Abstract
Caffeine intake has been associated with a decreased risk of Parkinson’s disease (PD) in men but the effect in women is less clear, and appears to be modified by use of post-menopausal estrogens. In a nested case-control study within the Nurses Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), we examined associations between single nucleotide polymorphisms (SNPs) of caffeine metabolizing genes (CYP1A2 and NAT2) and estrogen receptors (ESR1 and ESR2), their interaction with caffeine intake and hormone replacement therapy (PMH) use (collected prospectively) and risk of PD. We matched 159 female cases to 724 controls and 139 male cases to 561 controls on birth year, source of DNA (blood or buccal smear), age and sex. The CYP1A2 rs762551 polymorphism (lower enzyme inducibility) was marginally associated with an increased risk of PD (RR, for increasing number of minor alleles = 1.34; 95% CI 1.02, 1.78 in women, but not in men. None of the NAT2 (classified as slow vs. fast acetylator), ESR1 or ESR2 polymorphisms were significantly associated with an altered risk of PD. Marginally significant interactions were observed between caffeine intake and the ESR1 polymorphism rs3798577 (p = 0.07) and ESR2 polymorphism rs1255998 (p=0.07). The observed increased risk of PD among female but not male carriers of the rs762551 polymorphism of CYP1A2 and the interactions of caffeine with ESR1 rs3798577 and ESR2 rs1255998 may provide clues to explain the relationship between gender, caffeine intake, estrogen status and risk of PD and need to be replicated.
Keywords: Parkinson’s Disease (PD), epidemiology, caffeine, estrogen, polymorphism
Introduction
Caffeine intake has been associated with a significantly reduced risk of Parkinson’s disease (PD) in men in several cohort [1, 2] and case-control studies.[3] In women, the association appears to be modified by use of post-menopausal hormones: non-users of PMH are at a decreased risk of Parkinson’s disease, whereas among users of PMH, caffeine has been associated with increased risk of PD.[4] In an attempt to understand the mechanisms behind the protective effect of caffeine on the risk of PD and the interaction with estrogen, we studied polymorphisms of genes involved in caffeine and estrogen metabolism.
Caffeine is metabolized to paraxanthine primarily by the P450 CYP1A2 enzyme, which is responsible for more than 90% of caffeine metabolism.[5] CYP1A2 also metabolizes estrogen.[6] Individuals carrying the (-163A->C) variant of CYP1A2 (rs762551) have lower CYP1A2 activity, presumably because this polymorphism reduces the enzyme inducibility.[7]
Another enzyme involved in caffeine clearance is NAT2, whose function is to catalyze the transformation of a broad range of xenobiotics.[8] Carriers of mutant alleles are slow acetylators and metabolize less efficiently certain endogenous compounds, including caffeine.[8] This enzyme has previously been studied with relation to risk of PD and in gene-environment interaction studies,[9] but with mixed results.
The observation in a mouse model of Parkinson disease that estrogen prevents the neuroprotective effects of low and moderated doses of caffeine [10] supports the hypothesis that the difference in the observed effect of caffeine in men and women is explained by an interaction with estrogen. Therefore, polymorphisms in estrogen receptors could modify the association between caffeine and risk of PD in women. Two estrogen receptors have been identified, estrogen receptor alpha (ESR1), which is primarily responsible for sexual development with functions in the endometrium and the hypothalamus[11], and estrogen receptor beta (ESR2), with functions in the kidney, brain, bone, heart, lungs, intestinal mucosa, prostate, and endothelial cells.[11] An association between ESR1 and risk of PD has been reported in some[12] but not all[13] studies. There are no data, however, on possible interactions between these genes and either caffeine or use of postmenopausal hormones.
We here report the results of a nested case-control study among participants in the Nurses Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) conducted to examine whether polymorphisms of the caffeine metabolizing genes NAT2 and CYP1A2, and of the estrogen receptors ESR1 and ESR2 are associated with altered risk of PD, and whether these polymorphisms interact with caffeine intake or PMH in determining PD risk.
Methods
Participants in this study were men and women who provided a blood or buccal smear sample from amongst those enrolled in the NHS and HPFS. The NHS began in 1976 when 121,700 nurses aged 30–55 returned mailed questionnaires regarding lifestyle factors and disease history. The HPFS began in 1986 when 51,529 male health professionals (dentists, optometrists, pharmacists, osteopaths, podiatrists and veterinarians) aged 40–75 years returned similar questionnaires. Biennial questionnaires are mailed to study participants to update information on risk factors and disease occurrence. A semi-quantitative food frequency questionnaire (SFFQ) is mailed every four years to both cohorts and includes questions on consumption of coffee and other caffeinated beverages.
All participants were invited to provide blood samples for investigations of biomarkers and disease outcomes. Blood was collected between 1989 and 1990 in the NHS (32,826 women) and between 1993 and 1995 in the HPFS (18,018 men). In the NHS, cases and controls who had not provided blood as part of the main study were sent a package including an invitation to provide a buccal smear, two cytobrushes (one for each cheek), instructions for use, a request of consent to examine genetic markers of disease and a stamped return envelope. Up to three additional packages were sent to non-responders emphasizing the importance of their participation. We were able to obtain genetic samples on 52% (139 of 266) of the confirmed PD cases in the HPFS and 58% (153 of 264) confirmed PD cases in the NHS. Of those for whom gnentics samples were obtainted, in the HPFS, 139 (100%) provided a blood sample and in the NHS, 63 (40%) cases had provided buccal smear samples and the rest provided blood samples.
Both the NHS and HPFS were approved by the Human Subjects Research Committees at Harvard School of Public Health and Brigham and Women's Hospital. All study subjects provided written informed patient consent.
Case ascertainment
The ascertainment of PD cases in these cohorts has been previously described.[14] Briefly, participants who reported a new diagnosis of PD were asked permission to contact their neurologist and review their medical records. After obtaining permission, neurologists were asked to fill out a questionnaire to determine certainty of the diagnosis (definite, probable, possible, or not PD), or to send a copy of the medical records. Diagnoses made by neurologists have been found to be as accurate as those obtained by applying specific diagnostic criteria and shown to correctly identify a higher proportion of PD cases.[15] If a neurologist was not involved or did not respond, a questionnaire was mailed to the patient’s internist or general physician. For deceased participants, deaths were reported by next of kin, coworkers, postal authorities or the National Death Index.
A total of 159 incident cases of definite and probable PD were documented between baseline and December 2000 among women and 139 cases were documented between baseline and December 2002 among men with available genetic samples.
Control selection
To maximize the sample size and power of the study, we used all available controls. In the NHS, for each case, four controls who had never reported a diagnosis of PD were randomly selected and matched on year of birth, source of DNA (blood or buccal smear), and, for cases with blood, an additional 2 controls were matched on time between blood collection, processing of the blood sample and fasting status; this last matching factor was included because we plan to use these case-control sets also for separate studies involving plasma biomarkers. Likewise, for HPFS, an initial 2 to 1 matching was performed followed by an additional 2:1 matching on plasma factors for all cases because blood was available for all cases in HPFS. Overall, the study comprised 1285 controls (724 women and 561 men).
Laboratory analysis
All SNPs in this study were chosen based on their potential functional impact, relevance to PD, and minor allele frequency greater than 10% by searching public databases and prior publications. The SNPs chosen for analysis are listed in Table 1; they included three SNPs (representing the slow acetylator phenotype) of the NAT2 gene (Ile114->Thr (rs1801280), Arg197->Gln (rs1799930) and Gly286->Gln (rs1799931) one SNP (−163A->C) of the CYP1A2 gene (rs762551), and, among women, four SNPs of the ESR1 gene (rs2077647, rs2228480, rs379857, rs1801132) and and five SNPs of the ESR2 gene (rs1256049, rs1256030, rs928554, rs1255998, rs1152579). Genotyping was performed on genomic DNA extracted from buffy coat with QIAmp (Qiagen Inc., Chatsworth, CA) using the TaqMan assay on the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA).
Table 1.
Risk of Parkinson’s disease associated with increasing number of minor alleles of the CYP1A2 polymorphism RS762551 in men and women.
| CYP1A2 | Genotype | Cases (n) | Controls (n) | RR (95% CI)1 | Adj RR (95% CI)2 | Interaction with Caffeine* |
Interaction with PMH@ |
|---|---|---|---|---|---|---|---|
| WOMEN | |||||||
| All | AA/AC/CC | 59/79/15 | 339/311/51 | 1.35 (1.02, 1.78) | 1.34 (1.02, 1.78) | 0.29 | 0.33 |
| PMH Users | AA/AC/CC | 37/53/10 | 210/191/26 | 1.53 (1.08, 2.16) | 1.51 (1.07, 2.13) | ||
| Never Users | AA/AC/CC | 16/18/3 | 93/79/19 | 1.05 (0.61, 1.82) | 1.10 (0.62, 1.97) | ||
| MEN | |||||||
| All | AA/AC/CC | 71/54/8 | 279/230/41 | 0.89 (0.66, 1.21) | 0.87 (0.64, 1.19) | 0.47 | |
Caffeine modeled as a linear variable in mg/day
PMH modeled as dichotomous (never/ever users)
adjusted for matching factors (age, source of DNA (blood or buccal smear), race)
adjusted for matching factors (age, source of DNA (blood or buccal smear), race) with additional adjustment for smoking in pack years. Analyses among ever and never users of PMH included an additional interaction term between smoking and use of PMH
Covariate assessment
Caffeine intake was obtained from the general cohort follow-up. All the questionnaires addressed the usual consumption during the previous 12 months of a specified amount foods and beverages containing caffeine (one cup for coffee and tea, one glass for soft drinks, one ounce for chocolate) and allowed nine possible response categories ranging from never to six or more per day. Caffeine intake was calculated using the US Department of Agriculture food composition sources. In these calculations, we assumed the caffeine content was as follows: 137 mg per cup of coffee, 47 mg per cup of tea, 46 mg per can or bottle of cola beverage and 7mg per serving of chocolate candy.(16) Validation studies revealed high correlation between self-reported coffee intake according to the SFFQ and consumption during 4 weeks of diet records (r = 0.78). High correlations were also observed for other caffeinated beverages (tea: 0.93; cola drinks: 0.84).[17] Information on use and type of hormone therapy was collected in the NHS beginning in 1976. PMH is presented as a binary variable, but interactions with cumulative use of PMH were also examined. The validity of reported PMH use in this study is supported by prior reports of strong significant associations between this variable and ovarian and breast cancer in the NHS cohort.[18, 19] Smoking history was also obtained as part of the general cohort follow-up. Caffeine intake and pack years of smoking used in the analyses were the cumulative average up to two years before disease onset. Information on PMH use was taken from the questionnaire two years preceding disease onset.
Statistical analysis
The assumption of Hardy-Weinberg equilibrium (HWE) was tested for all SNPs using a χ2 test comparing observed to expected genotype frequencies. To account for the matched design of the study, conditional logistic regression models controlling for all the matching factors and smoking in pack years were used to calculate relative risks (RRs) and 95% confidence intervals (CIs) to assess the relationship between the SNPs listed in Table 1 and risk of PD. Conditional logistic regression models with a term for the SNP, a term for PMH and an interaction between the SNP and PMH and between the SNP and smoking were used to calculate RRs in stratified analyses. Genotypes were coded as 0, 1, or 2 according to number of minor alleles (multiplicative model) to calculate the risk with increasing number of minor alleles. To assess the statistical significance of interactions, we used likelihood ratio tests, comparing a model including the interaction to an otherwise equivalent model without the interaction. We also examined higher order interactions with both caffeine and PMH status for each of the polymorphisms in the study.
All genotyped SNPs were used for haplotype analyses (except for CYP1A2, in which only one SNP was genotyped). Haplotypes for cases and controls were imputed using PROC HAPLOTYPE in SAS Genetics (SAS Institute, Cary, NC). The association between specific haplotypes and PD risk was assessed in conditional logistic regression models controlling for all the matching factors and smoking in pack years. Likelihood ratio tests were used to determine the overall significance of this model.
Results
The polymorphisms examined in this study are listed in the online appendix Table 1S. Genotype and allele frequencies were similar between cases and controls for all SNPs in this study (Table 1–Table 3).
Table 3.
Risk of Parkinson’s disease associated with increasing number of minor alleles of the ESR1 and ESR2 polymorphisms in women.
| PMH Status | Cases (n) | Controls (n) | Adj RR (95% CI)1 | Int with Caff* | Int with PMH@ | |
|---|---|---|---|---|---|---|
|
ESR1 | ||||||
| All | 38/75/40 | 215/305/183 | 1.07 (0.84, 1.36) | 0.47 | 0.53 | |
| 9 | Ever Users | 24/50/26 | 124/186/115 | 1.03 (0.75, 1.42) | ||
| RS2077647 | Never Users | 8/20/10 | 69/86/41 | 1.26 (0.74, 2.13) | ||
| All | 103/44/5 | 458/215/24 | 1.01 (0.73, 1.40) | 0.66 | 0.74 | |
| 10 | Ever Users | 68/28/4 | 270/137/16 | 0.93 (0.62, 1.39) | ||
| RS2228480 | Never Users | 23/13/1 | 126/62/7 | 1.16 (0.59, 2.27) | ||
| All | 44/67/43 | 187/343/178 | 1.05 (0.82, 1.34) | 0.07 | 0.58 | |
| 11 | Ever Users | 30/44/26 | 110/215/105 | 1.04 (0.76, 1.43) | ||
| RS3798577 | Never Users | 7/16/15 | 51/88/58 | 1.31 (0.79, 2.16) | ||
|
ESR2 | ||||||
| All | 143/10/0 | 644/58/0 | 0.70 (0.34, 1.44) | 0.26 | 0.03 | |
| 4 | Ever Users | 97/4/0 | 386/41/0 | 0.35 (0.12, 1.05) | ||
| RS1256049 | Never Users | 30/6/0 | 181/12/0 | 2.00 (0.63, 6.36) | ||
| All | 47/78/29 | 217/352/140 | 0.99 (0.77, 1.28) | 0.10 | 0.37 | |
| 12 | Ever Users | 32/52/17 | 129/213/88 | 0.88 (0.64, 1.20) | ||
| RS1256030 | Never Users | 12/17/8 | 62/99/34 | 1.25 (0.75, 2.09) | ||
| All | 50/79/23 | 243/337/122 | 1.02 (0.79, 1.32) | 0.48 | 0.28 | |
| 13 | Ever Users | 34/52/14 | 142/204/78 | 0.88 (0.64, 1.21) | ||
| RS928554 | Never Users | 11/19/6 | 72/93/32 | 1.38 (0.81, 2.33) | ||
| All | 126/29/2 | 545/154/9 | 0.81 (0.54, 1.22) | 0.07 | 0.68 | |
| 14 | Ever Users | 86/14/2 | 332/92/3 | 0.72 (0.41, 1.24) | ||
| RS1255998 | Never Users | 29/10/0 | 154/41/3 | 0.88 (0.37, 2.11) | ||
| All | 50/80/23 | 235/331/117 | 1.02 (0.78, 1.32) | 0.58 | 0.20 | |
| 15 | Ever Users | 34/53/13 | 140/200/74 | 0.88 (0.64, 1.22) | ||
| RS1152579 | Never Users | 11/19/7 | 69/92/31 | 1.47 (0.88, 2.45) | ||
Caffeine modeled as a linear variable in mg/day
PMH modeled as dichotomous (never/ever users)
adjusted for matching factors (age, source of DNA (blood or buccal smear), race) with additional adjustment for smoking in pack years Analyses among ever and never users of PMH included an additional interaction term between smoking and use of PMH
All the SNPs except ESR1 rs1801132 in women and all SNPs in men confirmed to the Hardy-Weinberg equilibrium (HWE). The ESR1 rs1801132 polymorphism in women violated the HWE assumption (p = 0.0005), and was removed from subsequent analyses.
The overall relative risk associated with each additional 100mg caffeine intake in a linear model was 0.92 (0.82, 1.04) in men and 0.97 (95% CI: 0.88, 1.07) in women. Among women, the overall relative risk of PD associated with use of post-menopausal hormones was 1.21 (95% CI: 0.79, 1.87).
The CYP1A2 rs762551 polymorphism was associated with a marginally increased risk of PD in women (RR = 1.34; 95% CI 1.02, 1.78), but not in men (Table 1). When stratified by use of PMH, the association between this SNP and PD appeared slightly stronger in users of PMH (RR = 1.51; 95% CI 1.07, 2.13) than among non-users (RR = 1.10; 95% CI 0.62, 1.97).
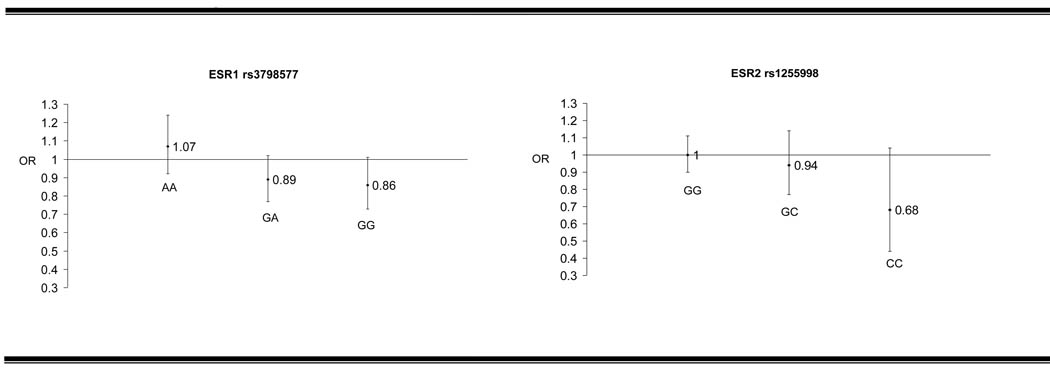
Neither the NAT2 slow acetylator phenotype nor any of the ESR1 or ESR2 polymorphisms in this study were associated with an increased risk of PD (Table 2 and Table 3). No statistically significant (p<0.05) interactions were observed between any of the polymorphisms in this study or the NAT2 acetylator phenotype and caffeine intake (Table 3). For polymorphisms where the p-interaction with caffeine was below 0.10 (ESR1 rs3798577 and ESR2 rs1255998), the effect of caffeine intake in increments of 100mg per day by genotype is shown in Figure 1.
Table 2.
Risk of Parkinson’s disease associated with NAT2 slow and fast acetylators.
| NAT2 | Genotype | %Cases (n) | %Controls (n) | RR (95% CI)1 | Adj RR (95% CI)2 | Interaction with Caffeine* |
Interaction with PMH@ |
|---|---|---|---|---|---|---|---|
| WOMEN | |||||||
| All | |||||||
| Fast | 40(59) | 44(298) | 1.00 (Ref) | 1.00 (Ref) | 0.32 | 0.85 | |
| Slow# | 60(90) | 57^(387) | 1.13 (0.77, 1.64) | 1.11 (0.76, 1.62) | |||
| PMH Ever | |||||||
| Users | |||||||
| Fast | 38 (37) | 44(183) | 1.00 (Ref) | 1.00 (Ref) | |||
| Slow# | 62(60) | 56(230) | 1.14 (0.72, 1.82) | 1.13 (0.71, 1.80) | |||
| Never | |||||||
| Users | |||||||
| Fast | 42 (16) | 40(76) | 1.00 (Ref) | 1.00 (Ref) | |||
| Slow# | 58 (22) | 60 (115) | 1.21 (0.55, 2.66) | 1.23 (0.55, 2.73) | |||
| MEN | |||||||
| Fast | 42 (54) | 39 (206) | 1.00 (Ref) | 1.00 (Ref) | 0.73 | ||
| Slow# | 58 (76) | 61 (327) | 0.87 (0.59, 1.30) | 0.84 (0.56, 1.26) | |||
Caffeine modeled as a linear variable in mg/day
slow acetylators defined as possessing 2 or more mutant alleles of NAT2 G286E (rs1799931), NAT2 I114T (rs1801280), NAT2 R197Q (rs1799930)
adjusted for matching factors (age, source of DNA (blood or buccal smear), race)
adjusted for matching factors (age, source of DNA (blood or buccal smear), race) with additional adjustment for smoking in pack years. Analyses among ever and never users of PMH included an additional interaction term between smoking and use of PMH
PMH modeled as dichotomous (never/ever users)
Numbers do not sum to 100% because of rounding.
Figure 1.
Effect of 100mg increase in caffeine intake1 according to genotype for ESR1 rs3798577 and ESR2 rs125599
1adjusted for matching factors (age, source of DNA (blood or buccal smear), race) with additional adjustment for smoking in pack years
A marginally significant interaction (p = 0.03) was observed between the ESR1 rs1256049 polymorphism and use of PMH among women. In ever users of PMH, this polymorphism was associated with a marginally decreased risk of PD (RR = 0.35; 95% CI 0.12, 1.05) and among never users of PMH this SNP was associated with a marginally increased risk of PD (RR = 2.00; 95% CI 0.63, 6.36). No significant interactions were observed between any of the other ESR1 and ESR2 polymorphisms (Table 3) nor the CYP1A2 rs762551 polymorphism (Table 1), or the NAT2 acetylator phenotype (Table 2) and use of PMH or No significant interactions were observed with duration of PMH use for any of the polymorphisms and the interaction of the ESR2 rs1256049polymorphism with duration of PMH use was not significant (p = 0.17).
In addition to it’s marginally significant interaction with caffeine (Table 3) we observed a significant three-way interaction with caffeine (linear) and PMH use (ever/never) and ESR2 rs1255998 (p= 0.05). Among ever-users of PMH, we observed a significant interaction of these polymorphism with caffeine (p = 0.04), whereas among never-users of PMH, the interaction was not significant. No other significant higher-order interactions were observed.
Haplotype analyses using all of the genotyped SNPs resulted in 3 haplotypes for NAT2 (men and women), 8 haplotypes for ESR1 (women only) and 3 haplotypes for ESR2 (women only) predicted to occur with greater than 5% frequency (Table 4). The haplotypes, individually or overall, were not associated with an increased risk of PD (Table 4).
Table 4.
Common NAT2, ESR1 and ESR2 haplotypes and risk of Parkinson’s disease in men and women.
| % Cases (n) | % Controls (n) | RR (95% CI)1 | Adj RR (95% CI)2 | ||
|---|---|---|---|---|---|
| WOMEN | |||||
| NAT2 | |||||
| G-C-G | 48 (153) | 45 (654) | Ref | Ref | |
| G-T-C | 27 (85) | 27 (386) | 0.93 (0.68, 1.27) | 0.95 (0.69, 1.30) | |
| G-T-G | 22 (69) | 26 (367) | 0.79 (0.57, 1.10) | 0.80 (0.58, 1.12) | |
| Other (<5%) | 3 (10) | 2 (33) | Model p-value†† | 0.04 | |
| ESR1 | |||||
| C-G-A-C | 22(70) | 20 (284) | Ref | Ref | |
| T-G-A-C | 18(56) | 18 (255) | 1.37 (0.69, 2.74) | 1.35 (0.65, 2.78) | |
| C-G-G-C | 13(41) | 12(167) | 1.29 (0.58, 2.90) | 1.29 (0.54, 3.04) | |
| T-G-A-C | 10(33) | 12(170) | 0.67 (0.33, 1.33) | 0.63 (0.31, 1.30) | |
| T-A-G-C | 8(24) | 8(113) | 2.96 (1.00, 8.72) | 3.11 (0.99, 9.76) | |
| T-G-A-G | 7(22) | 8(110) | 1.01 (0.42, 2.39) | 0.95 (0.39, 2.28) | |
| T-G-A-G | 7(23) | 7(97) | 0.61 (0.20, 1.84) | 0.46 (0.14, 1.49) | |
| T-G-G-G | 6(18) | 5(71) | 0.82 (0.23, 2.94) | 0.92 (0.25, 3.36) | |
| Other (<5%) | 10(31) | 12(175) | Model p-value†† | 0.99 | |
| ESR2 | |||||
| C-G-T-G-G | 46 (148) | 45(645) | Ref | Ref | |
| C-A-C-G-A | 41(130) | 41(587) | 0.93 (0.69, 1.25) | 0.95 (0.71, 1.28) | |
| C-G-T-C-G | 5(17) | 6(82) | 0.83 (0.39, 1.79) | 0.84 (0.39, 1.83) | |
| Other (<5%) | 7(23) | 9(125) | Model p-value†† | 0.41 | |
|
MEN | |||||
| NAT2 | |||||
| G-C-G | 46 (127) | 45(504) | Ref | Ref | |
| G-T-C | 28 (78) | 31(339) | 0.93 (0.67, 1.31) | 0.92 (0.65, 1.31) | |
| G-T-G | 22(61) | 23(250) | 0.94 (0.65, 1.35) | 0.98 (0.67, 1.42) | |
| Other (<5%) | 3(8) | 1(16) | Model p-value†† | 0.91 | |
Conditional logistic regression
adjusted for matching factors (age, source of DNA (blood or buccal smear), race)
adjusted for matching factors (age, source of DNA (blood or buccal smear), race) with additional adjustment for smoking in pack years
χ2 (2 d.f.) test comparing model without haplotypes (log additive coding) to one with all haplotypes predicted to occur with greater than 5% frequency
Discussion
We report a marginally increased risk of PD with increasing number of minor alleles (C allele) of the CYP1A2 rs762551 polymorphism. The CYP1A2 enzyme is part of the cytochrome P450 system. It is a hepatic enzyme primarily responsible for the metabolism of a number of substances, including drugs, caffeine, environmental toxins and estrogens.[20] This enzyme is responsible for more than 90 percent of caffeine metabolism.[21] In humans, the first step in the biotransformation of caffeine, the microsomal caffeine 3-demethylation in the liver, is catalyzed by CYP1A2.[22] CYP1A2 also metabolizes MPTP, a neurotoxin that induces Parkinsonism.[23] The rs762551 polymorphism of CYP1A2 is believed to either be a direct cause of decreased CYP1A2 activity, or to be linked to other genetic variants conferring lower inducibility and reduced caffeine clearance.[7] CYP1A2 rs762551 has also been linked to decreased serum estradiol levels,[20] suggesting that, given an association between this polymorphism and risk of PD, this enzyme may be implicated in the interaction between caffeine and estrogen use and risk of PD. Cornelis et al.[24] reported that this polymorphism modified the relationship between coffee intake and risk of myocardial infarction (MI). In carriers of at least one mutant allele of CYP1A2 rs762551, caffeine was associated with an increased risk of MI, whereas no association with caffeine was observed in non-carriers; the interaction between caffeine and PMH was not modified by CYP1A2. However, because we did not observe an interaction between CYP1A2 and either caffeine or PMH in this study, it is difficult to make a connection between this enzyme and the role of caffeine or estrogen in Parkinson disease,
Only one previous study has examined the association between this polymorphism and risk of PD. Tan et al.,[25] examined the effect of the CYP1A2 rs762551 polymorphism on risk of PD and the interaction of this SNP with caffeine intake in men and women. They observed an OR for PD for allele A vs. allele C of 0.827 (0.675, 1.104), p = 0.068, which correspond to an OR of 1.21 for allele C vs. allele A. This association is in the same direction as that observed among women in our study. Tan et al did not report significant interactions between caffeine and CYP1A2.
Forsyth et al[26] measured activity of CYP1A2 in PD patients and controls, in both treated and untreated PD patients, and in controls and did not find significant differences between these groups; genotypes were not reported.
We did not observe associations between the NAT2 acetylator phenotype and risk of PD. In some prior studies, NAT2 slow acetylators were reported to have an increased risk of PD,[9, 27] whereas other studies have been null.[28] In addition to combining the NAT2 SNPs into a slow/fast acetylator phenotype, we examined the effect of each individual NAT2 SNP (rs1799931, rs180280, and rs1799930) and did not observe significant associations with risk of PD or interactions with caffeine or PMH.
The interaction of ESR2 rs1256049 with PMH and the marginal interactions of ESR1 rs3798577 and ESR2 rs1255998 with caffeine deserve further investigation. The rs1256049 polymorphisms is a silent synonymous polymorphism and has been hypothesized to lead to changes in mRNA folding and differences in mRNA translation or stability. [29,30] To our knowledge, this polymorphism has not been previously associated with altered risk of Parkinson disease. Both ESR1 rs3798577 and ESR2 rs1255998 have previously been associated with lower serum estrogen concentrations.[31] Therefore, the observation that, in women carrying two mutant alleles of either of these polymorphisms, caffeine has a stronger protective effect then it does among women with no mutant alleles (Figure 1) supports the hypothesis that the protective effect of caffeine appears to be strongest in the relative absence of estrogen.
Important strengths of this study are the nested case-control design, which reduces bias due to selection of controls, the reliance on well-established cohorts with high rates of follow-up (response rates over 90%), and the availability of prospectively collected, updated, and validated information on caffeine consumption, PMH use or smoking.[2, 32] Furthermore, participants in the NHS and the HPFS have excellent access to health care, and the large majority of participants diagnosed with PD were followed by a neurologist or movement disorder specialist. This does not preclude diagnostic errors, and some level of misclassification is almost inevitable, but this is likely to be small, because using a neuropathological diagnosis as the standard, the positive predictive values for a clinical diagnosis of PD made by neurologists range from 85 to 98%.[15]
The main limitation of our study is the relatively small sample size. Whereas power was good for gene main effects – we estimated 80% power to detect odds ratios of greater than 1.18 for SNP with a minor allele frequency of 10% or greater. -- the power to detect interactions of moderate strength was modest, and therefore null results should be interpreted cautiously. Furthermore, some of the results may be due to chance. None of the p-values for the reported associations reached the statistical significance level that would be required if we apply a Bonferroni correction taking into account the 13 tests performed in women (significance p-value = 0.0038) and the 4 tests done in men (significance p-value = 0.0125).
In summary, we did not find convincing evidence that variations in CYP1A2, NAT2, ESR1 and ESR2 predict risk of PD or modify the association of caffeine with PD. The minor C allele of the rs762551 polymorphism of CYP1A2 was associated with an increased risk of PD among women, but not among men, but, even among women, this association was of marginal significance and could have occurred by chance. Possible interactions between PMH and ESR2 rs1256049 and between caffeine and ESR1 rs3798577 and ESR2 rs1256049 were of marginal significance.
Supplementary Material
Acknowledgements
This work was funded by NIH grant R01 NS048517. Natalia Palacios is supported by the Training Program in Environmental Epidemiology, NIH Kirshstein National Research Service Award, T32 ES07069. K. Simon is supported by NIH Kirshstein National Research Service Award T32 ES16645-01
The authors would like to thank Eilis O’Reilly for statistical and programming help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics
These studies [NHS and HPFS] were approved by the Human Subjects Research Committees at Harvard School of Public Health and Brigham and Women's Hospital.
N. Palacios performed the data analysis, wrote the first draft of the manuscript and edited subsequent drafts.M. Weisskopf provided scientific, analysis and statistical advice and reviewed in detail the manuscript drafts and the analysis.K. Simon provided analysis and statistical advice and reviewed drafts of the manuscript.M. Schwarzchild provided scientific guidance and reviewed drafts of the manuscript.A. Ascherio was responsible for securing funding, the concept and design of the study, assisting with data analysis and statistical support, and assisting with manuscript writing and editing.
References
(*The additional 2 references are included at the suggestion of the reviewers.)
- 1.Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. Jama. 2000 May 24–31;283(20):2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 2.Ascherio A, Zhang SM, Hernán MA, Kawachi I, Colditz GA, Speizer FE, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol. 2001;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 3.Benedetti MD, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, et al. Smoking, alcohol, and coffee consumption preceding Parkinson's disease: a case-control study. Neurology. 2000;55(9):1350–1358. doi: 10.1212/wnl.55.9.1350. [DOI] [PubMed] [Google Scholar]
- 4.Ascherio A, Weisskopf MG, O'Reilly EJ, McCullough ML, Calle EE, Rodriguez C, et al. Coffee consumption, gender, and Parkinson's disease mortality in the Cancer Prevention Study-II cohort: the modifying effects of estrogen. Am J Epidemiol. 2004;160(10):977–984. doi: 10.1093/aje/kwh312. [DOI] [PubMed] [Google Scholar]
- 5.Schwarzschild MA, Xu K, Oztas E, Petzer JP, Castagnoli K, Castagnoli N, Jr, et al. Neuroprotection by caffeine and more specific A2A receptor antagonists in animal models of Parkinson's disease. Neurology. 2003 Dec 9;61(11 Suppl 6):S55–S61. doi: 10.1212/01.wnl.0000095214.53646.72. [DOI] [PubMed] [Google Scholar]
- 6.Hong CC, Tang BK, Hammond GL, Tritchler D, Yaffe M, Boyd NF. Cytochrome P450 1A2 (CYP1A2) activity and risk factors for breast cancer: a cross-sectional study. Breast Cancer Res. 2004;6(4):R352–R365. doi: 10.1186/bcr798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachse C, Brockmoller J, Bauer S, Roots I. Functional significance of a C>A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. British Journal of Clinical Pharmacology. 1999;47(4):445–449. doi: 10.1046/j.1365-2125.1999.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum M, Demierre A, Grant DM, Heim M, Meyer UA. Molecular Mechanism of Slow Acetylation of Drugs and Carcinogens in Humans. Proceedings of the National Academy of Sciences. 1991 June 15;88(12):5237–5241. doi: 10.1073/pnas.88.12.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan DK, Lam MK, Wong R, Hung WT, Wilcken DE. Strong association between N-acetyltransferase 2 genotype and PD in Hong Kong Chinese. Neurology. 2003 Mar 25;60(6):1002–1005. doi: 10.1212/01.wnl.0000052787.87093.b8. [DOI] [PubMed] [Google Scholar]
- 10.Xu K, Xu Y, Brown-Jermyn D, Chen JF, Ascherio A, Dluzen DE, et al. Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J Neurosci. 2006 Jan 11;26(2):535–541. doi: 10.1523/JNEUROSCI.3008-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imamov O, Shim G-J, Warner M, Gustafsson J, Aring ke. Minireview. Estrogen Receptor beta in Health and Disease1. Biology of Reproduction. 2005 November 01;73(5):866–871. doi: 10.1095/biolreprod.105.043497. [DOI] [PubMed] [Google Scholar]
- 12.Ioannidis JP, Ralston SH, Bennett ST, Brandi ML, Grinberg D, Karassa FB, et al. Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. JAMA. 2004 Nov 3;292(17):2105–2114. doi: 10.1001/jama.292.17.2105. [DOI] [PubMed] [Google Scholar]
- 13.Maraganore DM, Farrer MJ, McDonnell SK, Elbaz A, Schaid DJ, Hardy JA, et al. Case-control study of estrogen receptor gene polymorphisms in Parkinson's disease. Mov Disord. 2002 May;17(3):509–512. doi: 10.1002/mds.1253. [DOI] [PubMed] [Google Scholar]
- 14.Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol. 2001 Jul;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 15.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson's disease. Neurology. 2001;57(8):1497–1499. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Stampfer MJ, Manson JE, Colditz GA, Rosner BA, Speizer FE, et al. Coffee consumption and coronary heart disease in women. A ten-year follow-up. JAMA. 1996;275:458–462. doi: 10.1001/jama.1996.03530300042038. [DOI] [PubMed] [Google Scholar]
- 17.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989 Dec;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 18.Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE, Stampfer MJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332(24):1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 19.Susan E Hankinson GACDJHWCWMJSBRCHHFES. A prospective study of reproductive factors and risk of epithelial ovarian cancer. Cancer. 1995;76(2):284–290. doi: 10.1002/1097-0142(19950715)76:2<284::aid-cncr2820760219>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Lurie G, Maskarinec G, Kaaks R, Stanczyk FZ, Le Marchand L. Association of Genetic Polymorphisms with Serum Estrogens Measured Multiple Times During a 2-Year Period in Premenopausal Women. Cancer Epidemiol Biomarkers Prev. 2005 June 1;14(6):1521–1527. doi: 10.1158/1055-9965.EPI-04-0746. [DOI] [PubMed] [Google Scholar]
- 21.Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang R, Puranam RS, Butler LS, Qian WH, He XP, Moyer MB, et al. Autoimmunity to munc-18 in Rasmussen's encephalitis. Neuron. 2000 Nov;28(2):375–383. doi: 10.1016/s0896-6273(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 23.Coleman T, Ellis SW, Martin IJ, Lennard MS, Tucker GT. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is N-demethylated by cytochromes P450 2D6, 1A2 and 3A4--implications for susceptibility to Parkinson's Disease. Journal of Pharmacology and Experimental Therapeutics. 1996;277(2):685–690. [PubMed] [Google Scholar]
- 24.Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA. 2006 Mar 8;295(10):1135–1141. doi: 10.1001/jama.295.10.1135. [DOI] [PubMed] [Google Scholar]
- 25.Tan EK, Chua E, Fook-Chong SM, Teo YY, Yuen Y, Tan L, et al. Association between caffeine intake and risk of Parkinson's disease among fast and slow metabolizers. Pharmacogenet Genomics. 2007 Nov;17(11):1001–1005. doi: 10.1097/FPC.0b013e3282f09265. [DOI] [PubMed] [Google Scholar]
- 26.Forsyth JT, Grunewald RA, Rostami-Hodjegan A, Lennard MS, Sagar HJ, Tucker GT. Parkinson's disease and CYP1A2 activity. Br J Clin Pharmacol. 2000;50(4):303–309. doi: 10.1046/j.1365-2125.2000.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandmann O, Vaughan J, Holmans P, Marsden CD, Wood NW. Association of slow acetylator geotype for N-acetyltransferase 2 with familial Parkinson's Disease. The Lancet. 1997;350:1136–1139. doi: 10.1016/s0140-6736(97)03495-8. [DOI] [PubMed] [Google Scholar]
- 28.van der Walt JM, Martin ER, Scott WK, Zhang F, Nance MA, Watts RL, et al. Genetic polymorphisms of the N-acetyltransferase genes and risk of Parkinson's disease. Neurology. 2003 Apr 8;60(7):1189–1191. doi: 10.1212/01.wnl.0000055929.84668.9a. [DOI] [PubMed] [Google Scholar]
- 29.Rexrode KM, Ridker PM, Hegener HH, Buring JE, Manson JE, Zee RYL. Polymorphisms and Haplotypes of the Estrogen Receptor-{beta} Gene (ESR2) and Cardiovascular Disease in Men and Women. Clin Chem. 2007 October 1;53(10):1749–1756. doi: 10.1373/clinchem.2007.091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, Gelernter J, et al. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003 February 1;12(3):205–216. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- 31.Sowers MR, Wilson AL, Karvonen-Gutierrez CA, Kardia SR. Sex steroid hormone pathway genes and health-related measures in women of 4 races/ethnicities: the Study of Women's Health Across the Nation (SWAN) Am J Med. 2006 Sep;119(9 Suppl 1):S103–S110. doi: 10.1016/j.amjmed.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Ascherio A, Chen H, Schwarzschild MA, Zhang SM, Colditz GA, Speizer FE. Caffeine, postmenopausal estrogen, and risk of Parkinson's disease. Neurology. 2003 Mar 11;60(5):790–795. doi: 10.1212/01.wnl.0000046523.05125.87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.