Abstract
Selenoproteins are a family of proteins which share the common feature of containing selenocysteine, ‘the 21st amino acid’. Selenocysteine incorporation occurs during translation of selenoprotein messages by redefinition of UGA codons, which normally specify termination of translation. Studies of the eukaryotic selenocysteine incorporation mechanism suggest that selenocysteine insertion is inefficient compared to termination. Nevertheless, Selenoprotein P and several other selenoproteins are known to contain multiple selenocysteines. The production of full length protein from these messages would seem to demand highly efficient selenocysteine incorporation due to the compounding effect of termination at each UGA codon. We present data demonstrating that efficient incorporation of multiple selenocysteines can be reconstituted in rabbit reticulocyte lysate translation reactions. Selenocysteine incorporation at the first UGA codon is inefficient but increases approximately 10 fold at subsequent downstream UGA codons. We find that ribosomes in the “processive” phase of selenocysteine incorporation (i.e. after decoding the first UGA codon as Selenocysteine) are fully competent to terminate translation at UAG and UAA codons, that ribosomes become less efficient at selenocysteine incorporation as the distance between UGA codons is increased, and that efficient selenocysteine incorporation is not dependent on cis-acting elements unique to Selenoprotein P. Further, we find that the percentage of ribosomes decoding a UGA codon as selenocysteine rather than termination can be increased three to five fold by placing the Murine Leukemia Virus UAG read-through element upstream of the first UGA codon or by providing a competing messenger RNA in trans. The mechanism of selenocysteine incorporation and selenoprotein synthesis are discussed in light of these results.
Keywords: Selenocysteine, Read-through, Redefinition, Termination, Selenoprotein
Introduction
Selenium has long been known for its beneficial antioxidant properties which is attributable to incorporation of this trace element into selenoproteins. Incorporation of selenium in the form of the 21st amino acid, selenocysteine, occurs co-translationally and is encoded in a subset of messages by UGA codons. The greater effectiveness of selenocysteine than cysteine for catalyzing redox reactions is likely the reason nature has evolved and maintained the specialized machinery required to produce and incorporate selenocysteine into proteins.
Ribosome reprogramming to decode a UGA codon as selenocysteine requires cis-acting signals in the RNA as well as a number of trans-acting factors. In eukaryotes, a secondary structure called the selenocysteine insertion sequence (SECIS) element is found in the 3’ untranslated region (3’ UTR) of selenoprotein messages. The presence of the SECIS element is necessary to direct message specific selenocysteine incorporation at UGA codons 1–3. Another cis-acting element which increases the efficiency of selenocysteine incorporation is a selenocysteine redefinition element (SRE), found immediately downstream of UGA codons in a subset of selenoprotein messages 4,5. A trans-acting factor, SECIS binding protein 2 (SBP2) has been shown to bind to eukaryotic SECIS elements 6,7 as well as the ribosome 7,8. SBP2 recruits EFsec 9,10, a specialized elongation factor 10,11 that ultimately delivers the Sec-tRNA[Ser]Sec to the decoding center of the ribosome. Other trans-acting factors have recently been implicated in the selenocysteine incorporation mechanism and it’s regulation including the ribosomal protein L30 12, nucleolin 13,14, and eIF4a3 15, each of which can interact directly with SECIS elements. However, the exact role of these latter proteins is not yet clear.
Of direct importance to the production and regulation of eukaryotic selenoproteins is the efficiency of selenocysteine incorporation. For selenoprotein genes with a single selenocysteine encoding UGA codon, termination appears to be the predominant event in E. coli 16, in rabbit reticulocyte in vitro translation reactions 17,18, and in transiently transfected mammalian cells 10,19. This suggests that selenocysteine incorporation is inherently inefficient relative to termination 19.
Several selenoprotein genes have now been identified with the potential to encode more than one selenocysteine residue. These include iodothyronine deiodinase 2 (DI2), an alternatively spliced isoform of selenoprotein N (SelN alternate transcript 1), selenoprotein L (SelL), and selenoprotein P (SelP). DI2, SelN transcript 1, and SelL each have two UGA codons. The total number of selenocysteines in SelP, ranges among species from 10 in humans to as many as 28 in sea urchin 20. The second and all downstream selenocysteine amino acids cluster near the Cterminus of SelP and appear to be required in mammals for delivery of selenium from the liver to various other tissues, most importantly the brain and testes 21. Purification of selenoprotein P from rat plasma revealed several isoforms of the protein. These isoforms were shown by carboxypeptidase sequencing 22 and mass spectrometry 23 to comprise a significant amount of full-length protein and several prematurely UGA terminated peptides. In contrast to the model of inefficient selenocysteine incorporation described above, production of full length SelP would require highly efficient selenocysteine incorporation due to the cumulative loss of product caused by termination at each UGA codon.
It has been suggested that SelP may utilize a special mechanism for selenocysteine incorporation due to the exceptional number of UGA codons and the observation that SelP messages are unique in having two conserved 3’ UTR SECIS elements. A recent study 24, suggests that each of the two SECIS elements in the zebrafish SelP message have different functions. In this model, the second SECIS, SECIS 2, serves to incorporate selenocysteine at the first UGA. It does so inefficiently, acting as a checkpoint for selenocysteine incorporation factors and to restrict the number of downstream ribosomes. The first SECIS, SECIS 1, is then dedicated for redefinition of the remaining 16 UGA codons near the 3’ end of the open reading frame. It was concluded that the SelP gene has evolved unique properties to accommodate the incorporation of multiple selenocysteines into one polypeptide.
The results presented here demonstrate that efficient selenocysteine incorporation can be reconstituted in rabbit reticulocyte lysate translation reactions. As previously reported for selenoprotein P sequences expressed in cultured cells 24, selenocysteine incorporation at the first UGA was inefficient but highly processive at downstream UGA codons. We demonstrate that processivity of selenocysteine incorporation is not dependent on sequence elements unique to SelP but can be recapitulated with SECIS elements from other selenoprotein messages or when the UGA codons are in non-SelP sequence contexts. A modified model for selenocysteine incorporation is proposed based on the results presented below.
Results
The efficiency of UGA codon redefinition to encode selenocysteine was measured using a dual luciferase reporter vector, pDluc, designed for expression of the reporter genes in rabbit reticulocyte lysate transcription and translation reactions or in cultured cells. The pDluc constructs used here contain both the Renilla and Firefly luciferase reporter genes flanking the UGA codon(s) of interest, and a SECIS element(s) inserted downstream of the Firefly luciferase gene in the 3’ UTR to support selenocysteine insertion (Materials and Methods). The following constructs were transcribed and translated in Rabbit Reticulocyte Lysate supplemented with the c-terminal 447 amino acids of SBP2 (SBP2-CT), which has been shown to be proficient for selenocysteine incorporation 7 . To determine the efficiency of selenocysteine insertion at each position during translation of messages containing more than one UGA codon, it is necessary to separate the resulting radio-labeled protein products by SDS polyacrylamide gel electrophoresis (SDS-PAGE). The products corresponding to termination at each UGA, UAA or UAG codon were quantified by phosphorimage analysis of SDS-PAGE gels and the efficiency of selenocysteine incorporation or ribosome read-through was determined by comparison of the quantified products. Where there are more than one UGA codons, the reported efficiency of selenocysteine incorporation at downstream UGA codons takes into account only those ribosomes which have read through the upstream UGA codons (Materials and Methods). Verification that read-through of a UGA codon was due to selenocysteine incorporation and not near-cognate tRNA decoding at the UGA codon was provided by the dependence of read-through on a SECIS element in the 3’UTR, the UGA codon, and the addition of SBP2-CT to the translation reaction. No read-through of UGA codons was observed when either the SECIS element(s) was lacking, the UGA codon was changed to UAG or UAA, or SBP2-CT was excluded from the reaction.
Selenocysteine incorporation efficiency during translation of messages containing more than one UGA codon
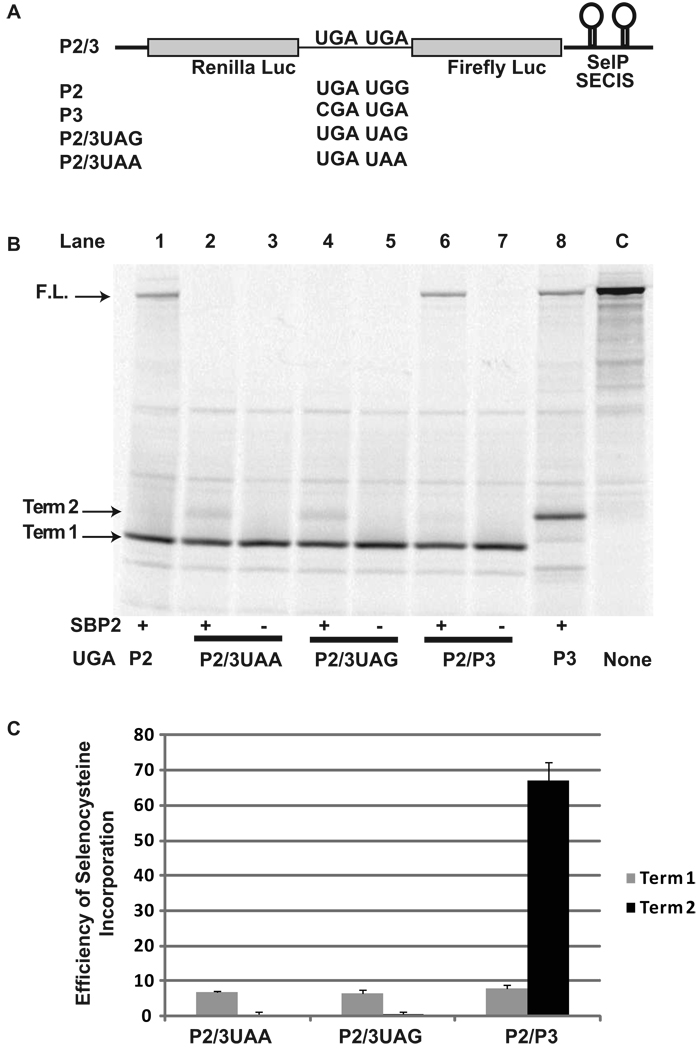
To determine the efficiency of selenocysteine incorporation when two UGA codons were present in one messenger RNA, we created a reporter construct P2/3 in which 130 nucleotides containing the second and third UGA codons of the human SelP gene were cloned between the Renilla and Firefly genes of pDluc containing SECIS 1 and SECIS 2 of SelP downstream of the Firefly coding sequence (Figure 1A) (See Materials and Methods, S1/S2). To determine if read-through at the second UGA codon was due to a general decrease in termination efficiency, two additional reporter constructs were made in which the second UGA codon was changed to either UAA or UAG, P2/3UAA and P2/3UAG respectively.
Figure 1.
Selenocysteine incorporation efficiency during decoding of a message with two UGA codons. A) Schematic representation of the reporter messages: P2/3 is shown in whole. P2, P3, P2/3UAG, and P2/3UAA are identical except for the indicated changes to the UGA codons. B) 35S labeled proteins from rabbit reticulocyte lysate transcription and translation reactions in the presence (+) or absence (−) of SBP2-CT (SBP2) were separated by SDS-PAGE. The messages translated were: Lane 1, P2; Lanes 2 and 3, P2/3UAA; Lanes 3 and 4, P2/3UAG; Lanes 5 and 6, P2/P3; Lane 6, P3 Lane 7, Control with no UGA codons. The position for protein products corresponding to termination at the first (Term 1) or second UGA/UAG/UAA (Term 2) and the full length (F.L.) protein are indicated by arrows. C) Quantitative analysis of selenocysteine incorporation efficiency at the first and second Selenocysteine/termination codons for P2/3UAA, P2/3UAG, P2/3. Bars are the average of two independent experiments (n=6). Error bars are the standard deviation from the mean.
These constructs were transcribed and translated in SBP2-CT supplemented Rabbit Reticulocyte Lysate (RRL) reactions. The resulting L-[35S]-Methionine labeled products were separated by SDS-PAGE (Figure 1B) and the termination and full length products quantified as described in Materials and Methods. Read-through of the first UGA was inefficient with approximately 7% selenocysteine incorporation (Figure 1B, lane 6 (term 1); Figure 1C). Read-through of the second UGA codon occurred with much higher efficiency, 67% (p < 0.001), a nearly 10 fold increase (Figure 1B, lane 6 (term2); Figure 1C). No full length product was detectable when the second UGA was changed to UAA or UAG (Figure 1B, lanes 2 and 4) or when SBP2-CT was excluded from the RRL transcription and translation reactions (Figure 1B, lanes 3, 5, and 7).
SECIS effects on Selenocysteine incorporation efficiency
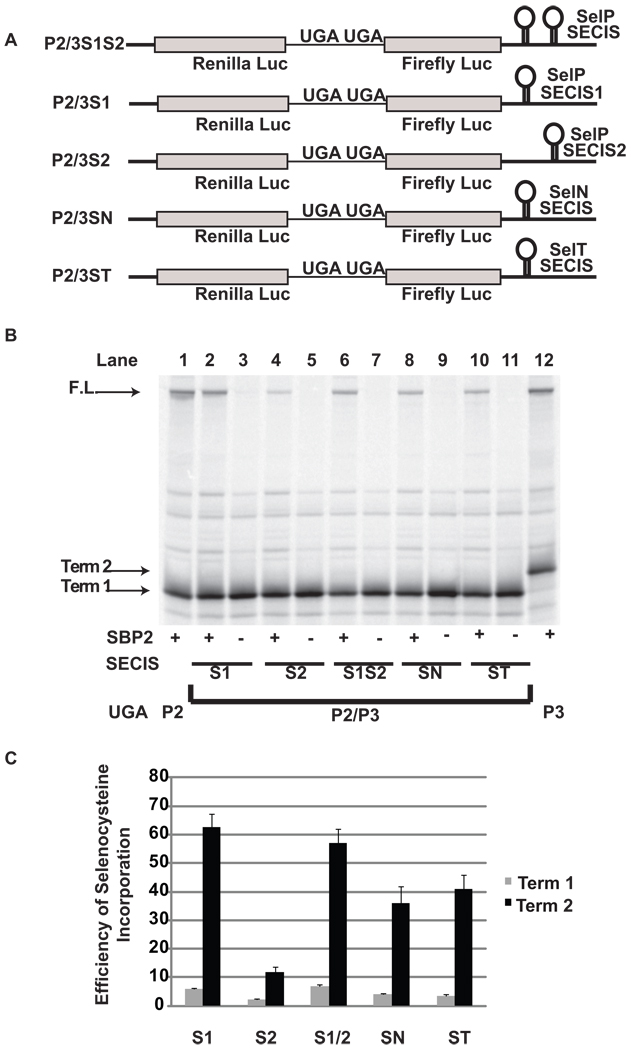
To determine the requirement for the two SelP SECIS elements to support high level decoding of the second UGA as selenocysteine, SECIS 1 and SECIS 2 (S1/S2) were replaced with either the first SECIS element alone (P2/3S1), the second SECIS element alone (P2/3S2), the SECIS element from SelN (P2/3SN), or the SECIS element from Selenoprotein T Sel T (P2/3ST) (Figure 2A). Selenocysteine insertion at the first UGA codon was inefficient ranging from 2 to 7% depending on the identity of the SECIS element(s), and in all cases selenocysteine insertion was significantly higher at the second UGA codon: P2/3S1 (6% and 63%), P2/3[S1/S2] (7% and 57%), P2/3SN (4% and 36%), P2/3ST (4% and 41%), P2/3S2 (2% and 12%) (Figure 2B, lanes 2, 4, 6, 8, and 10; Figure 2C) (p < 0.001). Consistent with previously published results 24, selenocysteine insertion efficiency for SECIS 1 from selenoprotein P was not significantly different than that observed with the combination of SECIS 1 and 2. It is notable that there was a strong positive correlation between the efficiency of read-through of the second UGA relative to the first UGA (See Discussion; Figure 8) which was dependent upon the identity of the SECIS element.
Figure 2.
SECIS effects on selenocysteine incorporation efficiency during decoding of a message with two UGA codons. A) Schematic representation of the reporter messages: P2/3 with the SECIS elements from SelP (2/3S1S2, P2/3S1, P2/3S2); SelN (P2/3SN); and SelT (P2/3ST) cloned into the 3’ UTR. B) 35S labeled proteins resulting from rabbit reticulocyte lysate transcription and translation reactions in the presence (+) or absence (−) of SBP2-CT (SBP2) were separated by SDS-PAGE . The messages translated were: Lane 1, P2; Lanes 2 and 3, P2/3S1; Lanes 4 and 5, P2/P3S2; Lanes 6 and 7, P2/P3S1S2; Lanes 8 and 9, P2/3SN, Lanes 10 and 11, P2/3ST; Lane 12, P3. The position for protein products corresponding to termination at the first (Term 1) or second (Term 2) UGA and the full length (F.L.) protein are indicated by arrows. C) Quantitative analysis of selenocysteine incorporation efficiency at the first and second Selenocysteine/termination codons. Bars are the average of two independent experiments (n=6). Error bars are the standard deviation from the mean.
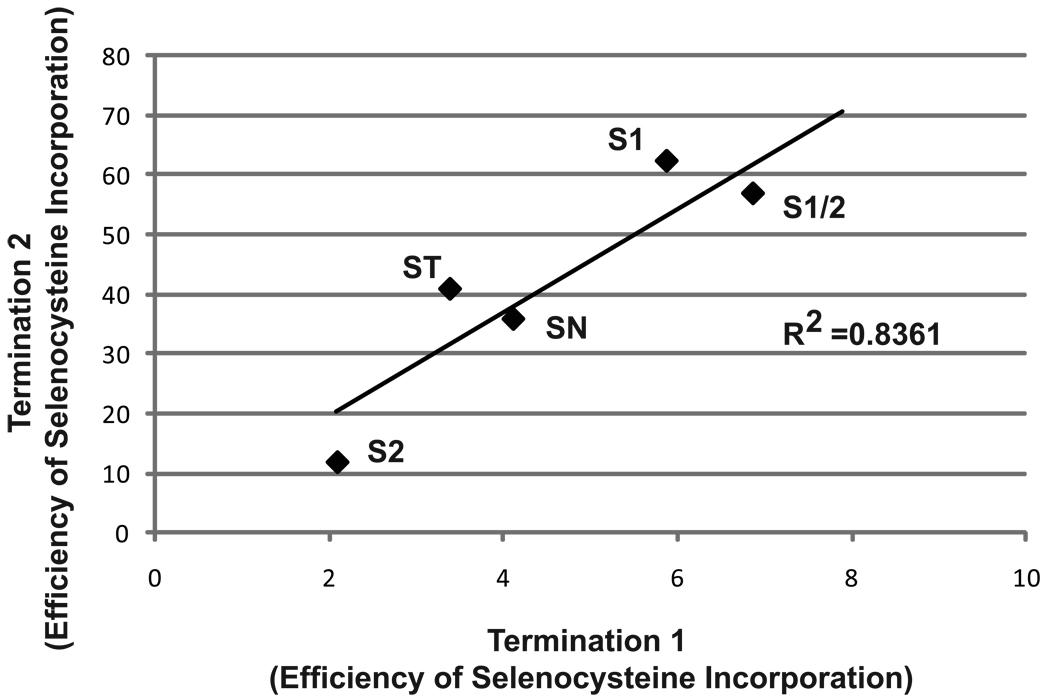
Figure 8.
Correlation of selenocysteine incorporation at the first and second UGA codons. The data from Figure 2 is shown with the efficiency of selenocysteine incorporation at the first UGA (Termination 1) on the X axis and the efficiency of selenocysteine incorporation at the second UGA on the Y axis (Termination 2). S1, S1/2, S2, ST, SN represent the data from analysis of the protein products produced from P2/3S1, P2/3S1S2. P2/3S2, P2/3ST, and P2/3SN.
Distance effects on selenocysteine incorporation efficiency
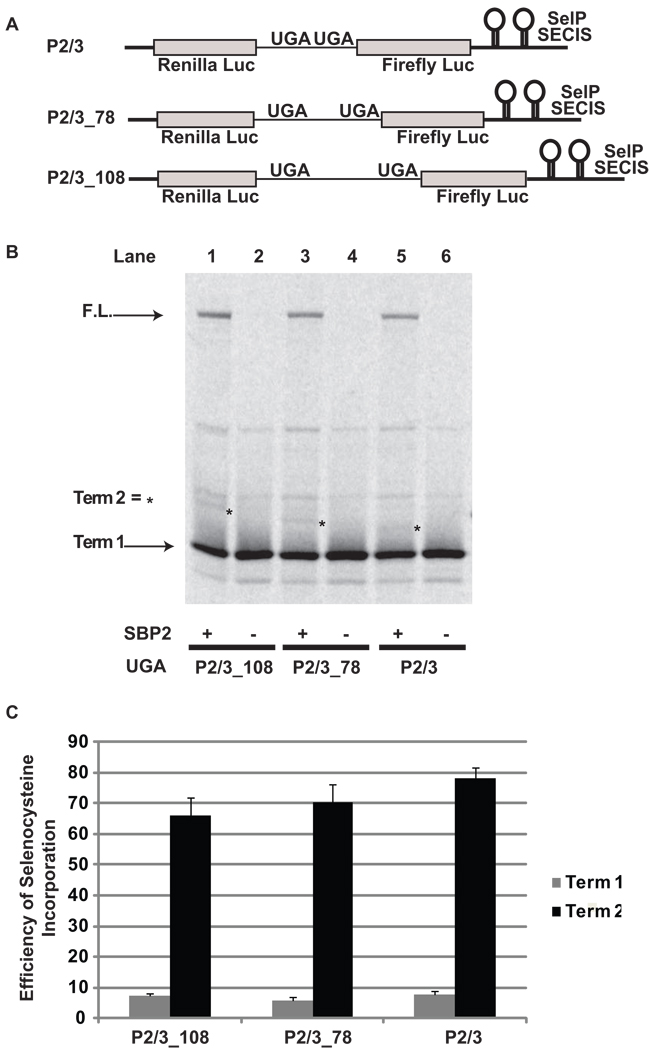
With the exception of the first selenocysteine-encoding UGA codon in SelP all downstream UGA codons are clustered near the 3’ end of the SelP message. To determine if the proximity of the downstream UGA codon relative to the upstream UGA codon could influence the efficiency of selenocysteine incorporation, we increased the distance between the two UGA codons in P2/3. The distance between the two UGA codons in P2/3 is 51 nts, as it is in the wildtype human SelP message. This distance was increased by insertion of random sequences to 78 nts and 108 nts to create the reporter constructs P2/3_78, and P2/3_108 respectively (Figure 3A). A small decrease in selenocysteine incorporation efficiency was observed when the distance between UGA codons was increased from 51 nts (78%) to 108 nts (66%) (p < 0.01) (Figure 3B, lanes 1, 3, and 5; Figure 3C).
Figure 3.
Distance effects on selenocysteine incorporation efficiency during decoding of a message with two UGA codons. A) Schematic representation of the reporter messages: P2/3, and with sequence insertions between the two UGA codons P2/3_78 and P2/3108. B) 35S labeled proteins resulting from rabbit reticulocyte lysate transcription and translation reactions in the presence (+) or absence (−) of SBP2-CT (SBP2) were separated by SDS-PAGE. The messages translated were: Lanes 1 and 2, P2/3_108; Lanes 3 and 4, P2/3_78; Lanes 5 and 6, P2/3. The position for protein products corresponding to termination at the first UGA (Term 1) and the full length (F.L.) protein are indicated by arrows. The positions for protein products arising from termination at the second UGA (Term 2) are indicated by asterisks. C) Quantitative analysis of selenocysteine incorporation efficiency at the first and second UGA codons. Bars are the average of two independent experiments (n=6). Error bars are the standard deviation from the mean.
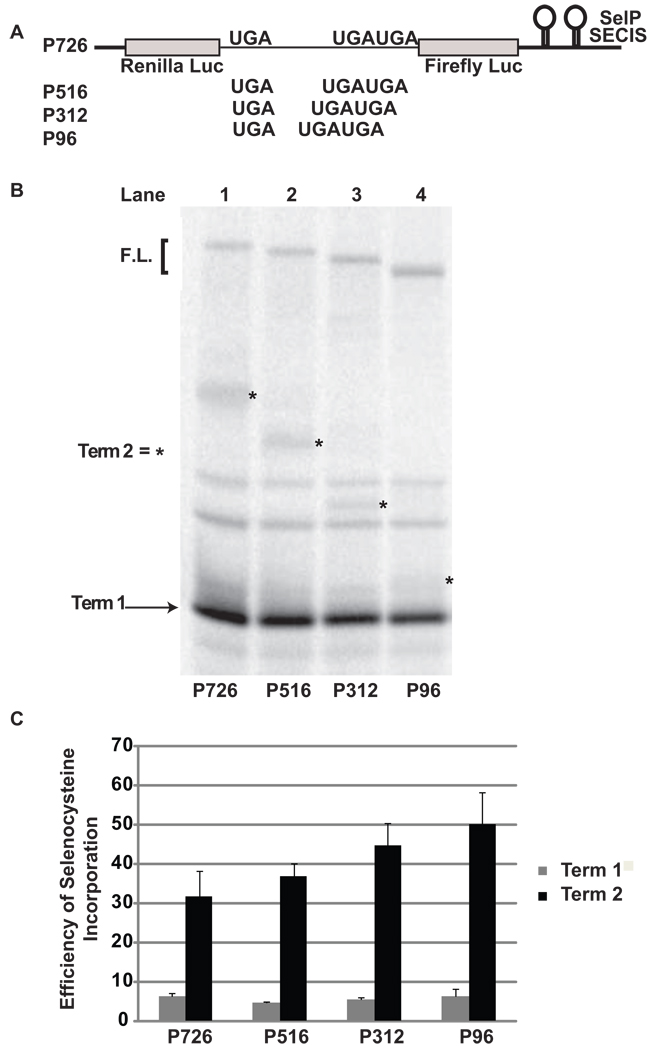
To examine the effect of greater distances between the first and second UGA codons, sequences from the beginning of SelP through SelP UGA 10 were cloned into pDlucS1/S2. UGA 3–9 were changed to sense codons (Figure 4A; and See Materials and Methods). In messages produced from the reporter construct P726, the distance between the first and second UGA codons is 726 nucleotides. This distance was reduced by making sequential deletions to 516 nts (P516), 312 nts (P312), and 96 nts (P96). The efficiency of selenocysteine incorporation at the first and second UGA codons was determined (Figure 4B) as described above; with the exception that termination at the third UGA (SelP UGA 10) was excluded from the calculation because no band could be detected. Selenocysteine incorporation of the second UGA codon was determined to be 32% (P726), 37% (P516), 45% (P312), and 50% (P96) (Figure 4B, lanes 1, 2, 3, and 4; Figure 4C). Increasing the distance between the first and second UGA codons by 630 nts reduced selenocysteine incorporation nearly 2-fold from 50% to 32% (p < 0.01). Selenocysteine incorporation at the first UGA codon was approximately 5% in all cases and unaffected by the distance between the first and second UGA codons.
Figure 4.
Distance effects on selenocysteine incorporation efficiency during decoding of a message with three UGA codons. A) Schematic representation of the reporter messages: P726, and with sequential deletions between the first two UGA codons P516 and P312, P96. B) 35S labeled proteins resulting from rabbit reticulocyte lysate transcription and translation reactions supplemented with SBP2-CT were separated by SDS-PAGE. The messages translated were: Lane1, P726; Lane 2, P516; Lane 3, P312 and Lane 4, P96. The position for protein products corresponding to termination at the first UGA (Term 1) and the full length (F.L.) protein are indicated by an arrow and bracket respectively. The positions for protein products arising from termination at the second UGA (Term 2) are indicated by asterisks. C) Quantitative analysis of selenocysteine incorporation efficiency at the first and second UGA codons for P726, P516, P312, and P96. Bars are the average of two independent experiments (n=6). Error bars are the standard deviation from the mean.
Sequence context effects on selenocysteine incorporation efficiency
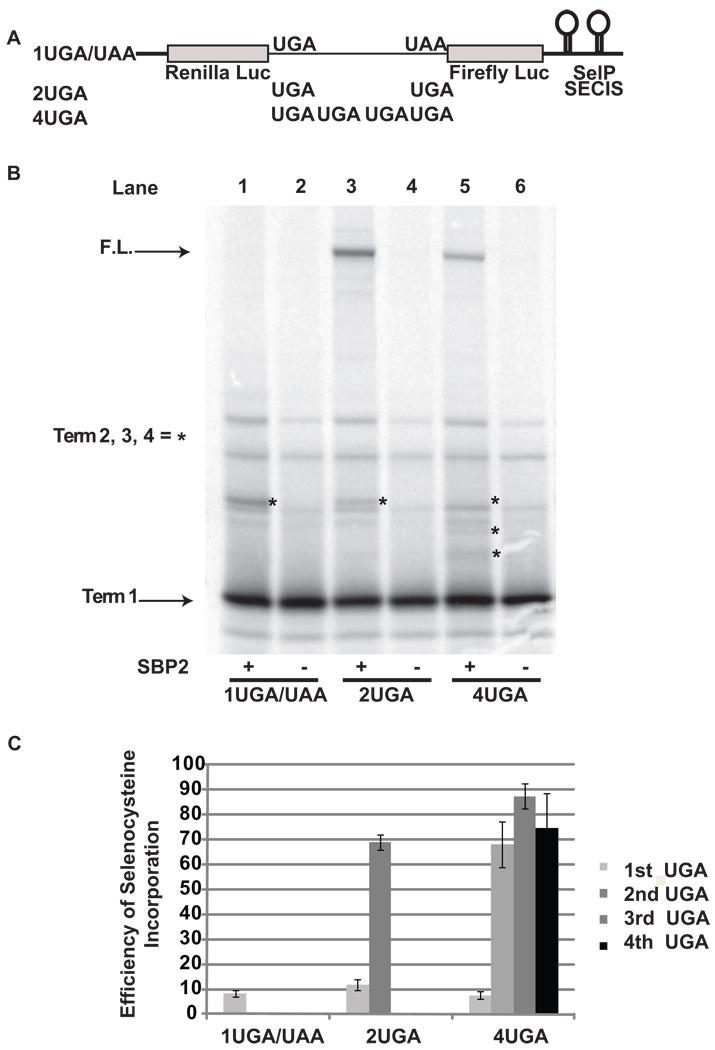
In the experiments described above, all UGA codons and their surrounding sequence contexts were derived from the human SelP gene, which has been postulated to have special signals or sequence contexts that contribute to highly efficient selenocysteine incorporation 24. To examine the effect of sequence context on selenocysteine incorporation efficiency, three reporter vectors with either one (1UGA/UAA), two (2UGA), or four TGAs (4UGA) in a random sequence were cloned into pDlucS1S2 between the Renilla and Firefly reporter genes (Figure 5A). These constructs were transcribed and translated and the radio-labeled products analyzed by SDS-PAGE (Figure 5B). As seen above, selenocysteine incorporation efficiency at the first UGA codon was inefficient (~10%) and highly efficient (between 68% and 87%) at all subsequent downstream UGA codons (Figure 5B, lanes 1, 3, and 5; Figure 5C). This finding demonstrates that although sequence context does have an influence on absolute levels of selenocysteine incorporation, special sequence contexts are not required for increased efficiency of selenocysteine incorporation following decoding at the first UGA codon.
Figure 5.
Local sequence context effects on selenocysteine incorporation efficiency during decoding of a message with multiple UGA codons. A) Schematic representation of the reporter messages: 1UGA/UAA contains a UGA codon and a UAA codon in a random sequence context, 2UGA and 4UGA contain two and four UGA codons respectively embedded in the same sequence context. B) 35S labeled proteins resulting from rabbit reticulocyte lysate transcription and translation reactions supplemented with (+) or without (−) SBP2-CT (SBP2) were separated by SDS-PAGE. The messages translated were: Lanes 1 and 2, 1UGA/UAA; Lanes 3 and 4, 2UGA, Lanes 5 and 6, 4UGA. The position for protein products corresponding to termination at the first and the full length protein are indicated by arrows. The positions for protein products arising from termination at the second, third or fourth UGA (or UAA) are indicated by asterisks. C) Quantitative analysis of selenocysteine incorporation efficiency at the UGA codons for each construct. Bars are the average of two independent experiments (n=6). Error bars are the standard deviation from the mean.
Read-through of the MuLV UAG redefinition element or addition of a competing messenger RNA can increase selenocysteine incorporation efficiency at UGA codons
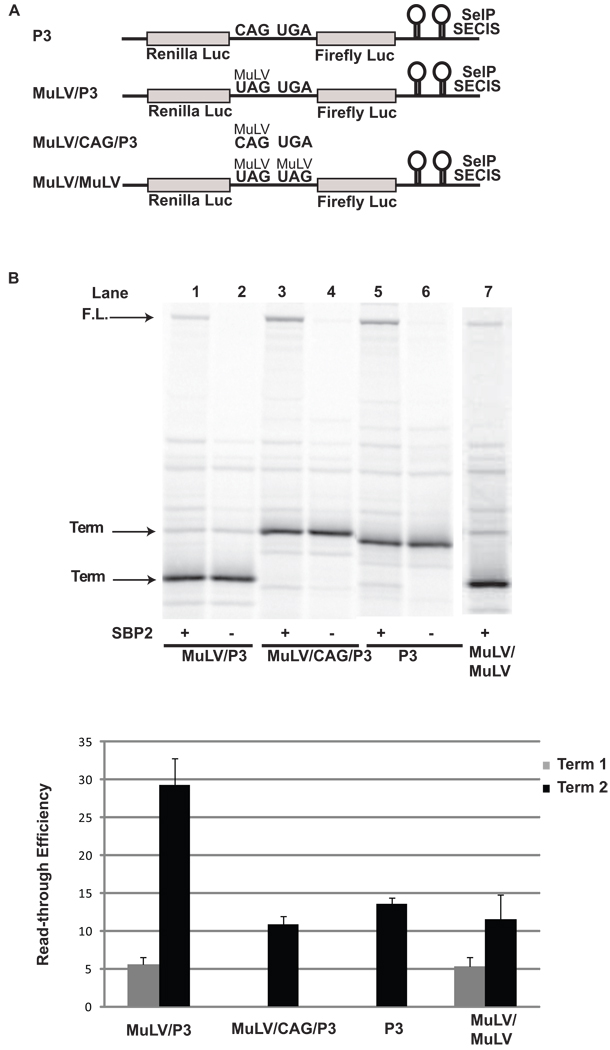
The results presented above demonstrate that under a variety of conditions the first UGA is decoded as selenocysteine with low efficiency and all subsequent UGA codons are read through with much higher efficiency. In order to determine if this effect is dependent upon selenocysteine insertion at the first UGA, the first UGA in P2/3 was replaced with approximately 80 nucleotides from the murine leukemia virus (MuLV read-through cassette) that includes a UAG codon and a pseudoknot structure that induces read-through of the UAG codon (MuLV/P3; Figure 6A). The murine leukemia virus relies on read-through of this UAG codon to express the gag-pol polyprotein. This case of stop codon redefinition is selenocysteine independent, and it has been shown that the UAG is decoded as glutamine 25 with approximately 5% efficiency 26,27. In good agreement with this value, we observed approximately 6% read-through of the UAG codon (Figure 6B, lane 1;Figure 6C) which was unaffected by the presence of SBP2-CT (Figure 6B, lane 2). Read-through of the UAG codon was sufficient to elevate selenocysteine incorporation during decoding of the downstream UGA codon to approximately 30%; three fold higher than the 10% (p < 0.001) selenocysteine incorporation efficiency observed when the UAG was changed to a CAG or P2 was changed to a sense codon (MuLV/CAG/P3, and P3). To determine if read-through of the MuLV UAG codon results in a general increase in stop codon redefinition efficiency, a dual MuLV construct was tested in which two MuLV read-through cassettes were inserted in tandem into pDlucS1S2 (MuLV/MuLV; Figure 6A). As with the MuLV/P3 construct, read-through efficiency of the second UAG codon increased nearly 2-fold (p < 0.01)(Figure 6B, lane 7; Figure 6C). Read-through of the UAG codons were shown to be unaffected by the addition of SBP2-CT (data not shown).
Figure 6.
Read-through of the MuLV UAG codon increases selenocysteine incorporation at a downstream UGA codon and read-through of a second downstream MuLV UAG codon. A) Schematic representation of the reporter messages: P3 contains a UGA codon, MuLV/P3 has the MuLV UAG codon and pseudoknot stimulator of read-through inserted upstream of the UGA codon, MuLV/CAG/P3 is the same as MuLV/P3 except that the UAG is mutated to a CAG, and MuLV/MuLV has two MuLV readthrough cassettes in tandem. B) 35S labeled proteins resulting from rabbit reticulocyte lysate transcription and translation reactions supplemented with (+) or without (−) SBP2-CT were separated by SDS-PAGE. The messages translated were: Lanes 1 and 2, MuLV/P3; Lanes 3 and 4, MuLV/CAG/P3, Lanes 5 and 6, P3, and Lane 7, MuLV/MuLV. The position for protein products arising from termination (Term) at the UAG codon, the UGA codon, and the full length (F.L.) protein are indicated by arrows. C) Quantitative analysis of selenocysteine incorporation or read-through efficiency at the UGA or UAG codons for each construct. Bars are the average of two independent experiments (n=6). Error bars are the standard deviation from the mean.
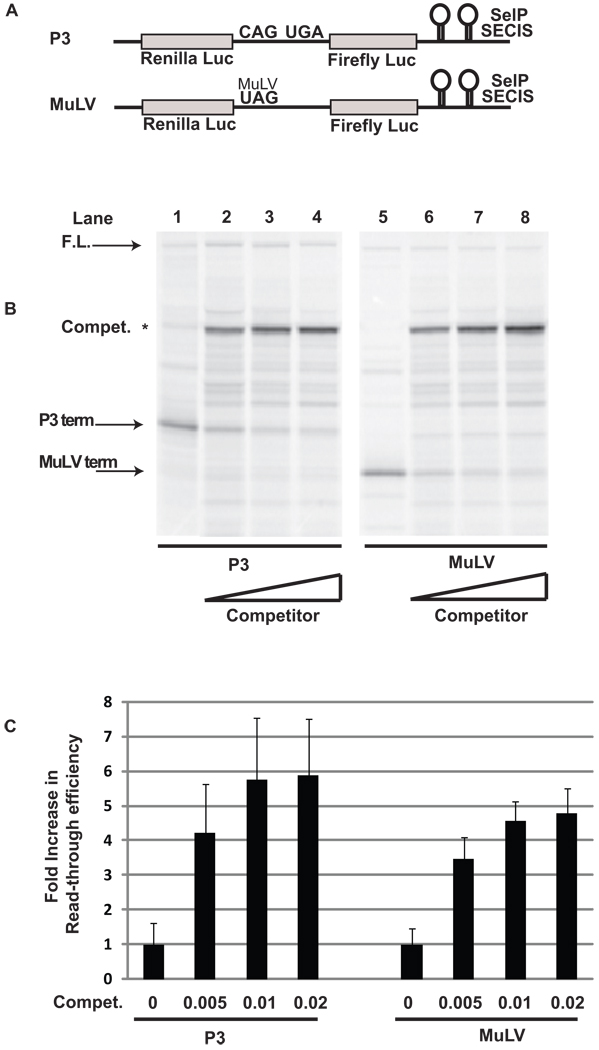
Inclusion of the MuLV read-through cassette in a message reduces the number of ribosomes translating downstream due to release of ribosomes that terminate translation. To determine if a general reduction in translation was sufficient to increase read-through efficiency, constructs containing either a single UGA codon (P3) or a single MuLV read-through cassette (MuLV) (Figure 7A) were transcribed and translated in the presence of increasing amounts of a competing messenger RNA (Figure 7B; Materials and Methods). As expected, increasing amounts of competitor resulted in decreased product produced from both the P3 and MuLV messages (Figure 7B, compare lanes 1–4 and 5–8 respectively). However, read-through efficiency increased up to 5 fold for both selenocysteine incorporation and UAG redefinition at the highest level of competitor tested (p < 0.001) (Figure 7C). These results demonstrate that inefficient decoding of a UAG codon, or reduced translation of messages due to competition with a messenger RNA provided in trans is sufficient to increase the efficiency of selenocysteine incorporation at downstream UGA codons.
Figure 7.
Competing messenger RNA increases the frequency of selenocysteine incorporation and MuLV UAG redefinition relative to termination. A) Schematic representation of the reporter messages: P3 contains a single UGA codon, MuLV contains a single MuLV UAG codon and pseudoknot stimulator of read-through inserted into pDlucS1S2. B) 35S labeled proteins resulting from rabbit reticulocyte lysate transcription and translation reactions supplemented with SBP2-CT and the indicated amounts (ug/ul rabbit reticulocyte lysate reaction) of Luciferase T7 plasmid. The messages translated were: Lanes 1 −4, P3 and Lanes 5–8, MuLV. The position for protein products arising from termination at the UAG codon, the UGA codon, and the full length protein are indicated by arrows. C) Quantitative analysis of the fold increase in selenocysteine incorporation or read-through efficiency at the UGA or UAG codons respectively (n=4).
Discussion
While significant advances have been made in our understanding of the selenocysteine insertion mechanism and its role in selenoprotein synthesis in eukaryotes, fundamental questions remain to be answered. The discovery of the SelP gene containing multiple UGA codons poses a conceptual challenge to the model of non-processive selenocysteine incorporation. How can two SECIS elements reprogram ribosomes to insert selenocysteine at multiple UGA codons in a single mRNA with sufficient processivity to produce full length protein?
Of central importance to this question is how the information in the 3’ UTR is conveyed to the ribosome during decoding of the UGA codons. Reprogramming of the ribosome to decode UGA as selenocysteine may occur during decoding of the first UGA codon whereby the SECIS element interacts with the ribosome via L30, SBP2 or yet to be identified factors. The initial interaction might be facilitated by ribosome pausing or possibly by cis-acting elements such as the SRE that reside near the UGA codon. Alternatively, it has been proposed that functional circularization of mRNAs through interactions between polyA binding protein and initiation factors may position the SECIS elements to interact with the ribosome early in translation, perhaps even during initiation. Intriguingly, the protein eIF4a3 shares homology to the translation initiation factor eIF4a1 and was recently shown to be a SECIS binding protein 15. Once the requisite interaction between the SECIS complex and ribosome has occurred, the SECIS element and associated factors may either remain associated with the ribosome during decoding of each UGA codon or the ribosome may be reprogrammed such that it maintains an altered state competent for redefinition of UGA codons without continued association of the SECIS. Either persistence of the SECIS complex association with the ribosome or a SECIS independent altered conformational state of the ribosome could account for highly processive selenocysteine incorporation at downstream UGA codons by ribosomes that transit the first UGA.
To Terminate or Incorporate
An increase in processivity could arise due to a ‘loss of function’ event in which the ribosome’s ability to terminate translation is reduced, thereby favoring selenocysteine incorporation. The data presented in Figure 1 demonstrate that after decoding of the first UGA codon in the message, a dramatic increase in selenocysteine incorporation efficiency is observed at a second downstream UGA codon. However, when the second UGA is changed to either a UAG or UAA codon, termination of translation is highly efficient. This result indicates that the ribosome’s ability to terminate translation remains intact and consequently a general reduction in termination efficiency is unlikely to account for enhanced selenocysteine incorporation at downstream UGA codons.
Role of the SECIS element
SelP genes are the only known selenoprotein genes to contain two SECIS elements, suggestive of a specialized role for each SECIS element during decoding of multiple UGA codons. However, when the two SECIS elements are replaced with SECIS 1 from SelP there was no significant change in the efficiency of selenocysteine incorporation at downstream UGA codons (Stoytcheva et al, 2006; and this study). The reason that two SECIS elements are conserved in SelP genes when one will suffice is unknown and requires further study. When the SelP SECIS elements were replaced with a single SECIS element from other selenoprotein genes, varying efficiencies of selenocysteine incorporation were observed at the first UGA. A key observation in this experiment was that the increased efficiency of selenocysteine incorporation at the second UGA correlated directly with, and was about 10 fold higher than read-through efficiency at the first UGA (Figure 8). As the second UGA codon is only decoded by ribosomes that do not terminate at the first UGA codon, this result demonstrates that the increase in processivity is observed regardless of which SECIS element is present. However, the absolute efficiency of incorporation at the downstream UGAs depends on the inherent ability of each SECIS element to deliver eEFSec and its associate tRNA to the ribosome.
The dependence of selenocysteine incorporation efficiency at both UGA codons on the identity of the SECIS element strongly suggests that the SECIS element interacts with the ribosome during decoding of each UGA codon in the message, and does not support a model in which the SECIS element acts by a singular event which recruits or creates a population of ribosomes programmed for selenocysteine incorporation without continued association with the SECIS element. If the latter were the case, then one would expect selenocysteine incorporation at the second UGA to be equal regardless of the identity of the SECIS element; i.e all ribosomes which incorporated selenocysteine at the first UGA would have an equal probability of incorporating selenocysteine at the second and all subsequent UGA codons.
Sequence context effects
The sequence context of UGA codons can modulate selenocysteine incorporation rates 4,28–30. In this study, UGA codons were placed in a random sequence context and selenocysteine incorporation rates were shown to be as high or higher (70–90%; Figure 6) than those found using UGA codons in a SelP context. However, a phylogenetically conserved stem loop structure, a potential SRE element, was found downstream of the first UGA codon in the P2/3 constructs (data not shown). Deletion of sequences comprising the 3’ side of the stem had a measurable effect on selenocysteine insertion at the second UGA codon (from 66% to 57% respectively). These results collectively indicate that special sequence signals from SelP are not required for, but most likely modulate, the increase in selenocysteine incorporation efficiency following decoding of the first UGA codon.
Implications for SelP protein expression
SelP consists of two domains, an amino-terminal domain containing the first selenocysteine residue with redox function and a C-terminal domain containing multiple selenocysteine residues necessary for the delivery of selenium through the plasma to various tissues. Purification of selenoprotein P from rat plasma revealed several isoforms of the protein. These isoforms were shown by carboxypeptidase sequencing 22 and mass spectrometry 23 to comprise full-length and several prematurely UGA-terminated proteins. Figures 3 and 4 demonstrate that selenocysteine incorporation efficiency at downstream UGA codons is reduced as the distance between the UGA codons is increased. This result may explain why the second and all subsequent UGA codons in SelP genes from a variety of organisms are found in close proximity near the C-terminal end of the open reading frame. If the UGA codons were positioned far apart, selenocysteine incorporation efficiency may not be sufficient to produce full length protein.
Analysis of SelP isoforms following transfection of the zebrafish gene into cultured cells indicated that there is significant termination at the first and second UGA codons but not at the subsequent fifteen UGA codons 24. In the zebrafish SelP gene, the distance between the first and second UGA is 621 nucleotides, which in accord with our data is a sufficient distance to reduce selenocysteine insertion efficiency at the second UGA codon by as much as 50%. In contrast, the second UGA and all remaining downstream UGA codons lie close together within a 291 nucleotide region of the open reading frame. Our data provides a viable explanation for the results observed in the Stoytcheva et al. study24; i.e. efficient termination at the first and second UGA codons with processive selenocysteine incorporation at the remaining UGA codons.
It is notable that zebrafish have a second SelP gene which has homology to the full length SelP gene lacking only the C-terminal domain containing the last 16 selenocysteine residues. Likewise, one isoform of SelP isolated from rats corresponded to termination at the second UGA codon. Based on these observations, it is possible that termination at the second UGA codon could produce a functional isoform of SelP containing a single selenocysteine residue with redox activity. Further studies are needed to address the potential redox function and biological role of this isoform.
Processive Selenocysteine incorporation
Based on the results presented here, we propose a simplified model for highly processive selenocysteine incorporation that invokes properties intrinsic to the mechanism of selenocysteine incorporation. In this model, the SECIS complex is loaded onto the ribosome prior to, or during, decoding of the first UGA codon. Once assembled, the ribosome and associated SECIS complex proceed to the next UGA codon primed for highly efficient selenocysteine incorporation. As discussed above, the dependence of selenocysteine incorporation efficiency at the first and also the downstream UGA codon on the identity of the SECIS element (Figures 2 and 8) argues that the SECIS is interacting with the ribosome at each UGA in the message. Although it cannot be strictly ruled out that the SECIS complex dissociates and then re-associates with the ribosome at each UGA codon, the simplest model is one in which the SECIS element and associated factors track with the ribosome following selenocysteine insertion at the first UGA and remain associated during decoding of all subsequent UGAs. In this scenario only recycling of eEFSec with an amino-acylated tRNA would be required for efficient selenocysteine incorporation.
One important implication of this model is that only one ribosome at a time can successfully translate through multiple UGA codons to produce full length protein. Meanwhile, any ribosomes translating to the first UGA codon will terminate. Only when the SECIS-associated ribosome has completed translation of the message can the SECIS element be recycled to a new ribosome. This aspect of the model explains why the first UGA and messages with only one UGA have low selenocysteine incorporation efficiency relative to termination. This model is also consistent with the observation that increasing the distance between UGAs diminishes selenocysteine incorporation efficiency at the second UGA codon (Figure 3). With increased codons to decode between the UGAs, the SECIS complex would have more time to inadvertently disassociate from the ribosome.
Reducing the number of ribosomes translating to the first UGA codon either by inclusion of an upstream MuLV read-through cassette or by addition of a competing messenger RNA (Figure 7) results in a higher percentage of ribosomes incorporating selenocysteine. One explanation for this result is that fewer ribosomes encounter the first UGA during the time it takes the SECIS associated ribosome to complete translation of the message and for the SECIS complex to re-associate with an upstream ribosome. This observation suggests that selenocysteine incorporation efficiency in vivo may be controlled by altering the number of ribosomes which initiate translation on selenoprotein messages.
Finally, the finding that the ability to decode multiple UGAs as selenocysteine is not unique to SelP sequences demonstrates that heterologous non-selenoprotein genes can be engineered to express proteins containing multiple selenocysteine residues.
Materials and Methods
Construction of pDluc reporter plasmid
The reporter plasmid pDluc was constructed by inserting the firefly luciferase coding sequence from pGL4.10 (Promega) into the Xba-I restriction site of pGL4.73 (Promega) downstream of the Renilla luciferase gene. A T7 promoter was inserted into the Hind III restriction site upstream of the Renilla gene. The region between the Renilla and Firefly luciferase genes was altered to include Xho-I, Hind-III, and Bgl-II cloning sites. In addition the native ATG start codon of Firefly was changed to ATT. Further details are available upon request.
Insertion of SECIS elements into pDluc
The following SECIS sequences were inserted into the Xba-1 site located downstream of the firefly luciferase gene in pDluc:
SECIS1: S1
GGTTAATATGTCTTTTTTTTTCTTTTTCCAGTGTTCTATTTGCTTTAATGAGAATAGAAACGTAAACTAT GACCTAGGGGTTTCTGTTGGATAATTAGCAGTTTAGAATGGAGGAAGAACAACAAAGAC
SECIS2: S2
TTGTCTGTCTCTATATTGCTTAGTAAGTATTTCCATAGTCAATGATGGTTTAATAGGTAAACCAAACCCTATAAACCTGACCTCCTTTATGGTTAATACTATTAAGCAAGAATG
SECIS1&2: S1/S2
GGTTAATATGTCTTTTTTTTTCTTTTTCCAGTGTTCTATTTGCTTTAATGAGAATAGAAACGTAAACTATGACCTAGGGGTTTCTGTTGGATAATTAGCAGTTTAGAATGGAGGAAGAACAACAAAGACATGCTTTCCATTTTTTTCTTTACTTATCTCTCAAAACAATATTACTTTGTCTTTTCAATCTTCTACTTTTAACTAATAAAATAAGTGGATTTTGTATTTTAAGATCCAGAAATACTTAACACGTGAATATTTTGCTAAAAAAGCATATATAACTATTTTAAATATCCATTTATCTTTTGTATATCTAAGACTCATCCTGATTTTTACTATCACACATGAATAAAGCCTTTGTATCTTTCTTTCTCTAATGTTGTATCATACTCTTCTAAAACTTGAGTGGCTGTCTTAAAAGATATAAGGGGAAAGATAATATTGTCTGTCTCTATATTGCTTAGTAAGTATTTCCATAGTCAATGATGGTTTAATAGGTAAACCAAACCCTATAAACCTGACCTCCTTTATGGTTAATACTATTAAGCAAGAATG
SelN SECIS: SN
AGTGGCTTCCCCGGCAGCAGCCCCATGATGGCTGAATCCGAAATCCTCGATGGGTCCAGCTTGATGTCTTTGCAGCTGCACCTATGGG
SelT SECIS: ST
GATCATTGCAAGAGCAGCGTGACTGACATTATGAAGGCCTGTACTGAAGACAGCAAGCTGTTAGTACAGA CCAGATGCTTTCTTGGCAGGCTCGTTGTACCTCT
Sequences containing one or more UGA codons were inserted into the respective pDluc vectors described in the text between the Xho-I and Bgl-II sites. Sequences are shown below. In-frame TGA, TAG, TAA codons and their respective changes are highlighted.
Reporter Constructs
SelP derived sequence inserts:
In order to examine the effect of varying the distance between the first and second UGA codons in the SelP message, sequences from the SelP gene starting just after the initiation ATG codon and including the first TAG were cloned into the Xho-I site of P2/10 described above. Sequential deletions were made in the region between the first and second TGAs. The sequences of those inserts are as follows:
The effect of distance between UGA codons was also investigated by inserting random sequence between TGA2 and 3 in our SelP2/3 constructs. Those constructs are as follows:
Non-SelP based sequence inserts
MuLV containing sequence inserts
Rabbit Reticulocyte Lysate Transcription and Translation
Rabbit reticulocyte translation reactions using Promega’s TNT Quick Coupled Transcription/Translation System were carried out according to the manufactor’s instructions in the presence of L-[35S]- Methionine, in the presence or absence of saturating levels of purified SBP2-CT as described in 5. In competition reactions, the plasmid Luciferase T7 (Promega) was included at the concentrations indicated and reporter DNAs were reduced 4 fold to 0.005ug/ul. 35S labeled protein products were separated by electrophoresis on 10% Bis-Tris NuPAGE denaturing gels in MOPs buffer according to manufacturers specifications (InVitrogen). Gels were fixed in 50% methanol:7.5% acetic acid and dried. Radiolabeled 35S labeled protein bands were visualized using the Typhoon Phosphorimager (GE Healthcare) and band intensities quantified using ImageQuant software.
Calculating efficiency of selenocysteine insertion
The intensity of all protein bands resulting from termination and the full length protein were determined using ImageQuant software and then each value was corrected for the number of methionines. To calculate percent termination, the intensity of the band corresponding to termination at that position was divided by the sum of that band and all bands arising from downstream termination at UGA (or UAG and UAA) codons. This value was then subtracted from 100 to determine the efficiency of selenocysteine incorporation (or UAG redefinition) as a percentage of ribosomes which encountered that UGA (UAG) codon. Each number represents the average value from at least two independent experiments (n=6 except where indicated) error bars represent the standard deviation from the mean, and p values were calculated by Student T-Test.
Acknowledgments
We thank John Atkins, Norma Wills, and Ray Gesteland for helpful discussions during the course of this work; and Norma Wills for providing the MuLV pDluc reporter plasmid. The authors declare that we have no conflict of interest. These studies were funded by the National Institutes of Health Grant GM077462 to MTH.
Abbreviations
- SECIS
Selenocysteine Insertion Sequence
- SBP2
SECIS binding protein 2
- SRE
Selenocysteine Redefinition Element
- UTR
Untranslated Region
- DI2
iodothyronine deiodinase 2
- SelL
selenoprotein L
- SelN
selenoprotein N
- SelT
Selenoprotein T
- SelP
selenoprotein P
- MuLV
Murine Leukemia Virus
- RRL
Rabbit Reticulocyte Lysate
- SDS-PAGE
SDS polyacrylamide gel electrophoresis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berry MJ, Banu L, Harney JW, Larsen PR. Functional characterization of the eukaryotic SECIS elements which direct selenocysteine insertion at UGA codons. Embo J. 1993;12:3315–3322. doi: 10.1002/j.1460-2075.1993.tb06001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill KE, Lloyd RS, Burk RF. Conserved nucleotide sequences in the open reading frame and 3' untranslated region of selenoprotein P mRNA. Proc Natl Acad Sci U S A. 1993;90:537–541. doi: 10.1073/pnas.90.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen Q, Chu FF, Newburger PE. Sequences in the 3'-untranslated region of the human cellular glutathione peroxidase gene are necessary and sufficient for selenocysteine incorporation at the UGA codon. J Biol Chem. 1993;268:11463–11469. [PubMed] [Google Scholar]
- 4.Howard MT, Aggarwal G, Anderson CB, Khatri S, Flanigan KM, Atkins JF. Recoding elements located adjacent to a subset of eukaryal selenocysteine-specifying UGA codons. Embo J. 2005;24:1596–1607. doi: 10.1038/sj.emboj.7600642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard MT, Moyle MW, Aggarwal G, Carlson BA, Anderson CB. A recoding element that stimulates decoding of UGA codons by Sec tRNA[Ser]Sec. Rna. 2007;13:912–920. doi: 10.1261/rna.473907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copeland PR, Fletcher JE, Carlson BA, Hatfield DL, Driscoll DM. A novel RNA binding protein, SBP2, is required for the translation of mammalian selenoprotein mRNAs. Embo J. 2000;19:306–314. doi: 10.1093/emboj/19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copeland PR, Stepanik VA, Driscoll DM. Insight into mammalian selenocysteine insertion: domain structure and ribosome binding properties of Sec insertion sequence binding protein 2. Mol Cell Biol. 2001;21:1491–1498. doi: 10.1128/MCB.21.5.1491-1498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caban K, Kinzy SA, Copeland PR. The L7Ae RNA binding motif is a multifunctional domain required for the ribosome-dependent Sec incorporation activity of Sec insertion sequence binding protein 2. Mol Cell Biol. 2007;27:6350–6360. doi: 10.1128/MCB.00632-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zavacki AM, Mansell JB, Chung M, Klimovitsky B, Harney JW, Berry MJ. Coupled tRNA(Sec)-dependent assembly of the selenocysteine decoding apparatus. Mol Cell. 2003;11:773–781. doi: 10.1016/s1097-2765(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 10.Tujebajeva RM, Copeland PR, Xu XM, Carlson BA, Harney JW, Driscoll DM, Hatfield DL, Berry MJ. Decoding apparatus for eukaryotic selenocysteine insertion. EMBO Rep. 2000;1:158–163. doi: 10.1093/embo-reports/kvd033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagegaltier D, Hubert N, Yamada K, Mizutani T, Carbon P, Krol A. Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation. Embo J. 2000;19:4796–4805. doi: 10.1093/emboj/19.17.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavatte L, Brown BA, Driscoll DM. Ribosomal protein L30 is a component of the UGA-selenocysteine recoding machinery in eukaryotes. Nat Struct Mol Biol. 2005;12:408–416. doi: 10.1038/nsmb922. [DOI] [PubMed] [Google Scholar]
- 13.Wu R, Shen Q, Newburger PE. Recognition and binding of the human selenocysteine insertion sequence by nucleolin. J Cell Biochem. 2000;77:507–516. doi: 10.1002/(sici)1097-4644(20000601)77:3<507::aid-jcb15>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Squires JE, Stoytchev I, Forry EP, Berry MJ. SBP2 binding affinity is a major determinant in differential selenoprotein mRNA translation and sensitivity to nonsense-mediated decay. Mol Cell Biol. 2007;27:7848–7855. doi: 10.1128/MCB.00793-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budiman ME, Bubenik JL, Miniard AC, Middleton LM, Gerber CA, Cash A, Driscoll DM. Eukaryotic initiation factor 4a3 is a selenium-regulated RNA-binding protein that selectively inhibits selenocysteine incorporation. Mol Cell. 2009;35:479–489. doi: 10.1016/j.molcel.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suppmann S, Persson BC, Bock A. Dynamics and efficiency in vivo of UGA-directed selenocysteine insertion at the ribosome. Embo J. 1999;18:2284–2293. doi: 10.1093/emboj/18.8.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry MJ, Banu L, Chen YY, Mandel SJ, Kieffer JD, Harney JW, Larsen PR. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3' untranslated region. Nature. 1991;353:273–276. doi: 10.1038/353273a0. [DOI] [PubMed] [Google Scholar]
- 18.Jung JE, Karoor V, Sandbaken MG, Lee BJ, Ohama T, Gesteland RF, Atkins JF, Mullenbach GT, Hill KE, Wahba AJ, Hatfield DL. Utilization of selenocysteyl-tRNA[Ser]Sec and seryl-tRNA[Ser]Sec in protein synthesis. J Biol Chem. 1994;269:29739–29745. [PubMed] [Google Scholar]
- 19.Nasim MT, Jaenecke S, Belduz A, Kollmus H, Flohe L, McCarthy JE. Eukaryotic selenocysteine incorporation follows a nonprocessive mechanism that competes with translational termination. J Biol Chem. 2000;275:14846–14852. doi: 10.1074/jbc.275.20.14846. [DOI] [PubMed] [Google Scholar]
- 20.Lobanov AV, Hatfield DL, Gladyshev VN. Reduced reliance on the trace element selenium during evolution of mammals. Genome Biol. 2008;9:R62. doi: 10.1186/gb-2008-9-3-r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burk RF, Hill KE. Selenoprotein P-expression, functions, and roles in mammals. Biochim Biophys Acta. 2009;1790:1441–1447. doi: 10.1016/j.bbagen.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Himeno S, Chittum HS, Burk RF. Isoforms of selenoprotein P in rat plasma. Evidence for a full-length form and another form that terminates at the second UGA in the open reading frame. J Biol Chem. 1996;271:15769–15775. doi: 10.1074/jbc.271.26.15769. [DOI] [PubMed] [Google Scholar]
- 23.Ma S, Hill KE, Caprioli RM, Burk RF. Mass spectrometric characterization of full-length rat selenoprotein P and three isoforms shortened at the C terminus. Evidence that three UGA codons in the mRNA open reading frame have alternative functions of specifying selenocysteine insertion or translation termination. J Biol Chem. 2002;277:12749–12754. doi: 10.1074/jbc.M111462200. [DOI] [PubMed] [Google Scholar]
- 24.Stoytcheva Z, Tujebajeva RM, Harney JW, Berry MJ. Efficient incorporation of multiple selenocysteines involves an inefficient decoding step serving as a potential translational checkpoint and ribosome bottleneck. Mol Cell Biol. 2006;26:9177–9184. doi: 10.1128/MCB.00856-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshinaka Y, Katoh I, Copeland TD, Oroszlan S. Murine leukemia virus protease is encoded by the gag-pol gene and is synthesized through suppression of an amber termination codon. Proc Natl Acad Sci U S A. 1985;82:1618–1622. doi: 10.1073/pnas.82.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wills NM, Gesteland RF, Atkins JF. Evidence that a downstream pseudoknot is required for translational read-through of the Moloney murine leukemia virus gag stop codon. Proc Natl Acad Sci U S A. 1991;88:6991–6995. doi: 10.1073/pnas.88.16.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wills NM, Gesteland RF, Atkins JF. Pseudoknot-dependent read-through of retroviral gag termination codons: importance of sequences in the spacer and loop 2. Embo J. 1994;13:4137–4144. doi: 10.1002/j.1460-2075.1994.tb06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grundner-Culemann E, Martin GW, 3rd, Tujebajeva R, Harney JW, Berry MJ. Interplay between termination and translation machinery in eukaryotic selenoprotein synthesis. J Mol Biol. 2001;310:699–707. doi: 10.1006/jmbi.2001.4809. [DOI] [PubMed] [Google Scholar]
- 29.Gupta M, Copeland PR. Functional analysis of the interplay between translation termination, selenocysteine codon context, and selenocysteine insertion sequence-binding protein 2. J Biol Chem. 2007;282:36797–36807. doi: 10.1074/jbc.M707061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCaughan KK, Brown CM, Dalphin ME, Berry MJ, Tate WP. Translational termination efficiency in mammals is influenced by the base following the stop codon. Proc Natl Acad Sci U S A. 1995;92:5431–5435. doi: 10.1073/pnas.92.12.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]