Abstract
The relationship between regulatory T cells (Tregs) and acute graft-versus-host disease (GVHD) in clinical allogeneic bone marrow transplantation (BMT) recipients is not well established. We conducted a prospective analysis of peripheral blood Tregs as determined by the frequency of CD4+CD25hiFOXP3+ lymphocytes in 215 BMT patients. Autologous BMT patients (N=90) and allogeneic BMT patients without GVHD (N=65) had similar Treg frequencies, whereas allogeneic patients with GVHD (N=60) had Treg frequencies that were 40% less than those without GVHD. Treg frequencies decreased linearly with increasing grades of GVHD at onset and correlated with eventual maximum grade of GVHD (p<0.001). In addition, frequency of Tregs at onset of GVHD predicted the response to GVHD treatment (p=0.003). Patients with Treg frequencies less than the median had higher non-relapse mortality than patients with Tregs greater than the median, but experienced equivalent relapse mortality, resulting in an inferior survival at two years (38% vs. 63%, p=0.03). Treg frequency may therefore have important prognostic value as a biomarker of acute GVHD.
Keywords: Allogeneic BMT, Acute graft-versus-host-disease, Regulatory T cells, Biomarker
INTRODUCTION
Allogeneic bone marrow transplantation (BMT) is a curative modality for many hematologic diseases, but its use is limited by mortality due to acute graft-versus-host-disease (GVHD). Naturally occurring regulatory T cells (Tregs) are mediators of immunologic tolerance that attenuate GVHD in experimental models (1, 2). Tregs were first defined by a CD4+CD25+ phenotype (3, 4), but subsequent studies identified forkhead box protein 3 (FOXP3) as a highly specific marker in both mouse and human T cells with regulatory function (5-7). High absolute numbers of CD4+FOXP3+ Tregs in the donor graft inversely correlated with acute GVHD incidence and overall survival of BMT recipients following myeloablative conditioning in one study (8), but not in a second (9). Higher numbers of CD4+FOXP3+ lymphocytes in stem cell products depleted of T cells have also been associated with a lower risk of developing GVHD (10, 11). Experimental models suggest that immunosuppressive therapies may adversely affect Treg reconstitution after BMT (12-14) and studies measuring Tregs following BMT have not consistently correlated with either GVHD occurrence or severity (10, 11, 15, 16); such discrepancies may be due to the sample size analyzed, timing of samples, and to various methodologies to quantify Tregs.
We prospectively analyzed Tregs using the CD4+CD25hiFOXP3+ phenotype within total lymphocytes in 215 recipients of autologous and allogeneic BMT to determine the utility of this measurement as a cellular biomarker for GVHD. FOXP3 is now accepted as a distinctive molecule for natural and peripherally derived Tregs and is constitutively expressed at high levels in humans (17). Although FOXP3 is expressed in CD4+ cells with low and intermediate levels of CD25, these populations contain effector cells capable of transient FOXP3 expression that may lack equivalent suppressive function compared to CD25hi (17-19). No stem cell sources in this study were manipulated ex vivo. The frequency of Tregs was analyzed at the onset of GVHD for its correlation to disease severity, non–relapse mortality (NRM), response to GVHD treatment, and overall survival (OS).
DESIGN AND METHODS
Study Population
Between July 2007 and July 2009, 215 BMT patients (125 allogeneic, 90 autologous) had peripheral blood samples collected under protocols approved by the University of Michigan institutional review board. After providing informed consent, samples were collected within 24 hours of clinical signs of acute GVHD onset and prior to initiation of corticosteroid therapy. In addition, samples were collected on days +20, +30, +60, and +100. Approximately 70% of all possible samples were collected. Reasons for missing samples include patient ineligibility (second BMT / donor lymphocyte infusion, untreated infections, or intercurrent illness requiring intensive care), relapsed leukemia, death, and failure to collect / technical problems. GVHD was histologically confirmed in 88% of patients and was staged per modified Gluksberg criteria (20). All allogeneic BMT patients received calcineurin inhibitors (>95% received tacrolimus) as part of their GVHD prophylactic regimen. Patients received unmanipulated grafts, and no patients analyzed received T cell-depleting antibodies or sera for conditioning.
Flow cytometry of Regulatory T cells
Fresh whole blood sample phenotyping of cell surface markers was performed using CD4-PerCP-Cy5.5 (clone SK3, BD Biosciences, San Jose, CA), CD25-PE (clone M-A251, BD Biosciences), and CD127-FITC (clone eBioRDR5, eBioscience) antibodies. Intracellular staining of FOXP3-APC (clone PCH101, eBioscience, San Diego, CA) was performed after fixation and permeabilization according to the manufacturer’s recommendation. We measured Treg frequency by three colors (CD4, CD25, FOXP3) or four colors (CD127, CD4, CD25, FOXP3) in 25% of samples. FACS analysis was performed using a Canto II flow cytometer (BD Biosciences) with FacsDiva software (BD Biosciences) to determine CD4+CD25hiFOXP3+ or CD127dimCD4+CD25hiFOXP3+ staining within total lymphocytes. Absolute Treg numbers were calculated by multiplying Treg frequency by the absolute lymphocyte count obtained by automated differential. Values are expressed as mean ± SEM.
Statistical Analysis
Differences in characteristics between patient groups were assessed with a Kruskal-Wallis test for continuous values and Fisher’s Exact Test for categorical values. Median frequencies of Tregs were compared using a Kruskal-Wallis test. Survival was modeled using Cox regression methods. Relapse mortality and NRM were modeled using cumulative incidence regression methods as described in Fine et al (21). The association between Treg frequency and response to therapy was calculated using chi-squared analysis. Wilcoxon signed rank tests were used to compare the Treg frequency at onset of GVHD and 4 weeks after treatment for 25 responders and 15 non-responders.
RESULTS
Phenotypic characterization of Tregs
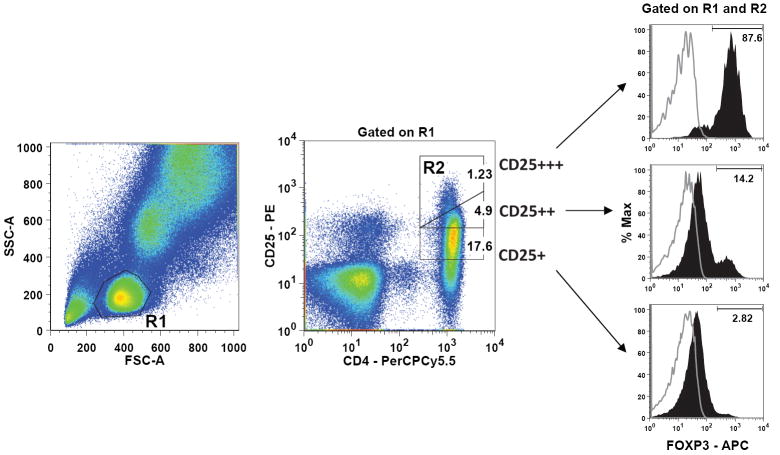
The population of CD4 lymphocytes expressing very high levels of CD25 has been shown to express high levels of FOXP3 and exert dose dependent inhibition on CD4+CD25- T cells (22). As expected, we observed the highest FOXP3 expression among the CD25+++ (CD25hi) lymphocyte subset, compared to CD25++ and CD25+ lymphocyte subsets in autologous and allogeneic patient samples (Figure 1). The mean FOXP3 expression in CD4+CD25hi cells was similar among recipients of autologous BMT (71.9 % ± 2.1), allogeneic BMT with no GVHD (68.9 % ± 2.5), and allogeneic BMT with GVHD (64.1% ± 3.2) (Figure S1). The CD4+CD25hiFOXP3+ phenotype had dim CD127 expression in greater than 95% of cells, consistent with prior reports (23, 24). But CD4+CD25+CD127dim T cells include cells that lack suppressive function and produce IL-17, IL-2 and IFN-γ (18, 25). We therefore used the CD4+CD25hiFOXP3+ phenotype to define the Treg population.
Figure 1. Phenotypic characterization of Tregs.
Representative flow data from a patient following allogeneic transplantation. Fresh whole blood was stained with CD4-PerCP-Cy5.5 and CD25-PE antibodies followed by intracellular staining for FOXP3-APC. Treg frequency was determined using a Canto II flow cytometer (BD Biosciences) to identify CD4+CD25hiFOXP3+ triple positive cells within the total lymphocyte population. Treg frequency was performed in triplicate for each sample. (A) The total lymphocyte population (R1) was identified by light scatter after backgating on the CD4+ population. On right are histograms of FOXP3+ expression (red) gated on CD4+CD25+, CD4+CD25++, CD4+CD25+++ (CD4+CD25hi) lymphocyte subsets. Analysis was restricted to the CD4+CD25hi lymphocyte subset (R2) which was identified in R1. The frequency of CD4+CD25hiFOXP3+ lymphocytes was calculated by multiplying the percentage of CD4+CD25hi cells by the percentage of FOXP3+ cells within CD4+CD25hi.
Treg frequencies following autologous and allogeneic BMT without GVHD are similar
We first compared Tregs by measuring CD4+CD25hiFOXP3+ cells from freshly acquired peripheral blood samples in autologous BMT patients (N = 90) and allogeneic BMT recipients without GVHD (N = 65). Characteristics of autologous and allogeneic populations are shown in Table 1A. High risk malignancy and age were significantly different between groups, but days to sample were similar. Frequencies of Tregs were similar in patients who did not develop GVHD after either autologous (1.09 ± 0.10) or allogeneic BMT (1.06 ± 0.10) (p = 0.84; Figure 2A). These frequencies were also similar to those obtained from six healthy donors (1.17 ± 0.16). However, absolute Treg numbers were reduced in allogeneic BMT patients with no GVHD compared to autologous BMT patients (p = 0.04, Figure 2B), as a result of lower absolute lymphocyte counts (ALC) in allogeneic BMT patients (Figure S2).
Table 1.
| A. Characteristics of autologous and allogeneic patients with no GVHD | ||||
|---|---|---|---|---|
| Characteristic | Auto | Allo | p-value | |
| Patients | 90 | 125 | ||
| Age, yr | Median | 55 | 50 | 0.004 |
| Range | 6-70 | 1-67 | ||
| Days to sample | Median | 32 | 29 | 0.14 |
| Range | 14-119 | 14-111 | ||
| Malignant disease, % (no.)1 | 98% (88) | 95 % (119) | 0.47 | |
| High risk malignancy, % (no.)2 | 14% (13) | 39% (49) | <0.001 | |
| B. Characteristics of GVHD positive and GVHD negative patients | ||||
| Characteristic | No GVHD | GVHD | p-value | |
| Patients | 65 | 60 | ||
| Age, yr | Median | 50 | 52 | 0.24 |
| Range | 1-67 | 1-66 | ||
| Related donor, % (no.) | 60% (39) | 33% (20) | 0.004 | |
| Matched donor, % (no.) | 86% (56) | 67% (40) | 0.01 | |
| Stem cell source, % (no.) | ||||
| Peripheral blood, % (no.) | 86% (56) | 78% (47) | ||
| Bone marrow, % (no.) | 11% (7) | 8% (5) | 0.12 | |
| Cord blood, % (no.) | 3% (2) | 13% (8) | ||
| Full intensity conditioning, % (no.) | 74% (48) | 70% (42) | 0.69 | |
| Malignant disease, % (no.) | 92% (60) | 98% (59) | 0.21 | |
| High risk malignancy, % (no.) | 32% (21) | 47% (28) | 0.12 | |
| GVHD prophylaxis, % (no.) | ||||
| TAC / MMF | 37% (24) | 42% (25) | ||
| TAC / MTX | 38% (25) | 25% (15) | 0.41 | |
| TAC / MMF or MTX / Etanercept | 11% (7) | 17% (10) | ||
| Other 3 | 14% (9) | 17% (10) | ||
| Days to sample | Median | 29 | 29 | 0.29 |
| Range | 16-106 | 14-111 | ||
| Days to GVHD onset | Median | 29 | ||
| Range | 14-111 | |||
| Onset GVHD grade, % (no.) | ||||
| Grade I | 46% (28) | |||
| Grade II | 44% (26) | |||
| Grade III-IV | 10% (6) | |||
| Maximum GVHD grade, % (no.) | ||||
| Grade I | 28% (17) | |||
| Grade II | 50% (30) | |||
| Grade III-IV | 22% (13) | |||
| Organ target at GVHD onset, % (no.) | ||||
| Skin only | 68% (41) | |||
| Gut only | 21% (13) | |||
| Liver only | 2% (1) | |||
| Skin + gut / liver | 9% (5) | |||
Malignant diseases include hodgkins disease (HD), chronic lymphocytic leukemia (CLL), acute myelogeneous leukemia (AML), myelodysplastic syndrome (MDS), non-hodgkin lymphoma (NHL), acute lymphoblastic leukemia (ALL), multiple myeloma (MM) and myeloproliferative disease (MPD).
AML and ALL >CR2, induction failure or refractory, AML arising from antecedent MDS, CML in blast or accelerated phases, HD and NHL > CR2 or refractory, progressive multiple myeloma.
Other GVHD prophylaxis include: TAC/SIRO (n = 3), TAC/MTX/SIRO (n=1), TAC/MMF/MTX (n=11), MMF/Cyclosporin (n=3), MTX/cyclosporin (n=1). TAC = Tacrolimus, MTX = Methotrexate, SIRO = Sirolimus, MMF = mycophenolate mofetil.
Figure 2. Regulatory T cells (Tregs) at GVHD onset.
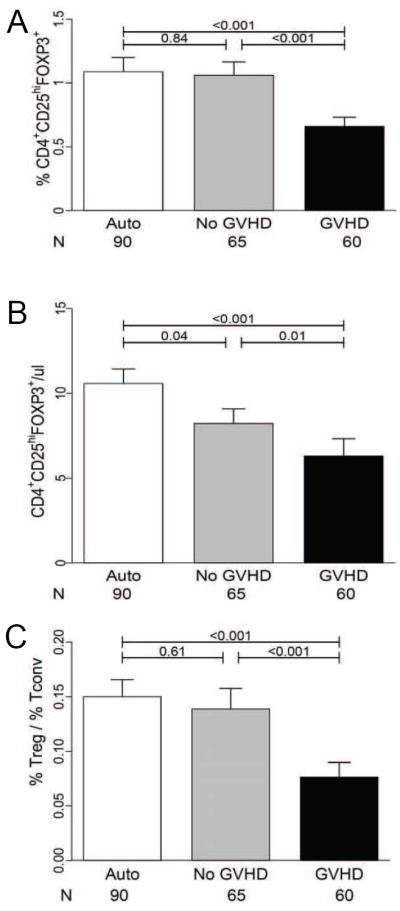
Fresh blood samples from autologous and allogeneic transplant patients (N=215) were acquired within 24hrs of acute GVHD onset or at equivalent timepoints following transplantation. Mean (A) Treg frequencies (B) Absolute Treg numbers and (C) Treg / Tconv frequencies for autologous BMT patients, allogeneic BMT patients with no GVHD, and allogeneic BMT patients with GVHD. Error bars represent the SEM.
Decreased Treg frequencies at time of GVHD onset and 3-14 days prior to GVHD
We compared samples at GVHD onset to samples from patients without GVHD, such that both groups were balanced for time of acquisition. Characteristics of allogeneic patients according to GVHD status are shown in Table 1B. Patients were not significantly different for age, nonmalignant disease, conditioning intensity, and median day of sample acquisition. As expected, recipients of grafts from donors who were not family members or who were not HLA-matched were overrepresented in the GVHD group (Table 2). Patients with all grades of GVHD had a 40 % lower Treg frequency than patients without GVHD (p < 0.001, Figure 2A). We calculated the absolute numbers of Tregs by multiplying the frequency of CD4+CD25hiFOXP3+ cells by the ALC, which was slightly higher in GVHD patients (1.13 ± 1.15) compared to patients without GVHD (Figure S2). The absolute Treg numbers remained lower in patients with GVHD compared to patients without GVHD (p = 0.01, Figure 2B). We also analyzed the ratio of Tregs to conventional T cells (Tconv) by dividing the CD4+CD25hiFOXP3+ frequency by the CD4+CD25-FOXP3- frequency. Tconv frequencies were similar in the two groups (11.8 ± 0.97 vs. 10.5 ± 0.91). The mean Treg/Tconv ratio was significantly lower in patients with GVHD (p < 0.001, Figure 2C).
Fourteen patients had paired samples available 3-14 days prior to GVHD onset, and these were compared to fifteen paired samples in patients without GVHD. Treg frequencies prior to acute GVHD significantly decreased in the majority of patients (p = 0.003, Figure 3A), while no decrease occurred over a similar time interval in allogeneic patients without GVHD (p = 0.7, Figure 3B).
Figure 3. Treg frequencies prior to GVHD onset.
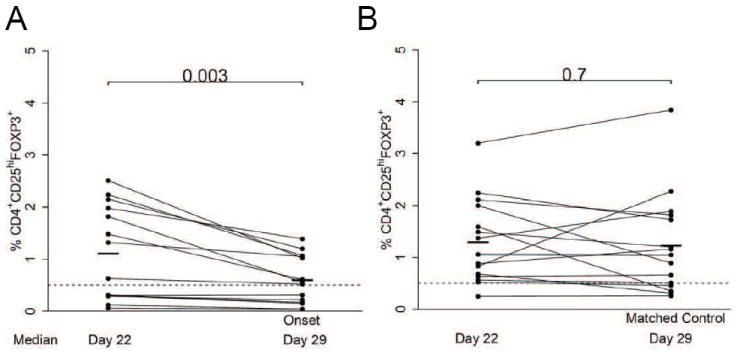
(A) Paired analysis in GVHD patients (N = 14) comparing Treg frequencies at GVHD onset to prior timepoints (3 to 14 days). (B) Treg frequencies from matched controls with no GVHD (N = 15) over an equivalent 3-14 day interval. Bars denote mean values.
Treg Frequency is correlated with GVHD severity at onset and eventual maximum GVHD grade
We next hypothesized that Treg frequency would correlate with GVHD severity at onset. Frequencies of Tregs decreased in an almost linear fashion with each increasing grade of GVHD, and were significantly reduced in patients with ≥ grade II GVHD compared to patients without GVHD (p < 0.001, Figure 4A). A similar pattern was observed with absolute Treg numbers, but the variability within groups was greater, reducing the statistically significant differences between groups (Figure 4B). We also assessed whether Treg frequency at onset correlated with eventual maximum overall GVHD. The median interval between GVHD onset and eventual maximum overall GVHD grade was 14 days, with 11/28 (39%) patients progressing from grade I to higher GVHD grades. Treg frequency at GVHD onset was significantly reduced in patients who eventually developed maximum grade II-IV GVHD compared to patients without GVHD or who remained at grade I GVHD (p<0.001, Figure 4C).
Figure 4. Treg frequencies and GVHD severity.
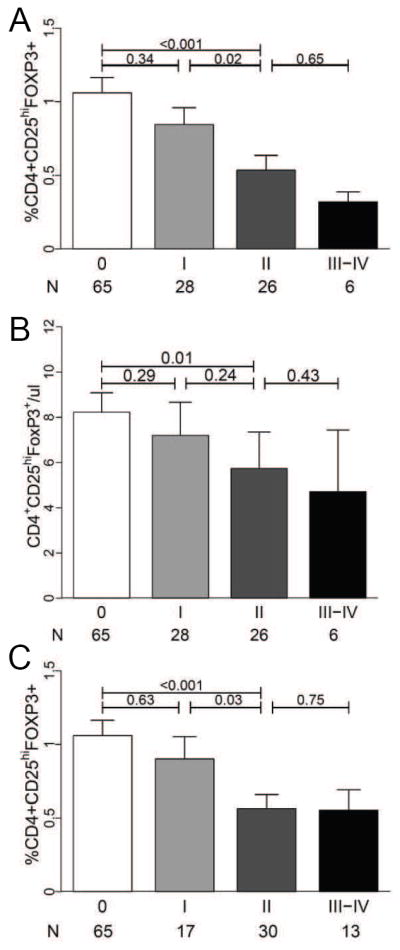
Fresh blood samples from allogeneic transplant patients with GVHD (N = 60) were acquired within 24hrs of acute GVHD onset and analyzed according to GVHD severity. (A) Mean Treg frequencies, (B) absolute Treg numbers by grade of GVHD at onset, and (C) mean Treg frequencies by eventual maximum overall GVHD grade. The median interval between GVHD onset and eventual maximum overall GVHD grade was 14 days. Error bars represent the SEM.
Treg frequency has prognostic value at GVHD onset
Given these correlations, we next evaluated whether Treg frequency at onset would predict outcomes in the 60 patients with GVHD by dividing patients according to their median Treg frequency (0.5%). As shown in Figure 5A, patients with GVHD whose Treg frequency was less than the median had a significantly greater NRM at one year (41% [95%CI, 23%-61%]) than patients with Treg frequencies equal to or greater than the median (7% [95%CI, 2%-24%]), p = 0.01. Acute GVHD was the major cause of death in patients with low Treg frequencies (27% vs. 3%, p = 0.03, Table S1).
Figure 5. Treg frequencies are correlated with clinical outcomes.
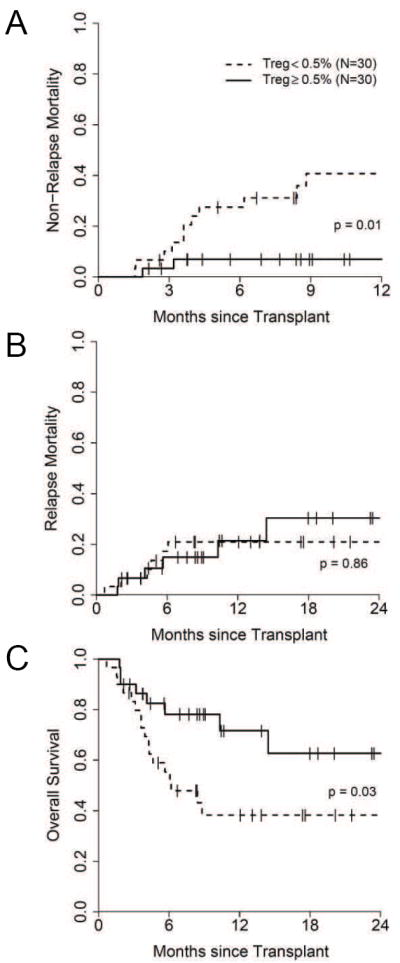
The median T reg frequency was 0.5% in allogeneic BMT patients at time of GVHD onset (N = 60). (A) non-relapse mortality, (B) relapse mortality and (C) overall survival in patients with GVHD divided according to the median Treg frequency (high Treg ≥ 0.5% (N=30) or low Treg < 0.5 (N=30).
Relapse mortality was similar: 21% (95% CI, 9-40) in patients with low Treg frequencies compared to 30% (95% CI, 12-57) in patients with high Treg frequencies (p=0.86, Figure 5B). The overall survival at two years was significantly less in patients with low Treg frequencies (38%, [95%CI, 23%-63%]) than in patients with high Treg frequencies (63%, [95%CI, 43%-91%])(p = 0.03, Figure 5C).
The difference in overall survival according to Treg frequency at GVHD onset remained significant after adjusting for absolute lymphocyte count at time of sample collection and other important prognostic factors such as, age, donor type, degree of HLA-match, and conditioning intensity (hazard ratio of 2.43 [95%CI: 1.01-5.87], p = 0.05). When both Treg frequency (≥ 0.5% vs. < 0.5%) and maximum GVHD grade (Grades I-II vs. III-IV) were analyzed in a simultaneous Cox regression analysis the Treg frequency remained significant (hazard ratio of 2.33 [95%CI: 0.96-5.66], p = 0.05).
Treg frequency and response to treatment
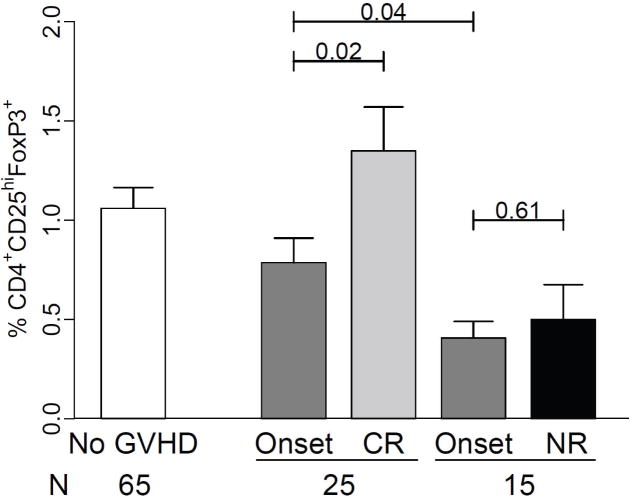
Since Treg frequency had prognostic value in GVHD, we analyzed if Treg frequency at GVHD onset predicted response to treatment. Four weeks following GVHD onset, patients with no GVHD (N = 29) or stage I GVHD of the skin (N =8) were classified as complete responders (N = 37), while patients with more advanced GVHD were classified as non-responders (N = 20). Three patients were not evaluable due to death or progressive disease. In patients with high Tregs (N = 28) at GVHD onset, 24 (86%) had complete responses, whereas in patients with low Tregs (N = 29) only 13 (45%) had complete responses (p=0.003). However, responders had more GVHD grade I at diagnosis (54% vs. 25%, p=0.05). When patients achieving a complete resolution of GVHD (N = 29) were evaluated, Treg frequency remained associated with treatment response (p=0.005). Paired samples at onset of GVHD and four weeks following treatment were available for 25/37 responding patients and 15/20 non-responding patients. As shown in Figure 6, responding patients showed a significant increase of their Treg frequency 4 weeks after treatment (p=0.02).
Figure 6. Treg frequencies at 4 weeks according to treatment response.
Mean (±SEM) Treg frequencies were measured on paired samples at onset and 4 weeks after treatment in 25 patients with complete response or near complete response (CR) and 15 non-responders (NR).
DISCUSSION
In this study we evaluated Treg as measured by the CD4+CD25hiFOXP3+ phenotype for its value as a cellular biomarker at GVHD onset. Because Tregs rely on exogenous IL-2 in order to function (17), situations characterized by impaired IL-2 production might alter Treg frequency. Calcineurin inhibitors, are frequently administered to allogeneic BMT patients as in this study, and have been shown to impair the expansion and suppressive function of Tregs in experimental models (12-14). Treg frequency at day + 30 after autologous and after allogeneic BMT in patients without GVHD were similar, suggesting that allogeneic transplant recipients receiving tacrolimus have similar Treg recovery to autologous transplant recipients without tacrolimus. However, absolute Tregs were less in allogeneic BMTs patients with no GVHD compared to autologous BMT patients, an effect attributed to lower absolute lymphocyte counts. This supports a study in which low dose cyclosporine had no effect on CD4+FOXP3+ cell frequencies at day +30 following T cell depleted BMT (10).
Previous studies measuring Tregs following BMT have not analyzed their frequency at time of GVHD onset. We observed that Treg frequencies measured within 24hrs of GVHD diagnosis were significantly less than allogeneic patients without GVHD, and correlated inversely with GVHD severity. This association supports an earlier report from Johns Hopkins University that demonstrated a correlation of FOXP3 mRNA with GVHD severity when using samples at GVHD onset (26). This finding has not been consistently confirmed in other studies (10, 11). As a single biomarker, Treg frequency at GVHD onset had only modest diagnostic value (AUC = 0.69), but the inverse relationship between Treg frequency and GVHD severity demonstrates its utility. It should also be noted that we measured Tregs in the peripheral blood, not in the target tissue, where they presumably exert their greatest effect. Interestingly, in a small cohort of patients we observed an overall decrease in Tregs prior to clinical onset of GVHD, suggesting that a decline in Treg frequency may herald onset of the disease. Although these analyses are correlative in nature, this observation indicates that systematic measurements of Treg frequencies may be able to predict the occurrence of GVHD and extends the findings of a previous study in which the frequency of CD4+CD25+ T cells decreased during the initial phases of GVHD (27).
Importantly, Treg frequency measured at GVHD onset may have prognostic value, which is a major feature of clinically relevant biomarkers (28). In our study, Treg frequency of less than 0.5% correlated with poor outcomes (maximum overall GVHD grade, NRM, OS). This finding suggests that prospective measurement of Treg frequency at GVHD onset may improve standard clinical GVHD grading and further risk stratify groups of patients. Identification of patients at high risk for severe GVHD early in their transplant course may impact clinical decisions to include more stringent monitoring and/or intense treatment.
Consistent with its prognostic value, Treg frequency at GVHD onset correlated with eventual treatment response at four weeks. It is not known whether altering immunosupression in patients with low Treg frequencies might subsequently improve outcomes through the in vivo expansion of Tregs. Recently, treatments such as rapamycin and extracorporeal photophoresis have been shown to increase Tregs and protect from experimental GVHD (29-31). Prospective studies of Treg expansion for the treatment and prevention of GVHD in high risk patients are therefore warranted.
Supplementary Material
Mean (±SEM) FOXP3 expression among CD4+CD25hi lymphocytes for autologous BMT patients and allogeneic BMT patients according to GVHD status. Error bars represent the SEM.
ALC by automated differential were obtained for autologous and allogeneic transplant patients within 24hrs of acute GVHD onset or at equivalent timepoints following transplantation. Error bars represent the SEM.
Causes of mortality in GVHD patients
Acknowledgments
Supported by grants from the National Institutes of Health P01-CA039542-20 and RCI-H2-101102, Cancer center care grant P30 CA46592, the American Cancer Society (CRP 08-224-01), the Leukemia and Lymphoma Society (#6013-09).
Footnotes
AUTHOR CONTRIBUTION J.M.M. and X.Q. performed research, analyzed data, and wrote the paper; C.E.R. designed experiments and performed research; I.T., M.S., D.B., K.L. and P.J. performed research; T.M.B. was the study statistician; D.J. managed human samples database; J.E.L., S.W.C., P.R., C.K. and S.M. contributed to patient accrual and quality control of the clinical endpoints, research discussion, and paper editing. J.E.L. also provided funding. J.L.M.F. and S.P. conceived and planned the study design, analyzed data, wrote the paper and provided funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 2.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 3.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 4.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–1254. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. doi: 10.1038/nature03725. [DOI] [PubMed] [Google Scholar]
- 6.Yagi H, Nomura T, Nakamura K, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 7.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 8.Wolf D, Wolf AM, Fong D, et al. Regulatory T-cells in the graft and the risk of acute graft-versus-host disease after allogeneic stem cell transplantation. Transplantation. 2007;83:1107–1113. doi: 10.1097/01.tp.0000260140.04815.77. [DOI] [PubMed] [Google Scholar]
- 9.Stanzani M, Martins SL, Saliba RM, et al. CD25 expression on donor CD4+ or CD8+ T cells is associated with an increased risk for graft-versus-host disease after HLA-identical stem cell transplantation in humans. Blood. 2004;103:1140–1146. doi: 10.1182/blood-2003-06-2085. [DOI] [PubMed] [Google Scholar]
- 10.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mielke S, Rezvani K, Savani BN, et al. Reconstitution of FOXP3+ regulatory T cells (Tregs) after CD25-depleted allotransplantation in elderly patients and association with acute graft-versus-host disease. Blood. 2007;110:1689–1697. doi: 10.1182/blood-2007-03-079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108:390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai M, Kitade H, Mathieu C, Waer M, Pirenne J. Inhibitory and stimulatory effects of cyclosporine A on the development of regulatory T cells in vivo. Transplantation. 2005;79:1073–1077. doi: 10.1097/01.tp.0000153505.73700.32. [DOI] [PubMed] [Google Scholar]
- 14.Coenen JJ, Koenen HJ, van Rijssen E, et al. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+ T cells. Bone Marrow Transplant. 2007;39:537–545. doi: 10.1038/sj.bmt.1705628. [DOI] [PubMed] [Google Scholar]
- 15.Noel G, Bruniquel D, Birebent B, et al. Patients suffering from acute graft-versus-host disease after bone-marrow transplantation have functional CD4+CD25hiFoxp3+ regulatory T cells. Clin Immunol. 2008;129:241–248. doi: 10.1016/j.clim.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez J, Casano J, Alvarez MA, et al. Kinetic of regulatory CD25high and activated CD134+ (OX40) T lymphocytes during acute and chronic graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol. 2004;126:697–703. doi: 10.1111/j.1365-2141.2004.05108.x. [DOI] [PubMed] [Google Scholar]
- 17.Bacchetta R, Gambineri E, Roncarolo MG. Role of regulatory T cells and FOXP3 in human diseases. J Allergy Clin Immunol. 2007;120:227–235. doi: 10.1016/j.jaci.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pzrepiorka D, Weisdorf D, Martin P, et al. Consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–828. [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22.Miyara M, Amoura Z, Parizot C, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006;203:359–370. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Battaglia M, Roncarolo MG. The fate of human Treg cells. Immunity. 2009;30:763–765. doi: 10.1016/j.immuni.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Miura Y, Thoburn CJ, Bright EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104:2187–2193. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 27.Schneider M, Munder M, Karakhanova S, Ho AD, Goerner M. The initial phase of graft-versus-host disease is associated with a decrease of CD4+CD25+ regulatory T cells in the peripheral blood of patients after allogeneic stem cell transplantation. Clin Lab Haematol. 2006;28:382–390. doi: 10.1111/j.1365-2257.2006.00825.x. [DOI] [PubMed] [Google Scholar]
- 28.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 29.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 31.Gatza E, Rogers CE, Clouthier SG, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112:1515–1521. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean (±SEM) FOXP3 expression among CD4+CD25hi lymphocytes for autologous BMT patients and allogeneic BMT patients according to GVHD status. Error bars represent the SEM.
ALC by automated differential were obtained for autologous and allogeneic transplant patients within 24hrs of acute GVHD onset or at equivalent timepoints following transplantation. Error bars represent the SEM.
Causes of mortality in GVHD patients