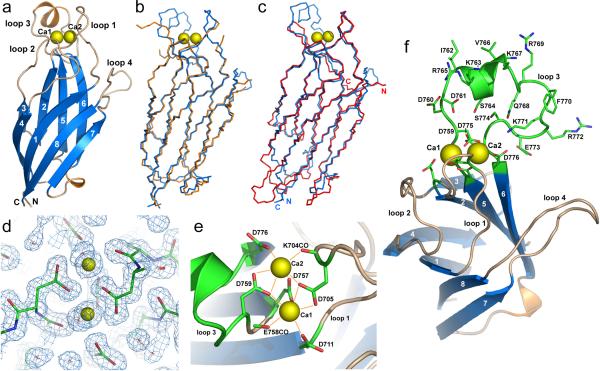
Figure 2. Three-dimensional structures of the Ca2+-free and Ca2+-bound Munc13-1 C2B-domain.
a. Ribbon diagram of the crystal structure of the Ca2+-bound Munc13-1 C2B-domain (blue = β-strands; orange = α-helices). Bound Ca2+-ions are shown as yellow spheres; β-strands are numbered from 1 to 8. The top loops are labeled loop 1 – loop 4; N and C indicate N- and C-termini, respectively. See Supplementary Fig. 2 for analysis of crystal contacts.
b. Backbone superposition of the Ca2+-free (orange) and Ca2+-bound (blue) Munc13-1 C2B-domains.
c. Backbone superposition of the crystal structures of the Ca2+-bound Munc13-1 C2B-domain (blue) and the Ca2+-free synaptotagmin-1 C2A-domain (red; PDB accession code 1rsy). N- and C-termini of both domains are indicated with letters of the corresponding color.
d. 2Fo−Fc electron density map contoured at 1σ of the Ca2+-binding region of the Munc13-1 C2B-domain superimposed with a stick model of the protein. Ca2+-ions and water molecules are represented by yellow spheres and red stars, respectively. In this and the following panels, protein atoms are color coded: green, carbon; blue, nitrogen; red, oxygen.
e. Ribbon-and-stick diagram summarizing the Ca2+-binding mode of the Munc13-1 C2B-domain. The water molecules are not shown for simplicity. All other Ca2+-ligands are shown as stick models and labeled; K704CO and E758CO denote the backbone carbonyl group of the corresponding residues. Ca2+-ions are labeled Ca1 and Ca2.
f. Ribbon-and-stick diagram of the Munc13-1 C2B-domain illustrating the amphipathic character of the α-helix of loop 3. The side chains of the Ca2+ ligands and of all residues in loop 3 are shown as stick models.
The Ca2+-free and Ca2+-bound C2B-domains contained a typical C2-domain β-sandwich fold with two four-stranded β-sheets and a type II C2-domain topology (Figs. 2a and 2b)32. Ca2+ did not cause major changes in the β-sandwich, as described for synaptotagmin-1 C2-domains31,33,34, but induced large changes in the top loops. In the Ca2+-free C2B-domain, substantial parts of loops 1, 3 and 4 exhibited little electron density, indicating that the Ca2+-free loops are disordered (Fig. 2b). In the Ca2+-bound C2B-domain, however, we observed well-defined electron densities for all four top loops (Figs. 2a and 2b), for two bound Ca2+-ions, and for multiple water molecules (partly illustrated in Fig. 2d). The Ca2+-binding region is likely stabilized by Ca2+ binding, similar to the synaptotagmin-1 C2-domains33,34. In addition, the top loops in the Ca2+-bound C2B-domain structure were involved in extensive crystal contacts that may have contributed to the stabilization of the top loops.
Structural comparisons using DALI35 revealed that several structures of other type II C2-domains in the Protein Data Bank (PDB) exhibited close similarity to the Munc13-1 C2B-domain, including structures of the C2-domains from PKCζ, PLC-δ1 and cPLA2 (PDB accession codes 1gmi, 1djx and 1rlw, respectively). However, the lowest rms deviation from the Ca2+-bound Munc13-1 C2B-domain (1.1 Å for 97 equivalent Cα carbons) was observed for the synaptotagmin-1 C2A-domain structure (PDB accession codes 1rsy and 1byn), despite the fact that it exhibits a type I topology. Backbone superpositions confirmed the similarity of the synaptotagmin-1 C2A-domain and the Munc13-1 C2B-domain (Fig. 2c). Strikingly, the major difference between the Munc13-1 C2B-domain and all other C2-domains is an extended loop 3 of the Munc13 C2B-domain that contains a unique protruding α-helix, and is absent from other C2-domains (Figs. 2a,f).
Ca2+-binding mode of the Munc13 C2B-domain
C2-domains commonly bind two or three Ca2+-ions through five conserved aspartate side chains from loop 1 (aspartates D1 and D2) and loop 3 (aspartates D3–D5)30,36. The crystal structure of the Ca2+-bound Munc13-1 C2B-domain revealed two bound Ca2+-ions (Figs. 2), consistent with the NMR data (Figs. 1d and 1e). The two Ca2+-ions are coordinated by the five canonical aspartate residues, accounting for the block of Ca2+-binding by the DN-mutation (Fig. 1b). In addition, the Ca2+-ions were coordinated by two backbone carbonyl oxygens and two water molecules (Figs. 2d and 2e). Loop 3 of the Munc13-1 C2B-domain contains additional acidic residues that did not participate in Ca2+-binding but are oriented towards the Ca2+-binding sites, and may increase its Ca2+-affinity (Fig. 2f). Moreover, exposed basic and hydrophobic residues confer an amphipathic character onto the unique α-helix of loop 3 in the Munc13 C2B-domain. This amphipathic character could be increased by extending the α-helix towards the C-terminus to include R769 to R772, and the helical structure may have been partially distorted by crystal contacts (Supplementary Fig. 2). Hence, a longer α-helix spanning residues 762–772 may be formed by loop 3 of the Munc13 C2B-domain in solution and/or upon binding of the C2B-domain to phospholipid bilayers (see below).
The Munc13 C2B-domain is a Ca2+/phospholipid-binding module
We next examined whether Munc13 C2B-domains bind to phospholipids in a Ca2+-dependent manner. First, we measured fluorescence resonance-energy transfer (FRET) from the Munc13-1 C2B-domain to dansyl-labeled liposomes containing PIP and PIP2. Ca2+ increased FRET only when both protein and liposomes were present, and this increase was reversed by EGTA (Fig. 3a and Supplementary Fig. 3). FRET may be mediated by the conserved tryptophan residue near loop 3 of the Munc13 C2B-domain (Fig. 1a), as this residue is in the phospholipid-interacting sequence of C2-domains.
Figure 3. Ca2+-dependent binding of the Munc13 C2B-domain to PIP/PIP2-containing liposomes.
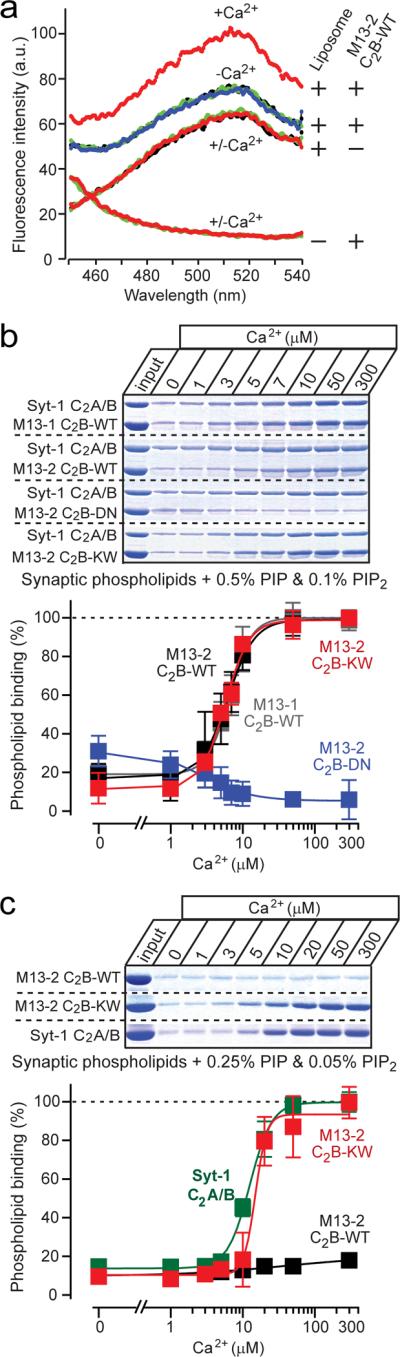
a. FRET assays of Ca2+-dependent binding of the Munc13 C2B-domain to dansyl-labeled `synaptic' liposomes containing 0.5% PIP and 0.1% PIP2 (0.03 mg/ml; total volume = 0.6 ml). Fluorescence spectra (excitation = 282 nm) were monitored in solutions containing either only the C2B-domain, liposomes, or both as indicated on the right34. Spectra were first recorded in Ca2+-free buffer (black traces, covered by overlying green, red, or blue traces), then after addition of 2 mM Mg2+ (blue traces, under the overlying green or red traces), then after addition of 0.2 mM Ca2+ (red traces), then again after further addition of 1 mM EGTA (green trace, done only for the samples containing both liposomes and C2B-domain protein). Data show a representative experiment repeated multiple times; see Supplementary Fig. 3 for individual spectra.
b, c. Centrifugation assays of Ca2+-dependent Munc13 C2B-domain binding to `synaptic' liposomes containing 0.5% PIP and 0.1% PIP2 (b), or 0.25% PIP and 0.05% PIP2 (c). GST-fused Munc13 C2B-domains and the synaptotagmin-1 C2A/B-domain fragment (used as an internal control) were bound to liposomes at the indicated free Ca2+-concentrations clamped with Ca2+/EGTA buffer containing 2 mM Mg2. Co-pelleted Munc13 and synaptotagmin-1 C2-domains were analyzed by SDS-PAGE and Coomassie Blue staining, and quantified by scanning (top panels = representative experiments; bottom panels = summary graphs (means ± SEMs [n=3]); data were normalized to binding at the highest Ca2+-concentration; quantitations for synaptotagmin-1 for panel b are shown in Supplementary Fig. 4).
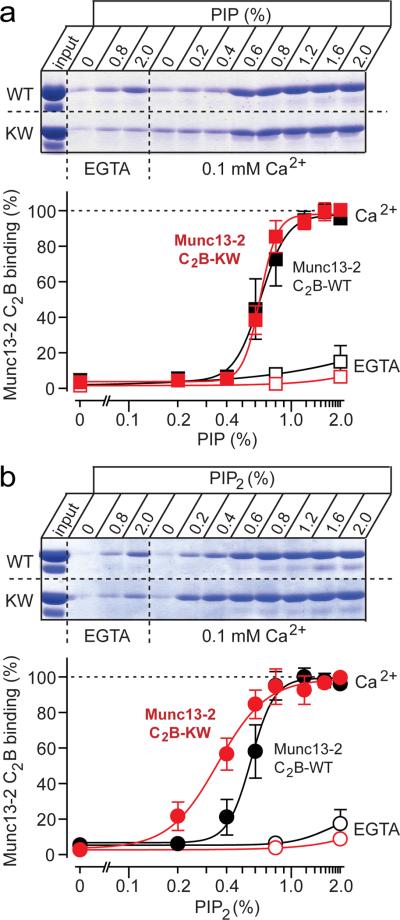
Next, we employed a centrifugation assay with liposomes containing a synaptic phospholipid composition with 0.5% PIP and 0.1% PIP2 (ref. 37). Besides the wild-type Munc13-1 and 13-2 C2B-domains and the DN-mutant Munc13-2 C2B-domain, we examined an additional Munc13-2 C2B-domain mutant called the KW-mutant, in which lysine630 in loop 1 is exchanged for tryptophan (Fig. 1a). The KW-mutant was designed to render the Munc13 C2B-domain more similar to the synaptotagmin-1 C2A-domain. Synaptotagmin-1 contains at this position a hydrophobic methionine (Fig. 1a) that inserts into the phospholipid bilayer in a Ca2+-dependent manner38, and enhances the Ca2+-dependent phospholipid binding affinity of the synaptotagmin-1 C2A-domain39–41. Tryptophan was used instead of methionine to maximize the membrane penetration of the Munc13 C2B-domain, as previously shown for the synaptotagmin-1 C2A-domain40,41.
The Munc13-1 and 13-2 C2B-domains bound poorly to synaptic liposomes in the absence of Ca2+, but strongly in the presence of Ca2+ (Fig. 3b). Quantitation of Coomassie-blue stained SDS-gels revealed that the Munc13-1 and 13-2 C2B-domains exhibited similar apparent Ca2+-affinities (Munc13-1: EC50 = 5.5±0.9 μM Ca2+ [n=3]; Munc13-2: EC50 = 5.3±0.8 μM Ca [n=3]; means ± SEMs) that were indistinguishable from that of the synaptotagmin-1 C2A/B-domain fragment (EC50 = 6.4 ± 0.5 μM Ca2+ [n=4; means ± SEMs]; Supplementary Fig. 4). The DN-mutation blocked all Ca2+-dependent phospholipid binding (EC50 >1 mM Ca2+), whereas the KW-mutation did not alter the extent or apparent Ca2+-affinity of Ca2+-dependent phospholipid binding under these conditions (EC50 = 5.4±0.9 μM Ca2+ [n=3; means ± SEM]; Fig. 3b).
Unusual phospholipid specificity of the Munc13 C2B-domain
We next examined whether decreasing the PIP- and PIP2-concentrations alters Ca2+-dependent phospholipid binding by wild-type or mutant Munc13 C2B-domains. An only two-fold decrease of the PIP- and PIP2-concentrations (to 0.25% PIP and 0.05% PIP2) abolished Ca2+-dependent liposome binding of the wild-type Munc13 C2B-domain, but not of the synaptotagmin-1 C2-domains (Fig. 3c). Strikingly, the KW-mutation converted the Munc13 C2B-domain into a synaptotagmin-like domain, with full Ca2+-induced binding to the liposomes containing reduced PIP- and PIP2-concentrations (Munc13-2 KW: EC50 = 18.8±2.6 μM Ca2+ [n=4]; Syt-1: EC50 = 17.2±2.4 μM Ca2+ [n=3]; means ± SEMs; Fig. 3c). Thus, the Munc13 C2B-domain is more sensitive to the PIP- and PIP2-concentrations than the synaptotagmin-1 C2-domains, but a single amino-acid substitution renders the lipid-binding properties of the Munc13 C2B-domain similar to those of synaptotagmin-1.
Assays of Ca2+-dependent binding of wild-type Munc13-2 C2B-domain to liposomes containing increasing concentrations of PIP or PIP2 revealed that both phosphatidylinositolphospholipids equally promoted Ca2+-dependent binding (Fig. 4 and Supplementary Fig. 5). This behavior was unexpected because Ca2+-dependent binding of the synaptotagmin-1 C2-domains to liposomes exhibits a strong preference for PIP2 due to its higher negative charge42,43. Again, the KW-mutant Munc13 C2B-domain preferentially bound to the PIP2-containing liposomes, similar to the synaptotagmin-1 C2-domains (Fig. 4).
Figure 4. PIP- and PIP2-dependence of Ca2+-induced liposome binding to Munc13 C2B-domains.
a, b Quantitation of Ca2+-dependent Munc13 C2B-domain binding to `synaptic' liposomes as a function of the PIP- (a) or PIP2-concentration (b). Binding assays were carried out using the centrifugation assay (Figs. 3b and 3c) in the absence (open symbols) or presence of 0.1 mM Ca2+ (filled symbols) as a function of the concentration of PIP (a) or PIP2 (b) in the liposomes. The top panels display representative experiments, and the bottom panels summary graphs (means ± SEMs [n=3]; data were normalized to binding at the highest free Ca2+ concentration). Wild-type and KW-mutant C2B-domains are not significantly different for the PIP titration (a), but are significantly different for the PIP2 titration (b; p=0.0016 using a 2-way ANOVA test; see Supplementary Fig. 5 for direct comparison of the binding of the WT C2B-domain to PIP- or PIP2-containing liposomes).
In synaptotagmin-1, C2-domains not only bind to phospholipids, but also to SNARE proteins43–46. However, pull-down experiments with solubilized brain proteins uncovered Ca2+-dependent binding of the Munc13 C2B-domains only to tubulin (which as an abundant protein binds non-specifically to many proteins), but not to SNARE proteins, suggesting that the Munc13 C2B-domain does not interact with SNARE proteins (Supplementary Fig. 6).
Role of Munc13-2 C2B-domain in neurotransmitter release
In order to assess the functional importance of Ca2+-binding to the Munc13 C2B-domain, we analyzed synaptic transmission in autapses formed by hippocampal neurons that were cultured on micro-islands of glia cells. The neurons were isolated from mice that lack Munc13-1 and 13-2, and were rescued by viral expression of the `ubMunc13-2' variant of Munc13-2, used because of its pronounced effects on short-term synaptic plasticity24,47.
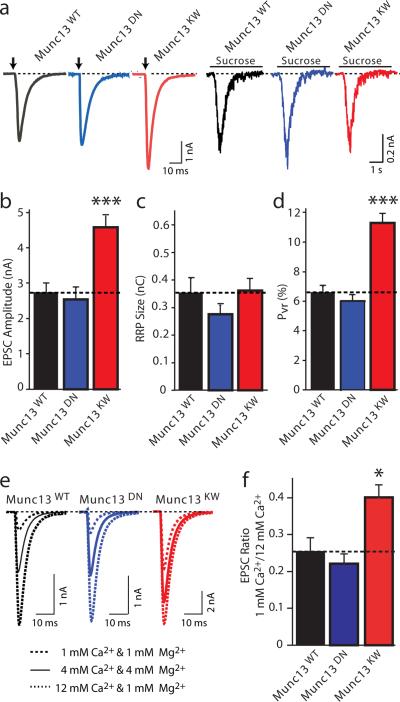
We first analyzed synaptic transmission induced by isolated action potentials. Wild-type, DN-mutant, and KW-mutant Munc13-2 rescued the loss of synaptic transmission induced by deletion of Munc13-1 and 13-2 (Fig. 5a). Rescue with WT and DN-mutant Munc13-2 caused no major change in EPSC amplitudes, whereas rescue with KW-mutant Munc13-2 increased the EPSC amplitudes almost two-fold (Fig. 5b). To test whether this change is due to a difference in the size of the RRP, we measured the RRP by application of hypertonic sucrose48, but detected no significant change (Fig. 5c). We also determined the vesicular release probability (Pvr) for each neuron expressing WT or mutant Munc13-2 by calculating the ratio of integrated EPSC and RRP charges. The DN-mutation did not alter the vesicular release probability, whereas the KW-mutation nearly doubled it (Fig. 5d).
Figure 5. Effect of Munc13-2 C2B-domain mutations on release induced by isolated action potentials.
All experiments in this figure and Fig. 6 were performed in hippocampal autaptic neurons cultured from Munc13-1/-2 double KO mice. Neurons were infected with recombinant Semliki Forest Virus expressing WT, DN-mutant, or KW-mutant Munc13-2, and excitatory postsynaptic currents (EPSCs) were recorded in whole-cell mode.
a, Representative EPSCs evoked by isolated action potentials (left) or 0.5 M sucrose (right) in neurons expressing WT (black), DN-mutant (blue), or KW-mutant Munc13-2 (red).
b–d, Mean EPSC amplitudes (b), RRP size (c, measured as the response to 0.5 M sucrose, integrating the transient current component for 4 s); and vesicular release probability (d, calculated as the ratio of the charge of evoked responses to that of the RRP). Data shown are means ± SEMs (n= (WT: n=58; DN: n=57; KW: n=79; ***, p<0.001 by paired t-test).
e, Representative EPSCs evoked by isolated action potentials in neurons expressing WT (black), DN-mutant (blue), or KW-mutant Munc13-2 (red) at three different Ca2+-concentrations as indicated.
f, Mean ratio of the EPSC amplitudes monitored at low vs. high Ca2+ in neurons expressing WT (black), DN-mutant (blue), or KW-mutant Munc13-2 (red; WT, n=16; DN, n=14; KW, n=16; *, p<0.05; see Supplementary Table 2 for a numerical listing of all electrophysiologically results.
To confirm that the KW- but not the DN-mutation of the C2B-domain of Munc13 alters the release probability during islated action potentials, we monitored the relative EPSC amplitudes of synapses expressing WT, DN-, or KW-mutant Munc13-2 at low (1 mM) or high (12 mM) extracellular Ca2+-concentrations (Fig. 5e). Consistent with an unchanged basal release probability, WT and DN-mutant Munc13-2 exhibited the same relative Ca2+-dependent changes in ESPC amplitudes. In contrast, KW-mutant Munc13-2 displayed a relative increase in EPSC amplitude at the low ambient Ca2+-concentration, confirming the hypothesis (Fig. 5f).
In increasing release, KW-mutant Munc13-2 could act either as a Ca2+-sensor for triggering release analogous to synaptotagmin, or as an auxiliary Ca2+-regulator of Ca2+-triggering by synaptotagmin. To differentiate between these two possibilities, we tested whether wild-type or KW-mutant Munc13-2 confer Ca2+-triggered neurotransmiter release onto synapses from synaptotagmin-1 KO mice that lack almost all such release49. However, neither WT nor KW-mutant Munc13-2 rescued the loss of Ca2+-induced synchronous release in synaptotagmin-deficient synapses, suggesting that Munc13-2 functions as an auxiliary Ca2+-regulator in release (Supplementary Fig. 7).
The Munc13-2 C2B-domain in short-term plasticity
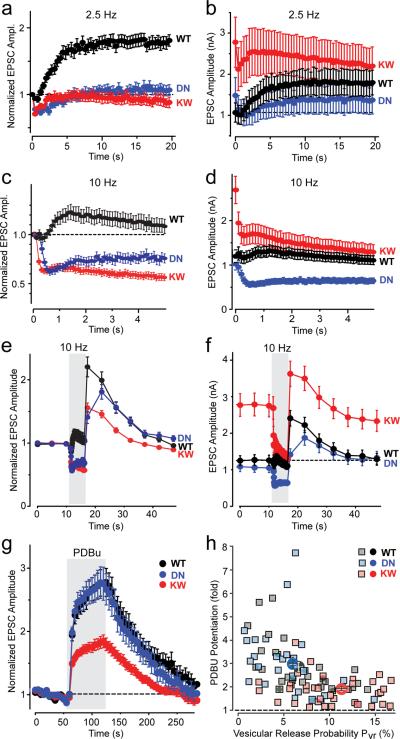
We next monitored synaptic responses induced by 2.5, 10, and 40 Hz stimulus trains in synapses expressing WT or mutant Munc13-2. Plots of normalized responses revealed that as expected, synapses expressing WT Munc13-2 exhibited strong facilitation at 2.5 Hz stimulation, and less facilitation at 10 Hz stimulation (Figs. 6a–6d; and Supplementary Fig. 8). In contrast, synapses expressing DN- or KW-mutant Munc13-2 both displayed no facilitation, but transient depression during the 2.5 Hz stimulation, and persistent depression during the 10 Hz stimulation (Figs. 6a and 6c).
Figure 6. Ca2+-binding to the Munc13 C2B-domain regulates release during high-frequency action potential trains.
a, b. Mean normalized (left panels) and absolute (right panels) EPSC amplitudes in response to a 2.5 Hz (a) or 10 Hz (b) action potential train in Munc13-deficient neurons expressing WT (black), DN-mutant (blue), or KW-mutant Munc13-2 (red; means ± SEMs). In the normalized plots (left panels), p<0.001 for WT compared to DN- and KW-mutant Munc13-2; in the absolute responses (right panels), the initial responses are significantly larger (p<0.01) for the KW-mutant Munc13-2 compared to the WT or DN-mutant protein, whereas the final responses are significantly smaller (p<0.001) for the DN-mutant compared to the WT and KW-mutant Munc13-2 (2.5 Hz, WT n=18; KW n=21; DN; n=16; 10 Hz, WT n=50; DN n=41; KW n=64).
c., d. Normalized (c) and absolute EPSC amplitudes (d) in response to a low-frequency stimulus train (0.2 Hz) that is interrupted by a 5 sec 10 Hz stimulus train to induce augmentation (gray area)47. Munc13-deficient neurons expressing WT (black), DN-mutant (blue), or KW-mutant Munc13-2 (red) were analyzed (for normalized responses, degree of augmentation is significantly higher (p<0.001) for WT compared to DN- and KW-mutant Munc13-2; for absolute responses, all three Munc13 forms differ significantly from each other at the p<0.001 level (WT n=50; DN n=41; KW n=64)).
e. Relative potentiation by PDBu (1 μM) of EPSC amplitudes evoked at 0.2 Hz in Munc13-deficient neurons expressing WT (black), DN-mutant (blue), or KW-mutant Munc13-2 (red). The relative PDBu potentiation was significantly lower (p<0.001) in synapses expressing KW-mutant Munc13-2 than in synapses expressing WT or DN-mutant Munc13-2 (WT, n=30; DN, n=31; KW, n=43).
f. Plot of the degree of PDBu potentiation as a function of the initial vesicular release probability (Pvr) in individual neurons. Each individual data point represents a Munc13-deficient neuron expressing WT (black), DN-mutant (blue), or KW-mutant Munc13-2 (red). The solid symbols represent the mean values for each group.
Strikingly, plots of absolute synaptic responses indicated that the analysis of normalized responses is misleading. Specifically, synapses expressing KW-mutant Munc13-2 started off with a much higher absolute EPSC value than synapses expressing WT Munc13-2, and exhibited continuously larger EPSCs, whereas synapses expressing DN-mutant Munc13-2 stated off with an unchanged EPSC value, but experienced more severe synaptic depression during the stimulus trains (right panels, Figs. 6b and 6d). Thus, in synapses containing KW-mutant Munc13-2, the initially increased release probability leads to a faster depletion of the RRP and apparent depression. In contrast, in synapses expressing DN-mutant Munc13-2, the initial release probability is normal, and depression develops because the accumulating Ca2+ that normally augments release by binding to the Munc13 C2B-domain can no longer bind to the domain.
To confirm these conclusions, we analyzed a second, related form of short-term synaptic plasticity: augmentation of synaptic responses observed after a short high-frequency stimulus train. We measured synaptic responses before and after application of a 5 s, 10 Hz stimulus train and again analyzed normalized and absolute EPSC amplitudes (Figs. 6e and 6f). Plots of normalized responses showed that augmentation was largest in synapses expressing wild-type Munc13-2 (2.3 ± 0.1 fold, n=77), but was impaired in synapses expressing DN- or KW-mutant Munc13-2 (1.3 ± 0.1, n=71, p<0.001; 1.5 ± 0.1, n=93, p<0.001). Plots of absolute responses, however, showed that DN-mutant expressing synapses exhibited a true loss of augmentation, whereas the apparent loss of augmentation in KW-mutant expressing synapses was not present since the synapses start from an enhanced `plateau' (Fig. 6f). Interestingly, the loss of augmentation in synapses expressing DN-mutant Munc13-2 applied only to the very initial phase; later in the stimulus train, responses recovered, consistent with the notion that multiple Ca2+-regulators mediate augmentation13,16–20,50.
Our data suggest that the main mechanism by which Ca2+-binding to the Munc13 C2B-domain mediates synaptic augmentation involves a change in vesicle release probability, as the gain-of-function KW mutant has a two-fold higher initial release probability. To test further whether additional effects on vesicle repriming could explain the phenotype, we measured the recovery of the RRP after vesicle depletion by a high-frequency stimulus train (40 Hz for 2.5 s). Although there was a trend towards a slower EPSC recovery in synapses expressing DN- or KW-mutant Munc13-2, this effect was not statistically significant (Supplementary Fig. 8).
Synaptic but not non-synaptic Munc13 isoforms contain adjacent C1- and C2-domains similar to PKC, where they cooperate with each other51. At a synapse, phorbol esters increase the presynaptic vesicle release probability without changing the RRP size47,52, at least in part by activating Munc13 (refs. 52 and 53). To test whether the Munc13 C1- and C2-domains also cooperate, we analyzed the effect of phorbol esters (1 μM PDBu applied for 1 min). We found that the relative potentiation by PDBu was significantly decreased in synapses expressing KW-mutant Munc13-2, whereas synapses expressing WT or DN-mutant Munc13-2 displayed similar degrees of potentiation (Figs. 6g). Plots of the relative potentiation of release by PDBu against the vesicular release probability for individual synapses revealed an inverse correlation (Fig 6h), indicating that the increased vesicular release probability caused by the KW-mutation occludes the PDBu potentiation.
DISCUSSION
Munc13's are essential components of the synaptic release machinery that prime synaptic vesicles for exocytosis, and regulate short-term plasticity of synaptic exocytosis10,13,47,53,54, but their mechanisms of action remain unclear. Here, we show that the central C2B-domain of Munc13's exhibits unusual Ca2+- and PIP/PIP2-dependent phospholipid binding properties. These properties structurally correlate with a unique accessory α-helix of the Munc13 C2B-domain that is part of its Ca2+-binding site. The unusual properties of the Munc13 C2B-domain enable Munc13's to mediate Ca2+-dependent augmentation of synaptic vesicle exocytosis during high-frequency trains of action potentials. As described below, we believe that this augmentation is likely based on the Ca2+-dependent binding of the Munc13 C2B-domain to the plasma membrane, which in turn is enabled by increased synthesis of PIP and PIP2 induced by accumulating Ca2+ during a high-frequency action potential train.
Properties of the Munc13 C2B-domain
Structurally, the Munc13 C2B-domain is composed of a standard C2-domain β-sandwich in which aspartate residues in the `top' loops coordinate two Ca2+-ions (Figs. 1 and 2). A distinctive feature of the Munc13 C2B-domain is Ca2+-binding loop 3, which includes an extended sequence that folds into an amphipathic α-helix (Fig. 2). Biochemically, the Munc13 C2B-domain binds to phospholipids in a Ca2+-dependent manner similar to other C2-domains, but differs from other known C2-domains, such as those from synaptotagmin, in that Ca2+-dependent phospholipid binding requires relatively high concentrations of PIP or PIP2 (Figs. 3 and 4). This unusual biochemical property is likely mediated, at least in part, by the unique α-helix formed by Ca2+-binding loop 3, which contains a highly positively charged region that may act similarly to the PIP2-dependent amphipathic α-helix observed in epsin55.
Our data show that in addition to the amphipathic α-helix, the conserved, positively charged K630 residue in Ca2+-binding loop 1 is a major determinant of the unusual phospholipid binding properties of the Munc13 C2B-domain. In the Ca2+-binding loops of synaptotagmin-1 C2-domains, the residues analogous to K630 of Munc13 are hydrophobic (M173 and V304; Fig. 1a). During Ca2+-dependent phospholipid binding of synaptotagmin-1 C2-domains, M173 and V304 insert into the phospholipid bilayer, and contribute to the relatively non-specific but tight Ca2+-dependent phospholipid-binding of these C2-domains38–41. Moreover, exchanging M173 and V304 in the synaptotagmin-1 C2-domains for tryptophan further enhances their Ca2+-dependent phospholipid binding, indicating that tryptophan increases Ca2+-dependent phospholipid binding mediated by hydrophobic residues40,41,56. These observations suggested to us that K630 in the Munc13 C2B-domain may contribute to its unique specificity for high PIP/PIP2-concentrations. To test this hypothesis, we substituted K630 of the Munc13 C2B-domain for tryptophan, resulting in the KW-mutation. The KW-mutation rendered the Munc13 C2B-domain responsive to PIP2 at a concentration at which WT Munc13 is inert but synaptotagmin-1 is active, and thus confers synaptotagmin-like properties onto the Munc13 C2B-domain (Fig. 4). As a result, the KW-mutation constitutes a gain-of-function mutation that enables Ca2+-dependent binding of the Munc13 C2B-domain to phospholipid membranes containing lower PIP/PIP2-concentrations than the Munc13 C2B-domain would normally bind to.
The C2B-domain regulates synaptic plasticity
Strikingly, abolishing Ca2+-binding to the Munc13-2 C2B-domain with the DN-mutation did not alter vesicle exocytosis triggered by isolated action potentials (Fig. 5), but impaired facilitation of synaptic vesicle exocytosis induced by repeated action potentials (Fig. 6). Thus, the Munc13 C2B-domain acts as a Ca2+-regulator of short-term synaptic plasticity, consistent with the notion that synaptic facilitation during high-frequency stimulus trains is not passively caused by residual Ca2+, but actively induced by Ca2+-binding to the Munc13 C2B-domain (and other Ca2+-binding proteins). The KW-mutation, in contrast, increased the amount of Ca2+-triggered release during single and repeated action potentials (Figs. 5 and 6), without itself acting as a Ca2+-sensor for release (Supplementary Fig. 7).
Viewed together, our data suggests that during isolated action potentials, the lower PIP/PIP2-content at rest prevents Ca2+-dependent binding of the WT Munc13 C2B-domain to the membrane. During stimulus trains, a Ca2+-dependent phosphatidylinositol kinase may increase the presynaptic PIP/PIP2-content, allowing residual Ca2+ to activate Munc13 and thereby to boost release. Consistent with this hypothesis, Ca2+ stimulates PIP and PIP2 synthesis in neuroendocrine cells57, and depolarization of neurons activates presynaptic PIP kinase Iγ by dephosphorylation, thereby increasing the plasma membrane PIP/PIP2-content58–60. Our hypothesis explains why the DN-mutation has no effect on Munc13 function during isolated action potentials, but interferes with Munc13 function during repeated action potentials. The hypothesis also accounts for the gain-of-function effect of the KW-mutation, since the PIP/PIP2-content at rest is proposed to be too low to allow Ca2+-activation of the WT Munc13 C2B-domain, but may suffice for Ca2+-activation of the KW-mutant C2B-domain.
Mechanism of the Munc13 C2B-domain action
In boosting release during a stimulus train, Ca2+-binding to the Munc13 C2B-domain likely increases vesicle priming by enhancing the priming function of Munc13. The KW-mutation may enable the Munc13 C2B-domain to perform the same activity even at rest, possibly because the KW-mutant C2B-domain binds to the plasma membrane even without the increase in PIP and PIP2 concentrations that is thought to occur during repeated action potentials57–60.
However, two of our findings with KW-mutant Munc13 appear to argue against the hypothesis that the Munc13 C2B-domain boosts the priming function of Munc13:
the KW-mutation selectively increased the Ca2+-sensitivity of release induced by isolated action potentials without increasing the size of the RRP (Fig. 5)
the KW-mutation did not significantly alter the rate by which evoked release recovered after depletion of the RRP, although there was a trend towards acceleration of priming (Supplementary Fig. 8)
Despite these findings, we believe that the priming hypothesis is correct for the following reasons: Priming likely involves a partial, if not complete assembly of SNARE-complexes between vesicles and the plasma membrane61. The number of assembled SNARE complexes per primed vesicle may determine (among others) the apparent Ca2+-affinity of synaptic vesicle fusion62. Thus, Ca2+-binding to the Munc13 C2B-domain may increase the ability of Munc13 to catalyze SNARE-complex assembly of docked vesicles during priming, which would result in the appearance of an increased Ca2+-sensitivity of release for the KW-mutation. This hypothesis is consistent with the fact that KW-mutant Munc13 does not act as a Ca2+-sensor for exocytosis itself (Supplementary Fig. 7). Although plausible, the priming hypothesis cannot be tested directly until its underlying tenet – namely that Munc13 mediates vesicle priming by catalyzing SNARE-complex assembly – has been confirmed63.
How does increased phospholipid binding induced by Ca2+-binding to the Munc13 C2B-domain potentiate Ca2+-triggered release, be it via priming or otherwise? As a component of the biochemically insoluble active zone, Munc13 already is normally close to the plasma membrane. Thus, Ca2+-dependent C2B-domain binding to the plasma membrane would not re-localize Munc13, but rather pull on the adjacent plasma membrane and stretch it. Such an activity may, analogous to what has been proposed for the mechanism of action of synaptotagmin64, promote exocytosis by decreasing the energy requirement for Ca2+-triggered fusion-pore opening. DAG-binding to the C1-domain of Munc13 – which also induces Munc13 plasma membrane binding – may potentiate release by an analogous, but Ca2+-independent mechanism. An alternative hypothesis is that the Munc13 C1- and C2B-domains are normally inhibitory, and that DAG- and Ca2+-binding reverse this inhibition54. This second hypothesis would require that the C1- and the C2B-domain have additional unknown biochemical interactions beyond lipid binding which are altered by the various mutations, a possibility that remains to be explored.
Munc13 as a computational unit for synaptic transmission
The activity of synaptic Munc13 isoforms is regulated via three distinct, adjacent signaling motifs: the previously described calmodulin-binding sequence and C1-domain12,13, and the C2B-domain we characterize here (Fig. 7). All three motifs are directly or indirectly activated by Ca2+, and have profound roles in controlling neurotransmitter release during short-term plasticity, but differ from each other in their mechanisms of activation and action. The C2B-domain is directly Ca2+-activated by Ca2+-influx during action potentials, but is presumably only stimulated after accumulating residual Ca2+ has induced the synthesis of PIP/PIP2. In contrast, the calmodulin-binding sequence is indirectly activated by binding of accumulating residual Ca2+ to calmodulin, which likely acts on many synaptic targets simultaneously. The C1-domain is activated indirectly via Ca2+-dependent induction of phospholipase C. Thus, the Ca2+-concentration dependence and time course of activation of the three regulatory motifs likely differ, leading to a common readout (synaptic potentiation) that results from the integration of multiple signals acting differentially on the three signaling motifs. Consistent with this model, the KW-mutation of Munc13 occludes the effect of phorbol esters on release triggered by isolated action potentials, whereas the DN-mutation (which has no effect on release triggered by isolated action potentials) has no effect on the phorbol ester potentiation under those conditions (Fig. 6). Moreover, at least mutations in the calmodulin-binding motif and the C2B-domain act additively during short-term synaptic plasticity (Supplementary Fig. 9).
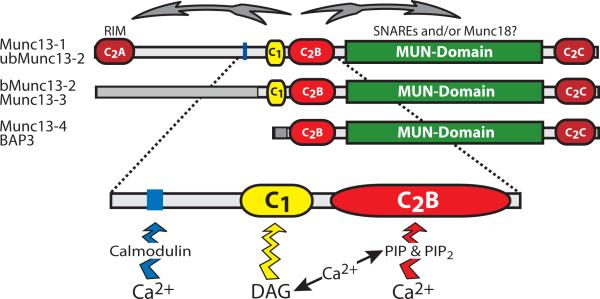
Figure 7. Model for the Ca2+-regulation of short-term plasticity by Munc13.
The top diagrams depict the domain structures of the three sub-families of Munc13 proteins, the two classes of long Munc13s expressed primarily in brain, and the class of short Munc13s expressed primarily in peripheral organs. Top arrows illustrate a possible regulation of the N-terminal RIM-binding sequences and the C-terminal MUN-domain of Munc13s by ligand-binding to the central C1- and C2-domains. The central regulatory domains of Munc13's are illustrated below the domain diagrams: the calmodulin-binding sequence found in Munc13-1 and bMunc13-2, the DAG-binding C1-domain found in all variants of Munc13-1, -2, and -3 but not the ubiquitous Munc13 isoforms, and the Ca2+-binding C2B-domain that is universally present in all neuronal and ubiquitous Munc13 isoforms. Note that in addition to binding to the C2B-domain, Ca2+ also serves to stimulate the production of DAG from PIP2 on the one hand, and the synthesis of DAG on the other hand.
In summary, our data establish that the Munc13 C2B-domain operates as a Ca2+-regulator of short-term synaptic plasticity. Apart from the importance of the Munc13 C2B-domain Ca2+-binding properties for synaptic exocytosis, our results also suggest that this domain may act as a Ca2+-regulator of exocytosis for non-synaptic Munc13 isoforms, which likely function in other forms of exocytosis (such as Munc13-4 in lymphocyte exocytosis27).
METHODS
Plasmids
Four different types of plasmids encoding rat Munc13-1 and 13-2 were used for this study: i. pGEX-KT derived plasmids for bacterial expression. ii. pFastBac™1 (Invitrogen life technologies) derived baculovirus expression plasmids into which we inserted the GST-coding region. The resulting plasmid was used for cloning pFastBac-GST-Munc13-1 C2B (residues 675–820; numbering based on U24070); pFastBac-GST-Munc13-2 C2B (residue 599–744; numbering based on AF159706); pFastBac-GST-Munc13-2 C2B-DN (D629N and D635N); and pFastBac-GST-Munc13-2 C2B-KW (K630W); iii. Semliki Forest Virus expression plasmids (pSFV ubMunc13-2 WT, pSFVubMunc13-2 C2B-D629N,D635N, pSFVub Munc13-2K630W) for neuronal cultures. iv. Lentiviral expression plasmid pFUW-Munc13-2 K603W that encodes ubMunc13-2 with the KW mutation.
Production of recombinant proteins
1. Bacterial expression
GST fusion proteins were expressed at 25 °C in E. coli BL21, isolated by affinity chromatography on glutathione-Sepharose followed by on-resin cleavage with thrombin, and cleaved proteins were further purified by ion-exchange and gel-filtration chromatography on MonoS and S75 columns (Amersham). Uniform 15N-labeling was achieved by growing the bacteria in 15NH4Cl as the sole nitrogen source.
2. Bacuolovirus expression
Munc13 C2B-domain proteins were generated using Bac-to-Bac® Baculovirus Expression System (Invitrogen Life Technologies). Recombinant GST-Munc13 C2B proteins were generated by infecting 400 ml of Sf9 cells (~2×106 cells/ml) in a 2 liter flask after inoculating 20 ml of baculovirus (~107 pfu/ml) for 3 days at 28 °C. Recombinant GST-fusion proteins were purified using glutathione-Sepharose™ 4B beads (Amersham Biosciences).
Antibodies were either described previously2,3 or obtained from Synaptic Systems GmbH (Göttingen, Germany), except when stated otherwise. SDS-PAGE and immunoblotting were performed as described3.
Fluorescence spectroscopy
Fluorescence spectra were recorded at 290 nm excitation in an LS55 luminescence spectrometer (PerkinElmer Life Sciences) with 0.3 μM purified Munc13 C2B-domains in 0.5 ml of 20 mM HEPES-NaOH pH 7.2 and 0.1 M NaCl, with the indicated additions. All buffers were passed through the AG MP-50 resin (Bio-Rad) to eliminate contaminating Ca2+.
NMR spectroscopy was carried out at 25 °C on Varian INOVA500 or INOVA600 spectrometers with samples containing ~120 μM Munc13-1 C2B-domain (residues 675–820) in 20 mM MES-NaOH pH 6.2, 0.1 M NaCl, and 0.5 mM TCEP. Ca2+-titrations were monitored by 1H-15N HSQC experiments as described30. All NMR data were processed with NMRPipe65 and analyzed with NMRView66.
X-ray crystallography
Purified Munc13-1 C2B-domain (residues 675–820; in 20 mM MES-NaOH pH 6.2, 0.1 M NaCl and 0.5 mM TCEP) was concentrated to 20 mg/ml, and crystallized in 30% (w/v) PEG-MME 2000 and 0.1 M Bis-Tris-Propane pH 6.8 at 20 °C without Ca2+ or with 0.01–0.1 M CaCl2 using the hanging-drop vapor-diffusion method. Ca2+-free Munc13-1 C2B-domain crystals appeared in two days, grew to a final size of 0.08 × 0.05 × 0.15 mm within a week, and were transferred into a solution of 35% (w/v) PEG-MME 2000, 0.1 M Bis-Tris-Propane pH 6.8 and 5% (v/v) glycerol, and flash-cooled in liquid propane. Ca2+-bound Munc13-1 C2B-domain crystals appeared within one week as needle-clusters and gradually transformed into a diamond-like shape over 3–4 weeks, with a final size of ~0.1 mm, after which they were transferred into a solution of 32% PEG-MME 2000, 0.1 M Bis-Tris-Propane pH 6.8, 0.1 M NaCl, 10% glycerol, 1 mM CaCl2 and flash-cooled in liquid propane. Diffraction data were collected at the Structural Biology Center beamlines 19BM and 19ID of the Advanced Photon Source at 100K. The diffraction of these crystals was highly anisotropic, leading to a gradual decrease in the completeness at resolutions above 2.2 Å. Crystals of the Ca2+-free Munc13-1 C2B-domain diffracted to a dmin of ~1.89 Å (space group C2221; unit cell parameters a = 43 Å, b = 101 Å, c = 68 Å; 1 molecule per asymmetric unit). Crystals of the Ca2+-bound Munc13-1 C2B-domain diffracted to a Bragg spacing (dmin) of ~1.37 Å (space group P43212; unit cell parameters a = 57 Å, b = 57 Å, c = 90 Å, 1 molecule per asymmetric unit). Data were processed in the HKL2000 program suite67. The structure of the Ca2+-free Munc13-1 C2B-domain was determined via molecular replacement with the program AMoRe68 using the synaptotagmin-1 C2A-domain (PDB code 1rsy) as search model. The Ca2+-bound Munc13-1 C2B-domain structure was determined via molecular replacement with the program Phaser69 using the final structure of Ca2+-free Munc13-1 C2B-domain as search model. Models were completed using the program Arp/Warp70, followed by manual adjustments with the program O71, and refinements with the program Refmac72 of the CCP4 package73, with a random subset of all data set aside for the calculation of free R factors. After complete refinement, solvent molecules were added where chemically reasonable. The final model for the Ca2+-bound Munc13-1 C2B-domain contains residues 673–675 and 687–819, two Ca2+-ions, two Cl−-ions, one glycerol molecule, and 161 water molecules (final R = 17.0; Rfree = 19.3; overall B-factor = 16.7). The final model for the Ca2+-free Munc13-1 C2B-domain contains residues 678–705, 708–763, 773–801 and 807–819, one molecule of Bis-Tris-Propane, 2 Cl−-ions, and 63 water molecules (final R = 21.7; Rfree = = 28.2; overall B-factor = 41.0). For data collection and refinement statistics, see Table 1.
Phospholipid binding assays
1. Centrifugation assay
Phospholipids and cholesterol (obtained from Avanti) were dissolved in chloroform:methanol (1:1; cholesterol and PIPs) or chloroform (PS, PI, PC, and PE), mixed in a `synaptic' composition (41% PC, 32% PE, 12% PS, 5% PI, and 10% cholesterol with or without additional PIP and PIP2; phospholipid composition approximates that of synaptic vesicles13–15), and dried under nitrogen. Lipids were resuspended by vortexing 175 mg lipid mixture for 20 min in 3.5 ml HEPES buffer (50 mM HEPES-NaOH pH 6.8, 0.1 M NaCl, and 4 mM EGTA) containing 0.5 M sucrose. Lipids were sonicated for 5 min in a bath sonicator (model G112SP1G; Laboratory Supply Co. Inc.), 14 ml HEPES buffer without sucrose were added, and liposomes were centrifuged at 100,000g for 30 min to separate heavy liposomes from free phospholipids. Liposomes were washed once, and repelleted (20,800g for 10 min). Binding assays were performed in 1 ml with 10 μg recombinant GST-fusion proteins (~0.125 μM) and 100 μg liposomes in HEPES buffer containing 2 mM MgCl2 and various Ca2+-concentrations that result in the indicated free [Ca2+] as calculated with EqCal software (Biosoft, Ferguson, MI). Binding reactions were incubated 10 min at 30 °C with 800 rpm shaking, pelleted by centrifugation (20,800g for 10 min), and washed three times with 1 ml of the corresponding buffers. Final liposome pellets were dissolved in chloroform:methanol (1:2, v/v). Precipitated proteins were recovered by centrifugation (20,800g for 15 min), resuspended in 30 μl of 2× SDS sample buffer, and analyzed by SDS-PAGE and Coomassie Blue staining2,16. Bound proteins were quantified analysis of scanned Coomassie-stained gels with the Image Quant program (version 5.2, Molecular Dynamics).
2. FRET assays
were performed essentially as described17 at room temperature in 0.5 ml of 20 ml HEPES-NaOH pH 7.2, 0.1 M NaCl, with 1 μM of Munc13-2 C2B-domain protein and 30 μg/ml liposomes containing 41% PC, 22% PE, 10% dansyl-PE, 12% PS, 5% PI, 10% cholesterol, 0.5% PIP, and 0.1% PIP2. Emission spectra (excitation: 282 nm) were first recorded without metal ions, then after addition of 2 mM MgCl2, then after addition of 0.2 mM CaCl2, and then after further addition of 1 mM EGTA.
GST pulldowns were performed as described2. One unstripped rat brain (~1.5 g/brain; Pel-Freez Biologicals, Rogers, Arkansas) was homogenized with a tissue homogenizer (Thomas Scientific, Philadelphia, Pennsylvania) in 30 ml HEPES buffer containing 1 mM DTT, 1 mM PMSF, 5 μg/ml leupeptin, and 2 μg/ml aprotinin. 1% Triton X-100 was added, proteins were extracted for 1 hr at 4 °C with rotation, insoluble proteins were removed by centrifugation (100,000g for 1 hr), and the supernatant was used for pulldowns using 30 μg GST-Munc13 C2B-domain proteins attached to glutathione-Sepharose, and 0.5 ml rat brain lysate. Pulldown reactions (1 ml volume) in HEPES buffer containing 2 mM MgCl2 and 0.5% Triton X-100 were incubated 1.5 hr at 4 °C with rotation, washed six times with corresponding buffer, and bound proteins were analyzed by SDS-PAGE and immunoblotting.
Electrophysiology
Microdot neuronal cultures were prepared and electrophopysiological analyses were performed at 22–25 °C as described18–20,74. Currents were recorded using an Axopatch 200B amplifier (Molecular Devices). Series resistance was within 10 MΩ and was electronically compensated at least 70%. Data were analyzed using AXOGRAPH software (version 4.9, Molcular Devices). For analyses of Syt-1 KO mice, primary cortical neurons from Syt-1 KO and littermate wild-type control mice were cultured in Modified Eagle Medium (MEM, Gibco) supplemented with B27 (Gibco), glucose, transferrin, fetal bovine serum, and Ara-C (Sigma)74. To monitor synaptic responses, whole-cell patch-clamp recordings were made with neurons at 14–16 days in vitro. Synaptic responses were triggered by a 1 ms current pulse (900 μA) through a local extracellular electrode (FHC, Inc.), and recorded in whole-cell voltage-clamp mode using a Multiclamp 700B amplifier (Axon Instruments, Inc.)74. Data were analyzed using Clampfit 9.02 (Axon Instruments, Inc) or Igor 4.0 (Wavemetrics).
Statistical analyses were performed using paired Student's t-test or ANOVA.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ms. Izabela Kornblum, Ina Herfort, and Hui Deng for excellent technical support. This paper was supported by grants from the NIH (NS051262 to C.R.; NS40944 to J.R.) and the Deutsche Forschungsgemeinschat (to C.R.).
Footnotes
DATA BANK ACCESSION NUMBERS To be added in proofs
REFERENCES
- 1.Katz B. The release of neuronal transmitter substances. Liverpool University Press; Liverpool, UK: 1969. [Google Scholar]
- 2.Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- 3.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 4.Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319:1543–1546. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- 5.Brose N, Hofmann K, Hata Y, Südhof TC. Mammalian homologues of C. elegans unc-13 gene define novel family of C2-domain proteins. J. Biol. Chem. 1995;270:25273–25280. doi: 10.1074/jbc.270.42.25273. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Okamoto M, Schmitz F, Hofman K, Südhof TC. RIM: A putative Rab3-effector in regulating synaptic vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- 7.Betz A, et al. Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron. 2001;30:183–96. doi: 10.1016/s0896-6273(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 8.Schoch S, et al. RIM1α forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- 9.Dulubova I, et al. A Munc13/RIM/Rab3 Tripartite Complex: From Priming to Plasticity? EMBO J. 2005;24:2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustin I, et al. The cerebellum-specific Munc13 isoform Munc13-3 regulates cerebellar synaptic transmission and motor learning in mice. J. Neurosci. 2000;21:10–17. doi: 10.1523/JNEUROSCI.21-01-00010.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlüter O, Schmitz F, Jahn R, Rosenmund C, Südhof TC. A complete genetic analysis of neuronal Rab3 function. J. Neurosci. 2004;24:6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee S-R, et al. Phorbol ester- and diacylglycerol-induced augmentation of neurotransmitter release from hippocampal neurons is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- 13.Junge HJ, et al. Calmodulin and Munc13 form a Ca2+ sensor/effector complex that controls short-term synaptic plasticity. Cell. 2004;118:389–401. doi: 10.1016/j.cell.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Basu J, et al. A minimal domain responsible for Munc13 activity. Nat. Struct. Mol. Biol. 2005;12:1017–1018. doi: 10.1038/nsmb1001. [DOI] [PubMed] [Google Scholar]
- 15.Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat. Rev. Neurosci. 2005;6:267–76. doi: 10.1038/nrn1647. [DOI] [PubMed] [Google Scholar]
- 16.Zühlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
- 17.Lee A, et al. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 1999;99:155–159. doi: 10.1038/20194. [DOI] [PubMed] [Google Scholar]
- 18.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 19.Wayman GA, Lee YS, Tokumitsu H, Silva A, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32:1119–1131. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- 21.Huang YY, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 22.Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:878–882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- 23.Augustin I, Rosenmund C, Südhof TC, Brose N. Munc-13 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- 24.Varoqueaux F, et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc. Natl. Acad. Sci. USA. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiratsuchi T, et al. Cloning and characterization of BAP3 (BAI-associated protein 3), a C2 domain-containing protein that interacts with BAI1. Biochem. Biophys. Res. Commun. 1998;251:158–165. doi: 10.1006/bbrc.1998.9408. [DOI] [PubMed] [Google Scholar]
- 26.Koch H, Hofmann K, Brose N. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem. J. 2000;349:247–253. doi: 10.1042/0264-6021:3490247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldmann J, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 28.Madison JM, Nurrish S, Kaplan JM. UNC-13 interaction with syntaxin is required for synaptic transmission. Curr. Biol. 2005;15:2236–2242. doi: 10.1016/j.cub.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 29.Stevens DR, et al. Identification of the minimal protein domain required for priming activity of Munc13-1. Curr. Biol. 2005;15:2243–2248. doi: 10.1016/j.cub.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 30.Ubach J, Zhang X, Shao X, Südhof TC, Rizo J. Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2-domain? EMBO J. 1998;17:3921–3930. doi: 10.1093/emboj/17.14.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutton RB, Davletov BA, Berghuis AM, Südhof TC, Sprang SR. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 32.Rizo J, Südhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. J. Biol. Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 33.Shao X, Fernandez I, Südhof TC, Rizo J. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: does Ca2+ induce a conformational change? Biochemistry. 1998;37:16106–16115. doi: 10.1021/bi981789h. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez I, et al. Three-dimensional structure of the synaptotagmin 1 C2B-domain. Synaptotagmin 1 as a phospholipid binding machine. Neuron. 2001;32:1057–1069. doi: 10.1016/s0896-6273(01)00548-7. [DOI] [PubMed] [Google Scholar]
- 35.Holm L, Sander C. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 1993;233:123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- 36.Shao X, Davletov BA, Sutton RB, Südhof TC, Rizo J. Bipartite Ca2+-binding motif in C2 domains of synaptotagmin and protein kinase C. Science. 1996;273:248–251. doi: 10.1126/science.273.5272.248. [DOI] [PubMed] [Google Scholar]
- 37.Benfenati F, Greengard P, Brunner J, Bahler M. Electrostatic and hydrophobic interactions of synapsin I and synapsin I fragments with phospholipid bilayer. J. Cell Biol. 1989;108:1851–1862. doi: 10.1083/jcb.108.5.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapman ER, Davis AF. Direct interaction of a Ca2+ binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 1998;273:13995–14001. doi: 10.1074/jbc.273.22.13995. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Rizo J, Südhof TC. Mechanism of phospholipid binding by the C2A-domain of synaptotagmin I. Biochemistry. 1998;37:12395–12403. doi: 10.1021/bi9807512. [DOI] [PubMed] [Google Scholar]
- 40.Gerber SH, Rizo J, Südhof TC. Role of electrostatic and hydrophobic interactions in Ca2+-dependent phospholipid binding by the C2A-domain from synaptotagmin I. Diabetes. 2002;Suppl 1:2–8. doi: 10.2337/diabetes.51.2007.s12. [DOI] [PubMed] [Google Scholar]
- 41.Shin OH, Rizo J, Südhof TC. Synaptotagmin function in dense core vesicle exocytosis studied in cracked PC12 cells. Nat. Neurosci. 2002;5:649–656. doi: 10.1038/nn869. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez-Chacon R, et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 43.Pang ZP, Shin OH, Meyer AC, Rosenmund C, Südhof TC. A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J. Neurosci. 2006;26:12556–12565. doi: 10.1523/JNEUROSCI.3804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 45.Li C, Ullrich B, Zhang ZZ, Anderson RGW, Brose N, Südhof TC. Ca2+-dependent and Ca2+-independent activities of neural and nonneural synaptotagmins. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 46.Chapman ER, Hanson PI, An S, Jahn R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J Biol Chem. 1995;270:23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- 47.Rosenmund C, et al. Differential control of vesicle priming and short-term plasticity by Munc13 Isoforms. Neuron. 2002;33:411–425. doi: 10.1016/s0896-6273(02)00568-8. [DOI] [PubMed] [Google Scholar]
- 48.Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 49.Geppert M, et al. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 50.Chapman PF, Frenguelli BG, Smith A, Chen CM, Silva AJ. The α-Ca2+/calmodulin kinase II: a bidirectional modulator of presynaptic plasticity. Neuron. 1995;14:591–597. doi: 10.1016/0896-6273(95)90315-1. [DOI] [PubMed] [Google Scholar]
- 51.Corbalan-Garcia S, Gomez-Fernandez JC. Protein kinase C regulatory domains: the art of decoding many different signals in membranes. Biochim. Biophys. Acta. 2006;1761:633–654. doi: 10.1016/j.bbalip.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 52.Wierda KD, Toonen RF, de Wit H, Brussaard AB, Verhage M. Interdependence of PKC-dependent and PKC-independent pathways for presynaptic plasticity. Neuron. 2007;54:275–290. doi: 10.1016/j.neuron.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Rhee SR, et al. Phorbol ester- and DAG-induced augmentation of neurotransmitter release from hippocampal neurons is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- 54.Basu J, Betz A, Brose N, Rosenmund C. Munc13-1 C1 domain activation lowers the energy barrier for synaptic vesicle fusion. J. Neurosci. 2007;27:1200–1210. doi: 10.1523/JNEUROSCI.4908-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ford MG, et al. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 56.Rhee J-S, et al. Augmenting neurotransmitter release by enhancing the apparent Ca2+-affinity of synaptotagmin 1. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18664–18669. doi: 10.1073/pnas.0509153102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eberhard DA, Holz RW. Calcium promotes the accumulation of polyphosphoinositides in intact and permeabilized bovine adrenal Chromaffin cells. Cell. Mol. Neurobiol. 1991;11:357–370. doi: 10.1007/BF00713279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wenk MR, et al. PIP kinase Iγ is the major PI(4,5)P2 synthesizing enzyme at the synapse. Neuron. 2001;32:79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 59.Itoh T, Ishihara H, Shibasaki Y, Oka Y, Takenawa Autophosphorylation of type I phosphatidylinositol phosphate kinase regulates its lipid kinase activity. J. Biol. Chem. 2000;275:19389–19394. doi: 10.1074/jbc.M000426200. [DOI] [PubMed] [Google Scholar]
- 60.Park SJ, Itoh T, Takenawa T. Phosphatidylinositol 4-phosphate 5-kinase type I is regulated through phosphorylation response by extracellular stimuli. J. Biol. Chem. 2001;276:4781–4787. doi: 10.1074/jbc.M010177200. [DOI] [PubMed] [Google Scholar]
- 61.Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 62.Gerber SH, et al. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science. 2008;321:1507–1510. doi: 10.1126/science.1163174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat. Struct. Mol. Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 65.Delaglio F, et al. Nmrpipe - A Multidimensional Spectral Processing System Based on Unix Pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 66.Johnson BA, Blevins RA. NMR View - A Computer-Program for the Visualization and Analysis of NMR Data. J. Biomol. NMR. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- 67.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Macromolecular Crystallography, Pt A. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 68.Navaza J. Amore - An Automated Package for Molecular Replacement. Acta Crystallographica Section A. 1994;50:157–163. [Google Scholar]
- 69.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Likelihood-enhanced fast translation functions. Acta Crystallogr. D. Biol. Crystallogr. 2005;61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 70.Perrakis A, Harkiolaki M, Wilson KS, Lamzin VS. ARP/wARP and molecular replacement. Acta Crystallogr. D. Biol. Crystallogr. 2001;57:1445–1450. doi: 10.1107/s0907444901014007. [DOI] [PubMed] [Google Scholar]
- 71.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved Methods for Building Protein Models in Electron-Density Maps and the Location of Errors in These Models. Acta Crystallographica Section A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 72.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallographica Section D-Biological Crystallography. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 73.Collaborative Computational Project No. 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 74.Pyott SJ, Rosenmund C. The effects of temperature on vesicular supply and release in autaptic cultures of rat and mouse hippocampal neurons. J. Physiol. 2002;539:523–535. doi: 10.1113/jphysiol.2001.013277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maximov A, Pang Z, Tervo DGR, Südhof TC. Monitoring Synaptic Transmission in Primary Neuronal Cultures Using Local Extracellular Stimulation. J. Neurosci. Methods. 2007;161:75–87. doi: 10.1016/j.jneumeth.2006.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.