Abstract
Aims
The cardiovascular system is an important target of estrogenic compounds. Considering the recent studies that question previously reported cardio-protective effects of estrogen, there is a growing concern that estrogenic environmental compounds may contribute to the pathology of vascular lesion formation.
Main methods
Real-time quantitative PCR was used to monitor the expression of genes involved in vascularization. Using Bayesian network modeling, we determined a gene network that estrogenic chemicals modulate in human vascular endothelial cells.
Key findings
We showed that planar and co-planar polychlorinated biphenyls (PCBs) induce the expression of different genes compared to estradiol. Non-planar PCB congener 153 induced NOTCH3 which is a new finding as well as CCL2 and IL8 similar to what has been reported by other non-planar PCBs in endothelial cells. Our gene network indicated that experimental treatments signal a network containing TGF-β receptor and NOTCH3; molecules biologically relevant to signaling pulmonary vascular lesions.
Significance
We report in the present study that exposure of vascular endothelial cells to environmentally relevant concentrations of estrogenic PCBs induce gene networks implicated in the process of inflammation and adhesion. Our data suggest that PCBs can promote vascular lesion formation by activating gene networks involved in endothelial cell adhesion, cell growth, and pro-inflammatory molecules which were different from natural estrogen. Since inflammation and adhesion are a hallmark in the pathology of endothelial cell dysfunction, reconstructing gene networks provide insight into the potential mechanisms that may contribute to the vascular risks associated with estrogenic environmental chemicals.
Keywords: polychlorinated biphenyls (PCBs), estrogen, vascular lesion
Introduction
The cardiovascular system is an important target of estrogenic compounds through genomic and non-genomic pathways (Chambliss et al., 2002). The estrogen receptor (ERα/β) has been shown in human coronary arteries, endothelial cells, and cardiomyocytes (Kim-Schulze et al., 1996;Yang et al., 2004). Recent studies question previously reported beneficial effects of estrogen on the cardiovascular system. The Women's Health Initiative, indicated that long-term use of estrogen did not decrease, and may have increased, the risk of cardiovascular disease (CVD) (Rossouw, 2005). However, the use of estrogens to prevent CVD has never been without controversy. For instance, The Coronary Drug Project was discontinued because of a significant increase of coronary heart disease among men receiving estrogens (1970;1973). Estrogen therapy in diabetic women was shown to be associated with a worsening of coronary atherosclerosis and exacerbation of inflammatory markers (Howard et al., 2004). And the use of the synthetic estrogen diethylstilbestrol to treat prostate cancer was associated with increased cardiovascular morbidity and mortality in men (Cox and Crawford, 1995).
Pulmonary arterial hypertension (PAH) is roughly twice as common in women compared to men (Newman et al., 2004); whether this occurrence may be attributed to the hormone estrogen is not clear. In the United States, PAH afflicts approximately 100,000 individuals and is the cause of death in 20,000 people each year (Hyduk et al., 2005). Evidence suggests that intra-luminal proliferation of endothelial cells causes PAH. Vascular lesions of severe PAH consist of actively proliferating endothelial cells (Sakao et al., 2009). Since estrogen is a known mitogen of endothelial cells, we postulate that pulmonary vascular lesions are a consequence of excess or unopposed estrogen. Although beneficial effects of estrogen on the endothelium have been reported (Cid et al., 2002); differences between endothelial cell types could account for greater susceptibility of pulmonary arteries to estrogen-induced inflammation and vascular lesion formation. Widespread contaminants in the environment such as arsenic, tobacco smoke, and polychlorinated biphenyls (PCBs) have been reported to possess estrogenic activity (Davey et al., 2007;Meek and Finch, 1999;Bitman and Cecil, 1970;Tavolari et al., 2006). Since the effect of estrogens on pulmonary vasculature is not well studied, research elucidating the role of these compounds in the development of pulmonary vascular lesions is highly relevant to cardiovascular public health.
Although estrogen-induced gene expression has been well studied in endothelial cells, there is a lack of knowledge about how the expression profile differs from estrogenic environmental chemicals. Several epidemiological studies have shown a link between PCB exposure and increased risk of CVD (Gustavsson and Hogstedt, 1997;Hay and Tarrel, 1997;Goncharov et al., 2008;Tokunaga and Kataoka, 2003;Sergeev and Carpenter, 2005). Considering the recent studies that question the previously reported cardio-protective effects of estrogens, there is a growing concern that estrogenic PCBs may increase the risk for PAH by inducing the formation of pulmonary vascular lesions. The present study aims to investigate the differential effects of estrogens on candidate genes involved in vascularization in human vascular endothelial cells exposed to environmentally relevant concentrations (ppb) of PCB126, PCB153, and physiological concentrations of 17β-estradiol.
Materials and Methods
Cell Culture
A telomerase-immortalized human microvascular endothelium (TIME) cell line was obtained from American Type Culture Collection (Manassas, VA) to be referred throughout this study as HMVEC. These endothelial cells have retained many of the phenotypic characteristics of the primary endothelial cells from which they were derived including normal cell morphology and the capacity to form tubules in vitro (Venetsanakos et al., 2002). Cells were grown in Endothelial Cell Basal Medium-2 (EBM-2) supplemented with EGM 2-MV Single-Quots from Lonza (Walkersville, MD). Estrogen treatments were studied under the following culture conditions: 75–80% confluent cultures were washed and serum starved in phenol red-free medium for 3h. Thereafter, the cells were treated with either PCB126, PCB153, or 17β-estradiol in phenol red-free medium supplemented with 10% charcoal/dextran-treated fetal bovine serum to avoid any estrogenic activity and maintained for 24 hours.
Estrogenic chemical treatments
Stock solutions of PCB126, PCB153, and 17β-estradiol were prepared in dimethyl sulfoxide (DMSO). The same amount of DMSO as in PCB and estradiol-treated cells were added to control cultures. The level of DMSO in experimental media was less than 0.1%. PCB blood levels have been reported to reach approximately 1000ng/ml (~3 M) in occupationally exposed individuals (Wassermann et al., 1979). Our unpublished data showed a significant increase in PCB-induced vascularization with PCB153 concentrations of 10–100ng/ml. Based on known PCB blood levels from occupational exposure and our preliminary results; we chose a 10-fold lower PCB dose of 100ng/ml (~0.3μM) to expose endothelial cells. Our previous studies also showed endothelial cell proliferation and vascular tube formation at physiological doses of 17β-estradiol (Felty, 2006;Felty and Porther, 2008); therefore HMVEC were exposed to estradiol at 1ng/ml (~3.6nM). PCB congeners 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153) and 3,3',4,4',5-pentachlorobiphenyl (PCB126) were purchased from AccuStandard (New Haven, CT) and dissolved in dimethyl sulfoxide (DMSO). All other chemicals and reagents were purchased from Sigma (St. Louis, MO).
Real-time RT PCR
Total RNA was isolated and purified using the RNeasy Mini Kit from Qiagen (Valencia, CA). RNA sample quality was verified by electrophoresis on a denaturing agarose gel and then reverse transcribed into cDNA using the RT2 First Strand Kit from SuperArray Bioscience Corporation (Frederick, MD) according to the manufacturer's protocol. The PCR reactions using cDNA were performed in a Applied Biosystems 7300 Real-Time PCR System using RT2 SYBR Green/ROX qPCR Master Mix and the manufacturer's thermal cycler protocol (95°C for 10 min, followed by 40 cycles at 95°C for 15s, 60°C for 1 min). The threshold cycle (CT) from each well was determined using ABI 7300 SDS software.
PCR array
PCR array analysis of cDNA from RNA isolated from HMVEC treated with vehicle control (0.1% DMSO), PCB126, PCB153, or 17β-estradiol for 24h was performed in triplicate using RT2 Profiler PCR array for Human Angiogenesis from SABiosciences, in accordance with the manufacturer's instructions. PCR arrays were used to compare the relative expression of 84 genes for each array. Relative changes in gene expression between the treated and untreated HMVEC RNA were calculated by the ΔΔCT method as previously described (Livak and Schmittgen, 2001). First, target genes in each array were normalized to the endogenous control or housekeeping genes: RPL13A, GAPDH, or ACTB. Next, each treatment group was normalized to the untreated group (vehicle control, 0.1% DMSO). Using the SABiosciences PCR Array Data Analysis Web Portal, fold changes in gene expression were analyzed between groups using the equation 2−ΔΔCT, where ΔΔCT= [CTtarget gene−Avg. CThousekeeping genes]treated group−[CTtarget gene−Avg. CThousekeeping genes]control group. Each value is a repeat of three independent experiments. ANOVA and Fisher's least significant difference test were used for multi-group comparisons, with a P value of less than 0.05 considered significant.
Gene Network Analysis
Given the emergence of datasets in medicine and biology with large number of variables, Bayesian Networks have been successful in developing efficient algorithms that scale up to datasets involving hundreds of variables in learning high quality predictive models from genomic data (Yoo and Cooper, 2004). A Bayesian Network is a directed acyclic graph in which each node represents a variable and each arc represents probabilistic influence. In Bayesian Networks, each arc is interpreted as a direct influence between a parent node (variable) and a child node, relative to the other nodes in the network.
Results
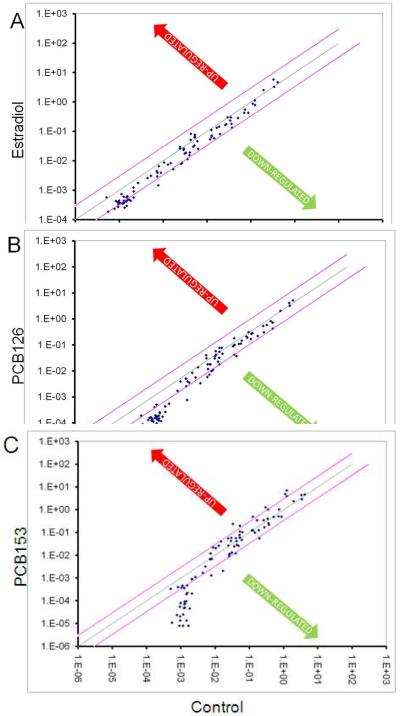
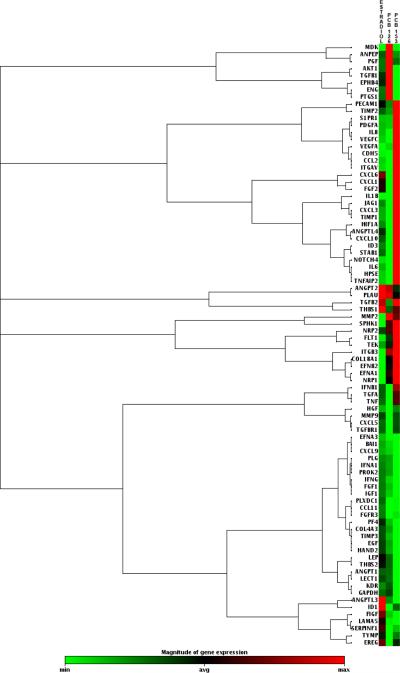
Although estradiol–induced gene expression in endothelial cells has been well studied, the question of whether estrogenic PCB-induced gene expression differs from the natural hormone 17β-estradiol has not been tested until now. Our previous studies have shown that physiological concentrations of estradiol induce the formation of reactive oxygen species (ROS) involved in signaling cell proliferation and vascularization (Felty, 2006;Felty and Porther, 2008). To further study this phenotype, we used a PCR array to compare 17β-estradiol to PCB-induced endothelial cell gene expression. We chose PCB153 because it was shown to bind the ERα and induce vessel formation in endothelial cells (Tavolari, Bucci, Tomasi, and Guarnieri, 2006). We chose a coplanar PCB (PCB126) to compare with the non-coplanar PCB153; in order to determine whether the gene expression profiles between these two PCB congeners were specific. Using the RT2 Profiler PCR array for Human Angiogenesis, we have monitored the mRNA levels of 84 different genes involved in vascularization. This array was selected because we previously reported estrogen-induced redox sensitive signaling in vascular tube formation. Human endothelial cells were treated with vehicle control (0.1% DMSO), 17β-estradiol, or PCB congeners 126 and 153 for 24h. Fig. 1 depicts a log transformation plot indicating the relative expression level of each gene. The red lines indicate a 3-fold change in gene expression compared to control. In Fig. 1A, PCB153 treatment increased the expression of six of the 84 genes by more than 3-fold. Expression of two genes, CCL2 and IL-8, was corroborated by other studies that reported the same genes to be induced by non-coplanar PCB congener 104 in endothelial cells (Choi et al., 2003;Eum et al., 2004). In Fig. 1B, PCB126 did not show a 3-fold increase in any of the genes studied, instead approximately 90% of the genes were less than or equal to the control. In Fig. 1C, estradiol treatment also did not show a 3-fold increase in gene expression and followed a trend similar to PCB126 treatment. Hierarchical clusters were used to analyze these expression profiles from the different treatments (Fig. 2). The hierarchical clustering method performs a cluster analysis using a set of dissimilarities for the gene profiles being clustered. Initially, each object is assigned to its own cluster and then the algorithm proceeds iteratively, at each stage joining the two most similar clusters, continuing until there is just a single cluster. At each stage distances between clusters are recomputed by the Lance-Williams dissimilarity update formula according to the particular clustering method being used. Among the treatment groups, we identified 5 clusters. The largest cluster consisted mainly of genes related to growth factors and proteases. While the second largest cluster showed a majority of cytokines and chemokines.
Fig. 1.
Scatter plot report for RT2 Profiler PCR array for Human Angiogenesis analysis of cDNA from RNA isolated from untreated HMVEC or HMVEC treated for 24h with (A) 17β-estradiol 1ng/ml, (B) PCB126 100ng/ml, and (C) PCB153 100ng/ml. The figure depicts a log transformation plot of the relative expression level of each gene between the estrogen treated and untreated HMVEC. Changes in mRNA expression were calculated by the ΔΔCT method. The figure is labeled with estrogen treatment on y-axis and untreated x-axis. The red lines indicate a 3-fold change in gene expression.
Fig. 2.
Hierarchical cluster of HMVEC gene expression changes in response to estrogen treatments. Each row represents an individual gene, and each column represents three independent experiments (n=3, estradiol, PCB126, PCB153). Up- (red) or Down- (green) regulated.
Genes regulated by estrogen treatments were organized by function to better understand their profile (Table 1). The manufacturer's categories: Adhesion Molecules, Cytokines & Chemokines, Growth Factors & Receptors, Proteases, Inhibitors, & Matrix Proteins, and Transcription Factors; were used to organize the genes by function. In Table 1, genes that exhibit at least a 2-fold regulation in expression in the estradiol, PCB126, and PCB153 treatments are highlighted in red if up-regulated and green if down-regulated. This classification showed that PCB153 up-regulated significantly greater number of genes when compared to estradiol. These genes included adhesion molecules CDH5, ITGAV; cytokines and chemokines IL8, IL6, CCL2, CXCL10; growth factors and receptors S1PR1, PDGFA, TEK, TGFB2, VEGFA, VEGFC; and from the remaining categories ANGPTL4 and NOTCH4. Endothelial cells depend on processes of migration, adhesion, and proliferation when forming vessels. PCB153 significantly up-regulated: growth factors, adhesion molecules, and pro-inflammatory factors relevant to the process of vascularization. When we compared the coplanar PCB congener 126 to PCB153 we observed a significantly different profile. PCB126 down-regulated many genes that were similar to PCB153; however, PCB126 exposed endothelial cells showed more cytokines and chemokines significantly down-regulated compared to both PCB153 and estradiol treated cells. In the estradiol treatment; TGFB2 up-regulation was similar to PCB153 treatment. The majority of down-regulated genes in the estradiol treatment were common to both PCB congeners.
Table 1.
Up/Down-Regulated Genes
| Functional Groups | Description | Up/Down Regulation (Compared to control) | ||
|---|---|---|---|---|
| Adhesion Molecules | ||||
| Estradiol | PCB126 | PCB153 | ||
| ANGPTL3 | Angiopoietin-like 3 | 0.05 | −1.93 | −5.27 |
| BAI1 | Brain-specific angiogenesis inhibitor 1 | −3.32 | −5.46 | −10.45 |
| CDH5 | Cadherin 5, type 2 | −1.20 | −1.29 | 5.05 |
| COL4A3 | Collagen, type XVII, alpha 1 | −1.74 | 0.03 | 1.58 |
| COL4A3 | Collagen, type IV, alpha 3 | −2.35 | −5.02 | −7.46 |
| ENG | Endoglin | 0.11 | 0.51 | −0.46 |
| IL8 | Interleukin 8 | −1.60 | −2.60 | 6.07 |
| ITGAV | Integrin, alpha V | 0.02 | −1.20 | 4.12 |
| ITGB3 | Integrin beta 3 | 0.44 | 0.76 | 0.55 |
| LAMA5 | Laminin, alpha 5 | −0.51 | −1.77 | −2.73 |
| NRP1 | Neuropilin 1 | 0.01 | 0.33 | 0.80 |
| NRP2 | Neuropilin 2 | 0.06 | 0.29 | 1.32 |
| STAB1 | Stabilin 1 | −0.50 | −2.47 | 1.43 |
| THBS1 | Thrombospondin 1 | 1.70 | 1.23 | 1.44 |
| THBS2 | Thrombospondon 2 | −1.80 | −3.30 | −40.40 |
| Cytokines & Chemokines | ||||
| Estradiol | PCB126 | PCB153 | ||
| CCL11 | Chemokine (C-C motif) ligand 11 | −2.18 | −7.37 | −8.28 |
| CCL2 | Chemokine (C-C motif) ligand 2 | −1.14 | −1.60 | 4.20 |
| CXCL1 | Chemokine (C-X-C motif) ligand 1 | −0.14 | −2.16 | 0.64 |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 | −1.14 | −3.72 | 4.17 |
| CXCL3 | Chemokine (C-X-C motif) ligand 3 | −1.66 | −2.38 | 0.85 |
| CXCL5 | Chemokine (C-X-C motif) ligand 5 | −1.43 | −2.21 | −1.47 |
| CXCL6 | Chemokine (C-X-C motif) ligand 6 | 0.02 | −2.71 | 1.05 |
| CXCL9 | Chemokine (C-X-C motif) ligand 9 | −1.58 | −0.74 | −1.29 |
| IFNA1 | Irterferon, alpha 1 | −2.43 | −3.68 | −17.44 |
| IFNB1 | Irterferon, beta 1, fibroblast | −1.53 | −3.40 | −0.55 |
| IFNG | Interferon, gamma | −2.58 | −4.87 | −8.20 |
| IL1B | Interleukin 1, beta | −2.42 | −2.41 | 0.98 |
| IL6 | Interleukin 6 (interferon, beta 2) | −1.76 | −3.02 | 2.44 |
| MDK | Midkine | −0.01 | 2.18 | −1.15 |
| TNF | Tumor necrosis factor | −2.04 | −5.30 | −0.85 |
| Growth Factnrs & Receptors | ||||
| Estradiol | PCB126 | PCB153 | ||
| ANGPT1 | Angiopoietin 1 | −3.45 | −5.49 | −42.88 |
| ANGPT2 | Angiopoietin 2 | 1.40 | 1.32 | 0.08 |
| ANPEP | Alanyl(membrane) aminopeptidase | −0.15 | 0.53 | 0.53 |
| TYMP | Tnymidine phosphorylase | −1.53 | −5.74 | −2.95 |
| S1PR1 | Sphingosine-1-phosphate receptor 1 | 1.17 | 1.15 | 2.56 |
| EFNA1 | Ephrin-A1 | −0.37 | 0.36 | 1.68 |
| EFNA3 | Ephrin-A3 | −2.50 | −3.14 | −4.45 |
| EFNB2 | Ephrin-B2 | −0.69 | 0.09 | 1.30 |
| EGF | Epidermal growth factor | −2.15 | −4.39 | −16.62 |
| EPHB4 | EPH receptor B4 | 0.16 | 0.92 | −1.78 |
| EREG | Epiregulin | −0.21 | −3.46 | −1.79 |
| FGF1 | Fibroblast growth factor 1 (acidic) | −2.54 | −4.14 | −10.13 |
| FGF2 | Fibroblast growth factor 2 (basic) | 0.11 | −1.61 | 0.34 |
| FGFR3 | Fibroblast growth factor receptor 3 | −2.29 | −4.53 | −6.61 |
| FIGF | C-fos induced growth factor | −0.15 | −1.97 | −2.28 |
| FLT1 | Fm5-related tyrosine kinase 1 | −0.26 | 0.11 | 1.94 |
| HGF | Hepatocyte growth factor | −2.25 | −2.68 | −2.30 |
| IGF1 | Insulin-like growth factor 1 | −2.44 | −4.56 | −7.82 |
| JAG1 | Jagged 1 (Alagille syndrome) | −0.94 | −2.81 | 0.86 |
| KDR | Kinase insert domain receptor | −0.25 | −0.17 | −1.65 |
| PDGFA | Platelet-derived growth factor alpha | 0.06 | 0.00 | 3.41 |
| PGF | Placental growth factor | −0.05 | 1.60 | 0.34 |
| PLXDC1 | Plexin domain containing 1 | −1.86 | −4.23 | −5.34 |
| TEK | TEK tyrosine kinase, endothelial | −0.52 | 0.00 | 3.95 |
| TGFA | Transforming growth factor, alpha | −1.76 | −4.80 | −0.81 |
| TGFB1 | Transforming growth factor, beta 1 | −0.35 | 0.45 | −2.20 |
| TGFB2 | Transforming growth factor, beta 2 | 2.39 | 0.26 | 4.14 |
| TGFBR1 | Transforming growth factor, beta receptor 1 | −1.39 | −2.06 | −1.15 |
| VEGFA | Vascular endothelial growth factor A | 0.06 | 0.15 | 2.81 |
| VEGFC | Vascular endothelial growth factor A | 0.06 | 0.15 | 4.45 |
| Proteases, inhibitors, other matrix proteins | ||||
| Estradio | PCB126 | PCB153 | ||
| ANGPTL4 | Angiopoietin-like 4 | 0.55 | −1.38 | 2.70 |
| LECT1 | Leukocyte cell derived chemotaxin 1 | −2.31 | −3.65 | −18.68 |
| LEP | Leptin | −2.11 | −4.14 | −34.01 |
| MMP2 | Matrix metallopeptidase 2 | −0.76 | 0.32 | 0 48 |
| MMP9 | Matrix metallopeptidase 9 | −1.94 | −6.80 | −2.60 |
| PECAM1 | Platelet/endothelial cell adhesion molecule | 1.40 | 0.31 | 0.84 |
| PF4 | Platelet factor 4 | −1.83 | −3.74 | −6.94 |
| PLAU | Plasminogen activator, urokinase | 0.22 | 0.21 | −0.13 |
| PLG | Plasminogen | −2.48 | −3.53 | −12.35 |
| PROK2 | Prokineticin 2 | −2.45 | −4.30 | −50.87 |
| SERPINF1 | Serpin peptidase inhibitor, clade F | −0.29 | −1.78 | −1.84 |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | −1.55 | −1.82 | 0.65 |
| TIMP2 | TIMP metallopeptidase inhibitor 2 | 0.03 | −0.10 | 1.63 |
| TIMP3 | TIMP metallopeptidase inhibitor 3 | −2.73 | −6.13 | −15.82 |
| TNFAIP2 | Tumor necrosis factor, alpha-induced protein 2 | −1.01 | −2.23 | 1.94 |
| Transcription factors & Others | ||||
| Estradiol | PCB126 | PCB153 | ||
| AKT1 | V-akt murine thymoma viral oncogene homolog 1 | −0.64 | 0.45 | −1.92 |
| HAND2 | Heart and neural crest derivatives expressed 2 | −2.46 | −7.73 | −71.51 |
| HIF1A | Hypoxia inducible factor 1, alpha subunit | 0.02 | −0.23 | 1.69 |
| HPSE | Heperanase | −0.28 | −1.45 | 1.32 |
| ID1 | Inhibitor of DNA binding 1 | 0.21 | −0.22 | −0.50 |
| ID3 | Inhibitor of DNA binding 3 | −1.30 | −1.76 | 1.45 |
| NOTCH4 | Notch homolog 4 (Drosophila) | −1.97 | −2.44 | 2.59 |
| PTGS1 | Prostaglandin-endoperoxide synthase 1 | 1.11 | 1.75 | −0.68 |
| SPHK1 | Sphingosine kinase 1 | −0.56 | −0.06 | −0.19 |
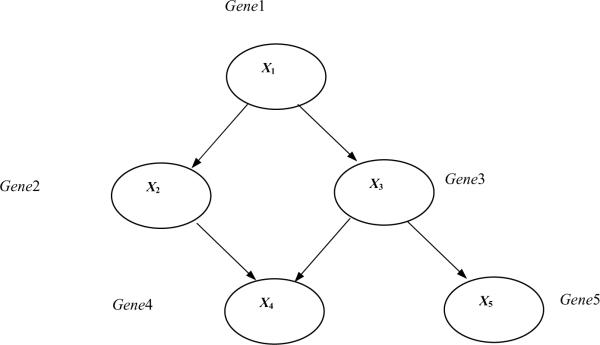
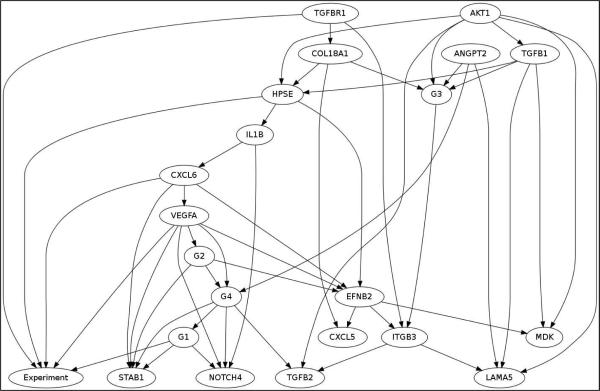
We have applied Bayesian Networks learning method to further understand interactions among the genes that respond to the treatments. Bayesian Networks is a knowledge representation formalism that has been widely used in translational biomedical research for constructing statistical graphical models and for generating causal hypotheses from data (Yoo and Cooper, 2004). Since we wanted to identify interactions among genes that respond to the treatments, we have categorized each gene's expression level to low (if the expression level is one standard deviation lower than the mean expression level), no change, and high (if the expression level is one standard deviation higher than the mean expression level), based on the normalized expression level of all the genes in control and treatments. To learn Bayesian networks among the genes, we then used a computer software called Banjo. Figure 3 illustrates the structure of a hypothetical Bayesian Network structure that contains five nodes. The probabilities associated with this network structure are not shown. The network structure in Fig. 3 indicates, for example, that Gene1 can regulate (influence) the expression level of Gene3, which in turn can regulate the expression level of Gene5. The Markov condition gives the conditional independence relationships that are specified by a Bayesian Network: A node is independent of its non–descendants (e.g., non–effects) i.e., given its its parents (i.e., its direct influences). Categorizing each gene's expression level to low, no change, and high, based on the normalized expression level of all the genes in control and treatments, we have identified the following genes that show identical expression pattern based on the categorization of the genes (Table 2). Bayesian network inference with Java objects (Banjo) software was used to analyze PCR array data of the estrogen treatments. Bayesian gene networks were reconstructed using the expression profile of all 84 genes from the RT2 Profiler PCR Array for Human Angiogenesis. Figure 3 shows the best Bayesian network reported by Banjo. The node labeled “Experiment” in the figure represents the experiment performed, i.e. Control, estradiol, PCB126, or PCB153. Other than the Experiment node, each node either represents a group of genes previously mentioned or a single gene. The lines between the genes denote inferred interactions. A continuous line means a direct relationship between the two genes, whereas a discontinuous line indicates an indirect association. The most significant network regulated by experimental treatments revealed 31 significant genes. The network identified that the following genes to have direct influence of the Experiment node, i.e., the following genes are the Markov Blanket of“Experiment” node: G1, VEGFA, CXCL6, HPSE, and TGFBR1.
Fig. 3.
A Bayesian Network representing a hypothetical gene-regulation pathway. This structure of a hypothetical Bayesian Network contains five nodes. The probabilities associated with this network structure are not shown. The network structure indicates that Gene1 can regulate (influence) the expression level of Gene3, which in turn can regulate the expression level of Gene5. The Markov condition gives the conditional independence relationships that are specified by a Bayesian Network: A node is independent of its non-descendants (e.g., non-effects) given its parents (i.e., its direct influences).
Table 2.
Gene Groups Identified by Bayesian Network
| Group Name | Genes |
|---|---|
| G1 | ANPEP, IL8, NRP2, PGF, TEK, TIMP2 |
| G2 | PLAU, HPRT1 |
| G3 | IL6, VEGFC, CXCL10 |
| G4 | FLT1, FGF2, PDGFA |
Discussion
Clinically, PAH causes an increase in pulmonary arterial pressure that leads to right ventricular failure and death. Pulmonary hypertension has been reported to be associated with the use of oral contraceptives (Kleiger et al., 1976). Estrogens found in oral contraceptives are known to produce an increased incidence of pulmonary embolism (Ramos K.S., Jr., 1996); however, evidence suggests that intra-luminal proliferation of endothelial cells rather than pulmonary embolism causes PAH. Since estrogen is a known mitogen of endothelial cells, we postulate that pulmonary vascular lesions are a consequence of excess or unopposed estrogen. In support of this concept, clinical cases of pulmonary intimal vascular lesions have been associated with the use of oral contraceptives (Irey and Norris, 1973).
In this study, we examined whether exposure of human vascular endothelial cells to environmentally relevant concentrations (100ppb) of estrogenic compounds PCB153 and PCB126 differed from the natural hormone 17β-estradiol with respect to effects on genes involved in vascularization. Our findings show considerably different responses of estrogenic PCB153 compared to estradiol treated endothelial cells. We have identified an increase in the expression of adhesion molecules cadherin 5 and integrin α5; cytokines and chemokines IL8, IL6, CCL2, CXCL10; growth factors and receptors such as TGFB2, VEGFA, VEGFC; and NOTCH4 in endothelial cells exposed to PCB153. Interestingly, PCB126 exposed endothelial cells showed a down-regulation of cytokines and chemokines compared to both PCB153 and estradiol treatments. TGFB2 was the only up-regulated gene that was shared between PCB153 and estradiol. PCB153 has been shown to induce vascularization and inflammation in human vascular endothelial cells. A functional change in CVD such as atherosclerosis is the activation of the endothelium that is initiated by an increase in the expression of specific cytokines and adhesion molecules. Since endothelial cell adhesion depends on molecules such as integrin α5 and cadherin, the gene network we reported to be stimulated by PCBs may explain the mechanism through which estrogenic chemicals can promote the development of vascular lesions.
Our gene network indicated that the estrogenic treatments may signal a network containing TGFBR2, IL6, IL8, and growth factors such as VEGFA, PDGFA, and FGF2. Based on previous studies, our results may be biologically relevant to signaling pulmonary vascular lesions. For instance, inherited susceptibility to PAH occurs in families with mutations in genes of the TGF-beta family of receptors (Davies and Morrell, 2008). Levels of growth factors and IL6 in the pulmonary circulation of patients with PAH have been implicated in vascular remodeling (Selimovic et al., 2009). Our gene network also showed a connection to the NOTCH homolog 4 (Drosophila) also known as NOTCH3. A recent study reported NOTCH3 signaling promotes the development of pulmonary arterial hypertension (Li et al., 2009). Human pulmonary hypertension was characterized by overexpression of NOTCH3 in small pulmonary arteries; furthermore the severity of PAH in humans and rodents correlated with the amount of NOTCH3 protein in the lung.
Our data suggest that PCBs can promote vascular lesion formation by activating gene networks involved in endothelial cell adhesion and inflammatory response which were different from natural estrogen. Previous studies have reported anti-inflammatory effects of estradiol on the endothelium. Vascular endothelial cells exposed to estrogen at pharmacological doses decreased cytokine-induced adhesion molecule expression (Caulin-Glaser et al., 1996). Our findings that show estrogen-induced expression of inflammatory and adhesion molecules is consistent with what others have reported in the literature. For example, at physiological doses (1–2ng/ml); estradiol was reported to increase β1, α5 and α6 integrin mRNA in subconfluent endothelial cells (Cid et al., 1999). Furthermore, estrogen was shown to amplify the expression of adhesion molecules in cytokine stimulated endothelial cells (Cid et al., 1994). These dual effects of estrogen may depend on the dose and time of exposure.
ER-mediated signaling pathways are considered to support cell proliferation; however, discrepancies between the binding affinity of various estrogens to the ER and their growth potency both in vitro and in vivo have been reported (DuMond, Jr. et al., 2001;Bocchinfuso et al., 1999). Although selective ER modulators (SERMS) such as tamoxifen and antiestrogens such as ICI 182,780 prevent the growth of estrogen sensitive tissue, the contribution of other mechanisms cannot be ruled out as these compounds also block metabolism and redox cycling of estrogen, and are free radical scavengers (Arteaga et al., 2003). Even though the estrogenic potency of PCBs are weak compared to 17β-estradiol, this study has reported significant fold differences in PCB-induced gene expression compared to the natural hormone. These differences may be explained by redox signaling pathways. Our previous studies have shown that physiological concentrations of estradiol induce the formation of ROS involved in signaling cell proliferation and vascularization (Felty, 2006;Felty and Porther, 2008). Coplanar PCBs like PCB126 bind with a high affinity to the aryl hydrocarbon receptor (AhR) and induce AhR mediated gene expression of cytochrome P450 1A1 (Hennig et al., 2002). This increase in CYP1A1 was implicated as a mechanism for the oxidative stress and inflammatory response in PCB126 treated endothelial cells reported by Hennig et al. Non-coplanar PCBs like PCB153 are not good ligands for the AhR and their mechanisms of action are not completely understood. Although these compounds have been reported to possess estrogenic activity, the observed profile differences clearly show a different mechanism of vascular gene expression for each chemical treatment. Estradiol-induced gene expression will be different based on nuclear ER mediated transcription. PCB126 expression changes will differ from estradiol via AhR mediated transcription. PCB153 does not bind with high affinity to either the nuclear ER or AhR. Even though PCB153 is considered to be estrogenic; the changes in gene expression cannot be explained by nuclear ER mediated signaling. There is evidence for PCB153 mediated activation of NADPH oxidase (Eum et al., 2009). Since NADPH oxidase is a known source of cellular ROS; it is possible that redox sensitive signaling pathways are responsible for the different gene changes observed in endothelial cells exposed to PCB153 when compared to PCB126 and estradiol treatment groups.
Estrogen produces both beneficial and adverse effects on cardiovascular health via mechanisms that remain unclear. The present study provides the first gene expression analysis comparing estrogenic chemicals PCB153 and PCB126 with the natural hormone 17β-estradiol in human vascular endothelial cells. Growing evidence implicates the overproduction of ROS such as hydrogen peroxide (H2O2), superoxide anion (O2•−), and peroxynitrite (ONOO−) in the development of CVD. Thus, identification of redox signaling pathways that mediate vascularization may add to the knowledge base of how estrogenic environmental chemicals influence the vascular wall. We believe that these gene profiles may lead to insights into the pathophysiological mechanisms of vascular lesion formation involved in PAH and provide a target pathway for therapeutic intervention.
Fig. 4.
Gene networks regulated by estrogen treatment. The node labeled “Experiment” in the figure represents the experiment performed, i.e., control, estradiol, PCB126, or PCB153. Other than the Experiment node, each node either represents a group of genes previously mentioned or a single gene. The network identified that the following genes to have direct influence of the Experiment node, i.e., the following genes are the Markov Blanket of “Experiment” node: G1, VEGFA, CXCL6, HPSE, and TGFBR1.
Acknowledgements
This work was supported in part by a grant from NIH SC3GM084827 (Felty, Q.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- The Coronary Drug Project. Initial findings leading to modifications of its research protocol. JAMA. 1970;214(7):1303–1313. [PubMed] [Google Scholar]
- The Coronary Drug Project. Findings leading to discontinuation of the 2.5-mg day estrogen group. The coronary Drug Project Research Group. JAMA. 1973;226(6):652–657. [PubMed] [Google Scholar]
- ARTEAGA E, VILLASECA P, BIANCHI M, ROJAS A, MARSHALL G. Raloxifene is a better antioxidant of low-density lipoprotein than estradiol or tamoxifen in postmenopausal women in vitro. Menopause. 2003;10(2):142–146. doi: 10.1097/00042192-200310020-00005. [DOI] [PubMed] [Google Scholar]
- BITMAN J, CECIL HC. Estrogenic activity of DDT analogs and polychlorinated biphenyls. J.Agric.Food Chem. 1970;18(6):1108–1112. doi: 10.1021/jf60172a019. [DOI] [PubMed] [Google Scholar]
- BOCCHINFUSO WP, HIVELY WP, COUSE JF, VARMUS HE, KORACH KS. A mouse mammary tumor virus-Wnt-1 transgene induces mammary gland hyperplasia and tumorigenesis in mice lacking estrogen receptor-alpha. Cancer Res. 1999;59(8):1869–1876. [PubMed] [Google Scholar]
- CAULIN-GLASER T, WATSON CA, PARDI R, BENDER JR. Effects of 17beta-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J.Clin.Invest. 1996;98(1):36–42. doi: 10.1172/JCI118774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBLISS KL, YUHANNA IS, ANDERSON RG, MENDELSOHN ME, SHAUL PW. ERbeta has nongenomic action in caveolae. Mol.Endocrinol. 2002;16(5):938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- CHOI W, EUM SY, LEE YW, HENNIG B, ROBERTSON LW, TOBOREK M. PCB 104-induced proinflammatory reactions in human vascular endothelial cells: relationship to cancer metastasis and atherogenesis. Toxicol.Sci. 2003;75(1):47–56. doi: 10.1093/toxsci/kfg149. [DOI] [PubMed] [Google Scholar]
- CID MC, ESPARZA J, SCHNAPER HW, JUAN M, YAGUE J, GRANT DS, URBANO-MARQUEZ A, HOFFMAN GS, KLEINMAN HK. Estradiol enhances endothelial cell interactions with extracellular matrix proteins via an increase in integrin expression and function. Angiogenesis. 1999;3(3):271–280. doi: 10.1023/a:1009023329294. [DOI] [PubMed] [Google Scholar]
- CID MC, KLEINMAN HK, GRANT DS, SCHNAPER HW, FAUCI AS, HOFFMAN GS. Estradiol enhances leukocyte binding to tumor necrosis factor (TNF)-stimulated endothelial cells via an increase in TNF-induced adhesion molecules E-selectin, intercellular adhesion molecule type 1, and vascular cell adhesion molecule type 1. J.Clin.Invest. 1994;93(1):17–25. doi: 10.1172/JCI116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CID MC, SCHNAPER HW, KLEINMAN HK. Estrogens and the vascular endothelium. Ann.N.Y.Acad.Sci. 2002;966:143–157. doi: 10.1111/j.1749-6632.2002.tb04211.x. [DOI] [PubMed] [Google Scholar]
- COX RL, CRAWFORD ED. Estrogens in the treatment of prostate cancer. J.Urol. 1995;154(6):1991–1998. [PubMed] [Google Scholar]
- DAVEY JC, BODWELL JE, GOSSE JA, HAMILTON JW. Arsenic as an endocrine disruptor: effects of arsenic on estrogen receptor-mediated gene expression in vivo and in cell culture. Toxicol.Sci. 2007;98(1):75–86. doi: 10.1093/toxsci/kfm013. [DOI] [PubMed] [Google Scholar]
- DAVIES RJ, MORRELL NW. Molecular mechanisms of pulmonary arterial hypertension: role of mutations in the bone morphogenetic protein type II receptor. Chest. 2008;134(6):1271–1277. doi: 10.1378/chest.08-1341. [DOI] [PubMed] [Google Scholar]
- DUMOND JW, JR., SINGH KP, ROY D. Regulation of the growth of mouse Leydig cells by the inactive stereoisomer, 17alpha-estradiol: Lack of correlation between the elevated expression of ERalpha and difference in sensitivity to estradiol isomers. Oncol.Rep. 2001;8(4):899–902. [PubMed] [Google Scholar]
- EUM SY, ANDRAS I, HENNIG B, TOBOREK M. NADPH oxidase and lipid raft-associated redox signaling are required for PCB153-induced upregulation of cell adhesion molecules in human brain endothelial cells. Toxicol.Appl.Pharmacol. 2009;240(2):299–305. doi: 10.1016/j.taap.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUM SY, LEE YW, HENNIG B, TOBOREK M. VEGF regulates PCB 104-mediated stimulation of permeability and transmigration of breast cancer cells in human microvascular endothelial cells. Exp.Cell Res. 2004;296(2):231–244. doi: 10.1016/j.yexcr.2004.01.030. [DOI] [PubMed] [Google Scholar]
- FELTY Q. Estrogen-induced DNA synthesis in vascular endothelial cells is mediated by ROS signaling. BMC.Cardiovasc.Disord. 2006;6:16. doi: 10.1186/1471-2261-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELTY Q, PORTHER N. Estrogen-induced redox sensitive Id3 signaling controls the growth of vascular cells. Atherosclerosis. 2008;198(1):12–21. doi: 10.1016/j.atherosclerosis.2007.12.048. [DOI] [PubMed] [Google Scholar]
- GONCHAROV A, HAASE RF, SANTIAGO-RIVERA A, MORSE G, MCCAFFREY RJ, REJ R, CARPENTER DO. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ Res. 2008;106(2):226–239. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAVSSON P, HOGSTEDT C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Am J.Ind.Med. 1997;32(3):234–239. doi: 10.1002/(sici)1097-0274(199709)32:3<234::aid-ajim8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- HAY A, TARREL J. Mortality of power workers exposed to phenoxy herbicides and polychlorinated biphenyls in waste transformer oil. Ann.N.Y.Acad.Sci. 1997;837:138–156. doi: 10.1111/j.1749-6632.1997.tb56871.x. [DOI] [PubMed] [Google Scholar]
- HENNIG B, HAMMOCK BD, SLIM R, TOBOREK M, SARASWATHI V, ROBERTSON LW. PCB-induced oxidative stress in endothelial cells: modulation by nutrients. Int.J.Hyg.Environ Health. 2002;205(1–2):95–102. doi: 10.1078/1438-4639-00134. [DOI] [PubMed] [Google Scholar]
- HOWARD BV, HSIA J, OUYANG P, VAN VL, LINDSAY J, SILVERMAN A, ALDERMAN EL, TRIPPUTI M, WATERS DD. Postmenopausal hormone therapy is associated with atherosclerosis progression in women with abnormal glucose tolerance. Circulation. 2004;110(2):201–206. doi: 10.1161/01.CIR.0000134955.93951.D5. [DOI] [PubMed] [Google Scholar]
- HYDUK A, CROFT JB, AYALA C, ZHENG K, ZHENG ZJ, MENSAH GA. Pulmonary hypertension surveillance--United States, 1980–2002. MMWR Surveill Summ. 2005;54(5):1–28. [PubMed] [Google Scholar]
- IREY NS, NORRIS HJ. Intimal vascular lesions associated with female reproductive steroids. Arch.Pathol. 1973;96(4):227–234. [PubMed] [Google Scholar]
- KIM-SCHULZE S, MCGOWAN KA, HUBCHAK SC, CID MC, MARTIN MB, KLEINMAN HK, GREENE GL, SCHNAPER HW. Expression of an estrogen receptor by human coronary artery and umbilical vein endothelial cells. Circulation. 1996;94(6):1402–1407. doi: 10.1161/01.cir.94.6.1402. [DOI] [PubMed] [Google Scholar]
- KLEIGER RE, BOXER M, INGHAM RE, HARRISON DC. Pulmonary hypertension in patients using oral contraceptives. A report of six cases. Chest. 1976;69(2):143–147. doi: 10.1378/chest.69.2.143. [DOI] [PubMed] [Google Scholar]
- LI X, ZHANG X, LEATHERS R, MAKINO A, HUANG C, PARSA P, MACIAS J, YUAN JX, JAMIESON SW, THISTLETHWAITE PA. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat.Med. 2009;15(11):1289–1297. doi: 10.1038/nm.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIVAK KJ, SCHMITTGEN TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- MEEK MD, FINCH GL. Diluted mainstream cigarette smoke condensates activate estrogen receptor and aryl hydrocarbon receptor-mediated gene transcription. Environ.Res. 1999;80(1):9–17. doi: 10.1006/enrs.1998.3872. [DOI] [PubMed] [Google Scholar]
- NEWMAN JH, FANBURG BL, ARCHER SL, BADESCH DB, BARST RJ, GARCIA JG, KAO PN, KNOWLES JA, LOYD JE, MCGOON MD, MORSE JH, NICHOLS WC, RABINOVITCH M, RODMAN DM, STEVENS T, TUDER RM, VOELKEL NF, GAIL DB. Pulmonary arterial hypertension: future directions: report of a National Heart, Lung and Blood Institute/Office of Rare Diseases workshop. Circulation. 2004;109(24):2947–2952. doi: 10.1161/01.CIR.0000132476.87231.6F. [DOI] [PubMed] [Google Scholar]
- RAMOS KSCEAD., JR. Toxic Responses of the Heart and Vascular Systems. In: Klaassen CD, editor. Casarett & Doull's Toxicology: The Basic Science of Poisons. McGraw-Hill; New York: 1996. pp. 487–527. [Google Scholar]
- ROSSOUW JE. Coronary heart disease in menopausal women: implications of primary and secondary prevention trials of hormones. Maturitas. 2005;51(1):51–63. doi: 10.1016/j.maturitas.2005.02.015. [DOI] [PubMed] [Google Scholar]
- SAKAO S, TATSUMI K, VOELKEL NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir.Res. 2009;10:95. doi: 10.1186/1465-9921-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELIMOVIC N, BERGH CH, ANDERSSON B, SAKINIENE E, CARLSTEN H, RUNDQVIST B. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur.Respir.J. 2009;34(3):662–668. doi: 10.1183/09031936.00174908. [DOI] [PubMed] [Google Scholar]
- SERGEEV AV, CARPENTER DO. Hospitalization rates for coronary heart disease in relation to residence near areas contaminated with persistent organic pollutants and other pollutants. Environ Health Perspect. 2005;113(6):756–761. doi: 10.1289/ehp.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAVOLARI S, BUCCI L, TOMASI V, GUARNIERI T. Selected polychlorobiphenyls congeners bind to estrogen receptor alpha in human umbilical vascular endothelial (HUVE) cells modulating angiogenesis. Toxicology. 2006;218(1):67–74. doi: 10.1016/j.tox.2005.10.008. [DOI] [PubMed] [Google Scholar]
- TOKUNAGA S, KATAOKA K. A longitudinal analysis on the association of serum lipids and lipoproteins concentrations with blood polychlorinated biphenyls level in chronic "Yusho" patients. Fukuoka Igaku Zasshi. 2003;94(5):110–117. [PubMed] [Google Scholar]
- VENETSANAKOS E, MIRZA A, FANTON C, ROMANOV SR, TLSTY T, MCMAHON M. Induction of tubulogenesis in telomerase-immortalized human microvascular endothelial cells by glioblastoma cells. Exp.Cell Res. 2002;273(1):21–33. doi: 10.1006/excr.2001.5424. [DOI] [PubMed] [Google Scholar]
- WASSERMANN M, WASSERMANN D, CUCOS S, MILLER HJ. World PCBs map: storage and effects in man and his biologic environment in the 1970s. Ann.N.Y.Acad.Sci. 1979;320:69–124. doi: 10.1111/j.1749-6632.1979.tb13137.x. [DOI] [PubMed] [Google Scholar]
- YANG SH, LIU R, PEREZ EJ, WEN Y, STEVENS SM, JR., VALENCIA T, BRUN-ZINKERNAGEL AM, PROKAI L, WILL Y, DYKENS J, KOULEN P, SIMPKINS JW. Mitochondrial localization of estrogen receptor beta. Proc.Natl.Acad.Sci.USA. 2004;101(12):4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOO C, COOPER GF. An evaluation of a system that recommends microarray experiments to perform to discover gene-regulation pathways. Artif.Intell.Med. 2004;31(2):169–182. doi: 10.1016/j.artmed.2004.01.018. [DOI] [PubMed] [Google Scholar]