Abstract
Chronic kidney disease (CKD) is now an accepted long term complication of allogeneic hematopoietic stem cell transplantation. Calcineurin inhibitors (CNI) which are used for prophylaxis and treatment of graft versus host disease have been associated with the development of nephrotoxicity. Hypertension (HTN) and thrombotic microangiopathy (TMA) are two comorbidities linked to CKD. T-cell depletion (TCD) of stem cell grafts can obviate the need for the use of CNI. We conducted a retrospective analysis of 100 patients who underwent TCD transplantation: 30 in group A were conditioned without total body radiation (TBI) and 70 in group B received a TBI containing regimen. None of the patients received CNI. The median age was 55.5 and 45 years for groups A and B respectively. Eleven patients developed TMA – all in group B. The two-year cumulative incidence of sustained CKD was 29.2 % and 48.8% in group A and group B respectively, with a mean follow up of at least 21 months. CKD free survival was better in non-TBI group (p=0.046). Multivariable survival analysis revealed that exposure to TBI, older age and TMA were risk factors for CKD. The incidence of new onset or worsening HTN was 6.7% and 25.7% (p=0.03) in group A and B, respectively. The use of TBI (p=0.0182) and diagnosis of TMA (p=0.0006) predisposed patients to the development of HTN using univariable logistic regression models. Thus, despite the absence of CNI, a proportion of these older patients in both groups developed CKD and HTN.
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (HSCT) is widely used in the treatment of hematologic disorders. Although the number of patients undergoing HSCT yearly is only ~8000 (1), that number has been increasing and it is expected to continue to rise. Long-term survival after transplantation has improved over the years as a consequence of molecular human leukocyte antigen (HLA) typing, tailoring transplantation to particular patient populations and diseases, improvements in peri-transplant supportive care, and advancements in infectious disease monitoring and treatment. As the number of HSCT survivors continues to increase, however, the medical community becomes more aware of transplantation-related health issues which can impact on quality of life and healthcare costs of these survivors (2–3).
Chronic kidney disease (CKD) is one of the long-term complications of HSCT which has recently been addressed in several retrospective studies (4–7). Although now well recognized, its incidence, etiology and clinical course remain controversial. While it may develop as a consequence of acute kidney injury (AKI), it has also been associated with older age, lower pretreatment glomerular filtration rate (GFR), female gender, use of total body irradiation (TBI) and fludarabine in the conditioning regimen, graft versus host disease (GVHD), use of calcineurin inhibitors (CNI), and a variety of other factors (4–7). Hypertension (HTN) has also been recognized as a late complication of HCST with incidence of HTN linked to the development of CKD (5).
A more serious renal complication of transplantation is thrombotic microangiopathy (TMA). The clinical manifestations of this syndrome include renal insufficiency, microangiopathic hemolytic anemia (MAHA), thrombocytopenia, HTN and in some cases neurologic deficits. Unfortunately, controversies in the criteria that define the syndrome have complicated the reporting of its actual incidence. (8). TMA has also been linked to many similar risk factors as CKD including older age, female gender, use of TBI, immunosuppression that includes the use of CNI, GVHD and infections. Nevertheless, no specific risk factor has been consistently supported by available reports in the allogeneic transplantation setting (8).
In appropriately selected patients, T-cell depleted (TCD) HSCT has similar survival outcomes to those of unmodified HSCT. TCD of hematopoietic stem cell (HSC) grafts can obviate the need for CNI as prophylaxis for GVHD. As a consequence, it offers a unique opportunity to study the effects of TBI and other risk factors on the incidence of CKD, TMA and HTN without the confounding effects of CNI exposure. Therefore, we retrospectively analyzed the incidence of CKD, HTN and TMA in two cohorts of patients transplanted at our center with allogeneic TCD grafts who were never exposed to CNI. One cohort of patients was conditioned only with chemotherapy, and the second cohort received conditioning with a TBI containing regimen.
PATIENTS AND METHODS
Patients
Data collection and analysis were performed with the approval of the Institutional Review Board of Memorial Sloan-Kettering Cancer Center, New York, NY. Protected health information was coded in accordance with requirements of Health Insurance Accountability and Portability Act. Between January 2001 and December 2006, 159 patients underwent transplantation on two protocols utilizing TCD allogeneic HSC grafts. Sixty patients received conditioning with high-dose chemotherapy alone (group A) and 99 patients were conditioned with high-dose chemotherapy plus TBI (group B). Patients younger than 18 years of age, those who did not survive beyond 180 days after transplantation or who underwent second HSCT, and those who received CNI for treatment of GVHD were excluded from the analysis. One patient in group B who received sirolimus for treatment of GVHD was also excluded.
Baseline and follow up demographic, clinical, and laboratory data were collected on patients by a review of the medical records. Serum creatinine (SCr) and blood pressure (BP) measurements were obtained from 0, 6, 12, 18 and 24 month post-transplantation clinic visit notes. In addition, each patient record was reviewed in detail for evidence of TMA as described below.
GVHD evaluation
Acute GVHD was assessed and graded according to the criteria of Glucksberg et al. (9) and modified by Martin et al. (10). Chronic GVHD was diagnosed and graded according to the criteria of Sullivan et al. (11).
Renal function, HTN and TMA assessment
Renal function at baseline and follow up was determined by calculating GFR using the Modified Diet in Renal Disease (MDRD) equation (12). Severity of CKD was graded as moderate (stage III) defined as GFR of <60ml/min/1.73m2 and severe (stage IV or worse) as GFR of <30ml/min/1.73m2 according to Kidney Disease Outcomes Improvement Initiative (13). Sustained CKD was a decline of GFR below 60 ml/min/1.73m2 at least 6 months after HSCT which persisted until last follow up or death. Patients with pretreatment GFR <60ml/min/1.73m2 were excluded from the study. AKI, which eventually resolved with a return to baseline kidney function, was not considered in the analysis.
New cases of HTN were defined in baseline normotensive individuals as the development of sustained BP greater than 140/90 and/or the need for treatment of HTN with medication. In patients with HTN at baseline, worsening HTN was defined as a sustained increase in BP over baseline levels requiring increased doses and/or addition of new classes of anti-HTN medications. Sustained increases were those that were either observed over at least 3 months or required initiation of anti-HTN therapy and were still present at last non-terminal followup.
TMA was diagnosed either by kidney biopsy or by the identification of a clinical syndrome that included some combination of the following criteria: concomitant development of renal dysfunction, either new or worsening HTN, evidence of MAHA (based on anemia, schistocytosis, elevated lactate dehydrogenase and low haptoglobin levels in the absence of disseminated intravascular coagulation), and thrombocytopenia. Other causes of renal disease were ruled out by appropriate laboratory and radiologic investigations.
Preparative regimens, donors and grafts
In group A the chemotherapy regimen consisted of busulphan 0.8 mg/kg/dose infused intravenously (IV) over 2 hrs, every 6 hrs for 10 doses at d -9 to -7, melphalan 70 mg/m2/d IV over 30 minutes daily d-7 and -6, and fludarabine on d -6 to -2 at 25 mg/m2/d IV infused over 30 minutes. Antithymocyte globulin (ATG), usually rabbit (2.5 mg/kg) and in a few cases equine (30 mg/kg), were administered on d -3 and -2 to all patients. In group B, the radiation containing regimen was comprised of hyperfractionated TBI (HFTBI) followed by thiotepa and fludarabine. HFTBI was administered in 11 fractions of 125 cGy over 4 d, to a total dose of 1375 cGy on d -9 to -6. All patients had protective lung shielding, and overlying ribs received an additional 600 cGy boost. The kidneys were not shielded. Male patients with acute leukemia received an additional 400 cGy testicular boost in a single fraction. After completion of HFTBI, thiotepa at 5 mg/kg/d was administered over 4 hours on d -5 and -4 with no adjustment for weight. Fludarabine at 25 mg/m2/d was administered over 30 minutes on d -5 through -1. Two doses of equine (30 mg/kg) or rabbit (2.5 mg/kg) ATG were administered on the same 2 days as the thiotepa only to patients receiving matched unrelated or any mismatched graft. The similarity in the two regimens was the use of equivalent doses of fludarabine in both. Differences were thiotepa in the TBI regimen and busulphan plus melphalan in the all chemotherapy regimen.
Donors included matched siblings or matched/mismatched unrelated donors recruited via the National Marrow Donor Program. Mobilization of peripheral blood stem cells (PBSC) from donors was performed by administration of granulocyte colony stimulating factor (G-CSF) followed by apheresis on the 5th and, if needed, the 6th days. Selection of CD34+ stem cells was accomplished using the ISOLEX 300i Magnetic Cell Separator, followed by sheep red blood cell (sRBC)-rosette depletion of T cells. This method achieved an approximate 5 log10 depletion of CD3+ cells (14). If bone marrow (BM) was used, based on donor preference, T cells were removed by sequential soybean agglutination and sRBC-rosette depletion (15). Fresh BM or PBSC grafts were infused through a central venous catheter 24–48 hours after completion of conditioning. TCD was the only GVHD prophylaxis; no other GVHD prophylaxis was given.
Supportive care
Patients were managed clinically according to MSKCC standard guidelines and as previously described (16). First line treatment for GVHD, when it occurred, was topical steroids (including budesonide) or systemic steroids.
Biostatistics
Continuous data were compared using a t-test or the Mann-Whitney test for non-normal data, while categorical variables were compared using a chi-squared test, and, where appropriate, Fisher’s Exact Test. Average GFR was compared between groups at each followup time using t-tests. Kaplan-Meier techniques were used to calculate survival probabilities for the development of CKD, and survival curves were compared between groups using a log-rank test. To examine the correlation between outcomes and potential predictors, univariable and multivariable analyses were done using Cox proportional hazards models for two CKD outcomes (sustained and severe) and logistic regression models for HTN. TBI treatment group and factors significantly associated with the outcomes in the univariable analysis were included in the multivariable models. Due to the low number of events for severe CKD, multivariable analysis was not performed for this outcome. The following parameters were considered: TBI treatment group, race, age at treatment, sex, AKI during the first month posttransplantation, development of GVHD, development of TMA, degree of HLA match, donor type, baseline HTN or diabetes, and disease. Event times for CKD were measured from date of transplantation to date of death or last contact. Statistical significance was defined by a p-value less than 0.05. All analyses were performed using SAS software version 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Thirty patients in group A and 70 patients in group B were included in the analysis. Baseline characteristics of patients in both groups are listed in Table 1. Patients in group A were significantly older (p<0.0001), and had a lower baseline GFR (p=0.0116). There were differences in the distribution of diagnoses between the two groups (p=0.004). Gender, length of follow up, pre-existing co-morbidities such as HTN, diabetes mellitus and childhood hemolytic uremic syndrome, mismatched donors and incidence of acute or chronic GVHD showed no statistically significant differences between the two groups (Table 1).
Table 1.
Patient Characteristics.
| Group A | Group B | p-value | |
|---|---|---|---|
| N = 30 | N = 70 | ||
| Median age | 55.5 | 45.0 | <0.0001 |
| Gender | NS† | ||
| Male | 15(50.0%) | 41(58.6%) | |
| Female | 15(50.0%) | 29(41.4%) | |
| Diagnosis | 0.004 | ||
| Acute myelogenous leukemia | 20(66.7%) | 33(47.1%) | |
| Acute lymphocytic leukemia | 1(3.3%) | 19(27.1%) | |
| Chronic myelogenous leukemia | 0(0.0%) | 7(10.0%) | |
| Non-Hodgkin's lymphoma | 4(13.3%) | 7(10.0%) | |
| Myelodysplastic syndrome | 5(16.7%) | 4(5.7%) | |
| Donor-Recipient Match | NS | ||
| Matched | 24(80.0%) | 60(85.7%) | |
| Mismatched | 6(20.0%) | 10(14.3%) | |
| Donor-Recipient Relationship | 0.01 | ||
| Related | 11(36.7%) | 45(64.3%) | |
| Unrelated | 19(63.3%) | 25(35.7%) | |
| Pre-treatment GFR*(mean) | 87.8 (SD‡=25.9) | 102.3 (SD=23.9) | 0.012 |
| Other Baseline Co-morbidities | |||
| Hypertension | 5(16.7%) | 7(10.0%) | NS |
| Diabetes | 0(0.0%) | 2(2.9%) | NS |
| Childhood hemolytic uremic syndrome | 0(0.0%) | 1(1.4%) | NS |
| Months Follow-up (mean) | 21 (SD=4.9) | 22 (SD=4.7) | NS |
| AKI** (first month post transplant) | 4(13.3%) | 21(30.0%) | NS |
| Alive at last follow up | 26(86.7%) | 56(80.0%) | NS |
| Acute GVHD*** | |||
| Grade 1 | 2(6.7%) | 4(5.7%) | 0.76943 |
| Grade 2 | 3(10.0%) | 4(5.7%) | |
| Grade 3 | 0(0.0%) | 2(%2.9) | |
| No Acute GVHD | 25(83.3%) | 60(85.7%) | |
| Chronic GVHD | |||
| Extensive | 1(%) | 3(%) | 0.23953 |
| Limited | 0(%) | 6(%) | |
| No Chronic GVHD | 29(%) | 61(%) |
Not Statistically Significant
Standard Deviation
Glomerular Filtration Rate
Acute Kidney Injury
Graft versus Host Disease
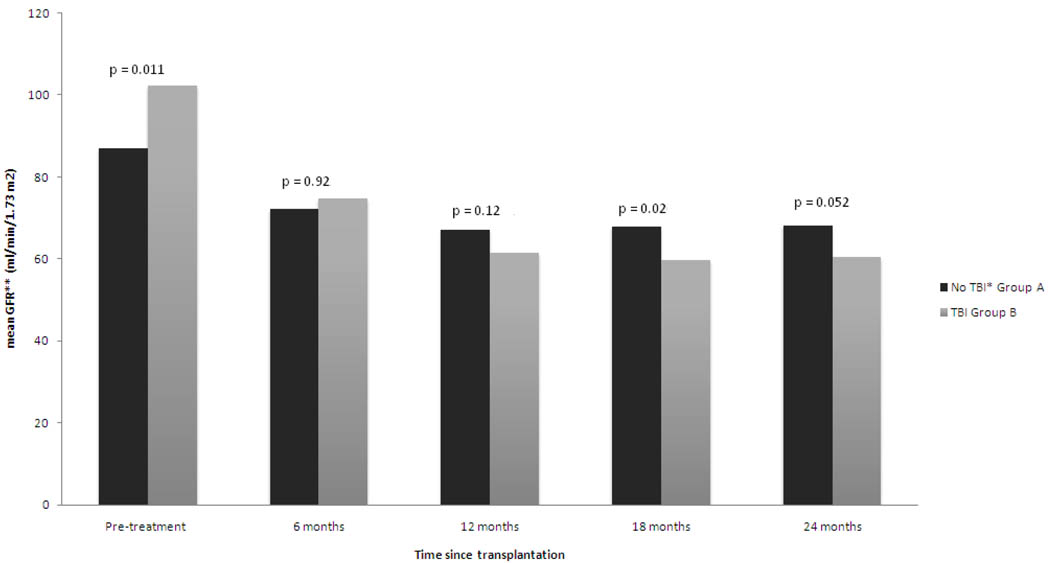
As illustrated in Figure 1, the mean GFR declined in both groups over the study period but remained at or above the 60 ml/min/1.73m2. Notably, however, despite a significantly higher mean GFR at baseline for group B, the mean GFR was lower at the 18 and 24 month follow up for group B (p=0.02 and p=0.052, respectively) compared with that for group A. The decline in mean GFR between baseline and last available follow up was also more significant in group B −42.0 ml/min/1.73m2 vs −20.7 ml/min/1.73m2 in group A (p=0.0002). The decline appeared to plateau between the 18 and 24 month points for both groups.
Figure 1.
Comparison of mean glomerular filtration rate between two groups during 2-yr follow up.
*-Total body irradiation
**-Glomerular filtration rate
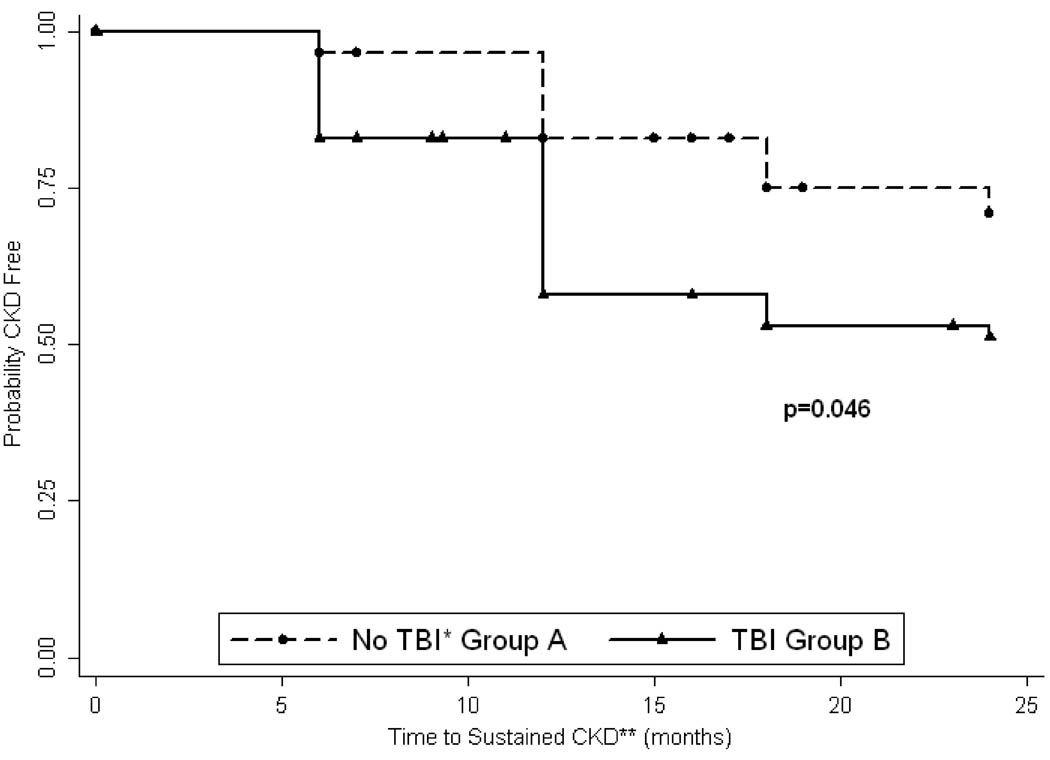
Sustained CKD developed in 8 patients in group A and 32 patients in group B, but this difference was not statistically significant. Likewise, the proportion of patients developing severe CKD was not statistically different between the two groups. On the other hand, new or worsening HTN was significantly higher in group B. Comparison of the time to development of sustained CKD between group A and B is illustrated by Kaplan-Meier survival curves and indicate significant difference in CKD-free survival between two groups (Figure 2). Of note and not shown, is the observation that several patients had GFR < 60ml/min/1.73m2 at some point during the 2 year period but recovered to normal renal function at last follow up, while no patient who progressed to severe CKD recovered.
Figure 2.
Kaplan-Meier estimate of sustained CKD free survival
* - Total body irradiation
** - Chronic kidney disease
Univariable analyses conducted to determine the effects of patient characteristics suggest that age (p=0.032) and TMA (p=0.0068) predicted development of sustained CKD. The only significant predictor of severe CKD was TMA (p<0.0001). Both treatment in the TBI group and development of TMA predicted development of HTN (p=0.0182 and p=0.0006, respectively).
The multivariable model predicting sustained CKD suggested that treatment with TBI (group B vs. A, p=0.0286)), age at transplant (p=0.001), and development of TMA (p=0.0012) were significantly associated with the outcome. The risk of developing sustained CKD is 2.71 (95% CI: 1.11, 6.60) times higher for the patients receiving TBI (group B) than the patients not receiving TBI (group A). The hazard ratio for age was 1.05 (95% CI: 1.02, 1.09) with a 1-year increase in a patient’s age raising his/her risk of developing CKD by 5%. The hazard ratio for TMA was 4.27 (95% CI: 1.78, 10.24), with the risk of developing CKD 4.3 times higher for patients with TMA than for patients without TMA. Donor status was considered as the fourth and final variable in the multivariable model but did not significantly predict the development of CKD.
The cumulative incidence probability at 2 years for development of sustained CKD was 29.2% (CI: 15.7, 50.2) for group A and 48.8% (CI: 37.4, 61.6) for group B. Similarly, the cumulative incidence probability of severe CKD was 6.5% (CI: 1.7, 23.5) for group A and 12.8% (CI: 6.6, 24.0) for group B.
TBI treatment group (group B) and development of TMA were included in the multivariable logistic regression model for the development of HTN. After adjusting for treatment group, patients with TMA were 9.0 times more likely to have HTN than patients without TMA (95% CI: 2.2, 39.84; p=0.0025).
Eleven patients developed TMA in group B while none were identified in group A (p=0.031). The clinical findings of TMA are summarized in Table 2. The mean time of onset of CKD and signs of hemolysis were 11 months post-transplantation with a range of 6 to 20 months. Seven of these patients developed new or worsening HTN and 3 developed edema. Renal dysfunction of varying degrees was present in all of these patients and 1 patient progressed to end stage renal disease (ESRD) requiring dialysis 30 months after HSCT. Patient 2 (Table 2) had a normal SCr 1.3 mg/dl (0.6–1.3 mg/dl) at the time of diagnosis but it was increased from the patient’s baseline of 0.8 mg/dl. Four patients underwent kidney biopsy to confirm the diagnosis of TMA. All biopsies showed glomerular capillary wall thickening and basement membrane double contours, as well as other features characteristic of TMA. Seven of the 11 patients with TMA survived and were evaluated at 24 months. The mean GFR in these patients was 41 ml/min/1.73 m2. Additionally, patient 11 (Table 2) was known to be alive at 24 months but did not have blood work evaluation recorded at our center beyond 18 months. At that time the GFR was 24 ml/min/1.732.
Table 2.
Clinical Features of Patients with Thrombotic Microangiopathy
| Pt1 No |
Time of onset (month) |
Haptoglobin | LDH2 | Schistocytes | Plt3 | Hgb4 | Creat5 | Proteinuria | Hematuria | Kidney Biopsy |
Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | <6 | 376 | no | 157 | 8.8 | 2.1 | yes | yes | yes | new HTN6 |
| 2 | 8 | <6 | 391 | no | 15 | 5.8 | 1.3 | yes | yes | (−)7 indirect Coombs | |
| 3 | 12 | 36 | 227 | no | 83 | 7.7 | 1.7 | yes | yes | yes | |
| 4 | 9 | not done | 309 | yes | 48 | 9.2 | 2.4 | yes | yes | new HTN | |
| 5 | 12 | 42 | 212 | no | 137 | 11.8 | 1.5 | yes | no | worsening HTN | |
| 6 | 14 | 62 | 268 | no | 223 | 9.4 | 2.6 | no | no | yes | ESRD8 |
| 7 | 12 | <6 | 303 | no | 113 | 10.8 | 2.4 | yes | no | new HTN | |
| 8 | 20 | 315 | 459 | not done | 126 | 9.7 | 2.3 | yes | no | new HTN | |
| 9 | 7 | <6 | 220 | no | 106 | 9.2 | 1.7 | yes | yes | new HTN; edema | |
| 10 | 10 | <6 | 501 | yes | 84 | 8.2 | 1.6 | yes | yes | edema | |
| 11 | 6 | <6 | 400 | yes | 72 | 10.6 | 1.5 | yes | yes | yes | edema; new HTN |
Patients;
Lactade Dehydrogenase (normal range: 60–200Units/L);
Platelets (normal range: 160–400K/mcL);
Hemoglobin (normal range: 13–17g/dl in males and 11.5–16g/d in females);
Creatinine (normal range: 0.6–1.3mg/dl);
Hypertension;
Negative;
End Stage Renal Disease.
DISCUSSION
The cumulative incidence in HSCT patients for development of moderate and severe CKD has been reported to be 12–29% and 3–3.6%, respectively, in retrospective cohort analyses (4–6). These rates are markedly higher than those observed in the general population of 4.3% and 0.2%, respectively (13). Furthermore, the incidence of ESRD was also reported to be 16 times higher than that in an age-matched population (17). Renal function also has been shown to decline significantly following HSCT, even if patients do not develop overt CKD. In that regard, Weiss et al. described a >25% decline in the renal function of 66% of patients who underwent a non-myeloablative HSCT which included low dose TBI and CNI prophylaxis for GVHD (18). A variety of risk factors have been associated with the renal complications of HSCT, including the use of CNI and TBI (4, 7). Our analysis of the two groups of patients who underwent TCD transplantation without the administration of CNI afforded the opportunity to assess the impact of TBI alone on renal function after HSCT.
In the present study, the renal function declined in both patient groups. The greater decline, however, occurred in the patients treated with TBI, group B. Interestingly, this group was comprised of younger patients, median age 45.0 yrs vs. 56.5 yrs (for group A), who had significantly higher baseline GFRs. Since both groups received similar dosing of fludarabine, and thiotepa is not considered a nephrotoxin, this finding supports an adverse effect of radiation therapy, even when the TBI is administered using hyperfractionation, albeit without kidney shielding. Our patients in group B all received a dose of 1375cGy which falls within the range previously associated with an increased risk of posttransplantation renal dysfunction. Miralbell et al. described a dose dependent increase in the incidence of renal dysfunction at 18 months post TCD transplantation, with a 52% incidence for patients who received 1350 cGy TBI (7). Although the patients in that study received short-term cyclosporine prophylaxis post-transplantation, their median age was only 32.5 yrs. The two-year cumulative incidence of sustained CKD of 48.8% (group B) versus 29.2% (group A) and of severe CKD of 12.8% (group B) and 6.5% (group A), confirms a greater nephrotoxic effect in the group that received TBI vs. the group that received only chemotherapy. Additionally, Kaplan-Meier analysis demonstrated a statistically significant difference in the development of sustained CKD between the two groups, and multivariable analysis showed that TBI is a risk factor for the development of CKD, after adjusting for age and TMA.
Despite the absence of CNI in the treatment regimens, both groups of patients in this analysis had a higher incidence of sustained and severe CKD than reported by a number of other investigations (4–6). This supports the contribution of other clinical factors. TCD is an especially attractive modality for older patients undergoing transplantation because of the reduced incidence of GVHD. Patients normally experience some decline in renal function with aging, which can be compounded by comorbidities and medications. Delgado et al. previously reported on 3 groups of patients receiving TBI and TCD grafts. Risk factors strongly predicting for CKD were age at transplantation, <30 yrs vs. ≥ 30 yrs (HR 6.58) and the presence of fludarabine in the conditioning regimen (HR 2.57). The median age of patients in the 3 groups were 27, 30, 38.5 yrs. Furthermore, a recent report showed that 69% of patients who developed CKD after HSCT were older then 40 years of age (5). Older age was confirmed to be a risk factor for the development of sustained CKD in this study as well. Patients in group A were significantly older than patients in group B and overall the median age in both groups (55.5 and 45.0 yrs) was significantly higher than that in previously reported cohorts receiving similar conditioning regimens. This may also explain why the incidence of sustained CKD was not statistically different in the 2 groups. After adjusting for treatment group, a 1-year increase in a patient’s age at the time of transplantation resulted in a 5% increased risk of CKD. This is contrasted to only a ~8% decline in GFR of healthy individuals with each decade of life beginning at age 30 (19).
AKI in the early post-transplantation period, female gender, GVHD and fludarabine have been reported as risk factors for CKD following HCST (4–6). The current study was unable to analyze fludarabine as a risk factor since patients in both cohorts received this agent as part of their conditioning. Therefore, a contribution of fludarabine to the higher incidence of CKD in both groups or an additive effect to that of the TBI cannot be excluded. Melphalan, used in the conditioning regimen of group A, has been reported to cause AKI in patients undergoing autologous PBSCT for treatment of systemic amyloidosis (20). Patients who developed AKI in that study were more likely to progress to ESRD. Notably, patients had baseline proteinuria in the nephrotic range and low baseline GFRs, indicating significant underlying renal pathology. It is therefore difficult to assess the true impact of the melphalan on the development of CKD. Although the current study cannot exclude a contribution of melphalan to the overall outcome in CKD for patients in group A, the incidence of AKI was low in that group and AKI was shown not to be a predictor of poor renal outcomes. In the univariable analysis neither gender nor AKI in the first month after transplantation were found to be significant risk factors for CKD. GVHD also did not predict development of CKD. This finding may be confounded, however, by the exclusion of patients with higher grade GVHD requiring treatment with CNI.
Thrombotic microangiopathy (TMA) has been recognized as one of the causes of CKD in HSCT patients. Histologic features of post-HSCT TMA in the kidneys have been well described (21). In a classic review by Pettitt and Clark, TMA following transplantation was divided into four distinct but overlapping subtypes based on association, clinical presentation and prognosis (22). The first type, called ‘multifactorial fulminant TMA’, occurs in the first 100 days in patients receiving CNI for active GVHD, patients developing CMV infection and following intensive pre-transplant conditioning. This diagnosis carries a poor prognosis. The second type, ‘conditioning related TMA’, includes patients who develop TMA later in the clinical course, around 6 months after transplantation. In most patients the conditioning regimen includes TBI. The remaining two subtypes are strongly associated with CNI use and manifest either as nephrotoxicity or neurotoxicity of these agents.
In this study, all cases of TMA occurred in patients in group B, supporting an association with the use of TBI. Time of presentation and clinical course of patients with TMA was consistent with the ‘conditioning related’ type. Interestingly, only 3 patients had schistocytes on peripheral smear, and haptoglobin was normal in 4 patients. Furthermore, LDH was only mildly elevated in this group with a mean value of 333 (60–200) U/L. Two patients with kidney biopsy findings of TMA had a normal haptoglobin and no schistocytes. This lack of some of the clinical criteria usually employed to make a diagnosis of TMA, especially in the absence of renal biopsies, could account for a lower incidence reported in some previous studies and a higher incidence in this study. This phenomenon of isolated renal involvement, however, has been described in both non-HSCT and HSCT patients with secondary TMA (23–25).
In recent years, two consensus statements have been published in an effort to define clinical features and diagnostic criteria of post-transplantation TMA. Unfortunately, even these two expert panels did not agree on all of the criteria. Presence of schistocytes on peripheral smear, elevated LDH level, concurrent renal and/or neurologic dysfunction and negative direct and indirect Coombs test have been selected as diagnostic criteria for TMA by the Blood and Marrow Transplant Clinical Trials Network Toxicity Committee (26). The International Working Group’s (IWG-an initiative of the European Group for Blood and Marrow Transplantation and the European LeukemiaNet) definition of transplant-associated microangiopathy (TAM) includes the presence of schistocytes, thrombocytopenia, increased LDH level, decrease in hemoglobin concentration or increased red blood cell transfusion requirement and decrease in serum haptoglobin (27). Interestingly, neither group addressed the Pettit et al categorization of time posttransplantation to development of TMA. Thus the criteria remain variable and pathologic findings on kidney biopsy may be present despite the absence of some of the clinical criteria. As observed in this study, only 1 of 4 patients, who had a positive kidney biopsy, satisfied all diagnostic criteria of the IWG consensus. Identifying other signs which are common in patients with TMA may assist in diagnosing this condition. The majority of patients with TMA in this study demonstrated proteinuria, hypertension and renal dysfunction. In a recent case cohort TMA syndrome was characterized by hypertension, renal dysfunction and anemia (28). Therefore new or worsening hypertension, edema, proteinuria and renal dysfunction may characterize patients developing late TMA even if the hematologic findings of MAHA are absent.
The importance of recognizing TMA as a late complication of HCST was underscored by the fact that TMA was the only risk factor identified in univariable analysis to be associated with development of severe CKD. It was also identified as a risk factor for development of new or worsening HTN in multivariable analysis. Since HTN and CKD are significant co-morbidities as well as risk factors for heart disease, even a more indolent form of TMA may impact on long term mortality of patients undergoing HSCT.
HTN has been reported as a complication of HSCT and has been associated with the development of CKD (5). In our study, HTN was strongly associated with exposure to TBI and development of TMA, and not to other potential risk factors that were evaluated.
Finally, of concern is the higher overall incidence of sustained and severe CKD observed in this study compared to previous reports. We propose a few potential explanations. First, the population of patients in both groups was older in this study than in the previous reports. We and others have confirmed the impact of age at transplantation on the incidence of CKD and the accelerated deterioration above that reported for the natural aging process. Second, criteria for diagnosing CKD have been variable across many published reports. Some studies have utilized an increase in SCr above normal for the diagnosis of CKD. This can lead to an underestimation of the diagnosis in patients with reduced muscle mass such as older individuals and those debilitated by previous chemotherapy. Third, a medication or treatment common to both groups, such as fludarabine, antithymocyte globulin or one of the prophylactic medications routinely used could have contributed to the age and TBI components.
Recently available data also supports our findings of high incidence of CKD after HSCT. In a cohort of 100 patients who underwent myeloablative allogeneic HSCT and survived for >100 days the incidence of CKD, defined as GFR <60 ml/min/1.73 m2 lasting for >3 months, was 64%. The risks for the development of CKD in this group were ARF within 100 days after HSCT and age >40 years (29).
Regardless of the specific etiologies, however, this study reflects the transplantation community’s continued interest in addressing the growing population of over 40 year olds who are undergoing HSCT and developing new medical and psychosocial issues. Myelodysplastic syndrome/secondary AML and non-Hodgkins lymphoma are diseases of older patients. Physicians have a better understanding of the lack of curability of patients with these diseases with chemotherapy alone and are now expanding the application of transplantation and referring more patients with these diagnoses. In most of the patients in this study with sustained CKD there was no impact on quality of life related to the condition. It remains to be seen whether CKD becomes a greater issue in healthcare as these patients age, acquire additional comorbidities that impact on renal function, or require treatments that can negatively impact renal function. For the present these findings support the development of clinical trials to proactively reduce renal damage in HSCT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: The authors have nothing to disclose.
REFERENCES
- 1.Progress Report January–December 2008. Center for International Blood and Marrow Transplant Research. 2008
- 2.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 3.Ferry C, Gemayel G, Rocha V, et al. Long-term outcomes after allogeneic stem cell transplantation for children with hematological malignancies. Bone Marrow Transplant. 2007;40:219–224. doi: 10.1038/sj.bmt.1705710. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hazzouri A, Cao Q, Burns LJ, Weisdorf DJ, Majhail NS. Similar risks for chronic kidney disease in long-term survivors of myeloablative and reduced-intensity allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:658–663. doi: 10.1016/j.bbmt.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Kersting S, Hene RJ, Koomans HA, Verdonck LF. Chronic kidney disease after myeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:1169–1175. doi: 10.1016/j.bbmt.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Delgado J, Cooper N, Thomson K, et al. The importance of age, fludarabine, and total body irradiation in the incidence and severity of chronic renal failure after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:75–83. doi: 10.1016/j.bbmt.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 7.Miralbell R, Bieri S, Mermillod B, et al. Renal toxicity after allogeneic bone marrow transplantation: the combined effects of total-body irradiation and graft-versus-host disease. J Clin Oncol. 1996;14:579–585. doi: 10.1200/JCO.1996.14.2.579. [DOI] [PubMed] [Google Scholar]
- 8.George JN, Li X, McMinn JR, Terrell DR, Vesely SK, Selby GB. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome following allogeneic HPC transplantation: a diagnostic dilemma. Transfusion. 2004;44:294–304. doi: 10.1111/j.1537-2995.2004.00700.x. [DOI] [PubMed] [Google Scholar]
- 9.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 11.Sullivan KM, Shulman HM, Storb R, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–276. [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 13.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 14.Collins NH, Fernandez J, Bleau S, et al. Comparison of bone marrow and G-CSF mobilized peripheral blood progenitor cells from single normal donors before and after T cell depletion. Cytotherapy. 1999;1:223. [Google Scholar]
- 15.Collins NH, Bleau SA, Kernan NA, O'Reilly RJ. T Cell Depletion of Bone Marrow by Treatment with Soybean Agglutinin and Sheep Red Blood Cell Rosetting. In: Areman E, Deeg HJ, Sacher RA, editors. Bone Marrow and Stem Cell Processing: Manual of Current Techniques. Philadelphia, PA: Davis; 1992. p. 171. [Google Scholar]
- 16.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen EP, Drobyski WR, Moulder JE. Significant increase in end-stage renal disease after hematopoietic stem cell transplantation. Bone Marrow Transplantation. 2007;39:571–572. doi: 10.1038/sj.bmt.1705643. [DOI] [PubMed] [Google Scholar]
- 18.Weiss AS, Sandmaier BM, Storer B, Storb R, McSweeney PA, Parikh CR. Chronic kidney disease following non-myeloablative hematopoietic cell transplantation. Am J Transplant. 2006;6:89–94. doi: 10.1111/j.1600-6143.2005.01131.x. [DOI] [PubMed] [Google Scholar]
- 19.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 20.Leung N, Slezak JM, Bergstralh EJ, et al. Acute renal insufficiency after high-dose melphalan in patients with primary systemic amyloidosis during stem cell transplantation. American Journal of Kidney Diseases. 2005;45:102–111. doi: 10.1053/j.ajkd.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Siami K, Kojouri K, Swisher KK, Selby GB, George JN, Laszik ZG. Thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation: an autopsy study. Transplantation. 2008;85:22–28. doi: 10.1097/01.tp.0000297998.33418.7e. [DOI] [PubMed] [Google Scholar]
- 22.Pettitt AR, Clark RE. Thrombotic microangiopathy following bone marrow transplantation. Bone Marrow Transplant. 1994;14:495–504. [PubMed] [Google Scholar]
- 23.Schwimmer J, Nadasdy TA, Spitalnik PF, Kaplan KL, Zand MS. De novo thrombotic microangiopathy in renal transplant recipients: a comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis. 2003;41:471–479. doi: 10.1053/ajkd.2003.50058. [DOI] [PubMed] [Google Scholar]
- 24.Raife TJ, Lager DJ. Chronic thrombotic microangiopathy associated with antineoplastic therapy with minimal hematologic effects. Mayo Clin Proc. 2002;77:323–328. doi: 10.4065/77.4.323. [DOI] [PubMed] [Google Scholar]
- 25.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:571–575. doi: 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Ruutu T, Barosi G, Benjamin RJ, et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92:95–100. doi: 10.3324/haematol.10699. [DOI] [PubMed] [Google Scholar]
- 28.Kersting S, Verdonck LF. Stem cell transplantation nephropathy: a report of six cases. Biology of Blood & Marrow Transplantation. 2007;13:638–643. doi: 10.1016/j.bbmt.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Cohen EP, Sumaili EK, Krzesinski JM, Delanaye P, Cavalier E, Beguin Y. Chronic Kidney Disease after Hematopoietic Stem Cell Transplantion: Incindence, Risk Factors and Survival. J Am Soc Nephrol. 2009;20:244A. Abstract Issue. [Google Scholar]