Abstract
Introduction
Dobutamine (DOB) stress in animal models of heart disease has been imaged so far using echocardiography and magnetic resonance imaging. The purpose of this study was to assess normal response to DOB stress in rats using anatomical and functional data using microcomputed tomography (CT).
Methods
Ten normal adult male rats were first injected with a liposomal-based blood pool contrast agent and next infused with DOB via a tail vein catheter. Using prospective gating, 5 pairs of systole/diastole micro-CT images were acquired (a) pre-infusion baseline; (b) at heart rate plateau during infusion of 10 µg/kg/min DOB; (c) at post-DOB infusion baseline; (d) at heart rate plateau during infusion of 30 µg/kg/min DOB; and (e) after post-infusion return to baseline. Heart rate, peripheral and breathing distensions were monitored by oximetry. Micro-CT images with 88-micron isotropic voxels were segmented to obtain cardiac function based on volumetric measurements of the left ventricle.
Results
DOB stress increased heart rate and cardiac output with both doses. Ejection fraction increased above baseline by an average of 35.9% with the first DOB dose and 18.4% with the second dose. No change was observed in relative peripheral arterial pressures associated with the significant increases in cardiac output.
Discussion
Micro-CT proved to be a robust imaging method able to provide isotropic data on cardiac morphology and function. Micro-CT has the advantage of being faster and more cost- effective than MR and is able to provide higher accuracy than echocardiography. The impact of such an enabling technology can be enormous in evaluating cardiotoxic effects of various test drugs.
Keywords: cardiac, small animal imaging, lung, micro-CT, stress, dobutamine
1. Introduction
Dobutamine (DOB) stress is commonly used with echocardiographic imaging in contemporary medical practice to evaluate myocardial viability and contractile reserve in patients with different cardiac diseases like myocardial ischemia and valvular disorders (Krahwinkel, et al., 1997). DOB stress echocardiography has also been used to study cardiac disease in large laboratory animals such as dogs and pigs (Handke, et al., 2003; McEntee, et al., 1998; McEntee, et al., 1996), but has also been used more recently in rodents (Plante, et al., 2005).
Cardiac studies in small animals are very challenging from an imaging perspective. Currently, two modalities—echocardiography and MR—are used to measure cardiac function in rodents. Although echocardiography has been the most widely used method, results are very operator-dependent. Moreover, echocardiography uses model-based estimation of the ventricle volumes that relies on geometric assumptions that work well only in normal hearts (Camici, 2003; Hoit & Walsh, 2001).
Alternatively, the gold standard for cardiac imaging in rodents has been considered to be MR microscopy (MRM), which refers to MR imaging applied at the microscopic scale. MRM has been applied to assess both cardiac morphology and function in rodents (Brau, Hedlund, & Johnson, 2004; Franco, Dubois, Peshock, & Shohet, 1998; Ruff, et al., 1998; Slawson, Roman, Williams, & Koretsky, 1998; Wiesmann, et al., 2000) . Moreover, MRM has also been used to measure dose-responsive changes in cardiac function with graded doses of dobutamine (Daire, et al., 2008). However, with some exceptions (Bucholz, Ghaghada, Qi, Mukundan, & Johnson, 2008) , most of the MRM and echocardiography studies are not providing isotropic resolution, and therefore multiplanar reformations require interpolations to account for non-cubic voxels.
We proposed a micro-CT-based method appropriate for in vivo characterization of cardiac structure and function in mice (C Badea, Fubara, Hedlund, & Johnson 2005), where true fourdimensional (4D) datasets are collected with an isotropic spatial resolution less than 100 microns and a temporal resolution of 10 ms. We have shown that cardiac micro-CT using high flux x-ray tubes, careful physiologic gating, and blood pool contrast agents can produce image sets with 10–50 times higher spatial resolution than with MR (Nahrendorf, et al., 2001; Wiesmann, et al., 2000). Higher resolutions allow for more accurate segmentation, and therefore better accuracy in the volumetric measurements. Measurements of left ventricular (LV) volumes at different points in the cardiac cycle can provide indices of cardiac function, such as ejection fraction (EF) and cardiac output (CO). Our goals in this study were to develop and validate a protocol for assessing cardiac function in vivo in rats using micro-CT and to use of this technology for detecting a DOB-induced change in cardiac function.
2. Materials and Methods
2.1 Animal preparation
All animal procedures were approved by the Duke University Institutional Animal Care and Use Committee. Ten male SD SAS rats (Charles-River, Wilmington, MA) 6–8 week old (average 214 g, 200–250 g) were used. Animals were anesthetized with isoflurane (1.5%) mixed with 50% oxygen and balanced with nitrogen that was delivered by nose cone. ECG was monitored with electrodes (Blue Sensor, Medicotest, UK) taped to the footpads and body temperature was maintained using heat lamps, a rectal probe, and feedback controller (Digi- Sense®, Cole Parmer, Chicago, IL). A pneumatic pillow on the thorax was used to monitor breathing. All physiologic signals were processed with Coulbourn modules (Coulbourn Instruments, Allentown, PA) and displayed on a computer monitor using a custom-written LabVIEW application (National Instruments, Austin, TX). Additionally, heart and breathing rates, pulse and breathing distentions, and oxygen saturation were monitored by a pulse oximeter placed on the hind paw (MouseOx, StarrLife Sci, PA). Pulse distention (PD) is a surrogate of arterial pressure and provides a relative measure of peripheral arterial pressure. Breathing distention (BD) is a surrogate of intrapleural pressure reflecting breathing effort (MouseOx User Manual, Rev.4.0). Since the distinction between blood and myocardium in micro-CT is low, contrast agents are essential. An intravascular catheter (24 ga, BD Medical Systems, UT) was placed in the lateral tail vein for iodinated contrast injection and DOB infusion. DOB infusions were controlled by syringe pump (Harvard Apparatus, MA). Before the start of imaging, a liposomal-based blood pool contrast agent (Mukundan, et al., 2006) (12 ml/kg, 80 mg Iodine/ml) was hand-injected into the tail vein. For imaging, the animal was placed in an acrylic cradle containing a nose cone for isoflurane delivery, upper incisor clamp, and pneumatic pillow for recording breathing motion (Howles, Nouls, Qi, & Johnson, 2009). This cradle assembly was then seated vertically in a rotatable base controlled by a stepper motor.
2.2 Imaging
Imaging was performed using our dual tube/detector micro-CT system, which (C. Badea, et al., 2008) describes in detail. The x-ray tubes and the detectors are arranged orthogonally. The system includes: (a) 2x G-297 x-ray tubes (Varian Medical Systems, Palo Alto, CA) with 0.3/0.8 mm focal spot size; (b) 2x Epsilon High Frequency x-ray generators (EMD Technologies, Quebec, Canada); (c) 2x XDI-VHR 2 CCD x-ray detectors (Photonic Science, East Sussex, UK) with a Gd2O2S phosphor and 22-micron pixels, which are typically binned to 88 microns. The sampling is controlled by a sequencing application written in LabVIEW. Only one of the imaging chains has been used in this study.
The imaging parameters were 80 kVp, 160 mA, with 10 ms exposures. Each 3D image set consisted of 250 projections acquired over 190-degree arc. The acquisition time was 4–5 minutes. Imaging was cardio-respiratory gated using a prospective strategy during end-expiration and at two phases of the cardiac cycle—at diastole at time of the R peak and during systole after a delay from the R peak equal to 35% of the RR interval. The projection images were used to reconstruct tomograms with a Feldkamp algorithm using Parker weighting (Feldkamp, Davis, & Kress, 1984). For this purpose, we used the Cobra EXXIM software package (EXXIM Computing Corp, Livermore, CA). 3D image arrays (5123) were produced with a voxel size of 883 microns.
The imaging protocol consisted of acquiring 5 pairs of systole/diastole images from 10 animals at (i) pre-infusion baseline (Pre10); (ii) at heart rate plateau during infusion of 10 µg/kg/min DOB (PK10); (iii) at post-DOB infusion baseline (Pre30); (iv) at heart rate plateau during infusion of 30 µg/kg/min DOB (PK30); and (v) after post-infusion return to baseline (Post30). DOB infusion lasted 15 minutes with imaging starting during the middle of these periods when the heart rate plateau has been reached. The return to baseline heart rates post DOB infusions occurred within 10 to 15 minutes. The total radiation dose per animal was 0.6 Gy.
2.3 Image Analysis
Quantitative analysis of cardiac function based on the left ventricle (LV) volumetric measurements was performed using ImageJ <http://rsb.info.nih.gov/ij/> and the Segmentation Assistant plugin. The segmentation procedure is semi-automatic. 3D stacks were imported into ImageJ, where the images were cropped to include only slices through the heart. The top of the LV was defined as the first slice caudal to the aortic root. The threshold-based segmentation used the contrast difference between the contrast enhanced blood and the myocardium. After the segmentation, the LV volume was computed as the total number of voxels corresponding to blood multiplied by voxel volume. The LV volumetric measurements were performed for both end-diastolic volume (EDV) and end-systolic volume (ESV). Stroke volume (SV) and ejection fraction (EF) were calculated with the EDV and ESV (SV= EDV- ESV; EF=SV/EDV). For cardiac output (CO), SV was multiplied by heart rate (HR).
3. Results
Representative coronal micro-CT images during diastole and systole and corresponding to the 5 stages of the imaging protocol are shown in Fig. 1. Because the micro-CT datasets have isotropic resolution (88-micron voxels), the same image quality is possible in any imaging plane. Note the dramatic decrease in LV volume at end-systole during the DOB infusion (Figs. 1B and D) and return to baseline volume after infusion (Figs. 1C and E).
Figure 1.
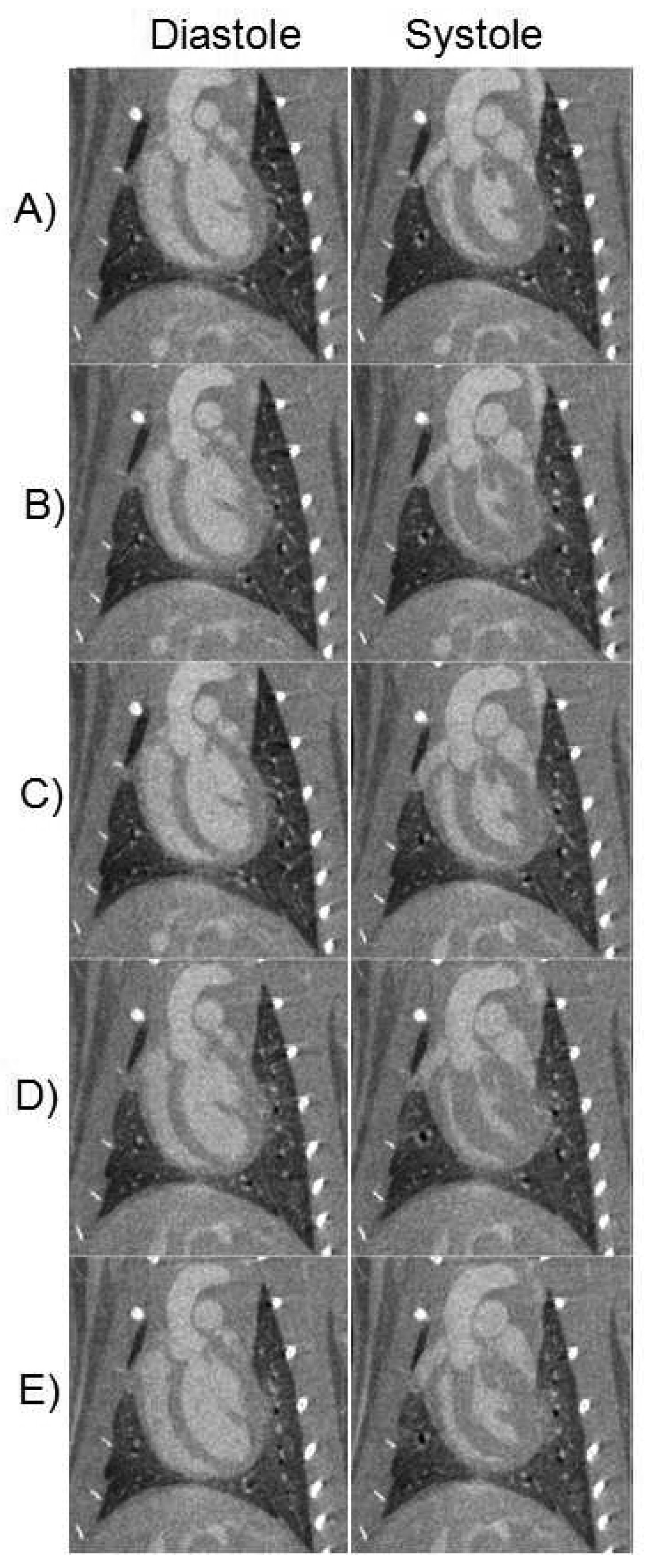
Representative micro-CT images reconstructed in diastole and systole, during Pre10 (A), PK10 (B), Pre30 (C), PK30 (D), and Post30 (E).
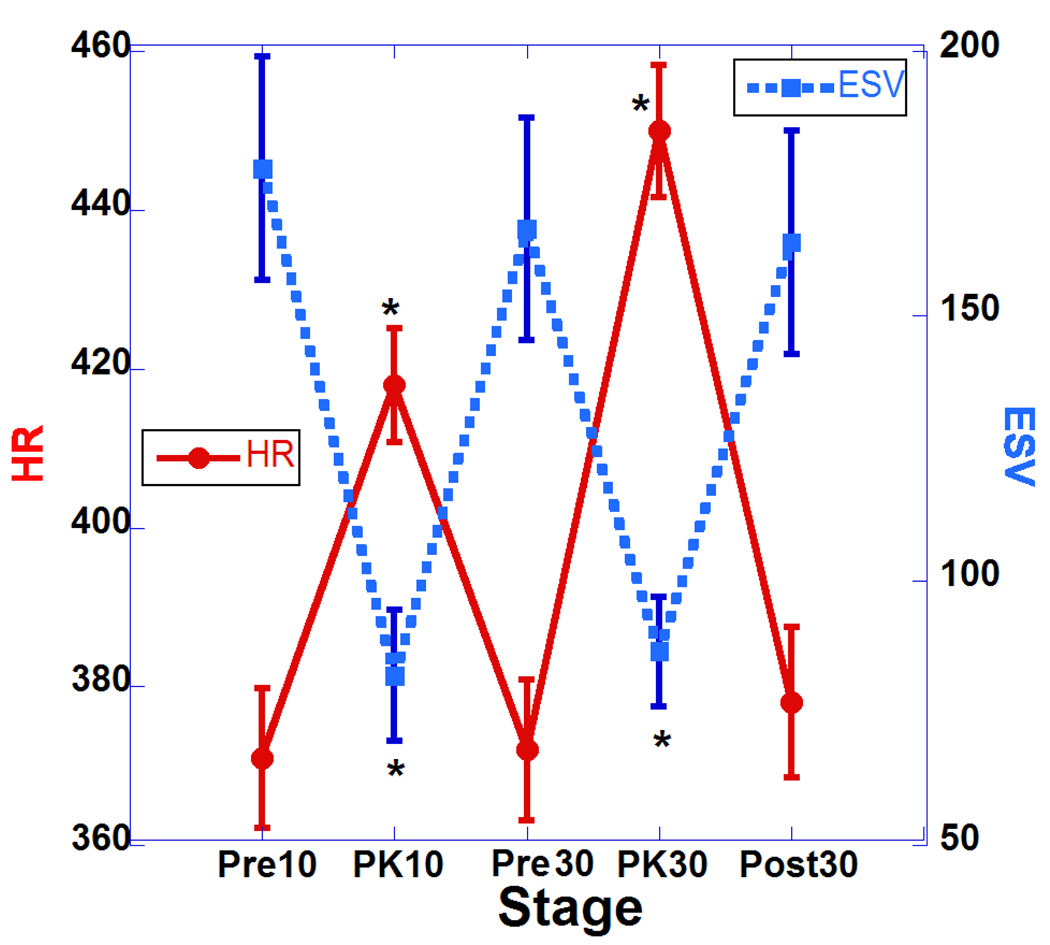
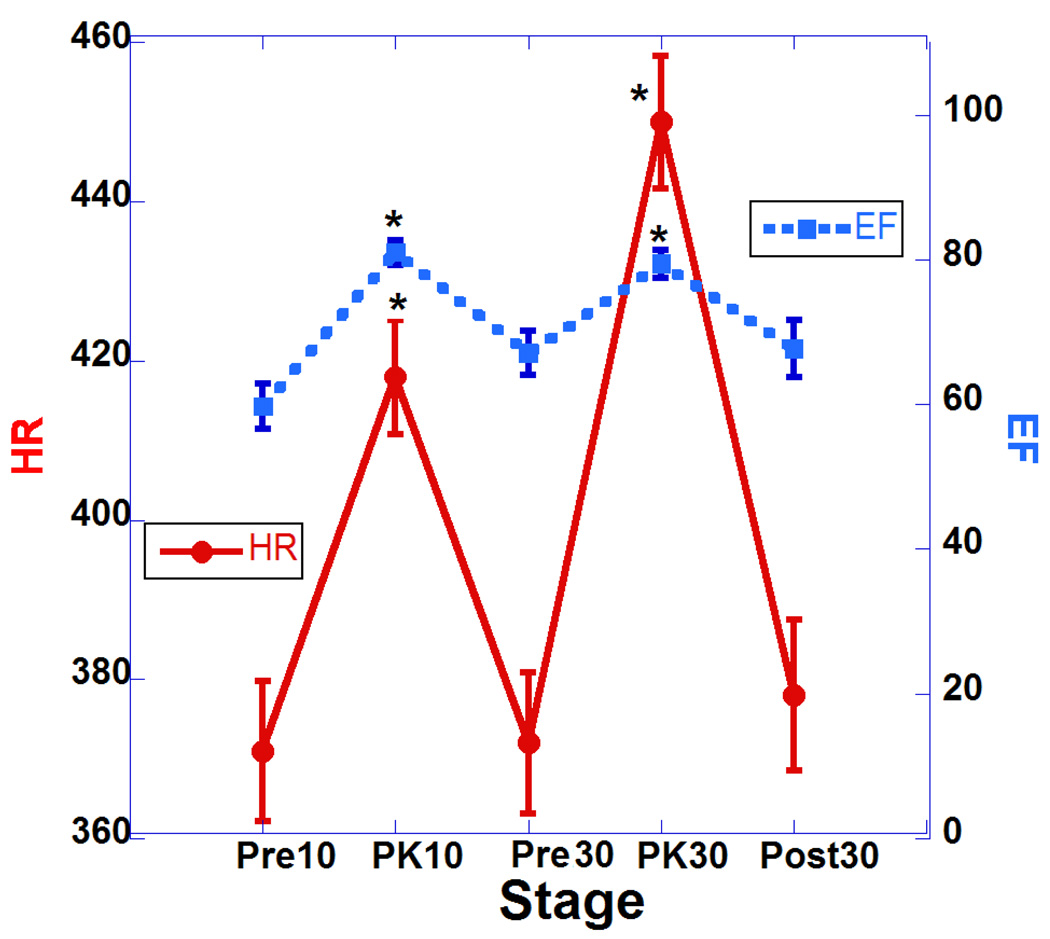
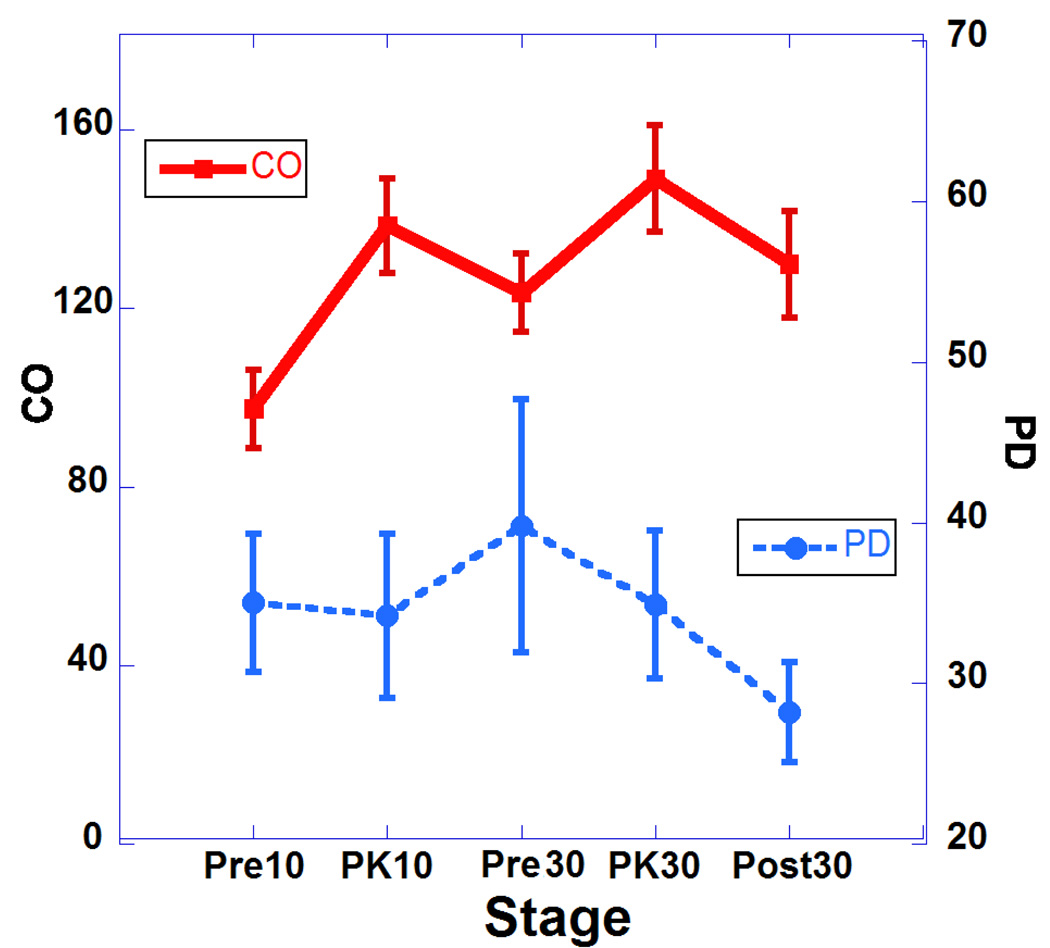
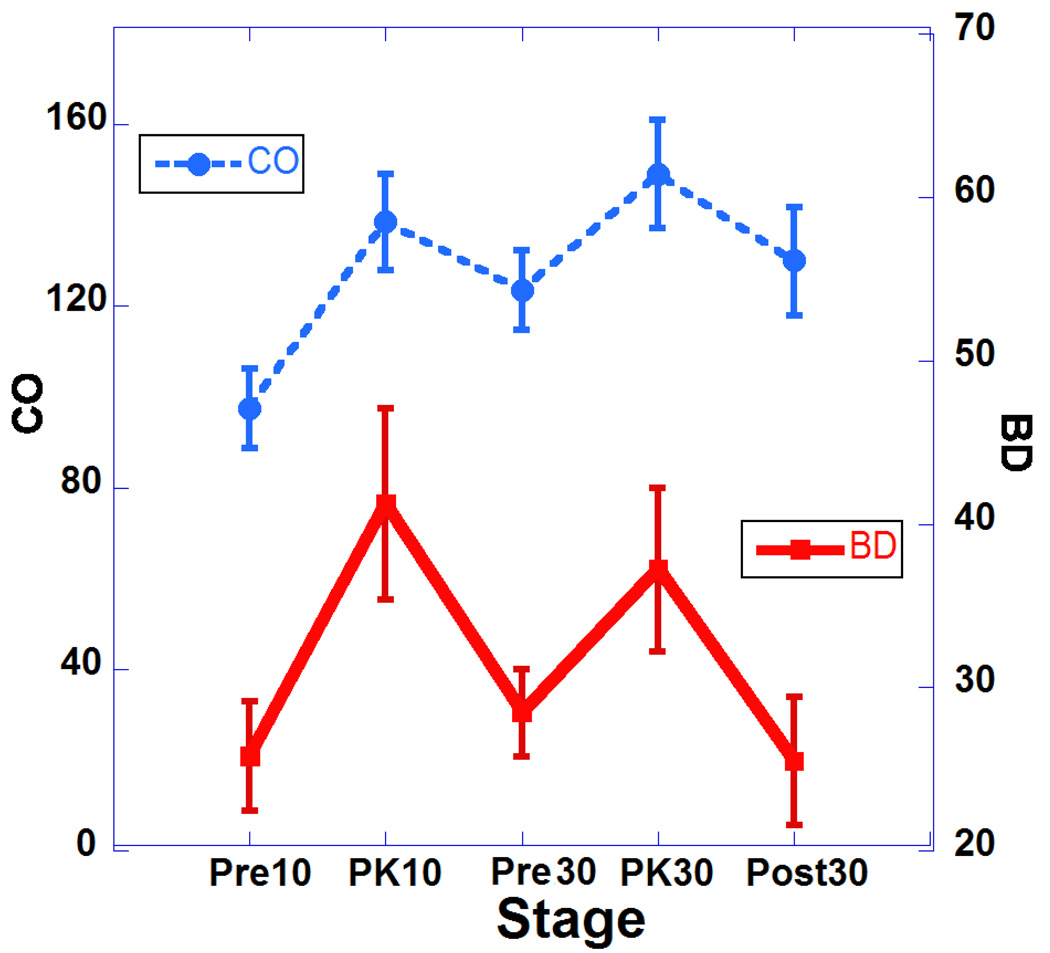
Table 1 summarizes the major changes of cardiac function after two doses of DOB (10 and 30 µg/kg). Results are presented as means, and standard errors of the mean. For statistical comparisons, we performed one-way analysis of variance (ANOVA). Statistical significance was set for Bonferroni corrected p values of 0.05 or less. There was a statistically significant increase in heart rate (HR) during infusion of both doses and this was associated with increases in cardiac output (Fig. 2; HR vs. CO) but without reaching statistical significance. These changes indicate the drug has a strong chronotropic effect. Also associated with the increases in HR with both doses are significant decreases in ESV and but non-significant decreases in EDS volumes (see Fig. 3; HR vs. ESV and Fig. 4; HR vs. EDV). Also, we observed significant increases in EF associated with the increase in HR indicating a strong inotropic effect of the drug at both doses (see Fig. 5; HR vs. EF). The plot of CO vs. pulse distension (PD) (Fig. 6) and Table 1 indicate there is no statistically significant change in peripheral arterial pressure associated with the significant increases in cardiac output. It is well established that DOB has a peripheral vasodilatory effect that essentially blocks any increase in arterial pressure associated with the increases in CO (Segreti, Marsh, Polakowski, & Fryer, 2008). There were statistically non-significant increases in breath distention (BD) during both DOB doses (see Fig.7).
Table 1.
Cardiovascular parameters from rats before, during, and after perfusion of 10 and 30 µg/kg/min of dobutamine.
| HR | EDV | ESV | SV | CO | EF | PD | BD | |
|---|---|---|---|---|---|---|---|---|
| b/min | µl | µl | µl | ml/min | % | |||
| Pre10 | 370.5 | 443.0 | 177.9 | 265.1 | 98.2 | 59.7 | 35.0 | 25.8 |
| SEM | 8.8 | 37.1 | 21.1 | 26.9 | 8.8 | 3.1 | 4.3 | 3.356 |
| PK10 | 418.3 | 415.5 | 82.0 | 333.5 | 139.5 | 81.0 | 34.2 | 41.23 |
| SEM | 7.2 | 37.4 | 12.4 | 27.7 | 10.7 | 1.7 | 5.1 | 5.87 |
| %ChgPrePK | 12.9 | −6.2 | −53.9 | 25.8 | 42.0 | 35.9 | −2.1 | 59.8 |
| Pre30 | 372.3 | 502.2 | 166.5 | 335.7 | 125.0 | 67.1 | 39.8 | 28.467 |
| SEM | 8.8 | 37.1 | 21.1 | 26.9 | 8.8 | 3.1 | 7.9 | 2.675 |
| PK30 | 450.4 | 420.1 | 86.6 | 333.5 | 150.2 | 79.4 | 34.9 | 37.21 |
| SEM | 8.3 | 35.0 | 10.4 | 28.5 | 11.9 | 1.9 | 4.6 | 5.019 |
| %ChgPrePK | 21.0 | −16.4 | −48.0 | −0.6 | 20.7 | 18.4 | −12.3 | 30.7 |
| Post30 | 377.5 | 513.6 | 163.9 | 349.7 | 132.0 | 67.7 | 28.2 | 25.48 |
| SEM | 9.5 | 38.9 | 21.2 | 36.9 | 11.9 | 3.9 | 3.1 | 3.9 |
| p values for F | 0.0001 | 0.2110 | 0.0002 | 0.3052 | 0.0179 | 0.0001 | 0.6973 | 0.0411 |
| Bonferroni Corrected p values | ||||||||
| Pre10/PK10 | 0.0013 | 1 | 0.0046 | 1 | 0.0911 | 0.0001 | 1 | 0.1555 |
| Pre30/PK30 | 0.0001 | 1 | 0.0291 | 1 | 0.9646 | 0.0451 | 1 | 1 |
| Pre10/Pre30 | 1 | 1 | 1 | 1 | 0.9079 | 0.7889 | 1 | 1 |
| Pre30/Post30 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
HR=heart rate; EDV=end-diastolic volume; ESV=end-systolic volume; SV=stroke volume; CO=cardiac output; EF=ejection fraction; PD=pulse distention, BD=breath distention. Data presented for n=10 animals with the exception of PD and BD where n=9.
Figure 2.
Plot of heart rate (HR) and cardiac output (CO) at 5 points of the study; baseline (Pre10), during peak effect of 10 µg infusion (PK10), baseline after 10 µg/kg/min DOB (Pre30), during peak effect of 30 10 µg/kg/min DOB infusion (PK30), and return to baseline after 30 µg/kg/min DOB (Post30). *Asterisks indicate significant difference compared to pre-challenge baseline (p <0.05, actual p values are shown in Table 1). Error bars represent standard errors of the mean.
Figure 3.
Plot of heart rate (HR) and end-systolic volume (ESV) at 5 points of the study. *Asterisks indicate significant difference compared to pre-challenge baseline (p <0.05, actual p values are shown in Table 1). Error bars represent standard errors of the mean.
Figure 4.
Plot of heart rate (HR) and end-diastolic volume (EDV) at 5 points of the study. *Asterisks indicate significant difference compared to pre-challenge baseline (p <0.05, actual p values are shown in Table 1). Error bars represent standard errors of the mean.
Figure 5.
Plot of heart rate (HR) and ejection fraction (EF) at 5 points of the study. *Asterisks indicate significant difference compared to pre-challenge baseline (p <0.05, actual p values are shown in Table 1). Error bars represent standard errors of the mean.
Figure 6.
Plot of cardiac output (CO) and pulse distention (PD) at 5 points of the study. Error bars represent standard errors of the mean.
Figure 7.
Plot of cardiac output (CO) and breath distention (BD) at 5 points of the study. Error bars represent standard errors of the mean.
4. Discussion
Evaluating the response of the small animal heart to stress in vivo is a challenging imaging task due to the demands for higher spatial and temporal resolution. In this study, we showed that micro-CT can be used to detect and measure DOB stress-induced changes in cardiac function in the rat. DOB infusion is safe, quick, and yields important information about the rat’s response to adrenergic stress.
The micro-CT methods used in this study provided multiple 3D datasets with isotropic resolution of 88 microns (voxel volume=0.6 µl), with relatively rapid acquisition (<10 min for two phases of the heart), and provided what we believe to be the most accurate imaging method currently available for measuring cardiac function. Echocardiography, while relatively inexpensive and easily performed, only provides a cross-section of the heart. Volume calculations rely on assumptions about the cardiac shape and results are very dependent on the operator. MR has been used to study DOB cardiac effects (Daire, et al., 2008), but the spatial resolution in this previous study (0.28 mm2 in-plane by 2 mm slice) was not isotropic. Moreover, the 156-µl nonisotropic voxels were more than 250-times larger than the (0.6 µl) voxels in this study. To minimize radiation dose, we acquired only end-diastolic and end-systolic images and not multiple phases of the entire cardiac cycle. Multiple phases of the complete cycle could be obtained with micro-CT using retrospective gating and fast 4D acquisitions (Drangova, Ford, Detombe, Wheatley, & Holdsworth, 2007). However, unlike MR cardiac imaging, CT does not allow wall tagging for analysis of the circumferential wall strain. But wall motion analysis is possible with 4D micro-CT based on registration and analysis of deformation fields (Schreibmann, Badea, & Fox, 2008) .
The MR imaging study (Daire, et al., 2008) performed in Sprague Dawley rats reported results similar to ours. The SV was reported to be increased by 19% and the cardiac output by 45% from baseline at a DOB dose of 10 µg/kg/min. In our study, the changes in SV and CO were 25.8% and 42%, respectively. DOB stress echocardiography has been used for noninvasive, serial evaluation of the heart in Wistar rats (Plante, et al., 2005). DOB was infused intravenously at a constant rate of 10 µg/kg/min until a plateau was reached in the LV EF for at least 2 minutes. The echocardiography-based study found that the effects of DOB were more prominent on end-systolic dimensions. The study reported a 24% increase in EF from baseline versus 35% in our work (see Table 1). The difference may be associated with variation in dose response between the different strains of animals, but we also believe it may be due to the inaccuracies associated with the M-mode measurements on which this study was based (C. T. Badea, et al., 2007).
One limitation of micro-CT versus other imaging modalities is the use of ionizing radiation. The dose associated with micro-CT methods is not negligible. In the application reported here, the dose is approximately 12-times less than the whole body lethal dose (LD 50/30) in rats of approximately 7.1 Gy (Kouzmanova, Ivanov, Nankova & Traykov, 1997). We are exploring a number of interesting possibilities for dose reduction that include iterative reconstruction algorithms. In reference (Song, Liu, Johnson, & Badea, 2007), we introduced a sparseness prior-based iterative reconstruction based on total variation, which provided adequate image quality with as low as 95 projections with a random angular distribution.
Cardiovascular safety is an important concern in contemporary drug development and a significant contributor to safety-related attrition of novel drugs in development. In vivo studies in laboratory rodents are used early in drug development to identify drug safety issues that may impact clinical development of a novel molecule. Accordingly, sensitive methods for identifying and characterizing cardiovascular safety concerns in rodents are necessary. The best methods are those that integrate both structural and functional endpoints, as well as those that have predictive capability (where “predictive” refers to the ability to identify long-term risks in short-duration studies). Micro-CT imaging of the functioning heart under stress meets these needs. Micro-CT has extraordinary spatial resolution that allows precise quantitation of anatomic structures throughout the cardiac cycle. The addition of dobutamine permits evaluation of functional cardiac reserve increasing opportunity to identify drug-related effects in the heart that may progress with continued dosing or have deleterious effects in a patient with pre-existing cardiac disease.
5. Conclusions
Dobutamine (DOB) stress micro-CT is a sensitive and potentially predictive method to evaluate cardiac function and functional reserve in rats. Micro-CT proved to be a robust imaging method able to provide isotropic data on cardiac morphology and function. Micro-CT has the advantage of being faster and more cost-effective than MR and is able to provide higher accuracy than echocardiography (C. T. Badea, et al., 2007). The potential impact of such an enabling technology can be enormous in evaluating cardiotoxic effects of new pharmaceuticals.
Acknowledgements
All work was performed at the Duke Center for In Vivo Microscopy, NCRR National Biomedical Technology Research Center (P41 RR005959), with additional support from NCI (U24 CA092656), and a collaborative research agreement with GlaxoSmithKline Safety Assessment. We thank our collaborators Drs. Ketan B. Ghaghada and Ananth Annapragada (University of Texas) for providing the liposomal blood pool contrast agent used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cristian T. Badea, Email: cristian.badea@duke.edu.
Laurence W. Hedlund, Email: laurence.hedlund@duke.edu.
James Cook, Email: jjc29@duke.edu.
Brian R. Berridge, Email: brian.x.berridge@gsk.com.
G. Allan Johnson, Email: gjohnson@duke.edu.
References
- Badea C, Fubara B, Hedlund L, Johnson G. 4D micro-CT of the mouse heart. Molecular Imaging. 2005;4:110–116. doi: 10.1162/15353500200504187. [DOI] [PubMed] [Google Scholar]
- Badea C, Johnston S, Johnson B, De Lin M, Hedlund LW, Johnson GA. JS Hsieh, Ehsan . SPIE, Medical Imaging. Vol. 6913. San Diego, CA: SPIE; 2008. A Dual Micro-CT System for Small Animal Imaging; pp. 691342–691310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea CT, Hedlund LW, Mackel JF, Mao L, Rockman HA, Johnson GA. Cardiac micro-computed tomography for morphological and functional phenotyping of muscle LIM protein null mice. Molecular Imaging. 2007;6:261–268. [PMC free article] [PubMed] [Google Scholar]
- Brau ACS, Hedlund LW, Johnson GA. Cine magnetic resonance microscopy of the rat heart using cardiorespiratory-synchronous projection reconstruction imaging. Journal of Magnetic Resonance Imaging. 2004;20:31–38. doi: 10.1002/jmri.20089. [DOI] [PubMed] [Google Scholar]
- Bucholz E, Ghaghada K, Qi Y, Mukundan S, Johnson GA. Four-dimensional MR microscopy of the mouse heart using radial acquisition and liposomal gadolinium contrast agent. Magnetic Resonance in Medicine. 2008;60:111–118. doi: 10.1002/mrm.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camici PG. Gated PET and ventricular volume. Journal of Nuclear Medicine. 2003;44:1662. [PubMed] [Google Scholar]
- Daire JL, Jacob JP, Hyacinthe JN, Croisille P, Montet-Abou K, Richter S, Botsikas D, Lepetit-Coiffe M, Morel D, Vallee JP. Cine and tagged cardiovascular magnetic resonance imaging in normal rat at 1.5 T: a rest and stress study. Journal of Cardiovascular Magnetic Resonance. 2008;10:48. doi: 10.1186/1532-429X-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drangova M, Ford NL, Detombe SA, Wheatley AR, Holdsworth DW. Fast Retrospectively Gated Quantitative Four-Dimensional (4D) Cardiac Micro Computed Tomography Imaging of Free-Breathing Mice. Investigative Radiology. 2007;42:85–94. doi: 10.1097/01.rli.0000251572.56139.a3. [DOI] [PubMed] [Google Scholar]
- Feldkamp LA, Davis LC, Kress JW. Practical cone-beam algorithm. Journal of the Optical Society of America. 1984;1:612–619. [Google Scholar]
- Kouzmanova M, Ivanov S, Nankova V, Traykov L. Effects of ionizing and nonionizing radiation on rat hemopoiesis, Electro- and Magnetobiology. 1997;16(2):161–168. [Google Scholar]
- Franco F, Dubois SK, Peshock RM, Shohet RV. Magnetic resonance imaging accurately estimates LV mass in a transgenic mouse model of cardiac hypertrophy. American Journal of Physiology. 1998;274:H679–H683. doi: 10.1152/ajpheart.1998.274.2.H679. [DOI] [PubMed] [Google Scholar]
- Handke M, Heinrichs G, Magosaki E, Lutter G, Bode C, Geibel A. Threedimensional echocardiographic determination of cardiac output at rest and under dobutamine stress: comparison with thermodilution measurements in the ischemic pig model. Echocardiography. 2003;20:47–55. doi: 10.1046/j.1540-8175.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- Hoit BD, Walsh RA. Cardiovascular Physiology in the Genetically Engineered Mouse. Kluwer Academic Publishers; 2001. [Google Scholar]
- Howles GP, Nouls JC, Qi Y, Johnson GA. Rapid production of specialized animal handling devices using computer-aided design and solid freeform fabrication. J Magnetic Resonance Imaging. 2009;30:466–471. doi: 10.1002/jmri.21821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahwinkel W, Ketteler T, Godke J, Wolfertz J, Ulbricht LJ, Krakau I, Gulker H. Dobutamine stress echocardiography. European Heart Journal. 1997;18 Suppl D:D9–D15. doi: 10.1093/eurheartj/18.suppl_d.9. [DOI] [PubMed] [Google Scholar]
- McEntee K, Amory H, Clercx C, Soyeur D, Michaux C, Vanhaeverbeek O, Jacqmot O, Henroteaux M. Physiologic response to dobutamine infusion during cardiac stress testing of dogs. American Journal of Veterinary Research. 1998;59:1160–1165. [PubMed] [Google Scholar]
- McEntee K, Clercx C, Pypendop B, Peeters D, Balligand M, D'Orio V, Henroteaux M. Cardiac performance in conscious healthy dogs during dobutamine infusion. Research in Veterinary Science. 1996;61:234–239. doi: 10.1016/s0034-5288(96)90070-3. [DOI] [PubMed] [Google Scholar]
- Mukundan S, Ghaghada K, Badea C, Hedlund L, Johnson G, Provenzale J, Bellamkonda R, Annapragada A. A Nanoscale, Liposomal Contrast Agent for Preclincal MicroCT Imaging of the Mouse. American Journal of Roentgenology. 2006;186:300–307. doi: 10.2214/AJR.05.0523. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Wiesmann F, Hiller KH, Hu K, Waller C, Ruff J, Lanz TE, Neubauer S, Haase A, Ertl G, Bauer WR. Serial cine-magnetic resonance imaging of left ventricular remodeling after myocardial infarction in rats. Journal of Magnetic Resonance Imaging. 2001;14:547–555. doi: 10.1002/jmri.1218. [DOI] [PubMed] [Google Scholar]
- Plante E, Lachance D, Drolet MC, Roussel E, Couet J, Arsenault M. Dobutamine stress echocardiography in healthy adult male rats. Cardiovascular Ultrasound. 2005;3:34. doi: 10.1186/1476-7120-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff J, Wiesmann F, Hiller K-H, Voll S, von Kienlin M, Bauer WR, Rommel E, Neubauer S, Haase A. Magnetic resonance microimaging for noninvasive quantification of myocardial function and mass in the mouse. Magnetic Resonance in Medicine. 1998;40:43–48. doi: 10.1002/mrm.1910400106. [DOI] [PubMed] [Google Scholar]
- Schreibmann E, Badea C, Fox T. AAPM: Medical Physics. 2008. Registration Based Automatic Segmentation and Wall Motion Analysis for 4D Cardiac Micro-CT in Mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segreti JA, Marsh KC, Polakowski JS, Fryer RM. Evoked Changes in Cardiovascular Function in Rats by Infusion of Levosimendan, OR-1896 [(R)-N-(4-(4- Methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl)acetamide], OR-1855 [(R)-6-(4- Aminophenyl)-5-methyl-4,5-dihydropyridazin-3(2H)-one], Dobutamine, and Milrinone: Comparative Effects on Peripheral Resistance, Cardiac Output, dP/dt, Pulse Rate, and Blood Pressure. Vol. 325. 2008. pp. 331–340. [DOI] [PubMed] [Google Scholar]
- Slawson SE, Roman BB, Williams DS, Koretsky AP. Cardiac MRI of the normal and hypertrophied mouse heart. Magnetic Resonance in Medicine. 1998;39:980–987. doi: 10.1002/mrm.1910390616. [DOI] [PubMed] [Google Scholar]
- Song J, Liu QH, Johnson GA, Badea CT. Sparseness prior based iterative image reconstruction for retrospectively gated cardiac micro-CT. Medical Physics. 2007;34:4476–4483. doi: 10.1118/1.2795830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann F, Ruff J, Hiller KH, Rommel E, Haase A, Neubauer S. Developmental changes of cardiac function and mass assessed with MRI in neonatal, juvenile, and adult mice. American Journal of Physiology, Heart and Circulatory Physiology. 2000;278:H652–H657. doi: 10.1152/ajpheart.2000.278.2.H652. [DOI] [PubMed] [Google Scholar]