Abstract
Objective and design
The pro-oxidative and pro-inflammatory pathways in vascular endothelium have been implicated in the development of atherosclerosis. In the present study, we investigated effect of interleukin-4 (IL-4) on monocyte chemoattractant protein-1 (MCP-1) expression in vascular endothelium and examined the role of distinct sources of reactive oxygen species (ROS) in this process.
Methods and results
Real-time reverse transcriptase-polymerase chain reaction and enzyme-linked immunosorbent assay showed that IL-4 significantly up-regulated mRNA and protein expression of MCP-1 in human aortic endothelial cells (HAEC) and C57BL/6 mice. A significant and dose-dependent inhibition of IL-4-induced MCP-1 expression was observed in HAEC pre-treated with antioxidants, such as pyrrolidine dithiocarbamate and epigallocatechin gallate, indicating that IL-4-induced MCP-1 expression is mediated via a ROS-dependent mechanism. Additionally, pharmacological inhibitors of NADPH oxidase (NOX) significantly attenuated IL-4-induced MCP-1 expression in HAEC. Furthermore, the disruption of the NOX gene dramatically reduced IL-4-induced MCP-1 expression in NOX knockout mice (B6.129S6-Cybbtm1Din/J). In contrast, overexpression of MCP-1 in IL-4-stimulated HAEC was not affected by inhibiting other ROS generating pathways, such as xanthine oxidase and the mitochondrial electron transport chain.
Conclusions
These results demonstrate that IL-4 up-regulates MCP-1 expression in vascular endothelium through NOX-mediated ROS generation.
Keywords: NADPH oxidase, IL-4, MCP-1, Reactive oxygen species, Vascular endothelium
Introduction
Monocyte chemoattractant protein-1 (MCP-1) is the best characterized member of the CC chemokine family and plays a key role in recruitment of monocytes into the arterial wall and transmigration into the subendothelial regions of the vessels. MCP-1 is expressed and released by various types of cells within the vessel wall, including endothelial cells and smooth muscle cells, in response to a number of extracellular stimuli such as cytokines and platelet-derived growth factor (PDGF) [1–6]. Compelling evidence has demonstrated the pivotal role of MCP-1 in the pathogenesis of atherosclerosis. For example, both MCP-1 mRNA and protein expression have been detected in early atherosclerotic lesions by immunostaining, northern blot analysis, and in situ hybridization [7–9]. Additionally, MCP-1 deficiency significantly reduced atherosclerosis in low density lipoprotein (LDL) receptor-deficient mice and human apolipoprotein B (ApoB)-expressing transgenic mice [10, 11]. It was also found that the deletion of CCR2, the corresponding receptor for MCP-1, markedly decreased atherosclerotic lesion formation in ApoE-deficient mice [12]. On the other hand, Aiello et al. [13] reported that enhanced expression of MCP-1 by transplantation of MCP-1 overexpressing bone marrow cells accelerated atherosclerosis in ApoE-deficient mice. Furthermore, human clinical studies have shown that patients with coronary artery disease had significantly elevated MCP-1 serum levels [14]. Finally, anti-MCP-1 gene therapy successfully attenuated atherosclerosis in ApoE-deficient mice [15, 16], suggesting MCP-1 as a potential therapeutic target in atherosclerosis. These studies clearly demonstrate that MCP-1 plays a fundamental role in the pro-inflammatory pathways in vascular endothelium and serves as a contributing factor to atherosclerosis.
Although the contribution of T-helper 1 (Th1) and T-helper 2 (Th2) cell responses to the development of atherosclerosis remains unclear, pro-inflammatory cytokines secreted by Th1 cells (Th1 cytokines) have been implicated in the initiation and progression of atherosclerosis. For example, interferon-γ (INF-γ) is a well-known pro-atherogenic cytokine, and pathophysiological role of INF-γ in atherosclerosis has been extensively investigated both in vitro and in vivo [17]. Recent evidence, however, has shown that the development of atherosclerosis was not completely abolished even though disruption of INF-γ gene significantly diminished the severity of this disease in LDLR-deficient mice [18]. These findings suggest that other pathways may contribute to the disease progression. Indeed, there is evidence that Th2 cells might play a role while little is known concerning the possible contribution of Th2 cytokines to vascular inflammation and atherosclerosis [1, 2, 19–35].
Interleukin-4 (IL-4) is a pleiotropic immunomodulatory cytokine secreted by Th2 cells (Th2 cytokines) and was traditionally considered as an anti-inflammatory cytokine [31, 33]. However, a growing body of evidence has suggested that IL-4 is pro-atherogenic and may play a critical role in the progression of atherosclerosis. For example, recent studies from our laboratory and others have demonstrated that IL-4 induces pro-inflammatory environments by overexpressing a number of pro-inflammatory mediators, such as vascular cell adhesion molecule-1 (VCAM-1), E-selectin, MCP-1, and interleukin-6 (IL-6) in human vascular endothelial cells [1, 2, 22–24, 26–29, 34, 35]. It was also found that IL-4 synergistically increases LL-1β-, TNF-α-, or lipopolysaccharide (LPS)-induced VCAM-1 expression in vascular endothelium [19, 20, 30]. In addition to in vitro cell culture studies, pro-atherogenic effects of IL-4 have been investigated in animal models of atherosclerosis. King et al. [25] have shown that transplantation of bone marrow stem cells isolated from IL-4-deficient (IL-4−/−) mice led to decreased atherosclerotic lesion formation in LDL receptor-deficient (LDLR−/−) mice. Furthermore, a significant reduction in atherosclerotic plaque area was observed in IL-4−/−/ApoE−/− mice compared to ApoE−/− mice [21]. These findings suggest that there may be potential, novel pathways by which IL-4 exerts its pro-atherogenic effects. The molecular signaling mechanisms responsible for IL-4-induced pro-inflammatory pathways in vascular endothelium, however, remain largely unknown.
In the present study, we investigated the effect of IL-4 on MCP-1 expression in vitro and in vivo. We also examined the potential role of distinct sources of reactive oxygen species (ROS) in this process. We demonstrated that IL-4 significantly up-regulates mRNA and protein expression of MCP-1 in vascular endothelium through NADPH oxidase (NOX)-mediated ROS generation.
Materials and methods
Cell culture
Primary human aortic endothelial cells (HAEC) were purchased from Cascade Biologics™ (Portland, OR) and cultured in medium 200 supplemented with low serum growth supplement (LSGS) in a 37°C, 5% CO2/95% air, humidified cell culture incubator.
Interleukin-4
Recombinant human and mouse IL-4 were purchased from R&D Systems Inc. (Minneapolis, MN) and dissolved in sterile phosphate buffered saline (PBS). Based on previous literature data from our group and others [1, 2, 19, 20, 22–24, 26–30, 34–40], concentrations of 0.1–10 ng/ml and 100 µg/kg of IL-4 were employed for in vitro and in vivo studies, respectively.
Animals
Male C57BL/6 mice (6 weeks old) were purchased from Harlan (Indianapolis, IN) and maintained under environmentally controlled conditions, and subject to a 12 h light/dark cycle with food and water ad libitum. Additionally, NOX knockout mice (NOX gp91phox−/− or B6.129S6-Cybbtm1Din/J, male, 6 weeks old) and matched wild-type controls with the same genetic background (C57BL/6J, male, 6 weeks old) were obtained from The Jackson Laboratory (Bar Harbor, ME). Animals (n = 4–5) received a single intraperitoneal injection of either PBS or 100 µg/kg of IL-4, and were humanely killed by CO2 inhalation. The aortas from mice in each group were collected and dissected gently free of adhering tissues. Isolated aortic samples were frozen and stored at −80°C until analysis. In addition, blood was obtained by cardiac puncture, and blood plasmas were prepared, aliquoted, frozen, and stored at −80°C. Freshly thawed blood plasmas were analyzed immediately. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85–23, revised 1996) and this study was approved by the Virginia Tech Institutional Animal Care and Use Committee (IACUC).
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
The aortas of mice were homogenized with 1.6 mm stainless steel beads and 1 ml of TRI Reagent (Sigma-Aldrich, St. Louis, MO) using a setting of 8 for 15 min in a tissue homogenizer (The Bullet Blender™, Next Advance Inc., Averill Park, NY), and total RNA was isolated from tissue homogenates as described previously [35, 41]. In cell culture studies, total RNA was isolated from HAEC using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the protocol of the manufacturer. One µg of total RNA was reverse-transcribed at 25°C for 15 min, 42°C for 45 min, and 99°C for 5 min in 20 µl of 5 mM MgCl2, 10 mM Tris–HCl, pH 9.0, 50 mM KCl, 0.1% Triton X-100, 1 mM dNTP, 1 U/µl of recombinant RNasin ribonuclease inhibitor, 15 U/µg of AMV reverse transcriptase, and 0.5 µg of random hexamers. Amplifications of individual genes were performed on ABI 7300 Sequence Detection System (Applied Biosystems, Foster City, CA) using TaqMan® Universal PCR Master Mix, gene-specific TaqMan PCR probes and primers, and a standard thermal cycler protocol (50°C for 2 min before the first cycle, 95°C for 15 s, and 60°C for 1 min, repeated 45 times). For specific probes and primers of PCR amplifications, TaqMan® Gene Expression Assay Reagents for human MCP-1, human glyceraldehyde-3-phosphate dehydrogenase (GAPDH), mouse MCP-1, and mouse GAPDH were obtained from Applied Biosystems. The threshold cycle (CT), which indicates the fractional cycle number at which the amount of amplified target gene reaches a fixed threshold, from each well was determined by the Applied Biosystems Sequence Detection Software v1.2.3. Relative quantification, which represents the change in gene expression from real-time quantitative PCR experiments between the treated group and untreated control group, was calculated by the comparative CT method as described previously [35, 42–44]. The data were analyzed using the equation 2−ΔΔCT, where ΔΔCT = [CT of target gene − CT of housekeeping gene]treated group − [CT of target gene − CT of housekeeping gene]untreated control group. For the treated samples, evaluation of 2−ΔΔCT indicates the fold change in gene expression, normalized to a housekeeping gene (GAPDH), and relative to the untreated control.
Enzyme-linked immunosorbent assay (ELISA)
MCP-1 concentrations in cell culture supernatants, mouse aortic homogenates, and mouse blood plasmas were measured with a Mouse Quantikine® ELISA Kit (R&D Systems Inc.).
Monocyte migration assay
Migration of monocytes was determined by QCM™ Chemotaxis 5 µm 96-Well Cell Migration Assay Kit (Millipore, Billerica, MA) as we described previously [43]. It provides a quick and efficient system for quantitative determination of monocyte/macrophage migration. The migratory cells on the bottom of the insert membrane were dissociated from the membrane when incubated with cell detachment buffer. These cells were subsequently lysed and detected by the CyQuant GR dye. This green fluorescent dye exhibits strong fluorescence enhancement when bound to cellular nucleic acids. The fluorescence was measured by a fluorescence multi-well plate reader with excitation and emission wavelengths of 480 and 520 nm.
Detection of ROS
The intracellular levels of ROS were measured by DCF fluorescence staining using a Zeiss AXIO Imager A1m fluorescence microscope equipped with AxioCam MRc5 Digital Imaging System (Carl Zeiss MicroImaging, Inc., Thornwood, NY). Briefly, HAEC were grown on the glass slide in the Lab-Tek® II Chamber Slide™ System (Nalge Nunc International Corp., Naperville, IL). After treatment with IL-4, the cells were loaded with carboxy-H2DCF-DA (Invitrogen Corp., Carlsbad, CA) at a concentration of 5 µM in PBS for 30 min at 37°C in 5% CO2/95% air, humidified cell culture incubator. HAEC monolayers were washed with PBS and examined on a Zeiss AXIO Imager A1m fluorescence microscope. Cell images were acquired with 20× objective by AxioCam MRc5 Digital Imaging System.
Statistical analysis
Statistical analysis of data was completed using SigmaStat 3.5 (Systat Software, Inc., Point Richmond, CA). One-way ANOVA was used to compare mean responses among the treatments. For each endpoint, the treatment means was compared using the Bonferroni least significant difference procedure. Differences among the means were considered significant at p < 0.05.
Results
IL-4 up-regulates MCP-1 expression in HAEC and mice
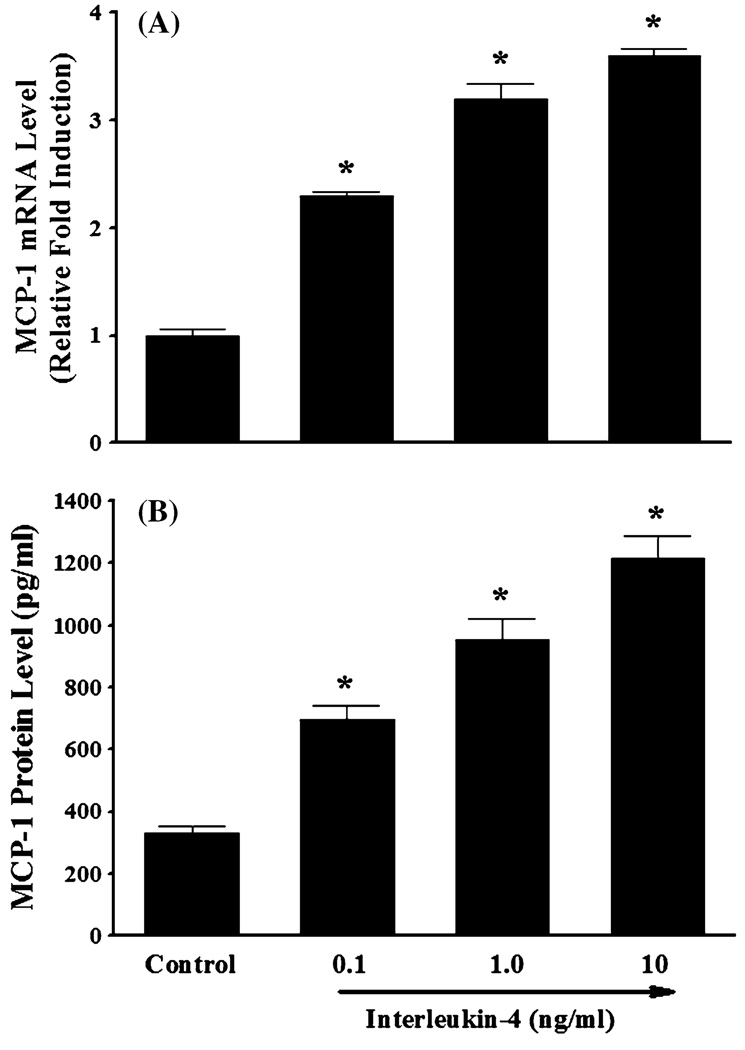
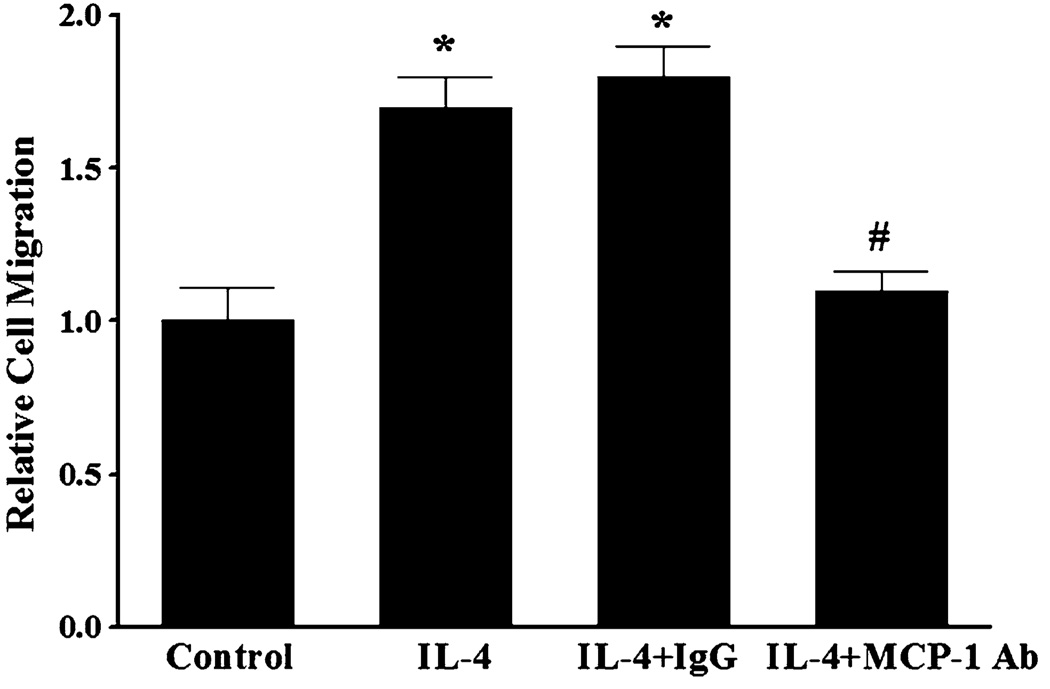
The recruitment of blood leukocytes and their migration throughout the vascular endothelium are thought to be critical early pathologic events in atherogenesis. These processes are directly facilitated by enhanced expression of pro-inflammatory chemokines and chemokine receptors in activated endothelial cells and leukocytes [45–49]. In the present study, we investigated the effect of IL-4 on MCP-1 expression in HAEC and monocyte migration. Quantitative real-time RT-PCR showed that treatment of HAEC with increasing concentrations of IL-4 (0.1, 1.0, and 10 ng/ml) significantly and dose-dependently up-regulated mRNA expression of MCP-1 (Fig. 1a). Consistent with the data on gene expression, exposure of HAEC to IL-4 resulted in a significant and dose-dependent up-regulation of MCP-1 protein expression (Fig. 1b). To verify the functional integrity of MCP-1 induced by IL-4-activated HAEC, monocyte migration assays were conducted. As shown in Fig. 2, treatment of HAEC with IL-4 significantly increased the migration of THP-1 human monocytic leukemia cells. To examine the critical role of MCP-1 in IL-4-induced monocyte migration, conditioned media (CM) from IL-4-treated HAEC were pre-incubated with either non-immune isotype control IgG (negative control) or neutralizing anti-MCP-1 antibody. Enhanced migration of THP-1 cells was significantly attenuated by pre-incubation of the CM with neutralizing anti-MCP-1 antibody, while it was not affected by pre-incubation of the CM with nonimmune isotype control IgG (Fig. 2). These data clearly demonstrated that IL-4-induced MCP-1 expression in HAEC is critically involved in stimulation of monocyte migration.
Fig. 1.
IL-4 up-regulates mRNA and protein expression of MCP-1 in human aortic endothelial cells. HAEC were treated with either PBS (control) or indicated concentrations of IL-4 for 4 h (a) or 16 h (b). The mRNA and protein expression levels of MCP-1 were determined by real-time RT-PCR (a) and ELISA (b). Values represent mean ± SEM (n = 4). *p < 0.05 versus control
Fig. 2.
Treatment of human aortic endothelial cells with IL-4 stimulates monocyte migration. Conditioned media (CM) were collected 16 h after treatment of HAEC with either PBS (control) or 10 ng/ml of IL-4. CM from IL-4-treated HAEC were pre-incubated with 10 µg/ml of non-immune isotype control IgG2B (negative control) (IL-4 + IgG) or 10 µg/ml of neutralizing anti-MCP-1 antibody (IL-4 + MCP-1 Ab) for 1 h at 37°C. Chemotactic activities of CM were measured by migration of THP-1 cells using the Chemicon 96-Well Cell Migration Assay Kit. Values represent mean ± SEM (n = 4). *p < 0.05 versus control; #p < 0.05 versus IL-4
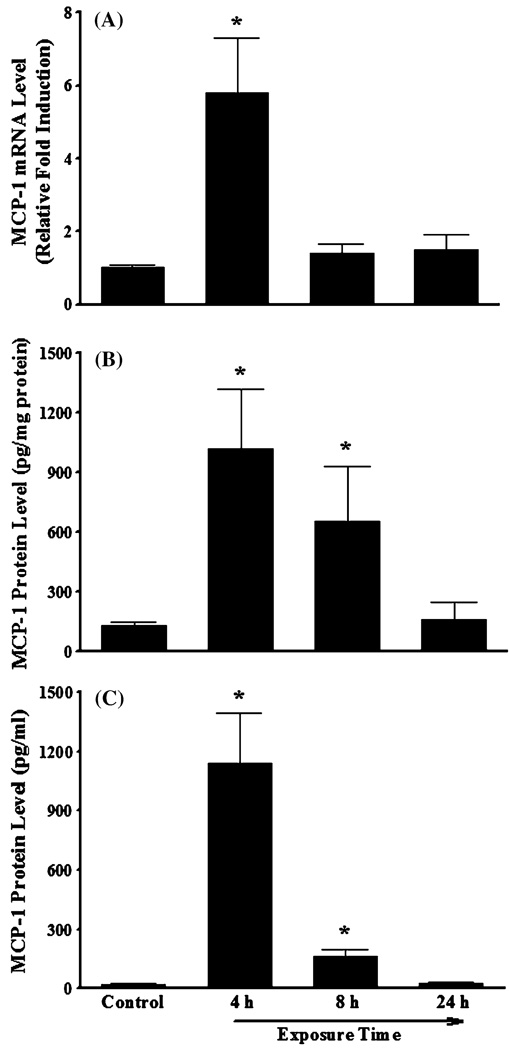
We also performed a series of animal experiments to examine whether IL-4 induces MCP-1 expression in vivo. As depicted in Fig. 3a, real-time RT-PCR analysis demonstrated a significant up-regulation of MCP-1 mRNA expression in mouse aortas collected 4 h after intraperitoneal administration of 100 µg/kg of IL-4. In addition, a significantly enhanced expression of MCP-1 protein was observed in both aortas and blood plasmas collected from mice 4 to 8 h after IL-4 injection (Fig. 3b, c). These results provide the first direct evidence demonstrating that IL-4 induces MCP-1 expression in both HAEC and mice.
Fig. 3.
IL-4 up-regulates mRNA and protein expression of MCP-1 in vivo. Mice were administered a single intraperitoneal injection of either PBS (control) or 100 µg/kg of IL-4, and exposed for 4, 8, and 24 h. The mRNA and protein expression levels of MCP-1 in mouse aortas (a, b) and blood plasma (c) were determined by real-time RT-PCR (a) and ELISA (b, c). Values represent mean ± SEM (n = 4). *p < 0.05 versus control
Antioxidants attenuate IL-4-induced MCP-1 expression in HAEC
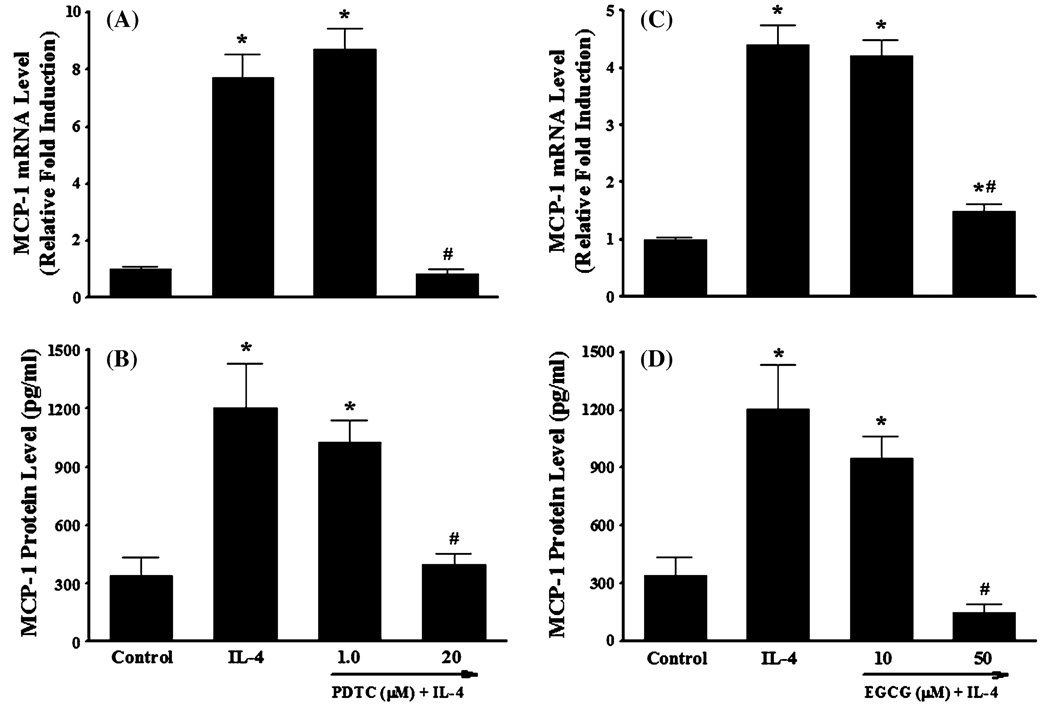
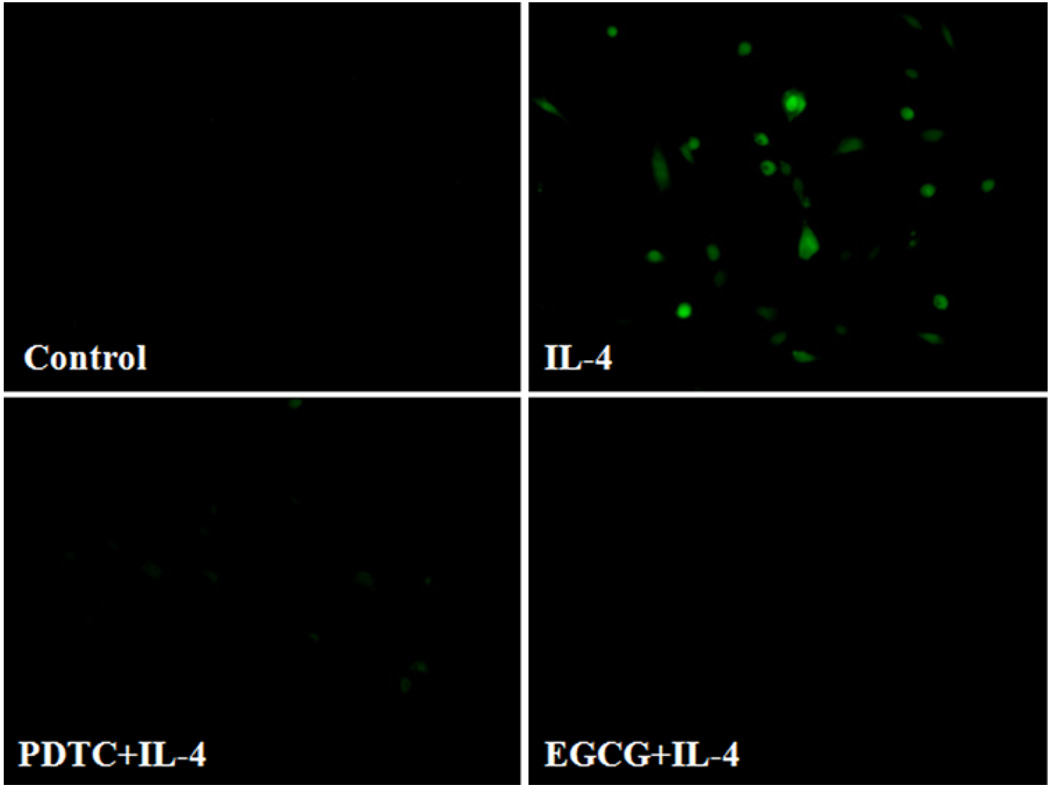
It is generally accepted that ROS play a critical role in atherogenesis by regulating the expression of pro-inflammatory mediators such as cytokines, chemokines, and adhesion molecules in vascular endothelium [1, 3, 28, 34, 35, 50]. To determine whether IL-4-induced MCP-1 expression in HAEC is mediated through ROS generation, we examined the effect of antioxidants on mRNA and protein expression of MCP-1 in IL-4-treated HAEC. Pre-treatment of HAEC with the antioxidant pyrrolidine dithiocarbamate (PDTC) markedly attenuated IL-4-mediated up-regulation of MCP-1 mRNA and protein expression (Fig. 4a, b). A similar effect was also observed in IL-4-activated HAEC pre-treated with epigallocatechin gallate (EGCG) (Fig. 4c, d). Both PDTC and EGCG have been frequently used as antioxidant compounds to investigate redox regulation of the intracellular signaling pathways and of cell function [1, 28, 35, 51, 52]. In addition, we employed DCF fluorescence assay to examine if the antioxidants used in the present study decrease the IL-4-induced ROS generation in HAEC. As illustrated in Fig. 5, IL-4-mediated increase in ROS production was completely abolished by pre-treatment of HAEC with antioxidants such as PDTC and EGCG. These results suggest that IL-4-induced MCP-1 expression in HAEC is mediated via a ROS-dependent mechanism.
Fig. 4.
Antioxidants attenuate IL-4-induced MCP-1 expression in human aortic endothelial cells. HAEC were pre-treated with indicated concentrations of PDTC or EGCG for 1 h and then exposed to 10 ng/ml of IL-4 for 4 h (a, c) or 16 h (b, d). The mRNA and protein expression levels of MCP-1 were determined by real-time RT-PCR (a, c) and ELISA (b, d). Values represent mean ± SEM (n = 4). *p < 0.05 versus control; #p < 0.05 versus IL-4
Fig. 5.
Antioxidants attenuate IL-4-induced ROS generation in human aortic endothelial cells. HAEC were pre-treated with 20 µM PDTC or 50 µM EGCG for 30 min, and then incubated with 10 ng/ml of IL-4 for 60 min. Intracellular levels of ROS generation were determined by DCF fluorescence staining. Results are representative of four independent experiments
Role of distinct sources of ROS in IL-4-induced MCP-1 expression
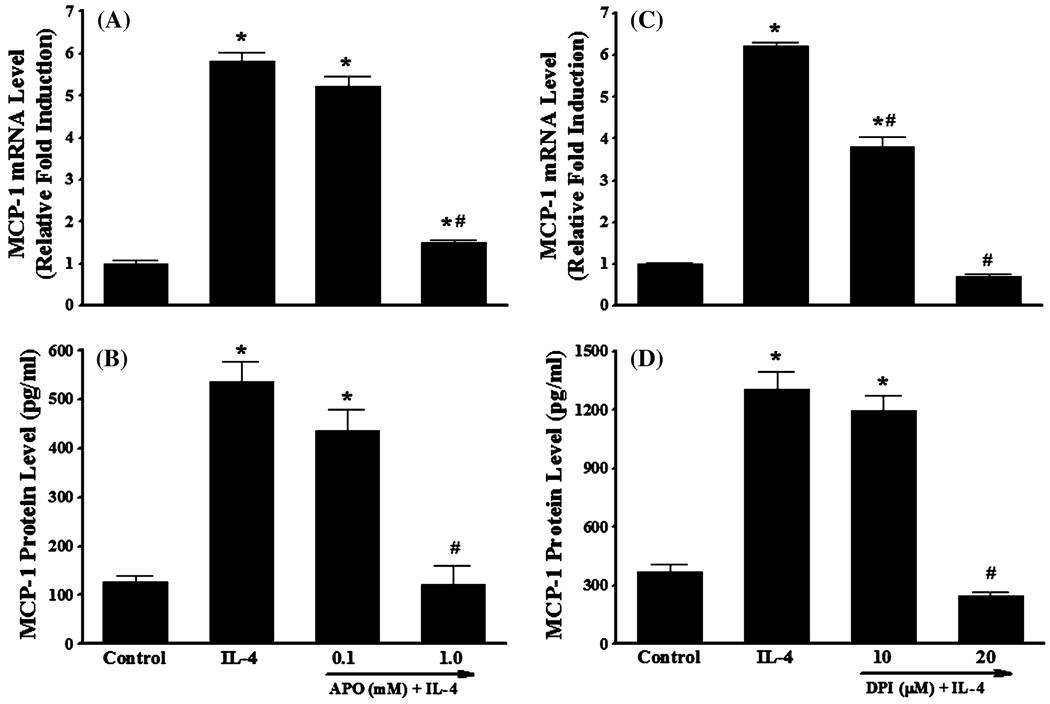
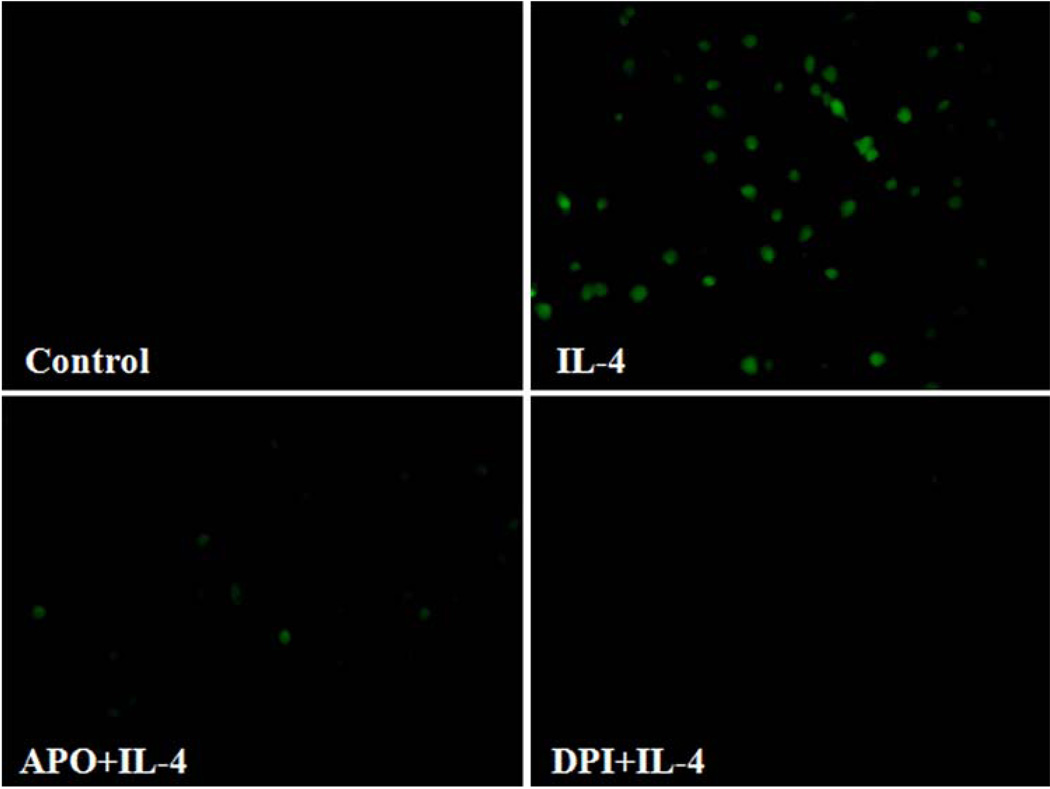
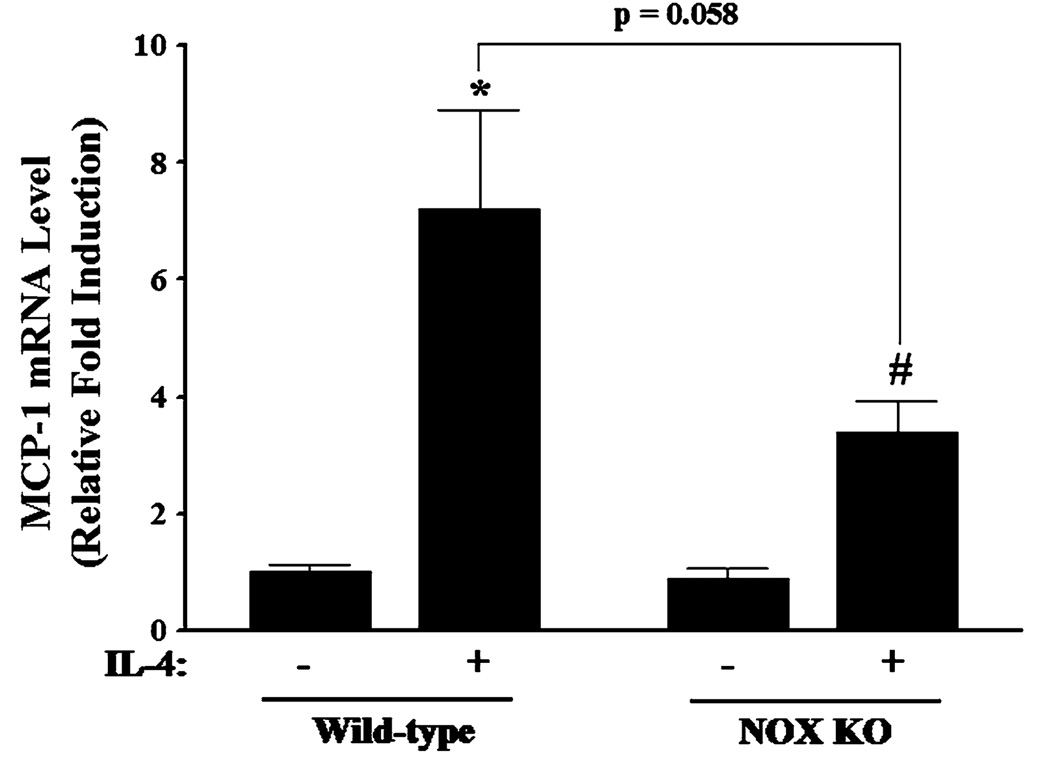
Previous studies have shown that NOX is a key source of enzymatic generation of ROS in a variety of cell types including endothelial cells [53]. To determine whether NOX is involved in IL-4-induced MCP-1 expression in vascular endothelium, HAEC were pre-treated with apocynin, an inhibitor of NOX, for 30 min and then further incubated with 10 ng/ml of IL-4. Figure 6 depicted that inhibition of NOX by apocynin significantly attenuated IL-4-induced MCP-1 mRNA and protein expression in HAEC (Fig. 6a, b). We also employed diphenylene iodonium (DPI) to further elucidate the potential role of NOX in IL-4-induced MCP-1 expression in HAEC. As demonstrated in Fig. 6c, d, a significant reduction of IL-4-mediated up-regulation of MCP-1 mRNA and protein expression was observed in HAEC pre-treated with increasing concentrations of DPI (10 and 20 µM). In addition, pre-treatment of HAEC with NOX inhibitors such as apocynin and DPI markedly suppressed IL-4-induced ROS generation as assessed by DCF fluorescence staining (Fig. 7), indicating that IL-4 up-regulates MCP-1 expression in HAEC through NOX-mediated ROS generation. Furthermore, NOX knockout mice and matched wild-type controls were used to examine the crucial role of NOX in IL-4-induced MCP-1 expression in vivo. Consistent with the data on IL-4-induced up-regulation of MCP-1 expression in mice (Fig. 3), IL-4 administration resulted in a significant increase in the mRNA expression of MCP-1 in wild-type mice. On the other hand, the disruption of NOX gp91phox gene markedly attenuated IL-4-induced MCP-1 expression in NOX knockout mice (Fig. 8). These results strongly suggest that NOX plays a pivotal role in IL-4-induced MCP-1 expression in vitro and in vivo.
Fig. 6.
NADPH oxidase inhibitors attenuate IL-4-induced MCP-1 expression in human aortic endothelial cells. HAEC were pre-treated with indicated concentrations of apocynin (APO) or diphenylene iodonium (DPI) for 30 min, and then incubated with 10 ng/ml of IL-4 for 4 h (a, c) or 16 h(b, d). The mRNA and protein expression levels of MCP-1 were determined by real-time RT-PCR (a, c) and ELISA (b, d). Values represent mean ± SEM (n = 4). *p < 0.05 versus control; #p < 0.05 versus IL-4
Fig. 7.
NADPH oxidase inhibitors attenuate IL-4-induced ROS generation in human aortic endothelial cells. HAEC were pre-treated with 1.0 mM apocynin (APO) or 20 µM diphenylene iodonium (DPI) for 30 min, and then incubated with 10 ng/ml of IL-4 for 60 min. Intracellular levels of ROS generation were determined by DCF fluorescence staining. Results are representative of four independent experiments
Fig. 8.
Effect of IL-4 on MCP-1 expression in wild-type and NADPH oxidase knockout (NOX KO) mice. Mice were administered a single intraperitoneal injection of either PBS or 100 µg/kg of IL-4, and exposed for 4 h. The mRNA expression levels of MCP-1 in mouse aortas were determined by real-time RT-PCR. Values represent mean ± SEM (n = 5). *Statistically significant compared with the wild-type control (p < 0.05). #Statistically significant compared with NOX KO control (p < 0.05)
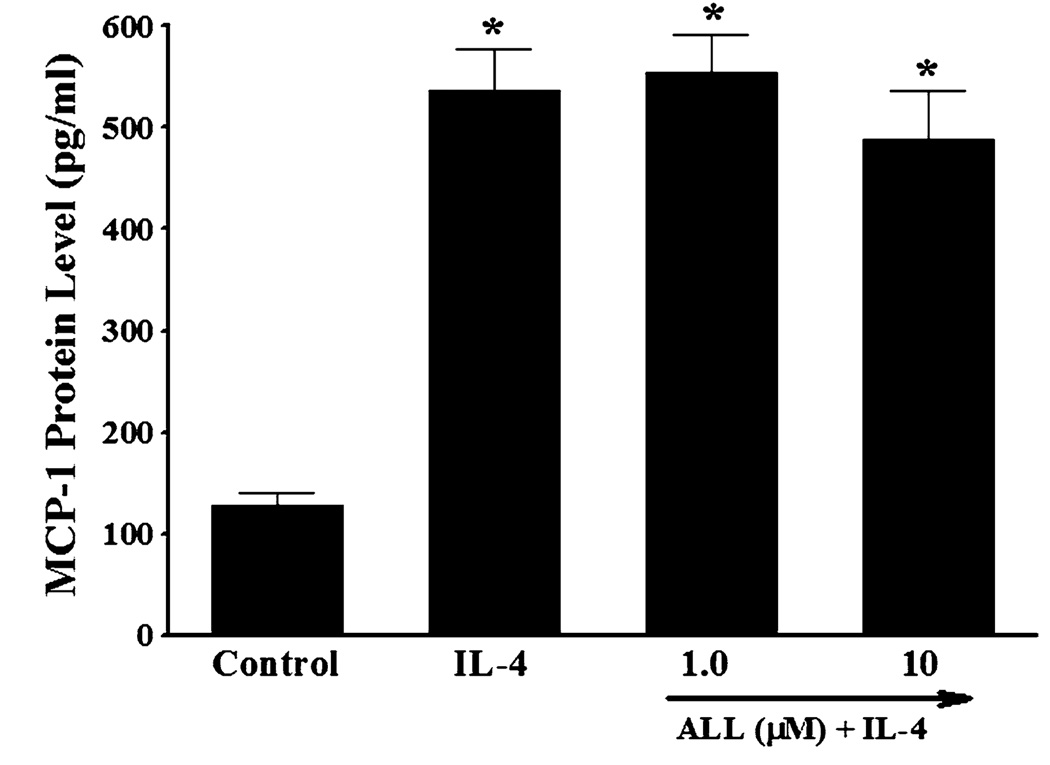
Another enzymatic source of ROS generation is xanthine oxidase [53]. To investigate the potential contribution of xanthine oxidase-mediated ROS generation to MCP-1 expression in IL-4-activated vascular endothelium, HAEC were pre-treated with allopurinol, an inhibitor of xanthine oxidase, for 30 min and then incubated with 10 ng/ml of IL-4 for 16 h. Pre-treatment of HAEC with allopurinol at 1.0 and 10 µM did not affect IL-4-mediated up-regulation of MCP-1 expression (Fig. 9), indicating that xanthine oxidase-mediated ROS generation is not associated with IL-4-induced MCP-1 expression in HAEC.
Fig. 9.
Effect of xanthine oxidase inhibitor on IL-4-induced MCP-1 expression in human aortic endothelial cells. HAEC were pre-treated with indicated concentrations of allopurinol (ALL) for 30 min, and then incubated with 10 ng/ml of IL-4 for 16 h. The protein expression levels of MCP-1 were determined by ELISA. Values represent mean ± SEM (n = 4). *p < 0.05 versus control
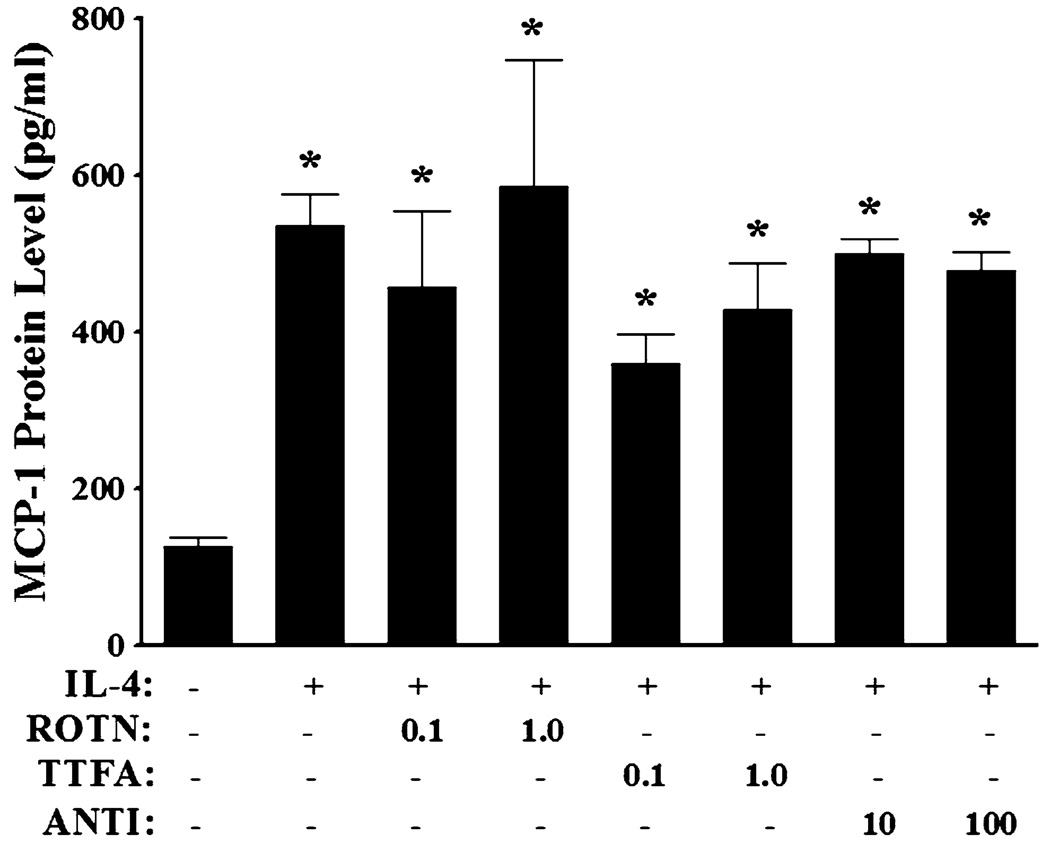
It is generally accepted that the mitochondrial electron transport chain is another major source of intracellular ROS production [53]. To determine the involvement of mitochondria in ROS-mediated MCP-1 overexpression in response to IL-4, HAEC were pre-treated with the mitochondrial electron transport chain inhibitors, such as rotenone, thenoyltrifluoroacetone (TTFA), or antimycin A, for 30 min to selectively block the electron flows between mitochondrial respiratory chain complexes, and then incubated with 10 ng/ml of IL-4 for 16 h. As shown in Fig. 10, inhibitions of mitochondrial electron transport chain did not exert any significant effects on MCP-1 expression in IL-4-activated HAEC. These results suggest that mitochondrial electron transport chain-mediated ROS generation is not involved in IL-4-induced MCP-1 expression in HAEC.
Fig. 10.
Effect of selective inhibitors of mitochondrial electron transport chain on IL-4-induced MCP-1 expression in human aortic endothelial cells. HAEC were pre-treated with rotenone (ROTN; 0.1 and 1.0 µM), thenoyltrifluoroacetone (TTFA; 0.1 and 1.0 µM), or antimycin A (ANTI; 10 and 100 µM) for 30 min, and then incubated with 10 ng/ml of IL-4 for 16 h. The protein expression levels of MCP-1 were determined by ELISA. Values represent mean ± SEM (n = 4). *p < 0.05 versus control
Discussion
The present study demonstrated that IL-4 induces MCP-1 expression in human aortic endothelial cells and mouse aortas. However, we cannot exclude possible involvement of other cell types, such as smooth muscle cells and macrophages, in IL-4-induced MCP-1 expression in aortas. Therefore, we are currently conducting a series of immunofluorescence staining with dual-labeling procedures to further investigate the potential contribution of specific type of cells, such as endothelial cells, smooth muscle cells, and macrophages, to the induction of MCP-1 expression in mouse aortas after treatment with IL-4.
Oxidative stress has been implicated in a number of inflammatory vascular diseases including atherosclerosis [3, 54]. For example, increased production of superoxide was observed in atherosclerotic human coronary arteries [55, 56]. It has become apparent that endothelial dysfunction is closely associated with an increased risk of atherosclerosis and vascular endothelial cells are particularly sensitive to oxidative stress [57, 58]. Heitzer et al. [59] demonstrated that elevated production of vascular ROS is linked to impaired endothelial function and progression of atherosclerosis in patients with coronary artery disease. In addition, oxidative stress up-regulates expression of pro-inflammatory mediators such as cytokines, chemokines, and adhesion molecules in vascular endothelium, which is one of the earliest steps in the development of atherosclerotic lesion formation [3, 60, 61].
Recent studies by our group and others highlight that IL-4 may be considered a pro-oxidative cytokine which increases the oxidizing potential of target cells [1, 28, 29, 35, 62]. For example, treatment with IL-4 resulted in a dose-dependent increase in intracellular ROS and subsequent overexpression of redox-responsive genes such as VCAM-1 and MCP-1 in human umbilical vein endothelial cells [1, 26, 28]. The distinct sources of ROS involved in the IL-4-induced MCP-1 expression, however, have not been reported. Therefore, we examined effects of various pharmacological inhibitors of ROS generating pathways, such as NOX, xanthine oxidase, and the mitochondrial electron transport chain, on IL-4-induced MCP-1 expression in HAEC. We used apocynin and allopurinol to selectively inhibit NOX and xanthine oxidase. Additionally, we employed rotenone as an inhibitor of complex I which blocks the electron flow from NADH dehydrogenase (complex I) to ubiquinone. TTFA was used as a complex II inhibitor which interferes with the electron transport from succinate dehydrogenase (complex II) to ubiquinone. We also used antimycin A to selectively inhibit the electron flow at complex III. Among these ROS generating pathways, the present study demonstrated that the inhibition of NOX by apocynin significantly attenuated IL-4-induced MCP-1 expression in HAEC while it was not affected by inhibiting other ROS generating pathways, such as xan-thine oxidase and the mitochondrial electron transport chain. The potential role of NOX in IL-4-induced MCP-1 expression was also confirmed by pre-treatment of HAEC with DPI, a structurally unrelated NOX inhibitor. Even though pharmacological inhibitors of NOX have been widely used in a number of previous in vitro and in vivo studies [32, 53, 63], it should be noted that they are not highly selective or specific. In addition to the pharmacological approach using NOX inhibitors such as apocynin and DPI, the genetic approach with NOX knockout mice further provided robust evidence in support of a central role for NOX in IL-4-induced MCP-1 expression in vascular endothelium. These findings suggest that at least one distinct ROS generating pathway mediates MCP-1 induction in human aortic endothelial cells and mouse aortas by IL-4.
In conclusion, we have demonstrated the first direct evidence indicating that IL-4 significantly up-regulates mRNA and protein expression of MCP-1 in vascular endothelium through NOX-dependent pathways. The present study will contribute to a better understanding of the molecular signaling mechanisms by which IL-4 mediates vascular inflammation and development of atherosclerosis. It will also provide insights to novel therapeutic approaches for atherosclerosis specifically targeted against pro-oxidative and pro-inflammatory pathways in vascular endothelium.
Acknowledgments
This study was supported in part by Grants from National Institutes of Health/National Heart, Lung, and Blood Institute (HL085229) and National Science Foundation Macromolecular Interfaces with Life Sciences-Integrative Graduate Education and Research Traineeship (MILES-IGERT).
Contributor Information
Yong Woo Lee, Laboratory of Vascular Biology, Department of Biomedical Sciences and Pathobiology, School of Biomedical Engineering and Sciences, Virginia Polytechnic Institute and State University (Virginia Tech), ICTAS Building (MC-0298), Stanger Street, Blacksburg, VA 24061, USA, ywlee@vt.edu; School of Biomedical Engineering and Sciences, Virginia Polytechnic Institute and State University (Virginia Tech), Blacksburg, VA 24061, USA.
Won Hee Lee, School of Biomedical Engineering and Sciences, Virginia Polytechnic Institute and State University (Virginia Tech), Blacksburg, VA 24061, USA.
Paul H. Kim, Laboratory of Vascular Biology, Department of Biomedical Sciences and Pathobiology, School of Biomedical Engineering and Sciences, Virginia Polytechnic Institute and State University (Virginia Tech), ICTAS Building (MC-0298), Stanger Street, Blacksburg, VA 24061, USA
References
- 1.Lee YW, Hennig B, Toborek M. Redox-regulated mechanisms of interleukin-4-induced MCP-1 expression in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2003;284:H185–H192. doi: 10.1152/ajpheart.00524.2002. [DOI] [PubMed] [Google Scholar]
- 2.Rollins BJ, Pober JS. Interleukin-4 induces the synthesis and secretion of MCP-1/JE by human endothelial cells. Am J Pathol. 1991;138:1315–1319. [PMC free article] [PubMed] [Google Scholar]
- 3.Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13:1231–1238. [PubMed] [Google Scholar]
- 4.Strieter RM, Wiggins R, Phan SH, Wharram BL, Showell HJ, Remick DG, et al. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989;162:694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- 5.Taubman MB, Rollins BJ, Poon M, Marmur J, Green RS, Berk BC, et al. JE mRNA accumulates rapidly in aortic injury and in platelet-derived growth factor-stimulated vascular smooth muscle cells. Circ Res. 1992;70:314–325. doi: 10.1161/01.res.70.2.314. [DOI] [PubMed] [Google Scholar]
- 6.Wung BS, Cheng JJ, Hsieh HJ, Shyy YJ, Wang DL. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ Res. 1997;81:1–7. doi: 10.1161/01.res.81.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991;88:1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yla-Herttuala S, Lipton BA, Rosenfeld ME, Sarkioja T, Yoshimura T, Leonard EJ, et al. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci USA. 1991;88:5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeya M, Yoshimura T, Leonard EJ, Takahashi K. Detection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Hum Pathol. 1993;24:534–539. doi: 10.1016/0046-8177(93)90166-e. [DOI] [PubMed] [Google Scholar]
- 10.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 11.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 13.Aiello RJ, Bourassa PK, Lindsey S, Weng W, Natoli E, Rollins BJ, et al. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1518–1525. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]
- 14.Martinovic I, Abegunewardene N, Seul M, Vosseler M, Horstick G, Buerke M, et al. Elevated monocyte chemoattractant protein-1 serum levels in patients at risk for coronary artery disease. Circ J. 2005;69:1484–1489. doi: 10.1253/circj.69.1484. [DOI] [PubMed] [Google Scholar]
- 15.Ni W, Egashira K, Kitamoto S, Kataoka C, Koyanagi M, Inoue S, et al. New anti-monocyte chemoattractant protein-1 gene therapy attenuates atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2001;103:2096–2101. doi: 10.1161/01.cir.103.16.2096. [DOI] [PubMed] [Google Scholar]
- 16.Inoue S, Egashira K, Ni W, Kitamoto S, Usui M, Otani K, et al. Anti-monocyte chemoattractant protein-1 gene therapy limits progression and destabilization of established atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2002;106:2700–2706. doi: 10.1161/01.cir.0000038140.80105.ad. [DOI] [PubMed] [Google Scholar]
- 17.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 18.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-γ on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 19.Barks JL, McQuillan JJ, Iademarco F. TNF-α and IL-4 synergistically increase vascular cell adhesion molecule-1 expression in cultured vascular smooth muscle cells. J Immunol. 1997;159:4532–4587. [PubMed] [Google Scholar]
- 20.Blease K, Seybold J, Adcock IM, Hellewell PG, Burke-Gaffney A. Interleukin-4 and lipopolysaccharide synergize to induce vascular cell adhesion molecule-1 expression in human lung microvascular endothelial cells. Am J Respir Cell Mol Biol. 1998;18:620–630. doi: 10.1165/ajrcmb.18.5.3052. [DOI] [PubMed] [Google Scholar]
- 21.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galéa P, Thibault G, Lacord M, Bardos P, Lebranchu Y. IL-4, but not tumor necrosis factor-alpha, increases endothelial cell adhesiveness for lymphocytes by activating a cAMP-dependent pathway. J Immunol. 1993;151:588–596. [PubMed] [Google Scholar]
- 23.Hong HY, Lee HY, Kwak W, Yoo J, Na MH, So IS, et al. Phage display selection of peptides that home to atherosclerotic plaques: IL-4 receptor as a candidate target in atherosclerosis. J Cell Moll Med. 2008;12:2003–2014. doi: 10.1111/j.1582-4934.2008.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H, Lavoie-Lamoureux A, Moran K, Lavoie JP. IL-4 stimulates the expression of CXCL-8, E-selectin, VEGF, and inducible nitric oxide synthase mRNA by equine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1147–L1154. doi: 10.1152/ajplung.00294.2006. [DOI] [PubMed] [Google Scholar]
- 25.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb Vasc Biol. 2002;22:456–461. doi: 10.1161/hq0302.104905. [DOI] [PubMed] [Google Scholar]
- 26.Lee YW, Eum SY, Chen KC, Hennig B, Toborek M. Gene expression profile in interleukin-4-stimulated human vascular endothelial cells. Mol Med. 2004;10:19–27. doi: 10.2119/2004-00024.lee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YW, Hirani AA. Role of interleukin-4 in atherosclerosis. Arch Pharm Res. 2006;29:1–15. doi: 10.1007/BF02977462. [DOI] [PubMed] [Google Scholar]
- 28.Lee YW, Kühn H, Hennig B, Neish AS, Toborek M. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J Mol Cell Cardiol. 2001;33:83–94. doi: 10.1006/jmcc.2000.1278. [DOI] [PubMed] [Google Scholar]
- 29.Lee YW, Kühn H, Kaiser S, Hennig B, Daughterty A, Toborek M. Interleukin 4 induces transcription of the 15-lipoxygenase I gene in human endothelial cells. J Lipid Res. 2001;42:783–791. [PubMed] [Google Scholar]
- 30.Masinovsky B, Urdal D, Gallatin WM. IL-4 acts synergistically with IL-1β to promote lymphocyte adhesion to microvascular endothelium by induction of vascular cell adhesion molecule-1. J Immunol. 1990;145:2886–2895. [PubMed] [Google Scholar]
- 31.Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–1870. [PubMed] [Google Scholar]
- 32.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ Res. 2001;89:408–411. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 33.Rocken M, Racke M, Shevach EM. IL-4-induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease. Immunol Today. 1996;17:225–331. doi: 10.1016/0167-5699(96)80556-1. [DOI] [PubMed] [Google Scholar]
- 34.Walch L, Massade L, Dufilho M, Brunet A, Rendu F. Pro-atherogenic effect of interleukin-4 in endothelial cells: modulation of oxidative stress, nitric oxide and monocyte chemoattractant protein-1 expression. Atherosclerosis. 2006;187:285–291. doi: 10.1016/j.atherosclerosis.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Lee YW, Lee WH, Kim PH. Oxidative mechanisms of IL-4-induced IL-6 expression in vascular endothelium. Cytokine. 2010;49:73–79. doi: 10.1016/j.cyto.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee YW, Kühn H, Hennig B, Daughterty A, Toborek M. IL-4 induces apoptosis of endothelial cells through the caspase-3-dependent pathway. FEBS Lett. 2000;485:122–126. doi: 10.1016/s0014-5793(00)02208-0. [DOI] [PubMed] [Google Scholar]
- 37.Sasaguri T, Arima N, Tanimoto A, Shimajiri S, Hamada T, Sasaguri Y. A role for interleukin 4 in production of matrix metalloproteinase 1 by human aortic smooth muscle cells. Atherosclerosis. 1998;138:247–253. doi: 10.1016/s0021-9150(97)00296-7. [DOI] [PubMed] [Google Scholar]
- 38.Chen CC, Manning AM. TGF-β1, IL-10 and IL-4 differentially modulate the cytokine-induced expression of IL-6 and IL-8 in human endothelial cells. Cytokine. 1996;8:58–65. doi: 10.1006/cyto.1995.0008. [DOI] [PubMed] [Google Scholar]
- 39.Bennett BL, Cruz R, Lacson RG, Manning AM. Interleukin-4 suppression of tumor necrosis factor α-stimulated E-selectin gene transcription is mediated by STAT6 antagonism of NF-κB. J Biol Chem. 1997;272:10212–10219. doi: 10.1074/jbc.272.15.10212. [DOI] [PubMed] [Google Scholar]
- 40.Wright PS, Cooper JR, Kropp KE, Busch SJ. Induction of vascular cell adhesion molecule-1 expression by IL-4 in human aortic endothelial cells is not associated with increased nuclear NF-κB levels. J Cell Physiol. 1999;180:381–389. doi: 10.1002/(SICI)1097-4652(199909)180:3<381::AID-JCP9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 41.Toborek M, Lee YW, Kaiser S, Hennig B. Measurement of inflammatory properties of fatty acids in human endothelial cells. Methods Enzymol. 2002;352:198–219. doi: 10.1016/s0076-6879(02)52020-6. [DOI] [PubMed] [Google Scholar]
- 42.Deng X, Li H, Tang YW. Cytokine expression in respiratory syncytial virus-infected mice as measured by quantitative reverse-transcriptase PCR. J Virol Methods. 2003;107:141–146. doi: 10.1016/s0166-0934(02)00211-2. [DOI] [PubMed] [Google Scholar]
- 43.Lee YW, Lee WH. Protective effects of genistein on pro-inflammatory pathways in human brain microvascular endothelial cells. J Nutr Biochem. 2008;19:819–825. doi: 10.1016/j.jnutbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 45.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 46.Gu L, Tseng SC, Rollins BJ. Monocyte chemoattractant protein-1. Chem Immunol. 1999;72:7–29. doi: 10.1159/000058723. [DOI] [PubMed] [Google Scholar]
- 47.Bursill CA, Channon KM, Greaves DR. The role of chemokines in atherosclerosis: recent evidence from experimental models and population genetics. Curr Opin Lipidol. 2004;15:145–149. doi: 10.1097/00041433-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Sheikine YA, Hansson GK. Chemokines as potential therapeutic targets in atherosclerosis. Curr Drug Targets. 2006;7:13–27. doi: 10.2174/138945006775270240. [DOI] [PubMed] [Google Scholar]
- 49.Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97:714–721. [PubMed] [Google Scholar]
- 50.Gimbrone MA, Bevilacqua MP, Cybulsky MI. Endothelial-dependent mechanisms of leukocyte adhesion in inflammation and atherosclerosis. Ann NY Acad Sci. 1990;598:77–85. doi: 10.1111/j.1749-6632.1990.tb42279.x. [DOI] [PubMed] [Google Scholar]
- 51.Iseki A, Kambe F, Okumura K, Niwata S, Yamamoto R, Hayakawa T, et al. Pyrrolidine dithiocarbamate inhibits TNF-α-dependent activation of NF-κB by increasing intracellular copper level in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2000;276:88–92. doi: 10.1006/bbrc.2000.3452. [DOI] [PubMed] [Google Scholar]
- 52.Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord Drug Targets. 2007;7:135–144. doi: 10.2174/187152907780830905. [DOI] [PubMed] [Google Scholar]
- 53.Basta G, Lazzerini G, Del Turco S, Ratto GM, Schmidt AM, De Caterina R. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol. 2005;25:1401–1407. doi: 10.1161/01.ATV.0000167522.48370.5e. [DOI] [PubMed] [Google Scholar]
- 54.Hennig B, Toborek M, McClain CJ, Diana JN. Nutritional implications in vascular endothelial cell metabolism. J Am Coll Nutr. 1996;15:345–358. doi: 10.1080/07315724.1996.10718609. [DOI] [PubMed] [Google Scholar]
- 55.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, et al. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 56.Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, et al. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:333–339. doi: 10.1161/01.ATV.0000196651.64776.51. [DOI] [PubMed] [Google Scholar]
- 57.Hennig B, Chow CK. Lipid peroxidation and endothelial cells injury: implication in atherosclerosis. Free Radical Biol Med. 1988;4:99–106. doi: 10.1016/0891-5849(88)90070-6. [DOI] [PubMed] [Google Scholar]
- 58.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 59.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 60.Yla-Herttuala S. Gene expression in atherosclerotic lesions. Hertz. 1992;17:270–276. [PubMed] [Google Scholar]
- 61.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 62.Brinckmann R, Topp MS, Zalan I, Heydeck D, Ludwig P, Kühn H, et al. Regulation of 15-lipoxygenase expression in lung epithelial cells by interleukin-4. Biochem J. 1996;318:305–312. doi: 10.1042/bj3180305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bedard K, Krause K. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]