Abstract
Neuroimaging with iron-sensitive MR sequences [gradient echo T2* and susceptibility-weighted imaging (SWI)] identifies small signal voids that are suspected brain microbleeds. Though the clinical significance of these lesions remains uncertain, their distribution and prevalence correlates with cerebral amyloid angiopathy (CAA), hypertension, smoking, and cognitive deficits. Investigation of the pathologies that produce signal voids is necessary to properly interpret these imaging findings. We conducted a systematic correlation of SWI-identified hypointensities to tissue pathology in postmortem brains with Alzheimer’s disease (AD) and varying degrees of CAA. Autopsied brains from eight AD patients, six of which showed advanced CAA, were imaged at 3T; foci corresponding to hypointensities were identified and studied histologically. A variety of lesions was detected; the most common lesions were acute microhemorrhage, hemosiderin residua of old hemorrhages, and small lacunes ringed by hemosiderin. In lesions where the bleeding vessel could be identified, β-amyloid immunohistochemistry confirmed the presence of β-amyloid in the vessel wall. Significant cellular apoptosis was noted in the perifocal region of recent bleeds along with heme oxygenase 1 activity and late complement activation. Acutely extravasated blood and hemosiderin were noted to migrate through enlarged Virchow–Robin spaces propagating an inflammatory reaction along the local microvasculature; a mechanism that may contribute to the formation of lacunar infarcts. Correlation of imaging findings to tissue pathology in our cases indicates that a variety of CAA-related pathologies produce MR-identified signal voids and further supports the use of SWI as a biomarker for this disease.
Keywords: Alzheimer’s disease, Cerebral amyloid angiopathy, Microbleeds, Microinfarcts, Susceptibility-weighted imaging, Blooming effect, Complement C6
Introduction
In vivo evidence of cerebral amyloid angiopathy (CAA) was limited until the introduction of gradient echo T2* (GRE-T2*) weighted magnetic resonance (MR) imaging, which remains the clinical standard for detection of brain microbleeds (BMB) which often result from CAA [3]. BMB are detected as focal signal intensity losses, presumably secondary to iron-containing hemosiderin residua of hemoglobin breakdown. Though recent reviews of the BMB literature have attempted to codify the interpretation of these findings, the inconsistency of data sets, the lack of pathological confirmation and the need for better designed prospective studies to determine their clinical significance has been emphasized [8, 10, 41]. Detection of BMB is improved by new, high-resolution, 3D GRE-T2* and susceptibility-weighted imaging (SWI). SWI is an advance in T2*-weighted brain MR imaging that enhances contrast from local susceptibility tissue variations [18, 20]. At 1.5T, the SWI sequence was found to be fourfold more sensitive for detection of traumatic BMB than conventional GRE-T2* and recent data in mild cognitive impairment subjects indicates again at least a fourfold increase in BMB recognition by SWI compared to conventional GRE-T2* imaging [19, 33, 34, 40].
To date, punctate signal voids have been observed in a number of diseases; by far the most common are hypertension and CAA. Those associated with hypertensive vasculopathy tend to be localized to basal ganglia, internal capsule, brain stem, and cerebellum, whereas those associated with CAA are generally smaller with a posterior lobar predilection [27, 42]. CAA is comorbid with Alzheimer’s disease (AD) in as many as 95% of AD cases [22]. In this condition the β-amyloid peptide is deposited along the cerebral and meningeal vasculature in the walls of small and medium-sized arterioles. This peptide appears to induce a local inflammatory response ranging from subtle changes to, in extreme cases, a granulomatous angiitis with apoptotic death of vascular smooth muscle cells [1, 2]. Studies of CAA autopsy material have confirmed these findings and the presence of other (non-β amyloid) proteins such as cysteine protease inhibitor (cystatin) in CAA vessel walls [2]. Vascular wall infiltration with these proteins appears to be associated with a structural instability of arterioles accounting for BMB and associated MR signal voids; however, the biologic and molecular mechanisms for β-amyloid accumulation, inflammatory and oxidative responses, and vascular weakening are unclear at present.
Recent evidence has shown that the presence of BMB predicts reduced global cognitive function and is a risk factor for progression of mild cognitive impairment to outright dementia [23, 45]. Additionally, one study noted a significant correlation between patients with at least one hypointensity in GRE-T2* imaging and those homozygous for the apolipoprotein E ε4 gene, a well-known risk factor for Alzheimer’s disease [35]. Remarkably, there have been very few verifications of the histopathology of radiologically identified BMB, and none utilizing the newer sensitive sequences [8]. There are currently three reports in the published literature that describe the pathology of hypointensities in MR images; all three rely upon GRE-T2* imaging and most evaluate hypertensive patients. A recent case report isolated eight microbleeds from a single, elderly hypertensive patient [37]. Tanaka and colleagues [36] studied hypointensities in three relatively young autopsy cases, two involving hypertension and one a large mass in the brain indicating that hemosiderin granules were found corresponding to hypointensities, except in one case where a microaneurysm was found. The largest correlative study evaluated 11 cases, but was only able to correlate imaging findings to pathologic abnormalities in 6. The hypointensities in that study were associated with hematomas and/or hemosiderin granules. However, only two of the cases had CAA and one of them was also hypertensive; neither of the patients were identified as having been clinically demented during life [14].
More recently, SWI has been used to study trauma; in this venue, numerous papers have shown SWI to be particularly sensitive for detecting blood products [39, 40]. Still, none of the many works in this area have correlated the imaging findings seen in trauma with histology. Our study represents the first correlation of SWI focal hypointensities to tissue pathology in post mortem human CAA-affected brain.
Materials and methods
Patient characteristics
Post mortem tissue was obtained from the Alzheimer’s Disease Research Center Brain Bank at the University of California, Los Angeles (Harry V. Vinters). All patients or their surrogates had consented to participate in research protocols prior to tissue donation. The research protocol was approved by the Institutional Review Board of Loma Linda University Medical Center (approval #54174). The average age for the dementia cases was 79.9 years with a standard deviation of 9.3 years; four were male, four female. AD pathology was classified by Braak and Braak staging guidelines and CAA pathology was graded by Vonsattel’s criteria. Briefly, grade 1 CAA involves β-amyloid deposition primarily in a fine rim on the basement membrane. Grade 2 disease extends to allocortical and cerebellar vessels and deposition of β-amyloid among smooth muscle cells partially replacing the tunica media. Grade 3 disease involves total replacement of arteriolar vascular smooth muscle [17]. Autopsy findings confirmed Braak and Braak pathology at stage VI and Vonsattel pathology at grade 3 for four of the brains. Two cases were found to have severe CAA pathology (grade 3) out of proportion to AD pathology. These are included as relatively “pure” cases of CAA. Two had stage VI AD pathology, but negligible CAA; these were included as AD controls. In addition to the eight demented cases, two neurologically normal, aged control brains were scanned along with the others and no hypointensities were detected in them. One of the patients with severe CAA and AD died of an acute intracerebral hemorrhage; the affected lobe was excluded from analysis. None of the patients had a history of hypertension, traumatic brain injury or other causes of BMB. Cases with Lewy bodies detected on pathologic examination were excluded. Patient demographics, clinical duration of dementia (when known) and the imaging findings are listed in Table 1.
Table 1.
Patient demographics and locations of microbleeds on MR
| Case#, age/sex | Clinical severity of dementia |
B&B/Vonsattel grade |
Lesion locations |
Cause of death | ||
|---|---|---|---|---|---|---|
| Cortical grey matter |
Deep grey matter |
White matter |
||||
| 1, 71/M | Neurologically normal | 0/0 | – | – | – | Sepsis, ischemic colitis |
| 2, 93/F | Neurologically normal | 0/0 | – | – | – | Heart failure, senile cardiac amyloidosis |
| 3, 89/M | 14 years/severe | VI/0 | – | – | – | Pneumonia |
| 4, 81/M | Severe/slow progression | VI/0 | – | – | – | Pneumonia |
| 5, 61/M | 2 years/rapid progression | IV/3+ | 1 | – | 4 | Complications of malnutrition |
| 6, 85/F | Slow progression | V/3+ | – | 1 | 4 | Coronary artery disease |
| 7, 73/F | Severe | VI/3 | – | – | 3 | Unknown |
| 8, 90/M | Severe/slow progression | VI/3 | – | 1 | 4 | Emphysema/pneumonia |
| 9, 74/F | Severe/slow progression | VI/3 | 7 | 2 | 2 | Massive intracerebral hemorrhage in the right frontal/parietal lobes |
| 10, 86/F | 8 years/slow progression | VI/3 | 4 | 1 | 4 | Pneumonia |
B&B = Braak and Braak score
Tissue preparation
Three, 1 cm coronal sections were obtained from each of ten human brains at autopsy; slices were taken through the frontal, temporo-parietal and occipital lobes. Because lesions frequently occur near the periphery of tissue specimens in CAA, they are easily obscured in post mortem imaging by the artifact produced at the air–tissue interface. To eliminate this interface and movement artifacts in imaging and to improve the ease of handling delicate tissue, the formalin-fixed brain slices were embedded in blocks of 4% agarose gel. Agarose is an aqueous suspension that effectively contrasts to the fixed tissue in MR images and eliminates the phase disturbance. The encased tissue is well preserved, and the gel block is easier to orient within the MR scanner and eliminates tissue movement, which was encountered when specimens were imaged in liquids. Agarose did not penetrate the tissue and was neatly separated from the specimen after imaging was completed. Air bubbles mimic the signal voids of focal iron, so care was taken to thoroughly remove any bubbles which might complicate interpretation of the MR scans.
Imaging parameters
MR imaging was performed on a 3T scanner in the coronal plane (Siemens). The following parameters were used: echo time, 20 ms; repetition time, 30 ms; flip angle, 15°; slice thickness, 2 mm; matrix size 256 × 256 mm; in plane resolution, 0.5 × 0.5 mm. The images were reviewed by two experienced interpreters blinded to autopsy diagnoses and any conflicts were settled by the senior neuroradiologist at Loma Linda University Medical Center, Dr. Daniel Kido. An explanation of hypointensity reporting criteria has been published previously and is comparable to other established protocols [9, 23]. Briefly, hypointensities were identified as regions 5.7 mm or less in diameter of relative signal void [16]. Additionally, hypointensities were evaluated for continuity with blood vessels, and location within a sulcus. If either were present, the hypointensity was interpreted as vascular in origin or an artifact from an air bubble and was not counted.
Dissection and histology
The focal points of interest were located within the tissue block by orienting to tissue architecture and cutting at the depth estimated from the slice thickness of the MR images, thus enabling accurate dissection and consistent recovery of the lesion. Thirteen, well-dissected microbleeds, five cortical and eight at the grey–white junction with at least one specimen from each CAA case, were measured with precision calipers through the widest point to record their diameters for comparison to apparent size in the MR images.
For immunohistochemistry, markers were observed on adjacent sections for each of ten histological specimens; representing two acute bleeds with intact erythrocytes, five old hematomas, two cavitary specimens and one bleed in the basal ganglia. These specimens were embedded in paraffin in the usual fashion. Serial sections were cut at a thickness of 8 µm through the region of interest and mounted on slides for hematoxylin and eosin staining, immunohistochemical and fluorescent studies. The sections were deparaffinized in three exchanges of xylene, rehydrated through serial alcohol exchanges, and permeabilized in 0.01% TritonX in PBS. Antigen retrieval was performed by heating sections in a microwave three times for 3 min each while submerged in a 0.01% citrate solution. The sections were then treated with 1% hydrogen peroxide in PBS and incubated with a blocking solution of 1.5% normal serum (also in PBS). Sections were then incubated with the primary antibody of choice in 4% normal serum overnight at 4°C. After washing with PBS, sections were incubated with the secondary antibody for 2 h, washed in additional exchanges of PBS and treated with diaminobenzidine/ hydrogen peroxide (DAB) for 10 min (Vector Laboratories). They were then rinsed for 3 min in PBS, dehydrated through serial alcohols, cleared to xylene and coverslipped with mounting resin (Permount, Fisher). Antibodies used were those against Aβ1-42 (1:100, Abcam), CD68 (1:100, Dako), HO-1 (1:250, Biomol), complement C6 (1:500, Quidel), CD3 (1:300, Abcam) and CD20 (1:800, Dako).
For visualizing iron, sections were stained by the Prussian blue method by immersing the sections in a mixture of equal parts of 20% hydrochloric acid and 10% potassium ferrocyanide solution for 20 min. All sections were washed in three changes of distilled water and when enhancement of the staining was necessary, sections were incubated with DAB (Vector Laboratories) for 5 min. They were counterstained with hematoxylin for 2 min, rinsed twice in distilled water, and dehydrated through 95 and 100% alcohol. Slides were cleared in xylene, and mounted with resinous mounting medium.
Fluorescent studies were performed on four microbleed specimens: two acute bleeds and two old hematomas. In order to more thoroughly characterize heme oxygenase 1 (HO-1) levels around lesions, we double labeled sections for HO-1 and MAP-2, a marker of neurons. Sections (8 µm) cut through the lesion were deparaffinized in three changes of xylene and washed in PBS for 15 min. They were then treated with 0.3% hydrogen peroxide in methanol for 30 min and incubated with a blocking solution of 2% normal goat serum in PBS. Subsequently, sections were incubated overnight at 4°C with mAb to HO-1 (1:100, BioMol) followed with FITC-conjugated goat anti-mouse secondary antibody (1:500) for 30 min at room temperature. After three 5-min rinses in PBS, sections were blocked with 5% normal mouse serum. After 2 h incubation with MAP-2 monoclonal antibody (1:500, Abcam) sections were rinsed three times in PBS for 5 min each and incubated with Texas Red conjugated goat anti-mouse secondary antibody in PBS for 30 min temperature. Sections were then rinsed in PBS, coverslipped with Vectashield containing DAPI (Vector Laboratories) and observed under a confocal microscope (Olympus). Apoptosis detection was performed using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) technique (Vector Laboratories). Five fields at a magnification of 100× were captured adjacent to each lesion and compared to fields at least five diameters away from the lesion. Statistically significant differences were determined by the Student t test.
Results
Radiologic–histopathologic correlation
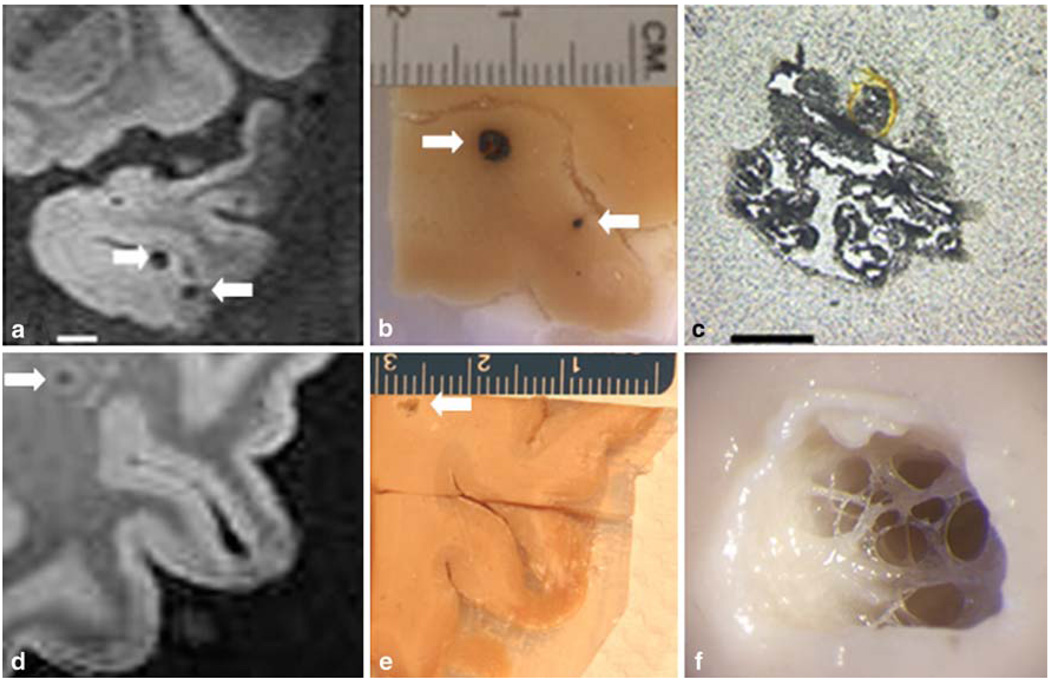
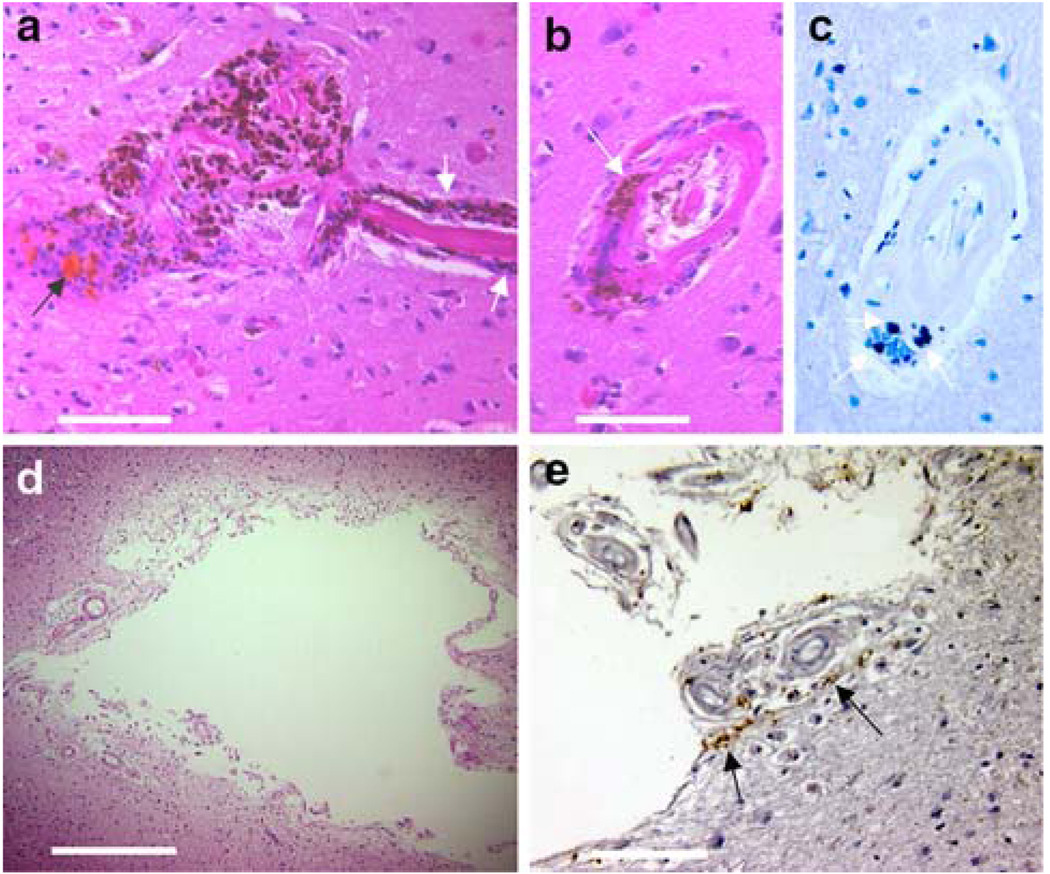
Thirty-eight lesions were detected in SWI images from our cases (Fig. 1; Table 1). The lesions ranged from 0.5 to 5 mm in diameter, and in ten lesions intact erythrocytes were found. In 16 specimens, old hematomas were found which contained dark brown cellular debris positive for Prussian blue staining. In seven of the specimens, small cavities were found at the site indicated by the MR image. At three sites, no pathology was visible on gross inspection, but hemosiderin granules and hematoidin deposition were detected at microscopy. At one site a dissection in the wall of a grossly distended vessel was discovered and blood within the vessel wall apparently produced the hypointensity on SWI. One additional hypointensity was caused by a microaneurysm (Fig. 2). The vast majority of lesions (79%) appeared near the cortical ribbon in two key distributions, just beneath the grey–white junction (18 lesions, recorded in Table 1 as white matter lesions) and in the superficial cortex where pial arterioles penetrate the grey matter (12 lesions). Representative examples of each are present in the adjacent lesions in Fig. 1a–c. Deep white matter lesions were rarely observed (3 of the 38 total specimens) and all had an atypical cavitary appearance. The appearance of the hypointensities was not affected by the morphology of the lesion, except for a few of the cavitary lesions which had a hyperintense halo about the hypointensity (Fig. 1d). This halo was present for all three of the deep white matter cavities, and one of the of the four superficial white matter cavities.
Fig. 1.
Correlation of hypointensities and tissue pathology. The stepwise isolation of two lesions is illustrated above. In image a, a hypointensity in SWI is noted in the left temporal lobe. The corresponding lesion is shown in image b. This hematoma is typical of those located in grey matter, the blood does not diffuse into the tissue, but remains encapsulated within a pseudocapsule. A second lesion is present in this tissue block; the size of this lesion is overestimated by SWI, illustrating the “blooming effect” of this technology (arrows in a and b). Another hypointensity located in the white matter of the left parietal lobe is indicated in image d (scale is equal to image a). The corresponding lesion has been dissected in image e and is shown under a dissecting microscope in image f. The final image shows a cavitary lesion trabeculated by vascular elements. Further histologic workup of the lesion demonstrated hemosiderin granules within a gliotic capsule (see Fig. 6). Scale bars a 5 mm, c 1 mm
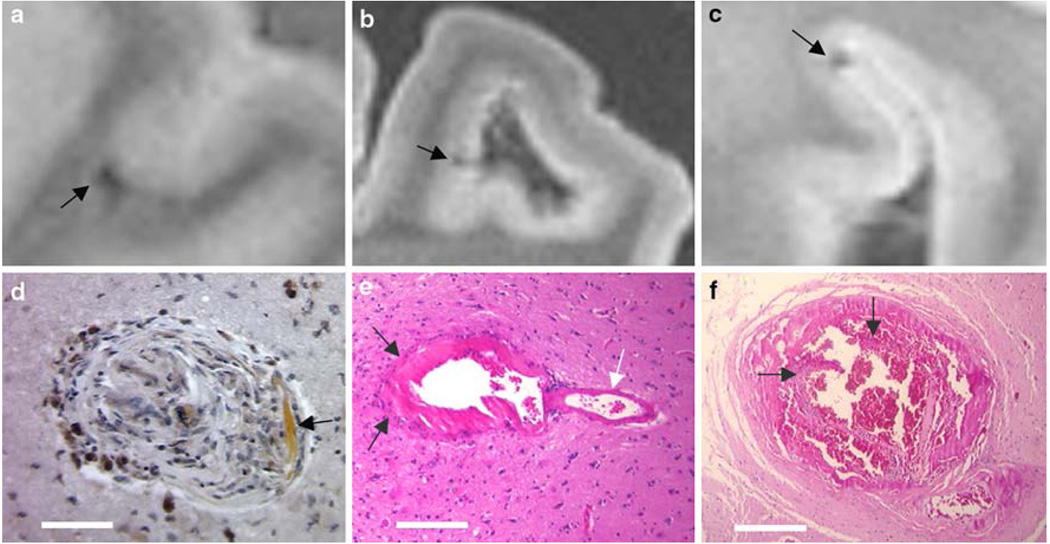
Fig. 2.
MR hypointensities without grossly visible pathology. The vast majority of hypointensities were associated with hemorrhages visible upon dissection; however, a few required more extensive investigation. Images a, b, c are MR images corresponding to the lesions shown in d, e, f, respectively. Shown in image d is a well-healed lesion consisting of focal scarring with hemosiderin deposits stained by DAB-enhanced Prussian blue stain. Hematoidin deposition is also noted in the lesion (white arrow). Image e shows an arteriolar aneurysm (arrows indicate the dome of the aneurysm; white arrow indicates the “parent” artery). Image f shows a severely dilated vessel with a dissection in the endothelium and blood in the vessel wall (arrows point the ruptured endothelial layer). Scale bars d 100 µm, e 250 µm, f 500 µm
Figure 3 compares the size of lesions to their apparent size in SWI. SWI consistently overestimated the diameter of small lesions in what has previously been termed the “blooming effect”; hypointensities in this study were 1.57 ± 0.75 times the size of the corresponding lesion. This discrepancy was most notable with smaller lesions where the hypointensity could appear more than three times the diameter of the actual lesion. This effect is illustrated in images a and b of Fig. 1 which show one lesion which appeared nearly accurately sized on SWI and another which was significantly magnified by the MR image. This effect may make quantifying the volume of hemorrhage from these MR images unreliable for BMB on the order of the voxel size.
Fig. 3.
The “blooming effect” of signal voids induced by hemosiderin-iron. The theoretical equality of lesion size to MR hypointensity size is graphed in red. The actual measurements are shown in the scatter plot and almost all the lesions are smaller than their associated MR finding. The signal voids averaged 1.58 ± 0.75 times the size of the associated lesion
CAA-related vascular damage
In several hemorrhages it was possible to determine which vessel ruptured. In these cases specific observation of the underlying vascular pathology was possible. We also examined the vasculature near sites of hemorrhage when examination of the ruptured vessel was not possible (Fig. 4). β-amyloid immunostaining demonstrated that β-amyloid deposition was present in the walls of involved vessels. Hematoxylin and eosin staining revealed significant morphologic changes in vessels associated with hemorrhage and the surrounding arterioles. The vessel walls were noted to be thickened, profoundly acellular, and lacking evidence of a muscularis layer. The pathology primarily involved arterioles, capillaries were only sparsely affected.
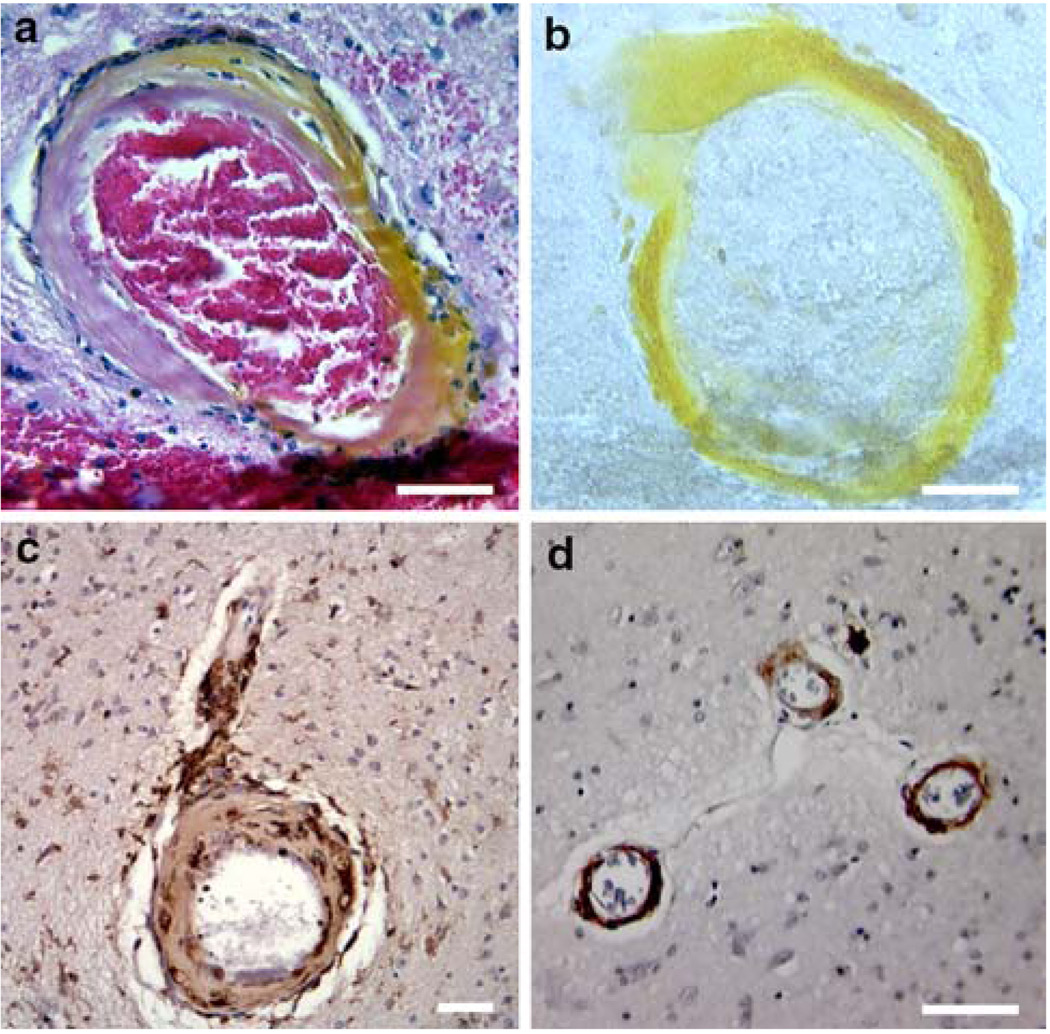
Fig. 4.
Vascular damage associated with CAA and hemorrhage. Images a and b show the vessel associated with the lesion in Fig. 1c. The vessel wall is hypocellular, eosinophilic and is surrounded by prominent macrophages. The arteriole is stained a brilliant yellow by the endogenous pigment hematoidin (a breakdown product of biliverdin indicating the presence of heme oxygenase activity). Image b represents an immunohistochemical stain of the same vessel demonstrating the presence of β-amyloid in the vessel wall (non-specific staining of the hemorrhage is present inferiorly in the photo). Image c represents an immunohistochemical stain against CD68, a marker of macrophages and microglia, demonstrating intense microglial activation around vascular elements and macrophages in the vessel wall. Image d shows immunohistochemistry for complement C6. The microvessels in CAA stain strongly for C6 in the tunica media. Scale bars a and b 50 µm, c and d 100 µm
CD68 immunostaining revealed activated microglia or macrophages on nearly all of the larger parenchymal vessels in a cortical/subcortical distribution. Little vascular staining was present in the deep grey matter. Complement activation on vessels was assessed with an antibody against complement component C6, the first component of the membrane attack complex (MAC) that stably inserts into the cell membrane. Once C6 is bound to C5b in the cell membrane, the MAC is activated, inevitably leading to cell lysis. The arterioles in CAA brain stained intensely against the C6 marker. This staining was associated with evidence of medial intima degeneration, the characteristic target-shaped vessel morphology associated with severe CAA. Arterioles in the basal ganglia did not react with the C6 antibody.
Evidence of peri-hematoma inflammation
Evidence for the presence of HO-1, an inducible, pro-oxidative enzyme that catalyzes the degradation of heme into biliverdin, carbon monoxide and free iron, was noted around many hematomas in the form of hematoidin, a bright yellow pigment. This pigment intensely stained the injured vessels in numerous lesions as illustrated in Fig. 1. Representative microbleeds were evaluated with immunohistochemical staining against HO-1. Intense staining of the hematoma was noted in every case along with variable reactivity of the adjacent parenchyma. More prominent staining of this adjacent tissue was noted near recent bleeds; however, some HO-1 activity was noted even around old bleeds without evidence of intact erythrocytes (see Fig. 5a). With fluorescent staining against HO-1, numerous non-neuronal cells in the perihematomal region stained strongly positive for HO-1 around both old and recent hematomas. No heme oxygenase activity was noted around the cavitary lesions. CD68 is a marker for cells of monocyte lineage and will stain microglia, macrophages and neutrophils. While intense reactivity of this antibody was noted in the vessels associated with each lesion, acute bleeds stained less intensely than old hematomas. Old hematomas were stained throughout the lesions and in perivascular spaces in which hemosiderin was deposited. CD20 and CD3 staining identified inflammatory cells in the vicinity of hemorrhage. Little evidence for B lymphocytes was present; however, significant CD3 staining of lymphocytes was noted near lesions. Finally, TUNEL staining for apoptotic cells was undertaken on four of the lesions (images not shown). Because formalin-archived tissue was used in this study, significant auto-fluorescence was present in the sections. To be considered apoptotic, a cell had to be both marked with the TUNEL probe and demonstrate a pyknotic or fragmented nucleus by DAPI staining. The region immediately adjacent to the hematoma was noted to contain significantly more apoptotic cells than the background, a nearly fourfold increase. The apoptosis rate in the background tissue of severe CAA/AD patients was 0.93% (±0.39%); 3.9% (± 1.6%) of the cells adjacent to the hematomas met criteria for apoptosis (p < 0.01).
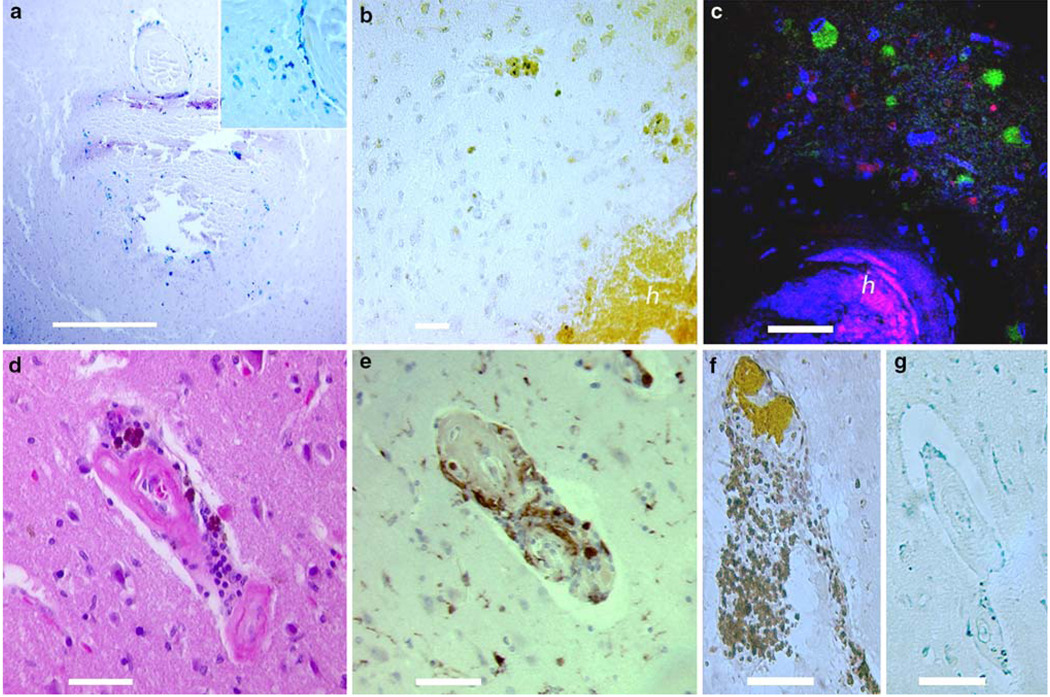
Fig. 5.
The local tissue reaction. Immunohistochemical staining highlights the local inflammatory response produced by a hematoma. Image a is a Prussian blue stain showing hemosiderin-laden macrophages infiltrating a hematoma. Iron-loaded macrophages are also prominent around the injured vessel (inset). Image b is stained by DAB over an anti-HO-1 primary antibody and illustrates intense heme oxygenase 1 (HO-1) reactivity within a hematoma and extending into the surrounding parenchyma (h indicates hematoma). Image c is a merged fluorescent study with MAP2/TexasRed staining in red to mark neurons, DAPI in blue to mark all cell nuclei and anti-HO-1/ FITC in green showing the presence of HO-1; the hematoma is visible in the inferior portion of the photo as non-specific staining (again labeled h). Perinuclear HO-1 expression is noted in the perifocal zone in non-neuronal cells. Image d shows an H&E of a vessel with perivascular heme-degradation products and surrounding inflammatory cells. Image e shows CD68 reactivity indicating a prominent microglial response around the same vessel. CD3 and CD20 staining of the same vessel are shown in images f and g, respectively. These images show that the inflammatory cells are primarily T lymphocytes, with negligible evidence of B cells. Scale bars b and c 50 µm, all others = 100 µm
Formation of secondary ischemia and white matter lesions
Cavitary, lacune-like lesions were present in one of seven deep grey matter lesions (14%), and six of 21 white matter lesions (29%). Three of these lesions occurred in deep white matter, three were in subcortical white matter. These sites were ringed by scarred vascular elements and a gliotic capsule containing hemosiderin granules and hemosiderinladen macrophages. Cavitary lesions in the deep white matter were larger (3–5 mm in diameter) than the subcortical cavitary lesions (1–2 mm). Numerous microinfarcts were encountered in the tissue which did not appear on SWI as hypointensities.
In acute bleeds in the white matter, hemorrhage products were noted to seep into the surrounding tissue, whereas hematomas discovered in grey matter were generally well-circumscribed lesions surrounding an injured vessel. Blood was noted to propagate along perivascular spaces away from the hemorrhage, bathing the regional vessels in blood and blood degradation products. These vessels were surrounded by T lymphocytes (as indicated by strongly positive CD3 and absent CD20 immunostaining) and numerous macrophages/activated microglia (Fig. 5). Similar pathologic abnormalities were found around the larger vascular components entering sites of ischemic cavitary lesions. No intact erythrocytes or evidence of acute blood was detected in or near any of the cavitary lesions. However, granular hemosiderin deposits and hemosiderin-laden macrophages were noted in the gliotic capsule around the lesion, so the lesions were interpreted as old, healed hemorrhage sites. Vessels were noted to cross nearly all of the cavitary lesions, but they appeared scarred, emerged from a fibrotic matrix and contained no residual blood (Fig. 6). Whether these lesions represent a primary ischemic pathology with secondary hemorrhage or tissue necrosis secondary to hemorrhage cannot be determined from this postmortem examination. Because CAA is primarily a cortical disease process, the most likely explanation is that cavitary lesions in the subcortical region represent secondary ischemia after a CAA-related microbleed. Cavities in the deep white matter are more likely secondary hemorrhage into a lacunar infarct, because CAA generally spares the deep white matter. Moreover, the white matter injury from a lacunar infarct could account for the hyperintense halo in SWI observed around these BMB.
Fig. 6.
Perivascular hemosiderin deposition may contribute to subsequent ischemic changes. Image a shows a hemorrhage around a degenerated arteriole with hematoidin deposition (yellow/orange material at black arrow), significant local inflammation and hemosiderin both in the lesion and tracking in the perivascular space along the arteriole (white arrow). Image b shows another vessel, ~500 µm distant from the lesion in image a, with extensive perivascular hemosiderin (arrow). Unenhanced Prussian blue staining of the same vessel is shown in image c. Hemosiderin was present around both capillaries and arterioles at a distance of more than twice the diameter of the lesion and was accompanied by inflammatory cells. Image d shows another lesion that appeared as a hypointensity in SWI (the cavitary lesion shown in Fig. 1d–f) and on pathological examination proved to be a lacunar infarct. Image e shows DAB-enhanced Prussian blue staining of the capsule around the lesion. The vessels are also surrounded by numerous inflammatory cells and hemosiderin-laden macrophages (arrows). Scale bars a and e 200 µm, b 100 µm, d 1 mm
Discussion
Post mortem MRI is valuable both as a research method and in clinical pathology [26]. Several approaches to post mortem MR imaging have been utilized, from scanning native, unfixed tissue to scanning fixed tissues bathed in liquid to embedding tissues in gels [14, 32]. Embedding tissues improves control and quality of the images and protects and preserves the specimens [28]. As neuroimaging techniques advance, it is important that imaging findings are carefully correlated to tissue pathology. Post mortem imaging enables a definitive approach to this process. While fixation of the brain certainly alters its magnetic resonance properties, these changes have been well studied and can be accommodated within standard imaging protocols [4, 7]. The architecture of the brains in our study remains clearly visualized with good signal-to-noise ratio at the current parameters. Moreover, iron pools have been shown to be stably preserved in formalin-archived tissue, so it is unlikely that the lesions of interest in this study were compromised by the fixation process [5].
In this study, blood and/or hemosiderin was encountered corresponding to every signal void in susceptibility-weighted images, but the size of the signal void did not reflect closely the size of the hemorrhage. There is wide variation in BMB size, location, and presumed clinical significance. Very small BMB with diameters of 50– 200 µm are widespread in the AD brain, but BMB visible by 1.5T GRE-T2* range from 3–10 mm in diameter in the MR image [11]. There is general agreement among radiologists that BMB are homogeneous round signal losses with diameters less than 5 mm, though some reports include lesions up to 10 mm in apparent diameter [25]. A recent report determined that hemorrhages fall in a bimodal distribution between macro- and micro-hemorrhages with the critical separating value being 5.7 mm [16]. Those detectable by SWI at 3T in this study are as small as 1 mm in apparent diameter on the scans, and 0.3–0.4 mm measured diameter in the tissue. SWI consistently overestimated the size of bleeds in what has been termed a “blooming effect.” In our specimens, the blooming effect magnified the smallest bleeds by as much as 300%. Because of this phenomenon, the apparent size of the lesion on MR is an unreliable estimate of the extent of tissue injury.
The underlying pathologic lesions we discovered correlating to hypointensities were quite varied. The vessels associated with bleeding demonstrated pathologies classically associated with CAA including vessel wall thickening, β-amyloid replacement of vascular smooth muscle, microaneurysms and, most frequently, frank hemorrhage. β-amyloid has been shown in in vitro studies to be particularly toxic to vascular smooth muscle cells, which may explain the relative acellularity of the muscularis layer in the involved arterioles [12]. Complement activation was also noted as a prominent part of the vascular pathology, and may be a mechanism of or contribute to β-amyloid toxicity in the vessel wall. The presence of late complement component C6 surrounding diseased vessels and hemorrhages is a notable finding. Numerous studies have examined the role of the complement system in Alzheimer’s disease; it has been established that β-amyloid binds to both C1q and C3 of the classical complement pathway, activating the early portion of the cascade [38, 46]. However, the cascade is only activated through C3 in the tissue parenchyma; the lytic portion has not been noted to play a major role in the inflammation induced by β-amyloid plaques [6]. We found that the lytic pathway is observed prominently in the vessel wall. Cultured human cerebrovascular cells have been shown to secrete all the components of the late complement cascade and C6 in particular is upregulated in the presence of β-amyloid [43]. The Alzheimer’s brain is primed for a complement reaction; with elevated parenchymal levels of activated early complement, the introduction of the terminal proteins through vascular injury and hemorrhage may trigger significant cellular loss. The presence of activated MAC in this pathology may make this cascade of future therapeutic interest.
Several sites thought to be microvascular bleeding on SWI were found to be associated with cavitary lesions resembling small lacunar infarcts. Iron deposits were found in the gliotic capsule around these lesions which presumably produced the signal void appreciated on SWI (Fig. 6d, e). In a few cases, particularly larger lesions, a hyperintense halo was present around the hypointensity which may represent superimposed white matter scarring (Fig. 1). A correlation between microbleeds and lacunar infarcts has been established by a number of imaging studies; in fact, one recent study found the presence of a lacunar infarct the strongest predictor of microbleeds in neurologically healthy adults, stronger than all traditional vascular risk factors [21]. The cause of this phenomenon is not entirely clear from previous studies. Central nervous system microvasculopathies are well associated with lacunar infarcts, causing a disturbance in the penetrating arteries feeding white matter tracts, interrupting this important terminal blood supply [15]. Recently, the severity of CAA pathology was shown to strongly correlate with the prevalence of microinfarcts in both grey and white matter in a post mortem study of patients with varying degrees of CAA [34]. Moreover, while the prevalence of microinfarcts (diameter < 5 mm, on the order of the size of microbleeds) correlates to CAA severity, the prevalence of larger lacunar infarcts does not [13, 34]. Several observations here may help clarify the pathological mechanism underlying these lesions and why they are so closely associated with microhemorrhages. The tissue response to microhemorrhage is an intense inflammatory reaction. We found that activated microglia and T lymphocytes are prominent around hemorrhage, complement is activated and HO-1 is induced. This ultimately results in significantly increased cell death including the loss of neurons about the lesions. It has been well described that extracellular fluids in the brain migrate along the vascular bed in a retrograde fashion through perivascular spaces and are reabsorbed at the level of the arterioles [31]. These Virchow–Robin spaces are significantly dilated in CAA which could facilitate the movement of perivascular contents [30]. As shown in Fig. 3, blood from hemorrhagic lesions propagates through the perivascular space delivering an inflammatory reaction along a significant portion of the local vasculature. This pattern of spread also has been noted in MR studies looking at the symptomatic perforating artery adjacent to a lacunar infarct [44]. Additionally, the toxic milieu is confined differently in white matter and grey matter. The Virchow–Robin spaces in grey matter are invested with two meningeal membranes: one basal lamina closely investing the vessel, one investing the glia limitans at the parenchymal surface. In the white matter, the parenchymal membrane is absent, which seems to facilitate the migration of hemorrhagic blood products through the white matter, while it is generally confined to a hematoma in the grey [29]. This may result in a more aggressive inflammatory response in the white matter, increasing the probability of spasm or scarring of the vascular supply. These features could conceptually account for the ischemic appearance of some lesions, particularly in the white matter. We observed significant scarring in vessels surrounded by hemosiderin as they enter the lacune-like portion of BMB. It is also possible that an ischemic insult precipitates secondary hemorrhage. This explanation is particularly likely for the hypointensities found in the deep white matter, as CAA pathology is generally limited to the cortical ribbon and the immediate subcortical white matter.
We did not attempt to quantitatively assess the sensitivity of the technique. The size of the lesions encountered enables a rough estimate of the detection limit, but does not address lesions which may have been missed by the MRI. We also did not control for false-positive findings, that is, that blood correlating to MR findings may be non-specific bleeding present in any given specimen of aged brain. However, all but 3 of the 38 lesions were readily visible upon inspection of the tissue prior to microscopy and those detected at microscopy were 300 µm in diameter or more; since these lesions are significantly larger than the traces of blood products which are frequently observed around aged vessels, we feel confident that they do not represent false-positive findings. With the current detection limit of the 3T clinical instrument, incidental, non-specific microbleeding is unlikely to be detected and the risk of false-positive findings is quite low. Finally, we encountered a somewhat greater number of recent bleeds (26% contained intact erythrocytes) than would be anticipated. This over-representation may be explained by peri-mortem trauma or gradual resolution of old microbleeds over time; however, we cannot exclude postmortem bleeding as an explanation for some of these acute bleeds [23].
This study is an important step in validating the interpretation of hypointensities in SWI as brain microbleeds. A number of studies now argue in favor of the use of these signal hypointensities as a biomarker of CAA. This may be justified, but we offer a few cautions. First, while hypointensities in this study were associated with CAA pathologies (microbleeding, microaneurysm and endothelial defect) there are conceivable causes for hypointensities that are unrelated to this pathology, including microthrombi, calcifications and air emboli. Additionally, while the distribution of lesions in CAA is characteristic, other disease processes can also produce microhemorrhages. In particular, chronic, severe hypertension is known to cause BMB, but these are primarily located in the basal ganglia and deep structures of the brain, while CAA-related lesions predominate in a lobar distribution. The presence of hypointensities should be interpreted in light of the clinical setting and the presence of a single hypointensity in a patient who is cognitively normal may be of little importance in evaluating CAA [24]. However, the presence of multiple BMBs is rarely, if ever, benign and likely does represent microvascular bleeding. Moreover, detectable bleeding in a distribution consistent with CAA in a patient with cognitive deficits likely indicates significant additional bleeding below the threshold currently detectable by clinical imaging studies [11].
Brain microbleeds are clearly an important “lesion.” Some studies indicate that over 95% of AD patients are found at autopsy to have some degree of CAA pathology [17]. Roughly one-third of patients with AD have MR-detectable microbleeds, and patients whose cognitive loss is primarily associated with this sort of bleeding may respond to different sorts of treatment modalities. These lesions have also been noted early in the process of cognitive loss and have a prognostic value for the progression of mild cognitive impairment to dementia. When viewed as an ongoing, progressive process, it is not difficult to believe that CAA and microvascular bleeding play a significant role in the cognitive dysfunction ascribed to Alzheimer’s disease. Ultimately, we continue to wait for effective therapeutic strategies for dementia to emerge, but this imaging technique offers in vivo diagnostic potential to a significant portion of Alzheimer’s patients and accurate diagnosis is a prelude to therapy.
Acknowledgments
This research was funded by the National Institutes of Health (AG20948). Harry V. Vinters is supported in part by P01 AG12435, P50 AG16570 and the Daljit S. and Elaine Sarkaria Chair in Diagnostic Medicine. E. Mark Haacke is a consultant to Siemens Corporation. None of the other authors have real or potential conflicts of interest related to this work. We thank Zachary Taylor who was an undergraduate summer researcher in our lab, as well as Cindy Dickson, April Dickson and Jackie Knecht for administrative assistance and for editing the manuscript.
Abbreviations
- GRE-T2*
Gradient echo T2*
- SWI
Susceptibility-weighted imaging
- MR
Magnetic resonance
- BMB
Brain microbleed
- CAA
Cerebral amyloid angiopathy
- AD
Alzheimer’s disease
- HO-1
Heme oxygenase 1
Contributor Information
Matthew Schrag, Neurosurgery Center for Research, Training and Education, Loma Linda University, Coleman Pavilion, Suite 11113, 11175 Campus St, Loma Linda, CA 92350, USA.
Grant McAuley, Neurosurgery Center for Research, Training and Education, Loma Linda University, Coleman Pavilion, Suite 11113, 11175 Campus St, Loma Linda, CA 92350, USA.
Justine Pomakian, Department of Pathology and Laboratory Medicine, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA; Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Arshad Jiffry, Neurosurgery Center for Research, Training and Education, Loma Linda University, Coleman Pavilion, Suite 11113, 11175 Campus St, Loma Linda, CA 92350, USA.
Spencer Tung, Department of Pathology and Laboratory Medicine, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA; Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Claudius Mueller, Neurosurgery Center for Research, Training and Education, Loma Linda University, Coleman Pavilion, Suite 11113, 11175 Campus St, Loma Linda, CA 92350, USA; Center for Applied Proteomics and Molecular Medicine, George Mason University, Manassas, VA, USA.
Harry V. Vinters, Department of Pathology and Laboratory Medicine, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
E. Mark Haacke, The Magnetic Resonance Imaging Institute for Biomedical Research, Detroit, MI, USA; Department of Radiology, Loma Linda University School of Medicine, Loma Linda, CA, USA; Department of Radiology, Wayne State University, Detroit, MI, USA.
Barbara Holshouser, The Magnetic Resonance Imaging Institute for Biomedical Research, Detroit, MI, USA.
Daniel Kido, The Magnetic Resonance Imaging Institute for Biomedical Research, Detroit, MI, USA.
Wolff M. Kirsch, Neurosurgery Center for Research, Training and Education, Loma Linda University, Coleman Pavilion, Suite 11113, 11175 Campus St, Loma Linda, CA 92350, USA, wkirsch@llu.edu
References
- 1.Aliev G, Smith MA, Seyidov D, et al. The role of oxidative stress in the pathophysiology of cerebrovascular lesions in Alzheimer’s disease. Brain Pathol. 2002;12:21–35. doi: 10.1111/j.1750-3639.2002.tb00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anders K, Wang Z, Kornfeld M, et al. Giant cell arteritis in association with cerebral amyloid angiopathy: immunohistochemical and molecular studies. Hum Pathol. 1997;89:1237–1246. doi: 10.1016/s0046-8177(97)90196-9. [DOI] [PubMed] [Google Scholar]
- 3.Atlas SW, Mark AS, Grossman RI, Gomori JM. Intracranial hemorrhage: gradient-echo MR imaging at 1.5 T. Comparison with spin-echo imaging and clinical applications. Radiology. 1988;168:803–807. doi: 10.1148/radiology.168.3.3406410. [DOI] [PubMed] [Google Scholar]
- 4.Blamire A, Rowe J, Styles P, McDonald B. Optimising imaging parameters for post mortem MR imaging of the human brain. Acta Radiol. 1999;40:593–597. doi: 10.3109/02841859909175593. [DOI] [PubMed] [Google Scholar]
- 5.Bush V, Moyer T, Batts K, Parisi J. Essential and toxic element concentrations in fresh and formalin-fixed human autopsy tissues. Clin Chem. 1995;41(2):284–294. [PubMed] [Google Scholar]
- 6.Cadmen E, Puttfarcken P. Beta-amyloid peptides initiate the complement cascade without producing a comparable effect on the terminal pathway in vitro. Exp Neurol. 1997;146:388–394. doi: 10.1006/exnr.1997.6540. [DOI] [PubMed] [Google Scholar]
- 7.Carvlin M, Asato R, Hackney D, Kassab E, Joseph P. High-resolution MR of the spinal cord in humans and rats. Am J Neuroradiol. 1989;10:13–17. [PMC free article] [PubMed] [Google Scholar]
- 8.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130:1988–2003. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- 9.Cordonnier C, Potter G, Jackson C, Doubal F, Sodlow C, Wardlaw J, Al-Shahi Salman R. Improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale. Stroke. 2008;40:94–99. doi: 10.1161/STROKEAHA.108.526996. [DOI] [PubMed] [Google Scholar]
- 10.Cordonnier C, van der Flier WM, Sluimer JD, Leys D, Barkhof F, Scheltens P. Prevalence and severity of microbleeds in a memory clinic setting. Neurology. 2006;66:1356–1360. doi: 10.1212/01.wnl.0000210535.20297.ae. [DOI] [PubMed] [Google Scholar]
- 11.Cullen KM, Kocsi Z, Stone J. Pericapillary haem-rich deposits: evidence for microhaemorrhages in aging human cerebral cortex. J Cereb Blood Flow Metab. 2005;25:1656–1667. doi: 10.1038/sj.jcbfm.9600155. [DOI] [PubMed] [Google Scholar]
- 12.Davis J, Cribbs DH, Cotman CW, Van Nostrand WE. Pathogenic amyloid-beta protein induces apoptosis in cultured human cerebrovascular smooth muscle cells. Amyloid. 1999;6:157–164. doi: 10.3109/13506129909007321. [DOI] [PubMed] [Google Scholar]
- 13.Ellis R, Olichney J, Thal L, et al. Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, Part XV. Neurology. 1996;46:1592–1596. doi: 10.1212/wnl.46.6.1592. [DOI] [PubMed] [Google Scholar]
- 14.Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher C. Lacunar infarcts—a review. Cerebrovasc Dis. 1991;1:311–320. [Google Scholar]
- 16.Greenberg S, Nandigam R, Delgado P, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke. 2009;40:2382–2386. doi: 10.1161/STROKEAHA.109.548974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg S, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Stroke. 1997;28:1418–1422. doi: 10.1161/01.str.28.7.1418. [DOI] [PubMed] [Google Scholar]
- 18.Haacke EM, Cheng NY, House MJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging. 2005;23:1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Haacke EM, DelProposto ZS, Chaturvedi S, et al. Imaging cerebral amyloid angiopathy with susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2007;28:316–317. [PMC free article] [PubMed] [Google Scholar]
- 20.Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- 21.Igase M, Tabara Y, Igase K, et al. Asymptomatic cerebral microbleeds seen in healthy subjects have a strong association with asymptomatic lacunar infarction. Circ J. 2009;73:530–533. doi: 10.1253/circj.cj-08-0764. [DOI] [PubMed] [Google Scholar]
- 22.Jellinger KA, Lauda F, Attems J. Sporadic cerebral amyloid angiopathy is not a frequent cause of spontaneous brain hemorrhage. Eur J Neurol. 2007;14:923–928. doi: 10.1111/j.1468-1331.2007.01880.x. [DOI] [PubMed] [Google Scholar]
- 23.Kirsch W, McAuley G, Holshouser B, et al. Serial susceptibility weighted MRI measures brain iron and microbleeds in dementia. J Alzheimer’s Dis. 2009;17:599–609. doi: 10.3233/JAD-2009-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 25.Koennecke HC. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology. 2006;66:165–171. doi: 10.1212/01.wnl.0000194266.55694.1e. [DOI] [PubMed] [Google Scholar]
- 26.Nicholl R, Balasubramanian V, Urguhart D, Sellathurai N, Rutherford M. Postmortem brain MRI with selective tissue biopsy as an adjunct to autopsy following neonatal encephalopathy. Eur J Paediatr Neurol. 2007;11:167–174. doi: 10.1016/j.ejpn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Rosand J, Muzikansky A, Kumar A, et al. Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann Neurol. 2005;58:459–462. doi: 10.1002/ana.20596. [DOI] [PubMed] [Google Scholar]
- 28.Pfefferbaum A, Sullivan E, Adalsteinssom E, Garrick T, Harper C. Postmortem MR imaging of formalin-fixed human brain. Neuroimage. 2004;21:1585–1595. doi: 10.1016/j.neuroimage.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 29.Pollock H, Hutchings M, Weller RO, Zhang ET. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat. 1997;191:337–347. doi: 10.1046/j.1469-7580.1997.19130337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roher A, Kuo Y, Esh C, et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer’s disease. Mol Med. 2003;9:112–122. [PMC free article] [PubMed] [Google Scholar]
- 31.Schley D, Carare-Nnadi R, Please C, Perry V, Weller R. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol. 2006;238:962–974. doi: 10.1016/j.jtbi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Schmierer K, Wheeler-Kingshott C, Boulby P, et al. Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage. 2007;35:467–477. doi: 10.1016/j.neuroimage.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sehgal V, Delproposto Z, Haacke EM, et al. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005;22:439–450. doi: 10.1002/jmri.20404. [DOI] [PubMed] [Google Scholar]
- 34.Soontornniyomkij V, Lynch M, Mermash S, et al. Cerebral microinfarcts associated with severe cerebral beta-amyloid angiopathy. Brain Path. 2009 doi: 10.1111/j.1750-3639.2009.00322.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79:1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka A, Ueno Y, Nakayama Y, Takano K, Takebayashi S. Small chronic microhemorrhages and ischemic lesion in association with spontaneous intracerebral hematomas. Stroke. 1999;30:1637–1642. doi: 10.1161/01.str.30.8.1637. [DOI] [PubMed] [Google Scholar]
- 37.Tatsumi S, Shinohara M, Yamamoto T. Direct comparison of histology of microbleed with postmortem MR images: a case report. Cerebrovasc Dis. 2008;26:142–146. doi: 10.1159/000139661. [DOI] [PubMed] [Google Scholar]
- 38.Terai K, Walker D, McGeer E, McGeer P. Neurons express proteins of the classical complement pathway in Alzheimer disease. Brain Res. 1997;769:385–390. doi: 10.1016/s0006-8993(97)00849-4. [DOI] [PubMed] [Google Scholar]
- 39.Tong KA, Ashwal S, Holshouser BA, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology. 2003;227:332–339. doi: 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- 40.Tong KA, Ashwal S, Holshouser BA, et al. Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Ann Neurol. 2004;56:36–50. doi: 10.1002/ana.20123. [DOI] [PubMed] [Google Scholar]
- 41.Viswanathan A, Chabriat H. Cerebral microhemorrhage. Stroke. 2006;37:550–555. doi: 10.1161/01.STR.0000199847.96188.12. [DOI] [PubMed] [Google Scholar]
- 42.Walker DA, Broderick DF, Kotsenas AL, Rubino FA. Routine use of gradient-echo MRI to screen for cerebral amyloid angiopathy in elderly patients. AJR Am J Roentgenol. 2004;182:1547–1550. doi: 10.2214/ajr.182.6.1821547. [DOI] [PubMed] [Google Scholar]
- 43.Walker D, Dalsing-Hernandez J, Lue L. Human postmortem brain-derived cerebrovascular smooth muscle cells express all genes of the classical complement pathway: a potential mechanism for vascular damage in cerebral amyloid angiopathy and Alzheimer’s disease. Microvasc Res. 2008;75:411–419. doi: 10.1016/j.mvr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wardlaw J, Dennis M, Warlow, Sandercock P. Imaging appearance of the symptomatic perforating artery in patients with lacunar infarction: 1 occlusion or other vascular pathology. Ann Neurol. 2001;50:208–215. doi: 10.1002/ana.1082. [DOI] [PubMed] [Google Scholar]
- 45.Yakushiji Y, Nishiyama M, Yakushiji S, et al. Brain microbleeds and global cognitive function in adults without neurological disorder. Stroke. 2008;39:3323–3328. doi: 10.1161/STROKEAHA.108.516112. [DOI] [PubMed] [Google Scholar]
- 46.Yasojima K, Schwab C, McGeer E, McGeer P. Up-regulated production and activation of the complement system in Alzheimer’s disease brain. Am J Pathol. 1999;154:927–936. doi: 10.1016/S0002-9440(10)65340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]