Abstract
A fibrin/heparin-based delivery system was used to provide controlled delivery of PDGF-BB in an animal model of intrasynovial flexor tendon repair. We hypothesized that PDGF-BB, administered in this manner, would stimulate cell proliferation and matrix remodeling, leading to improvements in the sutured tendon’s functional and structural properties. Fifty-six flexor digitorum profundus tendons were injured and repaired in 28 dogs. Three groups were compared: 1) controlled delivery of PDGF-BB using a fibrin/heparin-based delivery system, 2) delivery system carrier control, and 3) repair only control. The operated forelimbs were treated with controlled passive motion rehabilitation. The animals were euthanized at 7, 14, and 42 days, at which time the tendons were assessed using histologic (hyaluronic acid content, cellularity, and inflammation), biochemical (total DNA and reducible collagen crosslink levels), and biomechanical (gliding and tensile properties) assays. We found that cell activity (as determined by total DNA, collagen crosslink analyses, and hyaluronic acid content) was accelerated due to PDGF-BB at 14 days. Proximal interphalangeal joint rotation and tendon excursion (i.e., tendon gliding properties) were significantly higher for the PDGF-BB treated tendons compared to the repair alone tendons at 42 days. Improvements in tensile properties were not achieved, possibly due to sub-optimal release kinetics or other factors. In conclusion, PDGF-BB treatment consistently improved the functional but not the structural properties of sutured intrasynovial tendons through 42 days following repair.
Keywords: flexor tendon, growth factor, delivery system, tendon healing, controlled delivery
INTRODUCTION
One-third of all acute injuries in workers are to the upper extremity, many of which are open hand wounds requiring extensive tendon surgery.1 While advances in treatment over the past three decades have resulted in steady improvements in clinical outcomes, a large percentage of these injuries are debilitating, leading to a loss of hand function estimated to equal over 3 million restricted activity days and over 1.5 million days lost from work per year.2 The factors that most often lead to functional loss following injury and repair are the formation of adhesions within the digital sheath and the development of repair site elongation and rupture. Postoperative controlled passive motion (CPM) rehabilitation improves range-of-motion and strength of repair versus immobilization,3,4 but attempts to enhance the outcomes further by modifying rehabilitation variables have been largely unsuccessful. The immature repair site appears unresponsive to increasing levels of applied in vivo load or higher levels of intrasynovial repair site excursion.5–7
A potential for enhancing tendon healing by the delivery of growth factors was demonstrated in recent experimental studies. The growth factor PDGF-BB is particularly promising, as it led to increased cell proliferation and matrix synthesis in vivo and in vitro.8–12 In the context of flexor tendon repair, increased matrix synthesis may lead to improved structural (e.g. strength) and functional (e.g. range-of-motion) properties. Delivery of growth factors to healing tendons can be difficult, however, as factors delivered with a bolus injection are removed rapidly from the repair site.13 Therefore, it is important to develop controlled methods for delivering growth factors through the use of well-tolerated, biodegradable delivery systems.
PDGF-BB can be delivered in a controlled manner using a fibrin/heparin-based delivery system (HBDS).10,12 Further, 100ng of PDGF-BB delivered with a HBDS can stimulate cell proliferation, extracellular matrix synthesis, and collagen remodeling in the earliest intervals after tendon suture.8,10,12 This increased biologic activity led to improved tendon gliding (i.e., range-of-motion) in vivo.9 However, while PDGF-BB delivery can increase total repair site DNA, collagen crosslink activity, and range-of-motion, it has been ineffective in improving structural properties. In these prior studies, a low dose of PDGF-BB (100ng) was selected to avoid over-stimulation of local cells and to minimize the risk of adhesion formation between the tendon and the digital sheath. PDGF-BB at this dose led to increased biologic activity and enhanced tendon gliding without deleterious side effects, but it did not improve repair site strength at 21 days. In addition, the previous studies did not rule out the possibility that the positive effects observed may have been due to the delivery system rather than PDGF-BB alone.
The lack of structural property improvements9,10 may have been due to dosage, release rate, and/or healing duration. Therefore, in the current study we increased the dosage and slowed the release rate to promote higher biologic activity for a longer duration. We delivered the maximum dosage at the slowest release rate allowable by HBDS (i.e., a 5-fold increase in dose and a 2-fold decrease in the release rate). In our previous studies, we assessed the repair only at early timepoints (≤21 days post-operatively).9,10 Since there is a delay between biologic (e.g., increased matrix synthesis) and structural (e.g., increased failure load) effects, the increased biologic activity we saw at the early timepoints might not manifest into structural effects until later. Thus, we chose to examine the repair at 42 days, the earliest timepoint when we have observed increased failure load compared to time-zero in prior studies5,7,14.
We hypothesized that prolonged delivery of a higher dosage of PDGF-BB over a longer interval following repair would promote cell proliferation and collagen remodeling, leading to sustained improvements in biomechanical properties (both strength and gliding) of repaired intrasynovial flexor tendons. We further hypothesized that the delivery system alone would have no effect on the healing tendon.
METHODS
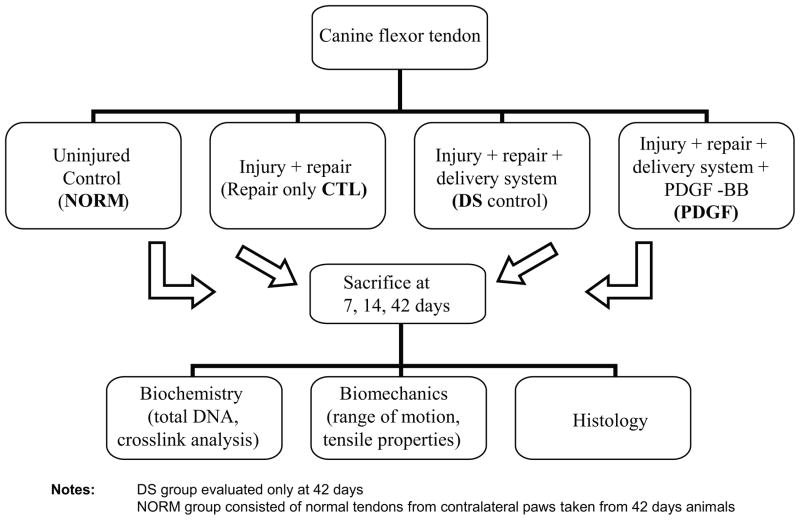
Fibrin matrices with a HBDS loaded with 500ng (1.25μg/mL) PDGF-BB were implanted between repaired flexor tendon stumps of 28 dogs (“PDGF” group). All animal procedures were approved by the Washington University Animal Studies Committee. The adjacent flexor tendon in each dog was injured and repaired and received either fibrin matrices without growth factor (delivery system carrier control, “DS” group) or no additional treatment (repair only control, “CTL” group) (Fig. 1). Uninjured normal tendons (“NORM” group) were also used for some assays. Animals were sacrificed 7, 14, or 42 days after surgical repair. Tendons were evaluated using histologic, biochemical, and biomechanical assays.
Figure 1.
Functional properties (range-of-motion) were evaluated at 7, 14, and 42 days and structural properties (stiffness, ultimate load) at 42 days. Each specimen was processed for multiple assays. For example, specimens at 7 days were tested for range-of-motion, split longitudinally, and processed for either biochemistry or histology. At 42 days, N=8 specimens were examined per group for range-of-motion, structural properties, and biochemistry. For earlier timepoints, N=4 samples were examined for range-of-motion (non-destructively), biochemistry, and histology.
Fibrin/heparin-based delivery system
The fibrin/heparin-based delivery system (HBDS) included a bi-domain peptide with a Factor XIIIa substrate derived from α2-plasmin inhibitor at the N-terminus, and a C-terminal heparin-binding domain.15 The bi-domain peptide is covalently crosslinked to a fibrin matrix during coagulation by the transglutaminase activity of Factor XIIIa. The peptide immobilizes heparin electrostatically to the matrix which, in turn, immobilizes heparin-binding growth factor (e.g. PDGF-BB), preventing its diffusion from the matrix. Fibrin matrices (30 μl matrix volume) were made with the following final component concentrations: 500 ng PDGF-BB (R&D Systems), 10 mg/ml fibrinogen concentration (preparation as previously described – Sigma),15 6.5 mM CaCl2, 13 units/ml thrombin (Sigma), 2.6 mM ATIII peptide15 (dLNQEQVSPK(βA)FAKLAARLYRKA-NH2, where dL denotes dansyl leucine) and heparin (627 μM, Sigma), in Tris-buffered saline (TBS, 137mM NaCl, 2.7mM KCl, 33mM Tris, pH 7.4). Our prior studies using this system demonstrated that PDGF-BB can be delivered in a controlled manner over a period of about 10 days.10,12 Based on our previous in vitro results12, the rate of release of PDGF-BB in the current in vivo study was one half the rate of our previous in vivo studies9,10. About 250ng of PDGF-BB were released in the first 48 hours and 25ng of PDGF-BB were released per day thereafter.
Animal model
The canine model was chosen because of the similarity of canine flexor tendon apparati to those of humans16 and to allow comparison to our previous studies.3–5,7–10,12,17 Fifty-six flexor digitorum profundus (FDP) tendons from the right forelimbs of 28 adult mongrel dogs (20 to 30 kg body mass) were transected and immediately repaired. For surgery, the animals were anesthetized, intubated, and maintained on anesthesia. Surgery was performed under tourniquet control. The sheaths of the 2nd and 5th digits in the region between the annular pulleys proximal and distal to the proximal interphalangeal joint were exposed through midlateral incisions. The sheaths were entered, and the FDP tendons were cut transversely. To create space for the delivery system, transversely oriented defects were created in the free ends of both tendon stumps with a scalpel; this was also done for the CTL group tendons. The tendons were repaired with an eight-strand core-suture technique consisting of two orthogonally placed, four-strand repairs performed with use of a continuous loop of 4-0 Supramid suture.18 After the suture had been inserted into one tendon stump, a sterile fibrin matrix with PFGF-BB (“PDGF group”) or without PDGF-BB (“DS group”) was incorporated into the repair site of either the medial or lateral forepaw flexor digitorum profundus tendon. The remaining medial or lateral flexor digitorum profundus tendon was repaired without the fibrin matrix and served as a paired control (“CTL group”). Groups were assigned randomly to either the medial or lateral tendon in each dog. The sheaths were not repaired, and the skin was closed with use of a running suture (3-0 Nylon). After surgery, the right forelimbs were immobilized with use of fiberglass shoulder spica casts with the elbows flexed 90° and the wrists flexed 70°. The distal ends of the casts were removable to allow for CPM during two five-minute rehabilitation sessions performed five days a week starting on the first postoperative day. Dogs were euthanized at designated timepoints (Fig. 1) for histologic, biochemical, or biomechanical assessment.
Histology
We examined N=4 samples for PDGF at 7 and 14 days and N=2 for PDGF and DS at 42 days. As with all assays, each PDGF and DS sample had a paired control (CTL group). Samples for histologic analysis were fixed in 4% paraformaldehyde overnight, processed for paraffin embedded sections, cut at 5μm, and stained with H&E or processed for hyaluronic acid binding protein (HABP) immunohistochemistry19. For HABP localization, sections were incubated with 10mM Sodium Citrate, 0.05% Tween-20, pH 6.0 reaction buffer and then digested with Chondroitinase ABC (US Biological; C5068-05). After blocking endogenous peroxidase activity with methanol, sections were incubated with 1:500 dilution HABP (1μg/m) (US Biological; H7980-35) overnight. Finally, sections were incubated with 1:500 dilution Streptavidin-HRP followed by Zymed DAB (diaminobenzidine). Histologic sections were qualitatively evaluated by two investigators, blinded to group and timepoint, for HABP staining intensity, adhesions/epitenon reaction, cellularity, acute inflammatory cells (neutrophils), chronic inflammatory cells (lymphocytes, macrophages, and plasma cells), and fibrous healing between tendon ends.
Biochemical assays
DNA concentration in the tendons was assessed as described previously20 (N=4 for 7 and 14 days, N=8 for 42 days). Briefly, 7–10mg (dry) of H2O-washed and lyophilized tissue were solubilized by incubation for 2h in 1N NaOH at 37°C. Quantification of the released deoxyribose was determined using a colorimetric assay (results expressed as μg DNA per mg of dry tissue). The reducible collagen crosslink, DHNL, indicative of collagen remodeling, was quantified in tissues using cation-exchange HPLC as described previously20 (N=4 for 7 and 14 days, N=8 for 42 days). Briefly, 5–10mg of tissue (dry weight) was reduced with tritiated sodium borohydride in PBS and hydrolyzed in 6N HCl for 24h at 108°C. Following hydrolysis an aliquot was submitted to cation-exchange HPLC coupled with in-line liquid scintillation spectrometry to isolate and quantitate DHLNL (results expressed as cpm per picomole of total tissue collagen).
Biomechanical assays
The 2nd and 5th digits from the non-operated (left) and operated (right) forelimbs were disarticulated at the metacarpophalangeal joint, and the flexor digitorum profundus tendons were transected proximally at the musculotendinous junction. The range-of-motion of both the non-operated (uninjured control, i.e., NORM group) and the repaired digits with fibrin matrix (CTL, PDGF, and DS groups) was assessed using a motion-analysis system21 (PC-Reflex; Qualisys, Glastonbury, CT) (N=4 for 7 and 14 days, N=8 for 42 days). Two pairs of reflective hemispherical markers were pinned to the middle and distal phalanges of each toe nail. The proximal phalanx was clamped in a vertical orientation, and the coordinates of the markers were sampled first with the digit in a flexed position and then in an extended position as previously described.7,9 Based on differences between the positions, we computed rotations of the proximal interphalangeal joint (PIP) and linear displacement of the flexor tendon.
The flexor tendons (with attached distal phalanges) were then isolated from the repaired digits and tested to failure in tension using a servohydraulic materials testing system (Instron 8500R, Canton, MA) as previously described7,9 (N=8 for 42 days). Each distal phalanx was held rigidly in a custom fixture and the proximal tendon stump held in a soft tissue clamp such that the tendon-bone specimen was in approximately neutral alignment. A pair of reflective markers were attached to the tendon, one on each side of the repair-site. The distal phalanx was then displaced vertically to apply a 1N pre-load. After five pre-conditioning cycles in load control (triangle waveform, 1 to 5 N, 0.25Hz), the specimens were displaced using a linear ramp at 0.375mm/s until failure. From the marker positions we computed repair-site elongation-per-length (strain, mm/mm), where the unit length was the initial vertical distance between the markers. We determined peak (ultimate) force, repair-site stiffness (slope of the linear portion of the force-elongation curve), repair-site rigidity (slope of the linear portion of the force-strain curve), and repair-site strain at 20 N force.
Statistics
Our experiment was designed based on a sample size of N = 8 being adequate for detecting a between group difference in ultimate force of ≥20% at a significance level of 0.05 and power of 0.8. Comparisons between repaired tendons with the HBDS (PDGF and DS groups) versus repair only tendons (CTL group) were made using paired t-tests. Comparisons to uninjured controls (NORM group) and differences over time were tested using a repeated measures ANOVA followed by a Fisher’s Least Squares Differences post-hoc test (significance level set at 0.05).
RESULTS
Gross observations and histology/immunohistochemistry
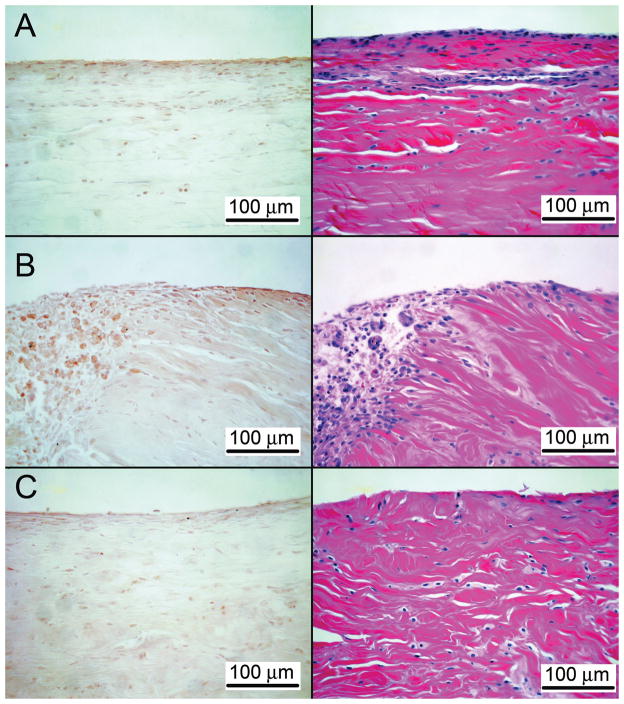
At dissection, all repairs were intact without gap formation. Adhesions were either minimal to moderate or were absent on gross and on histological sections. The PDGF-BB group displayed qualitatively higher fibroblast density at 7 days compared to CTL. Slightly more neutrophils appeared to be present in the PDGF-BB group at 7 days compared to CTL. At 14 days, there appeared to be more fibrous connections between the tendon ends in the PDGF-BB group compared to CTL. No differences were found between groups at 42 days. No obvious differences were noted in chronic inflammatory cells between groups at any timepoint. Positive staining for hyaluronic acid was seen in all groups. Hyaluronic acid staining intensity was higher in the PDGF-BB group compared to the CTL group at all timepoints (Fig. 2). Hyaluronic acid staining intensity was similar when comparing the PDGF-BB group to the NORM group.
Figure 2.
Representative sections for hyaluronic acid binding protein immunohistochemistry for the 7 day timepoint (40x). The epitenon surface is at the top of each micrograph (A: NORMAL, B: PDGF, C: CTL). Hyaluronic acid staining intensity (brown staining) was higher in the PDGF-BB group compared to the CTL group. The edge of the repair site can be seen on the left of panel B for the PDGF sample.
Biochemical assays
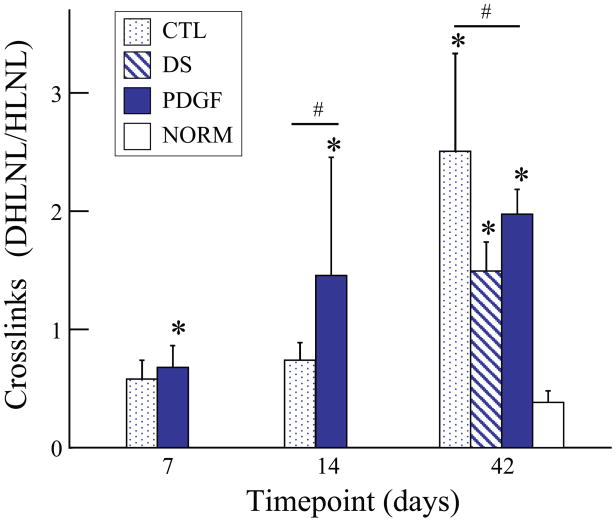
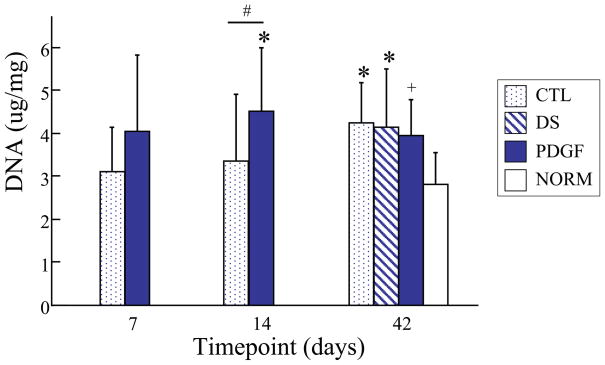
Uninjured flexor tendons contain low levels of the reducible collagen crosslink, DHLNL. After injury and repair, both untreated and treated tendons contained significantly elevated levels of DHLNL at 42 days, whereas only treated (PDGF) tendons had elevated DHLNL at 7 and 14 days (Fig. 3). Compared to the CTL group, PDGF treated tendons had a higher concentration of DHLNL at 14 days and a lower concentration of DHLNL at 42 days (Fig. 3). DNA concentration in the PDGF group at 14 and 42 days and in the DS at 42 days was increased relative to uninjured tendons (NORM) (Fig. 4). PDGF treated tendons had a higher DNA concentration than CTL at 14 days. These results suggest enhanced cellularity secondary to PDGF treatment during the early phase following injury and repair.
Figure 3.
At 14 days, DHLNL concentration was significantly higher in the PDGF group compared to the CTL group. At 42 days, DHLNL concentration was significantly higher in the CTL group compared to the PDGF group. Compared to the NORM group, DHLNL concentration was significantly higher at all timepoints in the PDGF group and at 42 days in the CTL and DS groups. [# p<0.05 vs. CTL, * p<0.05 vs. NORM].
Figure 4.
At 14 days, total DNA was significantly higher in the PDGF group compared to the CTL group. Compared to the NORM group, total DNA was significantly higher at 14 days in the PDGF group and at the 42 days timepoint in the CTL and DS groups. [# p<0.05 vs. CTL, + p<0.07 vs. CTL, * p<0.05 vs. NORM].
Biomechanics
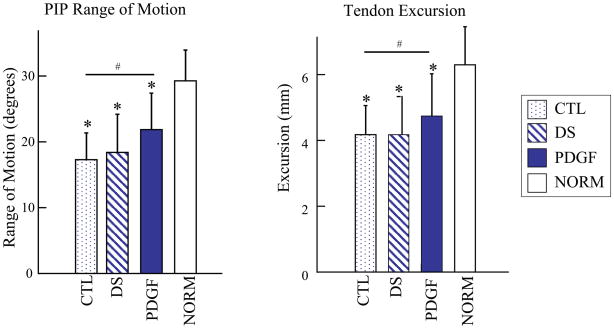
PIP joint rotation and flexor tendon excursion were significantly higher in the PDGF-BB treated tendons compared to the repair alone (CTL) tendons at 42 days (Fig. 5). Results for the delivery system control (DS) were similar to those for the CTL group, demonstrating that the improvements in range-of-motion in the PDGF group were due to the growth factor and not the delivery system. No significant differences in tensile properties were found when comparing PDGF-BB or DS to repair-alone (CTL) tendons (Table 1).
Figure 5.
Range-of-motion and tendon excursion were significantly higher in the PDGF group compared to the CTL group. Compared to the NORM group, the CTL, DS, and PDGF groups had significantly lower range-of-motion and tendon excursion. [# p<0.05 vs. CTL, * p<0.05 vs. NORM].
TABLE 1.
Structural properties at 42 days
| Max. Load (N) | Rigidity (N/mm/mm) | Stiffness (N/mm) | Strain at 20N (Unitless) | |
|---|---|---|---|---|
| PDGF | 92.7 ± 23.5 | 1212.4 ± 348.0 | 19.3 ± 5.5 | 0.03 ± 0.02 |
| Repair CTL | 103.7 ± 25.7 | 1448.3 ± 630.9 | 19.1 ± 5.2 | 0.03 ± 0.02 |
| DS CTL | 93.1 ± 23.1 | 1184.9 ± 425.5 | 16.7 ± 6.0 | 0.03 ± 0.01 |
DISCUSSION
Clinical and experimental studies have indicated that both repair site deformation and loss of gliding properties occur regularly in the early stages after tendon repair and can lead to rupture and loss of finger function.22–25 Repair site deformation is likely due to low levels of matrix formation and remodeling at the healing tendon interface leading to a lack of strength accrual. Loss of gliding properties, on the other hand, is likely due to post repair edema and the activity of repair site fibroblasts on the tendon surface forming extracellular matrix connections (adhesions) between the tendon and its sheath. Growth factor delivery for enhanced flexor tendon healing is designed to stimulate the cells at the healing interface and on the gliding surface of tendon without stimulating an increase in the formation of fibrovascular adhesions. This goal was achieved in part in our study through the local delivery of PDGF-BB. The growth factor was effective in stimulating cell activity and improving gliding properties, but ineffective in improving the structural properties of the repair.
Based on the results of the delivery system control group presented here, the improved gliding was shown to be attributable to PDGF-BB treatment and not due to any component of the delivery system. Range-of-motion in the delivery system control group was similar to the repair-only control group, while range-of-motion in the PDGF-BB group was significantly improved. These results also demonstrate that the previously reported improvements in gliding at 21 days due to PDGF-BB9 are maintained for at least 42 days after surgical repair.
The improvement in tendon gliding due to PDGF-BB seen in this experimental study was consistent with our previous in vivo study and with the in vitro studies of others.26–29 A number of extracellular matrix proteins may have been upregulated due to PDGF-BB, leading in improved tendon gliding. Studies showed that many lubricants found at articulating joint surfaces (e.g., hyaluronic acid, phospholipids, and lubricin) are also found in flexor tendons.30,31 One recent study showed that the gliding resistance of canine FDP tendons increased after treatment with hyaluronidase, phospholipase, lipid solvent, and/or trypsin.31 These results suggest that phospholipids, hyaluronic acid, and protein components are all involved in maintaining the low gliding resistance of flexor tendons. In addition, the application of hyaluronic acid resulted in a restoration of the gliding surface of repaired intrasynovial tendons in a number of experimental studies.32,33 In our study, PDGF-BB may have promoted hyaluronic acid production leading to improved tendon gliding. This is consistent with in vivo and in vitro studies showing that PDGF-BB stimulates hyaluronic acid biosynthesis in animal and human fibroblasts.26–29 Our qualitative immunohistochemical analysis revealed higher levels of hyaluronic acid in tendons treated with PDGF-BB. These results are consistent with the quantitative improvements in range-of-motion due to PDGF-BB treatment. Further study is necessary to elucidate the mechanism which led to improved gliding.
The tensile properties of the repaired tendons were not improved with local PDGF-BB delivery. Failure to achieve increases in ultimate load and stiffness and reductions in strain in the treated group may have been due to a number of factors. PDGF-BB, while successful in stimulating fibroblast proliferation, may be less effective for stimulating collagen synthesis in a timely way. Even if the growth factor stimulated matrix synthesis, there may have been matrix defects at the microstructural (e.g., poor cross linking) and/or macrostructural (e.g., poor fibril organization) levels. A different growth factor (e.g., bFGF, BMP-12) or a combination of growth factors may be necessary to stimulate both cell proliferation and matrix synthesis successfully. In addition, there may be insufficient numbers of local fibroblasts in the early period after surgical repair to respond to the growth factor(s). Populating the HBDS with tendon fibroblasts or mesenchymal stromal cells may be more effective for enhancing flexor tendon healing than PDGF-BB alone. These groups will be evaluated in future studies.
This study has several limitations. We lack in vivo information regarding the release kinetics of PDGF-BB. Our in vitro experiments showed that we were able to provide controlled release of the PDGF-BB and that we were able to control the release kinetics with a HBDS.10,12 However, the wound site in vivo is more complex and includes multiple cell types that may degrade the fibrin matrix and increase the release rate. The specific in vivo release rate will be examined in future studies. While we propose that a stimulation of hyaluronan synthesis is a possible mechanism for improved functional properties in the PDGF-BB treated group, we have not established that this was the operative mechanism in this model. Future studies will seek to identify hyaluronic acid up-regulation in PDGF-BB treated tendons compared to control.
In summary, we further demonstrated the potential for biologic enhancement of flexor tendon repair with PDGF-BB treatment. We were able to promote cellular activity at the repair site and sustained improvement in range-of-motion in vivo without promoting adhesion formation at the tendon surface. These results hold promise for improving flexor tendon repair after recent failed attempts to enhance the repair using mechanical perturbations.
Acknowledgments
Funding for this project was provided by NIAMS (NIH RO1 5RO1AR033097). The authors also thank Tim Morris for his assistance during the canine surgeries and Matthew Wood, Angela Guzman, and Nicole Moore for assistance with peptide synthesis.
References
- 1.Feuerstein M, Miller VL, Burrell LM, Berger R. Occupational upper extremity disorders in the federal workforce. Prevalence, health care expenditures, and patterns of work disability. J Occup Environ Med. 1998;40:546–555. doi: 10.1097/00043764-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Kelsey JL. Upper extremity disorders: frequency, impact and cost. Churchill Livingstone; New York, NY: 1997. [Google Scholar]
- 3.Woo SL, Gelberman RH, Cobb NG, et al. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop Scand. 1981;52:615–622. doi: 10.3109/17453678108992156. [DOI] [PubMed] [Google Scholar]
- 4.Takai S, Woo SL, Horibe S, et al. The effects of frequency and duration of controlled passive mobilization on tendon healing. Journal of Orthopaedic Research. 1991;9:705–713. doi: 10.1002/jor.1100090510. [DOI] [PubMed] [Google Scholar]
- 5.Silva MJ, Brodt MD, Boyer MI, et al. Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. J Orthop Res. 1999;17:777–783. doi: 10.1002/jor.1100170524. [DOI] [PubMed] [Google Scholar]
- 6.Lieber RL, Amiel D, Kaufman KR, et al. Relationship between joint motion and flexor tendon force in the canine forelimb. J Hand Surg [Am] 1996;21:957–962. doi: 10.1016/S0363-5023(96)80299-1. [DOI] [PubMed] [Google Scholar]
- 7.Boyer MI, Gelberman RH, Burns ME, et al. Intrasynovial flexor tendon repair. An experimental study comparing low and high levels of in vivo force during rehabilitation in canines. J Bone Joint Surg Am. 2001;83-A:891–899. [PubMed] [Google Scholar]
- 8.Thomopoulos S, Harwood FL, Silva MJ, et al. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg [Am] 2005;30:441–447. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, et al. The early effects of sustained platelet-derived growth factor administration on the functional and structural properties of repaired intrasynovial flexor tendons: an in vivo biomechanic study at 3 weeks in canines. J Hand Surg [Am] 2007;32:373–379. doi: 10.1016/j.jhsa.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Thomopoulos S, Zaegel M, Das R, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25:1358–1368. doi: 10.1002/jor.20444. [DOI] [PubMed] [Google Scholar]
- 11.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Medicine. 2003;33:381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 12.Sakiyama-Elbert S, Das R, Gelberman RH, et al. Controlled release kinetics and biologic activity of PDGF-BB for use in flexor tendon repair. Journal of Hand Surgery [American] 2008 doi: 10.1016/j.jhsa.2008.05.030. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson SN, Talmadge JE. Sustained release of growth factors. In Vivo. 2002;16:535–540. [PubMed] [Google Scholar]
- 14.Gelberman RH, Boyer MI, Brodt MD, et al. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg Am. 1999;81:975–982. doi: 10.2106/00004623-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 16.Potenza AD. Tendon healing within the flexor digital sheath in the dog. J Bone Joint Surg Am. 1962;44-A:49–64. [PubMed] [Google Scholar]
- 17.Lieber RL, Silva MJ, Amiel D, Gelberman RH. Wrist and digital joint motion produce unique flexor tendon force and excursion in the canine forelimb. J Biomech. 1999;32:175–181. doi: 10.1016/s0021-9290(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 18.Winters SC, Gelberman RH, Woo SL, et al. The effects of multiple-strand suture methods on the strength and excursion of repaired intrasynovial flexor tendons: a biomechanical study in dogs. J Hand Surg [Am] 1998;23:97–104. doi: 10.1016/s0363-5023(98)80096-8. [DOI] [PubMed] [Google Scholar]
- 19.Ohashi M, Ide S, Sawaguchi A, et al. Histochemical localization of the extracellular matrix components in the annular ligament of rat stapediovestibular joint with special reference to fibrillin, 36-kDa microfibril-associated glycoprotein (MAGP-36), and hyaluronic acid. Med Mol Morphol. 2008;41:28–33. doi: 10.1007/s00795-007-0394-3. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu C, Yoshioka M, Coutts RD, et al. Long-term effects of hyaluronan on experimental osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1998;6:1–9. doi: 10.1053/joca.1997.0086. [DOI] [PubMed] [Google Scholar]
- 21.Silva MJ, Boyer MI, Ditsios K, et al. The insertion site of the canine flexor digitorum profundus tendon heals slowly following injury and suture repair. Journal of Orthopaedic Research. 2002;20:447–453. doi: 10.1016/S0736-0266(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C, Amadio PC, Paillard P, et al. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Joint Surg Am. 2004;86-A:320–327. doi: 10.2106/00004623-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 23.May EJ, Silfverskiold KL. Rate of recovery after flexor tendon repair in zone II. A prospective longitudinal study of 145 digits. Scand J Plast Reconstr Surg Hand Surg. 1993;27:89–94. doi: 10.3109/02844319309079789. [DOI] [PubMed] [Google Scholar]
- 24.Silfverskiold KL, May EJ. Gap formation after flexor tendon repair in zone II. Results with a new controlled motion programme. Scand J Plast Reconstr Surg Hand Surg. 1993;27:263–268. [PubMed] [Google Scholar]
- 25.Boyer MI, Goldfarb CA, Gelberman RH. Recent progress in flexor tendon healing. The modulation of tendon healing with rehabilitation variables. J Hand Ther. 2005;18:80–85. doi: 10.1197/j.jht.2005.02.009. quiz 86. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki M, Asplund T, Yamashita H, et al. Stimulation of hyaluronan biosynthesis by platelet-derived growth factor-BB and transforming growth factor-beta 1 involves activation of protein kinase C. Biochem J. 1995;307 (Pt 3):817–821. doi: 10.1042/bj3070817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartold PM. Platelet-derived growth factor stimulates hyaluronate but not proteoglycan synthesis by human gingival fibroblasts in vitro. J Dent Res. 1993;72:1473–1480. doi: 10.1177/00220345930720110301. [DOI] [PubMed] [Google Scholar]
- 28.Papakonstantinou E, Karakiulakis G, Roth M, Block LH. Platelet-derived growth factor stimulates the secretion of hyaluronic acid by proliferating human vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1995;92:9881–9885. doi: 10.1073/pnas.92.21.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiedemann K, Malmstrom A, Westergren-Thorsson G. Cytokine regulation of proteoglycan production in fibroblasts: separate and synergistic effects. Matrix Biol. 1997;15:469–478. doi: 10.1016/s0945-053x(97)90020-2. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Berger EJ, Zhao C, et al. Expression and mapping of lubricin in canine flexor tendon. J Orthop Res. 2006;24:1861–1868. doi: 10.1002/jor.20239. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Chen MY, Zhao C, et al. The effect of hyaluronidase, phospholipase, lipid solvent and trypsin on the lubrication of canine flexor digitorum profundus tendon. J Orthop Res. 2008;26:1225–1229. doi: 10.1002/jor.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller JA, Ferguson RL, Powers DL, et al. Efficacy of hyaluronic acid/nonsteroidal anti-inflammatory drug systems in preventing postsurgical tendon adhesions. J Biomed Mater Res. 1997;38:25–33. doi: 10.1002/(sici)1097-4636(199721)38:1<25::aid-jbm4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 33.Zhao C, Sun YL, Amadio PC, et al. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic Acid. An in vivo canine model. J Bone Joint Surg Am. 2006;88:2181–2191. doi: 10.2106/JBJS.E.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]