Abstract
Purpose
Osteosarcoma (OS) remains an incurable and ultimately fatal disease in many patients, and novel forms of therapy are needed. Improved models of OS that more closely mimic human disease would provide more robust information regarding the utility of novel therapies. Spontaneous OS in dogs may provide such a model. Pharmacologic inhibition of histone deacetylase (HDAC) enzymes has a variety of anti-tumor effects but may demonstrate the most utility when utilized in combination with standard cytotoxic therapies. We sought to determine the in vitro and in vivo effects of the HDAC inhibitor valproic acid (VPA) on doxorubicin (DOX) sensitivity in canine and human OS.
Methods
We evaluated the in vitro anti-proliferative and apoptotic effects of VPA/DOX combination treatment, alterations in histone acetylation and nuclear DOX accumulation resulting from VPA treatment, and the in vivo efficacy of combination therapy in a xenograft model.
Results
Treatment of canine and human OS cell lines with clinically achievable VPA concentrations resulted in increased histone acetylation but modest anti-proliferative effects. Pre-incubation with VPA followed by doxorubicin (DOX) resulted in significant growth inhibition and potentiation of apoptosis, associated with a dose-dependent increase in nuclear DOX accumulation. The combination of VPA and DOX was superior to either monotherapy in a canine OS xenograft model.
Conclusion
These results demonstrate a rationale for the addition of HDAC inhibitors to current protocols for the treatment of OS and illustrate the similarities in response to HDAC inhibitors between human and canine OS, lending further credibility to the canine OS model.
Keywords: Valproic acid, Osteosarcoma, HDAC, Canine model, Doxorubicin
Introduction
A multitude of pathways have been identified as targets of aberrant gene silencing via epigenetic mechanisms, including cell cycle control, apoptosis, developmental and differentiation pathways, DNA damage repair, and cell adhesion and migration [1]. Post-translational modification, including acetylation, of core histone proteins has been shown to be a major determinant of chromatin structure, thereby serving as a primary regulator of gene transcription [2]. Histone acetylation is dependent upon the balance between enzymes with histone acetyltransferase (HAT) activity and those with histone deacetylase (HDAC) activity. Altered expression of genes that encode the HAT and HDAC enzymes or their binding partners has been clearly linked to carcinogenesis [3–6]. Moreover, aberrant expression of HDAC enzymes has been linked to prognosis in a variety of cancers [4, 7, 8]. Combination therapies utilizing HDAC inhibitors and conventional cytotoxic drugs have shown superior in vitro efficacy versus mono-therapy in a variety of tumor types [9–13]. In case of agents that directly interact with DNA, the conformational changes in chromatin resulting from exposure to HDAC inhibitors may be partially responsible for enhancing anti-tumor effects [14]. Valproic acid (VPA) is a short chain fatty acid historically used for the treatment of epilepsy and bipolar disorder and can have anti-neoplastic effects through inhibition of HDAC at low millimolar concentrations [15]. While much of the initial work with VPA as a cancer therapy was performed on hematologic disorders such as acute myelogenous leukemia and myelodysplastic syndrome, recent evidence has shown efficacy in a number of solid malignancies, particularly when used in combination with demethylating agents, cytotoxic chemotherapy, and radiation therapy [10, 11, 13, 14, 16, 17]. Recent studies on the effect of HDAC inhibition in OS have found an increased sensitivity to Fas-mediated cell death occurring through downregulation of Fas-inhibitory molecules and/or increased expression of Fas-ligand [18–20]. In addition, other reports have documented the ability of various HDAC inhibitors to induce apoptosis in a caspase-dependent manner in OS cell lines [21, 22].
Osteosarcoma (OS) is the most common primary bone cancer in humans, primarily affecting pediatric patients. It typically demonstrates invasive and rapid growth with frequent occurrence of pulmonary metastasis. Current combinatorial therapies include surgery and multimodal chemotherapy, and a clear correlation between histologic necrosis following neoadjuvant chemotherapy and survival has been documented [23]. While cure rates approach 65% for patients with localized disease, those presenting with metastasis have a worse prognosis, and no improvements in survival for these patients have been achieved in the past two decades [24].
The dog is an excellent translational model for the investigation of novel anti-neoplastic therapies. Unlike murine models, dogs are relatively outbred, immunocompetent animals with spontaneously occurring tumors experiencing spontaneous metastasis and therapy resistance, representing a spectrum of tumor histotypes that have biology similar to that found in humans. The relatively large size of canine tumors, when compared with murine tumors, more closely approximates human solid tumors with respect to important biological factors such as hypoxia and clonal variation, and allows for multiple samplings of tumor tissue over time. The relatively rapid time course of disease progression, when compared with human cancer, allows for more rapid assessment of therapeutic endpoints than is possible in many human clinical trials [25, 26].
We hypothesized that treatment of canine and human OS cells with clinically achievable concentrations of VPA prior to DOX treatment would yield superior anti-tumor effects compared to DOX alone. Our results demonstrate that pre-treatment of OS cells with VPA leads to decreased proliferation and increased apoptosis in vitro and an improved anti-tumor effect in an in vivo xenograft model, providing a rationale for further investigation into combination therapies involving HDAC inhibitors in the treatment of OS in humans and in dogs as a pre-clinical model.
Materials and methods
Cell lines and conditions
The D17 canine OS cell line and the SAOS-2 human OS cell lines were purchased from the American Type Culture Collection (Rockville, MD). The Abrams canine OS cell line was kindly provided by Dr. William Dernell (Colorado State University, Fort Collins, CO). SJSA1 human OS cells were kindly provided by Dr. Lia Gore (University of Colorado Health Sciences Center, Denver, CO). Species authentication of canine cell lines was performed by evaluation of prepared metaphase spreads. Cells were serially passaged by trypsinization in C/10 [Minimum Essential Medium Eagle (Lonza, Walkersville, MD) supplemented with 1X MEM vitamin solution (Cellgro, Henderson, VA), 2 mM L-glutamine (Cellgro), 1 mM sodium pyruvate (Cellgro), 1X non-essential amino acid solution (Cellgro), 1X antibiotic/antimycotic (Cellgro), and 10% heat-inactivated fetal bovine serum (FBS) (Atlas, Fort Collins, CO)]. For experimental procedures, cells were incubated in a humidified atmosphere with 5% CO2 at 37°C.
Chemicals and antibodies
Valproic acid was purchased from Sigma (St. Louis, MO) and dissolved in tissue culture medium immediately prior to use. Anti-acetyl-histone H3 and total histone H3 antibodies were purchased from Upstate Biotechnology (Waltham, MA). Horseradish peroxidase-conjugated goat anti-rabbit IgG antibody was purchased from Pierce (Rockford, IL). Doxorubicin (DOX) was purchased from Bedford Laboratories (Bedford, OH).
Growth inhibition
For single agent VPA evaluation, cells were plated in C/10 at 2 × 103 per well in 96-well plates and allowed to adhere overnight. The following day, the plates were washed and the media replaced with C/10 containing increasing concentrations of VPA (0–10 mM). After 48 h, relative viable cell number was determined using a bioreductive fluoro-metric assay (Alamar Blue™, Promega, Madison, WI) according to manufacturer directions. Fluorescence was determined using a microplate reader (Synergy™ HT, Bio-Tek, Winooski, VT) with excitation/emission spectra of 530 and 590 nm, respectively. For combination DOX/VPA assays, cells were either co-incubated in 0, 0.5, or 1 mM VPA and increasing concentrations of DOX (0–1,000 ng/mL Abrams, D17, SAOS2; 0–3,000 ng/mL SJSA1), or pre-incubated for 48 h in VPA, followed by a 48 h dose of DOX. Relative viable cell numbers were determined as mentioned earlier. For the clonogenic assay, Abrams cells were pre-incubated in 0, 0.5, or 1 mM VPA for 48 h, and incubated in media containing 0 or 20 ng/mL DOX for 24 h. Cells were then trypsinized and washed followed by plating in single cell suspension into 6 well plates in drug-free C/10. After 7 days, colonies were stained with crystal violet and counted.
Apoptosis
Caspase activity
Apoptosis of OS cells was evaluated using the SensoLyte® Homogenous AMC Caspase-3/7 Assay Kit (AnaSpec, San Jose, CA) according to manufacturers’ directions. Briefly, canine and human OS cells were incubated in 0, 0.5, or 1.0 mMVPAfor 48 h prior to the addition of 0 or 100 ng/mL DOX (200 ng/mL for SJSA1) for an additional 48 h. Cells were lyzed in 1X lysis buffer (AnaSpec) and transferred to 1.5-mL Eppendorf tubes. Tubes containing lysates were placed on a rotating apparatus at 4°C for 30 min. Lysates were then centrifuged at 2500g for 10 min at 4°C. Supernatants were collected and 60 17/2/2010L was added to wells of a 384-well plate, followed by 20 lL of Caspase 3/7 substrate solution (AnaSpec). Reagents were mixed by shaking on a plate shaker for 60 min at 200 rpm. Fluorescence was determined at 360/460 nm, and results were reported as relative fluorescence units for each treatment condition.
Annexin V/Propidium Iodide staining
Apoptosis results from caspase 3/7 activity assay were validated with a flow cytometry-based assay. Treatment conditions were identical to those listed for the caspase assay. After incubation, cells were harvested by trypsinization and washed three times in PBS. Apoptosis was then determined using the BD Pharmingen™ Annexin V-FITC Apoptosis Detection Kit 1 (BD Biosciences, San Diego, CA) according to manufacturers recommendations. Results were analyzed using Summit v4.3.02 software (Beckman Coulter, Inc., Fullerton, CA).
In vitro histone acetylation
Western analysis
Human and canine OS cells were incubated in 0, 0.5, or 1.0 mM VPA for 48 h and then harvested by trypsinization. Cells were added to lysis buffer (M-PER Protein Extraction Reagent (Pierce), 1 mM NaVO4, 1 mM PMSF, Complete Mini protease inhibitor tablet (Roche, Indianapolis, IN), and 1% SDS), transferred to 1.5 mL microfuge tubes and passed through a 25 gauge needle 7–10 times before centrifugation at 10,000g for 10 min. Supernatants were transferred to new 1.5-mL tubes and protein concentration was determined via BCA assay (Pierce). Lysates were loaded into a denaturing 4–12% Bis–Tris gel (Invitrogen, Carlsbad, CA) and electrotransferred to a polyvinylidene difluoride (PVDF) membrane. After three washes in TBST (40 mM Tris–HCl, pH 7.6, 300 mM NaCl, and 0.5% Tween-20), membranes were blocked with 5% non-fat dry milk in TBST and incubated in a 1:4000 dilution of rabbit polyclonal anti-acetyl-H3 in blocking solution overnight at 4°C. After three washes in TBST, membranes were incubated in a 1:10,000 dilution of horseradish peroxidase-conjugated goat anti-rabbit IgG for 1.5 h at room temperature. Immunoreactive proteins were detected using SuperSignal® West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) and analyzed by autoradiography. Densitometry was performed using Image J software freely available online (NIH).
Doxorubicin accumulation
Abrams OS cells were plated in 150-cm2 culture flasks and treated with 0, 0.5, or 1 mM VPA for 48 h, followed by a 4-h incubation in 20 ng/mL DOX. Cells were washed, harvested by trypsinization, and placed into lysis buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl, 10 mM KCl, 0.5 mM DTT, 2 mM NaVO4, 5 mM NaF, 0.2 mM PMSF, 0.1% nonidet-P40 and protease inhibitor cocktail tablet) at 2.5 × 107 cells/mL in 1.5-mL microfuge tubes. Cells were centrifuged at 16,000g for 5 min at 4°C. Cytosolic supernatants were removed, and the nuclear pellet was solubilized in lysis buffer (20 mM HEPES, pH 7.9, 1.5 mM MgCl, 420 mM NaCl, 0.5 mM DTT, 2 mM NaVO4, 5 mM NaF, 0.2 mM PMSF, 25% glycerol and protease inhibitor cocktail tablet) at 1 × 108 cells/mL in 1.5-mL tubes. Tubes were vortexed for 10 s and incubated on ice for 20 min, followed by centrifugation at 16,000g for 5 min at 4°C. Supernatants were aliquotted into fresh ice-cold tubes and stored at −80°C. Protein concentration of the nuclear extracts was determined using the Qubit Fluorometer (Invitrogen, Carlsbad, CA). Fifty micrograms of protein from each treatment condition was added, in triplicate, to a 96-well plate, and fluorescence intensity of DOX was assessed (excitation 480/20 nm, emission 590/30 nm).
Animal studies
All animal experiments were performed under an Institutional Animal Care and Use Committee-approved protocol and institutional guidelines for animal welfare. Four- to 6-week old nu/nu athymic male mice were obtained from Harlan Laboratories (Indianapolis, IN) and housed in ventilated caging. After a 1-week acclimatization period, Abrams canine OS cells (1 × 106 in 0.9% NaCl) were injected s.c. on the right flank. Seven days after tumor inoculation, mice were size-matched, divided into 4 groups and treated in the following cohorts: saline only, VPA only, DOX only, and VPA followed by DOX. Mice receiving VPA were injected with 500 mg/kg VPA in 0.1 mL saline i.p. twice daily for 5 doses. Doxorubicin (3 mg/kg in 0.05 mL saline) was injected intravenously by tail-vein once after the last dose of VPA. These treatments were repeated 2 weeks later. Tumor growth (8 mice per cohort) was monitored three times per week by measuring two perpendicular tumor diameters with a caliper. Tumor volumes were calculated as V = (L × W2)/2. Animals were sacrificed when the largest tumor diameter reached 10 mm or when the tumor became ulcerated. In a separate experiment, groups of mice (n = 3) bearing established Abrams OS tumors were treated as mentioned earlier and killed 48 h following DOX treatment. Tumors were removed, placed in formalin, and paraffin embedded for immunohistochemistry (IHC).
Immunohistochemistry
Proliferation
Tissue samples were cut in 5-µm sections and mounted onto positively charged slides. Sections were deparaffinized in xylene, followed by rehydration in graded ethanol to water. Intrinsic peroxidase activity was blocked with 3% H2O2 in methanol for 15 min at room temperature. The sections were then incubated in monoclonal mouse anti-human Ki67 antibody clone MIB-1 (Dako, Carpinteria, CA) at 1:50, overnight at 4°C. Antibody binding was detected with a goat anti-mouse HRP antibody (Pierce) at 1:250 for 1.5 h at room temperature. Immunoreactive complexes were detected using diaminobenzidine (DAB) (Vector Labs), lightly counterstained with hematoxylin, and examined under light microscope. Canine lymph node was used as a control tissue. Images were obtained using a Zeiss Axioplan 2 microscope coupled with a Zeiss AxioCam HRc camera and results were calculated by counting the number of Ki67-positive nuclei per 20× field in 7 random fields per tissue section.
Histone acetylation
Deparaffinized sections of VPA treated and control tumors were stained for histone H3 acetylation after antigen retrieval using DakoCytomation Target Retrieval Solution pH9 (Dako). Prepared sections were incubated with anti-AcH3 at 1:50 overnight at 4°C followed by goat anti-rabbit HRP at 1:250 for 1.5 h at room temperature followed by DAB staining and hematoxylin counterstain.
Apoptosis
Slides were deparaffinized as described earlier and TUNEL staining was performed after antigen retrieval (low-pH) using DakoCytomation Target Retrieval Solution Citrate pH 6 (Dako). Slides were stained using the In Situ Cell Death Detection Kit (Roche, Indianapolis, IN) as per manufacturer’s recommendations and mounted using VectaShield plus DAPI® (Vector Labs). Images were obtained and results were reported as the number of dual-positive cells (TUNEL and DAPI) per 40× field.
Statistical analysis
In order to determine whether the addition of VPA to cytotoxic chemotherapy synergistically enhanced anti-proliferative activity, the Bliss independence model was utilized. Briefly, the Bliss criterion is described by the following equation:
where E(x) is the fractional inhibition of concentration × of VPA (between 0 and 1), E(y) is the fractional inhibition of concentration y of chemotherapy, and E(x,y) is the combined effect. Theoretical growth inhibition curves were constructed using this equation, and standard deviations were estimated by error propagation of experimental SD. Differences between treatment groups (Bliss theoretical vs. experimental) were assessed using two-way ANOVA and a Bonferroni post-test. Using this model, if the experimental fractional inhibition across concentration was significantly higher than the theoretical value, the interaction was considered synergistic. Comparison of tumor volumes among groups in the xenograft study was done by two-way ANOVA. Survival curves, with maximal tumor diameter (10 mm) or tumor ulceration as endpoints, were generated using the Kaplan–Meier method, and differences between treatment groups compared using log-rank (Mantel-Cox) analysis. Ki67 and TUNEL immunoreactivity was compared between groups using one-way ANOVA with Bonferroni multiple comparison test. In vitro apoptotic values were compared using one-way ANOVA. Statistical analysis was performed using GraphPad Prism® (GraphPad Software, La Jolla, CA). For all comparisons, a P-value less than 0.05 was considered significant.
Results
Valproic acid enhances osteosarcoma chemosensitivity
We first evaluated the anti-proliferative effect of increasing concentrations of VPA as a single agent against human and canine OS cells. In all four cell lines, VPA was able to significantly reduce proliferation at suprapharmacologic concentrations (10 mM). However, there was only a modest anti-proliferative effect in the dose range that is considered clinically achievable (0.1–2 mM) (Online Resource 1). We next evaluated the anti-proliferative effect of VPA when combined with DOX, an intercalating agent belonging to the anthracycline class commonly utilized in the treatment of canine and human OS. While co-incubation of VPA and DOX led to measurable chemosensitization, pre-incubation with VPA for 48 h followed by exposure to DOX clearly led to superior chemosensitization (Table 1). Proliferation results were confirmed by clonogenic assay in the Abrams cell line (Online Resource 2).
Table 1.
Summary of DOX IC50 values for cell growth inhibition in ng/mL
| Co-incubation | Pre-incubation | |||||
|---|---|---|---|---|---|---|
| [VPA], mM | 0 | 0.5 | 1.0 | 0 | 0.5 | 1.0 |
| Abrams | 60 | 24.3 | 13 | 74.6 | 26.1 | 10.3* |
| D17 | 17 | 10 | 9 | 758 | 193* | 66* |
| SAOS2 | 225.5 | 117.4* | 120* | 111 | 64.7* | 53.4* |
| SJSA1 | 104 | 109.7 | 77.06 | 271.3 | 134.3* | 92.4* |
Cells were plated in 96-well plates and treated with DOX ranging from 0–1,000 ng/mL (Abrams, D17, SAOS2) or 0–3,000 ng/mL (SJSA1). Relative viable cell numbers were determined by Alamar blue reduction and fluorescence detection. For co-incubation, cells were treated for 48 h with VPA and DOX. For pre-incubation, cells were treated with VPA for 48 h prior to 48 h incubation in VPA and DOX. There are larger reductions in IC50 with VPA pre-incubation than co-incubation, with synergism seen only after pre-incubation in three of four cell lines.
synergistic interaction (P < 0.05) determined by Bliss analysis as described in Methods
HDAC inhibition enhances doxorubicin-induced apoptosis
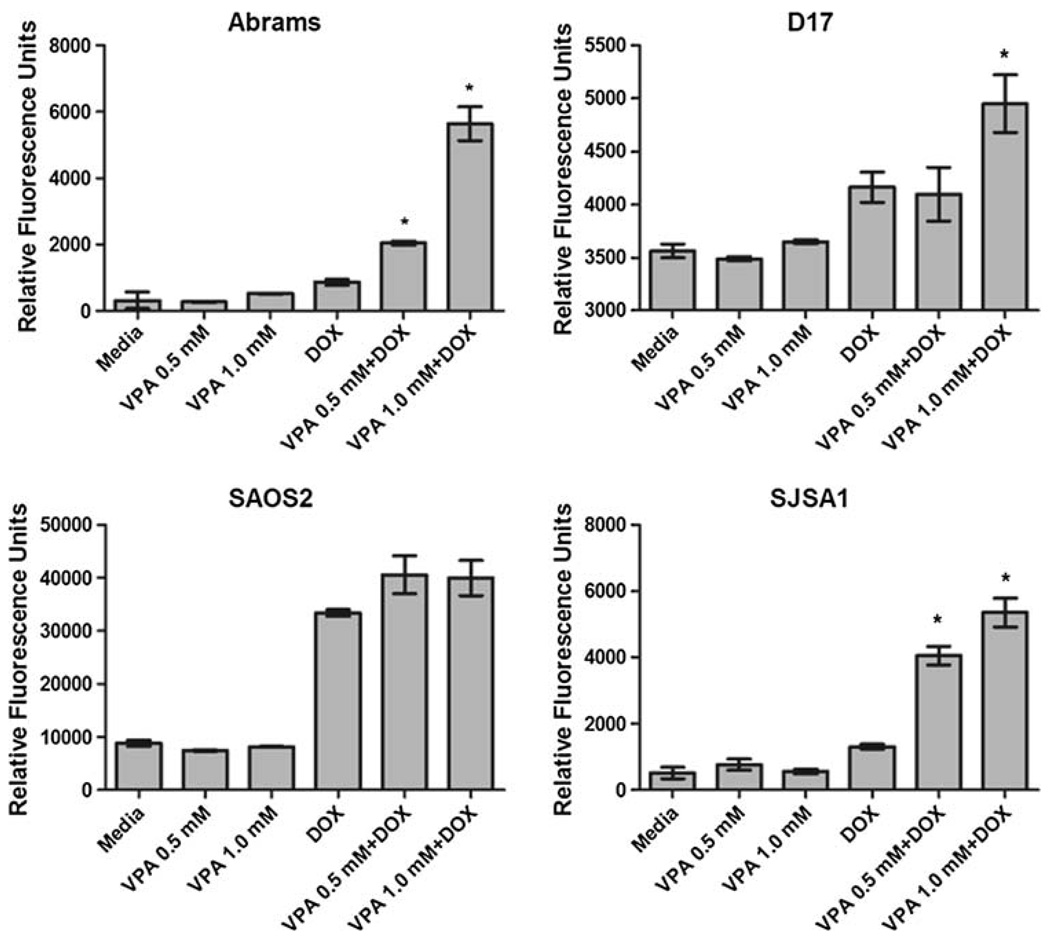
The bioreductive methodology applied earlier cannot distinguish between growth arrest and cell death. To determine whether the combination treatment was inducing apoptosis, all four cell lines were pre-incubated in 0, 0.5 or 1.0 mMVPA for 48 h followed by 48 h exposure to DOX(100 ng/mL) and VPA, followed by apoptosis assessment by caspase 3/7 activity. An increase in apoptosis was seen in the cells pretreated with VPA compared to DOX alone in all four cell lines (Fig. 1). These results were confirmed by apoptosis evaluation via AnnexinV/Propidium iodide staining and flow cytometric analysis (Online Resource 3).
Fig. 1.
Evaluation of in vitro apoptosis in canine and human OS. Cells were pre-incubated in VPA for 48 h followed by 48 h of combination treatment using 100 ng/mL DOX (Abrams, D17, SAOS2) or 200 ng/mL DOX (SJSA1). Pre-treatment with VPA results in enhanced apoptotic effect compared to DOX alone * P < 0.05
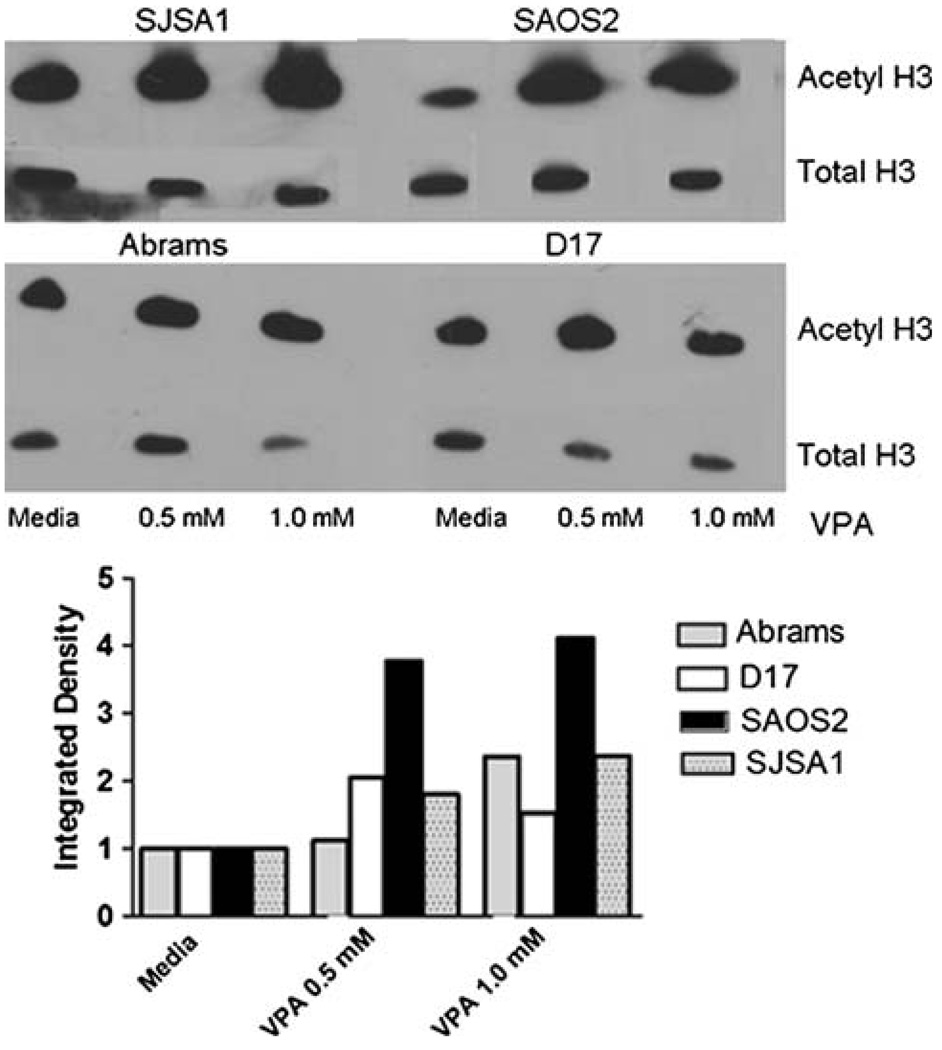
VPA increases histone acetylation in canine and human OS
We next compared the ability of VPA to induce histone hyperacetylation in human and canine OS cell lines treated in vitro. For tumor cell lines, 48-h incubation in 0, 0.5, or 1 mM VPA was followed by cell lysis and western analysis for acetylated histones H3, using total H3 as a loading control. A dose-dependent increase in acetylated histones is seen in VPA-treated canine and human OS cells (Fig. 2). These results demonstrate that VPA effectively hyper-acetylates histones in both canine and human OS, at concentrations that are clinically achievable.
Fig. 2.
Western blot analysis of acetylated histones in human and canine OS cells treated with 0, 0.5, or 1.0 mM VPA for 48 h. Densitometry normalized to total histone H3 reveals a dose-dependent increase in acetylated histones with VPA treatment
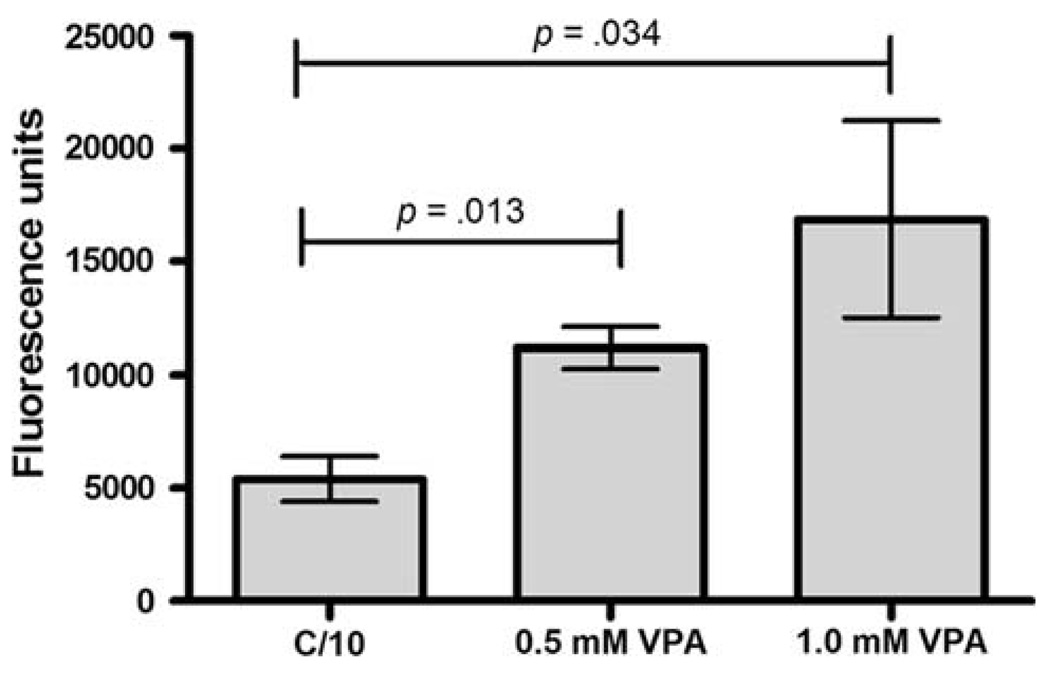
Valproic acid enhances nuclear doxorubicin accumulation
To address whether HDAC inhibition and the resultant alteration in chromatin structure might increase access of DNA intercalating molecules leading to enhanced activity, we next evaluated whether the VPA-induced sensitization of OS cells to DOX was associated with an increased nuclear accumulation of DOX. We measured the nuclear accumulation of DOX in VPA pre-treated Abrams OS cells after a 4-h DOX exposure. Nuclei were extracted and DOX fluorescence measured and standardized to protein concentration as described above in Methods. The VPA pretreatment resulted in a significant and dose-dependent increase in nuclear DOX accumulation (Fig. 3), presumably as a result of chromatin decondensation secondary to histone hyperacetylation.
Fig. 3.
Evaluation of nuclear DOX accumulation. Abrams OS cells were incubated with 0, 0.5, or 1 mM VPA for 48 h and then exposed to a 4 h pulse of DOX. Fluorescence of nuclear extracts at 485/595 nM indicates VPA pre-exposure increases nuclear accumulation of DOX
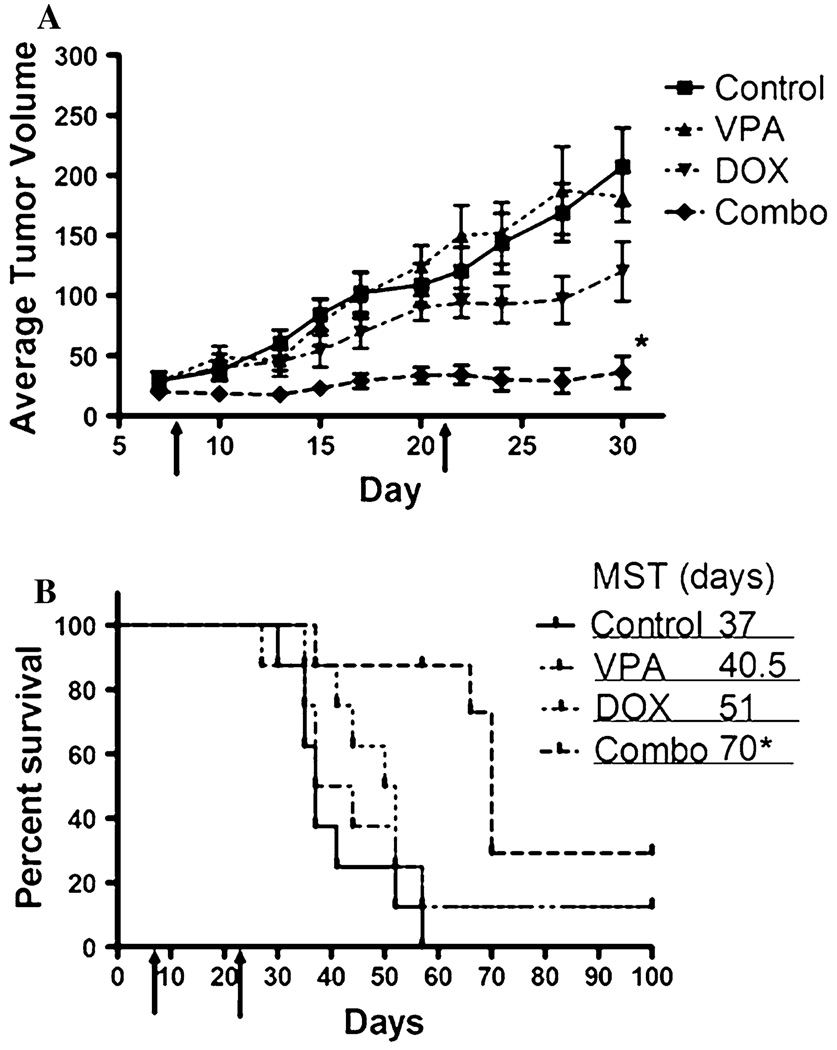
In vivo evaluation of VPA-DOX combination therapy
Based on the promising in vitro results obtained when combining VPA and DOX, we next evaluated the in vivo efficacy in a canine OS subcutaneous xenograft model. Mice bearing established Abrams OS tumors were treated with VPA alone, DOX alone, VPA followed by DOX, or saline. VPA treatment was initiated 48 h prior to DOX administration in the combination group and the treatment cycles were given on days 7–9 and 21–23. Mice were weighed and monitored for treatment related morbidity during the entire study. Combination-treated mice exhibited moderate weight loss (less than 10%) during treatment but regained weight between treatment cycles (data not shown). Tumor volumes from the VPA alone treatment were similar to saline-treated animals, indicating that short-term single agent therapy with VPA is not effective in this model. Doxorubicin-treated mice exhibited a modest reduction in tumor growth, while the combination-treated mice had a significantly reduced tumor growth (P < 0.05) when compared to all other treatment groups (Fig. 4). This translated into a significant increase in survival, compared to all other groups, when growth to 10 mm or ulceration was used as the endpoint (Fig. 4). These results suggest that the addition of VPA to standard chemotherapy for OS can improve the anti-tumor effects of cytotoxic chemotherapy.
Fig. 4.
Growth of subcutaneous Abrams OS xenografts implanted into athymic mice (n = 8 per group). Arrows indicate treatment cycles. a Average tumor volumes were measured for each group and tumor growth curves over time were compared using ANOVA. A significant reduction in tumor growth is observed in the VPA/DOX combination-treated mice when compared to all other groups; * P < 0.05 b Kaplan–Meier survival curve showing a statistically significant improvement in overall survival for the VPA pre-treated group
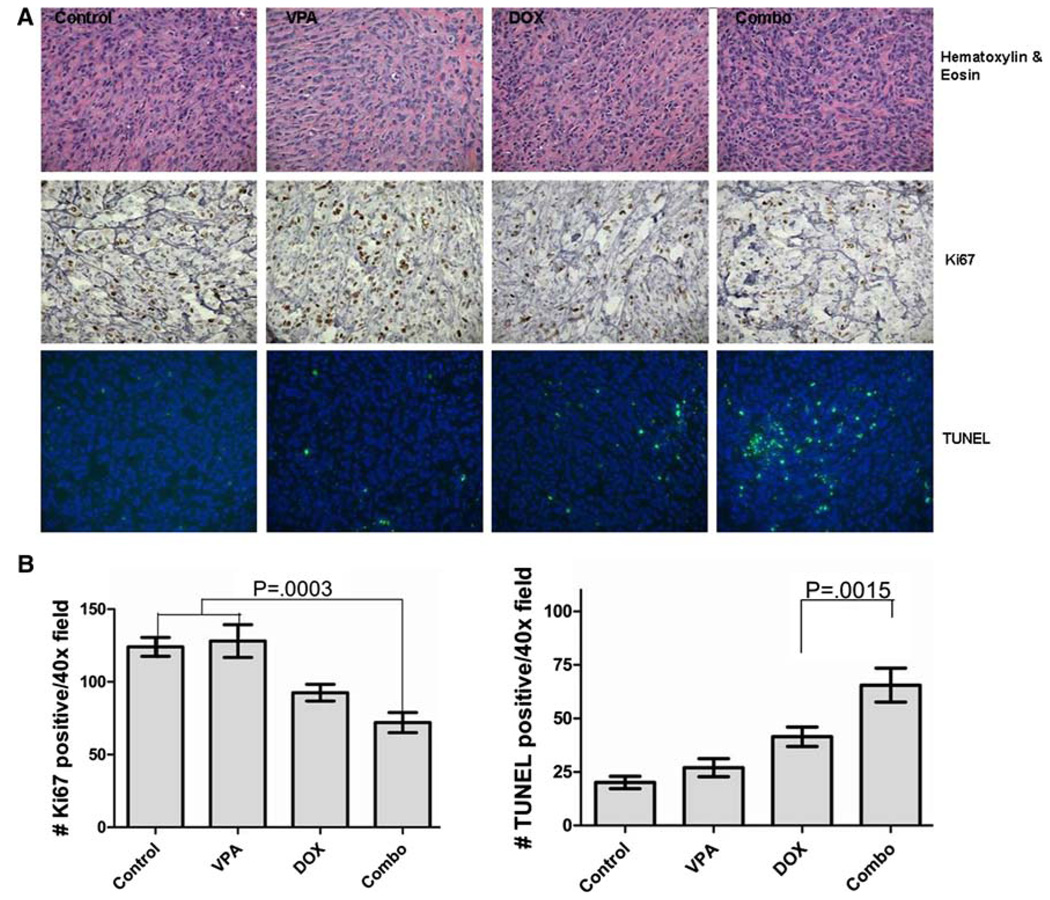
Immunohistochemical analysis of OSA xenografts
We next utilized IHC to evaluate the xenograft tumors for changes in histone acetylation, apoptosis, and proliferation. Mice were divided into treatment groups (n = 3 per group) identical to those described earlier and killed 24 h after the first DOX administration. Tumors were removed and formalin fixed prior to IHC analysis. As previously demonstrated in vitro, there was a statistically significant increase in apoptosis (P < 0.0001) and a decrease in proliferation (P = 0.0003) in the combination-treated mice (Fig. 5). In addition, histone hyperacetylation was evident by IHC in the VPA-treated mice (Online Resource 4). Taken together, these results indicate that the addition of VPA to standard cytotoxic chemotherapy results in tumor cell histone hyperacetylation, increased apoptosis, and decreased proliferation in OS when compared to either drug administered alone.
Fig. 5.
a Photomicrographs of Abrams OS xenografts. Formalin fixed, paraffin embedded tumors were evaluated for changes in proliferation (Ki67 immunoreactivity) and apoptosis (TUNEL) between treatment groups. b Graphical representation of Ki67 and TUNEL immunoreactivity. Proliferation is significantly decreased in tumors from combination-treated mice (left) accompanying a significant increase in apoptotic cells in this same group (right)
Discussion
Osteosarcoma remains the most common primary bone tumor, developing most commonly in the long bones during adolescence [24]. The cause of death for the vast majority of these patients continues to be the development of metastases, primarily to the lungs, as local failure in patients with appendicular tumors has been minimized by improvements in primary tumor management [27]. While the development of multi-modality chemotherapy protocols for OS in the 1980s provided significant improvements in long-term outcome for patients presenting with localized disease, this has not held true for patients in whom metastases are present at the time of diagnosis, and fewer than 30% of these patients survive [24, 27]. Factors that have been correlated with long-term survival in OS patients include the degree of necrosis of the primary tumor following an intensified, multi-modality chemotherapy protocol prior to surgical amputation or limb salvage procedure [28]. Unacceptable toxicities prevent further dose intensification from providing any further improvement in long-term survival. The use of agents targeting epigenetic changes is one example of the recent trend in cancer therapy to combine biologically targeted agents with cytotoxic chemotherapy.
In our present study, we found that the HDAC inhibitor VPA can sensitize human and canine OS cells to the anti-tumor effects of the topoisomerase-II inhibitor DOX. As a single agent, VPA demonstrated only modest anti-proliferative effects in the concentration range that is considered achievable in vivo. While simultaneous treatment with both agents was able to produce an increase in DOX sensitivity, the most profound chemosensitization was seen with 48-h VPA pre-treatment. This might be explained by decondensation of chromatin induced by VPA, allowing increased access of DOX to the DNA. This schedule dependence has also been reported by others evaluating different combinations of HDAC inhibitors and intercalating macromolecules in vitro and in vivo [29]. Interestingly, the partial charge neutralization brought about by hyperacetylation of histone tails may only be partly responsible for the decondensation of chromatin following exposure to HDAC inhibitors. Alterations in expression levels of proteins that are important for maintaining the structure of chromatin (SMCs) have been implicated in this conformational change [14]. The combination of decreased associations of histone tails with DNA and reduction in levels of proteins that maintain chromatin structure may explain the reductions in DOX IC50 in the VPA pre-treated OS cells compared to those that were co-incubated.
We also found that the decreased viable cell number observed with combination therapy was, at least in part, due to an increase in apoptosis. Caspase and Annexin V/PI assays revealed that the addition of VPA to DOX treatment significantly increased the apoptotic index of OS cells in vitro, and this was associated with increased nuclear DOX accumulation in these same cells. We found identical results in vivo, with the addition of VPA providing a significant increase in the percentage of TUNEL-positive tumor cells over mice treated with DOX alone. These results are particularly noteworthy in light of the fact that increased tumor cell death in primary OS tumors following neoadjuvant chemotherapy is associated with improved long-term survival in human patients [28]. Similarly, a direct correlation between percentage necrosis following DOX therapy and survival time has also been described in dogs [30]. In addition to enhanced apoptotic activity, our results from the evaluation of the proliferation marker Ki-67 show that the combination of VPA and DOX also has a greater anti-proliferative effect than either agent alone.
We demonstrated by western analysis that in vitro treatment of canine and human OS cells with VPA results in similar hyperacetylation of histone H3 using concentrations that are achievable in patients. Hyperacetylation was observed in vivo as well, although no increased anti-tumor activity was observed with short-term single agent VPA treatment compared to controls. Although it has not been shown to be a predictor of anti-tumor activity in single agent HDACi-treated cells, evaluation of tumor histone acetylation in vivo may be useful in evaluating pharmacodynamics of VPA when determining optimal dosing in combination with DOX, as chromatin decondensation and increased access to DNA may require histone hyperacetylation.
The relatively low incidence rate of OS in humans is a significant obstacle in developing and rigorously evaluating novel treatment combinations and designing clinical trials that will generate meaningful outcome data. In contrast, the incidence of spontaneously occurring OS in canine patients is approximately 8–12 times higher [31]. These canine tumors are histologically indistinguishable from their human counterparts and share common features such as biological aggressiveness, response rates, propensity to metastasize to the lungs, anatomic site predilections, and prognostic factors [31–33]. Studies in canine patients with spontaneous OS have proven useful in developing novel therapeutic strategies for humans. A randomized, double-blind study in canine patients using a liposome encapsulated form of the macrophage-activating compound muramyl tripeptide phoshphatidylethanolamine (L-MTPPE) demonstrated a significant improvement in event-free survival following amputation [34], the results of which led to a large, randomized phase-III trial in human OS patients [35]. Our current study illustrates the similarity between canine and human OS cells in their molecular responses to HDAC inhibition by VPA, providing further evidence that spontaneously occurring OS in dogs may provide a robust model to develop novel epigenetic strategies that may further improve long-term outcomes.
In conclusion, we have demonstrated that VPA is capable of effective inhibition of HDAC in canine and human OS cells, resulting in histone hyperacetylation. In addition, pre-treatment of these cells with VPA results in enhanced sensitivity to DOX in vitro and profound tumor growth inhibition in vivo. We also found that decreases in markers of proliferation and increases in apoptosis were sequelae of VPA-DOX combination therapy in a xenograft model of canine OS. This study provides further support to the use of HDAC inhibitors as a means of chemosensitization in the treatment of cancer and, more specifically, the integration of HDAC inhibitors into cytotoxic chemotherapy protocols in OS. Spontaneous canine OS may serve as a novel translational bridge for the evaluation of these combinations.
Supplementary Material
Acknowledgments
Morris Animal Foundation (CSU Fund #5354500), National Institutes of Health NCRR T32-RR-007072-06, American Cancer Society RSG-04-219-01.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00280-010-1287-z) contains supplementary material, which is available to authorized users.
Conflict of interest statement None.
Contributor Information
Luke A. Wittenburg, Email: lwittenb@colostate.edu, Department of Clinical Sciences, James L. Voss Veterinary Teaching Hospital, Colorado State University Animal Cancer Center, Fort Collins, CO 80525, USA; Department of Cell and Molecular Biology, James L. Voss Veterinary Teaching Hospital, Colorado State University Animal Cancer Center, Fort Collins, CO 80525, USA.
Liam Bisson, Department of Clinical Sciences, James L. Voss Veterinary Teaching Hospital, Colorado State University Animal Cancer Center, Fort Collins, CO 80525, USA.
Barbara J. Rose, Department of Clinical Sciences, James L. Voss Veterinary Teaching Hospital, Colorado State University Animal Cancer Center, Fort Collins, CO 80525, USA
Christopher Korch, University of Colorado Cancer Center (UCCC), University of Colorado Denver Anschutz Medical Campus, Aurora, CO 80045, USA.
Douglas H. Thamm, Department of Clinical Sciences, James L. Voss Veterinary Teaching Hospital, Colorado State University Animal Cancer Center, Fort Collins, CO 80525, USA Department of Cell and Molecular Biology, James L. Voss Veterinary Teaching Hospital, Colorado State University Animal Cancer Center, Fort Collins, CO 80525, USA; University of Colorado Cancer Center (UCCC), University of Colorado Denver Anschutz Medical Campus, Aurora, CO 80045, USA.
References
- 1.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer-a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 2.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 3.Mai A, Massa S, Rotili D, Cerbara I, Valente S, Pezzi R, Simeoni S, Ragno R. Histone deacetylation in epigenetics: an attractive target for anticancer therapy. Med Res Rev. 2005;25:261–309. doi: 10.1002/med.20024. [DOI] [PubMed] [Google Scholar]
- 4.Ouaissi M, Sielezneff I, Silvestre R, Sastre B, Bernard JP, Lafontaine JS, Payan MJ, Dahan L, Pirro N, Seitz JF, Mas E, Lombardo D, Ouaissi A. High histone deacetylase 7 (HDAC7) expression is significantly associated with adenocarcinomas of the pancreas. Ann Surg Oncol. 2008;15:2318–2328. doi: 10.1245/s10434-008-9940-z. [DOI] [PubMed] [Google Scholar]
- 5.Zhu P, Martin E, Mengwasser J, Schlag P, Janssen KP, Gottlicher M. Induction of HDAC2 expression upon loss of APC in colorectal tumorigenesis. Cancer Cell. 2004;5:455–463. doi: 10.1016/s1535-6108(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 6.Ropero S, Ballestar E, Alaminos M, Arango D, Schwartz S, Jr, Esteller M. Transforming pathways unleashed by a HDAC2 mutation in human cancer. Oncogene. 2008;27:4008–4012. doi: 10.1038/onc.2008.31. [DOI] [PubMed] [Google Scholar]
- 7.Weichert W, Roske A, Gekeler V, Beckers T, Stephan C, Jung K, Fritzsche FR, Niesporek S, Denkert C, Dietel M, Kristiansen G. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer. 2008;98:604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weichert W, Roske A, Gekeler V, Beckers T, Ebert MP, Pross M, Dietel M, Denkert C, Rocken C. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol. 2008;9:139–148. doi: 10.1016/S1470-2045(08)70004-4. [DOI] [PubMed] [Google Scholar]
- 9.Carraway HE, Gore SD. Addition of histone deacetylase inhibitors in combination therapy. J Clin Oncol. 2007;25:1955–1956. doi: 10.1200/JCO.2006.09.8293. [DOI] [PubMed] [Google Scholar]
- 10.McIntyre J, Angels-Moral M, Bozzo J. Combination therapy with valproic acid in cancer: initial clinical approach. Drugs Future. 2007;32(1):45. [Google Scholar]
- 11.Marchion DC, Bicaku E, Daud AI, Sullivan DM, Munster PN. In vivo synergy between topoisomerase II and histone deacetylase inhibitors: predictive correlates. Mol Cancer Ther. 2005;4:1993–2000. doi: 10.1158/1535-7163.MCT-05-0194. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 2003;63:7291–7300. [PubMed] [Google Scholar]
- 13.Catalano MG, Fortunati N, Pugliese M, Poli R, Bosco O, Mastrocola R, Aragno M, Boccuzzi G. Valproic acid, a histone deacetylase inhibitor, enhances sensitivity to doxorubicin in anaplastic thyroid cancer cells. J Endocrinol. 2006;191:465–472. doi: 10.1677/joe.1.06970. [DOI] [PubMed] [Google Scholar]
- 14.Marchion DC, Bicaku E, Daud AI, Sullivan DM, Munster PN. Valproic acid alters chromatin structure by regulation of chromatin modulation proteins. Cancer Res. 2005;65:3815–3822. doi: 10.1158/0008-5472.CAN-04-2478. [DOI] [PubMed] [Google Scholar]
- 15.Kostrouchova M, Kostrouch Z, Kostrouchova M. Valproic acid, a molecular lead to multiple regulatory pathways. Folia Biol (Praha) 2007;53:37–49. [PubMed] [Google Scholar]
- 16.Munster P, Marchion D, Bicaku E, Schmitt M, Lee JH, DeConti R, Simon G, Fishman M, Minton S, Garrett C, Chiappori A, Lush R, Sullivan D, Daud A. Phase I trial of histone deacetylase inhibition by valproic acid followed by the topoisomerase II inhibitor epirubicin in advanced solid tumors: a clinical and translational study. J Clin Oncol. 2007;25:1979–1985. doi: 10.1200/JCO.2006.08.6165. [DOI] [PubMed] [Google Scholar]
- 17.Zhu WG, Otterson GA. The interaction of histone deacetylase inhibitors and DNA methyltransferase inhibitors in the treatment of human cancer cells. Curr Med Chem Anticancer Agents. 2003;3:187–199. doi: 10.2174/1568011033482440. [DOI] [PubMed] [Google Scholar]
- 18.Yamanegi K, Yamane J, Hata M, Ohyama H, Yamada N, Kato-Kogoe N, Futani H, Nakasho K, Okamura H, Terada N. Sodium valproate, a histone deacetylase inhibitor, decreases the secretion of soluble Fas by human osteosarcoma cells and increases their sensitivity to Fas-mediated cell death. J Cancer Res Clin Oncol. 2009;135:879–889. doi: 10.1007/s00432-008-0522-z. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe K, Okamoto K, Yonehara S. Sensitization of osteosarcoma cells to death receptor-mediated apoptosis by HDAC inhibitors through downregulation of cellular FLIP. Cell Death Differ. 2005;12:10–18. doi: 10.1038/sj.cdd.4401507. [DOI] [PubMed] [Google Scholar]
- 20.Imai T, Adachi S, Nishijo K, Ohgushi M, Okada M, Yasumi T, Watanabe K, Nishikomori R, Nakayama T, Yonehara S, Toguchida J, Nakahata T. FR901228 induces tumor regression associated with induction of Fas ligand and activation of Fas signaling in human osteosarcoma cells. Oncogene. 2003;22:9231–9242. doi: 10.1038/sj.onc.1207184. [DOI] [PubMed] [Google Scholar]
- 21.Roh MS, Kim CW, Park BS, Kim GC, Jeong JH, Kwon HC, Suh DJ, Cho KH, Yee SB, Yoo YH. Mechanism of histone deacetylase inhibitor Trichostatin A induced apoptosis in human osteosarcoma cells. Apoptosis. 2004;9:583–589. doi: 10.1023/B:APPT.0000038037.68908.6e. [DOI] [PubMed] [Google Scholar]
- 22.Jaboin J, Wild J, Hamidi H, Khanna C, Kim CJ, Robey R, Bates SE, Thiele CJ. MS-27–275, an inhibitor of histone deacetylase, has marked in vitro and in vivo antitumor activity against pediatric solid tumors. Cancer Res. 2002;62:6108–6115. [PubMed] [Google Scholar]
- 23.Hamada K, Tomita Y, Inoue A, Fujimoto T, Hashimoto N, Myoui A, Yoshikawa H, Hatazawa J. Evaluation of chemotherapy response in osteosarcoma with FDG-PET. Ann Nucl Med. 2009;23:89–95. doi: 10.1007/s12149-008-0213-5. [DOI] [PubMed] [Google Scholar]
- 24.Felx M, Guyot M-C, Isler M, Turcotte RE, Doyon J, Khatib A-M, Leclerc S, Moreau A, Moldovan F. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-κB in human osteosarcoma. Clin Sci. 2006;110:645–654. doi: 10.1042/CS20050286. [DOI] [PubMed] [Google Scholar]
- 25.Hansen K, Khanna C. Spontaneous and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer. 2004;40:858–880. doi: 10.1016/j.ejca.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 26.Kimmelman J, Nalbantoglu J. Faithful companions: a proposal for neurooncology trials in pet dogs. Cancer Res. 2007;67:4541–4544. doi: 10.1158/0008-5472.CAN-06-3792. [DOI] [PubMed] [Google Scholar]
- 27.Khanna C. Novel targets with potential therapeutic applications in osteosarcoma. Curr Oncol Rep. 2008;10:330–358. doi: 10.1007/s11912-008-0054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Federman N, Bernthal N, Eilber FC, Tap WD. The multidisciplinary management of osteosarcoma. Curr Treat Options Oncol. 2009;10:82–93. doi: 10.1007/s11864-009-0087-3. [DOI] [PubMed] [Google Scholar]
- 29.Marchion DC, Bicaku E, Daud AI, Richon V, Sullivan DM, Munster PN. Sequence-specific potentiation of topoisomerase II inhibitors by the histone deacetylase inhibitor suberoylanilide hydroxamic acid. J Cell Biochem. 2004;92:223–237. doi: 10.1002/jcb.20045. [DOI] [PubMed] [Google Scholar]
- 30.Berg J, Weinstein MJ, Springfield DS, Rand WM. Results of surgery and doxorubicin chemotherapy in dogs with osteosarcoma. J Am Vet Med Assoc. 1995;206:1555–1560. [PubMed] [Google Scholar]
- 31.Mueller F, Fuchs B, Kaser-Hotz B. Comparative biology of human and canine osteosarcoma. Anticancer Res. 2007;27:155–164. [PubMed] [Google Scholar]
- 32.Vail D, Thamm D. Spontaneously occurring tumors in companion animals as models for drug development. In: Teicher B, Andrews P, editors. Cancer drug discovery and development guide: preclinical screening, clinical trials, and approval. Totowa: Humana Press Inc; 2004. pp. 259–284. [Google Scholar]
- 33.Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, Yeung C, Gorlick R, Hewitt SM, Helman LJ. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- 34.MacEwen EG, Kurzman ID, Rosenthal RC, Smith BW, Manley PA, Roush JK, Howard PE. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst. 1989;81:935–938. doi: 10.1093/jnci/81.12.935. [DOI] [PubMed] [Google Scholar]
- 35.Kleinerman ES, Gano JB, Johnston DA, Benjamin RS, Jaffe N. Efficacy of liposomal muramyl tripeptide (CGP 19835A) in the treatment of relapsed osteosarcoma. Am J Clin Oncol. 1995;18:93–99. doi: 10.1097/00000421-199504000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.