Abstract
Developmental ethanol exposure damages the hippocampus, causing long-lasting learning and memory deficits. Synaptic plasticity mechanisms (e.g. long-term potentiation—LTP), contribute to synapse formation and refinement during development. We recently showed that acute ethanol exposure inhibits glutamatergic synaptic transmission and N-methyl-D-aspartate (NMDA) receptor-dependent LTP in the CA1 hippocampal region of postnatal day (P) 7–9 rats. The objective of this study was to further characterize the effect ethanol on LTP in the developing CA1 hippocampus during the third trimester-equivalent. To more closely model human ethanol exposure during this period, rat pups were exposed to ethanol vapor (2 or 4.5 g/dL in air; serum ethanol concentrations = 96.6 – 147.2 or 322 – 395.6 mg/dL) from P2–9 (4 hrs/day). Brain slices were prepared immediately after the end of the 4-hr exposure on P7–9 and extracellular electrophysiological recordings were performed 1–7 hrs later under ethanol-free conditions to model early withdrawal. LTP was not different than group-matched controls in the 96.6 – 147.2 mg/dL group; however, it was impaired in the 322 – 395.6 mg/dL group. Neither α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/NMDA receptor function nor glutamate release was affected in the 322 – 395.6 mg/dL ethanol exposure group. These data suggest that repeated in vivo exposure to elevated ethanol doses during the third trimester-equivalent period impairs synaptic plasticity, which may alter maturation of hippocampal circuits and ultimately contributeto the long-lasting cognitive deficits associated with fetal alcohol spectrum disorder.
Keywords: Fetal alcohol syndrome, development, plasticity, glutamate, synaptic
Introduction
Ethanol exposure during fetal development can result in a spectrum of clinical findings (termed fetal alcohol spectrum disorder—FASD), ranging from relatively mild central nervous system impairments, such as learning and memory deficits, to the most severe manifestation of the disorder—fetal alcohol syndrome (FAS). The clinical hallmarks of FAS include characteristic facial dysmorphology, growth retardation, and impaired central nervous system function. Ethanol has been shown to affect many parts of the brain, including the hippocampus, a region involved in learning and memory (Goodlett and Johnson, 1997; Hamilton et al., 2003; Livy et al., 2003; O’Leary-Moore et al., 2006; Thomas et al., 2002; Thomas et al., 2004; Uecker and Nadel, 1998). Studies with laboratory animals have shown that exposure during the first and second trimester-equivalents of human pregnancy canproduce long-lasting hippocampal alterations (reviewed in Berman and Hannigan, 2000). In addition, persistent hippocampal dysfunction has been observed in animal models of ethanol exposure during the third trimester-equivalent (approximately postnatal day (P) 0–12 in rats), and this is illustrated by the results of two behavioral studies that assessed mnemonic performance in rats. Goodlett and colleagues (1997) demonstrated impaired place learning and probe trial search patterns in P26–31 rats that were exposed to ethanol on P7–9 (blood ethanol concentration ~265 mg/dL). In addition, Thomas and colleagues (2002) showed that 6 g/Kg of ethanol administered on P6 in a binge-like fashion resulted in impaired performance in a spatial discrimination reversal learning task at P40–42. The developmental mechanisms by which ethanol exposure during the third trimester-equivalent produces these long-lasting deficits in hippocampal function are not fully understood.
During the third trimester-equivalent period, intense glutamatergic synapse formation and refinement occur in the hippocampus and these processes are thought to be mediated by long-term potentiation (LTP)-like, activity-dependent plasticity mechanisms (Constantine-Paton and Cline, 1998; Durand et al., 1996; Lauri et al., 2007; Leinekugel, 2003; Yasuda et al., 2003; Zhu et al., 2000). Experimental evidence suggests that LTP is involved in strengthening of hippocampal synaptic connections, leading to their stabilization during this developmental period (Constantine-Paton and Cline, 1998; Durand et al., 1996; Lauri et al., 2007). We hypothesized that one mechanism by which ethanol could impair hippocampal development is via alterations in synaptic plasticity mechanisms involved in the refinement of neuronal circuitry. This hypothesis is supported by our recent finding that acute ethanol exposure inhibits ionotropic glutamate receptor-mediated responses and LTP induction in the CA1 hippocampal region of P7–9 rat pups (Puglia and Valenzuela, 2010). The objective of this study was to further characterize the effect ethanol on LTP in the developing CA1 hippocampus during the third trimester-equivalent. To more closely model human ethanol exposure during this period, we used an in vivo ethanol vapor exposure paradigm where rat dams and pups were repeatedly exposed to ethanol vapor from P2–9. In this paradigm, blood ethanol concentrations gradually rise and fall, lasting for several hours, as opposed to acute exposure of slices to ethanol where neurons are only briefly exposed to ethanol concentrations that increase and decrease rapidly (Galindo and Valenzuela, 2006). The ethanol vapor exposure paradigm is also advantageous because it minimizes stress in dams and neonates by eliminating the need for maternal separation. Additionally, this paradigm is unlikely to significantly alter maternal care as ethanol levels in the dams are low or undetectable (Galindo and Valenzuela, 2006; Puglia and Valenzuela, 2009). Acute brain slices were prepared from P7–9 rat pups exposed to air or ethanol vapor, and slice electrophysiological techniques were used to investigate LTP and ionotropic glutamate receptor-mediated synaptic transmission.
Experimental Procedures
Ethanol vapor chamber exposure paradigm
Animal procedures were approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center and conformed to National Institutes of Health guidelines. Timed-pregnant Sprague-Dawley rats (gestational days 9–17) were obtained from Harlan Laboratories Inc. (Indianapolis, IN). Neonatal rat pups and dams were exposed to 2 or 4.5 g/dL of ethanol vapor, as previously described (Galindo and Valenzuela, 2006; Puglia and Valenzuela, 2009). Briefly, litters were culled to 10 pups on P2 and dams and rat pups were exposed daily for 4 hrs per day until P9 (Fig. 1A). Exposures were started at 07:00 hrs (lights on at 06:00 hrs and lights off at 18:00 hrs). Ninety-five percent ethanol (Cat #801VWR Tarr, Phoenix, AZ) was vaporized with a heating flask that was regulated with a peristaltic pump. Ethanol vapormixed with air, or air alone, was delivered to the exposure chamber for the ethanol and control groups, respectively. Control and ethanol litters were paired based upon exposure round, where birthdays were 0–3 days apart, and further referred to as pair-matched. The only mortality in any of the 6 pair-matched groups was 3 pups in one litter from the 322 – 395.6 mg/dL ethanol exposure group. Qualitatively, pup motor activity was indistinguishable between the control and the 2 g/dL ethanol group and only slight impairments were observed in the 4.5 g/dL group, consistent with previous observations of resistance to the depressant effects of ethanol in neonatal rats (Fang et al., 1997; Galindo and Valenzuela, 2006; Hollstedt et al., 1980; Silveri and Spear, 1998).
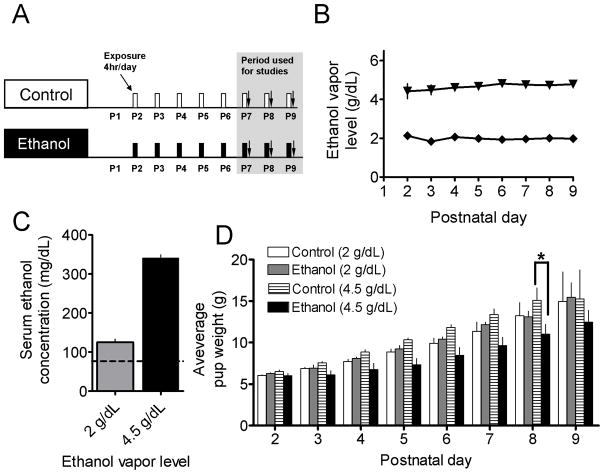
Figure 1.
Third trimester-equivalent ethanol exposure paradigm. (A) Schematic representation of the exposure paradigm where rat pups and their respective dams were repeatedly exposed to ethanol vapor or air (control) for 4 hrs per day, starting on P2 and continuing to P9. Brain slices for electrophysiology studies were prepared from P7, P8 or P9 rat pups, immediately after the 4 hr exposure period. (B) Ethanol concentrations in the exposure (vapor) chamber. Note that in most cases error bars are smaller than the symbol. (C) Serum ethanol concentrations measured from trunk blood samples collected immediately after euthanasia. The dashed line represents the legal intoxication limit (80 mg/dL = 17.4 mM). (D) Average pup weight of control and ethanol vapor-exposed groups as a function of postnatal day. *p<0.05, by two-way ANOVA followed by Bonferroni post hoc test.
Tissue preparation and solutions
Unless otherwise indicated, chemicals were purchased from Sigma (St. Louis, MO) or Tocris Cookson (Ellisville, MO). Rat pups (male and female) were deeply anesthetized with ketamine (250 mg/Kg of body weight) and euthanized by decapitation immediately after the 4 hr exposure to air or ethanol on P7–9. Serum was obtained by collecting trunk blood samples, allowing them to coagulate and centrifuging at 2.3 × g for 15 min at 4° C. Serum ethanol concentrations (SECs) were determined using an alcohol dehydrogenase-based assay as previously described (Puglia and Valenzuela, 2009). Coronal brain slices (400 μm) were prepared using a vibratome, as previously described (Mameli et al., 2005). After a recovery period of 45 min at 35–36°C, slices were maintained for 1–7 hrs at room temperature. Artificial cerebrospinal fluid (ACSF) contained the following (in mM): 126 NaCl, 2 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1 MgSO4, 2 CaCl2, 10 glucose, and 0.01 gabazine (also known as SR-95531) equilibrated with 95%O2/5%CO2. When indicated, the ACSF also contained 50 μM DL-2-amino-5-phosphonovaleric acid (AP5), and/or 10 μM 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX). For NMDA EPSP recordings, the ACSF contained 0 mM Mg2+ and 3 mM Ca2+.
Electrophysiological recordings
Recordings were performed in the CA1 stratum radiatum with a perfusion rate of 2 ml/min at 32°C using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Recording micropipette glass electrodes had resistances of 3–5 MΩ and were filled with ACSF. A concentric bipolar electrode (inner pole 25 μm; outer pole 125 μm; Frederick Haer Company, Bowdoinham, ME) was placed in the vicinity of the Schaffer collateral fibers, approximately 200 μm from the recording electrode, to evoke fEPSPs. Input-output curves were measured at the start of all recordings, and, for subsequent experiments, the stimulation intensity was set at 40–50% of that required to produce maximal responses. Stimulus duration was 75 μs and stimuli were delivered at 0.033 Hz. LTP was induced via tetanic stimulation (100 Hz train for 1 s; this train was repeated a total of 3 times at 5 s intervals). For paired-pulse studies, the interpulse interval was 50 ms.
Data Analysis
Data were acquired and analyzed with the aid of pClamp 9 (Molecular Devices) and GraphPad Prizm 4.0 Softwares (San Diego, CA). The short time interval between the presynaptic volley and the fEPSP prevented accurate measurement of the slope in most experiments; therefore, data were analyzed using the fEPSP amplitude. For each experiment, baseline was defined as the average of 20 fEPSPs immediately before drug application. For LTP quantification, the percent change from baseline was measured after ≥35 min from induction and was the average of 10 fEPSPs.
Statistical analyses of pooled data were performed using GraphPad Prism. A p ≤ 0.05 was considered to be statistically significant. Shown data are presented as mean ± SEM, and the number of determinations (n) represents the number of recordings, which were performed in slices from 6–10 pups from 2–3 different litters per treatment condition.
Results
To model ethanol exposure during the equivalent to the human third trimester of pregnancy, rat dams and pups were exposed to air (control) or ethanol via an inhalation paradigm (Fig 1A). Exposures occurred for 4 hrs/day and chamber ethanol vapor levels were 2 or 4.5 g/dL (Fig. 1B), which yielded SECs of 124.2 ± 7.4 (96.6 – 147.2) and 340.4 ± 8.7 (322 – 395.6) mg/dL, respectively (Fig. 1C). This exposure paradigm did not affect rat pup weight gain in the 96.6 – 147.2 mg/dL group; however, in the 322 – 395.6 mg/dL group, there was a trend towards reduced pup weights that reached significance at P8 (Fig. 1D; p<0.05 by two-way ANOVA followed by Bonferroni post hoc test.).
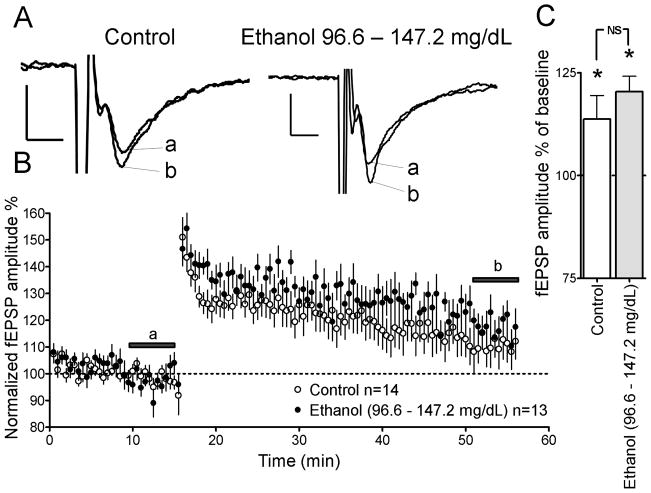
We assessed the effects of repeated in vivo ethanol exposure on LTP in brain slices of P7–9 rat pups in the presence of gabazine (10 μM; Figs. 2–3). In the 96.6 – 147.2 mg/dL ethanol group, a high frequency LTP induction paradigm significantly increased AMPAR-mediated fEPSP amplitude to 120.4 ± 3.7 % of baseline (at t = 50–55 min; Figs. 2A–C; p<0.05 by one-sample t-test from 100%). Group matched controls similarly showed an increase to 113.7 ± 5.7 % in AMPAR-mediated fEPSP amplitude with respect to baseline (at t = 50–55 min; Figs. 2A–C; p<0.05 by one-sample t-test from 100%). The magnitude of LTP induction was not significantly different between 96.6 – 147.2 mg/dL group ethanol group and group-matched controls (Fig. 2C; by unpaired t-test).
Figure 2.
Repeated in vivo ethanol exposure does not inhibit LTP in rat pups with SECs of 96.6 – 147.2 mg/dL. (A) Sample traces of glutamatergic fEPSP-responses from brain slices of control and ethanol-exposed rat pups. Note that letters correspond to time points indicated by the lines in Panel B. Scale bars 0.2 mV/5 ms. (B) Time course of AMPAR-mediated fEPSPs before and after LTP induction in the ethanol and control groups. (C) Summary of AMPAR-mediated fEPSP amplitude after LTP induction (t = 51–56 min). *p<0.05 from one-sample t-test from 100%. NS, not significant by unpaired t-test.
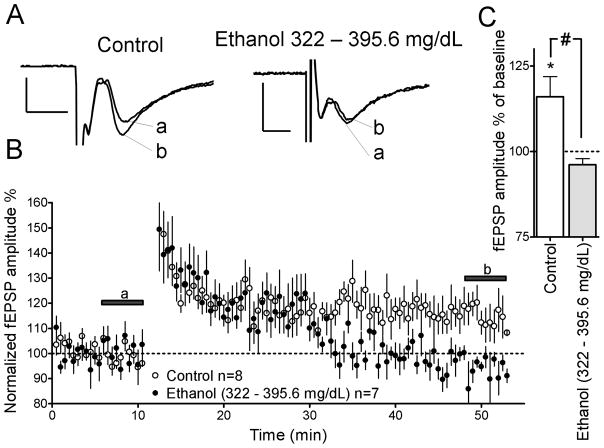
Figure 3.
Repeated in vivo ethanol exposure inhibits LTP in rat pups with SECs of 322 – 395.6 mg/dL. (A) Sample traces of glutamatergic fEPSP-responses from brain slices of control and ethanol-exposed rat pups. Note that letters correspond to time points indicated by the lines in Panel B. Scale bars 0.2 mV/5 ms. (B) Time course of AMPAR-mediated fEPSPs before and after LTP induction in the ethanol and control groups. (C) Summary of AMPAR-mediated fEPSP amplitude after LTP induction (t = 48–53 min). *p<0.05 from one-sample t-test from 100%. #p<0.05 by unpaired t-test.
In rat pups where repeated in vivo ethanol exposure led to SECs of 322 – 395.6 mg/dL, LTP was significantly impaired, with AMPAR-mediated fEPSP amplitude being 96.1 ± 1.8 % of baseline (at t = 50–55 min; Figs. 3A–C; NS from 100% by one sample t-test). Group-matched controls showed a significant increase to 116.0 ± 5.9 % in AMPAR-mediated fEPSP amplitude with respect to baseline (at t = 50–55 min; Figs. 3A–C; p<0.05 by one-sample t-test from 100%). The magnitude of LTP induction was significantly different between the 322 – 395.6 mg/dL ethanol group and group-matched controls (Fig. 3C; p<0.05 by unpaired t-test).
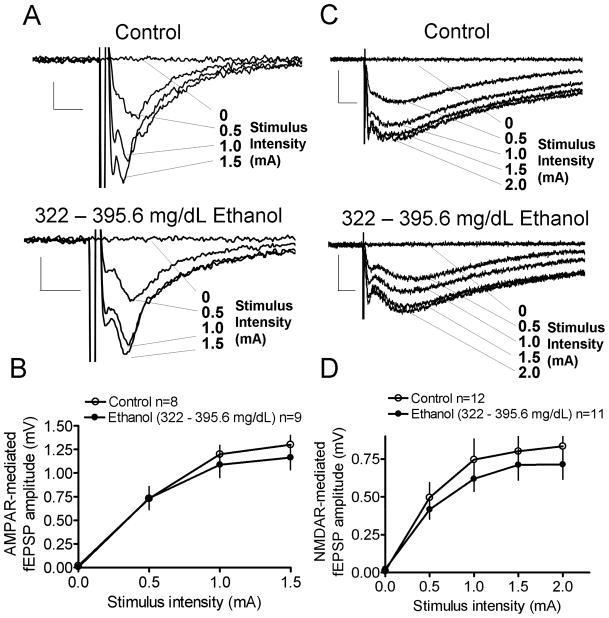
To investigate whether the deficits in LTP induction in the 322 – 395.6 mg/dL ethanol group were a consequence of alterations in ionotropic glutamatergic signaling, NMDAR- and AMPAR-mediated input-output curves were recorded (Fig. 4). AMPAR-mediated input-output curves were recorded in the presence of gabazine (10 μM) and AP5 (50 μM; Fig. 4A). There were no significant differences in the input-output curves between the ethanol and group-matched controls (Fig. 4B; by two-way ANOVA). NMDAR-mediated input-output curves were recorded in the presence of gabazine (10 μM), NBQX (10 μM) and in the absence of Mg 2+ (Fig. 4C). There were no significant differences in the input-output curves between the ethanol and group-matched controls (Fig. 4D; by two-way ANOVA).
Figure 4.
Repeated in vivo ethanol exposure does not alter AMPAR- and NMDAR-mediated input-output curves in rat pups with SECs of 322 – 395.6 mg/dL. Sample traces and input-output curves for AMPAR- (A, B; scale bar = 0.2 mV/5 ms) and NMDAR-mediated fEPSPs (C, D; scale bar = 0.5 mV/25 ms; NS by two-way ANOVA).
Lastly, we investigated whether the impaired LTP in the 322 – 395.6 mg/dL ethanol group was a result of alterations in glutamate release by measuring paired-pulse plasticity of AMPAR-mediated fEPSPs (Valenzuela et al., 2008). The paired-pulse ratio is typically inversely related to the probability of glutamate release (Mameli et al., 2005). Paired-pulse ratios were not significantly different between the ethanol and group-matched controls (Fig 5; by unpaired t-test).
Figure 5.
Repeated in vivo ethanol exposure does not affect AMPAR-mediated fEPSP paired-pulse ratios. Sample traces of control (A) and ethanol (B) groups. Scale bars 0.2 mV/10 ms (C) Scatter plot of paired-pulse ratios (AMPAR-mediated fEPSP2/fEPSP1) (NS by unpaired t-test).
Discussion
This study was undertaken to determine if repeated in vivo ethanol exposure during the third trimester-equivalent period in rat pups alters glutamatergic signaling and plasticity. The main findings of this study are that exposure to high levels of ethanol inhibits LTP in the developing CA1 hippocampal region and that this is not a consequence of alterations in glutamatergic synaptic efficacy or glutamate release.
Ethanol exposure leading to SECs of 96.6 – 147.2 mg/dL did not significantly affect pup weight gain over the course of postnatal days 2–9; however, exposures leading to SECs of 322 – 395.6 mg/dL trended towards reduced weight gain over this period and was significant on P8, potentially modeling one aspect FAS, where growth retardation is a requisite for diagnosis. These results are consistent with previous vapor-inhalation paradigms that caused growth retardation following high levels of ethanol exposure during the third trimester-equivalent period (Bellinger et al., 2002). In contrast, the lower exposure dose did not affect weight, possibly mimicking the less severe end of FASD.
We found that LTP was blunted during the early withdrawal period following repeated ethanol exposure. The blunted LTP was observed only in the ethanol exposure group with SECs of 322 – 395.6 mg/dL, suggesting that relatively high doses of ethanol exposure are needed to impair synaptic plasticity mechanisms during the third trimester-equivalent. These results are consistent with studies of acute ethanol application in brainslices from P7–9 rat pups, where 368 mg/dL (80 mM) application (but not 20 or 40 mM application) inhibited the induction of LTP (Puglia and Valenzuela, 2010). In addition, acute ethanol (276 mg/dL) application inhibited CA1 hippocampal LTP in slices from P15–25 rats, but this effect was not observed in P70–100 rats (Swartzwelder et al., 1995). Furthermore, LTP was not impaired in P40–80 guinea pigs gestationally exposed to ethanol during the third trimester-equivalent period (maternal blood ethanol concentration 245 mg/dL) (Byrnes et al., 2004). Collectively, these studies suggest that LTP is sensitive to acute ethanol exposure in preparations from young rats and that, in the case of neonatal rats, repeated ethanol exposure causes an LTP deficit that persists during withdrawal.
We found no difference in synaptic strength measured by AMPAR- and NMDAR-mediated input-output curves. This was surprising in light of studies showing that NMDAR antagonists (administered during the withdrawal period) were protective in learning tasks of P40–42 rats that were exposed to ethanol during third trimester-equivalent period in a binge like fashion (Thomas et al., 2002; Thomas et al., 2004). These data suggest that the proposed enhanced excitation occurring during the withdrawal period is not observed in our vapor chamber ethanol exposure paradigm, and further indicates that synaptic strength is preserved with our repeated ethanol exposure paradigm, possibly through homeostatic plasticity mechanisms (Carpenter-Hyland and Chandler, 2006; Hou et al., 2008; Swann, 2004; Turrigiano and Nelson, 2004). These homeostatic plasticity mechanisms do not appear to be engaged when ethanol is administered in a binge-like fashion to neonatal rats, where excitotoxicity mediated by NMDAR upregulation is the likely mechanism of action of ethanol (Thomas et al., 2002). Future studies should examine whether exposure paradigms involving gradual administration of ethanol, result in persistent learning and memory alterations that can be ameliorated by NMDAR antagonists. Importantly, previous studies in P45–60 rats exposed to similar levels of ethanol (approximately 368 mg/dL) during the third trimester-equivalent period (P4–9), exhibited a persistent decrease in fEPSP input-output curves in the CA1 hippocampal region of (Bellinger et al., 1999). Taken together with our results, these finding suggest that homeostatic plasticity mechanisms are only able to transiently maintain glutamatergic synaptic strength and that it eventually becomes impaired during the juvenile period of rat development.
The lack of an effect on glutamate release measured by paired-pulse ratios is consistent with our previously reported effects of acute ethanol in the CA1 hippocampal region in brain slices from P7–9 rat pups (Puglia and Valenzuela, 2010). The results of this study, along with those presented here, suggest that acute or repeated ethanol exposure during the third trimester-equivalent period does not affect glutamate release in the CA1 hippocampal region. However, acute ethanol exposure has been shown to inhibit glutamate release in the CA3 hippocampal region of P3–10 rat pups (but not >P15) by means of inhibition of an N-type Ca2+ channel, indicating that there are region specific differences in the effects of ethanol on presynaptic function (Mameli et al., 2005).
We investigated the effects of ethanol on glutamatergic synaptic strength, as well as presynaptic glutamate release, and found that third trimester-equivalent ethanol exposure did not affect either of these parameters. How then might ethanol be impairing LTP? The time-course of LTP showed that for the first 15–20 min after LTP induction, there was no difference between the ethanol (322 – 395.6 mg/dL) and control group; however, after about 20 min, the fEPSPs in the ethanol group rapidly decayed to or below baseline levels, while those in the control group remained potentiated. This indicates that ethanol potentially impairs a process involved in LTP expression and/or maintenance. A candidate factor that has been shown to be involved in LTP induction and maintenance during this developmental period is protein kinase A (PKA) (Yasuda et al., 2003). The dependence of LTP on PKA activity has been shown to be developmentally regulated, where inhibition of this signaling enzyme leads to impaired LTP in P7–9 rat pups, but not in more mature animals (Yasuda et al., 2003). The time course of LTP in the presence of a PKA inhibitor closely resembles the time course of LTP in the ethanol group (322 – 395.6) (Yasuda et al., 2003). Moreover, ethanol has been shown to affect PKA signaling, potentially implicating this molecule as a target of developmental ethanol exposure (Yao et al., 2002). However, other mechanisms of action of ethanol such as altered Ca2+ influx and intracellular signaling pathways, as well as impaired AMPAR trafficking into the postsynaptic density should also be considered (Durand et al., 1996; Elias et al., 2008; Grosshans et al., 2002; Kerchner and Nicoll, 2008). Furthermore, it also remains a possibility that deficits in nourishment may have contributed to the plasticity alterations, as a significant difference in pup weight was found on P8 in the 322 – 395.6 mg/dL ethanol group.
Future studies should address the mechanism by which this third-trimester ethanol exposure paradigm leads to impaired LTP in brain slices from P7–9 rat pups. We chose to focus on the third trimester-equivalent period because it is associated with intense synaptic formation and refinement; however, since women who consume ethanol are likely to do so throughout pregnancy, future studies should model ethanol exposure through all trimesters of pregnancy. Furthermore, previous studies in mature animals have shown that effects of ethanol on GABAergic signaling can play a role in alterations in LTP (Schummers and Browning, 2001). Thus, future studies should address developmental effects of ethanol with GABAergic transmission intact, keeping in mind that GABAA receptors exert dual excitatory and inhibitory effects on hippocampal neurons during the neonatal period of rat development (Ben-Ari et al., 2007).
In conclusion, the studies presented here suggest that a third trimester-equivalent ethanol exposure paradigm leads to impaired synaptic plasticity in the developing CA1 hippocampal region. In addition, these data indicate that not all third trimester-equivalent ethanol exposure paradigms result in enhanced NMDAR function during the withdrawal period. Synaptic plasticity mechanisms are thought to be fundamental for the generation of appropriate synaptic connections during development. Alterations in these processes present one mechanism that could underlie the long-term deficits associated with FASD.
Acknowledgments
This work was supported by NIH grants RO1-AA15614, T32-AA14127, F30-AA017813, and the UNM-SOM M.D./Ph.D program. We thank Drs. L. D. Partridge, K. K. Caldwell, R. H. Glew, and D. J. Vanderjagt for critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Bellinger FP, Bedi KS, Wilson P, Wilce PA. Ethanol exposure during the third trimester equivalent results in long-lasting decreased synaptic efficacy but not plasticity in the CA1 region of the rat hippocampus. Synapse. 1999;31:51–58. doi: 10.1002/(SICI)1098-2396(199901)31:1<51::AID-SYN7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Davidson MS, Bedi KS, Wilce PA. Neonatal ethanol exposure reduces AMPA but not NMDA receptor levels in the rat neocortex. Brain Res Dev Brain Res. 2002;136:77–84. doi: 10.1016/s0165-3806(02)00363-2. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Byrnes ML, Richardson DP, Brien JF, Reynolds JN, Dringenberg HC. Spatial acquisition in the Morris water maze and hippocampal long-term potentiation in the adult guinea pig following brain growth spurt–prenatal ethanol exposure. Neurotoxicol Teratol. 2004;26:543–551. doi: 10.1016/j.ntt.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Homeostatic plasticity during alcohol exposure promotes enlargement of dendritic spines. Eur J Neurosci. 2006;24:3496–3506. doi: 10.1111/j.1460-9568.2006.05247.x. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT. LTP and activity-dependent synaptogenesis: the more alike they are, the more different they become. Curr Opin Neurobiol. 1998;8:139–148. doi: 10.1016/s0959-4388(98)80017-2. [DOI] [PubMed] [Google Scholar]
- Costa ET, Savage DD, Valenzuela CF. A review of the effects of prenatal or early postnatal ethanol exposure on brain ligand-gated ion channels. Alcohol Clin Exp Res. 2000;24:706–715. [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc Natl Acad Sci U S A. 2008;105:20953–20958. doi: 10.1073/pnas.0811025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Ionescu P, Gong D, Kendig J, Harris A, Eger EI., 2nd Maturation decreases ethanol minimum alveolar anesthetic concentration in mice as previously demonstrated in rats: there is no species difference. Anesth Analg. 1997;85:160–163. doi: 10.1097/00000539-199707000-00029. [DOI] [PubMed] [Google Scholar]
- Galindo R, Valenzuela CF. Immature hippocampal neuronal networks do not develop tolerance to the excitatory actions of ethanol. Alcohol. 2006;40:111–118. doi: 10.1016/j.alcohol.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal Binge Ethanol Exposure Using Intubation: Timing and Dose Effects on Place Learning. Neurotoxicol Teratol. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res. 2003;143:85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Hollstedt C, Olsson O, Rydberg U. Effects of ethanol on the developing rat. II. Coordination as measured by the tilting-plane test. Med Biol. 1980;58:164–168. [PubMed] [Google Scholar]
- Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci U S A. 2008;105:775–780. doi: 10.1073/pnas.0706447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Palmer M, Segerstrale M, Vesikansa A, Taira T, Collingridge GL. Presynaptic mechanisms involved in the expression of STP and LTP at CA1 synapses in the hippocampus. Neuropharmacology. 2007;52:1–11. doi: 10.1016/j.neuropharm.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Leinekugel X. Developmental patterns and plasticities: the hippocampal model. J Physiol Paris. 2003;97:27–37. doi: 10.1016/j.jphysparis.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Mameli M, Zamudio PA, Carta M, Valenzuela CF. Developmentally regulated actions of alcohol on hippocampal glutamatergic transmission. J Neurosci. 2005;25:8027–8036. doi: 10.1523/JNEUROSCI.2434-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AE, Krahe TE. Neocortical plasticity deficits in fetal alcohol spectrum disorders: lessons from barrel and visual cortex. J Neurosci Res. 2008;86:256–263. doi: 10.1002/jnr.21447. [DOI] [PubMed] [Google Scholar]
- Medina AE, Krahe TE, Coppola DM, Ramoa AS. Neonatal alcohol exposure induces long-lasting impairment of visual cortical plasticity in ferrets. J Neurosci. 2003;23:10002–10012. doi: 10.1523/JNEUROSCI.23-31-10002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary-Moore SK, McMechan AP, Mathison SN, Berman RF, Hannigan JH. Reversal learning after prenatal or early postnatal alcohol exposure in juvenile and adult rats. Alcohol. 2006;38:99–110. doi: 10.1016/j.alcohol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Puglia MP, Valenzuela CF. Ethanol Acutely Inhibits Ionotropic Glutamate Receptor-Mediated Responses and Long-Term Potentiation in the Developing CA1 Hippocampus. Alcohol Clin Exp Res. 2010;34:594–606. doi: 10.1111/j.1530-0277.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MP, Valenzuela CF. AMPAR-mediated synaptic transmission in the CA1 hippocampal region of neonatal rats: unexpected resistance to repeated ethanol exposure. Alcohol. 2009;43:619–625. doi: 10.1016/j.alcohol.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Browning MD. Evidence for a role for GABA(A) and NMDA receptors in ethanol inhibition of long-term potentiation. Brain Res MolBrain Res. 2001;94:9–14. doi: 10.1016/s0169-328x(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Swann JW. The effects of seizures on the connectivity and circuitry of the developing brain. Ment Retard Dev Disabil Res Rev. 2004;10:96–100. doi: 10.1002/mrdd.20018. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Fleming SL, Riley EP. Administration of low doses of MK-801 during ethanol withdrawal in the developing rat pup attenuates alcohol’s teratogenic effects. Alcohol Clin Exp Res. 2002;26:1307–1313. doi: 10.1097/01.ALC.0000025888.60664.D9. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garcia GG, Dominguez HD, Riley EP. Administration of eliprodil during ethanol withdrawal in the neonatal rat attenuates ethanol-induced learning deficits. Psychopharmacology (Berl) 2004;175:189–195. doi: 10.1007/s00213-004-1806-x. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Uecker A, Nadel L. Spatial but not object memory impairments in children with fetal alcohol syndrome. Am J Ment Retard. 1998;103:12–18. doi: 10.1352/0895-8017(1998)103<0012:SBNOMI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Partridge LD, Mameli M, Meyer DA. Modulation of glutamatergic transmission by sulfated steroids: role in fetal alcohol spectrum disorder. Brain Res Rev. 2008;57:506–519. doi: 10.1016/j.brainresrev.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Arolfo MP, Dohrman DP, Jiang Z, Fan P, Fuchs S, Janak PH, Gordon AS, Diamond I. betagamma Dimers mediate synergy of dopamine D2 and adenosine A2 receptor-stimulated PKA signaling and regulate ethanol consumption. Cell. 2002;109:733–743. doi: 10.1016/s0092-8674(02)00763-8. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]