Abstract
This analysis examines the potential for the elderly to receive indirect protection from pneumonia and influenza (P&I) from vaccination of children. Using data from the Centers for Medicare and Medicaid Services, the National Immunization Survey, and the Behavioral Risk Factor Surveillance System, mixed-effects models were used to assess associations between vaccination coverage and P&I on the state level overall and by urbanicity and income. As vaccination coverage in children increased, the state-level P&I rates in seniors decreased (β= −0.040, −0.074--0.006), where β represents the expected change in the logged age-associated rate of disease increase for a one-percentage point increase in vaccination coverage. Increasing vaccination coverage in the elderly was associated with an increase in P&I rates (β= 0.045, 0.011–0.077) in seniors. The degree of association was more prominent in urban and high income areas. The consistent associations between influenza in the elderly and vaccination coverage in children suggest that routine vaccination of children may impart some indirect protection to the elderly.
Keywords: vaccine, herd immunity, pneumonia, elderly, children
Introduction
The purpose of this research project is to assess the potential for seniors to receive indirect protection from pneumonia and influenza (P&I) by vaccinating children. Influenza causes substantial morbidity and mortality, especially in the older population. Between 1976 and 1998, influenza was responsible for approximately 6,000 annual deaths, while influenza-related diseases caused over 36,000 deaths annually, with most deaths attributed to influenza-induced pneumonia,1 one of the most prevalent co-infections associated with influenza, especially in the elderly.2 In addition, there were nearly 1.7 million annual hospitalizations attributable to influenza and pneumonia, with 65% of those cases having pneumonia or influenza as the primary diagnosis.3
The mortality rate from P&I is 22.1 per 100,000 in the elderly which is nearly 100 times higher than the group with the next highest rate—0.3 per 100,000 in children under one year of age. Over 90% of the influenza-associated deaths between 1990 and 1998 occurred in the elderly (population age 65 and above).1 From 2000 to 2002, there were 670,000 average annual hospitalizations with a primary diagnosis of pneumonia in the elderly, and more than 1.2 million hospitalizations in that population with any-listed diagnosis of pneumonia.4 Influenza-associated complications increase rapidly with age in the older population. The population aged 85 and above experience the highest rates of hospitalization for these diseases—629 per 100,000 person-years.3 The present and future aging and subsequent growth of the elderly population will likely serve to magnify the impact of influenza and pneumonia in this vulnerable population and place a greater strain on the health care delivery system as a result.
Children are also severely impacted by pneumonia and influenza. During the winter months, hospitalization rates for P&I ranged from 220 per 100,000 children per year in the population aged 5 to 15 years to as high as nearly 4,500 per 100,000 children per year in the population aged less than 6 months.5 Although P&I in children is not as deadly as it is for the elderly, influenza mortality rates ranged from 0.11 per 100,000 in children 5 to 17 years of age to as high as 0.88 per 100,000 for children 0 to 6 months of age.6
Declining immune function with age is a well-documented phenomenon7,8 and is particularly problematic given that P&I increase rapidly with age in the elderly population. Recent research suggests potential patterns and pathways for transmission of influenza virus and associated infections between children and the elderly. Influenza vaccination coverage in children was associated with all-cause mortality and mortality from P&I in the older population in Japan,9 in addition to protecting the children themselves from death.10 A community-level, controlled study of mass influenza vaccination of children in Russia demonstrated a significant reduction of influenza-like illness in children, as well as a reduction in influenza-related diseases in the elderly.11 Simulation models suggest that vaccinating just 20% of the population aged 6 months to 18 years could reduce influenza in the total US population by 46%.12
These results suggest herd immunity, which occurs when vaccinating one population subgroup reduces potential exposure in another group,13 and has been demonstrated in other infectious diseases, including Haemophilus influenzae type b (Hib), a common co-infection associated with respiratory viruses. Hib is an important and potentially synergistic co-infection in patients with influenza.14,15 Vaccination to induce herd immunity has been shown for other diseases, such as measles16 and oral cholera.17 In a 1970 study by Monto et al.,18 a community with customary influenza vaccination practices experienced influenza incidence in the adult population that was substantially higher than in a neighboring community in which 85% of schoolchildren were vaccinated against influenza. Influenza incidence in children was three times higher in the community with customary vaccination practices, compared to the community with nearly complete vaccination coverage in children. The association between vaccination coverage in children and acute respiratory illness was strongest in the population aged 65 and older.19
The objective of this study is to assess the potential for herd immunity in the US elderly population against pneumonia and influenza using the most complete database of medical records of the elderly population—Medicare records. We explore associations between vaccination coverage in children and influenza and pneumonia hospitalizations in the elderly during the period 1991–2004. We also examine the effect of pneumonia and influenza vaccination coverage in the older population on P&I outcomes in the population age 65 and above. Specifically, we evaluated how vaccination coverage in children and the elderly affects the age-related acceleration in hospitalizations for pneumonia and influenza.
Methods
Data Sources
Outcome variables
We utilize Centers for Medicare and Medicaid Services (CMS) data containing all Medicare-eligible hospitalizations in the United States from July 1995 through June 2004. All claims records of hospitalizations associated with P&I (ICD-9CM codes 480 – 487)20 were abstracted from CMS databases for each of nine influenza years from 1995–96 to 2003–04, where an “influenza year” is defined as July through June of the following year, for the population age 65 to 99. For each state and season the records were summed and arranged as a single-year age distribution. Population counts were obtained for the population age 65 to 99 for single-years of age and single influenza year from the US Census Bureau Population Estimates Program. From hospitalization and population counts, the P&I rates were estimated by single-year of age, state, and season.
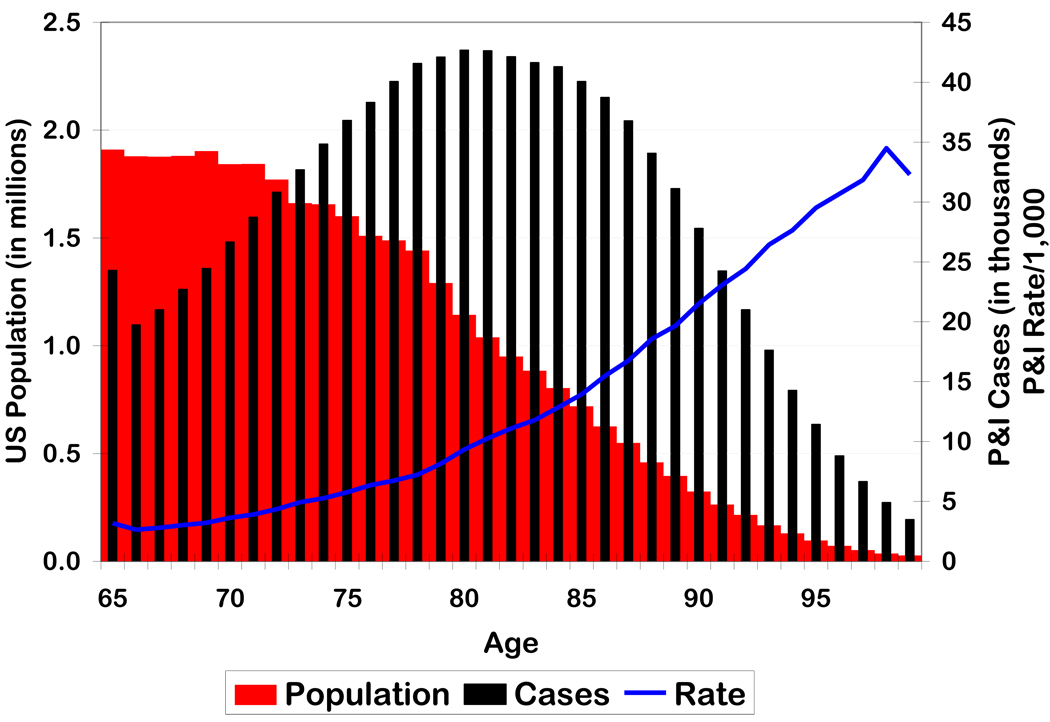
Figure 1 shows the age distribution of the US population, and P&I cases and rates in the population age 65 to 99. Age-specific population size generally decreased with age in the elderly population, with the largest proportion observed in the younger elderly. P&I cases peaked in the population at about age 80. This means that P&I rates steadily increased with age throughout the elderly population, with a slight leveling off at the oldest age groups. Though the population at age 98 was substantially smaller than the population at age 65, P&I rates were nearly 10 times higher for 98 year olds than for 65 year olds.
Figure 1.
Population, average annual pneumonia and influenza cases, and average annual pneumonia and influenza rate by age in the United States, 1998–2002
P&I rates increase nearly exponentially with age in the elderly population, therefore the main outcome variable is defined as the log of the rate of increase in disease rates with age. This variable, which we will call the age-related disease rate acceleration (ARAC), is a relative measure of disease burden in the elderly. The regression model used to estimate each state’s ARAC for each influenza year is:
The ARAC for each state and influenza year is the β1ij, or slope term. An ARAC of 0.02, for example, means that for each one-year increase in age, we expect P&I rates to increase by a factor of exp(0.02), or increase by approximately 2% per year. The measure takes advantage of having disease rates by single-year of age and captures the relationship between age and disease rates, which is important as the burden of P&I is highest in the oldest old.21 Standard approaches used to estimate disease burden in the elderly that utilize overall age-adjusted rates by default weight age groups with the highest populations, which is generally the youngest elderly, given that population size decreases with increasing age. Using age-acceleration of P&I rates, however, effectively weights each age equally reflecting P&I patterns across all ages simultaneously in the elderly population, including those at the highest risk of disease.22
Exposure Variables
Data on state-level influenza vaccination coverage in children were obtained from the National Immunization Program, which is a branch of the Centers for Disease Control and Prevention (CDC). The National Immunization Program conducts annual telephone surveys on influenza vaccination in children age 19 months to 35 months old through the National Immunization Survey (NIS).23 It is used to estimate immunization coverage on the national, state, and selected urban area levels, although in this analysis, only the state influenza vaccination coverage will be used. These data were tabulated by state and year and were available on the National Immunization Survey’s website. The variable used in this analysis is state coverage of the 4:3:1:3 vaccine series. This vaccine consists of at least four doses of diphtheria-tetanus toxoids-pertussis vaccine, three doses of poliovirus vaccine, at least one dose of measles vaccine, and three doses of Haemophilis influenzae type B vaccine, one of the primary causes of bacterial pneumonia.24
Pneumonia and influenza vaccination coverage in the elderly population was extracted from the Behavioral Risk Factor Surveillance System (BRFSS) database, a telephone survey administered by CDC to assess prevalence, trends, and spatial distributions of many disease and disease risk factors in the US and its territories. Vaccination status was asked of study participants age 65 and above and the aggregated state-level annual influenza vaccination coverage was abstracted for this analysis for each year from 1995 to 2004. Each of these surveys use complex weighting systems to develop their state specific samples, and thus are designed for public health surveillance at the State level, providing accurate state-level estimates of behaviors such as immunization.25
Statistical Analysis
Summary statistics were obtained for all variables, influenza and pneumonia vaccination coverage in the elderly, and 4:3:1:3 and influenza vaccination coverage in children at the state level. Pearson correlations were then estimated between each of the exposure-outcome pairs of variables for all states by season, and for all seasons by state. To model the relationship between vaccination coverage and P&I outcomes, linear mixed-effects models were implemented with state as the random effect to assess the role of state-level covariates in explaining between-state variation.
For combined P&I outcomes, these models were estimated using five sets of exposure variables: pneumonia vaccination coverage in the elderly alone, influenza vaccination coverage in the elderly alone, 4:3:1:3 vaccination coverage in children alone, vaccination coverage in children and pneumonia vaccination coverage in the elderly, and 4:3:1:3 vaccination coverage in children and influenza vaccination coverage in the elderly. For pneumonia-only outcomes, three exposure groups were used in the models: pneumonia vaccination coverage in the elderly alone, vaccination coverage in children alone, and pneumonia vaccination coverage in the elderly and 4:3:1:3 vaccination coverage in children together. Lastly, for influenza-only outcomes, models were estimated using three sets of exposure variables: influenza vaccination coverage in the elderly alone, vaccination coverage in children alone, and influenza vaccination coverage in the elderly and 4:3:1:3 vaccination coverage in children together.
Base model
All models were estimated separately using each observation with equal weight, and were weighted by the log of the population in each state. Model parameters are also displayed unadjusted, as well as adjusted for two potential confounding variables, median state income26 and log of population density.27 Based on the observed autocorrelations, first-order autoregressive covariance structures were used in all models which account for the observation that the temporal correlations were strongest for a one-season lag, and got progressively weaker as the lag increased. The equation used in this analysis is:
for each state i in influenza year j. β1 represents the coefficients of the explanatory variable vaccination coverage in the elderly, influenza or pneumonia. β2 represents the coefficients from 4:3:1:3 vaccination coverage in children. β i is the vector of effects of the potential confounding variables matrix Xi for each state i. The random effect of state is captured in the model and represented by the α i term, and the residual error term is represented by εij. The temporal correlation between a pair of measurements on the same state decreases toward zero as the seasonal lag increases, as described above. Since the observation times are equally spaced, each element of the variance-covariance matrix corrected for decay in autocorrelation ρ can be expressed as
where ρ is equal to exp(−φ), and φ is a constant representing the rate of decline and k is the seasonal lag, measured in seasons, k = 0 to 3, for all models, marginal and mixed effects.28
Stratified analysis
Additional analyses on combined P&I rates were conducted stratified by income and urbanicity to determine if the relationships between vaccination coverage and P&I rates depend upon these sociodemographic proxies which have been found to be potential effect modifiers in past studies.29 Stratification by income is designed to determine how the potential relationships between P&I and vaccination coverage vary by socioeconomic status. Vaccination coverage is positively associated with area-level income30 and we hypothesized that vaccination may have a greater impact in low income states than in high income areas. Likewise, these models were stratified by urbanicity because past studies have demonstrated a relationship between level of urbanicity and vaccination coverage31 to ascertain whether the association between vaccination coverage and P&I age acceleration differs by urbanicity. Three-way interaction terms using dummy variables for strata were used to determine the statistical significance of interaction parameters.
States were divided into tertiles, based on median state income and population density, and analyses on both outcome variables were repeated and compared for states in each tertile. All statistical analysis was performed using SAS version 9.0 (Cary, N.C.), and relevant figures were produced in Microsoft Excel (Redmond, Wash.) and SPSS version 15.0 (Chicago, Ill.).
Results
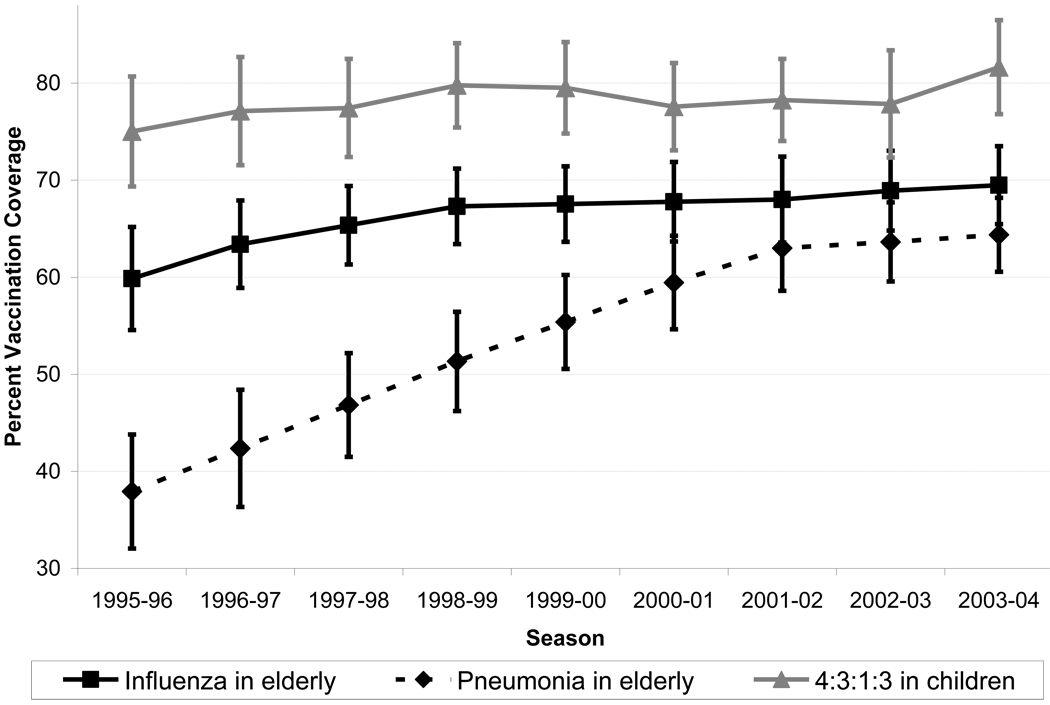
Summary statistics are displayed in Table 1. Vaccination coverage in the elderly for pneumonia was lower than influenza vaccination coverage, and influenza cases represented approximately 2% of all P&I cases. The state average log of the age acceleration in P&I rates was 0.078, meaning that for each single-year increase with age, P&I rates increased by approximately 8.1%. Figure 2 shows the temporal trends in vaccination coverage for elderly adults and children. Average state-level 4:3:1:3 vaccination coverage increased slightly from 1995 to 1998, remained steady, then increased from 78.9% to 81.2% from the 2002–03 to the 2003–04 season. Influenza vaccination coverage also increased slightly over the period, with smaller incremental increases over the most recent influenza years. Pneumonia vaccination coverage in the elderly doubled over the period, and was only five percentage points lower than influenza vaccination coverage in the 2003–04 season. For the initial models used to estimate the ARAC parameter, residuals were approximately normal with one potential outlier—Hawaii –in several models (standardized residuals as low as −2.9).
Table 1.
Descriptive statistics for outcome and exposure variables overall and by level of urbanicity and income
| Urbanicity | State Median Income | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Low | Medium | High | Low | Medium | High | |||
| 4:3:1:3 Vaccination Coverage in Children |
78.2 (5.2) | 75.8 (5.1) | 80.1 (4.5) | 78.7 (5.2) | abc | 77.7 (5) | 78.6 (5.1) | 78.5 (5.5) | |
| Influenza Vaccination Coverage in the Elderly |
66.4 (5.1) | 68.9 (4.3) | 65.6 (5.2) | 64.8 (5.0) | ab | 66.9 (5.3) | 66.6 (4.4) | 65.7 (5.6) | |
| Pneumonia Vaccination Coverage in the Elderly |
53.8 (10.5) | 56.2 (9.8) | 53.2 (10.4) | 52.1 (10.8) | ab | 52.9 (10.9) | 54.6 (9.6) | 53.9 (10.8) | |
| Pneumonia and Influenza Rate (per 100,000) |
3889 (968) | 3923 (1143) | 4289 (896) | 3432 (584) | abc | 4588 (940) | 3732 (879) | 3305 (531) | abc |
| Pneumonia rate (per 100,000) | 3812 (926) | 3819 (1079) | 4203 (872) | 3389 (565) | abc | 4471 (898) | 3664 (848) | 3258 (514) | abc |
| Influenza rate (per 100,000) | 77.6 (80.4) | 104 (113.6) | 85.2 (59.3) | 43 (35.6) | bc | 116.2 (106.4) | 67.7 (59.9) | 46.5 (39.7) | abc |
| Log of age increase rate (×100) | 7.80 (0.59) | 7.84 (0.47) | 7.58 (0.47) | 8.01 (0.73) | abc | 7.67 (0.48) | 7.76 (0.60) | 7.99 (0.64) | bc |
Significant (p < 0.05) difference between low and medium groups
Significant (p < 0.05) difference between low and high groups
Significant (p < 0.05) difference between medium and high groups
Figure 2.
Vaccination coverage for influenza and pneumonia in the elderly, and for Haemophilis influenzae (type b) (4:3:1:3) in children age 19 months to 35 months by influenza season (1995–2004)
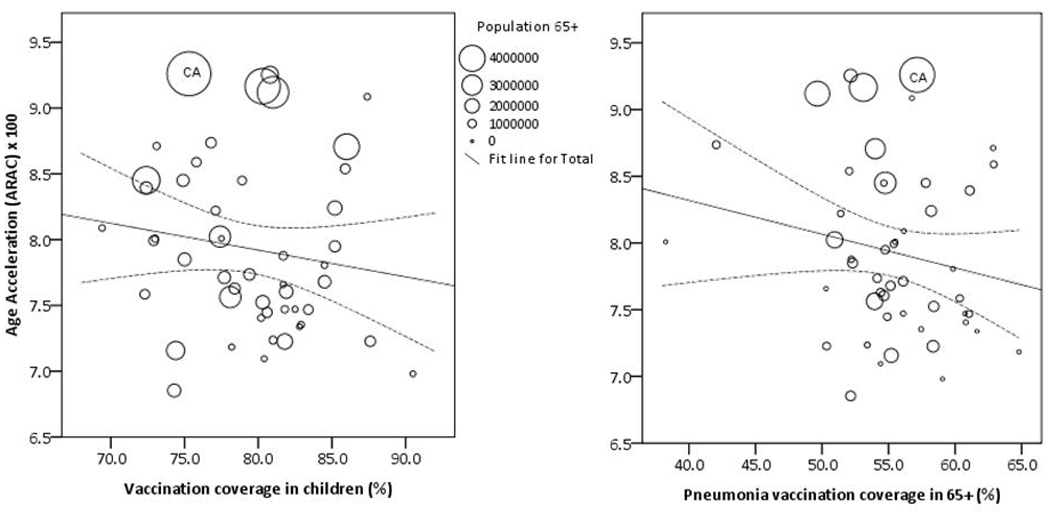
No significant association existed between average influenza vaccination coverage in the elderly and age acceleration in P&I (r = −0.048, p = 0.318), yet there was a weak, but significant and negative association found between 4:3:1:3 vaccination coverage in children and P&I ARAC (r = −0.196, p < 0.001). This means that for each standard deviation increase in 4:3:1:3 vaccination coverage, we observed a 0.196-standard deviation unit decrease in ARAC. The associations between ARAC for combined P&I and mean annual vaccination coverage in the elderly (r = 0.159, p = 0.277) and children (r = −0.256, p = 0.076) are shown in Figure 3.
The parameter estimates derived from the mixed-effects models are shown in Table 2. Significant negative associations were observed between age acceleration in pneumonia rates and 4:3:1:3 vaccination coverage in children, and these were consistent among all models examined. As 4:3:1:3 vaccination coverage in children increased, the age acceleration in influenza rates also significantly decreased in Model 3, which showed consistent positive associations between vaccination coverage in the elderly and the age acceleration in influenza. Positive associations were also evident between the age acceleration in influenza and influenza vaccination coverage in the elderly in Model 1. These relationships were generally consistent, regardless of the modeling procedure used (weighted, adjusted, weighted and adjusted, or unweighted and unadjusted).
Table 2.
Parameter estimates from mixed effects models analysis for three vaccination models of age acceleration, by disease, pneumonia or influenza, and type of model
| Model 1 | Model 2 | Model 3 | ||
|---|---|---|---|---|
| Influenza | Elderly Influenza Vaccination Coverage |
Child 4:3:1:3 Vaccination Coverage |
Elderly Influenza Vaccination Coverage |
Child 4:3:1:3 VaccinationCoverage |
| Unadjusted & Unweighted | 0.0448 (0.0172)** | −0.0252 (0.0164) | 0.0595 (0.0181)** | −0.0419 (0.0172)* |
| Unadjusted & Weightedb | 0.0480 (0.0171)** | −0.0231 (0.0163) | 0.0626 (0.0180)*** | −0.0410 (0.0171)* |
| Adjusteda & Unweighted | 0.0378 (0.0174)* | −0.0190 (0.0165) | 0.0534 (0.0184)** | −0.0401 (0.0172)* |
| Adjusteda & Weightedb | 0.0411 (0.0172)* | −0.0171 (0.0164) | 0.0564 (0.0183)** | 0.0392 (0.0169)* |
| Model 1 | Model 2 | Model 3 | ||
| Pneumonia | Elderly Pneumonia Vaccination Coverage |
Child 4:3:1:3 Vaccination Coverage |
Elderly Pneumonia Vaccination Coverage |
Child 4:3:1:3 Vaccination Coverage |
| Unadjusted & Unweighted | −0.0033 (0.0027) | −0.0150 (0.0045)*** | −0.0011 (0.0028) | −0.0123 (0.0040)** |
| Unadjusted & Weightedbc | −0.0036 (0.0026) | −0.0145 (0.0044)*** | −0.0015 (0.0029) | −0.0118 (0.0040)** |
| Adjusteda & Unweighted | −0.0032 (0.0027) | −0.0129 (0.0039)* | −0.0009 (0.0029) | −0.0128 (0.0041)** |
| Adjusteda & Weightedbc | −0.0035 (0.0028) | −0.0124 (0.0039)* | −0.0001 (0.0029) | −0.0123 (0.0040)** |
Adjusted for log of population density and median state income
Weighted by log of population size
p < 0.05,
p < 0.01,
p < 0.001
Explanatory variables:
Model 1: Elderly pneumonia vaccination coverage only (pneumonia models), or Elderly influenza coverage only (influenza models)
Model 2: 4:3:1:3 vaccination coverage in children
Model 3: Elderly pneumonia vaccination coverage only (pneumonia models), or Elderly influenza coverage only (influenza models) and 4:3:1:3 vaccination coverage in children
States were stratified by two key sociodemographic factors—state-level median income and degree of urbanicity—to assess the potential for effect modification for the observed relationships between vaccination coverage and P&I outcomes. The results of these models are displayed in Table 3. Influenza vaccination coverage in the elderly was not significantly associated with P&I ARAC, similar to the findings of pneumonia alone in the previous models. As was the case for P&I individually, states with higher 4:3:1:3 vaccination coverage in children tended to have lower levels of age acceleration in P&I rates in all models examined. This association depended upon urbanicity and, to a lesser degree, state median income level. Vaccination coverage in children was significantly and negatively related to age acceleration in P&I rates in the most rural and the most urban states, but this negative relationship was non-significant in the middle stratum in the model that included influenza vaccination coverage in the elderly and 4:3:1:3 vaccination coverage in children (Model 3A). Similar results were observed in Model 3B, which included pneumonia vaccination coverage in the elderly and vaccination coverage in children, except that the association between vaccination coverage in children and age accelerations in P&I rates was only significant in the most urban stratum, while this association was only marginally significant (p = 0.058) in the most rural stratum. There was also a significant negative relationship observed between vaccination coverage in children and age acceleration in P&I rates in those states with the highest median income. For any of the models examined, no significant associations were observed between age accelerations in P&I rates and vaccination coverage in the elderly for either pneumonia or influenza.
Table 3.
Parameter estimates (and standard errors) from models of pneumonia and influenza overall, and by urbanicity and median state income level
| Model 1A | Model 1B | Model 2 | |
|---|---|---|---|
| Elderly Influenza Vaccination Coverage |
Elderly Pneumonia Vaccination Coverage |
Child 4:3:1:3 Vaccination Coverage |
|
| All | 0.0018 (0.0063) | −0.0028 (0.0027) | −0.0130 (0.0039)*** |
| Urbanicity | MH | ||
| Low | 0.0063 (0.0122) | −0.0026 (0.0045) | −0.0144 (0.0074) |
| Medium | −0.0002 (0.0099) | −0.0018 (0.0049) | −0.0071 (0.0064) |
| High | −0.0016 (0.0110) | −0.0032 (0.0052) | −0.0149 (0.0060)* |
| Income | |||
| Low | −0.0105 (0.0092) | −0.0060 (0.0035) | −0.0131 (0.0069) |
| Medium | 0.0139 (0.0135) | 0.0007 (0.0062) | −0.0113 (0.0069) |
| High | 0.0046 (0.0103) | −0.0010 (0.0050) | −0.0127 (0.0064) |
| Model 3A | Model 3B | |||
|---|---|---|---|---|
| Elderly Influenza Vaccination Coverage |
Child 4:3:1:3 Vaccination Coverage |
Elderly Pneumonia Vaccination Coverage |
Child 4:3:1:3 Vaccination Coverage |
|
| All | 0.0089 (0.0066) | −0.0147 (0.0041)*** | −0.0005 (0.0029) | −0.0127 (0.0040)** |
| Urbanicity | LM, MH | LM, MH | ||
| Low | 0.0112 (0.0128) | −0.0158 (0.0076)* | −0.0002 (0.0050) | −0.0147 (0.0076) |
| Medium | 0.0018 (0.0100) | −0.0072 (0.0065) | −0.0011 (0.0050) | −0.0067 (0.0065) |
| High | 0.0121 (0.0117) | −0.0180 (0.0067)** | 0.0003 (0.0052) | −0.0149 (0.0063)* |
| Income | LM | LM, MH | LM | |
| Low | −0.0062 (0.0098) | −0.0119 (0.0071) | −0.0048 (0.0038) | −0.0108 (0.0071) |
| Medium | 0.0200 (0.0134) | −0.0140 (0.0071) | 0.0026 (0.0061) | −0.0117 (0.0071) |
| High | 0.0144 (0.0110) | −0.0161 (0.0069)* | 0.0017 (0.0052) | −0.0132 (0.0066)* |
Adjusted for log of population density and median state income
p < 0.05,
p < 0.01,
p < 0.001
LM = interaction term comparing Low and Medium states significant (p < 0.05)
LH = interaction term comparing Low and High states significant (p < 0.05)
MH = interaction term comparing Medium and High states significant (p < 0.05)
Explanatory variables:
Model 1A: Elderly influenza vaccination coverage only
Model 1B: Elderly pneumonia vaccination coverage only
Model 2: 4:3:1:3 vaccination coverage in children
Model 3A: Elderly influenza vaccination coverage and 4:3:1:3 vaccination coverage in children
Model 3B: Elderly pneumonia vaccination coverage and 4:3:1:3 vaccination coverage in children
Discussion
These results suggest a somewhat complex relationship between vaccination coverage in children and the elderly and P&I outcomes in the elderly. Consistent negative associations between 4:3:1:3 vaccination coverage in children and the age increase in P&I rates suggest that vaccinating children may have the potential to protect the oldest elderly against P&I. In models that included both elderly vaccination coverage and 4:3:1:3 vaccination coverage in children, increased vaccination coverage of children was generally associated with smaller age increases of influenza and pneumonia in the elderly. However, increased vaccination coverage in the elderly was not consistently associated with a decrease in the age increase of P&I in the older population, which is consistent with prior research.32–33 These results were consistent with those obtained through the use of marginal models using the generalized estimating equation,34 as well as when using the more traditional outcome of age-standardized disease rates.
The potential to protect the oldest elderly against P&I is particularly important when considering that, from 1998 to 2002, 73.6% of P&I hospitalizations in the 65+ population occurred in people age 75 and above, and more than half (53.6%) occurred in the population aged 80 years and older. Pneumonia and influenza, while prevalent in the entire population, are particularly problematic for the oldest elderly, who, consequently, also experience the highest mortality from these diseases. Relative measures, such as the age acceleration in P&I, supplement more traditional summary measures to provide a more comprehensive picture of disease patterns in the vulnerable elderly population than many traditional measures provide.
The fact that increased 4:3:1:3 vaccination coverage in children was related to reductions in P&I for the oldest elderly may be due to a true biological link between vaccination of children and elderly P&I outcomes, or because 4:3:1:3 vaccination coverage in children serves as a proxy for some intermediate variable that could mediate, modify, or confound the relationship. The potential for a relationship between vaccination coverage and area-level sociodemographic factors involved with this relationship merits future research. However, if the results truly reflect the biological relationship between vaccination and P&I outcomes in the elderly, it suggests that one potential means of protecting the oldest elderly against P&I may be to increase vaccination coverage in children, particularly in highly rural or urban areas.
The analysis is subject to several important limitations and caveats. The first involves the data used in this analysis, which covers pre-school children aged 19 months to 35 months. Previous research has suggested there may be a link between vaccinating school-aged children and P&I morbidity and mortality in the elderly9–11; however, vaccination coverage in school-aged children on the state level is not readily and publicly available from the National Immunization Survey or other related national data sources. But these findings on vaccinating young children are consistent with those of a household-based study that demonstrated a significant reduction in influenza infection in households in which children age 24 months to 60 months who attended day care were immunized.35 Additional research is needed to assess the most appropriate age groups of children to target for immunization. This leads to another limitation of the study: the exposure data was based not on all pneumonia vaccination itself, but rather on vaccination coverage of one of the primary organisms responsible for pneumonia.36 In addition, defining the exposure as the percent of children who have received the 4:3:1:3 series may under-report the true population coverage with actual Hib vaccines. Some children receive 3 doses of Hib vaccine without having all the other vaccines. In 1999, 93.5% of children 19–35 months of age received 3 doses of Hib but only 83.8% percent received 4 doses of diphtheria tetanus and pertussis vaccines.37
Estimates from both the National Immunization Survey and the Behavioral Risk Factor Surveillance System may are subject to potential bias due to the use of stratified weighted samples.38 In addition to bias, the sampling design may induce additional sampling variability, which may affect that precision of estimates that subsequently may affect the observed significance of the association in the regression models.
Another important limitation exists regarding the outcome selected, all pneumonia and influenza hospitalizations. The contribution of Hib to the overall burden of pneumonia in seniors was between 10 and 13%, and varied geographically and temporally. Although H. influenzae is a cause of pneumonia in seniors, it only causes a modest proportion of all pneumonia in seniors, consistent with prior studies.39,40 Consistent with these studies, in our Medicare claims database, only 5.0% of all pneumonia hospitalizations were due to H. influenzae. The proportion of H. influenzae pneumonias that are caused by type b H. influenzae will be smaller, and coding of the type of H. influenzae is not available in the Medicare hospitalization data. Thus, even if Hib vaccination of children is effective at preventing Hib pneumonia in the elderly, the expected benefit against all-cause pneumonia may only be modest.
It is important to note, however, that H. influenzae is a typical cause of a substantial amount of community-acquired pneumonia, specifically in the elderly population.2 Furthermore, laboratory testing of elderly individual for specific bacterial strains of pneumonia may not be complete. In this analysis, all pneumonia and influenza diagnoses were used because pneumonia diagnoses are often not based on laboratory testing, and such cases where the exact species-specific cause is not tested for, these cases may manifest as unspecified pneumonia hospitalizations in the Medicare hospitalization dataset.41
There are also important caveats to the types of models used in the analysis. The dominant circulating influenza virus strain and whether or not the proper vaccine for each influenza year was used were not taken into consideration. Effective matching of vaccine strains to the circulating strain could modulate the effectiveness of vaccinating elderly against influenza and pneumonia. Additionally, the models assessed whether vaccination coverage was associated with P&I outcomes in the concurrent influenza and pneumonia season. This strategy does not take into account the potential for immune memory to last for consecutive seasons. Supplemental research using transition models could further explore the effect of immunization on immune memory and whether its effects could last for multiple seasons. Another limitation of the models used involved the initial stage of modeling to obtain the initial ARAC values used as the outcome measure in the linear mixed effects models. Alternate approaches include Poisson regression, negative binomial or related generalized linear models and could have been used instead. A linear regression modeling approach with log-transformed outcomes (P&I rates) was chosen to make the outcome, the ARAC, more straightforward to interpret.
The choice of outcome measure used, the ARAC, is another consideration. The ARAC is not an absolute measurement, but rather a relative measure of disease burden, taking advantage of the population-level exponential increase in disease rates with age in the elderly population. Since the measure itself is derived from regression models, the ARAC has some standard error. It should be noted however, that on the state level, the standard error is minor, as the log-transformed age-specific rates follow an almost perfectly linear pattern.21
This study is ecological in design, which indicates that individual-level relationships cannot be inferred from these results. The objective of the study, however, was not to examine individual-level relationships within households or families but to look at population level associations. Such research is critical for the understanding of the transmissibility of influenza and pneumonia from child to adult and has been explored on a small scale in the US35 and in Europe.42 Instead, the objective of our study was to assess whether vaccination of children in the community could potentially reduce P&I in the elderly of the community to explore the potential for herd immunity, which is not applicable on the individual level.
Our approach also allowed for the characterization of pneumonia and influenza by age on a fine scale—namely by single-year of age—instead of using typical age groupings. A new summary measure of disease burden in the elderly population was derived from this age distribution of disease, based on age-related trends, the ARAC. Traditional measures of disease burden, such as age-standardized and age-specific rates, while useful, mathematically weight the age groups with the largest populations. The ARAC weights each age equally, which is particularly important given that the highest rates of disease are found in the oldest age groupings. Although itself subject to limitations, the ARAC captures an important disease process—the exponential increase in disease rates with age—for which other methods attempt to control or adjust. The oldest elderly are at the highest risk of complications and mortality from pneumonia and influenza, and the ARAC quantifies this effect in a demographically meaningful manner. The ARAC, however, is a relative measure, and is subject to the effect of the intercept, or the starting rate at age 65. However, there is substantially more variation in the ARAC than in the intercept.
Despite the limitations, this study is among the first to directly compare vaccination coverage in children and in the elderly simultaneously in relation to P&I outcomes in the elderly. In particular, this study focused on several aspects of P&I hospitalizations, analyzing P&I separately and combined as an outcome, and to examine these relationships over multiple seasons. Despite these limitations, there were consistent and significant associations between 4:3:1:3 vaccination coverage, which includes Hib, in children and age acceleration of pneumonia and combined P&I in the elderly. This could be due to the fact that nearly 99% of all P&I hospitalizations are due to pneumonia, so increasing vaccination coverage in children could decrease the likelihood of children having this disease, and, as a consequence, the potential for the elderly to contract one of the major causes of pneumonia from children may decrease. The outcome variables from this study are not based on samples or surveys; instead, they were derived from all Medicare-eligible hospitalizations in the population. The results of this preliminary study suggest that P&I in the elderly may be linked to vaccination coverage in children. Our results suggest that the issue of vaccine coverage in children could potentially have a dual benefit, not only for children themselves, but also for the elderly population, whose risk of contracting pneumonia may decrease as an indirect result, particularly in the oldest old. These associations deserve further research as a potentially viable means of reducing preventable respiratory infections in the elderly population.
Acknowledgments
The following funding agencies are thanked for their continual support: NIH-NIAID U19 A162627 and HHSN266200500024C. We also thank Drs. Kenneth Chui, Janet Forrester, Jyotsna Jagai, Siobhan Mor, and Ms. Julia Wenger of Tufts School of Medicine for their assistance with this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 2.Cunha BA. Pneumonia in the elderly. Clin Microbiol Infect. 2001;7:581–588. doi: 10.1046/j.1198-743x.2001.00328.x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 4.Fry AM, Shay DK, Holman RC, Curna AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons age 65 years or older in the United States, 1998–2002. JAMA. 2005;294:2712–2719. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 5.Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. New Eng J Med. 2000;342:225–231. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 6.Bhat N, Wright JG, Broder KR, Murra EL, Greenberg ME, Glover MJ, et al. Influenza-associated deaths among children in the United States, 2003–2004. New Eng J Med. 2005;353:2559–2567. doi: 10.1056/NEJMoa051721. [DOI] [PubMed] [Google Scholar]
- 7.Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: Reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 8.Webster RG. Immunity to influenza in the elderly. Vaccine. 2000;18:1686–1689. doi: 10.1016/s0264-410x(99)00507-1. [DOI] [PubMed] [Google Scholar]
- 9.Reichert TA, Sugaya N, Fedson DS, Glezan WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Eng J Med. 344:889–896. doi: 10.1056/NEJM200103223441204. [DOI] [PubMed] [Google Scholar]
- 10.Sugaya N, Takeucki Y. Mass vaccination of schoolchildren against influenza and its impact on the influenza-associated mortality rate among children in Japan. Clin Infect Dis. 2005;41:939–947. doi: 10.1086/432938. [DOI] [PubMed] [Google Scholar]
- 11.Ghendon YZ, Kaira AN, Elshina GA. The effect of mass influenza immunization in children on the morbidity of the unvaccinated elderly. Epidemiol Infect. 2006;134:71–78. doi: 10.1017/S0950268805005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wycker D, Edelsberg J, Halloran ME, Longini IM, Ziman A, Ciuryla V, Oster G. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005;23:1284–1293. doi: 10.1016/j.vaccine.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 13.Glezen WP. Herd protection against influenza. J Clin Virol. 2006;37:237–243. doi: 10.1016/j.jcv.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Brundage JF, Shanks GD. Deaths from bacterial pneumonia during 1918–19 influenza pandemic. Emerg Infect Dis. 2008;14:1193–1199. doi: 10.3201/eid1408.071313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath PT, McVernon J. The UK Hib vaccine experience. Arch Dis Child. 2002;86:396–399. doi: 10.1136/adc.86.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlenker TL, Bain C, Baughman AL, Hadler SC. Measles herd immunity: The association of attack rates with immunization rates in preschool children. JAMA. 1992;267:823–826. doi: 10.1001/jama.267.6.823. [DOI] [PubMed] [Google Scholar]
- 17.Emch M, Ali M, Park J, Yunus M, Sack DA, Clemens JD. Relationship between neighbourhood-level killed oral cholera vaccine coverage and protective efficacy: Evidence for herd immunity. Int J Epidemiol. 2006;35:1044–1050. doi: 10.1093/ije/dyl100. [DOI] [PubMed] [Google Scholar]
- 18.Monto AS, Davenport FM, Napier AJ, Francis T. Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. J Infect Dis. 1970;122:16–25. doi: 10.1093/infdis/122.1-2.16. [DOI] [PubMed] [Google Scholar]
- 19.Piedra PA, Gaglani MJ, Kozinetz CA, Herschler G, Riggs M, Griffith M, et al. Herd immunity in adults against influenza-related illness with use of the trivalent-live attenuated vaccine (CAIV-T) in children. Vaccine. 2005;23:1540–1548. doi: 10.1016/j.vaccine.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Viboud C, Bjornstad ON, Smith DL, Simonsen L, Miller MA, Grenfell BT. Synchrony, waves, and spatial hierarchies in the spread of influenza. Science. 2006;312:447–451. doi: 10.1126/science.1125237. [DOI] [PubMed] [Google Scholar]
- 21.Cohen SA, Chui KKH, Naumova EN. Measuring disease burden in the older population using the slope-intercept method for population log-linear estimation (SIMPLE) Stat Med. 2010 doi: 10.1002/sim.3886. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen SA, Naumova EN. Population dynamics in the elderly: The need for age-adjustment in national BioSurveillance systems. Lec Notes Comp Sci. 4506:47–58. [Google Scholar]
- 23.Centers for Disease Control and Prevention: National Immunization Program. Immunization Coverage in the US. [Accessed November 1, 2006];Atlanta, Georgia: US Department of Health and Human Services; Website: http://www.cdc.gov/nip/coverage/default.htm#chart.
- 24.Levine OS, Lagos R, Muñoz A, Villaroel J, Alvarez AM, Abrego P, Levine MM. Defining the burden of pneumonia in children preventable by vaccination against Haemophilus influenzae type b. Pediatr Infect Dis J. 1999;18:1060–1064. doi: 10.1097/00006454-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Nelson DE, Holtzman D, Bolen J, Stanwyck CA, Mack KA. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS) Soc Prev Med. 2001;46:S03–S42. [PubMed] [Google Scholar]
- 26.Armstrong K, Berlin M, Schwartz JS, Propert K, Ubel PA. Barriers to influenza immunization in a low-income urban population. Am J Prev Med. 2001;20:21–25. doi: 10.1016/s0749-3797(00)00263-4. [DOI] [PubMed] [Google Scholar]
- 27.Glezen WP. The changing epidemiology of respiratory syncytial virus and influenza: Impetus for new control measures. Pediatr Infect Dis J. 2004;23:S202–S206. doi: 10.1097/01.inf.0000144662.86396.07. [DOI] [PubMed] [Google Scholar]
- 28.Diggle PJ, Heagerty P, Liang K, Zeger SL. 2nd ed. New York (NY): Oxford University Press; 2002. Analysis of longitudinal data. [Google Scholar]
- 29.Singleton JA, Poel AJ, Lu P, Nichol KL, Iwane MK. Where adults reported receiving influenza vaccination in the United States. Am J Infect Control. 2005;33:563–570. doi: 10.1016/j.ajic.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman RK, Ahwesh ER, Mieczkowski TA, Block B, Janosky JE, Barker DW. Influence of family functioning and income on vaccination in inner-city health centers. Arch Pediatr Adolesc Med. 1996;150:1054–1061. doi: 10.1001/archpedi.1996.02170350056010. [DOI] [PubMed] [Google Scholar]
- 31.Luman ET, Barker LE, Simpson DM, Rodewald LE, Szilagyi PG, Zhao Z. National, state and urban-area vaccination-coverage levels among children aged 19–35 months, United States, 1999. Am J Prev Med. 2001;20:88–153. doi: 10.1016/s0749-3797(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 32.Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005;165(3):265–272. doi: 10.1001/archinte.165.3.265. [DOI] [PubMed] [Google Scholar]
- 33.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 34.Zeger SL, Liang K, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 35.Hurwitz ES, Haber M, Chang A, Shope T, Teo S, Ginsberg M, et al. Effectiveness of influenza vaccination of day care children in reducing influenza-related morbidity among household contacts. JAMA. 2000;284:1677–1682. doi: 10.1001/jama.284.13.1677. [DOI] [PubMed] [Google Scholar]
- 36.Adams WD, Dwaver KA, Cochi SL, Plikaytis BD, Zell ER, Broome CV, et al. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA. 1993;269:221–226. [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. National, state, and urban area vaccination coverage among children aged 19–35 months--United States, 2003. MMWR Morb Mortal Wkly Rep. 2004;53:658–661. [PubMed]
- 38.Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health. 1991;81:1166–1173. doi: 10.2105/ajph.81.9.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz M, Ewig S, Marcos MA, Martinez JA, Arancibia F, Mensa J, Torres A. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160:397–405. doi: 10.1164/ajrccm.160.2.9808045. [DOI] [PubMed] [Google Scholar]
- 40.Zalacain R, Torres A, Celis R, Blanquer J, Aspa J, Esteban L, et al. Community-acquired pneumonia in the elderly: Spanish multicentre study. Eur Respir J. 2003;21:294–302. doi: 10.1183/09031936.03.00064102. [DOI] [PubMed] [Google Scholar]
- 41.Naumova EN, Parisi SM, Castronovo D, Pandita M, Wenger J, Minihan P. Pneumonia and influenza hospitalizations in elderly people with dementia. J Am Geriatr Soc. 2009;57:2192–2199. doi: 10.1111/j.1532-5415.2009.02565.x. [DOI] [PubMed] [Google Scholar]
- 42.Viboud C, Boëlle P, Cauchemez S, Lavenus A, Valleron A, Flahault A, et al. Risk factors of influenza transmission in households. Brit J Gen Pract. 2004;54:684–689. [PMC free article] [PubMed] [Google Scholar]