Abstract
Stimulant abuse continues to be a problem, particularly for women. There is increasing preclinical and clinical evidence showing that the hormone progesterone attenuates the behavioral effects of cocaine, and this effect is primarily observed in females. The purpose of the present study was to determine if progesterone would also alter the behavioral effects of another stimulant, oral d-amphetamine (AMPH) in women. Eighteen normal non-drug abusing women completed eight outpatient sessions over two menstrual cycles. During the follicular phase of each cycle, women were administered AMPH (0, 10, 20 mg); in one cycle they were pretreated with oral micronized progesterone (200 mg) and in another cycle they were pretreated with placebo progesterone. Each session, participants completed a range of tasks including subjective measures of abuse liability, cognitive performance tasks, and behavioral measures of impulsivity and risk-taking. AMPH produced dose-related increases in positive subjective effects and these effects were enhanced by progesterone pretreatment. AMPH alone, or in combination with progesterone, had little effect on performance or behavioral measures of impulsivity. These results are in contrast with previous studies showing that progesterone attenuates the subjective response to cocaine and nicotine. Additional studies are needed to explore the modulatory role of progesterone on the effects of AMPH to determine whether progesterone has any clinical utility for AMPH abuse.
Keywords: Amphetamine, Impulsivity, Progesterone, Subjective Effects, Women
Introduction
Misuse of stimulants is an ongoing problem in the United States (e.g. Lord et al., 2009; McCabe et al., 2004; Kaloyanides et al., 2007), particularly in women. Among women surveyed in the United States, 11.9% have used cocaine and 7.6% have used other stimulants in their lifetime (NSDUH, 2008). Further, although the number of methamphetamine/d-amphetamine (AMPH) treatment admissions was similar for men and women (TEDS, 2007), women report a higher frequency of AMPH use and more emotional effects from AMPH use than men (Holdcraft & Iacono, 2004).
Although there is currently no effective treatment for stimulant abuse, recent data suggest that the hormone progesterone may attenuate the reinforcing effects of stimulants, especially in females (Evans, 2007; Carroll & Anker, in press). Preclinical studies have shown that stimulant-induced behaviors vary across the menstrual cycle in female primates and the estrous cycle in female rats, and this may be due to fluctuating progesterone levels. For example, self-administration of a low dose of cocaine was lower during the luteal phase, when progesterone levels are elevated, than during the follicular phase in monkeys (Mello et al., 2007). In rats, AMPH-stimulated behavior and striatal dopamine release (Becker & Cha, 1989), cocaine self-administration (Roberts et al., 1989) and cocaine seeking (Feltenstein & See, 2007) was higher during estrus, when progesterone is minimal, compared to other phases of the estrous cycle. The administration of progesterone attenuated cocaine-induced behaviors in female rats including locomotor activity (Niyomchai et al., 2006), reinstatement of cocaine-seeking behavior (Anker et al., 2007; Feltenstein et al., 2009), and cocaine self-administration (Jackson et al., 2006; Larson et al., 2007). Similarly, the progesterone metabolite allopregnanolone attenuated cocaine-induced (Anker et al., 2009) and stress-induced (Anker & Carroll, 2010) reinstatement to cocaine-seeking behavior in females, but not males. We are only aware of one preclinical study that examined the behavioral interaction of AMPH and progesterone; progesterone plus estradiol did not alter AMPH-induced stereotypy, but did alter AMPH-attenuated copulatory response (lordosis) compared to estradiol alone (Michanek & Meyerson, 1982). Together, preclinical studies suggest that progesterone attenuates stimulant-induced behaviors.
It has also been shown that progesterone plays a role in stimulant-induced behaviors in humans (Evans, 2007; Evans & Foltin, in press). During the normal menstrual cycle, the subjective effects of oral AMPH (Justice and de Wit, 1999; White et al., 2002) and cocaine (Evans et al., 2002; Sofuoglu et al., 1999) were attenuated in women during the luteal phase of the menstrual cycle, when progesterone levels are elevated. Stress-induced and drug-induced cocaine craving were also lower among women who had elevated progesterone levels (Sinha et al., 2007). Further, the administration of oral progesterone decreased the subjective effects of cigarettes (Sofuoglu et al., 2001) and cocaine (Evans & Foltin, 2006; Sofuoglu et al., 2002) in women during the follicular phase of the menstrual cycle. Although oral progesterone has been shown to attenuate subjective effects related to stress in men (Childs et al., 2010), oral progesterone did not attenuate the positive subjective effects of cocaine in men (Evans & Foltin, 2006) and did not decrease cocaine use in men in a pilot treatment trial (Sofuoglu et al., 2007). Thus, the existing preclinical and clinical data suggest that progesterone alters the effects of stimulants in females. However, similar to the paucity of studies in animals, to our knowledge, no human studies have assessed whether oral progesterone alters the effects of the stimulant AMPH.
To further explore progesterone’s potential utility as a treatment for stimulant abuse in women, this study examined whether oral micronized progesterone would alter the effects of oral AMPH on subjective measures, psychomotor performance, and other behaviors related to substance abuse, such as impulsivity, in normal healthy women in the follicular phase of the menstrual cycle. Previous studies have shown that AMPH decreased impulsivity in rats (Evenden & Ko, 2005, Rivalan et al., 2007; Wade et al., 2000; Winstanley et al., 2005) and normal healthy humans (de Wit et al., 2000; de Wit et al., 2002; but see Fillmore et al., 2005). Further, in rats, progesterone decreased marble burying, thought to be a measure of impulsivity in rats (Llaneza & Frye, 2009), but we are unaware of any studies examining the effects of progesterone on impulsivity in humans.
We hypothesized that oral AMPH would increase subjective measures related to abuse liability and based on the existing preclinical and clinical literature with cocaine, we expected that oral micronized progesterone would attenuate the positive subjective effects of oral AMPH. Further, we hypothesized that oral AMPH would decrease impulsivity and that oral micronized progesterone would produce further decreases in impulsivity. Lastly, we hypothesized that oral AMPH would improve performance, but given that progesterone can induce sedation (van Broekhoven et al., 2006; Frishman et al., 1995; Freeman et al., 1992; Söderpalm et al., 2004; de Wit et al., 2001), progesterone was expected to impair performance.
Methods
Participants
Women were recruited via advertisements posted on the internet, flyers and local newspapers to participate in a research study examining the effects of hormones and common medications on mood and performance. Out of 22 normal healthy women who started the study, 18 women completed the study. They had a mean age of 29, were predominately White (n = 10), had a mean of 16 years of education, were normal weight (BMI ≤ 23) and had normal menstrual cycles. Overall, women were light drinkers (approximately 4 drinks/week) and reported little or no other drug use. All women were medically and psychiatrically healthy based on a detailed medical history, laboratory tests, ECG, physical and clinical interview. None of the participants were pregnant (based on urine pregnancy tests), taking oral contraceptives, hormones or any other prescription medication. The Structured Clinical Interview for DSM-IV (SCID I, First et al., 1995) was conducted by a trained Master’s level clinical interviewer to rule out women with a current Axis I psychiatric disorder (including substance abuse or eating disorders). Lastly, none of the female participants suffered from premenstrual dysphoric disorder.
The Barratt Impulsiveness Scale, version 11 (BIS), the Impulsivity Questionnaire (IQ) and the Sensation-Seeking Scale (SSS) were completed during screening. The BIS is a 30-item questionnaire that measures three dimensions of impulsivity: attentional, motor, and non-planning and also generates a total impulsivity score (Patton et al., 1995). The IQ is a 54-item questionnaire that measures three dimensions of impulsivity: impulsiveness, venturesomeness, and emphathy, and also generates a total impulsivity score (Eysenck et al., 1985). The SSS is a 40-item questionnaire that measures four dimensions of sensation seeking: thrill and adventure seeking, experience seeking, disinhibition, and boredom susceptibility and also generates a total sensation-seeking score (Zuckerman et al., 1978). Total scores on these self-reports (reported as mean ± SD) were 63 (± 9) for the BIS, 22 (± 6) for the IQ, and 24 (± 6) for the SSS. These are all within the normal range of impulsivity (Patton et al., 1995; Petry, 2001).
The study was approved by the Institutional Review Board of the New York State Psychiatric Institute. Participants gave their written informed consent before beginning the study and were paid for their participation. Participants were told that the purpose of the study was to investigate the interaction of the hormone progesterone in combination with various prescription and over-the-counter medications on mood, ability to perform certain tasks, vital signs (heart rate and blood pressure), and hormone levels.
Procedures
The women participated as outpatients at the New York State Psychiatric Institute for a total of 8 sessions; each session was approximately 7 hr long. All participants had one practice session (usually during the late luteal phase of the menstrual cycle) to familiarize them with the routines to be followed; data from the practice session were not analyzed. The testing phase consisted of six sessions (sessions 2–7) scheduled during the follicular phases (days 4–10 after the onset of menstruation) of two different menstrual cycles; these sessions were scheduled based upon menstrual cycle length and the onset of menstruation. Mood, menstrual cycle length and onset of menstruation were obtained from the Daily Ratings Form (see Evans et al., 1998 for details) that participants filled out each evening throughout the study. During one follicular phase, half the women were pretreated with oral micronized progesterone (200 mg) to mimic progesterone levels observed during the midluteal phase and half the women were pretreated with the vehicle (olive oil). This order was reversed for the second follicular phase. Each phase, women also were administered oral AMPH (0, 10 or 20 mg) in a randomized order on separate sessions. The eighth session did not have to occur during the follicular phase because it was a lottery session (described below).
In the event that a woman began menstruating earlier than expected or a session could not be scheduled for other reasons, missed sessions were rescheduled during the correct phase of the next menstrual cycle.
d-Amphetamine and Progesterone Dosing
Oral AMPH (10 and 20 mg; Cardinal Health, Dublin, OH) was prepared as capsules (size 00) with lactose powder as filler; placebo capsules contained only lactose powder. Each session, participants ingested two identically appearing capsules, which were two placebo capsules, one 10 mg AMPH and one placebo capsule, or two 10 mg AMPH capsules (total of 20 mg).
The dose of oral micronized progesterone selected for the current study (200 mg) was chosen to produce progesterone levels comparable to those observed during the normal midluteal phase of the menstrual cycle. A pharmacokinetic study showed that 200 mg oral micronized progesterone produced progesterone levels of 13.8 ng/ml (Simon et al., 1993) and in a previous study from our laboratory, progesterone levels during the normal midluteal phase were similar (10.0 ng/ml; Evans et al., 2002). The Women’s International Pharmacy (Madison, WI) compounded oral micronized progesterone as 50 mg capsules with olive oil and participants were administered 4 capsules containing a total of 200 mg oral micronized progesterone or matching placebo (olive oil) each session.
Progesterone and AMPH capsules were given simultaneously because oral micronized progesterone (de Lignières, 1999) and oral AMPH (Asghar et al., 2003) plasma levels similarly peak at approximately 2–3 hrs. Capsules were ingested with 150 ml water under staff supervision. Both the staff and the participants were blind to study medication.
Hormone Assays
Each experimental day, approximately 1 hr after dosing, venous blood samples (approximately 6 ml) for estradiol and progesterone were drawn into tubes containing SST® gel and clot activator. Samples were centrifuged within 30 min of collection, yielding approximately 3 ml of plasma, and stored frozen until the time of analysis. Estradiol and progesterone levels were determined by Dr. Michel Ferin at the College of Physicians and Surgeons of Columbia University, Department of Obstetrics and Gynecology (New York, NY). Estradiol and progesterone were measured by a commercial solid-phase, chemiluminescent immunoassay (Immulite, Diagnostic Products Co., DPC, Los Angeles, CA). For estradiol, the assay sensitivity was 4 pg/ml and the intra- and interassay coefficients of variation were 4.3 and 10.5%. For progesterone, the assay sensitivity was 0.2 ng/ml and the intra- and interassay coefficients of variation were 4.8 and 9.1%.
Experimental Sessions
Participants reported to the laboratory at approximately 8:30 a.m. and remained until approximately 3:30 pm. They were instructed not to eat breakfast before reporting to the laboratory and to refrain from using alcohol and all psychoactive drugs (with the exception of tobacco and caffeinated products) the day before and the day of an experimental session. Upon arrival each session, a urine specimen was collected and analyzed for the presence of illicit drugs and a breath alcohol test was conducted to test for the presence of alcohol in expired air. Weekly, urine pregnancy tests were performed. Participants first filled out a Food Desirability Questionnaire (see Evans et al., 1999) and then were served a light breakfast approximately 30 min before the beginning of the session, including a caffeinated beverage for those women who regularly consumed caffeine. Participants were given the same breakfast on all subsequent sessions because the bioavailability of progesterone can be altered by food intake (Simon et al., 1993). After breakfast, participants completed a baseline assessment battery of various computerized questionnaires, impulsivity tasks and performance tasks. After the baseline assessment battery, participants were given two medication bottles. One bottle contained four capsules of oral micronized progesterone (200 mg) or vehicle and the other bottle contained two capsules of oral AMPH (10 or 20 mg) or placebo. Then, participants completed computerized questionnaires and tasks at specified times, described below. Approximately 2 hr after the beginning of the session, participants selected lunch for that day and 3.5 h after the beginning of the session, women were given 30 min to eat the lunch items they had selected. Between breakfast and lunch participants were only allowed to consume water.
At the end of each session, participants were evaluated prior to discharge for signs of intoxication and were required to pass a field sobriety test. As a safety precaution, they were not allowed to drive to and from the laboratory; they were provided subway fare at the end of each session. In the event a participant was still impaired, she either remained at the laboratory until the drug effects subsided or she was transported home in a taxicab. Participants were also instructed that they should not operate a vehicle for 8 hours after drug administration and should not take any medications or alcohol.
Measures
Abuse-Liability Measures
Drug Effects Questionnaire (DEQ)
This questionnaire (Evans et al., 1994) asked participants to rate “good effects” and “bad effects” from the drug on a 5-point scale from 0 (“no effect at all”) to 4 (“very much”), the strength of the drug effect from 0 (“no drug effect at all”) to 4 (“very strong effect”), as well as the degree they would be “willing to take the drug again” from 0 (“not at all”) to 4 (“very much”). Lastly, they rated how much they liked the drug effect on a 9-point scale: −4 indicated “dislike very much,” 0 indicated “feel neutral, or feel no drug effect,” and 4 indicated “like very much.” The DEQ was completed 0.25, 1, 2, 3, 4 and 5 hr after drug administration each session.
Multiple Choice Procedure
This procedure has been used as an efficient and valid method to assess the reinforcing effects of drugs in humans (Griffiths et al., 1993, 1996). Four times each session participants made a series of 9 discrete choices between the drug dose administered and various amounts of money, with the dollar value increasing from $0.25 to $64.00. Data from this procedure were analyzed as the maximum dollar amount that participants chose drug over money (i.e. the cross-over point). The last experimental session (Session 8) was a lottery session and participants randomly selected a poker chip; each poker chip had a number that corresponded to one of their previous choices from sessions 2–7. The choice corresponding to the number on the poker chip drawn was then implemented. In this study, “Drug” referred to the medication (placebo or AMPH, not the hormone). Regardless of the outcome, participants completed the standard experimental session on Session 8, similar to sessions 2–7. The Multiple Choice Procedure was completed 0.25, 1, 2, 3, 4 and 5 hr after drug administration each session.
Subject-Rated Questionnaires
Beck Depression Inventory II (BDI II)
This 21-item self-report questionnaire (Beck et al., 1996) was completed at baseline and 2 and 4 hr after drug administration each session.
State–Trait Anxiety Inventory (STAI)
The 20-item State component of this self-report questionnaire (Spielberger et al., 1970) was completed at baseline and 2 and 4 hr after drug administration each session.
Profile of Mood States (POMS)
For the 72-item Profile of Mood States questionnaire (POMS; McNair et al., 1971), 10 scales were analyzed (see Evans et al., 1998 for details). Participants rated each item on a 5-point scale from 0 (“not at all”) to 4 (“extremely”). To have all subscales on a similar 5-point scale, total scores for each subscale were divided by the number of items used to determine the subscale score. The POMS was completed at baseline and 0.25, 1, 2, 3, 4 and 5 hr after drug administration each session.
Biphasic Alcohol Effects Questionnaire (BAES)
The BAES (Martin et al., 1993) is a 14-item adjective rating scale that provides measures of alcohol’s effects on an 11-point scale from “not at all” (0) to “extremely” (10). The BAES contains 2 subscales measuring stimulant (BAES Stimulant) and sedative (BAES Sedative) effects. Although this questionnaire was originally designed to measure the effects of alcohol, it asks general questions about stimulant and sedative effects that could pertain to other stimulant and sedative drugs. The BAES was completed at baseline and 0.25, 1, 2, 3, 4 and 5 hr after drug administration each session.
Observer-Rated Questionnaire
Observer ratings were completed by trained research assistants who were blind to drug and medication conditions. The participant was rated on a 5-point scale, from 0 (normal) to 4 (extreme impairment or disruption) on seven dimensions representative of mood, motor changes, or a drug effect and a total score was generated. Observers were instructed to base their ratings on observation of the participant’s behavior rather than on the participant’s verbal reports or ratings. The primary measure was the total observer-rated score. Observer ratings were completed at baseline and 0.25, 1, 2, 3, 4 and 5 hr after drug administration each session.
Performance measures
Digit Symbol Substitution Test (DSST)
This 3-min task consists of nine random 3-row by 3-column squares (one square blackened per row) displayed across the top of the computer screen (McLeod et al., 1982). In each trial, a randomly generated number (1–9) appears at the bottom of the screen, indicating which of the arrays displayed at the top of the screen should be reproduced. Participants were instructed to press the keys in a 3-row by 3-column keypad that corresponded to the pattern associated with the randomly generated number. The primary dependent measure was total arrays correctly completed. The DSST was completed at baseline and 0.25, 1, 2, 3, 4 and 5 hr after drug administration each session.
Balance Task
This motor coordination task assessed the participant’s ability to stand upright for a maximum of 30 s on each foot while eyes are closed (Evans et al., 1994). The score was the total number of seconds the participant was able to balance (maximum of 60 s). The Balance task was completed at baseline and 0.25, 1, 2, 3, 4 and 5 hr after drug administration each session.
Impulsivity Tasks
Immediate Memory Task/Delayed Memory Task (IMT/DMT)
The IMT/DMT is a continuous performance task that yields a number of measures related to response initiation (Dougherty & Marsh, 2003; Dougherty et al., 2002, 2003a). In the IMT, a series of 5-digit numbers appeared successively on a computer monitor. Participants were instructed to respond when the stimulus on the monitor was identical to the one that preceded it. Each 5-digit number appeared for 500 msec, and successive numbers were separated by a 500-msec inter-trial interval. The DMT required the participant to remember a 5-digit number and then compare it with another that was presented 3.5 sec later. During the 3.5-sec interval, repetitive distracter stimuli (the 5-digit number 12345) were presented at the same rate and duration as the other stimuli. Participants were told to ignore the distracter stimuli and to remember and compare only the numbers spanning the distracter stimuli. The IMT/DMT consisted of one 5-min block of IMT followed by one 5-min block of DMT, with a 30-sec rest period between blocks. The primary dependent measure for each of these two tasks was the IMT ratio and DMT ratio, respectively. The ratio is defined as the proportion of commission errors to correct detections (Dougherty et al., 2002, 2008). The IMT/DMT was completed at baseline and 1, 2 and 4 hr after drug administration each session.
The GoStop Task
The GoStop task is a task that measures response inhibition (Dougherty et al., 2003b, 2005). In the GoStop task, a series of 5-digit numbers presented in black on a white background, with randomly generated 5-digit numbers appearing for 500 msec every 2 sec (500 msec on, 1500 msec off) were presented. Participants were told to respond when the number they saw was identical to the previous number. Half of all target trials feature a target-stop trial when the color of the matching target’s numerals changed from black to red at 50, 150, 250 and 350 msec after its presentation. Participants were instructed to respond to the identically matching numbers before the number disappeared from the screen, but not to respond to a number that turns red. The primary dependent measure was the 150 msec GoStop ratio, which is the number of response inhibition failures for the 150 msec delay relative to the number of responses to go trials (Dougherty et al., 2008). We also examined the percent of inhibition failures for the 150 msec delay. The GoStop task was completed at baseline and 1, 2 and 4 hr after drug administration each session.
The Delay Discounting Task (DDT)
The DDT, developed by Kirby & Marakovic (1996) and revised by Kirby et al. (1999), consisted of a fixed set of 27 choices between smaller immediate rewards and larger delayed rewards; reward values ranged between $11–$85 and delays ranged between 7 days to 6 months (Petry et al., 2002). The primary dependent measure is the k value, which determines the discount rate, or the steepness of the reduction in the present value of a reward with increases in delay to that reward (Kirby et al., 1999). Higher k values indicate higher levels of impulsivity. To encourage attentive responding, participants were informed that at the end of session 8, they would be given a 1 in 6 chance of receiving the reward that they chose on one of the trials. This was done by having participants role a die on session 8; if they rolled a 6, one question/response was selected out of a container of all of the questions/responses on all sessions, then they received whatever they chose in response to that question. If their choice had been an immediate amount of money, they received the money in cash immediately. If they selected delayed money, the money was given when the time had elapsed. The DDT was completed at baseline and 0.25, 1, 2 and 4 hr after drug administration each session.
Measure of Risk-Taking
Balloon Analogue Risk Task (BART)
This task, developed by Lejuez et al. (2002), involved displaying a small blue balloon on a computer screen. Each pump inflated the balloon and was accompanied by an accrual of 5 cents; the average pump break point was 64 pumps. When the balloon was pumped past its individual explosion point, a “pop” sound was generated and all money in the temporary bank was lost, then the next un-inflated balloon was displayed. However, at any point during each balloon trial, the participant could stop pumping the balloon and click the collect money button that transferred all money from the temporary bank to the permanent bank. Fifteen balloon trials were presented each time. The primary dependent measure was the number of adjusted pumps, defined as the average number of pumps excluding balloons that exploded (i.e. the average number of pumps on each balloon before money collection). To ensure consistent effort, participants were instructed that at the end of the study, they would receive a percentage of the actual money earned on this task. The BART was completed at baseline and 0.25, 1, 2 and 4 after drug administration each session.
Vital Signs
Heart rate and blood pressure were measured each session at baseline and 0.25, 1, 2, 3, 4 and 5 hr after drug administration using a Sentry II vital signs monitor (Model 6100; NBS Medical Services, Costa Mesa, CA).
Data Analysis
Analyses were based on the 18 women who completed the study. The results from session 1 (practice session) and session 8 (lottery session) were not included in the data analyses. For all measures assessed during the six testing sessions, peak data were analyzed. The direction of the peak effect (maximum or minimum) was based on initial inspection of the time-course data. Separate two-factor repeated measures within-subject analyses of variance (ANOVA) were conducted for each measurement. The two factors were pretreatment (progesterone vs. placebo) and AMPH dose (0, 10, 20 mg). Planned comparisons were used to compare 10 and 20 mg AMPH to 0 mg AMPH within each pretreatment and to compare pretreatments at each AMPH dose. For all analyses, results were considered statistically significant if p ≤ 0.05, using Huynh-Feldt corrections as a conservative measure to control for potentially uncorrelated within-subject data.
Results
Table 1 presents the results of the repeated-measures ANOVAs conducted on the measurements where peak effects were analyzed.
Table 1.
Summary of results of ANOVAs analyzing effects at peak time points
| Measure | Main Effect of Pretreatment | Main Effect of AMPH Dose | Pretreatment × AMPH Dose Interaction | |||
|---|---|---|---|---|---|---|
| F(1,17) | p | F(2,34) | p | F(2,34) | p | |
| Abuse Liability | ||||||
| DEQ | ||||||
| Good Drug Effect | 8.98 | 0.008* | 19.84 | 0.0001* | 2.11 | 0.14 |
| Drug Liking | 4.05 | 0.06 | 14.43 | 0.0001* | 1.03 | 0.37 |
| Strength of the Drug Effect | 4.20 | 0.06 | 5.74 | 0.007* | 1.02 | 0.37 |
| Take Again | 0.20 | 0.66 | 4.16 | 0.02* | 1.86 | 0.17 |
| Bad Drug Effect | 3.32 | 0.07 | 6.40 | 0.006* | 0.92 | 0.40 |
| Multiple Choice Procedure | 1.44 | 0.25 | 4.98 | 0.01* | 4.90 | 0.03* |
| Subjective Effects | ||||||
| BDI | 0.40 | 0.54 | 3.51 | 0.05* | 0.03 | 0.96 |
| State Anxiety | 0.007 | 0.93 | 3.72 | 0.04* | 1.43 | 0.26 |
| POMS | ||||||
| Positive Mood | 0.000001 | 1.00 | 20.69 | 0.0001* | 2.23 | 0.12 |
| Arousal | 0.08 | 0.78 | 15.21 | 0.0001* | 0.39 | 0.67 |
| Vigor | 0.003 | 0.96 | 18.76 | 0.0001* | 0.35 | 0.71 |
| Elation | 0.07 | 0.80 | 21.37 | 0.0001* | 2.46 | 0.10 |
| Friendly | 0.07 | 0.79 | 10.55 | 0.0003* | 1.60 | 0.22 |
| Fatigue | 0.16 | 0.70 | 15.33 | 0.0003* | 1.72 | 0.20 |
| Tension-Anxiety | 1.24 | 0.28 | 1.46 | 0.25 | 0.82 | 0.43 |
| Depression-Dejection | 1.79 | 0.20 | 0.57 | 0.57 | 2.88 | 0.07 |
| Confusion | 0.76 | 0.40 | 2.43 | 0.11 | 0.09 | 0.89 |
| Anger-Hostility | 4.61 | 0.05* | 0.10 | 0.90 | 0.87 | 0.43 |
| BAES | ||||||
| Stimulation | 0.05 | 0.82 | 8.05 | 0.001* | 0.85 | 0.41 |
| Sedation | 1.04 | 0.32 | 6.32 | 0.005* | 2.26 | 0.13 |
| Total observer-rated scores | 1.72 | 0.21 | 3.18 | 0.07 | 1.24 | 0.30 |
| Performance Tasks | ||||||
| DSST total arrays | 2.90 | 0.11 | 9.36 | 0.0008* | 7.40 | 0.003* |
| Balance task | 0.53 | 0.47 | 1.77 | 0.19 | 0.27 | 0.76 |
| Impulsivity and Risk-Taking | ||||||
| IMT ratio | 0.01 | 0.91 | 0.36 | 0.70 | 1.11 | 0.33 |
| DMT ratio | 1.68 | 0.21 | 0.21 | 0.77 | 5.81 | 0.01* |
| GoStop ratio | 0.07 | 0.79 | 0.54 | 0.56 | 0.59 | 0.56 |
| GoStop inhibition failures | 0.04 | 0.84 | 1.25 | 0.29 | 0.37 | 0.69 |
| BART adjusted average number of pumps | 0.74 | 0.40 | 3.33 | 0.05* | 1.16 | 0.33 |
| DDT k values | 0.56 | 0.46 | 1.86 | 0.19 | 0.90 | 0.39 |
| Vital Signs | ||||||
| Heart rate | 0.07 | 0.80 | 3.55 | 0.04* | 0.16 | 0.82 |
| Systolic pressure | 0.30 | 0.59 | 27.43 | 0.0001* | 3.18 | 0.07 |
| Diastolic pressure | 1.57 | 0.23 | 30.03 | 0.0001* | 6.23 | 0.005* |
denotes significant difference (p = 0.05).
Hormone levels
All women had ovulatory menstrual cycles that ranged from 26 to 32 days. When examining hormone levels as a function of pretreatment, estradiol levels were not significantly different after progesterone pretreatment compared to placebo pretreatment (55.64 ± 8.17 vs. 63.00 ± 9.78 pg/ml). However, progesterone levels were significantly higher after progesterone pretreatment than placebo pretreatment [10.36 ± 4.06 vs. 1.05 ± 0.09 ng/ml, F(1,17) = 5.51, p = 0.03].
Abuse Liability Measures
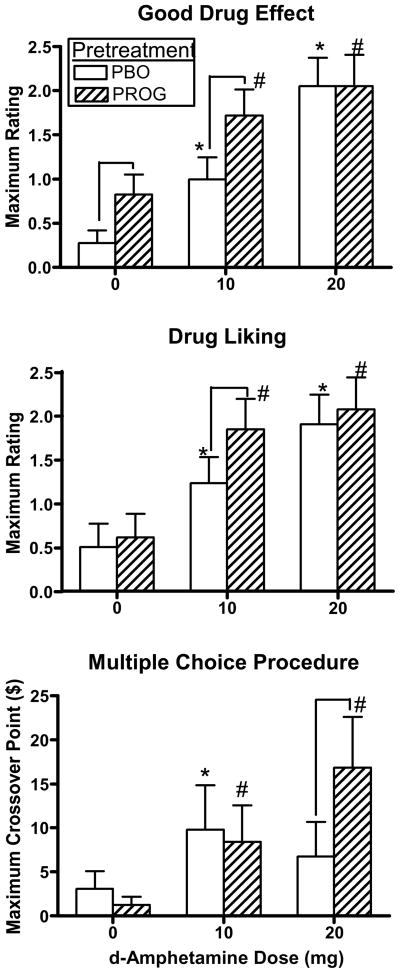
Fig. 1 documents peak ratings of Good Drug Effect and Drug Liking on the DEQ and the cross-over point for choosing drug over money on the Multiple Choice Procedure as a function of pretreatment and AMPH dose. Ratings of Good Drug Effect [AMPH dose effect: F(2,34) = 19.84, p = 0.0001], Drug Liking [AMPH dose effect: F(2,34) = 14.43, p = 0.0001], Strength of the Drug Effect [AMPH dose effect: F(2,34) = 5.74, p = 0.007; data not shown], and Take Again [AMPH dose effect: F(2,34) = 4.16, p = 0.02; data not shown] were increased in response to AMPH. Progesterone pretreatment alone increased ratings of Good Drug Effect (p ≤ 0.05) and Strength of the Drug Effect (p ≤ 0.05; data not shown), as indicated by elevated ratings under 0 mg AMPH, compared to placebo pretreatment. Lastly, progesterone pretreatment further increased ratings of Good Drug Effect [pretreatment effect: F(1,17) = 8.98, p = 0.008] and tended to increase ratings of Drug Liking [pretreatment effect: F(1,17) = 4.05, p = 0.06] and Strength of the Drug Effect [pretreatment effect: F(1,17) = 4.20, p = 0.06] compared to placebo pretreatment when combined with AMPH; based on the planned comparisons this was observed following 10 mg AMPH (p ≤ 0.05) for these measures. Ratings of Bad Drug Effect were correspondingly decreased in response to AMPH [AMPH dose effect: F(2,34) = 6.40, p = 0.006; data not shown], however there was no effect of pretreatment.
Fig. 1.
Peak ratings of Good Drug Effect and Drug Liking on the DEQ and peak crossover point for choosing drug over money on the Multiple Choice Procedure as a function of pretreatment and AMPH dose. * denotes a significant difference compared to 0 mg AMPH after placebo pretreatment (p ≤ 0.05). # denotes a significant difference compared to 0 mg AMPH after progesterone pretreatment (p ≤ 0.05). ⊓ denotes a significant difference between pretreatments at that AMPH dose (p ≤ 0.05). Error bars represent + 1 SEM.
As shown in Fig. 1, AMPH significantly increased the choice of drug over money as indicated by a higher cross-over point on the Multiple Choice Procedure [AMPH dose effect: F(2,34) = 4.98, p = 0.01]. Progesterone pretreatment further increased the crossover point following 20 mg AMPH compared to placebo pretreatment [AMPH dose x pretreatment interaction: F(2,34) = 4.90, p = 0.03].
Subjective Effects
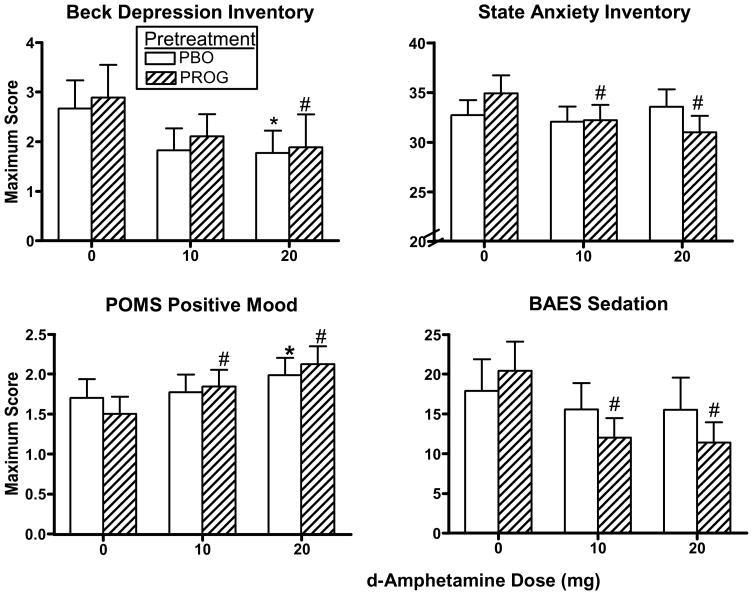
Fig. 2 documents peak BDI, State Anxiety, POMS Positive Mood and BAES Sedation scores as a function of pretreatment and AMPH dose. BDI scores were significantly decreased by AMPH to a similar extent after both pretreatments [AMPH dose effect: F(2,34) = 3.51, p = 0.05]. While there was a dose-related decrease in State Anxiety scores [AMPH dose effect: F(2,34) = 3.72, p = 0.04], planned comparisons revealed that progesterone pretreatment decreased State Anxiety scores when combined with AMPH (p ≤ 0.05).
Fig. 2.
Peak BDI, state anxiety, POMS Positive Mood and BAES Sedation scores as a function of pretreatment and AMPH dose. * denotes a significant difference compared to 0 mg AMPH after placebo pretreatment (p ≤ 0.05). # denotes a significant difference compared to 0 mg AMPH after progesterone pretreatment (p ≤ 0.05). Error bars represent + 1 SEM.
AMPH significantly increased Positive Mood (Fig. 2), Arousal, Vigor, Elation and Friendly scores on the POMS [AMPH dose effect: F(2,34) ≥ 10.55, p ≤ 0.0003]. Although there was not an AMPH dose x pretreatment interaction, planned comparisons showed that Positive Mood, Arousal, Vigor and Elation scores were increased after both pretreatments following 20 mg AMPH (p ≤ 0.05), but increased only after progesterone pretreatment following 10 mg AMPH (p ≤ 0.05), whereas Friendly scores were increased by both doses of AMPH when combined with progesterone pretreatment (p ≤ 0.05). AMPH significantly increased ratings of Stimulation on the BAES similarly after both pretreatments [AMPH dose effect: F(2,34) = 8.05, p = 0.001]. In contrast, Fatigue scores on the POMS were decreased by AMPH to a similar extent after both pretreatments [AMPH dose effect: F(2,34) = 15.33, p = 0.0003]. Correspondingly, Sedation scores on the BAES (Fig. 2) showed a significant dose-related decrease [AMPH dose effect: F(2,34) = 6.32, p = 0.005], however planned comparisons revealed that this decrease was only observed when AMPH was combined with progesterone pretreatment (p ≤ 0.05). There was a single pretreatment effect on the POMS [F(1,17) = 4.61, p = 0.05] where progesterone pretreatment significantly decreased scores of Anger-Hostility compared to placebo pretreatment when combined with AMPH, however scores were low in response to AMPH after placebo or progesterone pretreatment, indicative of very little anger in general. There was no effect of AMPH dose, pretreatment, or any interactions on peak Tension-Anxiety, Depression-Dejection or Confusion scores on the POMS.
While progesterone alone did not increase Fatigue scores on the POMS or Sedation scores on the BAES based on the peak analyses, inspection of the time course data revealed that, under 0 mg AMPH, progesterone alone tended to increase Fatigue scores (particularly at hours 3–5; p ≤ 0.07) and Sedation scores (particularly at hours 2–4; p ≤ 0.05), compared to placebo alone.
Observer Ratings
There was a trend for a dose-related decrease in observer-rated scores [AMPH dose effect: F(2,34) = 3.18, p = 0.07], however based on the planned comparisons this was related to the fact that progesterone alone increased observer-rated scores under 0 mg AMPH compared to placebo pretreatment (p ≤ 0.05), and when progesterone was combined with AMPH (p ≤ 0.05), these scores returned to similar levels observed after placebo pretreatment.
Performance Tasks
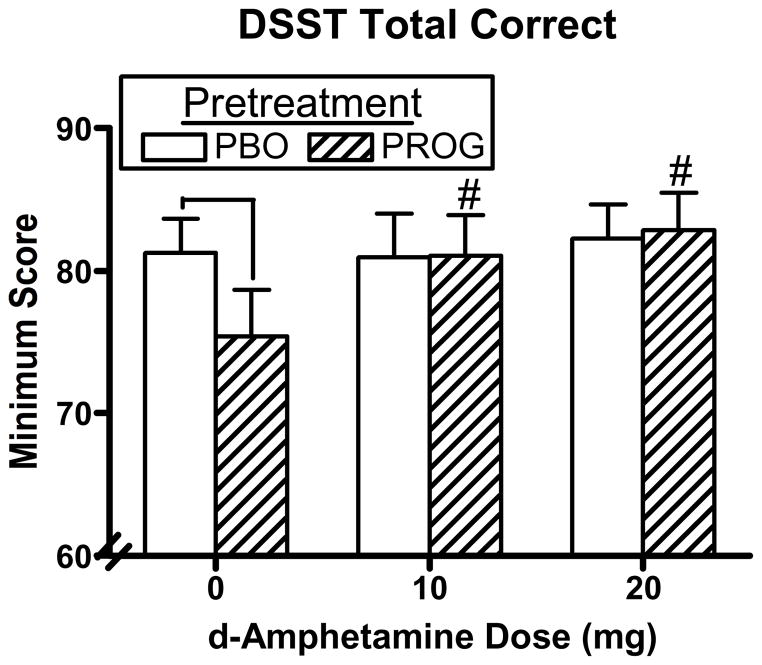
Figure 3 documents peak number correct on the DSST as a function of pretreatment and AMPH dose. DSST performance showed a significant dose-related increase [AMPH dose effect: F(2,34) = 9.36, p = 0.0008], but this increase was accounted for by the fact that progesterone pretreatment alone impaired DSST performance under 0 mg AMPH compared to placebo pretreatment and this impairment returned to baseline levels when progesterone was combined with AMPH [AMPH dose x pretreatment interaction: F(2,34) = 7.40, p = 0.003]. There were no effects of AMPH dose, pretreatment, or any interactions on the balance task.
Fig. 3.
Peak DSST correct arrays as a function of pretreatment and AMPH dose. # denotes a significant difference compared to 0 mg AMPH after progesterone pretreatment (p ≤ 0.05). ⊓ denotes a significant difference between pretreatments at that AMPH dose (p ≤ 0.05). Error bars represent + 1 SEM.
Impulsivity and Risk-Taking
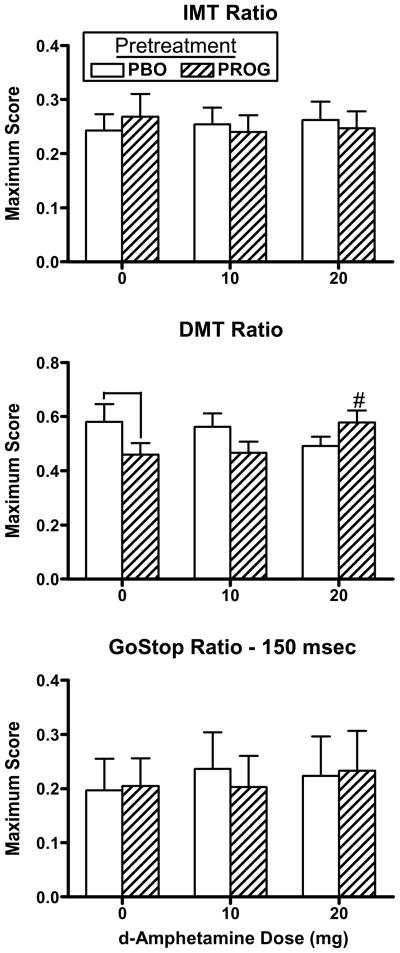
Figure 4 documents peak IMT ratio, DMT ratio and GoStop ratio as a function of pretreatment and AMPH dose. The DMT ratio (Fig. 4, middle panel) was significantly lower after progesterone pretreatment alone, under 0 mg AMPH, compared to placebo pretreatment [AMPH dose x pretreatment interaction: F(2,34) = 5.81, p = 0.01], although this was reversed by the high dose of AMPH (p ≤ 0.05). On the BART (data not shown), adjusted average number of pumps was significantly increased by AMPH [AMPH dose effect: F(2,34) = 3.33, p = 0.05], however there was no effect of pretreatment. There was no significant difference in IMT ratio, GoStop ratio, inhibition failures on the GoStop task, or DDT k values in response to AMPH dose or pretreatment.
Fig. 4.
Peak IMT ratio, DMT ratio and GoStop ratio as a function of pretreatment and AMPH dose. # denotes a significant difference compared to 0 mg AMPH after progesterone pretreatment (p ≤ 0.05). ⊓ denotes a significant difference between pretreatments at that AMPH dose (p ≤ 0.05). Error bars represent + 1 SEM.
Vital Signs
AMPH produced significant increases in heart rate [AMPH dose effect: F(2,34) = 3.55, p = 0.04], systolic pressure [AMPH dose effect: F(2,34) = 27.43, p = 0.0001] and diastolic pressure [AMPH dose effect: F(2,34) = 30.03, p = 0.0001]. In contrast, progesterone pretreatment alone decreased diastolic pressure under 0 mg AMPH compared to placebo pretreatment (p ≤ 0.05). Further, progesterone pretreatment attenuated the AMPH-induced increases in systolic pressure [AMPH dose x pretreatment interaction: F(2,34) = 3.18, p = 0.07] and diastolic pressure [AMPH dose x pretreatment interaction: F(2,34) = 6.23, p = 0.005] compared to placebo pretreatment; planned comparisons showed that these decreases were observed when progesterone was combined with10 mg AMPH (p ≤ 0.05).
Discussion
Positive Subjective Effects and Abuse Liability
To our knowledge, this is the first study examining the effects of oral micronized progesterone on oral AMPH in normally-cycling, non-drug using women. As expected, oral AMPH increased positive subjective effects and measures of abuse liability in normal healthy women, similar to what has been observed in previous studies using mixed gender samples (e.g. de Wit et al., 2002; Stoops et al., 2007). Contrary to our original hypothesis, oral micronized progesterone enhanced AMPH-induced positive subjective effects and other measures of abuse liability. These results are inconsistent with previous studies showing that progesterone decreased the effects of other stimulants. For example, in rodent studies, progesterone has been shown to attenuate behaviors related to cocaine administration, such as cocaine-seeking behavior (Feltenstein et al., 2009; Anker et al., 2007), cocaine-induced conditioned place preference (Russo et al., 2008), and cocaine self-administration (Jackson et al., 2006; Larson et al., 2007). In women, progesterone attenuated the positive subjective effects of nicotine (Sofuoglu et al., 2001, 2009) and cocaine (Sofuoglu et al., 2002, 2004; Evans & Foltin, 2006) during the follicular phase of the menstrual cycle. It has also been shown that the positive subjective effects of oral AMPH were dampened in the luteal phase compared to the follicular phase of normal healthy women (Justice & de Wit, 1999), suggesting that progesterone would attenuate the subjective effects of AMPH as well.
In addition to increasing positive subjective effects, AMPH increased positive mood in the current study, and this was even more apparent when AMPH was combined with progesterone. Again, these findings are inconsistent with previous studies conducted with cocaine users. For instance, Sofuolgu et al. (2002) did not observe an effect of oral progesterone in combination with cocaine on any POMS scores. Moreover, among non-drug users, White et al. (2002) showed that AMPH-induced Friendliness scores on the POMS were negatively correlated with progesterone levels, suggesting that progesterone would decrease AMPH-induced Friendliness. However, the effects of oral progesterone on AMPH have not been examined. Based on the present study, it appears that oral progesterone produces a different effect on AMPH in non-drug users than on cocaine in cocaine users.
Impulsivity and Risk-Taking
Oral AMPH had little effect on measures of impulsivity and risk-taking in the current study. Although one previous study reported no effect of i.v. AMPH on impulsivity or risk-taking (Kelly et al., 2006), other studies have shown that oral AMPH decreased impulsivity as measured by response inhibition tasks (de Wit et al., 2000, 2002) and the delay discounting task (de Wit et al., 2002) and altered risk-taking in males but not females (White et al., 2007). One important difference in the current study compared to previous studies examining oral AMPH on impulsivity is that the previous studies used mixed gender samples (e.g. 70% male in de Wit et al., 2000; 50% male in de Wit et al., 2002) whereas the current study used females only. Given that men tend to be more impulsive than women (e.g. Zuckerman et al., 1978; Petry et al., 2002), changes in impulsivity are more likely to be observed in a sample that consists predominantly of men. Further, menstrual cycle phase was not accounted for in the women in the previous studies. Although there are no studies in humans assessing changes in impulsivity across the menstrual cycle, one study in normally-cycling female rodents found that impulsive burying was reduced when progesterone and estrogen were elevated than when progesterone and estrogen were low (Llaneza & Frye, 2009), thus there may be differences in impulsivity across the menstrual cycle in women. This also suggests that progesterone would decrease impulsivity. This is supported by a second experiment by Llaneza & Frye (2009) where ovariectomized rats that were administered progesterone engaged in less impulsive burying than those treated with vehicle. In the current study, progesterone alone decreased impulsivity, but only as measured by the DMT. However, progesterone did not decrease impulsivity when given in combination with AMPH, as hypothesized. Thus, it appears that AMPH and progesterone have little impact on behavioral measures of impulsivity in non-drug using women.
Performance
Oral AMPH alone had no effect on performance in the current study. This was somewhat surprising, as other studies have shown that stimulants improve performance, specifically DSST performance. For example, i.v. cocaine produced a small but insignificant increase in DSST performance (Johnson et al., 1998). Further, oral (Hart, 2005) and intranasal (Hart et al., 2008) methamphetamine increased DSST performance. However, the existing literature on DSST performance in response to AMPH has been inconsistent. In mixed gender samples of non-drug users, one study found that AMPH increased DSST performance (Hamidovic et al., 2010), while another study found no effect (Makris et al., 2007). These differences across studies could be related to various factors including drug, dose, route of administration, gender, and the drug history of the participants.
As hypothesized, progesterone alone decreased performance on the DSST, but not on the balance task. These results are consistent with the time course data showing that progesterone produced sedation. Although progesterone did not alter sedation in cocaine users (Sofuoglu et al., 2004; Evans & Foltin, 2006), progesterone has been shown to increase sedation (van Broekhoven et al., 2006; Freeman et al., 1992) and impair DSST performance in non-drug using women (Freeman et al., 1992; but see de Wit et al., 2001). Similarly, among a mixed gender sample (Söderpalm et al., 2004), i.m. progesterone increased Fatigue scores on the POMS, but did not alter DSST performance. Taken together, progesterone administration produces mild performance impairment and sedation, even when administered intramuscularly, resulting in much higher progesterone levels than typically observed during a normal luteal phase (see de Wit et al., 2001; Söderpalm et al., 2004). In the present study, the mild sedation and performance impairment produced by progesterone were reversed by the administration of AMPH.
Cardiovascular Effects
Amphetamine produced dose-dependent increases in cardiovascular effects, as expected. In contrast, progesterone alone produced minimal changes in cardiovascular effects, confirming previous studies (Sofuoglu et al., 2001, 2002, 2004; Evans & Foltin, 2006; Söderpalm et al., 2004). However, progesterone decreased the AMPH-induced increases in diastolic and systolic pressure following an intermediate dose of AMPH. While some studies have shown that progesterone has no effect on stimulant-induced cardiovascular measures in stimulant users (Sofuoglu et al., 2001, 2002, 2009), others have found that progesterone decreased the cocaine-induced increases in diastolic pressure (Sofuoglu et al., 2004; Evans & Foltin, 2006) and heart rate (Evans & Foltin, 2006). This effect was observed in both males and females in the study by Evans and Foltin (2006). Thus, oral micronized progesterone may have a protective effect on the cardiovascular increases observed in response to stimulants, particularly cocaine and AMPH, but this needs to be further explored.
Strengths and Limitations
Importantly, this study had a number of procedural strengths. First, menstrual cycle was controlled for and women were all tested in the follicular phase. Second, multiple measures of impulsivity were used to examine the various characteristics of impulsivity. Third, we had a sample size of 18 women, which is the largest sample size to date in a controlled laboratory study examining the effects of progesterone on stimulants in women.
The present study also had several limitations. One obvious limitation is that men were not included to assess potential sex differences. However, our previous study in cocaine users showed that progesterone attenuates the subjective effects of cocaine in women, not men (Evans & Foltin, 2006), thus it seemed reasonable to initially assess the effects of progesterone on AMPH in women. A second limitation is that we did not use a loading dose of progesterone the evening before the sessions, as was done in some inpatient studies (e.g. Evans & Foltin, 2006; Sofuoglu et al., 2004). However, in the majority of outpatient laboratory studies examining progesterone (e.g. Freeman et al., 1992; de Wit et al., 2001; Childs et al., 2010), including those examining oral progesterone on the effects of stimulants (e.g., Sofuoglu et al., 2001; 2002; 2009), progesterone was given as a single acute dose. Further, acute progesterone administration in the laboratory on each outpatient day ensured that progesterone was taken soon after food consumption, since absorption of progesterone is affected by food intake. Additionally, acute progesterone administered after baseline measures allowed for examination of the effect of progesterone alone, amphetamine alone, and the combination of the two on these measures, compared to baseline measures in the absence of any drug or medication.
Lastly, another limitation is that the participants had minimal experience with stimulants other than caffeine. Different results might have been obtained among a group of women who abused amphetamines. It is possible that stimulant-dependent individuals, who would be the target treatment population for this medication, would be more sensitive to the effects of amphetamine, and that oral progesterone may increase these effects, similar to what was observed in non-drug users in the current study. Thus, oral progesterone may not be a good candidate for a treatment medication for this particular population. However, it is also possible that oral progesterone has a different effect in stimulant users than non-drug users and would decrease the effects of amphetamine in stimulant abusers, similar to the decrease in effects of cocaine or nicotine in users of those drugs. This may be due to stimulant users having a different neurochemical profile in the brain than non-drug users (e.g. Volkow et al., 2005; Martinez et al., 2009), and therefore progesterone, which interacts with systems such as the dopaminergic and GABAergic systems, may have a different impact in this population than non-drug users. However, it is important to note that the effects of oral progesterone on these neurochemical systems has not been fully elucidated in any population, and thus more research is needed to understand the effects of progesterone.
Conclusions
The most intriguing and important finding in the current study was that progesterone enhanced the positive subjective effects of AMPH in female non-drug users, whereas previous studies have shown that progesterone attenuated the effects of cocaine. The reasons for these findings are unclear. It has been suggested estrogen may enhance stimulant effects in rats (e.g. Carroll et al., 2004) and humans (e.g. Lile et al., 2007) and that estrogen’s effects on amphetamine-induced behavior in humans is dependent on progesterone levels (Justice & de Wit, 1999). Therefore, it is possible that an estrogen/progesterone interaction plays a role in the effects of oral progesterone on stimulants. However, estradiol levels are generally lower during the midfollicular phase compared to the luteal phase of the menstrual cycle, and estradiol levels were relatively low in the previous studies (Sofuoglu et al., 2002; Evans & Foltin, 2006) and the current study and therefore probably not contributing to the behavioral differences observed in these studies. Alternatively, progesterone may differentially alter cocaine, but not AMPH, due to their different mechanisms of action. For example, while cocaine and AMPH both block the reuptake of dopamine, AMPH is also a dopamine releaser. There is evidence that progesterone blocks AMPH-stimulated dopamine release in castrated male rats given estrogen (Dluzen & Ramirez, 1990), suggesting that an effect of progesterone on AMPH may be estrogen-dependent. However, another study found that progesterone increases dopamine release independent of estrogen in rats (Petitclerc et al., 1995), and thus progesterone may produce an additive effect on AMPH-induced dopamine release. It is also possible that progesterone is interacting with another neurotransmitter system that is producing the attenuation of effects observed with other stimulants, but not AMPH. For example, progesterone appears to interact with the serotonin system (Bethea et al., 2002), which may in turn alter the effects of cocaine, although exactly how this interaction works is still unclear. Further, the progesterone metabolite allopregnanolone has been shown to potentiate GABA receptors (Paul & Purdy, 1992), which may explain the attenuating effects of progesterone on cocaine (Evans & Foltin, 2006). Alternatively, it is possible that progesterone has a different effect among stimulant users compared to the non-drug users who participated in the present study. Lastly, AMPH was given orally in the current study, whereas in previous studies stimulants were administered either by the smoked or intravenous route, suggesting that progesterone may have a different effect based on the route of stimulant administration. In summary, additional studies are needed to explore the modulatory role of progesterone on the effects of AMPH in different populations of women and men to determine whether progesterone has any clinical utility for AMPH abuse.
Acknowledgments
This research was supported by Grants R01 DA009114 (SME), K02 000465 (FRL), and K01 022282 (SCR) from the National Institute on Drug Abuse. The authors gratefully acknowledge the assistance of the research and clinical staff. The Women’s International Pharmacy (Madison, WI) graciously provided the micronized progesterone and matching placebo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;107:264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Zlebnik N, Carroll ME. Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology. 2009;203:63–72. doi: 10.1007/s00213-008-1371-9. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 2007;15:472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- Asghar SJ, Tanay VA, Baker GB, Greenshaw A, Silverstone PH. Relationship of plasma amphetamine levels to physiological, subjective, cognitive and biochemical measures in healthy volunteers. Hum Psychopharmacol. 2003;18(4):291–299. doi: 10.1002/hup.480. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and –II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. doi: 10.1016/j.yhbeh.2009.10.001. In press. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Childs E, Van Dam NT, de Wit H. Effects of acute progesterone administration upon responses to acute psychosocial stress in men. Exp Clin Psychopharmacol. 2010;18:78–86. doi: 10.1037/a0018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lignières B. Oral micronized progesterone. Clin Ther. 1999;21(1):41–60. doi: 10.1016/S0149-2918(00)88267-3. [DOI] [PubMed] [Google Scholar]
- de Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neuroscience. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- de Wit H, Schmitt L, Purdy R, Hauger R. Effects of acute progesterone administration in healthy postmenopausal women and normally-cycling women. Psychoneuroendocrinology. 2001;26:697–710. doi: 10.1016/s0306-4530(01)00024-5. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD. In vitro progesterone modulates amphetamine-stimulated dopamine release from the corpus striatum of castrated male rats treated with estrogen. Neuroendocrinology. 1990;52:517–520. doi: 10.1159/000125637. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM. Immediate and delayed memory tasks (IMT/DMT 2.0): a research tool for studying attention, memory, and impulsive behavior [Manual] Neurobehavioral Research Laboratory and Clinic, University of Texas Health Science Center; Houston, Houston, TX: 2003. [Google Scholar]
- Dougherty DM, Bjork JM, Harper RA, Mathias CW, Moeller FG, Marsh DM. Validation of the immediate and delayed memory tasks in hospitalized adolescents with disruptive behavior disorders. Psychol Rec. 2003a;53:509–532. doi: 10.1111/1469-7610.00197. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, Mathias CW. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug Alcohol Depend. 2008;96:111–120. doi: 10.1016/j.drugalcdep.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Mathias CW. Immediate and delayed memory tasks: a computerized behavioral measure of memory, attention, and impulsivity. Behav Res Methods Instrum Comput. 2002;34:391–398. doi: 10.3758/bf03195467. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM. Neurobehavioral Research Laboratory and Clinic. University of Texas Health Science Center; Houston, Houston, TX: 2003b. GoStop impulsivity paradigm (version 1.0) [Manual] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Jagar AA. Laboratory behavioral measures of impulsivity. Behav Res Methods. 2005;37:82–90. doi: 10.3758/bf03206401. [DOI] [PubMed] [Google Scholar]
- Evans SM. The role of estradiol and progesterone in modulating the subjective effects of stimulants in humans. Exp Clin Psychopharmacol. 2007;15:418–426. doi: 10.1037/1064-1297.15.5.418. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav. doi: 10.1016/j.yhbeh.2009.08.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW, Fischman MW. Food “cravings” and the acute effects of alprazolam on food intake in women with premenstrual dysphoric disorder. Appetite. 1999;32:331–349. doi: 10.1006/appe.1998.0222. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Levin FR, Foltin RW, Fischman MW. Mood and performance changes in women with premenstrual dysphoric disorder: acute effects of alprazolam. Neuropsychopharm. 1998;19:499–516. doi: 10.1016/S0893-133X(98)00064-5. [DOI] [PubMed] [Google Scholar]
- Evans SM, Troisi JR, II, Griffiths RR. Tandospirone and alprazolam: comparison of behavioral effects and abuse liability in humans. J Pharmacol Exp Ther. 1994;271:683–694. [PubMed] [Google Scholar]
- Evenden J, Ko T. The psychopharmacology of impulsive behaviour in rats VIII: effects of amphetamine, methylphenidate, and other drugs on responding maintained by a fixed consecutive number avoidance schedule. Psychopharmacology. 2005;180:294–305. doi: 10.1007/s00213-005-2163-0. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Pearson PR, Easting G, Allsopp JF. Age norms for impulsiveness, venturesomeness and empathy in adults. Person Individ Diff. 1985;6:613–619. [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34:343–352. doi: 10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89(2–3):183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Kelly TH, Martin CA. Effects of d-amphetamine in human models of information processing and inhibitory control. Drug Alcohol Depend. 2005;77:151–159. doi: 10.1016/j.drugalcdep.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders - Patient Edition (SCID-I/P, version 2.0) Biometrics Research Department. New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Freeman EW, Weinstock L, Rickels K, Sondheimer SJ, Coutifaris C. A placebo-controlled study of effects of oral progesterone on performance and mood. Br J Clin Pharmacol. 1992;33:293–298. doi: 10.1111/j.1365-2125.1992.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishman GN, Klock SC, Luciano AA, Nulsen JC. Efficacy of oral micronized progesterone in the treatment of luteal phase defects. J Reprod Med. 1995;40:521–524. [PubMed] [Google Scholar]
- Griffiths RR, Rush CR, Puhala KA. Validation of the multiple-choice procedure for investigating drug reinforcement in humans. Exp Clin Psychopharmacol. 1996;4:97–106. [Google Scholar]
- Griffiths RR, Troisi JR, II, Silverman K, Mumford GK. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav Pharmacol. 1993;4:3–13. [PubMed] [Google Scholar]
- Hamidovic A, Dlugos A, Palmer AA, de Wit H. Polymorphisms in dopamine transporter (SLC6A3) are associated with stimulant effects of d-amphetamine: an exploratory pharmacogenetic study using healthy volunteers. Behav Genet. 2010;40:255–261. doi: 10.1007/s10519-009-9331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Gunderson EW, Perez A, Kirkpatrick MG, Thurmond A, Comer SD, Foltin RW. Acute physiological and behavioral effects of intranasal methamphetamine in humans. Neuropsychopharmacology. 2008;33:1847–1855. doi: 10.1038/sj.npp.1301578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Nasser J, Foltin RW. Combined effects of methamphetamine and zolpidem on performance and mood during simulated night shift work. Pharmacol Biochem Behav. 2005;81:559–568. doi: 10.1016/j.pbb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG. Cross-generational effects on gender differences in psychoactive drug abuse and dependence. Drug Alc Depend. 2004;74:147–158. doi: 10.1016/j.drugalcdep.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31(1):129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Johnson B, Overton D, Wells L, Kenny P, Abramson D, Dhother S, Chen YR, Bordnick P. Effects of acute intravenous cocaine on cardiovascular function, human learning, and performance in cocaine addicts. Psychiatry Res. 1998;77:35–42. doi: 10.1016/s0165-1781(97)00127-3. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Kaloyanides KB, McCabe SE, Cranford JA, Teter CJ. Prevalence of illicit use and abuse of prescription stimulants, alcohol, and other drugs among college students: relationship with age at initiation of prescription stimulants. Pharmacotherapy. 2007;27:666–674. doi: 10.1592/phco.27.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington NG, Rush CR. Individual differences in drug abuse vulnerability: d-amphetamine and sensation-seeking status. Psychopharmacology. 2006;189:17–25. doi: 10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Marakovic NN. Delay-discounting probabilistic rewards: rates decrease as amounts increase. Psychonom Bull Rev. 1996;3:100–104. doi: 10.3758/BF03210748. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15(5):461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong GR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Applied. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kendall SL, Babalonis S, Martin CA, Kelly TH. Evaluation of estradiol administration on the discriminative-stimulus and subject-rated effects of d-amphetamine in healthy pre-menopausal women. Pharmacol Biochem Behav. 2007;87:258–266. doi: 10.1016/j.pbb.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaneza DC, Frye CA. Progestogens and estrogen influence impulsive burying and avoidant freezing behavior of naturally cycling and ovariectomized rats. Pharmacol Biochem Behav. 2009;93:337–342. doi: 10.1016/j.pbb.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord S, Downs G, Furtaw P, Chaudhuri A, Silverstein A, Gammaitoni A, Budman S. Nonmedical use of prescription opioids and stimulants among student pharmacists. J Am Pharm Assoc. 2009;49:519–528. doi: 10.1331/JAPhA.2009.08027. [DOI] [PubMed] [Google Scholar]
- Makris AP, Rush CR, Frederich RC, Taylor AC, Kelly TH. Behavioral and subjective effects of d-amphetamine and modafinil in healthy adults. Exp Clin Psychopharmacol. 2007;15:123–133. doi: 10.1037/1064-1297.15.2.123. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, Slifstein M, Van Heertum R, Kleber HD. Lower level of endogenous dopamine in patients with cocaine dependence: Findings from PET imaging of D2/D3 receptors following acute dopamine depletion. Am J Psychiatry. 2009;166:1170–1177. doi: 10.1176/appi.ajp.2009.08121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ. The use, misuse and diversion of prescription stimulants among middle and high school students. Subst Use Misuse. 2004;39:1095–1116. doi: 10.1081/ja-120038031. [DOI] [PubMed] [Google Scholar]
- McLeod D, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instrum. 1982;14:463–466. [Google Scholar]
- McNair D, Lorr M, Droppleman LF. Profile of mood states. San Diego: Educational and Industrial Testing Services; 1971. [Google Scholar]
- Mello NK, Knudson IM, Mendelson JH. Sex and menstrual cycle effects on progressive ratio measures of cocaine self-administration in cynomolgus monkeys. Neuropsychopharmacology. 2007;32:1956–1966. doi: 10.1038/sj.npp.1301314. [DOI] [PubMed] [Google Scholar]
- Michanek A, Meyerson BJ. Influence of estrogen and progesterone on behavioral effects of apomorphine and amphetamine. Pharmacol Biochem Behav. 1982;16:875–879. doi: 10.1016/0091-3057(82)90039-9. [DOI] [PubMed] [Google Scholar]
- Niyomchai T, Akhavan A, Festa ED, Lin SN, Lamm L, Foltz R, Quiñones-Jenab V. Estrogen and progesterone affect cocaine pharmacokinetics in female rats. Brain Res Bull. 2006;68:310–314. doi: 10.1016/j.brainresbull.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Petitclerc M, Bédard PJ, Di Paolo T. Progesterone releases dopamine in male and female rat striatum: a behavioral and microdialysis study. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:491–497. doi: 10.1016/0278-5846(95)00029-u. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 2001;154(3):243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J Stud Alcohol. 2002;63:83–90. [PubMed] [Google Scholar]
- Rivalan M, Grégoire S, Dellu-Hagedorn F. Reduction of impulsivity with amphetamine in an appetitive fixed consecutive number schedule with cue for optimal performance in rats. Psychopharmacology. 2007;192:171–182. doi: 10.1007/s00213-007-0702-6. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Sun WL, Minerly AC, Weierstall K, Nazarian A, Festa ED, Niyomchai T, Akhavan A, Luine V, Jenab S, Quiñones-Jenab V. Progesterone attenuates cocaine-induced conditioned place preference in female rats. Brain Res. 2008;1189:229–235. doi: 10.1016/j.brainres.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Simon JA, Robinson DE, Andrews MC, Hildebrand JR, III, Rocci ML, Jr, Blake ME, Hodgen GD. The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone. Fertil Steril. 1993;60:26–33. [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong KI, Sofuoglu M, Morgan PT, Bergquist KT. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility. Exp Clin Psychopharmacol. 2007;15:445–452. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- Söderpalm AH, Lindsey S, Purdy RH, Hauger R, de Wit H. Administration of progesterone produces mild sedative-like effects in men and women. Psychoneuroendocrinology. 2004;29:339–354. doi: 10.1016/s0306-4530(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav. 2001;69:299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Mooney M. Progesterone effects on subjective and physiological responses to intravenous nicotine in male and female smokers. Hum Psychopharmacol. 2009;24:559–564. doi: 10.1002/hup.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Gonzalez G, Gonsai K, Oliveto A, Kosten TR. Progesterone effects on cocaine use in male cocaine users maintained on methadone: a randomized, double-blind, pilot study. Exp Clin Psychopharmacol. 2007;15:453–460. doi: 10.1037/1064-1297.15.5.453. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State-Trait Anxiety Inventory (“Self-Evaluation Questionnaire”) Palo Alto, CA: Consulting Psychologists Press, Inc; 1970. [Google Scholar]
- Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: influence of sensation-seeking status. Addict Behav. 2007;32:1177–1188. doi: 10.1016/j.addbeh.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies. National Survey on Drug Use and Health. (NSDUH); Rockville, MD: 2008. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies. Treatment Episode Data Set (TEDS); Rockville, MD: 2007. [Google Scholar]
- van Broekhoven F, Bäckström T, Verkes RJ. Oral progesterone decreases saccadic eye velocity and increases sedation in women. Psychoneuroendocrinology. 2006;31:1190–1199. doi: 10.1016/j.psyneuen.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: Relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- White TL, Lejuez CW, de Wit H. Personality and gender differences in effects of d-amphetamine on risk taking. Exp Clin Psychopharmacol. 2007;15:599–609. doi: 10.1037/1064-1297.15.6.599. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America: cross-cultural, age and sex comparisons. J Consult Clin Psychol. 1978;46:139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]