Abstract
Chronic Hydrocephalus (CH) is often associated with decreased cerebral blood flow (CBF) and oxygen levels. While the exact pathophysiology is not clear, vascular endothelial growth factor (VEGF) and its receptor-2 (VEGFR-2) may be involved. Because the choroid plexus (CP) is involved in cerebrospinal fluid (CSF) production and secretes numerous growth factors including VEGF, it is important to understand VEGF/VEGFR-2 levels in the CP–CSF circulatory system. Our results showed significant decreases in CBF and VEGFR-2 levels in frontal cortex (FC) in CH compared with SC; there were no significant changes in VEGF levels. CBF change in FC was positively correlated with VEGFR-2 levels (P=0.024). Immunohistochemistry (IHC) showed robust expression of VEGF/VEGFR-2 in CP. After CH induction, ventricular CSF volume and VEGF levels significantly increased. These results suggest that the decreased VEGFR-2 levels in FC may be contributed to decreased CBF and increased ventricular CSF-VEGF levels possibly reflected a hypoxic response and/or accumulation of VEGF from CP secretion after blockage of CSF outlet. Further investigation into CSF-VEGF levels in different sites may provide a better understanding of VEGF/VEGFR-2 modulation in the normal and hydrocephalic brain, and may represent a feasible approach to potential therapeutic options for hydrocephalus.
Keywords: Angiogenesis, Canine, Hypoxia, Ischemia
1. Introduction
Chronic hydrocephalus, characterized by obstruction of CSF flow or absorption with consequent ventriculomegaly and/or increased intracranial pressure, may be associated with cognitive dysfunction, impaired gait, and urinary incontinence [1]. Common features of cognitive impairment include a decline in memory, attention, information processing, psychomotor speed, executive functions, and visuospatial and visuoconstructional skills. The decline of this skill set is deemed “fronto-subcortical dementia” due to dysfunction of the frontal lobe and subcortical structures[2, 3], suggesting the frontal cortex is greatly affected in CH.
While the exact pathophysiology is not clearly understood, cognitive deficits observed in CH may be related to direct compression of brain tissue and blood vessels, fiber stretching, or reduced CBF and oxygen levels[4, 5]. Our previous studies showed a short-term decrease in vascularity in the frontal cortex[6] and decreased CBF [7] after induced obstructive hydrocephalus, both of which may contribute to cognitive impairment. These studies also showed a later increase in vascularity above baseline levels suggesting a possible adaptive angiogenesis[6].
Decreased CBF in CH may be related to increased ICP, decreased cardiac output, mechanical compression, or impaired metabolic activity[7, 8], however, the underlying mechanism still remains unclear. Vascular endothelial growth factor (VEGF) and its receptor-2 (VEGFR-2) may play an important role in alteration of vascularity and CBF in CH. As an angiogenic factor, VEGF is crucial for vascular development and maintenance through autocrine and/or paracrine pathways[9–12].
The choroid plexus, which is involved in CSF production and secretes numerous growth factors[13–16], normally have high levels of VEGF[12]. In the CSF, VEGF levels may reflect CP secreted ligand or migration of VEGF from surrounding brain tissue and play an active role in brain tissue angiogenesis. Alternatively, VEGF/VEGFR-2 levels in the CP–CSF circulatory system may reflect the activity of VEGF system in the brain, especially in periventricular areas. Previously, we have shown increased VEGFR-2 levels with corresponding increases in blood vessel density (BVD) in two periventricular areas, i.e. hippocampus and caudate nucleus[4, 17]. We also showed a decrease in short-term vascularity and CBF in frontal cortex, but lacked information concerning VEGF/VEGFR-2 expression after CH[6, 7]. In this study, we employed an experimental model of chronic obstructive hydrocephalus developed and investigated previously in our laboratory[4, 6, 7, 18] to investigate the relationship between CBF and VEGF/VEGFR-2 levels in the frontal cortex. Further, we quantified VEGF/VEGFR-2 levels in the choroid plexus, as well as soluble VEGF levels in ventricular CSF to elucidate the possible role and modulation mechanism of VEGF/VEGFR-2 system in CH.
2. Materials and methods
2.1. Animals
Thirteen young adult, male canines (canis familiaris) approximately 8 to 9 months of age, weighing 25–30 kg were used in this study. Animals were divided into three groups, Surgical Control (SC, 14–16 weeks, n=5), Short Term Chronic Hydrocephalus (ST-CH, 2–4 weeks, n=4), and Long Term Chronic Hydrocephalus (LT-CH, 14–16 weeks, n=4) according to condition and duration of insult. Animals were obtained from licensed suppliers and quarantined for a minimum of seven days before entering into the study. All animals were maintained in the Cleveland Clinic’s fully accredited Animal Care Facility in accordance with Public Health Service policy and the Health Research Extension Act (PL99-158) under the rules and regulations of the Guide for the Care and Use of Laboratory Animals.
2.2. Hydrocephalus induction
The surgical procedure used to induce chronic obstructive hydrocephalus was originally developed [18] and extensively studied [4, 6, 7] in our laboratory. In brief, anesthesia was induced with sodium pentothal and maintained under general gas anesthesia (1.0–1.5% isoflurane). Animals were placed in a prone position in a stereotaxic headframe. Under sterile conditions, a suboccipital craniectomy was performed to allow visualization of the dorsal cerebellar vermis and brainstem. A small opening was made in the dura where a flexible silicon catheter (1.5mm out diameter) was inserted into the fourth ventricle and 0.5 ml of cyanoacrylic gel was injected. The catheter was then cut below the level of the dura and left in position. Dura, muscle, fascia, and skin were sutured in a layered fashion. Surgical control animals were generated using the same procedures as those described for experimental CH animals with the exception of a saline injection into the fourth ventricle rather than cyanoacrylic gel.
2.3. ICP measurement
Intra-operative ICP measures were obtained for all animals at baseline and again at sacrifice as previously described[7, 18]. In brief, animals were placed in a prone position in a stereotaxic headframe and a femoral arterial line was established for monitoring and controlling hemodynamic conditions (PaCO2, 35–45mmHg; pH=7.4). A midline incision was made along the scalp, enabling the retraction of the right temporalis muscle to expose the frontal bone posterior to the coronal suture. Using a small twist-drill, a 5 mm frontal burr hole craniotomy was made above the dorsolateral frontal cortex of the right hemisphere. The dura was opened and ICP microsensor probe (Camino, #110-4BT; Integra NeuroSciences) was inserted approximately 2 mm below the cortical surface. Approximately 30 to 45 minutes were allowed for stabilization before ICP measurements were obtained.
2.4. Cerebral blood flow measurements
In each animal, the left atrium was cannulated (Access Technologies, Skokie, IL, #CNC-7IS) and a subcutaneous portal system (Access Technologies, #CP2) was implanted for CBF microsphere technique as previously described [7]. 4 ml (approximate 10 million) of stable isotope labeled microspheres (BioPal™, Medford, MA) were injected directly into the arterial blood circulation via left atrial access port to determine regional blood flow. At sacrifice, frontal cortex samples (~0.5g) were collected and stored at 4°C until processed by BioPhysics Assay Labs (Worcester, MA).
2.5. Post-operative care
All animals received Dexamethasone, Gentamicin and Cefazolin immediately postoperatively. Medical treatment for increased intracranial pressure included mannitol and acetazolamide. Due to acute fluctuations in intracranial pressure and fluid retention, post-operative orders for i.v. fluids were limited to a rate of 25 ml/hr until the animal was able to drink independently. Routine post-operative care was provided for pain, hydration and infection, and included regular examination of vitals, pupillary reflex, urine production, activity, and leg responsiveness. Neurological status evaluation was performed subjectively. Most animals exhibited signs of lethargy, weakness, and anorexia within a few days to 1 week post induction.
2.6. Magnetic resonance imaging (MRI) and volumetric analysis
Magnetic resonance images were collected prior to CH induction (i.e. baseline) and again at sacrifice for all animals and used to assess the anatomical severity of hydrocephalus. Routine spin-echo magnetic resonance images were acquired using a 1.5 T Siemens vision magnetom and archived onto optical disk for subsequent volumetric analyses. Separate measures for brain and ventricular volume were obtained by manually tracing their contours on approximately 60–80 sections in the coronal plane of 1 mm thick gapless sections from digital images using an image analysis system (Neurolucida, Microbrightfield, version 4.0, Colchester, VT, USA).
2.7. Sacrifice and sample collection
For sacrifice, animals were deeply anesthetized with sodium pentobarbital in combination with inhaled isoflurane and perfused via left atrial port access with 0.9% saline followed by 4% paraformaldehyde (PFA) in 0.1M PBS. The frontal cortex and choroid plexus were extracted en bloc and frozen at −80°C for the study of quantitative Western blot. For immunohistochemistry, the tissue was post-fixed in PFA and cryoprotected in graded sucrose solutions. CSF samples were collected during ICP monitoring at different time points for the study of enzyme immunoassay.
2.8. Immunohistochemistry (IHC)
After post-fixation and cryoprotection in graded sucrose solutions overnight, brains were blocked and frozen in optimal cutting temperature gel (Sakura Finetek Inc., Torrance, CA). Brains were serially sectioned in the coronal plane at 12 μm using a cryostat (Thermo Electron Corp., Cheshire, UK.). The sections were then thaw-mounted onto pre-cleaned superfrost plus micro slides and immunohistochemically stained for VEGF and VEGFR-2 with the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA) according to manufacturer’s instructions. In brief, after rinsing with PBS, sections were incubated in 0.002% proteinase K solution for 10 minutes at 37°C, followed by rinsing again and incubation in 1% H2O2 in methanol for 10 minutes. Tissue sections were then blocked with blocking serum for 20 minutes. Primary antibodies were diluted in blocking solution, including goat anti-canine VEGF (diluted in 15μm/ml, AF1603, R&D Systems, Inc., Minneapolis, MN) and rabbit anti-VEGFR-2 (diluted in 1:125, #2479, Cell Signaling Technology, Inc., Danvers, MA), and applied to sections in a humid chamber on a shaker for one hour at room temperature. Negative controls were performed by applying blocking solution alone to different sections on the same slide.
Sections were washed with PBS before diluted biotinylated secondary antibodies of rabbit anti-goat (for VEGF) or goat anti-rabbit (for VEGFR-2) were applied for 30 minutes at room temperature. Followed by PBS rinses, sections were incubated with avidin-HRP (ABC mix of VECTASTAIN Elite ABC Reagent) for 30 minutes at room temperature. Immunohistochemical staining was visualized with 3′3-diaminobenzidine (DAB) mixed solution (DAB Substrate Kit, Vector Laboratories, Burlingame, CA) for 5–10 minutes. Finally, slides were counterstained with Vector Methyl Green Solution (Vector Laboratories, Burlingame, CA) and coverslipped with Cytoseal-60 mounting medium (Richard-Allan Scientific, Kalamazoo, MI).
To outline vascularity, brains were sectioned in the coronal plane at 40 μm and immunohistochemically stained with VEGFR-2 antibody using the same procedures described above except counterstaining.
2.9. Quantitative western blot
Fresh tissue was snap frozen and preserved in −80°C until use. Tissue was rapidly homogenized and proteins were extracted in extraction buffer (FNN0021, Invitrogen Corporation, Carlsbad, CA). Samples were loaded onto a 4–12% Bis-Tris Gel (NP0335BOX, Invitrogen Corporation, Carlsbad, CA) and electrophoresed for the separation of proteins by SDS-PAGE.
Proteins were transferred onto a polyvinylidene fluoride (PVDF) microporous membrane (LC2005, Invitrogen Corporation, Carlsbad, CA) and probed with antibodies against VEGF (goat antibody, 1:1000 dilution, AF1603, R&D Systems, Inc., Minneapolis, MN) or VEGFR-2 (rabbit antibody, 1:1000 dilution, #2479, Cell Signaling Technology, Inc., Danvers, MA) and with second antibodies of mouse anti-goat IgG –HRP (for VEGF, 1:2000 dilution) or goat anti-rabbit IgG –HRP (for VEGFR-2, 1:5000 dilution, Santa Cruz Biotechnology, CA). The blot was stripped and reprobed with an antibody against tubulin as the protein loading control. The bands were scanned and the protein expression levels were quantitatively analyzed using Quantity One software (Version 4.6.2, Bio-Rad Laboratories, Hercules, CA).
2.10. Enzyme immunoassay
Soluble VEGF levels in ventricular CSF were evaluated using canine VEGF immunoassay kit/Quantikine according to the manufacturer’s instruction (CAVE00, R&D Systems, Inc., Minneapolis, MN). 200 μl of each sample or standards were used per evaluation. All samples and standards were assayed in duplicate and analyzed in 96-well microtiter plates.
2.11. Statistical analyses
All data was presented as mean ± sd and analyzed by ANOVA (one way analysis of variance) followed by subsequent two-tailed unpaired Student’s t-tests. P<0.05 was considered as the level of significance.
3. Results
3.1. Confirmation of hydrocephalic induction
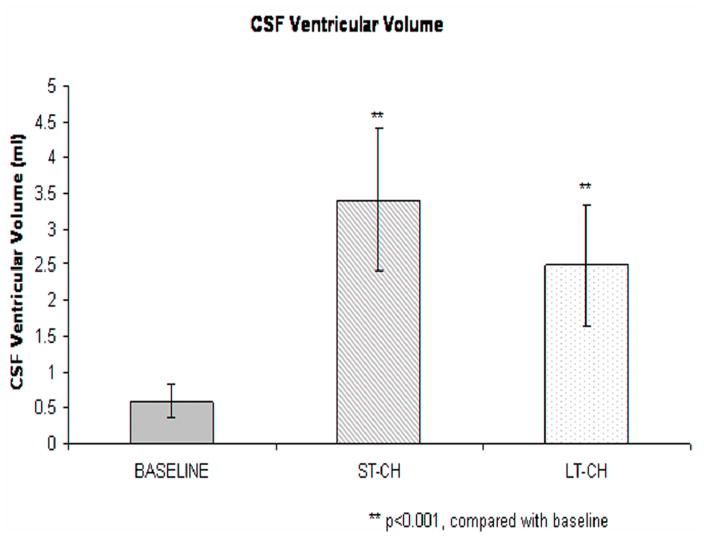
After induction of hydrocephalus, CSF total volume increased approximately twofold in CH animals compared to SC animals (p≤0.01) (Fig. 1). There was no significant difference in CSF volume between ST- and LT-CH groups. Changes in CSF volume in the lateral ventricles that were quantitated from MRI are graphically illustrated in Figure 2 for animals undergoing CH inductive surgery. Overall, after CH induction, CSF volume in the lateral ventricles increased from 0.60±0.24 cm3 (baseline) to 3.41±1.01 cm3 (ST-CH) at 2–4 weeks (P<0.001), then decreased slightly, but remained significantly higher in 14–16 weeks (2.49±0.86cm3, LT-CH) compared with baseline (p<0.001). Compared to significantly increased CSF volume, ICP gradually increased after CH induction as previously shown[7], but was not significantly different between CH and SC groups at the end point.
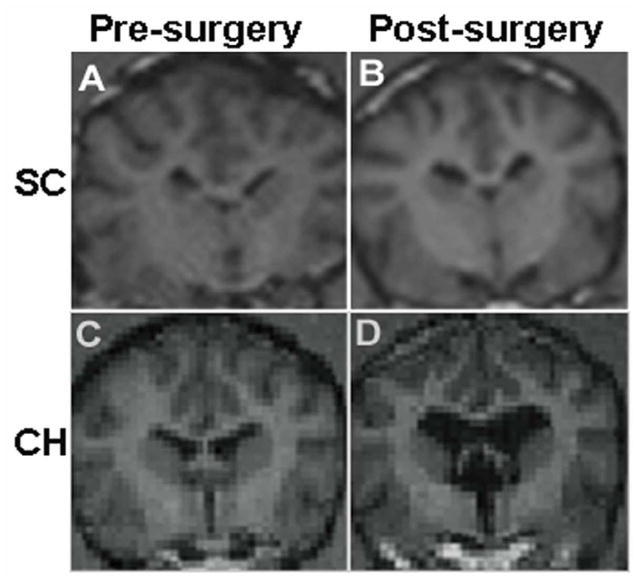
Figure 1.
MRI confirmation of CSF ventricular volume changes in Surgical Control (A, B) and Chronic Hydrocephalus (C, D) animals after CH induction. Overall, CSF volume increased approximately 200% from baseline in CH animals compared to 14% in SC animals as measured via 3D MRI volumetrics.
Figure 2.
Changes in CSF volume in the lateral ventricles for animals undergoing CH induction surgery. CSF volume increased significantly in both short term and long term hydrocephalic animals after CH induction compared with baseline (P<0.001) as measured via 3D MRI volumetrics.
There was no incidence of mortality or morbidity in any animal for either the experimental CH or control groups. Autopsy performed in each animal revealed no evidence of intracerebral or intraventricular bleeding, which corroborated with MRI data.
3.2. Cerebral blood flow in frontal cortex
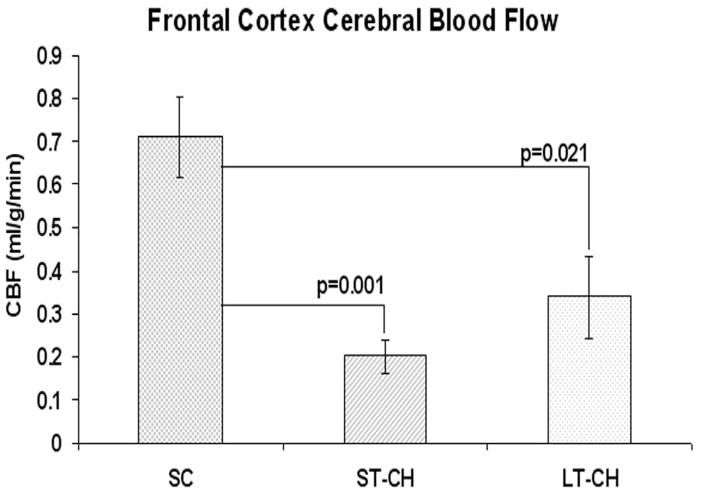
Baseline of CBF in frontal cortex was 0.782±0.251 ml/g.min. After induction of hydrocephalus, CBF in frontal cortex decreased significantly in ST-CH (0.181±0.048 ml/g.min) and LT-CH (0.339±0.095 ml/g.min), but did not change significantly in SC (0.711±0.092 ml/g.min) compared with baseline. Compared to SC animals, CBF in frontal cortex significantly decreased in both ST-CH (P=0.001) and LT-CH (P=0.021) animals, but there was no significant difference between ST-CH and LT-CH (P=0.188) (Fig. 3)
Figure 3.
Cerebral blood flow in frontal cortex after CH induction surgery. Compared with SC, CBF in frontal cortex showed significantly decrease in both ST-CH (P=0.001) and LT-CH (P=0.021). There was no significant difference in frontal cortex CBF between ST-CH and LT-CH (P=0.188).
3.3. VEGFR-2 expression in frontal cortex and VEGFR-2 positive BVD
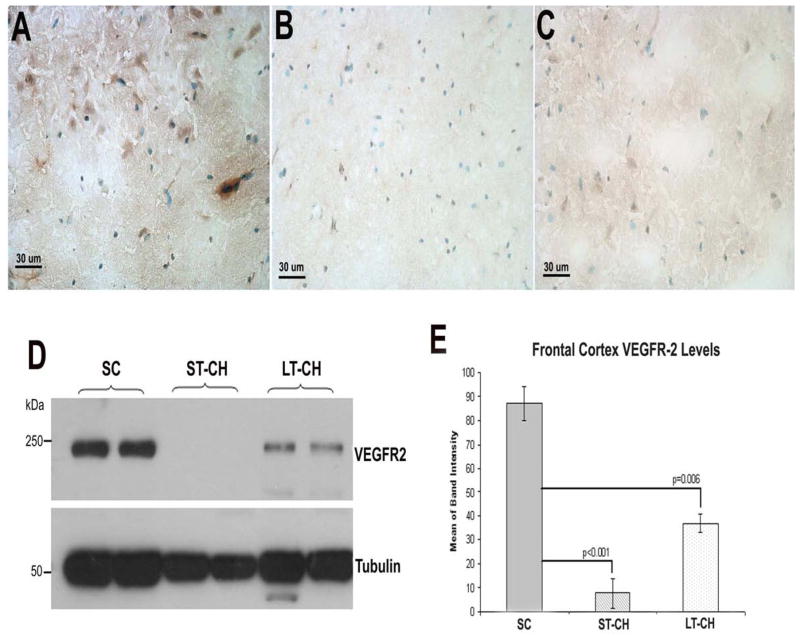
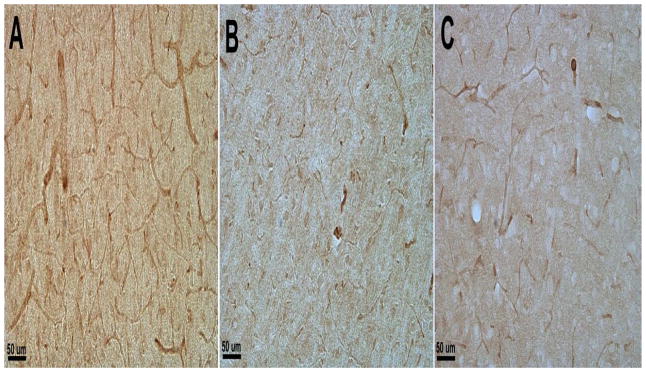
The expression levels of VEGFR-2 in frontal cortex were detected using both immunohistochemistry and Western blot. The band intensities of Western blot were further analyzed quantitatively. Overall, IHC staining with a monoclonal antibody against VEGFR-2 showed decreased staining in ST-CH and LT-CH animals compared with control animals (Fig. 4). Further analysis of VEGFR-2 levels in frontal cortex by Western blot showed a significant decrease in animals of ST-CH (p<0.001) compared with control animals, and slight increase in the LT-CH group, but were still significantly lower than the SC group (p<0.006, Fig. 4D, E). There was no significant difference in VEGFR-2 levels in frontal cortex between ST-CH and LT-CH groups.
Figure 4.
VEGFR-2 expression levels in frontal cortex shown by immunohistochemistry (A–C) and Western blot (D). (E) Quantitative analysis of band intensity in Western blot. Overall, IHC staining with anti-VEGFR-2 antibody showed decreased staining in ST-CH (B) and LT-CH (C) animals compared with SC animals (A). Corresponding to IHC, further analysis of VEGFR-2 expression levels in frontal cortex by Western blot showed a significant decrease in ST-CH (p<0.001) and LT-CH (p<0.006) compared with SC animals (D and E).
Blood vessel density demonstrated by positive VEGFR-2 staining showed decreased vascularity in ST-CH animals compared with SC and LT-CH animals (Fig 5), suggesting decreased VEGFR-2 level may be also related to decreased BVD.
Figure 5.
BVD demonstrated by positive VEGFR-2 staining showed decreased vascularity in ST-CH (B) compared with SC (A) and LT-CH (C) animals.
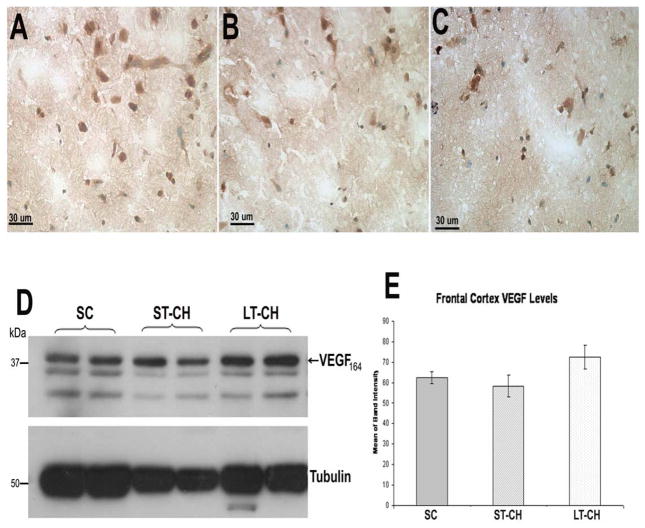
3.4. VEGF expression in frontal cortex
The expression levels of VEGF164 and isoforms in frontal cortex were detected using both immunohistochemistry and Western blot with a canine VEGF antibody. The results of IHC showed similar staining in frontal cortex of animals in SC, ST-CH and LT-CH groups (Fig. 6). Corresponding to IHC, the Western blot analysis demonstrated a similar result; there was no significant difference in VEGF levels in frontal cortex between SC and ST-CH or LT-CH groups (Fig. 6D, E).
Figure 6.
IHC with an antibody against canine VEGF164 showed similar staining in frontal cortex of animals in SC (A), ST-CH (B) and LT-CH (C) groups. Corresponding to IHC, the Western blot analysis demonstrated similar results. There was no significant difference in VEGF levels in frontal cortex between SC and ST-CH or LT-CH groups (D and E).
3.5. VEGF and VEGFR-2 expression in choroid plexus
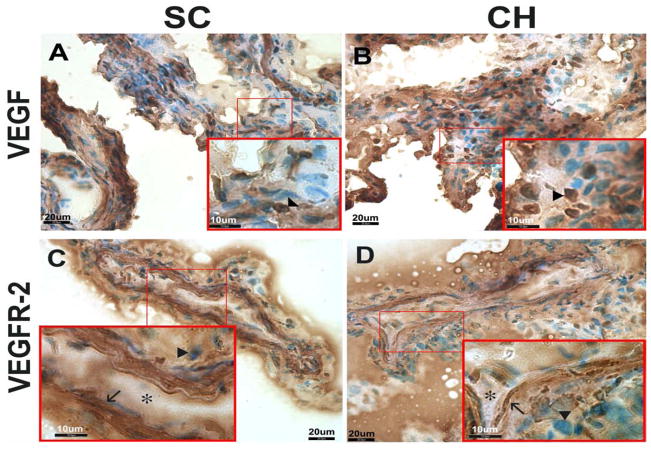
Consistent with previous studies[12, 19], our results showed similar robust expression of VEGF in the cobblestone-shaped epithelial cells[16] in CP in both control and hydrocephalic animals (Fig. 7). Although the CP samples in ST-CH animals were not collected, VEGF levels in CP of SC (Fig. 7A) and LT-CH (Fig. 7B) animals showed similar expression. Corresponding to VEGF, VEGFR-2 levels also showed similar expression in both control (Fig. 7C) and hydrocephalic (Fig. 7D) animals. VEGFR-2 levels showed robust expression in the spindle-shaped endothelial cells[16] compared to epithelial cells.
Figure 7.
IHC photomicrographs of VEGF (A, B) and VEGFR-2 (C, D) expression in choroid plexus. Cobblestone-shaped epithelial cells (arrowheads) showed similar expression of VEGF in both control (A) and hydrocephalic (B) animals. VEGFR-2 showed robust expression in the spindle-shaped endothelial cells (arrows) compared to the epithelial cells (arrowheads, C and D). When comparing control (C) and hydrocephalic (D) animals, VEGFR-2 showed similar expression. Capillary lumina are indicated by asterisks.
3.6. Soluble VEGF levels in ventricular CSF
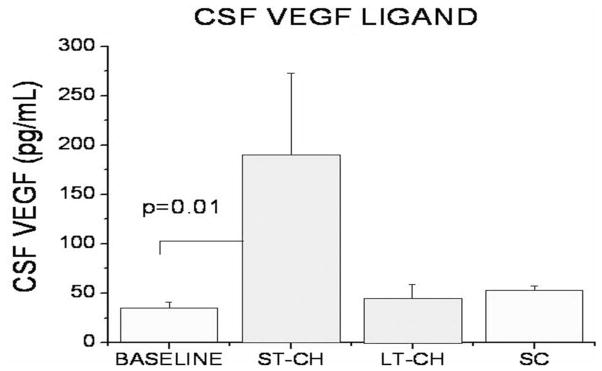
CSF samples of the lateral ventricles were collected prior to CH inductive surgery and immediately before sacrifice in all animals. The samples collected pre-induction served as baseline. The results showed a significant increase in soluble VEGF levels in ventricular CSF of animals in the ST-CH group compared with baseline (p=0.01, Fig. 8). There was no significant difference of soluble VEGF levels in ventricular CSF between baseline and SC or LT-CH animals (Fig. 8).
Figure 8.
The CSF samples collected prior to surgical induction of CH in all animals served as baseline. Results showed a significant increase in soluble VEGF levels in ventricular CSF of ST-CH animals compared with baseline. No significant difference in soluble VEGF levels in ventricular CSF between baseline and SC or LT-CH animals was observed.
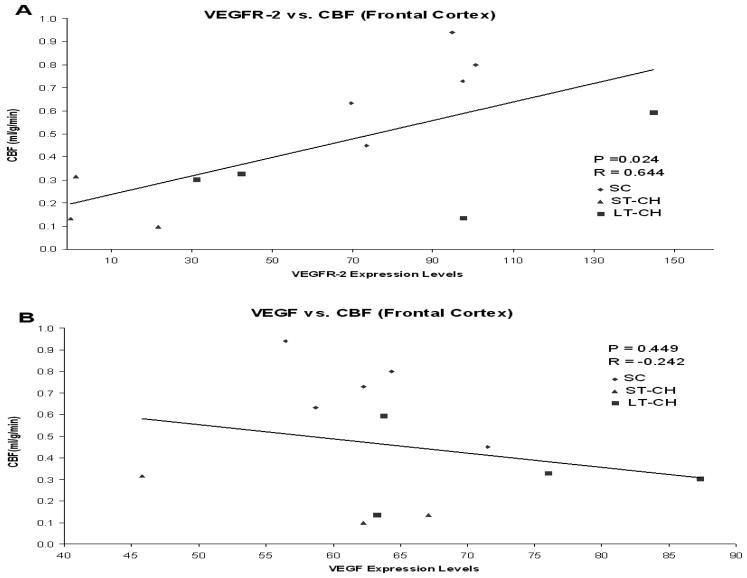
3.7. Relationships between severity of hydrocephalus, CBF, ICP, VEGF and VEGFR-2 expression levels
Cerebral blood flow was directly correlated with VEGFR-2 expression levels in frontal cortex (P=0.024, R=0.644), but did not correlate with VEGF expression levels (Fig. 9). There was no correlation between VEGF and VEGFR-2 expression levels in frontal cortex. In addition, changes in CSF ventricular volume and ICP did not correlate with either cerebral blood flow or VEGF/VEGFR-2 expression levels.
Figure 9.
Scatterplots showing significant correlation between cerebral blood flow and VEGFR-2 expression levels (P=0.024) (A), but no correlation with VEGF expression levels (B) in frontal cortex.
4. Discussion
The present study represents a continuation of our previous investigations of cerebrovascular and CBF changes and VEGF/VEGFR-2 responses in CH[4, 6, 7, 17]. Consistent with our previous finding of decreased CBF[7] and short-term decrease in vascularity[6], the results demonstrated a dramatic decrease in VEGFR-2 levels in frontal cortex of ST-CH compared to surgical control animals, which was correlated with decreased CBF after induced obstructive hydrocephalus. Frontal CBF and VEGFR-2 levels in LT-CH animals were slightly higher when compared to ST-CH, but remained significantly lower than control animals. Interestingly, the VEGF levels in frontal cortex did not show significant differences. However, soluble VEGF levels in ventricular CSF showed significant increases in ST-CH animals. Immunohistochemistry demonstrated robust expression of VEGF and VEGFR-2 in choroid plexus.
We have previously shown increased VEGFR-2 levels with corresponding increases in blood vessel density in periventricular areas of hippocampus and caudate nucleus[4, 17]. We also showed decreased vascularity and CBF in frontal cortex after induced CH and have suggested that these changes may be related to increased ICP, decreased cardiac output, or mechanical compression[6, 7]. However, the blood vessel density changes in chronic hydrocephalus have been observed to be in both directions and hence cannot be explained by simple spatial compression[4, 6, 20]. In the current study, we further explored the underlying mechanisms and demonstrated decreased VEGFR-2 levels, correlating with decreased CBF in frontal cortex after CH induction. Since VEGFR-2 receptors are dense in endothelial cells, the current finding of decreased VEGFR-2 in frontal cortex may be related to the decreased vascularity seen in this area after hydrocephalus. However, the relationship may also be based on VEGFR-2 effect on vascular density. The VEGF family and its receptors are important for vascular development and angiogenesis as well as for the maintenance of blood vessels[9]. Both in vitro and in vivo studies suggest a role for VEGF as a vessel survival factor[12, 21]. Loss of VEGFR-2 results in arrest of vascular and hematopoietic development[22, 23]. VEGF is expressed in many adult tissues including the brain and choroid plexus and acts to stabilize mature blood vessels[12, 24] and maintain vasculature and capillary number[10]. Furthermore, VEGFR-2 in these tissues is constitutively phosphorylated, indicating that VEGF is actively signaling, consistent with its role as a maintenance factor[11]. Overall, the integrity and function of mature blood vessels are dependent, at least in part, on the interactions between the endothelial cells and surrounding mural cells through paracrine VEGF and/or other growth factor signaling[12, 25]. In summary, although the decreased VEGF receptor level in frontal cortex may be the reflection of decreased vascularity, it is also possible that changes in the VEGF system may play an active role in vascularity changes in hydrocephalus.
The choroid plexus plays a key role in setting the extracellular milieu and supporting neuronal function by secreting CSF and various growth factors[13, 26]. Expressional studies of mRNA or protein revealed various growth factors, including VEGF, extant in the choroid plexus[27, 28]. Throughout the brain a low level of VEGF expression was observed in glial cells, whereas higher expressions were detected in the choroid plexus, cerebellum, and olfactory bulb in normoxic condition[29]. Moreover, using VEGF-lacZ adult mice, Maharaj et al. demonstrated that robust VEGF expression was seen in the choroid plexus in epithelial cells, whereas it was nonuniformly expressed in the cerebrum[12]. In addition to higher levels of growth factors in the CP, CSF growth factors secreted by choroid plexus have a role in modulating fluid dynamics in various regions of the developing or injured brain[30]. For example, elevation of TGF-β and FGF-2 in the CNS alters the extracellular milieu and causes hydrocephalus[31–33]. In addition, Alzheimer’s disease (AD) is known to be associated with a number of growth factor abnormalities, which may contribute to the pathogenesis of the hydrocephalus ex vacuo that is characteristically seen in AD[13]. Increased FGF-2 levels were detected in the brain parenchyma of AD patients, but no qualitative changes in the expression of FGF-2 and VEGF were observed in the AD choroid plexus compared to control samples[13, 34]. Though little is known about conditions that alter CSF titers of growth factors, it is likely that the choroid plexus plays a major role in the homeostasis of growth factor concentrations in brain extracellular fluid[13]. Consistent with these results, our results showed robust expression of VEGF and VEGFR-2 in choroid plexus. Even though there is a paucity of information about the presence and nature of growth factors in the choroid plexus, the demonstrated abundance of VEGF in the choroid plexus suggests that it may be secreted for the CP’s own maintenance or for distribution to target cells in the brain parenchyma[13, 29].
VEGF/VEGFR-2, involved in angiogenesis, vascular permeability and mitosis, are up-regulated in various tissues in hypoxic conditions[4, 35]. Although in vitro studies suggested endothelial cells as a production site for VEGF and thus postulated an autocrine mechanism of action, the general concept suggests that VEGF is expressed in parenchymal cells adjacent to vessels to stimulate endothelial cells in a paracrine fashion by means of its receptors[36, 37]. An embryonic study revealed the profile of VEGF expression in the ventricular neuroectoderm and of VEGF receptor expression in brain capillaries[19]. An in vivo study of brain cortical microvessels showed that endothelial cells did not express VEGF mRNA, either under control conditions or under hypoxia. On the other hand, the adjacent glial cells did express VEGF and increased the expression of VEGF and/or VEGFR-2 during hypoxic conditions, suggesting that in vivo VEGF acts in a paracrine rather than autocrine manner[4, 29]. Moreover, VEGF is a readily diffusible molecule[38]; normal serum and saliva contain free VEGF[39, 40], consistent with a potential juxtacrine role for VEGF. Our CP results showed that VEGF was predominantly expressed in epithelial cells, whereas VEGFR-2 expression was concentrated in endothelial cells, suggesting that CP may be a paracrine tissue involved in the regulation of VEGF/VEGF-receptor in the CP tissue and possible remote brain tissue through CSF circulation.
A recent study showed that following hypoxic insult the CP displayed increased expression of VEGF and other factors, as well as increased secretion demonstrated by extrusion of cytoplasmic fragments from the apical cell surfaces into the ventricular lumen[35]. Similarly, our results showed significant increases of VEGF levels in ventricular CSF in ST-CH animals, possibly reflecting the hypoxic response and/or the accumulation of VEGF from CP secretion after blockage of CSF outlet in obstructive hydrocephalus. Further investigation into VEGF levels in CSF from different sites and the regulatory role in its receptors will shed important insight to understand VEGF in adaptive mechanisms and its function in the normal and hydrocephalic brain. It will also allow modifications of therapies aimed at either neutralization or stimulation of the VEGF system.
Acknowledgments
This research was supported by grant from NIH RO1 NS041553-04
Abbreviations
- BVD
Blood Vessel Density
- CBF
Cerebral Blood Flow
- CH
Chronic Hydrocephalus
- CP
Choroid Plexus
- CSF
Cerebrospinal Fluid
- FC
Frontal Cortex
- ICP
Intracranial Pressure
- IHC
Immunohistochemistry
- LT
Long-Term
- MRI
Magnetic Resonance Imaging
- SC
Surgical Control
- ST
Short-Term
- VEGF
Vascular Endothelial Growth Factor
- VEGFR-2
Vascular Endothelial Growth Factor Receptor 2
Footnotes
Disclosure/Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Factora R, Luciano M. Normal pressure hydrocephalus: diagnosis and new approaches to treatment. Clin Geriatr Med. 2006;22:645–57. doi: 10.1016/j.cger.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141–51. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–54. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 4.Dombrowski SM, Deshpande A, Dingwall C, Leichliter A, Leibson Z, Luciano MG. Chronic hydrocephalus-induced hypoxia: increased expression of VEGFR-2+ and blood vessel density in hippocampus. Neuroscience. 2008;152:346–59. doi: 10.1016/j.neuroscience.2007.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owler BK, Pena A, Momjian S, Czosnyka Z, Czosnyka M, Harris NG, et al. Changes in cerebral blood flow during cerebrospinal fluid pressure manipulation in patients with normal pressure hydrocephalus: a methodological study. J Cereb Blood Flow Metab. 2004;24:579–87. doi: 10.1097/00004647-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Luciano MG, Skarupa DJ, Booth AM, Wood AS, Brant CL, Gdowski MJ. Cerebrovascular adaptation in chronic hydrocephalus. J Cereb Blood Flow Metab. 2001;21:285–94. doi: 10.1097/00004647-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Dombrowski SM, Schenk S, Leichliter A, Leibson Z, Fukamachi K, Luciano MG. Chronic hydrocephalus-induced changes in cerebral blood flow: mediation through cardiac effects. J Cereb Blood Flow Metab. 2006;26:1298–310. doi: 10.1038/sj.jcbfm.9600282. [DOI] [PubMed] [Google Scholar]
- 8.Kondziella D, Sonnewald U, Tullberg M, Wikkelso C. Brain metabolism in adult chronic hydrocephalus. J Neurochem. 2008;106:1515–24. doi: 10.1111/j.1471-4159.2008.05422.x. [DOI] [PubMed] [Google Scholar]
- 9.Breen EC. VEGF in biological control. J Cell Biochem. 2007;102:1358–67. doi: 10.1002/jcb.21579. [DOI] [PubMed] [Google Scholar]
- 10.Tang K, Breen EC, Gerber HP, Ferrara NM, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics. 2004;18:63–9. doi: 10.1152/physiolgenomics.00023.2004. [DOI] [PubMed] [Google Scholar]
- 11.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–76. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 12.Maharaj AS, Saint-Geniez M, Maldonado AE, D’Amore PA. Vascular endothelial growth factor localization in the adult. Am J Pathol. 2006;168:639–48. doi: 10.2353/ajpath.2006.050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stopa EG, Berzin TM, Kim S, Song P, Kuo-LeBlanc V, Rodriguez-Wolf M, et al. Human choroid plexus growth factors: What are the implications for CSF dynamics in Alzheimer’s disease? Exp Neurol. 2001;167:40–7. doi: 10.1006/exnr.2000.7545. [DOI] [PubMed] [Google Scholar]
- 14.Johanson CE, Palm DE, Primiano MJ, McMillan PN, Chan P, Knuckey NW, et al. Choroid plexus recovery after transient forebrain ischemia: role of growth factors and other repair mechanisms. Cell Mol Neurobiol. 2000;20:197–216. doi: 10.1023/a:1007097622590. [DOI] [PubMed] [Google Scholar]
- 15.Anthony SG, Schipper HM, Tavares R, Hovanesian V, Cortez SC, Stopa EG, et al. Stress protein expression in the Alzheimer-diseased choroid plexus. J Alzheimers Dis. 2003;5:171–7. doi: 10.3233/jad-2003-5301. [DOI] [PubMed] [Google Scholar]
- 16.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140:947–59. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deshpande A, Dombrowski SM, Leichliter A, Krajcir N, Zingales N, Inoue M, et al. Dissociation between vascular endothelial growth factor receptor-2 and blood vessel density in the caudate nucleus after chronic hydrocephalus. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.98. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MJ, Ayzman I, Wood AS, Tkach JA, Klauschie J, Skarupa DJ, et al. Development and characterization of an adult model of obstructive hydrocephalus. J Neurosci Methods. 1999;91:55–65. doi: 10.1016/s0165-0270(99)00072-2. [DOI] [PubMed] [Google Scholar]
- 19.Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–32. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- 20.Nakada J, Oka N, Nagahori T, Endo S, Takaku A. Changes in the cerebral vascular bed in experimental hydrocephalus: an angio-architectural and histological study. Acta Neurochir (Wien) 1992;114:43–50. doi: 10.1007/BF01401113. [DOI] [PubMed] [Google Scholar]
- 21.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–88. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Edholm D, Lanner F, Breier G, Farnebo F, Dimberg A, et al. Lentiviral rescue of vascular endothelial growth factor receptor-2 expression in flk1-/-embryonic stem cells shows early priming of endothelial precursors. Stem Cells. 2007;25:2987–95. doi: 10.1634/stemcells.2007-0397. [DOI] [PubMed] [Google Scholar]
- 23.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–6. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 24.Ng YS, Rohan R, Sunday ME, Demello DE, D’Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn. 2001;220:112–21. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1093>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 25.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–55. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 26.Spector R, Johanson CE. The mammalian choroid plexus. Sci Am. 1989;261:68–74. doi: 10.1038/scientificamerican1189-68. [DOI] [PubMed] [Google Scholar]
- 27.Ichimiya Y, Emson PC, Northrop AJ, Gilmour RS. Insulin-like growth factor II in the rat choroid plexus. Brain Res. 1988;464:167–70. doi: 10.1016/0169-328x(88)90009-5. [DOI] [PubMed] [Google Scholar]
- 28.Cuevas P, Carceller F, Reimers D, Fu X, Gimenez-Gallego G. Immunohistochemical localization of basic fibroblast growth factor in choroid plexus of the rat. Neurol Res. 1994;16:310–2. doi: 10.1080/01616412.1994.11740245. [DOI] [PubMed] [Google Scholar]
- 29.Marti HH, Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci U S A. 1998;95:15809–14. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez AM, Logan A, Ying W, Lappi DA, Berry M, Baird A. Fibroblast growth factor in the hypothalamic-pituitary axis: differential expression of fibroblast growth factor-2 and a high affinity receptor. Endocrinology. 1994;134:2289–97. doi: 10.1210/endo.134.5.8156932. [DOI] [PubMed] [Google Scholar]
- 31.Aliev G, Miller JP, Leifer DW, Obrenovich ME, Shenk JC, Smith MA, et al. Ultrastructural analysis of a murine model of congenital hydrocephalus produced by overexpression of transforming growth factor-beta1 in the central nervous system. J Submicrosc Cytol Pathol. 2006;38:85–91. [PubMed] [Google Scholar]
- 32.Pearce RK, Collins P, Jenner P, Emmett C, Marsden CD. Intraventricular infusion of basic fibroblast growth factor (bFGF) in the MPTP-treated common marmoset. Synapse. 1996;23:192–200. doi: 10.1002/(SICI)1098-2396(199607)23:3<192::AID-SYN8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Johanson CE, Szmydynger-Chodobska J, Chodobski A, Baird A, McMillan P, Stopa EG. Altered formation and bulk absorption of cerebrospinal fluid in FGF-2-induced hydrocephalus. Am J Physiol. 1999;277:R263–71. doi: 10.1152/ajpregu.1999.277.1.R263. [DOI] [PubMed] [Google Scholar]
- 34.Stopa EG, Gonzalez AM, Chorsky R, Corona RJ, Alvarez J, Bird ED, et al. Basic fibroblast growth factor in Alzheimer’s disease. Biochem Biophys Res Commun. 1990;171:690–6. doi: 10.1016/0006-291x(90)91201-3. [DOI] [PubMed] [Google Scholar]
- 35.Sivakumar V, Lu J, Ling EA, Kaur C. Vascular endothelial growth factor and nitric oxide production in response to hypoxia in the choroid plexus in neonatal brain. Brain Pathol. 2008;18:71–85. doi: 10.1111/j.1750-3639.2007.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ Res. 1995;77:638–43. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 37.Namiki A, Brogi E, Kearney M, Kim EA, Wu T, Couffinhal T, et al. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J Biol Chem. 1995;270:31189–95. doi: 10.1074/jbc.270.52.31189. [DOI] [PubMed] [Google Scholar]
- 38.Dvorak HF, Sioussat TM, Brown LF, Berse B, Nagy JA, Sotrel A, et al. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med. 1991;174:1275–8. doi: 10.1084/jem.174.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hetland ML, Christensen IJ, Lottenburger T, Johansen JS, Svendsen MN, Horslev-Petersen K, et al. Circulating VEGF as a biological marker in patients with rheumatoid arthritis? Preanalytical and biological variability in healthy persons and in patients. Dis Markers. 2008;24:1–10. doi: 10.1155/2008/707864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pammer J, Weninger W, Mildner M, Burian M, Wojta J, Tschachler E. Vascular endothelial growth factor is constitutively expressed in normal human salivary glands and is secreted in the saliva of healthy individuals. J Pathol. 1998;186:186–91. doi: 10.1002/(SICI)1096-9896(1998100)186:2<186::AID-PATH148>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]